Abstract
Context:
The relationship between generalized joint laxity and plantar pressure distribution of the foot and the potential implications for lower extremity injury have not been studied.
Objective:
To determine the relationship between generalized joint laxity and dynamic plantar pressure distribution. We hypothesized that individuals with greater generalized joint laxity, or hypermobility, would have greater dynamic medial midfoot pressure and loading during walking than nonhypermobile individuals.
Design:
Case control.
Setting:
Institutional biomechanics laboratory.
Patients or Other Participants:
Participants included 112 female soccer players between 11 and 21 years of age.
Main Outcome Measure(s):
Each participant was tested for generalized joint laxity using the Beighton and Horan Joint Mobility Index (BHJMI; range, 0–9) and was categorized as having either high (BHJMI score ≥4) or low (BHJMI score <4) generalized joint laxity. Peak pressure and maximum force were calculated from a dynamic, barefoot plantar pressure distribution system.
Results:
Peak pressure and maximum force were greater in the 27 participants categorized as having high generalized joint laxity than in the 85 participants categorized as having low generalized joint laxity. The midfoot region exhibited greater loading in participants with high generalized joint laxity than in the other participants. We found an effect of BHJMI classification in the medial midfoot; peak pressure in the dominant (F1,109 = 11.262, P = .001) and nondominant (F1,109 = 14.32, P < .001) sides and maximum force in the dominant (F1,109 = 7.88, P = .006) and nondominant (F1,109 = 9.18, P = .003) sides were greater in the high generalized joint laxity group than in the low generalized joint laxity group.
Conclusions:
Athletes classified as having high generalized joint laxity demonstrated increased midfoot loading. Delineation of risk factors for medial collapse of the foot, which include hypermobility in athletes, may help clinicians evaluate and prevent lower extremity injury with treatments, such as orthoses.
Keywords: hypermobility, foot pressure, maximum force, foot mechanics, patellofemoral pain
Key Points
Dynamic peak pressure and maximum force in the medial midfoot were greater in athletes classified as having high generalized joint laxity than in athletes classified as having low generalized joint laxity.
An athlete's score on the Beighton and Horan Joint Mobility Index may be a valuable indicator of her potential risk for lower extremity injury.
Our findings of increased medial pressure may be indicative of medial forefoot region collapse in the athlete with high generalized joint laxity, which has implications for the development and prevention of lower extremity injury.
The increased relative load of the midfoot region could contribute to increased acute or chronic injury to the lower extremity.
When evaluating a lower extremity injury in an athlete with high generalized joint laxity, the athletic trainer not only should treat that injury, but he or she also should be aware of mechanical and force alterations and should make necessary corrections to prevent future injuries.
Joint hypermobility in athletes may be an underlying factor contributing to lower extremity joint injuries and disorders. A joint is typically classified as hypermobile if its range of motion exceeds normal limits, taking into account age, sex, and ethnicity.1 First described by Kirk et al,2 hypermobility syndrome has evolved into benign joint hypermobility syndrome and has incorporated the Beighton and Horan Joint Mobility Index (BHJMI) scoring system that takes into account symptoms and extra-articular features.3 Joint hypermobility occurs most often in children and diminishes with age.1 Joint mobility is highest at birth, decreases in children around 9 to 12 years of age, peaks again in adolescent girls around the age of 15 years, and decreases in both sexes for the remainder of the lifespan.4 Researchers have suggested that the peak at 15 years in adolescent girls is due to hormonal changes that occur in puberty, such as the release of the hormone relaxin.4 Between 5% and 12% of individuals have some form of joint hypermobility,1,4 and it is 3 times more common in females than in males.5
Individuals with joint hypermobility may avoid strenuous physical activity because of a possible increased risk of injury.6 However, joint hypermobility can have a performance advantage in some sports, such as gymnastics and ballet, in which excessive range of motion may be desirable.7 Athletes with joint hypermobility often present with increased incidence of arthralgias, soft tissue lesions, recurrent joint dislocations or subluxations, sprains, and osteoarthritis.1,8 A higher incidence of joint sprain also is observed in individuals with joint hypermobility.1 They may have impaired proprioception, most notably of the finger and knee joints.8 This impaired proprioception may play a role in increased risk of lower extremity joint injury and disease.
The foot is the first link in the kinetic chain and can provide a first response to the imposed demands of lower extremity loads during dynamic tasks. Anatomic or mechanical impairment can affect the proximal links of the kinetic chain. The foot and ankle play important roles in the distribution of forces from heel strike through toe off of gait. The normal progression of peak foot pressure during gait is from heel to lateral midfoot, to lateral forefoot, and to medial forefoot.9 In the normal rear-strike loading pattern, a peak in pressure occurs at heel strike, and a second peak occurs at toe off at the end of the stance phase.10 The lowest pressure occurs in the midfoot, with less pressure in the medial than in the lateral midfoot.10 Deviations in this progression may impair the ability of the entire lower extremity to attenuate force and can result in repetitive stress injury.
During gait, the largest area of foot-ground contact occurs at the medial forefoot, which is followed by the lateral midfoot and the hindfoot.9 Area contact time generally is defined as the duration of contact in each foot region expressed as a percentage of the entire stance phase of gait. Typically, the heel and great toe are each in contact with the ground for approximately 54% to 64% of the stance phase, whereas the medial forefoot may hold contact for as much as 84% of the stance phase.9 Rear-strike runners tend to land in approximately 5° of inversion of the calcaneus, traveling into 10° of eversion by midstance.11 On the other hand, forefoot strikers land in a greater degree of inversion, prompting increased eversion excursion.11 In a forefoot-strike pattern, contact time and acceleration time are both shorter (12% and 25%, respectively) than in other strike patterns.12
The complex natures of the ligamentous, tendinous, and muscular articulations of the foot and ankle are important joint components that may be factors in lower extremity injury. Ankle sprain is the most common athletic injury, accounting for 15% to 20% of all sports injuries.13 In soccer and basketball, ankle sprains represent 45% and 31%, respectively, of all injuries.13 Athletes with hypermobile joints have a greater risk of ankle injury compared with athletes with normal joint mobility (26.1% and 9%, respectively).6 Individuals with joint hypermobility have a 93% incidence of ankle joint hypermobility and are susceptible to pes planus deformity.14 In dynamic situations (when the body is moving), the incidence of lower extremity stress fractures in individuals with pes planus deformity is nearly twice as high as in individuals with average arch height, and it is 20% greater than in individuals with pes cavus foot structure.15 Sullivan et al16 also reported that pes planus may be a predisposing condition for the development of stress fractures. Metatarsal stress fractures, which originally most commonly occurred in military service members and were nicknamed “march fractures,” have increased in prevalence among athletes, most notably long-distance runners, dancers, and jumping-sport athletes.17 In addition to anatomic and biomechanical risk factors for the development of stress fractures, sex may play a role. Female athletes are at greater risk than their male counterparts for developing stress fractures.18
Given the complex nature of hypermobility and the prevalence of lower extremity injury in athletes, the purpose of our study was to determine the relationship between generalized joint laxity and dynamic plantar pressure distribution. We hypothesized that, during walking, medial midfoot pressure and loading would be higher in the participants with hypermobile joints than in participants with normal joint mobility.
METHODS
Participants
Participants included 112 female soccer players between the ages of 11 and 21 years (age = 16.2 ± 2.8 years, height = 161.6 ± 6.6 cm, mass = 58.7 ± 10.0 kg). Athletes were included in the study if they were female and participated with an organized soccer team. Athletes were excluded if they had sustained a lower extremity injury within the year before the study. Each participant (if older than 17 years of age) or her guardian provided written informed consent, and the study was approved by the Cincinnati Children's Hospital Institutional Review Board.
Instrumentation
Dynamic plantar pressure distribution was obtained as each participant walked barefoot at a self-selected pace over a 6-m walkway. An emed-x system (Novel, Munich, Germany) was mounted in the middle of the walkway and level with the surface. The platform consisted of a 48 × 32-cm2 matrix of capacitive sensors (4 sensors/cm2), which collected data at 100 Hz.
To measure joint laxity, we used a standard goniometer (model 7514; Sammons Preston Rolyan, Bolingbrook, IL).
Procedures
Data were collected before the start of the athletes' competitive soccer seasons. Each participant was tested by the same certified athletic trainer (AT) (Mark V. Paterno), who demonstrated an intraclass correlation coefficient of 0.972 for intrarater reliability using the Beighton and Horan Joint Mobility Index (range, 0–9) to measure generalized joint laxity. Generalized joint laxity tests consisted of fifth-finger hyperextension greater than 90°, elbow hyperextension greater than 10°, thumb-to-forearm opposition, knee hyperextension greater than 10°, and palms to floor with knees fully extended (Figure 1). All tests were performed bilaterally except for trunk flexion, with goniometric measurements following placement guidelines described by Norkin and White19 and procedures described by Boyle et al.20 A 1-point value was given each time an athlete surpassed the designated laxity measure at each joint. An injury allowance point was given for a joint that did not surpass the designated laxity measure if the participant had a history of mobility-limiting injury to that joint (eg, elbow fracture) and if the participant displayed a positive test on the corresponding contralateral joint.21 Points were summed to give an overall BHJMI score. Participant composite scores were operationally categorized into 2 groups: low (range, 0–3; n = 85) and high (range, 4–9; n = 27) generalized joint laxity (Table 1). The high category represented the participants with greatest joint mobility.20
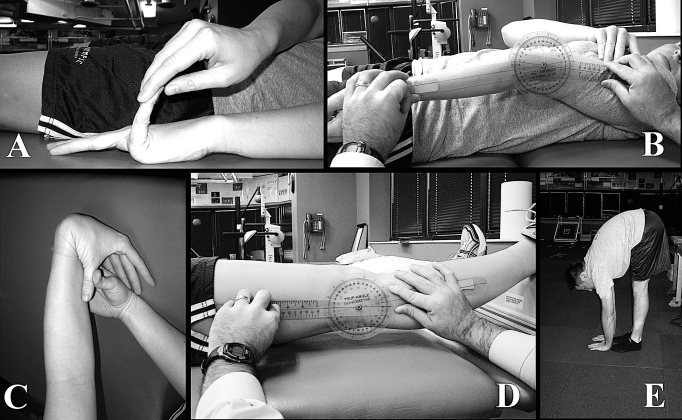
Figure 1. Tests to measure generalized joint laxity. A, Fifth-finger hyperextension test. Each participant's forearm, wrist, and fingers were stabilized on a flat table. The tester passively extended the participant's fifth finger as far as possible without pain. Hyperextension of 90° or greater resulted in a score of 1. Hyperextension of less than 90° resulted in a score of 0. B, Elbow hyperextension test. Each participant's shoulder was positioned with the forearm supinated. The axis of the goniometer was placed over the lateral epicondyle of the humerus, with the distal end aligned with the radial styloid process and the proximal arm aligned along the lateral midline of the participant's humerus. If elbow hyperextension was 10° or greater, a score of 1 was given. Hyperextension of less than 10° resulted in a score of 0. C, Thumb opposition test. Each participant flexed the wrist and pulled the thumb toward the forearm using the opposite hand. If the thumb could be abducted to touch the forearm, a score of 1 was given. Inability to touch the forearm resulted in a score of 0. D, Knee hyperextension test. Each participant was placed in a supine position with a box placed under both ankles. The axis of the goniometer was aligned with the lateral epicondyle of the femur. The distal arm was positioned with the lateral malleolus, and the proximal arm was aligned with the greater trochanter. Hyperextension of the knee to 10° or greater resulted in a score of 1; anything less than 10° resulted in a score of 0. E, Palms to floor test. Each participant was instructed to keep both knees extended and attempt to touch the floor with the palms flat to the floor. The ability to touch both palms flat on the floor resulted in a score of 1. If the participant was unable to place both palms flat on the floor, a score of 0 was given. Reprinted from Journal of Science and Medicine in Sport, 11(3), Quatman CE, Ford KR, Myer GD, Paterno MV, Hewett TE, The effects of gender and pubertal status on generalized joint laxity in young athletes, 257–263, 2008, with permission from Elsevier. http://www.sciencedirect.com/science/journal/14402440.
Table 1.
Participant Demographics (Mean ± SD)
Participants were instructed to walk normally at a self-selected speed22 over the walkway until 5 successful trials on each side (dominant, nondominant) were collected. A trial was accepted if the entire foot was within the sensor area. The walking speed was not quantified in this study. However, we calculated the related variable (stance time) for each group. Dominant side was operationally defined as the limb the athlete used to kick the soccer ball as hard as she could. No group differences existed in dominant-side (F1,110 = 0.96, P = .33) or nondominant-side (F1,110 = 0.614, P = .44) stance time. For the dominant side, stance times were 0.66 ± 0.06 seconds for the low generalized joint laxity group and 0.65 ± 0.05 seconds for the high generalized joint laxity group. For the nondominant side, the stance times were 0.66 ± 0.06 seconds for the low generalized joint laxity group and 0.65 ± 0.05 seconds for the high generalized joint laxity group. Participants performed the test barefoot to control for any discrepancies due to self-selected footwear. Fatigue was not considered to be a confounding factor during this test.
Data Reduction
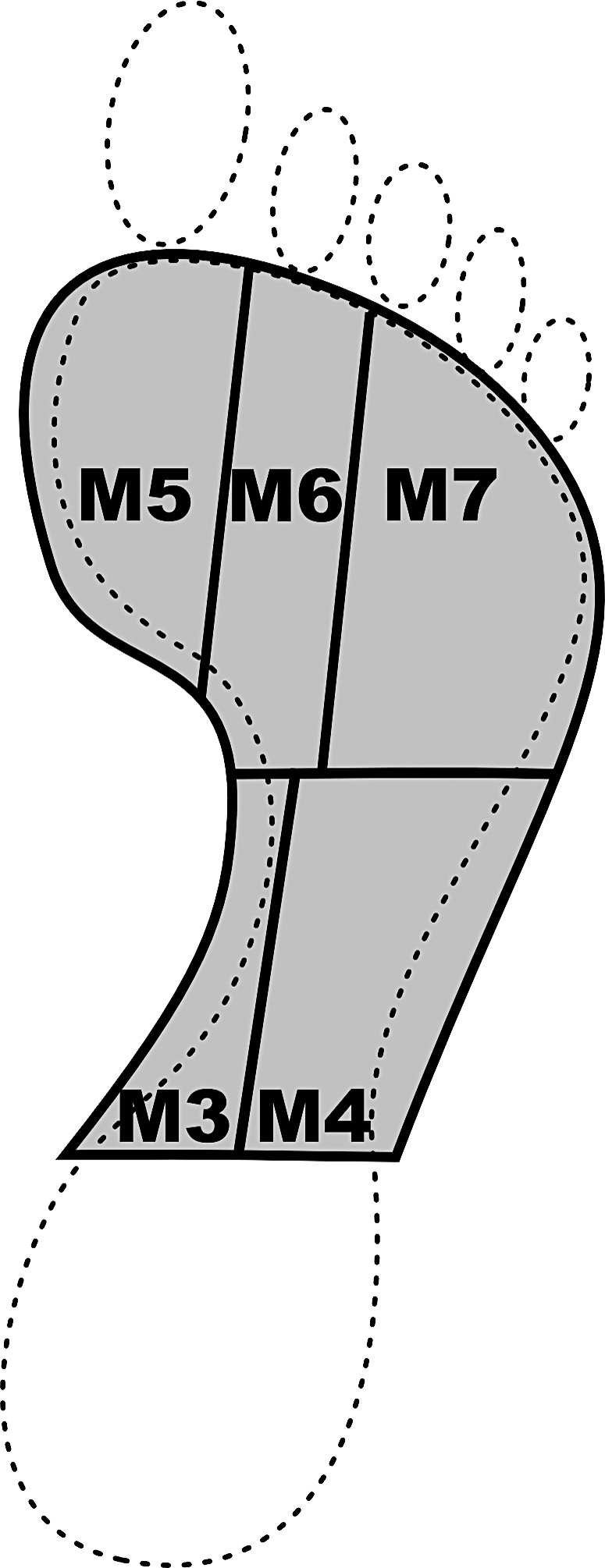
Pressure distribution trials were analyzed within a commercial software package (Projects; Novel). The emed-x system calculates the pressure in each calibrated sensor. Pressure is equal to force per unit area. Therefore, when the region of interest is determined, the total area and pressure are known, and total force within the region can be calculated. From each walking trial, the foot was subdivided into 10 anatomic regions as described by Cavanagh et al.23 These regions included the medial and lateral heel, medial and lateral midfoot, forefoot (first, second, and third through fifth metatarsal heads), hallux, second toe, and lateral (third through fifth) toes. For these analyses, we focused on the 5 regions of the midfoot and forefoot, which were designated M3 through M7 (Figure 2). The dynamic peak pressure and maximum force within the regions were calculated.
Figure 2. Analysis of the foot subdivided into 5 regions based on the description by Cavanagh et al.23 Regions include the medial midfoot (M3), lateral midfoot (M4), first metatarsal head (M5), second metatarsal head (M6), and lateral (third through fifth) metatarsal heads (M7).
Statistical Analyses
Means and SDs of the peak pressure and maximum force were calculated from each foot and were classified as either dominant or nondominant. A 1-way analysis of covariance was performed with the independent variable of BHJMI classification (high and low groups) and covariate of participant mass. The dependent variables were peak pressure and maximum force in each region. Bonferroni corrections for the different regions of the foot used in the analyses provided an adjusted α level of .01.
RESULTS
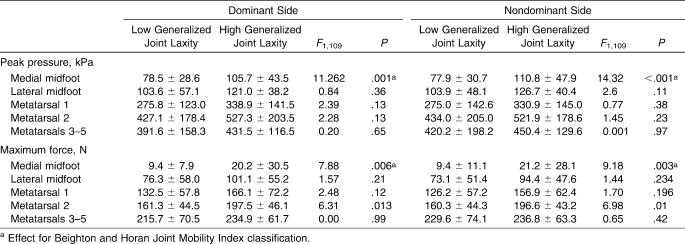
Differences in dynamic peak pressure were observed between high generalized joint laxity and low generalized joint laxity groups (Table 2). An effect of BHJMI classification was found in the medial midfoot. The high generalized joint laxity group displayed greater dynamic peak pressure on both the dominant (F1,109 = 11.262, P = .001) and nondominant (F1,109 = 14.32, P < .001) sides compared with the low generalized joint laxity group (Figure 3).
Table 2.
Peak Pressure and Maximum Force Within Each Region for the Dominant and Nondominant Sides in Athletes With Low Generalized Joint Laxity and Athletes With High Generalized Joint Laxity (Mean ± SD)
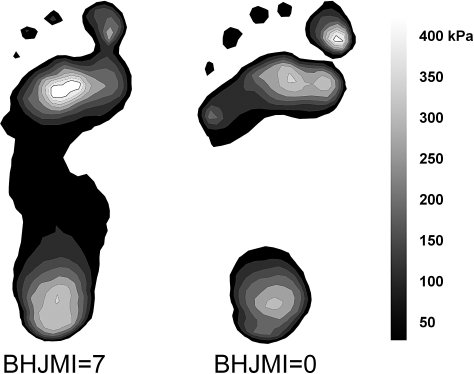
Figure 3. Representative trial of a participant with a Beighton and Horan Joint Mobility Index (BHJMI) score of 7 compared with a trial from a participant with a score of 0.
Differences in maximum force also were observed between high and low generalized joint laxity groups (Table 2). Compared with the other group, the high generalized joint laxity group displayed greater maximum force in the medial midfoot of both the dominant (F1,109 = 7.88, P = .006) and nondominant (F1,109 = 9.18, P = .003) sides. The high generalized joint laxity group also displayed greater maximum force in the second metatarsal region of both the dominant (F1,109 = 6.31, P = .013) and nondominant (F1,109 = 6.98, P = .01) sides compared with the other group. No other differences were observed within the other foot regions.
DISCUSSION
In our study, peak pressure and maximum force were higher in athletes with high generalized joint laxity than in athletes with low generalized joint laxity. Mechanically, this medial collapse of the midfoot combined with greater force production may result in alteration of functional biomechanics of the metatarsals during the pronation phase of gait, further promoting dissipation of energy at the forefoot.24 Ferrari and Watkinson24 found that this was more pronounced in girls than in boys. Medial collapse of the midfoot can alter the biomechanics of the foot during normal gait. Lees et al25 found that greater peak pressure and force could contribute to greater injury risk, because participants with a higher arch index related to medial collapse of the forefoot were more prone to stress fractures in the metatarsal bones.
Medial collapse can result in increased incidence of stress fractures in the metatarsal bones, as well as problems with force distribution and dissipation when measured dynamically in individuals with pes planus.15 Clinically, the athlete with joint hypermobility may benefit from the use of orthoses as a corrective measure for pes planus to offset this medial collapse. Hockenbury17 showed that the use of orthoses decreased the incidence of metatarsal stress fractures in military recruits with pes planus. Brophy et al26 drew a similar conclusion after examining 44 National Football League athletes and finding a correlation between increased medial forefoot pressure and the development of turf toe.
During gait, typical foot pressure progresses from the heel to the lateral midfoot, to the lateral forefoot, and to the medial forefoot.9 We found that peak pressure in the medial midfoot was higher in athletes with high generalized joint laxity than in athletes with low generalized joint laxity. Therefore, medial foot collapse during gait is more prevalent in athletes with high generalized joint laxity than in athletes with low generalized joint laxity. Ferrari and Watkinson24 documented similar findings, noting that the center-of-pressure trajectory was more medial in girls than in boys, who demonstrated a centrally placed center-of-pressure trajectory. They found that, because of this medial trajectory, the first metatarsal head was loaded more in girls than in boys.24 Clinically, this may help explain why females are more susceptible than males to hallux valgus disorders.24 This medial collapse of the midfoot region and resultant pes planus with increased loading at the first metatarsal head also may alter the mechanical advantage of the peroneus longus muscle, leading to decreased eversion and plantar flexion.27
Our results also showed that maximum force under the second metatarsal region was higher in the high generalized joint laxity group than in the low generalized joint laxity group. This trend was evident in both the dominant and nondominant sides. Thijs et al28 examined 102 female runners and the correlation between patellofemoral pain and altered foot mechanics, and they found that runners who developed patellofemoral pain had a predisposing factor of a higher peak force under the second metatarsal when compared with other runners. We found that athletes with high generalized joint laxity also showed this increased pressure under the second metatarsal. Further research is warranted to investigate a link between high generalized joint laxity and the predisposition to patellofemoral pain.
We observed participants during walking. In comparison, Eils et al29 examined plantar pressure distribution patterns in men performing soccer-specific movements. They reported29 that during cutting maneuvers versus straight running, pressure load shifted to the heel, medial midfoot, medial forefoot, and hallux. During kicking versus running, the load was shifted to the lateral heel and lateral midfoot.29 Further investigation is under way to determine whether increased medial loading occurs during running, cutting, and kicking in female soccer players with joint hypermobility.
The high generalized joint laxity score may be a valuable indicator of potential injury risk in athletes.30 For example, Decoster et al6 found that the rate of ankle injuries was 26% higher in athletes with generalized joint hypermobility than in athletes with normal joint mobility. The demands placed on the joints of an athlete with joint hypermobility are important to consider prospectively, because the joints generally may tolerate repetitive activity better than tasks requiring stabilization.6 Athletes with joint hypermobility also may develop compensatory mechanisms, such as premature recruitment of hip muscles, when faced with ankle perturbation.31 These athletes, particularly females, are at greater risk than other athletes for developing hallux valgus deformity, because correlation between hypermobility and increased first metatarsal-phalangeal angles has been found.14
Clinically, our findings indicate that increased medial pressure may have led to collapse of the medial forefoot in athletes with high generalized joint laxity. This medial collapse may have important implications in the development and prevention of lower extremity injury. Medial forefoot collapse may increase the athlete's risk for developing conditions such as medial tibial stress syndrome, because the forces at the foot may not be transferred proximally in an optimal fashion. It also could lead to ankle instability, increased incidence of ankle sprain,6 and muscular problems due to the decreased mechanical advantage of the peroneus longus muscle. In addition, increased stress may move up the kinetic chain and may initiate patellofemoral pain, stress fracture, or medial tibial stress syndrome because of differences in force distribution or mechanical and anatomic changes resulting from the medial collapse.15
Primary goals of the AT should be to prevent injury and to minimize the development of secondary problems related to injury or impairment. Because pes planus and a medially displaced center-of-pressure trajectory can increase the risk of lower extremity injury, the athlete should be evaluated for joint hypermobility; conversely, an athlete who has joint hypermobility should be tested for signs of medial foot collapse. A relatively easy way to accomplish this is through footprint analysis. If the athlete demonstrates medial foot collapse, appropriate measures can be taken to correct these gait mechanics to prevent secondary injury. A primary example of external modification would be fitting the athlete with orthoses. Fitting an athlete who has joint hypermobility with rigid or semirigid orthoses to help decrease this medial collapse may potentially prevent the development of secondary lower extremity injury. Athletic trainers should examine a possible underlying mechanical cause of an injury when treating the injury itself.
The main limitation to our study is the reliability of measures for generalized joint laxity. Questions concerning the reliability of these measures are evident in conflicting results in numerous studies.4,21,32 We attempted to minimize this limitation by using 1 AT with many years of experience with the BHJMI to screen hundreds of athletes with high intrarater reliability. Despite this limitation, we found a relationship between generalized joint laxity and increased peak pressure and maximum force in the medial aspect of the forefoot.
CONCLUSIONS
Compared with athletes who have low BHJMI scores, athletes with high BHJMI scores also may have increased midfoot peak pressure and maximum force. The increased relative load of the midfoot region potentially could contribute to increased acute or chronic injury to the lower extremities. When evaluating a lower extremity injury in an athlete deemed to have joint hypermobility, the AT not only should treat that injury, but he or she also should be aware of mechanical and force alterations and should make necessary corrections to prevent future injuries. Further study should be dedicated to comparing plantar pressure distribution and joint hypermobility between sexes to provide additional insight into sex differences and how they may relate to the development of structural foot deformities and lower extremity injuries.
Acknowledgments
Funding for this study was provided by National Institutes of Health grant R01-AR049735. We thank Tiffany Evans for her assistance with preparation of the article. We also thank our journal club, including Jon Divine, Josh Takagishi, Jensen Brent, Rachael Heyl, Mark Paterno, Adrick Harrison, and Chad Cherny, for the stimulating discussion related to this study. We also thank Novel for loaning the pressure platform for this study.
Footnotes
Kim D. Barber Foss, MS, LAT, ATC, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. Kevin R. Ford, PhD, and Gregory D. Myer, MS, CSCS, contributed to conception and design; acquisition and analysis and interpretation of the data; and critical revision and final approval of the article. Timothy E. Hewett, PhD, contributed to conception and design; analysis and interpretation of the data; and critical revision and final approval of the article.
REFERENCES
- 1.Seçkin U, Tur B.S, Yilmaz O, Yağci I, Bodur H, Arasil T. The prevalence of joint hypermobility among high school students. Rheumatol Int. 2005;25(4):260–263. doi: 10.1007/s00296-003-0434-9. [DOI] [PubMed] [Google Scholar]
- 2.Kirk J.A, Ansell B.M, Bywaters E.G. The hypermobility syndrome: musculoskeletal complaints associated with generalized joint hypermobility. Ann Rheum Dis. 1967;26(5):419–425. doi: 10.1136/ard.26.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grahame R, Bird H.A, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS) J Rheumatol. 2000;27(7):1777–1779. [PubMed] [Google Scholar]
- 4.Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202–1206. doi: 10.1080/08035250410023971. [DOI] [PubMed] [Google Scholar]
- 5.Hakim A, Grahame R. Joint hypermobility. Best Pract Res Clin Rheumatol. 2003;17(6):989–1004. doi: 10.1016/j.berh.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Decoster L.C, Bernier J.N, Lindsay R.H, Vailas J.C. Generalized joint hypermobility and its relationship to injury patterns among NCAA lacrosse players. J Athl Train. 1999;34(2):99–105. [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack M, Briggs J, Hakim A, Grahame R. Joint laxity and the benign joint hypermobility syndrome in student and professional ballet dancers. J Rheumatol. 2004;31(1):173–178. [PubMed] [Google Scholar]
- 8.Smith R, Damodaran A.K, Swaminathan S, Campbell R, Barnsley L. Hypermobility and sports injuries in junior netball players. Br J Sports Med. 2005;39(9):628–631. doi: 10.1136/bjsm.2004.015271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X.C, Thometz J.G, Tassone C, Barker B, Lyon R. Dynamic plantar pressure measurement for the normal subject: free-mapping model for the analysis of pediatric foot deformities. J Pediatr Orthop. 2005;25(1):103–106. doi: 10.1097/00004694-200501000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Titianova E.B, Mateev P.S, Tarkka I.M. Footprint analysis of gait using a pressure sensor system. J Electromyogr Kinesiol. 2004;14(2):275–281. doi: 10.1016/S1050-6411(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 11.Stackhouse C.L, Davis I.M, Hamill J. Orthotic intervention in forefoot and rearfoot strike running patterns. Clin Biomech (Bristol, Avon) 2004;19(1):64–70. doi: 10.1016/j.clinbiomech.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Ardigò L.P, Lafortuna C, Minetti A.E, Mognoni P, Saibene F. Metabolic and mechanical aspects of foot landing type, forefoot and rearfoot strike, in human running. Acta Physiol Scand. 1995;155(1):17–22. doi: 10.1111/j.1748-1716.1995.tb09943.x. [DOI] [PubMed] [Google Scholar]
- 13.Nyska M, Shabat S, Simkin A, Neeb M, Matan Y, Mann G. Dynamic force distribution during level walking under the feet of patients with chronic ankle instability. Br J Sports Med. 2003;37(6):495–497. doi: 10.1136/bjsm.37.6.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamanli A, Sahin S, Ozgocmen S, Kavuncu V, Ardicoglu O. Relationship between foot angles and hypermobility scores and assessment of foot types in hypermobile individuals. Foot Ankle Int. 2004;25(2):101–106. doi: 10.1177/107110070402500211. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman K.R, Brodine S.K, Shaffer R.A, Johnson C.W, Cullison T.R. The effect of foot structure and range of motion on musculoskeletal overuse injuries. Am J Sports Med. 1999;27(5):585–593. doi: 10.1177/03635465990270050701. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan D, Warren R.F, Pavlov H, Kelman G. Stress fractures in 51 runners. Clin Orthop Relat Res. 1984;187:188–192. [PubMed] [Google Scholar]
- 17.Hockenbury R.T. Forefoot problems in athletes. Med Sci Sports Exerc. 1999;31(suppl 7):S448–S458. doi: 10.1097/00005768-199907001-00006. [DOI] [PubMed] [Google Scholar]
- 18.Omey M.L, Micheli L.J. Foot and ankle problems in the young athlete. Med Sci Sports Exerc. 1999;31(suppl 7):S470–S486. doi: 10.1097/00005768-199907001-00008. [DOI] [PubMed] [Google Scholar]
- 19.Norkin C.C, White D.J. Measurement of Joint Motion: A Guide to Goniometry. Philadelphia, PA: FA Davis; 1985. [Google Scholar]
- 20.Boyle K.L, Witt P, Riegger-Krugh C. Intrarater and interrater reliability of the Beighton and Horan Joint Mobility Index. J Athl Train. 2003;38(4):281–285. [PMC free article] [PubMed] [Google Scholar]
- 21.Decoster L.C, Vailas J.C, Lindsay R.H, Williams G.R. Prevalence and features of joint hypermobility among adolescent athletes. Arch Pediatr Adolesc Med. 1997;151(10):989–992. doi: 10.1001/archpedi.1997.02170470023005. [DOI] [PubMed] [Google Scholar]
- 22.Hennig E.M, Rosenbaum D. Pressure distribution patterns under the feet of children in comparison with adults. Foot Ankle. 1991;11(5):306–311. doi: 10.1177/107110079101100507. [DOI] [PubMed] [Google Scholar]
- 23.Cavanagh P.R, Rodgers M.M, Iiboshi A. Pressure distribution under symptom-free feet during barefoot standing. Foot Ankle. 1987;7(5):262–276. doi: 10.1177/107110078700700502. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari J, Watkinson D. Foot pressure measurement differences between boys and girls with reference to hallux valgus deformity and hypermobility. Foot Ankle Int. 2005;26(9):739–747. doi: 10.1177/107110070502600912. [DOI] [PubMed] [Google Scholar]
- 25.Lees A, Lake M, Klenerman L. Shock absorption during forefoot running and its relationship to medial longitudinal arch height. Foot Ankle Int. 2005;26(12):1081–1088. doi: 10.1177/107110070502601214. [DOI] [PubMed] [Google Scholar]
- 26.Brophy R, Gamradt S.C, Hillstrom H, Barnes R.P, Rodeo S.A, Warren R.F. The effect of turf toe on plantar contact foot pressures in professional American football players. Paper presented at: National Football League Physician's Society Scientific Meeting; February 22, 2008; Indianapolis, IN.
- 27.Ledoux W.R, Hillstrom H.J. The distributed plantar vertical force of neutrally aligned and pes planus feet. Gait Posture. 2002;15(1):1–9. doi: 10.1016/s0966-6362(01)00165-5. [DOI] [PubMed] [Google Scholar]
- 28.Thijs Y, De Clercq D, Roosen P, Witvrouw E. Gait-related intrinsic risk factors for patellofemoral pain in novice recreational runners. Br J Sports Med. 2008;42(6):466–471. doi: 10.1136/bjsm.2008.046649. [DOI] [PubMed] [Google Scholar]
- 29.Eils E, Streyl M, Linnenbecker S, Thorwesten L, Völker K, Rosenbaum D. Characteristic plantar pressure distribution patterns during soccer-specific movements. Am J Sports Med. 2004;32(1):140–145. doi: 10.1177/0363546503258932. [DOI] [PubMed] [Google Scholar]
- 30.Pasque C.B, Hewett T.E. A prospective study of high school wrestling injuries. Am J Sports Med. 2000;28(4):509–515. doi: 10.1177/03635465000280041101. [DOI] [PubMed] [Google Scholar]
- 31.Beckman S.M, Buchanan T.S. Ankle inversion injury and hypermobility: effect on hip and ankle muscle electromyography onset latency. Arch Phys Med Rehabil. 1995;76(12):1138–1143. doi: 10.1016/s0003-9993(95)80123-5. [DOI] [PubMed] [Google Scholar]
- 32.Biro F, Gewanter H.L, Baum J. The hypermobility syndrome. Pediatrics. 1983;72(5):701–706. [PubMed] [Google Scholar]