Abstract
In various organisms, high intracellular manganese provides protection against oxidative damage through unknown pathways. Herein we use a genetic approach in S. cerevisiae to analyze factors that promote manganese as an anti-oxidant in cells lacking Cu/Zn superoxide dismutase (sod1Δ). Unlike certain bacterial systems [1], oxygen resistance in yeast correlates with high intracellular manganese without a lowering of iron. This manganese for anti-oxidant protection is provided by the Nramp transporters Smf1p and Smf2p, with Smf1p playing a major role. In fact, loss of manganese transport by Smf1p together with loss of the Pmr1p manganese pump is lethal to sod1Δ cells in spite of normal manganese SOD2 activity. Manganese-phosphate complexes are excellent superoxide dimustase mimics in vitro [2], yet through genetic disruption of phosphate transport and storage, we observed no requirement for phosphate in manganese suppression of oxidative damage. If anything, elevated phosphate correlated with profound oxidative stress in sod1Δ mutants. The efficacy of manganese as an anti-oxidant was drastically reduced in cells that hyper-accumulate phosphate without effects on MnSOD activity. Non-SOD manganese can provide a critical backup for Cu/Zn SOD1, but only under appropriate physiologic conditions.
Keywords: Manganese, SOD1, Yeast, Phosphate, Nramp
INTRODUCTION
Superoxide dimustases (SOD) are the only eukaryotic enzymes known to remove toxic superoxide anions. Typically, the mitochondrial matrix harbors a manganese SOD2, while superoxide scavenging in all other compartments is covered by a single highly abundant Cu/Zn SOD1. In spite of the predicted importance of Cu/Zn SOD1, various genetic models have indicated that SOD1 is not essential for aerobic life, but does help guard against extensive oxidative damage. For example, mouse SOD1−/− mutants survive to adulthood, yet exhibit a high incidence of fatal liver carcinomas and loss in muscle mass [3–6]. Drosophila mutants of SOD1 survive throughout development, but have dramatically reduced lifespans as adults [7]. In S. cerevisiae, sod1Δ mutants are viable in atmospheric oxygen, yet exhibit numerous markers of oxidative stress, including damage to the lysine and methionine biosynthetic pathways, defects in vacuolar morphology and increased pools of labile iron [8–13].
A number of studies have indicated that high intracellular manganese can compensate for loss of SOD and provide protection against oxidative stress. Archibald and Fridovich observed that strains of Lactobaccilus planarum that lack SOD enzymes are oxygen tolerant due to intracellular accumulation of mM manganese [14–18]. More recently, elegant work by M. Daley has demonstrated that radiation and oxygen resistance in bacteria correlates with a high manganese to iron ratio [1, 19, 20]. In the nematode C. elegans, treatment with high manganese can prolong life and provide resistance to stress [21] and in the bakers yeast S. cerevisiae, elevated manganese is particularly effective in compensating for loss of Cu/Zn SOD1. Supplementing yeast cells with millimolar quantities of manganese salts will reverse all the aforementioned aerobic growth defects of sod1Δ mutants [22–24]. Moreover, sod1Δ deficiency can be reversed without manganese supplements when cells have mutations in either the PMR1 or BSD2 genes, both of which lead to increased accumulation of cellular manganese [23, 25–27].
Yeast cells express two Nramp metal transporters for manganese, Smf1p and Smf2p [28–30]. Smf1p is the more abundant of the two [30], yet earlier studies indicated that the less abundant Smf2p transporter accounts for much of the intracellular manganese and for activation of manganese enzymes in the mitochondria, Golgi and cytosol [31, 32]. The function of Smf1p was unclear. A possible role for Smf1p and/or Smf2p in manganese suppression of oxidative damage had not been rigorously tested, but studies with the aforementioned bsd2Δ suppressors of sod1Δ mutants suggest one or both transporters may be key. Bsd2p normally functions to down-regulate Smf1p and Smf2p by helping to target these proteins to the vacuole for degradation in a manner dependent on the Rsp5p ubiquitin ligase [27, 33–36]. As such, the steady state levels of Smf1p and Smf2p are greatly elevated in bsd2Δ mutants, correlating with suppression of oxidative damage [27, 33].
The nature of the anti-oxidant that accumulates with high manganese is not known, but it is not MnSOD. In bacteria and yeast cells that lack manganese containing SOD molecules, elevated manganese can still suppress oxidative damage [23, 26, 37, 38]. It is possible that specific manganese binding metabolites in cells can function as SOD-mimics. For example, recent studies by Valentine and Cabelli have shown that ortho-, but not pyro-phosphate complexes of manganese can serve as excellent SOD mimics [2] and a compound of this nature may serve as the manganese anti-oxidant in vivo. A manganese dependent superoxide scavenging activity has been identified in lysates from bacteria and yeast cells that hyperaccumulate the metal [14, 18, 24, 37], yet the precise compound has not been purified, perhaps due to re-distribution of manganese upon cell lysis [37].
Herein we employed a genetic strategy in the bakers yeast to analyze manganese suppression of oxidative damage. We observed that the Nramp metal transporter Smf1p serves as an important source of the manganese anti-oxidant, and that under certain instances, Smf1p is needed for aerobic viability of yeast sod1Δ mutants. Surprisingly, we observed no requirement for cellular phosphate in manganese suppression of oxidative damage. If anything, accumulation of high intracellular phosphate correlated with a loss in manganese anti-oxidant activity. Proper manganese bioavailability is critical for aerobic survival in cells lacking SOD1.
MATERIALS AND METHODS
Yeast strains, growth conditions and plasmids
Many yeast strains in this study were derived from EG103 (MATa, leu2-3, 112, his3Δ1, GAL+, trp1-289a, ura3-52) and include the previously reported sod1Δ::TRP1 KS107 [10], sod1Δ::TRP1 pmr1Δ::LEU2 KS111 [10], sod1Δ::TRP1 bsd2Δ::HIS3 XL110 [27], sod1Δ::TRP1 bsd2Δ::HIS3 smf1Δ::URA3 XL110 strains [27] and the sod1::URA3 sod2::TRP1 pmr1-1 VCSUP1 strain that contains a fully inactive allele of PMR1 [23]. Construction of the SL109 sod1Δ::TRP1 smf1Δ::URA3 [27], SL113 sod1Δ::TRP1 pmr1Δ::LEU2 smf1Δ::URA3, AR001 sod1Δ::TRP1 pmr1Δ::LEU2 smf2Δ::HIS3, MC123 sod1Δ::LEU2 smf2Δ::HIS3 and MC127 sod1Δ::TRP1 bsd2Δ::LEU2 smf2Δ::HIS3 strains were carried out using the previously published plasmids for deleting SOD1 [39], BSD2 [26, 27], PMR1 [23], SMF1 and SMF2 [27]. All other strains for this study were derived from BY4741 (MATa, leu2Δ0, met15Δ0, ura3Δ0, his3Δ1) and include commercially available kanMX4 deletion derivatives (Open Biosystems, www.openbiosystems.com) of bsd2, pmr1, pho80 and vph1, all verified by colony purification and gene sequencing. LJ283 (sod1Δ::LEU2 pmr1Δ::kanMX4) and LJ284 (sod1Δ::LEU2) were obtained by deleting SOD1 from the pmr1Δ::kanMX4 strain and BY4741 parent as described [39]. LJ346 (vtc4Δ::URA3), LJ285 (sod1Δ::LEU2 pmr1Δ::kanMX4 vtc4Δ::URA3) and LJ286 (sod1Δ::LEU2 vtc4Δ::URA3) represent vtc4Δ::URA3 derivatives of BY4741, LJ283 and LJ284, all created by using the vtc4 gene deletion plasmid pΔVTC4 described below. A pho84Δ::HIS3 version of BY4741 (strain LJ297) was created using the pLJ089 plasmid (see below). RS001 (sod1Δ::LEU2 pho84Δ::HIS3), RS002 and LR156 (sod1Δ::LEU2 pho80Δ::kanMX4) and MC130 (sod1Δ::LEU2 vph1Δ::kanMX4) represent sod1Δ::LEU2 [39] derivatives of LJ297, the pho80Δ::kanMX4 and vph1Δ::kanMX4 strains respectively.
The vtc4Δ::URA3 plasmid pΔVTC4 was constructed by PCR amplifying VTC4 sequences −449 to +157 and +1217 to +2574 using primers that introduced HinDIII sites at −449 and +2574, a BamHI site at +157 and a SalI site at +1217. The PCR products were digested at these sites and ligated in a trimolecular reaction to the SalI and BamHI sites of the URA3 integrating vector pRS306 [40]. The resulting plasmid, pΔVTC4, was linearized with HinDIII and used to delete chromosomal VTC4 sequences +158 to +1216. To create the pho84::HIS3 plasmid pLJ089, a XhoI-NotI fragment from plasmid pLJ246 [41] containing PHO84 upstream and downstream sequences was ligated into pRS403 cut with the same enzymes.
Yeast cells were grown at 30°C either in enriched yeast extract, peptone, dextrose medium (YPD) or synthetic complete (SC) medium lacking lysine as needed [42]. Experiments with sod1Δ pho84Δ mutants used a modified synthetic medium prepared with phosphate-free yeast nitrogen base (QBiogene) and supplemented with 1 mM monobasic potassium phosphate. This medium promoted the phosphate deficiency phenotype of a pho84 strain which is less apparent in standard SC medium containing ≈7 mM phosphate (Jensen and Culotta, unpublished). For anaerobic growth, medium was supplemented with 15 mg/L ergosterol and 0.5% Tween-80. All experiments employed cells freshly obtained from frozen stocks and cultured on YPD + ergosterol in oxygen depleted, CO2 enriched culture jars (GasPak, Becton Dickinson). For growth tests under “microaerobic” conditions, including assays for lysine and methionine dependence, cells were seeded in 1 ml cultures at O.D.600 = 0.05 and incubated at 30°C in air without shaking for 16 hours. For spot tests of oxygen sensitivity, 104 and 103 cells were spotted onto YPD plates and allowed to grow in air for 3 days. In liquid culture tests for oxygen sensitivity, cells were pre-grown in liquid YPD + ergosterol anaerobically for 16 hours for ≈ 4 doubling times. Cells were then diluted back to an O.D.600 = 0.1 or 0.5 in 5 ml YPD or SC medium and allowed to grow in air with shaking at 220 RPM for defined time points.
Biochemical assays
Inorganic phosphate (largely orthophosphate) levels were measured by molybdate reactivity [24, 43]. Cultures were grown in SC medium without shaking to O.D.600nm of ~2.0. 2×108 cells were harvested and washed twice with deionized H2O and resuspended in 500 μL 0.1% Triton X-100. Cells were lysed by glass bead homogenization and lysates were clarified by centrifugation for 10 minutes at 16,000×g. 100 μg protein from each sample or phosphoric acid standards were diluted in 3 mL deionized H2O followed by the sequential addition of 1 mL 0.1 M NaCl, 0.5 mL 0.5 M H2SO4, 0.5 mL 50 mM (NH4)6Mo7O24-4H2O, and 3 mL 1-butanol with vortexing between each addition. 2 mL of the upper organic phase was added to 1 mL of 9 mM SnCl2-H2O and mixed well by vortexing. The absorbance of the upper phase was measured at O.D700 nm and the inorganic phosphate levels were calculated based on standard curves generated from the phosphoric acid standards.
Polyphosphate was detected by gel electrophoresis and toluidine blue staining [44]. Cells isolated as above for orthophosphate analysis were resuspended in 250 μL 20 mM Tris-HCl pH 7.4, 20 mM KCl, 1 mM EDTA and 250 μL of phenol/chloroform/isoamyl alcohol (25:24:1) was added. Cells were subject to glass bead homogenization, followed by clarification through centrifugation for 5 minutes at 16,000×g. The upper phase was extracted with 250 μL chloroform, and total RNA concentration in the aqueous phase determined by absorbance at A260 nm. The equivalent of 80 μg of RNA was applied to 20% polyacrylamide gels buffered with 0.5X Tris-borate (45 mM Tris, 110 mM boric acid, pH 8). Gels were fixed with 10% acetic acid and 10% methanol followed by staining for polyphosphate using 0.5% Toluidine Blue O in 25% methanol, 5% glycerol and 5% acetic acid.
Atomic absorption spectroscopy (AAS) of total cellular manganese was carried as described [41]. Manganese SOD2 activity analysis was carried out by native gel electrophoresis and nitroblue tetrazolium (NBT) staining [45, 46], and SOD2 protein levels were monitored by immunoblot [46]; in both cases, 35 μg of total cell lysate protein was analyzed. For ICP-MS (Inductively coupled plasma mass spectrometry) analysis of manganese and iron, cells were cultured in SC medium to an O.D.600 of 3.0–4.0, harvested and washed in cold TE buffer (10 mM Tris-hydrochloride and 1mM EDTA, pH 8.0). 20 OD600 units of cells were digested overnight at 95°C in 1ml 20% nitric acid and subsequently diluted to 1% nitric acid with deionized water. Metal analysis was performed on an Agilent 7500ce ICP-MS according to manufacturer specification and values were converted to nmoles metal/109 cells.
RESULTS
The Nramp transporters and resistance to oxidative stress in yeast sod1Δ mutants
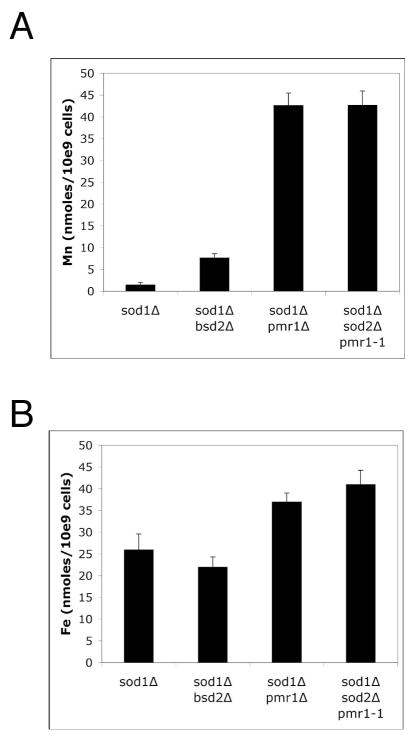
In bacteria, resistance to radiation and oxidative stress has been associated with accumulation of high manganese and low iron [1, 19, 20]. Elevated manganese can suppress oxidative damage in the eukaryotes C. elegans and S. cerevisiae [21–24] but potential effects of iron have not been examined. We examined whole cell metals in cases where oxidative damage in yeast sod1Δ mutants is suppressed by mutations in either pmr1 or bsd2 [23, 26]. As seen in Fig. 1A, suppression of oxidative damage in these strains correlated with increased manganese accumulation, even in strains lacking manganese containing SOD2, consistent with accumulation of a non-SOD manganese based anti-oxidant. Yet in all cases, iron levels were not lowered in the oxygen resistant strains. This was true not only of the genetic suppressors of sod1Δ (Fig. 1B), but also of sod1Δ mutants suppressed by treatment with mM concentrations of manganese salts (see ahead Fig. 6B). Suppression of oxidative damage in yeast cells is associated with elevated intracellular manganese without lowering iron.
Fig. 1.
Suppression of sod13 deficiency by changes in manganese, not iron.
The indicated strains were grown to an O.D.600 nm of 3.0 in SC medium. Whole cell manganese (A) and iron (B) was analyzed by ICP-MS as described in Materials and Methods. Values represent the average of three-four independent cultures with error bars representing standard deviation. Strains employed: sod1 Δ, KS107; sod1 Δ pmr1 Δ, KS111; sod1 Δ bsd2 Δ, XL110; sod1 Δ sod2 Δ pmr1-1, VCSUP1.
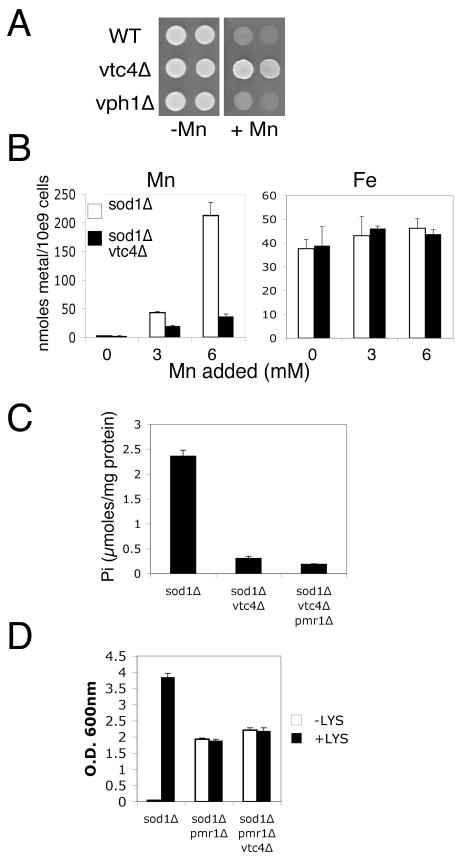
Fig. 6.
Studies with vtc4 Δ show a role for intracellular manganese but not phosphate in suppression of oxidative damage.
A) 105 and 104 cells of the indicated strains were spotted onto YPD plates supplemented where indicated with 5 mM MnCl2. Cells were allowed to grow for three days. B) Strains were grown in SC complete medium supplemented where indicated with the designated levels of MnCl2 prior to analysis of total cellular manganese (left) and iron (right) by AAS. In the absence of added manganese, the total manganese accumulation in the sod1 Δ and sod1 Δ vtc4 Δ mutants is 2.54 and 2.25 nmoles/109 cells respectively. C) Orthophosphate analysis of the indicated strains grown in SC medium was carried out as in Fig. 5B. D) Test for lysine independent growth as in Fig. 2A was conducted in the designated strains supplemented with the indicated levels of MnCl2. Strains employed include: WT, BY4741; vtc4 Δ and vph1 Δ, the corresponding kanMX4 derivates of BY4741; sod1 Δ, LJ284; sod1 Δ vtc4 Δ, LJ286; sod1 Δ vtc4 Δ pmr1 Δ, LJ285; sod1 Δ pmr1 Δ, LJ283. (B–D) results represent the averages of 3–4 independent cultures and error bars represent standard deviation.
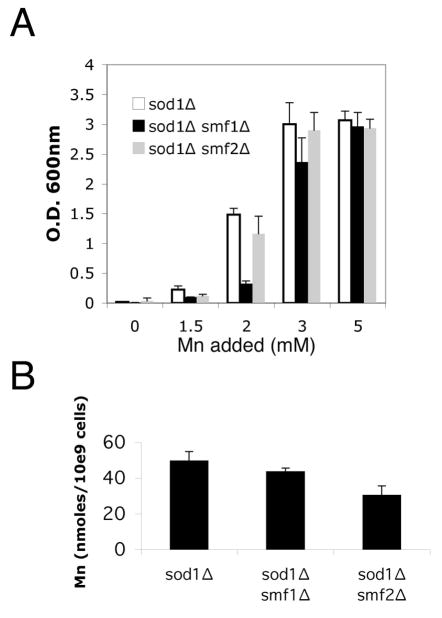
The effects of manganese but not iron on oxidative stress resistance led us to examine the role of the manganese Nramp transporters Smf1p and Smf2p. We first tested whether Smf1p and Smf2p are required for suppression of sod1Δ deficiency by mM manganese. Due to oxidative damage to the lysine biosynthetic pathway, sod1Δ mutants cannot grow in air without exogenous lysine [13, 25], and this defect was suppressed by supplementing the growth medium with ≈2–3 mM manganese (Fig. 2A). Manganese could still suppress oxidative damage in double sod1Δ smf1Δ and sod1Δ smf2Δ mutants. However, the sod1Δ smf1Δ mutant consistently required higher concentrations of the metal (Fig. 2A), even though intracellular manganese levels were not greatly affected by the smf1Δ mutation (Fig. 2B). This was the first indication that Smf1p is needed for efficient manganese suppression of sod1Δ.
Fig. 2.
The role of Nramp metal transporters in suppression of oxidative damage by mM manganese.
A) The indicated strains were grown in air without shaking in liquid SC medium lacking lysine and supplemented with the designated concentrations of MnCl2. Following 16 hours incubation, total cell growth was monitored as a function of absorbance at 600 nm. B) The indicated strains were grown as above in (A), except medium was supplemented with lysine and 3 mM MnCl2. Total cellular manganese was determined by AAS as described in Materials and Methods. Values represent the average of three independent cultures with error bars representing standard deviation. Strains employed sod1 Δ, KS107; sod1 Δ smf1 Δ, SL109; sod1 Δ smf2 Δ, MC123.
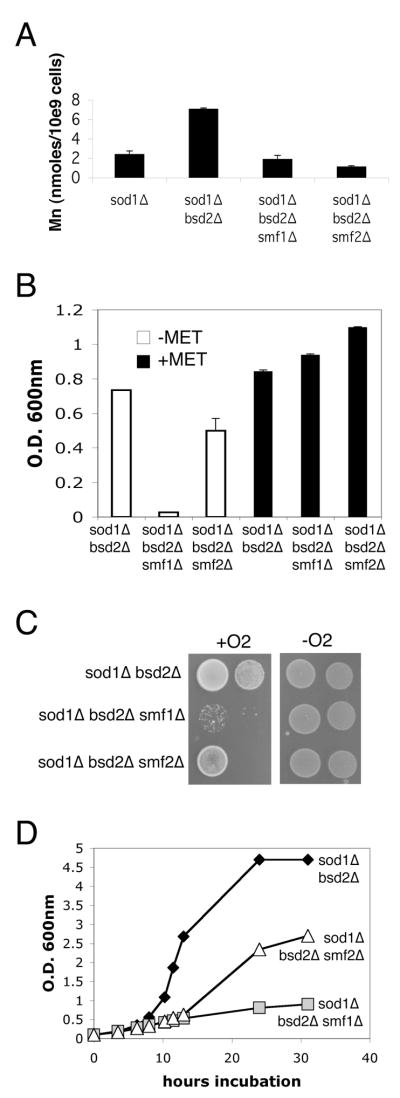
We also tested the role of Smf1p and Smf2p in suppression of oxidative damage when manganese levels are elevated without manganese supplements to the growth medium, i.e., in bsd2Δ and pmr1Δ mutants. Mutations in BSD2 are associated with a 2–3 fold increase in intracellular manganese and this was reversed by mutations in either smf1 or smf2 (Fig. 3A). Yet of the two Nramp transporters, Smf1p seems more critical for bsd2 suppression of oxidative damage. Mutations in smf1 greatly diminished the ability of bsd2 mutations to suppress the aerobic methionine deficiency of a sod1Δ mutant (Fig. 3B) [26]. The growth tests of Figs. 2A and 3B were conducted under “microaerobic” conditions (liquid cultures not aerated by shaking), where sod1Δ cells generally grow well in complete minimal medium supplemented with methionine and lysine (e.g., see Fig. 3B, right). A more severe oxidative stress is imposed when cells are directly exposed to atmospheric oxygen by growth on solid medium (as in Fig. 3C) or are well aerated in liquid cultures (Fig. 3D). In both cases, the strong aerobic growth of the sod1Δ bsd2Δ strain was drastically impaired by a smf1Δ mutation; the effects of smf2Δ mutations were less pronounced.
Fig. 3.
Smf1p and suppression of oxidative damage by bsd2 Δ mutations.
A) Total cellular manganese from three independent cultures was analyzed in the indicated strains grown in complete SC medium as described in Fig. 2B. B) The indicated strains were grown 15 hours non-shaking in SC medium containing lysine, but lacking or supplemented with methionine as designated. Total cell growth was monitored as a function of absorbance at 600 nm. Values represent the average of three independent cultures with error bars representing standard deviation. C) 104 and 103 cells of the indicated strains were spotted onto enriched YPD medium and incubated for 3 days either in air or in anaerobic culture jars. D) Strains were pre-grown overnight in complete SC medium under anaerobic conditions; cultures were then diluted to O.D.600nm = 0.1 in the same medium and monitored for aerobic growth under well-aerated conditions for the indicated time points. Strains utilized: sod1Δ, KS107; sod1 Δ bsd2 Δ, XL110; sod1 Δ bsd2 Δ smf1 Δ, XL111; sod1 Δ bsd2 Δ smf2 Δ, MC127.
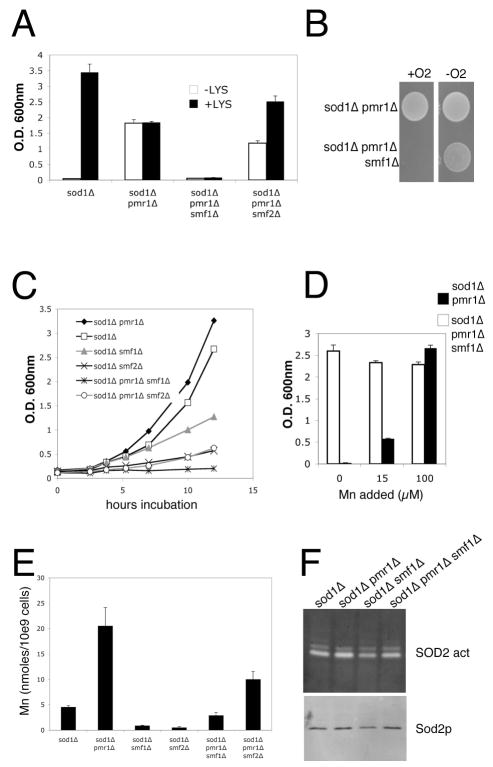
PMR1 encodes a Golgi pump for manganese [47, 48] and in yeast pmr1Δ mutants, the manganese that cannot enter the Golgi hyper-accumulates in the cytosol and other cellular locales [31, 49]. The elevated manganese in pmr1Δ mutants reverses the aerobic lysine deficiency of sod1Δ mutants [49], however this oxygen resistance was abrogated in the triple sod1Δ pmr1Δ smf1Δ mutant (Fig. 4A). Effects of the smf2Δ mutation were minor by comparison (Fig. 4A).
Fig. 4.
A combination of smf1 Δ and pmr1 Δ mutations causes severe oxidative stress
A) Yeast strains were grown in triplicate cultures as in Fig. 3B in medium supplemented with methionine and containing or lacking lysine as indicated. B) 104 cells of the indicated strains were grown as in Fig. 3C. C) Aerobic growth test in enriched YPD medium was conducted as described in Fig. 3D. D) Yeast strains were grown in triplicate cultures as in Fig. 3B in medium supplemented with both lysine and methionine and also with the indicated concentrations of MnCl2. E) The indicated cells pre-grown in enriched YPD medium under anaerobic conditions and switched to air for 1.5 hours were analyzed for total manganese content by AAS. Values represent averages of four independent cultures analyzed in two experimental trials; error bars represent standard deviation. F) The indicated strains grown as in (E) were lysed and assayed for mitochondrial manganese SOD2 activity by native gel electrophoresis and NBT staining (top) and for SOD2 polypeptide levels by immunoblotting (bottom). Strains utilized include: sod1 Δ, KS107; sod1 Δ pmr1 Δ, KS111; sod1 Δ smf1 Δ, SL109; sod1 Δ pmr1 Δ smf1 Δ, SL113; sod1 Δ pmr1 Δ smf2 Δ, AR001; sod1 Δ smf2 Δ, MC123.
Notably, the smf1Δ mutation had a profound effect on aerobic viability of the sod1Δ pmr1Δ strain. The triple sod1Δ pmr1Δ smf1Δ mutant failed to grow in complete medium under all aerobic conditions tested, including direct exposure to atmospheric oxygen (Fig. 4B), in well aerated liquid cultures (Fig. 4C) and under microaerobic conditions (Fig. 4A). The strong growth defect of the sod1Δ pmr1Δ smf1Δ strain was reversed by anaerobic conditions (Fig. 4B) or by treatments with micromolar levels of manganese (Fig. 4D). This rescue by low supplements of manganese would suggest that manganese is limiting in the sod1Δ pmr1Δ smf1Δ strain. The sod1Δ pmr1Δ smf1Δ strain does accumulate lower levels of manganese than the sod1Δ pmr1Δ parent, but this level approximates that of the sod1Δ parent and is still higher than the sod1Δ smf1Δ and sod1Δ smf2Δ strains that are all viable in air (Fig. 4E). As an additional marker of manganese, we tested for activity of manganese containing SOD2 in the mitochondria. As seen in Fig. 4F, SOD2 activity was not affected in the sod1Δ pmr1Δ smf1Δ strain. Hence a specialized pool of manganese distinct from that utilized by mitochondrial SOD2 supports aerobic life of sod1Δ cells and this pool becomes limiting with loss of Smf1p and Pmr1p. The data of Fig. 4F also addresses the non-SOD nature of the manganese antioxidant. The presence of robust SOD2 activity in the sod1Δ pmr1Δ smf1Δ strain underscores the notion that manganese promotes aerobic life in ways that are independent of the MnSOD enzyme.
The effects of phosphate on manganese suppression of oxidative damage
Manganese-phosphate complexes can scavenge superoxide in vitro [2], raising the intriguing possibility that manganese-phosphate is the anti-oxidant back up for Cu/Zn SOD1 in vivo. To address this, we introduced mutations in sod1Δ cells that disrupt phosphate uptake and storage and monitored effects on manganese suppression of oxidative damage.
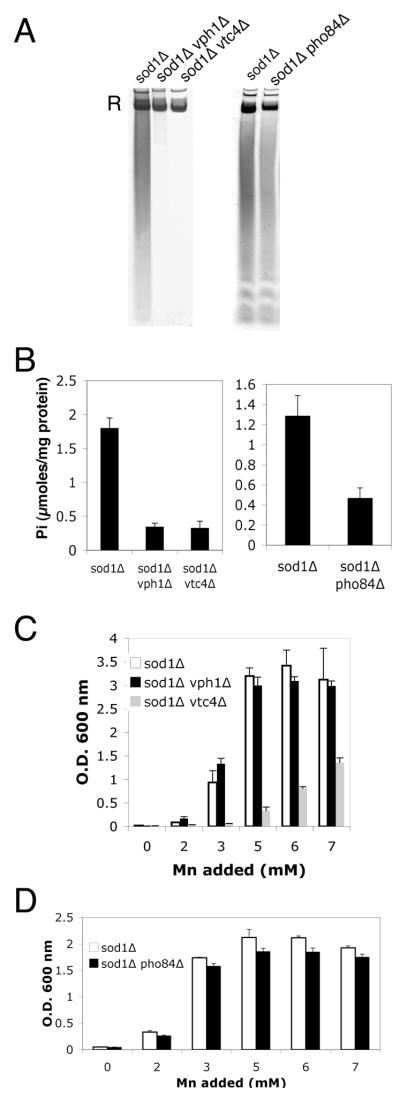
Mutations in vph1Δ and vtc4Δ mutants impair phosphate storage [50, 51], and both polyphosphate (Fig. 5A left) and orthophosphate (Fig. 5B left) were dramatically lowered in these mutants. Mutations in pho84Δ encoding the high affinity metal-phosphate transporter [41, 52] also reduce phosphate when grown in a defined medium containing 1 mM phosphate (normal synthetic medium contains close to 10 mM phosphate) (Fig. 5A, 5B, right). In spite of vastly different levels of intracellular phosphate, the sod1Δ, sod1Δ vph1Δ and sod1Δ pho84Δ strains all exhibited suppression of oxidative damage at ≥3.0 mM manganese, as monitored by reversal of the lysine biosynthetic defect (Fig. 5C, 5D). However, manganese suppression of the sod1Δ vtc4Δ strain was severely compromised and required at least 6–7 mM concentrations of the metal (Fig. 5C). This result was unexpected since vtc4Δ and vph1Δ mutations similarly lower phosphate even in cells grown in 3 mM manganese (Fig. 5A, 5B). We observed that vtc4 mutants specifically exhibit a resistance to manganese toxicity (Fig. 6A) that correlates with a dramatic lowering of intracellular manganese (Fig. 6B). When treated with 3 mM manganese, sod1Δ vtc4Δ cells accumulate approximately half the level of manganese seen in the parental sod1Δ strain (left), while iron levels are not affected (right). At 6 mM manganese where the sod1Δ vtc4Δ strain begins to exhibit suppression of oxidative damage (Fig. 5B), intracellular manganese levels more closely approximate that of the sod1Δ parent grown at 3 mM manganese. Manganese suppression of oxidative damage is clearly correlating with intracellular manganese, not phosphate.
Fig. 5.
Phosphate deficient mutants of S. cerevisiae and manganese suppression of oxidative damage.
A–B) The indicated cultures were grown to early stationary phase in minimal medium containing methionine and lysine and supplemented with 3 mM MnCl2 (manganese levels that typically suppress sod1 Δ deficiency). Cell lysates were prepared for analysis of polyphosphate (A) by polyacrylamide gel electrophoresis and toluidene blue staining, or for analysis of ortho phosphate (B) by molybdate reactivity as described in Materials and Methods. “R” = RNA staining by toluidene blue. Values of orthophosphate (Pi) are the averages of three independent cultures with error bars representing standard deviation. C,D) Suppression of the sod1 Δ lysine biosynthetic defect by mM manganese was examined in triplicate cultures of the indicated strains as described in Fig. 2A. Strains used: sod1 Δ, LJ284; sod1 Δ pho84 Δ, RS001; sod1 Δ vph1 Δ, MC130; sod1 Δ vtc4 Δ, LJ286. Standard SC medium containing ≈7 mM phosphate was used for A, B left panels and for C. A,B right panels and D employed a minimal medium containing 1 mM phosphate to maximize effects of the pho84 mutation (see Materials and Methods).
The effect of vtc4Δ mutations on lowering manganese is only seen with manganese salts added to the growth medium; sod1Δ vtc4Δ cells accumulate normal levels of the metal without manganese supplements (Fig. 6B left, see legend). As such, vtc4Δ mutants provided a good background to test the effects of lowering phosphate in the pmr1Δ suppression of oxidative damage that does not require manganese supplements. As seen in Fig. 6C, the triple sod1Δ pmr1Δ vtc4Δ mutant accumulates very low orthophosphate. However, there was no loss in pmr1Δ suppression of oxidative damage (Fig. 6D). Together the studies of Figs. 5–6 demonstrate that a lowering of intracellular phosphate does not preclude manganese suppression of oxidative damage by either mM manganese supplements or by the pmr1 suppressor of sod1Δ.
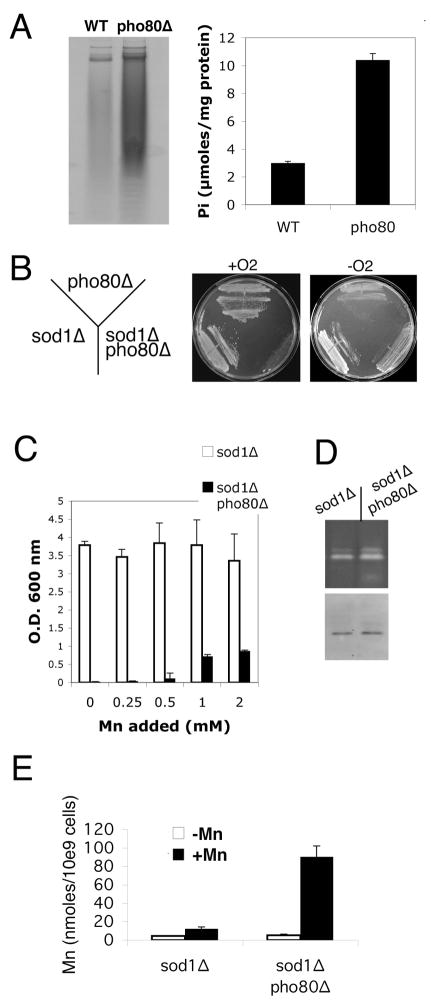
We also tested the effects of elevating intracellular phosphate using mutants of pho80 encoding a negative regulator of phosphate uptake and storage [52–s55]. As seen in Fig. 7A, pho80 mutants accumulate very high levels of polyphosphate (left) and orthophosphate (right). Since manganese orthophosphate complexes are good mimics of SOD [2], pho80 was expected to increase oxygen resistance in sod1Δ strains. Surprisingly however, the double sod1Δ pho80Δ exhibited a profound oxygen sensitivity (Fig. 7B, 7C). The strain failed to grow aerobically under all conditions tested, similar to what we observed with the sod1Δ pmr1Δ smf1Δ strain (see Fig. 4). The sod1Δ pho80Δ strain did exhibit growth under anaerobic conditions (Fig. 7B) or when manganese was supplemented to aerobic cultures (Fig. 7C). Yet compared to the sod1Δ pmr1Δ smf1Δ strain, rescue of sod1Δ pho80Δ required much higher concentrations of the metal (millimolar as opposed to micromolar in minimal medium) and manganese was only mildly effective in restoring aerobic growth (Fig. 7C).
Fig. 7.
Deleterious effects of pho80 mutations on cells lacking Cu/Zn SOD1.
A) The indicated strains were grown in YPD medium to an O.D. ≈ 6.0 and analyzed for polyphosphate and ortho phosphate as in Fig. 5A,B. Orthophosphate measurements were taking in three independent cultures with error bars representing standard deviation. B) Strains were plated onto YPD medium and allowed to grow in air or in anaerobic culture jars for three days. C) The indicated strains were grown in SC complete medium supplemented with the indicated concentrations of MnCl2 as in Fig. 4C. Results were averaged over three independent cultures, error bars represent standard deviation. D) The indicated strains were grown as in Fig. 4E and tested for SOD2 activity and protein levels as in Fig. 4F. E) Total cellular manganese was monitored in the indicated cells grown in enriched YPD medium as in Fig. 4E. Where indicated, cells were supplemented or not supplemented with 100 μM MnCl2, the concentration of manganese in enriched medium that supports maximal aerobic growth of the sod1 Δ pho80 Δ mutant (not shown). Results represent the averages of four cultures in two independent trials; error bars represent standard deviation. Strains employed: WT, BY4741; pho80, pho80 Δ::kanMX4 derivative of BY4741; sod1 Δ, LJ284; sod1 Δ pho80 Δ LR156C.
The poor rescue of oxidative damage by manganese in pho80 mutants cannot be explained by a general limitation in manganese ion bioavailability. The manganese containing SOD2 of the mitochondria was active (Fig. 7D). Moreover there was no deficiency in manganese accumulation (Fig. 7E). In fact with manganese supplements, the sod1Δ pho80 mutant accumulated 5–10 fold higher levels of the metal compared to the sod1Δ parent (Fig. 7E). Nevertheless, this very high intracellular manganese poorly rescues aerobic growth. Loss of phosphate control in pho80 cells correlates with a drastic reduction in the efficacy of manganese as an anti-oxidant. Overall, these findings do not support a role for manganese-phosphate compounds in oxidative stress protection.
DISCUSSION
High intracellular manganese has long been known to suppress oxidative stress [1, 14, 22–24], but the requisite cellular factors were unknown. Using a yeast model system, we now show that the Nramp metal transporters together with the Golgi transporter for manganese are critical for manganese suppression of oxidative damage. We also provide evidence for phosphate compounds altering the efficacy of manganese as an anti-oxidant. Disruptions in manganese homeostasis or elevations in cellular phosphate can cause aerobic lethality to cells lacking Cu/Zn SOD1 in spite of abundant manganese SOD2 activity. Independent of its role as a co-factor for SOD2, manganese serves as an important anti-oxidant backup for Cu/Zn SOD1.
Of the two Nramp manganese transporters in yeast, we observed that Smf1p is most critical for manganese suppression of oxidative damage. This represents the first described function for this Nramp transporter. Most of the manganese requirements of the cell, e.g., manganese activation of SOD2 in the mitochondria and of sugar transferasese in the Golgi, involve the other less abundant Nramp of yeast, Smf2p [31]. Evidently, the increased demand for manganese during oxidative stress cannot be met by Smf2p and the more abundant Smf1p Nramp comes into play.
When combined with mutations in pmr1 encoding the Golgi pump for manganese, loss of Smf1p is lethal to cells lacking SOD1. A fraction of Smf1p resides in the secretory pathway[33, 36], and Pmr1p and Smf1p are predicted to transport the metal in opposite directions. An intriguing possibility is that some of the manganese transported into the secretory pathway by Pmr1p is re-claimed by Smf1p transport of manganese back into the cytosol. This re-cycling of manganese between the secretory pathway and cytoplasm together with the Smf1p-cell surface uptake of manganese may be critical for accumulating manganese as an anti-oxidant.
Nramp transporters are well-conserved throughout nature and Smf1p-like molecules from other organisms are likely to be used for oxidative stress protection. The oxygen tolerant Lactobaccillus planarum expresses a number of Nramp transporters that may support the strong manganese anti-oxidant activity of this organism [56], and a loss of the Smf-3 Nramp transporter of C. elegans has been associated with superoxide sensitivity [57]. Mammals express two Nramp transporters that have the capacity to transport manganese [58–60] and either one may help support oxidative stress resistance.
Studies have shown that certain manganese-phosphate complexes can serve as excellent mimics for SOD in vitro [2, 15]. Yet in our genetic studies, we observed no requirement for cellular phosphate in manganese suppression of oxidative damage. If anything, high intracellular phosphate correlated with increased oxidative stress, and sod1Δ pho80Δ mutants that accumulate very high phosphate are inviable in air. In this manner, the sod1Δ pho80Δ cells phenocopy the aforementioned sod1Δ pmr1Δ smf1Δ mutants. Both are defective in their capacity to provide manganese anti-oxidant protection, but apparently through different mechanisms. The sod1Δ pmr1Δ smf1Δ mutants are limited for manganese, as the oxidative stress is fully reversed by supplementing the cells with low (micro molar in minimal medium) levels of the metal. By comparison, the sod1Δ pho80Δ strain is poorly rescued by even very high levels of manganese. Total cellular manganese is not limiting in pho80 strains, rather the metal appears non-reactive as an anti-oxidant backup for SOD1.
The pho80 mutants hyperaccumulate a wide array of phosphate compounds, and in vitro studies have shown that manganese binding to pyro and polyphosphate can inhibit superoxide scavenging activity of the metal [2, 14, 18]. In addition, inorganic phosphate and polyphosphate can inhibit manganese reactivity with H2O2 in the presence of bicarbonate [61]. It is possible that in pho80 mutants, manganese binding to one or more of these phosphate complexes or to another cellular metabolite interferes with the metal’s ability to guard against oxidative stress. While we cannot totally exclude the possibility that a certain manganese-phosphate complex acts as an SOD-mimic in vivo, our genetic studies of phosphate control suggest that other possibilities should be considered such as carboxylates [20].
Lastly, these studies address the enigma of whether Cu/Zn SOD1 is actually essential for aerobic life. In spite of the widespread indices of oxidative damage noted with various eukaryotic models of SOD1 deficiency, the organisms for the most part are viable in atmospheric oxygen [6, 7, 13]. Based on the studies presented here, we propose that the physiological levels of manganese that accumulate in all cells serves as a backup to Cu/Zn SOD1. Although SOD is the only enzyme known to scavenge superoxide, non-SOD complexes of manganese can act as a secondary means of removing reactive oxygen species.
Acknowledgments
We wish to thank Mark Carroll for generously providing yeast strains and Jana Mihalic for conducting ICP analyses. This work was funded by the JHU NIEHS center and by NIH grants GM 50016 and ES 08996. R.S was supported by training grant T35 ES007308, A. R and L.R. are funded by the JHU NIEHS training grant ES 07141 and A.N. by Royal Thai Government Scholarship.
LIST OF ABBREVIATIONS
- SOD
superoxide dismutase
- PCR
polymerase chain reaction
- YPD
yeast extract, peptone, dextrose medium
- SC
synthetic complete medium
- AAS
Atomic absorption spectroscopy
- NBT
nitroblue tetrazolium
- ICP-MS
Inductively coupled plasma mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 2.Barnese K, Gralla EB, Cabelli DE, Valentine JS. Manganous Phosphate Acts as a Superoxide Dismutase. J Am Chem Soc. 2008 doi: 10.1021/ja710162n. [DOI] [PubMed] [Google Scholar]
- 3.Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 4.Jackson MJ. Lack of CuZnSOD activity: a pointer to the mechanisms underlying age-related loss of muscle function, a commentary on “absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy”. Free Radic Biol Med. 2006;40:1900–1902. doi: 10.1016/j.freeradbiomed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, 2nd, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MFRHB, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn-SOD superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 7.Phillips J, Campbell S, Michard D, Charbonneau M, Hilliker A. Null mutations of copper/zinc superoxide in Drosophila confer hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci USA. 1989;83:3820–3824. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corson LB, Folmer J, Strain JS, Culotta VC, Cleveland DW. Oxidative stress and iron are implicated in fragmenting vacuoles of Saccharomyces cerevisiae lacking Cu, Zn Superoxide dismtuase. J Biol Chem. 1999;274:27590–27596. doi: 10.1074/jbc.274.39.27590. [DOI] [PubMed] [Google Scholar]
- 9.Chang E, Kosman D. O2-dependent methionine auxotrophy in Cu, Zn Superoxide dismutase deficient mutants of Saccharomyces cerevisiae. J Bacteriol. 1990;172:1840–1845. doi: 10.1128/jb.172.4.1840-1845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slekar KH, Kosman D, Culotta VC. The yeast copper/zinc superoxide dismutase and the pentose phosphate pathway play overlapping roles in oxidative stress protection. J Biol Chem. 1996;271:28831–28836. doi: 10.1074/jbc.271.46.28831. [DOI] [PubMed] [Google Scholar]
- 11.Freitas JMD, Liba A, Meneghini R, Valentine JS, Gralla EB. Yeast Lacking Cu-Zn Superoxide Dismutase Show Altered Iron Homeostasis. Role of oxidative stress in iron metabolism. J Biol Chem. 2000;275:11645–11649. doi: 10.1074/jbc.275.16.11645. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan SC, Liba A, Imlay JA, Valentine JS, Gralla EB. Yeast Lacking Superoxide Dismutase(s) Show Elevated Levels of “Free Iron” as Measured by Whole Cell Electron Paramagnetic Resonance. J Biol Chem. 2000;275:29187–29192. doi: 10.1074/jbc.M004239200. [DOI] [PubMed] [Google Scholar]
- 13.Bilinski T, Krawiec Z, Liczmanski L, Litwinska J. Is hydroxyl radical generated by the fenton reaction in vivo? Biochem Biophys Res Comm. 1985;130:533–539. doi: 10.1016/0006-291x(85)90449-8. [DOI] [PubMed] [Google Scholar]
- 14.Archibald FS, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:422–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archibald FS, Fridovich I. The scavenging of superoxide radical by manganous complexes in vitro. Arch Biochem Biophys. 1982;214:452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 16.Archibald FS, Fridovich I. Manganese, superoxide dismutase and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981;146:928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archibald FS, Duong MN. Manganese Acquistion by Lactobacillus plantarum. J Bacteriol. 1984;158:1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archibald FS, Fridovich I. Investigations on the state of the manganese in Lactobacillus plantarum. Arch biochm Biophys. 1982;215:589–596. doi: 10.1016/0003-9861(82)90120-5. [DOI] [PubMed] [Google Scholar]
- 19.Daly MJ. Modulating radiation resistance: Insights based on defenses against reactive oxygen species in the radioresistant bacterium Deinococcus radiodurans. Clin Lab Med. 2006;26:491–504. doi: 10.1016/j.cll.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Leapman RD, Lai B, Ravel B, Li SM, Kemner KM, Fredrickson JK. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 2007;5:e92. doi: 10.1371/journal.pbio.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin YT, Hoang H, Hsieh SI, Rangel N, Foster AL, Sampayo JN, Lithgow GJ, Srinivasan C. Manganous ion supplementation accelerates wild type development, enhances stress resistance, and rescues the life span of a short-lived Caenorhabditis elegans mutant. Free Radic Biol Med. 2006;40:1185–1193. doi: 10.1016/j.freeradbiomed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez RJ, Srinivasan C, Munroe WH, Wallace MA, Martins J, Kao TY, Le K, Gralla EB, Valentine JS. Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking CuZnSOD. J Biol Inorg Chem. 2005;10:913–923. doi: 10.1007/s00775-005-0044-y. [DOI] [PubMed] [Google Scholar]
- 23.Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang EC, Kosman DJ. Intracellular Mn(II)-associated superoxide scavenging activity protects Cu, Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J Biol Chem. 1989;264:12172–12178. [PubMed] [Google Scholar]
- 25.Liu XF, Elashvili I, Gralla EB, Valentine JS, Lapinskas P, Culotta VC. Yeast lacking superoxide dismutase: isolation of genetic suppressors. J Biol Chem. 1992;267:18298–18302. [PubMed] [Google Scholar]
- 26.Liu XF, Culotta VC. The requirement for yeast superoxide dismutase is bypassed through mutations in BSD2, a novel metal homeostasis gene. Mol Cell Biol. 1994;14:7037–7045. doi: 10.1128/mcb.14.11.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XF, Supek F, Nelson N, Culotta VC. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J Biol Chem. 1997;272:11763–11769. doi: 10.1074/jbc.272.18.11763. [DOI] [PubMed] [Google Scholar]
- 28.West AH, Clark DJ, Martin J, Neupert W, Hart FU, Horwich AL. Two related genes encoding extremely hydrophobic proteins suppress a lethal mutation in the yeast mitochondrial processing enhancing protein. J Biol Chem. 1992;267:24625–24633. [PubMed] [Google Scholar]
- 29.Cohen A, Nelson H, Nelson N. The family of SMF metal ion transporters in yeast cells. J Biol Chem. 2000;275:33388–33394. doi: 10.1074/jbc.M004611200. [DOI] [PubMed] [Google Scholar]
- 30.Portnoy ME, Liu XF, Culotta V. C. Saccharomyces cerevisiae expresses three functionally distinct homologues of the Nramp family of metal transporters. Mol Cell Biol. 2000;20:7893–7902. doi: 10.1128/mcb.20.21.7893-7902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luk E, Culotta VC. Manganese superoxide dismutase in S.cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transproter, Smf2p. J Biol Chem. 2001;276:47556–47562. doi: 10.1074/jbc.M108923200. [DOI] [PubMed] [Google Scholar]
- 32.Luk E, Carroll M, Baker M, Culotta VC. Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc Natl Acad Sci USA. 2003;100:10353–10357. doi: 10.1073/pnas.1632471100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu XF, Culotta VC. Post-translational control of Nramp metal transport in yeast: role of metal ions and the BSD2 gene. J Biol Chem. 1999;274:4863–4868. doi: 10.1074/jbc.274.8.4863. [DOI] [PubMed] [Google Scholar]
- 34.Hettema EH, Valdez-Taubas J, Pelham HR. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 2004 doi: 10.1038/sj.emboj.7600137. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stimpson HE, Lewis MJ, Pelham HR. Transferrin receptor-like proteins control the degradation of a yeast metal transporter. Embo J. 2006;25:662–672. doi: 10.1038/sj.emboj.7600984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan JA, Lewis MJ, Nikko E, Pelham HR. Multiple interactions drive adaptor-mediated recruitment of the ubiquitin ligase rsp5 to membrane proteins in vivo and in vitro. Mol Biol Cell. 2007;18:2429–2440. doi: 10.1091/mbc.E07-01-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Maghrebi M, Fridovich I, Benov L. Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch Biochem Biophys. 2002;402:104–109. doi: 10.1016/S0003-9861(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 38.Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–1186. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 39.Culotta VC, Joh HD, Lin SJ, Slekar KH, Strain J. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J Biol Chem. 1995;270:29991–29997. doi: 10.1074/jbc.270.50.29991. [DOI] [PubMed] [Google Scholar]
- 40.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces ceresiviae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen LT, Ajua-Alemanji M, Culotta VC. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J Biol Chem. 2003;278:42036–42040. doi: 10.1074/jbc.M307413200. [DOI] [PubMed] [Google Scholar]
- 42.Sherman F, Fink GR, Lawrence CW. Methods in yeast genetics. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1978. [Google Scholar]
- 43.Berenblum I, Cham EB. Biochem J. 1938;32:295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaneko Y, Toh-e A, Oshima Y. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol Cell Biol. 1982;2:127–137. doi: 10.1128/mcb.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flohe L, Otting F. Superoxide dismutase assays. In: Packer L, editor. Methods in enzymology: oxygen radicals in biological systems. New York: Academic press; 1984. pp. 93–104. [DOI] [PubMed] [Google Scholar]
- 46.Luk E, Yang M, Jensen LT, Bourbonnais Y, Culotta VC. Manganese activation of superoxide dismutase 2 in the mitochondria of Saccharomyces cerevisiae. J Biol Chem. 2005;280:22715–22720. doi: 10.1074/jbc.M504257200. [DOI] [PubMed] [Google Scholar]
- 47.Antebi A, Fink GR. The yeast Ca+2-ATPase homologue, PMR1, is responsible for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao JI, Moir DT. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca+2-ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 49.Lapinskas P. Characterization of genes involved in the homeostasis of oxygen free radicals and metal ions in Saccharomyces cerevisiae. Johns Hopkins University; 1995. [Google Scholar]
- 50.Ogawa N, DeRisi J, Brown PO. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell. 2000;11:4309–4321. doi: 10.1091/mbc.11.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castrol C, Koretsky A, Domach M. NMR-Observed phosphate trafficking and polyphosphate dynamics in wild-type and vph1-1 mutant Saccharomyces cerevisae in response to stresses. Biotechnol Prog. 1999;15:65–73. doi: 10.1021/bp9800743. [DOI] [PubMed] [Google Scholar]
- 52.Wykoff DD, O’Shea EK. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics. 2001;159:1491–1499. doi: 10.1093/genetics/159.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carroll AS, O’Shea EK. Pho85 and signaling environmental conditions. Trends Biochem Sci. 2002;27:87–93. doi: 10.1016/s0968-0004(01)02040-0. [DOI] [PubMed] [Google Scholar]
- 54.Lee YS, Mulugu S, York JD, O’Shea EK. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wykoff DD, Rizvi AH, Raser JM, Margolin B, O’Shea EK. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol Cell. 2007;27:1005–1013. doi: 10.1016/j.molcel.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groot MN, Klaassens E, de Vos WM, Delcour J, Hols P, Kleerebezem M. Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology. 2005;151:1229–1238. doi: 10.1099/mic.0.27375-0. [DOI] [PubMed] [Google Scholar]
- 57.Cho JH, Ko KM, Singaravelu G, Ahnn J. Caenorhabditis elegans PMR1, a P-type calcium ATPase, is important for calcium/manganese homeostasis and oxidative stress response. FEBS Lett. 2005;579:778–782. doi: 10.1016/j.febslet.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 58.Jabado N, Jankowski A, Dougaparsad A, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JA, Singleton S, Garrick LM. DMT1: a mammalian transporter for multiple metals. Biometals. 2003;16:41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- 60.Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stadtman ER, Berlett BS, Chock PB. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc Natl Acad Sci U S A. 1990;87:384–388. doi: 10.1073/pnas.87.1.384. [DOI] [PMC free article] [PubMed] [Google Scholar]