Abstract
Previous research into tooth crown dimensions and cusp proportions has proved to be a useful way to identify taxonomic differences in Pliocene and Pleistocene fossil hominins. The present study has identified changes in both M1 crown size and cusp proportions within the genus Homo, with M1 overall crown size reduction apparently occurring in two main stages. The first stage (a reduction of ca. 17%) is associated with the emergence of Homo ergaster and Homo erectus sensu stricto. The second stage (a reduction of ca. 10%) occurs in Homo sapiens, but the reduced modern human M1 tooth crown size was only attained in Upper Paleolithic times. The absolute sizes of the individual cusps are highly positively correlated with overall crown size and dental reduction produces a reduction in the absolute size of each of the cusps. Most of the individual cusps scale isometrically with crown size, but the paracone shows a negative allometric relationship, indicating that the reduction in paracone size is less than in the other M1 cusps. Thus, the phylogenetically oldest cusp in the upper molars also seems to be the most stable cusp (at least in the M1). The most striking change in M1 cusp proportions is a change in the relative size of the areas of the paracone and metacone. The combination of a small relative paracone and a large relative metacone generally characterizes specimens attributed to early Homo, and the presence of this character state in Australopithecus andParanthropus suggests it may represent the primitive condition for the later part of the hominin clade. In contrast, nearly all later Homo taxa, with the exception of Homo antecessor, show the opposite condition (i.e. a relatively large paracone and a relatively small metacone). This change in the relationship between the relative sizes of the paracone and metacone is related to an isometric reduction of the absolute size of the metacone. This metacone reduction occurs in the context of relative stability in the paracone as crown size decreases. Among later Homo taxa, both Homo heidelbergensis and Homo neanderthalensis show a further reduction of the metacone and an enlargement of the hypocone. Fossil and contemporary H. sapiens samples show a trend toward increasing the relative size of the protocone and decreasing the relative size of the hypocone. In Europe, modern human M1 cusp proportions are essentially reached during the Upper Paleolithic. Although some variation was documented among the fossil taxa, we suggest that the relative size of the M1 paracone and metacone areas may be useful for differentiating the earliest members of our genus from subsequent Homo species.
Keywords: cusp proportions, hominin, Homo, molar
Introduction
Studies of fossil hominin dental remains have led to the identification of species-specific patterns of dental morphology and have been useful for reconstructing the evolutionary relationships among fossil hominin taxa. Numerous studies have focused on tooth crown shape and on the details of occlusal and subocclusal morphology (Corruccini & McHenry, 1980; Wood & Abbott, 1983; Wood et al. 1983, 1988; Wood & Uytterschaut, 1987; Wood & Engleman, 1988; Brown, 1994; Bailey, 2004; Bailey & Lynch, 2005; Guatelli-Steinberg & Irish, 2005; Bailey & Wood, 2007; Gómez-Robles et al. 2007, 2008; Souday, 2008). Crown dimensions, as well as cusp base areas and their relative proportions have been shown to differentiate Plio-Pleistocene hominin taxa in both East and southern Africa (Wood & Uytterschaut, 1987; Wood & Engleman, 1988; Suwa et al. 1994, 1996; Boccone & Moggi-Cecchi, 2006; Moggi-Cecchi & Boccone, 2007). A number of studies have also considered the size of postcanine cusp areas in Pleistocene members of the genus Homo (Bermúdez de Castro & Nicolás, 1995; Bermúdez de Castro et al. 1999; Bailey, 2004; Bailey & Lynch, 2005; Trefný, 2005). The present study expands this line of research to a consideration of the M1 cusp areas in the entire genus Homo.
Recent discussions of crown shape and internal cusp arrangement in both the M1 and P4 have led to discussion about, and sometimes conflicting interpretations regarding, the polarity of certain morphological features (Bailey, 2004; Bailey & Lynch, 2005; Martinón-Torres et al. 2006; Gómez-Robles et al. 2007). For example, Bailey & Lynch (2005) proposed that the asymmetry of the Neandertal P4 was derived. This suggestion was based on the observation that the mean shape for contemporary modern humans was closer to that of Homo erectus than that of Neandertals. Unfortunately, their sample sizes precluded statistical analysis of this interpretation. In a subsequent study using larger sample sizes and a wider range of material (including Australopithecus and early Homo), Martinón-Torres et al. (2006) concluded that because asymmetry in the P4 is observed in Australopithecus, as well as certain early Homo and Homo erectus individuals, it most likely represents the primitive condition within the hominin clade. Moreover, they suggested that the internal arrangement of cusp tips contributed importantly to the overall shape of the tooth.
Bailey (2004) suggested that the skewed M1 shape found in Neandertals was likely derived for this group, but again lacked the samples of early hominins to formally test the hypothesis. Gómez-Robles et al. (2007) used a much larger sample that included Australopithecus and earlier Homo. Their assessment of polarity agreed with that of Bailey (2004), but they also found that, rather than it being exclusive to Neandertals, the same M1 shape was also found in earlier members of the Neandertal lineage (in which they would include Homo heidelbergensis, represented by specimens from Sima de los Huesos, Steinheim and Pontnewydd) as well as Homo antecessor. Studies such as these have highlighted the need to consider the relationship between internal cusp arrangement and the shape of the crown outline and also the necessity of including larger samples of a range of earlier and later hominins.
We focused our analysis on the M1 because, even within an individual, molars vary in both their morphological and metrical features (Bailey, 2002; Hlusko, 2002), such that combining molar types in a single analysis would not be warranted. The M1 was chosen for this study because it is considered to be the least variable of the upper molars (Dahlberg, 1945). In modern humans, at least, it is well understood that M3s are highly variable (Kraus et al. 1969) and the M1 has been found to be less variable (and more diagnostic) than the M2 in mid-late Pleistocene hominins (Bailey, 2002). Likewise, in baboons, heritability estimates for maxillary loph angle phenotypes are higher for the M1 than for the M2 (Hlusko et al. 2004). As such, the anatomical differences in the M1 should more closely reflect underlying genetic differentiation between populations, which is the soundest criterion for making evolutionary inferences in the fossil record (Lieberman, 1995). The recognition of consistent patterns of change in the dentition within the genus Homo could be useful for elucidating phylogenetic relationships among taxa and for clarifying the taxonomic identity of individual fossil hominin specimens, which are often fragmentary and/or consist only of isolated teeth.
Materials
The samples used in this study together with their sources are listed in Table 1. There is little consensus regarding the taxonomic allocations of specimens attributed to the earliest members of the genus Homo (Clarke & Howell, 1972; Rightmire, 1990; Tobias, 1991; Wood, 1991; Grine et al. 1993; Spoor et al. 2007). In addition, one of us (B.W.) has argued that the taxa Homo habilis and Homo rudolfensis should be removed from the genus Homo (Wood & Collard, 1999; Wood & Richmond, 2000; Collard & Wood, 2007). A recent study has also shown that some of the dental trends that characterize the genus Homo appear relatively late in human evolution (Bailey & Wood, 2007). Given the uncertainty surrounding which specimens should be included within the hypodigms of H. habilis and H. rudolfensis, we have taken a conservative approach and subsumed specimens from both East Africa and southern Africa into a pooled early Homo(non-ergaster) hypodigm.
Table 1.
Sample composition used in the present study
| Sample | Label | n | Specimens/Source |
|---|---|---|---|
| Australopithecus africanus | SAFGRA | 7 | Wood & Engleman (1988), Wood (1991) |
| Australopithecus afarensis | EAFGRA | 3 | Wood & Engleman (1988) |
| Paranthropus robustus | SAFROB | 17 | Wood & Engleman (1988), SK 14133, & SKX 3601 |
| Paranthropus boisei | EAFROB | 3 | Wood & Engleman (1988), Wood (1991) |
| Early Homo – South Africa | SAFHOM | 4 | SE 255, SK 27, SKX 268 & SKW 3114 |
| Early Homo – East Africa | EAFHOM | 14 | Wood & Engleman (1988), Wood (1991), A.L. 666-1, Omo P933-1 & Omo SH1-17 |
| Homo ergaster | EAFHERG | 1 | Bailey (2004) |
| Asian Homo erectus | ASIAHER | 5 | Bailey (2004), Sangiran 4, S7-3, S7-9, & S7-37 |
| Homo antecessor | HOMANT | 2 | Bermúdez de Castro et al. (1999) |
| Homo heidelbergensis(European Middle Pleistocene) | HOMHEID | 4 | Bailey (2004), Arago 31 |
| Homo neanderthalensis | NEAN | 21 | Bailey (2004), Arsuaga et al. (2007), St. Cesaire, Obi-Rakhmat, MonsempronPetit-Puymoyen, Pinilla del Valle, Arcy sur Cure 39 & 45 |
| Qafzeh | MPHSAP | 7 | Qafzeh 4, 5, 6, 9, 10, 11 & 15 |
| European Upper Paleolithic Homo sapiens | UPHSAP | 15 | Bailey (2004), Arsuaga et al. (2007), Parpalló 5, Abri Pataud #26.224 & #26.236Les Rois 19 & unnumbered, St. Germaine 2 & B7, Laugerie-Basse & Fontechevade |
| Contemporary Homo sapiens (Global sample) | HOMSAP | 59 | Bailey (2004) |
There is also considerable disagreement regarding which specimens should be assigned to H. erectus. Some scholars prefer to group African and Asian specimens together into a single variable species (Rightmire, 1990, 1998; Antón, 2002, 2003; Antón et al. 2007; Spoor et al. 2007), whereas others have recognized a specific distinction between the two groups (Andrews, 1984; Wood, 1994; Wood & Collard, 1999; Tattersall, 2007). In the present study, we restrict the use of the term H. erectus sensu stricto to the Asian specimens from Sangiran and Zhoukoudian and prefer H. ergaster as the taxonomic name for the earliest of the African fossils assigned to H. erectus. We recognize the European middle Pleistocene specimens assigned to H. heidelbergensis as forming part of the Neandertal evolutionary lineage (Arsuaga et al. 1993, 1997; Hublin, 1998), and although there are differences of opinion (Vandermeersch, 1981; Tillier, 1999; Schwartz & Tattersall, 2000, 2003), we have opted to assign the entire Qafzeh sample to Homo sapiens.
Data for specimens attributed to early Homo are drawn primarily from studies where original specimens were measured (Wood & Engleman, 1988; Wood, 1991). After cross-checking the published data with measurements taken independently on high-resolution casts we found only a single discrepancy (OH 21), and in this case our assessment of tooth orientation in the two photos led us to use the measurements taken on the cast. We also excluded the specimens KNM-ER 807 and KNM-ER 808 because of crown damage. We augmented our sample by including a number of specimens that were either not considered in earlier publications or discovered more recently (Table 1). From East Africa, these include A.L. 666-1, Omo P933-1 and Omo SH1-17, all of which have been attributed to Homo (Coppens, 1980; Howell et al. 1987; Kimbel et al. 1996). From southern Africa, we included SK 27, SKX 268 and SKW 3114 from Swartkrans and SE 255 from Sterkfontein, which have also been attributed to Homo (Clarke, 1977; Tobias, 1978; Grine, 1993, 2005; Grine & Strait, 1994). All of the latter data were collected on the original specimens.
Most of the data for later members of the genus Homo were taken from recent studies (Bermúdez de Castro et al. 1999; Bailey, 2002, 2004; Arsuaga et al. 2007). However, the present study also includes several H. erectus s. s. individuals (n = 4) from Sangiran whose cusp areas have, until now, been unpublished. In addition, the Qafzeh sample (n = 7), as well as those of H. neanderthalensis (n = 21) and Upper Paleolithic H. sapiens (n = 15) have been substantially augmented since an earlier study (Bailey, 2004). We have also included data from a geographically diverse sample (n = 59) of contemporary H. sapiens (Bailey, 2002). All the data was collected on original specimens.
To clarify the polarity of cusp areas and relationships within the genus Homo, data on early hominin specimens representing the genera Australopithecus and Paranthropus were also considered. Although there are important dental differences between Australopithecus andParanthropus, both show a similar trend toward trait intensification, including additional cusps on the maxillary molars (Bailey & Wood, 2007). Since our focus is on the genus Homo, we combined the Australopithecus and Paranthropus specimens within a single pooled early hominin sample to serve as an outgroup comparison for early Homo. We relied primarily on published data (Wood & Engleman, 1988; Wood, 1991) collected on original specimens. After cross-checking against measurements taken on high-resolution casts, only a single specimen (Sts 56) departed from the published measurements, and for this tooth we again used the cast measurements. We augmented the early hominin sample with data collected on the original specimens SK 14133 and SKX 3601, both of which have been attributed to Paranthropus robustus (Grine, 1989, 1993; Grine & Strait, 1994). We excluded some southern African specimens included in Wood & Engleman (1988): Sts 21 was judged to be too worn to reliably identify the intercusp fissures, and Sts 52a and Sts 57 show several large cracks in the enamel surface that affect the cusp base areas. We excluded TM 1512 because of damage to the crown outline and significant wear. Finally, we excluded LH 11 as in the original description of this tooth White (1977) classified it as an M2.
Methods
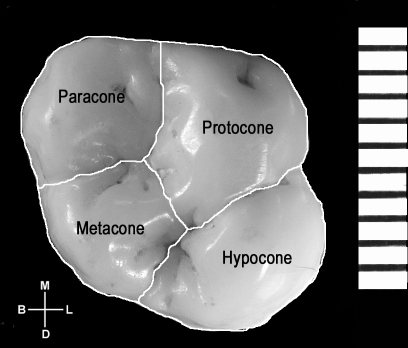
The technique for measuring the M1 cusp base areas has been outlined in previous studies (Wood & Engleman, 1988; Bailey, 2004). Briefly, an occlusal photograph of each tooth was taken with the cervical margin oriented perpendicular to the axis of the camera lens and a millimeter scale was included within each photo (Fig. 1). The scale included in each photograph was used to calibrate the image for measurement of the crown and cusp areas using either a planimeter (Wood & Engleman, 1988) or computer software (Bailey, 2004). This technique has been shown to produce reliable results between observers when applied to cusp base areas (Wood & Abbott, 1983; Bailey et al. 2004).
Fig. 1.
Occlusal photograph of the Krapina D171 M1 with the cusps outlined to illustrate the measurement methodology.
We measured individual cusp base areas by tracing the outline of the cusp, following the main fissures in the occlusal surface. We excluded teeth suffering from crown wear or damage that has obscured the fissure pattern or prevented an accurate assessment of crown area. Occasionally, wear obscured the course of the main fissures toward the edge of the tooth. In these cases, we estimated the course of the fissure by extrapolating a line from where the main fissure was eroded to the crown edge. The areas of accessory cusps were divided between the adjacent main cusps [e.g. the area of the metaconule (cusp 5) was divided between the metacone and the hypocone]. When both the right and left M1 were present and equally well preserved in the same individual, the data for the left side were used. Otherwise, the tooth best preserved and least worn was used. The total crown base area (TCBA) was calculated as the sum of the absolute cusp base areas. The relative size of each cusp was determined by dividing the absolute cusp area by the TCBA.
In addition, we examined the size of each of the four main cusps relative to each other to investigate changes in cusp relationships (e.g. paracone larger or smaller than the metacone). As individual cusp sizes in some specimens may actually differ very little, characterizing one cusp as larger than the other may be overemphasizing what is in reality a minor difference. To avoid this mischaracterization, we have considered cusps that differ by less than the known interobserver measurement error (Bailey et al. 2004) to be equal in size. The difference in relative cusp sizes recorded by Bailey et al. (2004) ranged from 0.1% for both the paracone and hypocone to 1.1% for the metacone. This suggests that, as a general rule, individual cusps that differ in their relative sizes by less than or equal to 1.0% (≤ 1.0%) should be considered equal in size. Thus, for example, the relative sizes of the paracone (23.7%) and metacone (24.1%) in SK 1591 (P. robustus) were deemed to be equal in size, as the difference between them is less than 1.0%. Throughout our analysis, we have relied on this criterion when discussing the size of one cusp relative to another.1 In the rest of the text we use ‘area’ as a proxy for the 2D areas of either the whole crown or of individual cusps.
We used basic descriptive statistics of crown and cusp base areas to investigate absolute and relative cusp size differences among hominin groups. The small sample sizes for some hominin taxa result in a higher probability that some groups depart from a normal distribution. Thus, the non-parametric Mann–Whitney test was used to determine statistical significance in the differences between groups.
The presence or absence of allometric trends in the absolute cusp areas in each Homo group (Table 1) was assessed following the techniques described in Hills et al. (1983). No correlation between TCBA and absolute cusp areas indicates isometry, significant positive correlation indicates positive allometry, and significant negative correlation indicates negative allometry. If changes in absolute cusp area are isometric, then relative cusp areas should remain relatively stable (apart from random variation), suggesting they are largely independent of TCBA. A value for the allometric coefficient can then be calculated from the slope and its standard error for the least squares regression line between log absolute cusp area and log TCBA (Hills et al. 1983). For the analysis of allometric trends across the genus Homo(i.e. between groups or taxa), mean values were used for each group due to differences in sample size.
Results
Total crown base area and absolute cusp size in the genus Homo
Within the pooled early Homo sample, the mean values for the TCBA and the absolute areas of individual cusps are larger in the southern African specimens than in their East African counterparts (Table 2). However, this difference is only significant (P < 0.05) for the paracone. In contrast, none of the relative cusp areas differs significantly between the southern African and East African specimens.
Table 2.
Total crown base area (TCBA) and absolute and relative M1 cusp areas in fossil and living hominins
| Relative |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample (n) | Total Crown Base Area (mm2)* Mean ± SD | Protocone Area (mm2) Mean ± SD | Paracone Area (mm2) Mean ± SD | Metacone Area (mm2) Mean ± SD | Hypocone Area (mm2) Mean ± SD | Protocone Area (%) Mean ± SD | Paracone Area (%) Mean ± SD | Metacone Area (%) Mean ± SD | Hypocone Area (%) Mean ± SD |
| SAFGRA (7) | 150.6 ± 17.4 | 45.9 ± 6.4 | 31.1 ± 2.6 | 36.6 ± 3.4 | 36.9 ± 8.3 | 30.5 ± 2.6 | 20.7 ± 1.2 | 24.5 ± 2.2 | 24.3 ± 2.6 |
| EAFGRA (2) | 150.9 ± 11.3 | 49.6 ± 4.8 | 34.1 ± 0.3 | 36.0 ± 4.3 | 31.3 ± 2.5 | 32.8 ± 0.7 | 22.6 ± 1.9 | 23.8 ± 1.0 | 20.7 ± 0.1 |
| SAFROB (17) | 156.7 ± 14.0 | 45.1 ± 4.3 | 34.7 ± 3.8 | 39.9 ± 4.9 | 37.1 ± 5.0 | 28.8 ± 1.8 | 22.1 ± 1.7 | 25.4 ± 1.8 | 23.6 ± 2.0 |
| EAFROB (3) | 188.7 ± 13.4 | 56.7 ± 4.4 | 41.1 ± 2.6 | 46.4 ± 2.9 | 44.1 ± 7.1 | 30.1 ± 0.7 | 21.8 ± 2.1 | 24.6 ± 0.4 | 23.3 ± 2.2 |
| Pooled non-Homo (29) | 158.1 ± 17.7 | 46.8 ± 5.9 | 34.3 ± 4.2 | 39.3 ± 5.1 | 37.4 ± 6.3 | 29.6 ± 2.2 | 21.8 ± 1.7 | 25.0 ± 1.8 | 23.6 ± 2.2 |
| SAFHOM (4) | 144.7 ± 8.1 | 43.1 ± 6.6 | 34.4 ± 1.5 | 35.4 ± 2.0 | 31.8 ± 1.2 | 29.7 ± 3.0 | 23.8 ± 0.7 | 24.5 ± 0.8 | 22.1 ± 2.0 |
| EAFHOM (14) | 132.1 ± 15.9 | 38.4 ± 4.6 | 30.2 ± 3.3 | 32.9 ± 5.1 | 30.5 ± 6.1 | 29.1 ± 1.9 | 23.0 ± 2.4 | 24.9 ± 2.2 | 23.0 ± 2.5 |
| Pooled early Homo (18) | 134.9 ± 15.3 | 39.5 ± 5.3 | 31.1 ± 3.5 | 33.5 ± 4.6 | 30.8 ± 5.4 | 29.3 ± 2.1 | 23.2 ± 2.2 | 24.8 ± 2.0 | 22.8 ± 2.4 |
| EAFHERG (1) | 99.6 | 29.4 | 27.3 | 25.9 | 17.1 | 29.5 | 27.4 | 26.0 | 17.2 |
| ASIAHER (5) | 115.5 ± 6.8 | 34.5 ± 3.0 | 28.7 ± 2.7 | 26.5 ± 3.2 | 25.8 ± 1.6 | 29.9 ± 2.2 | 24.9 ± 2.3 | 22.9 ± 1.7 | 22.3 ± 0.4 |
| HOMANT (2)** | 120.5 | 35.31 | 27.59 | 29.88 | 27.47 | 29.3 | 22.9 | 24.8 | 22.8 |
| HOMHEID (4) | 115.5 ± 17.0 | 34.8 ± 6.8 | 28.3 ± 4.8 | 24.2 ± 4.8 | 28.2 ± 3.0 | 31.1 ± 3.7 | 24.8 ± 0.9 | 20.1 ± 2.6 | 24.0 ± 1.6 |
| NEAN (21) | 112.3 ± 16.6 | 33.7 ± 6.1 | 28.8 ± 4.2 | 22.9 ± 3.9 | 26.8 ± 5.1 | 29.9 ± 2.4 | 25.8 ± 2.1 | 20.6 ± 1.8 | 23.7 ± 2.1 |
| MPHSAP (7) | 111.3 ± 12.7 | 34.7 ± 2.0 | 27.5 ± 2.4 | 23.7 ± 3.7 | 25.8 ± 7.8 | 31.3 ± 2.3 | 24.8 ± 1.6 | 21.3 ± 2.5 | 22.8 ± 5.0 |
| UPHSAP (15) | 99.6 ± 10.2 | 31.7 ± 3.6 | 25.2 ± 2.4 | 22.6 ± 3.3 | 20.3 ± 3.7 | 31.8 ± 1.5 | 25.7 ± 2.3 | 22.4 ± 1.7 | 20.1 ± 3.0 |
| Pooled fossil later Homo (55) | 109.0 ± 14.5 | 33.3 ± 4.7 | 27.5 ± 3.6 | 23.5 ± 3.8 | 24.8 ± 5.5 | 30.6 ± 2.2 | 25.5 ± 2.1 | 21.5 ± 2.0 | 22.5 ± 3.1 |
| HOMSAP (59) | 96.6 ± 14.0 | 29.9 ± 4.8 | 24.8 ± 3.7 | 22.1 ± 3.9 | 19.7 ± 3.8 | 31.0 ± 2.0 | 25.8 ± 2.1 | 22.9 ± 1.8 | 20.4 ± 2.5 |
| Pooled later Homo (114) | 102.2 ± 15.4 | 31.4 ± 5.0 | 26.0 ± 3.8 | 22.7 ± 3.9 | 22.0 ± 5.3 | 30.8 ± 2.1 | 25.6 ± 2.1 | 22.2 ± 2.0 | 21.4 ± 3.0 |
Sample labels follow Table 1.
Total crown base area is equal to the sum of the individual cusp areas.
Absolute cusp areas calculated from the relative areas and TCBA in Bermúdez de Castro et al. (1999).
Although there is some overlap among specimens, the TCBA of the pooled early Homo sample is significantly larger (P < 0.05) than that of later members of the genus (Table 3). The only exception is the small H. heidelbergensis sample, which contains both large (Petralona = 134.5 mm2) and small (Pontnewydd 4 = 101.9 mm2) individuals. Within later archaic Homo(i.e. non-Homo sapiens) groups, the TCBAs do not differ significantly from one another, but they are significantly larger than that of most Homo sapiens groups (Tables 2 and 3). A notable exception is the single H. ergaster specimen (KNM-WT 15 000), which at 99.6 mm2 falls below the lower limit of the H. erectus s. s. range of variation (105.2–124.1 mm2) and is similar in size to Upper Paleolithic and contemporary H. sapiens. In contrast to these latter samples, the Qafzeh sample maintains the large TCBA of later archaic Homo.
Table 3.
P-values for the comparison of the total crown base area (TCBA) in the genus Homo based on the Mann–Whitney-U non-parametric tests
| Sample | Pooled early Homo | ASIAHER | HOMHEID | NEAN | HOMHEID + NEAN | Pooled archaic Homo | MPHSAP | UPHSAP |
|---|---|---|---|---|---|---|---|---|
| ASIAHER | ** | |||||||
| HOMHEID | N.S. | N.S. | ||||||
| NEAN | ** | N.S. | N.S. | |||||
| HOMHEID + NEAN | ** | N.S. | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | ** | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | ** | ** | N.S. | ** | ** | ** | * | |
| HOMSAP | ** | ** | N.S. | ** | ** | ** | ** | N.S. |
| UPHSAP + HOMSAP | ** | ** | * | ** | ** | ** | ** | – |
| Pooled Homo sapiens | ** | ** | N.S. | ** | ** | ** | – | – |
Significant at P < 0.05
significant at P < 0.01. Sample labels follow Table 1. Pooled early Homo includes both southern and East African specimens
Pooled archaic Homo includes all non-Homo sapiens specimens. Pooled Homo sapiens includes Qafzeh, Upper Paleolithic and contemporary samples.
Homo ergaster and Homo antecessor were not analyzed individually due to small sample size but were included in pooled archaic Homo sample
The results for the absolute cusp base areas are similar to those for TCBA. The pooled early Homo sample shows larger mean values for each of the cusp base areas compared to later members of the genus (Table 2). Generally speaking, these differences are significant (Table 4), although they are less pronounced (only significant for a single cusp) compared to H. erectus and H. heidelbergensis. Notably, one variable – the absolute metacone size – is significantly larger in the pooled early Homo sample than it is in all later Homo taxa. Conversely, among the later Homo taxa this variable showed the fewest significant differences (Table 4). The European Upper Paleolithic and contemporary H. sapiens samples have the smallest absolute cusp areas (Table 2), and are significantly different from most of the other later Homo groupings, particularly with respect to the size of the hypocone.
Table 4.
Significance of between-group comparisons of M1 absolute cusp size in the genus Homo based on Mann–Whitney U non-parametric tests
| Sample | Pooled early Homo | ASIAHER | HOMHEID | NEAN | HOMHEID + NEAN | Pooled archaic Homo | MPHSAP | UPHSAP |
|---|---|---|---|---|---|---|---|---|
| Protocone | ||||||||
| ASIAHER | N.S. | |||||||
| HOMHEID | N.S. | N.S. | ||||||
| NEAN | ** | N.S. | N.S. | |||||
| HOMHEID + NEAN | ** | N.S. | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | * | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | ** | N.S. | N.S. | N.S. | N.S. | * | * | |
| HOMSAP | ** | * | N.S. | ** | ** | ** | ** | N.S. |
| UPHSAP + HOMSAP | ** | * | N.S. | N.S. | N.S. | ** | N.S. | – |
| Pooled Homo sapiens | ** | * | N.S. | * | * | ** | – | – |
| Paracone | ||||||||
| ASIAHER | N.S. | |||||||
| HOMHEID | N.S. | N.S. | ||||||
| NEAN | * | N.S. | N.S. | |||||
| HOMHEID + NEAN | * | N.S. | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | * | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | ** | * | N.S. | ** | ** | ** | N.S. | |
| HOMSAP | ** | * | N.S. | ** | ** | ** | * | N.S. |
| UPHSAP + HOMSAP | ** | * | N.S. | N.S. | N.S. | ** | N.S. | – |
| Pooled Homo sapiens | ** | * | N.S. | ** | ** | ** | – | – |
| Metacone | ||||||||
| ASIAHER | * | |||||||
| HOMHEID | * | N.S. | ||||||
| NEAN | ** | * | N.S. | |||||
| HOMHEID + NEAN | ** | N.S. | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | ** | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | ** | * | N.S. | N.S. | N.S. | ** | N.S. | |
| HOMSAP | ** | * | N.S. | N.S. | N.S. | ** | N.S. | N.S. |
| UPHSAP + HOMSAP | ** | ** | N.S. | * | N.S. | ** | N.S. | – |
| Pooled Homo sapiens | ** | * | N.S. | N.S. | N.S. | ** | – | – |
| Hypocone | ||||||||
| ASIAHER | N.S. | |||||||
| HOMHEID | N.S. | N.S. | ||||||
| NEAN | * | N.S. | N.S. | |||||
| HOMHEID + NEAN | * | N.S. | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | ** | ** | * | ** | ** | ** | N.S. | |
| HOMSAP | ** | ** | ** | ** | ** | ** | * | N.S. |
| UPHSAP + HOMSAP | ** | ** | ** | ** | ** | ** | N.S. | – |
| Pooled Homo sapiens | ** | ** | ** | ** | ** | ** | – | – |
Significant at P < 0.05
significant at P < 0.01. Sample labels follow Table 1. Pooled early Homo includes both southern and East African specimens.
Pooled archaic Homo includes all non-Homo sapiens specimens. Pooled Homo sapiens includes Qafzeh, Upper Paleolithic and contemporary samples
Homo ergaster and Homo antecessor were not analyzed individually due to small sample size but were included in pooled archaic Homo sample
Relative cusp size in the genus Homo
When the relative cusp areas of early Homo are compared with those of later members of the genus, the most obvious difference is that relative metacone size is significantly smaller in all the later taxa (Tables 2 and 5). Although a statistical comparison cannot be made with H. ergaster (n = 1), the single individual included in this taxon shows a large relative metacone area. Thus, the decrease in relative metacone area begins with the appearance of H. erectus s. s. We also note a significant increase in the relative paracone area in most Homo taxa that postdate H. heidelbergensis samples. In contrast, the relative protocone area in the early Homo sample is only significantly different from the H. sapiens samples, whereas that of the hypocone differs only from Upper Paleolithic and contemporary H. sapiens. A notable departure from these general patterns is found in the smallH. antecessor sample (n = 2), which possesses relative cusp areas that are quite similar to early Homo (Table 2).
Table 5.
Significance of between-group comparisons of M1 relative cusp area in the genus Homo based on Mann–Whitney U non-parametric tests
| Pooled early Homo | ASIAHER | HOMHEID | NEAN | HOMHEID + NEAN | Pooled archaic Homo | MPHSAP | UPHSAP | |
|---|---|---|---|---|---|---|---|---|
| Protocone | ||||||||
| ASIAHER | N.S. | |||||||
| HOMHEID | N.S. | N.S. | ||||||
| NEAN | N.S. | N.S. | N.S. | |||||
| HOMHEID + NEAN | N.S. | N.S. | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | * | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | ** | N.S. | N.S. | ** | * | ** | N.S. | |
| HOMSAP | ** | N.S. | N.S. | * | N.S. | ** | N.S. | N.S. |
| UPHSAP + HOMSAP | ** | N.S. | N.S. | * | N.S. | ** | N.S. | – |
| Pooled Homo sapiens | ** | N.S. | N.S. | * | N.S. | ** | – | – |
| Paracone | ||||||||
| ASIAHER | N.S. | |||||||
| HOMHEID | N.S. | N.S. | ||||||
| NEAN | ** | N.S. | N.S. | |||||
| HOMHEID + NEAN | ** | N.S. | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | ** | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| HOMSAP | ** | N.S. | N.S. | N.S. | N.S. | ** | N.S. | N.S. |
| UPHSAP + HOMSAP | ** | N.S. | N.S. | N.S. | N.S. | ** | N.S. | – |
| Pooled Homo sapiens | ** | N.S. | N.S. | N.S. | N.S. | * | – | – |
| Metacone | ||||||||
| ASIAHER | * | |||||||
| HOMHEID | ** | N.S. | ||||||
| NEAN | ** | ** | N.S. | |||||
| HOMHEID + NEAN | ** | ** | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | ** | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | ** | N.S. | N.S. | ** | ** | N.S. | N.S. | |
| HOMSAP | ** | N.S. | * | ** | ** | N.S. | N.S. | N.S. |
| UPHSAP + HOMSAP | ** | N.S. | * | ** | ** | N.S. | N.S. | – |
| Pooled Homo sapiens | ** | N.S. | * | ** | ** | N.S. | – | – |
| Hypocone | ||||||||
| ASIAHER | N.S. | |||||||
| HOMHEID | N.S. | N.S. | ||||||
| NEAN | N.S. | N.S. | N.S. | |||||
| HOMHEID + NEAN | N.S. | N.S. | – | – | ||||
| Pooled archaic Homo | – | – | – | – | – | |||
| MPHSAP | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | ||
| UPHSAP | * | N.S. | * | ** | ** | ** | N.S. | |
| HOMSAP | ** | * | ** | ** | ** | ** | N.S. | N.S. |
| UPHSAP + HOMSAP | ** | * | ** | ** | ** | ** | N.S. | – |
| Pooled H. sapiens | ** | N.S. | * | ** | ** | ** | – | – |
Significant at P < 0.05;
significant at P < 0.01. Sample labels follow Table 1. Pooled early Homo includes both southern and East African specimens.
Pooled archaic Homo includes all non-Homo sapiens specimens. Pooled Homo sapiens includes Qafzeh, Upper Paleolithic and contemporary samples.
Homo ergaster and Homo antecessor were not analyzed individually due to small sample size but were included in pooled archaic Homo sample.
There are no significant differences in relative protocone area between H. erectus s. s. and the H. heidelbergensis/H. neanderthalensis lineage (Table 5), but there is a small increase in the relative size of the protocone in the H. sapiens samples (Table 2). This difference is not significant when the H. sapiens sample is compared with H. erectus s. s., but it does reach statistical significance when it is compared with both Neandertals and the pooled archaic Homo sample. The relative area of the paracone is considerably larger in the single H. ergaster specimen than in early Homo, and the remaining later Homo taxa also have a large relative paracone area (Tables 2 and 5). The only statistically significant difference in relative metacone area among later Homo is its small size in the H. heidelbergensis/H. neanderthalensis lineage. The relative area of the hypocone is largest in specimens that comprise the H. heidelbergensis/H. neanderthalensis lineage and smallest in Upper Paleolithic and contemporary H. sapiens samples.
Thus, within later Homo, H. erectus s. s. shows a reduction in the relative area of the metacone and an increase in the relative area of the paracone. With the exception of H. antecessor, the same pattern is seen in the other later Homo taxa, and a small metacone has been put forward as a possible derived feature of the H. heidelbergensis/H. neanderthalensis lineage (Bailey, 2004). Finally, a reduction in the relative area of the hypocone is not seen until the Upper Paleolithic and contemporary samples of H. sapiens.
Allometric trends in cusp size
In general, absolute cusp size is highly positively correlated with TCBA (0.83 < r < 0.97) across the genus Homo. So reductions in crown size are associated with reductions in the absolute size of each of the four main cusps. However, the changes in relative cusp size in the genus Homo discussed above indicate an allometric relationship between the absolute size of individual cusps and the TCBA. These allometric trends in M1 cusp proportions were assessed within each Homo group.
The only significant correlation between relative cusp size and TCBA in any Homo taxon was a strong negative correlation (r = –0.86) with the protocone in the Qafzeh sample. Although the sample size is small (n = 7), this relationship remains significant even when a Bonferroni correction is applied. Changes in crown size appear to account for about 74% of the variation in the relative size of the protocone. Thus, smaller teeth in the Qafzeh sample are associated with relatively larger protocones. The allometric coefficient of protocone area in the Qafzeh sample (0.42 ± 0.14) was calculated from the slope and its standard error for the least squares regression line between log absolute cusp area and log TCBA. None of the remaining Homo taxa showed any statistically significant departures from isometry in any of the individual cusps. This was also the case in the pooled early Homo sample, which may include more than one taxon.
Relationships of the relative cusp areas across the genus Homo were similarly assessed using mean group values (Tables 2 and 6). The relative areas of the anterior cusps show negative correlations with TCBA, whereas the posterior cusps show positive correlations with crown size. However, the only significant correlation between relative cusp size and TCBA was a strong negative correlation (r = –0.83) with the paracone. The results for the hypocone (r = 0.66; P = 0.051) are suggestive of a positive allometric relationship, but the present sample does not allow rejection of isometry. The slope and its standard error can be calculated from the least squares regression line between log absolute paracone area and log TCBA to provide a value (0.57 ± 0.17) for the allometric coefficient of the paracone across the genus Homo (Hills et al. 1983) (Table 7). Thus, as tooth size decreases, so does the absolute size of the paracone. However, the reduction in paracone size is less than in the other cusps in the M1, resulting in an increase in the relative paracone size as tooth size decreases (Table 7).
Table 6.
Correlations (r) between tooth size and relative cusp proportions in the genus Homo *
| Total crown base area (TCBA) | |
|---|---|
| Relative protocone area | –0.54 |
| Relative paracone area | –0.83 |
| Relative metacone area | 0.09 |
| Relative hypocone area | 0.66 |
Relies on group means (n = 9) (Table 2).
Values in bold indicate significance at P < 0.05.
Table 7.
Regression slopes and allometric scaling of log absolute cusp area (X) to log TCBA (M) in the genus Homo*
| Regression Line |
Correlation between |
||||||
|---|---|---|---|---|---|---|---|
| Cusp | Intercept | Slope | SE of Slope | X, M | X-M, M | Allometry ** | |
| Protocone | –0.17 | 0.83 | 0.09 | 0.97 | –0.63 | Isometry | |
| Paracone | 0.28 | 0.57 | 0.17 | 0.89 | –0.83 | – | |
| Metacone | –0.77 | 1.06 | 0.22 | 0.81 | 0.08 | Isometry | |
| Hypocone | –2.05 | 1.68 | 0.16 | 0.91 | 0.66 | Isometry | |
Homo groups included in analysis (n = 9) are those listed in Table 1.
The relationship was deemed to depart from isometry if the correlation between X-M and M was significant at P < 0.05.
Polarity of cusp area proportions in early Homo
Data for Australopithecus and Paranthropus provide some evolutionary context for interpreting differences in relative cusp areas between the early and later Homo samples. When the pooled non-Homo sample is compared with the pooled early Homo sample, only relative paracone size is significantly different, being larger in early Homo (Table 8). This suggests that, with the exception of a slight increase in the relative size of the paracone (Table 2), early Homo specimens preserve the likely primitive condition for the hominin clade. Later archaic Homo taxa further differ from the early hominins in showing a significantly smaller relative metacone area. Finally, when the pooled Homo and non-Homo samples are compared, the differences in relative cusp base area are significant (P < 0.05) for all four main cusps (Table 8). Compared with the non-Homo specimens, Homo has a relatively larger protocone and paracone and a relatively smaller metacone and hypocone. Of the four main cusps, temporally the paracone was the first to change its relative size, followed by the metacone, protocone and hypocone, in that order.
Table 8.
P-values for the comparison of the relative cusp areas between the pooled non-Homo group and those of Homo based on Mann–Whitney U non-parametric tests
| Pooled non-Homo (n = 29) vs. | PooledEarly Homo (n = 18) | Pooled laterArchaic Homo (n = 32)* | PooledHomo (n = 131) |
|---|---|---|---|
| Relative protocone size | 0.526 | 0.410 | 0.018 |
| Relative paracone size | 0.019 | < 0.001 | < 0.001 |
| Relative metacone size | 0.599 | < 0.001 | < 0.001 |
| Relative hypocone size | 0.294 | 0.823 | < 0.001 |
Values in bold indicate significance at P < 0.05.
MPHSAP, UPHSAP and HOMSAP samples have been removed.
The paracone/metacone shift
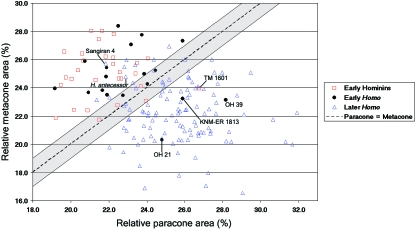
The early hominin genera Australopithecus and Paranthropus show a relative paracone area which is smaller than that of the metacone (Table 2). Despite a slight increase in the relative paracone area, early Homo specimens also generally show this same relationship. Thus, this would appear to represent the primitive condition for the genus Homo. In later taxa, including the single H. ergaster individual KNM-WT 15 000 and H. erectus s. s., the relative area relationships have shifted so that the relative area of the paracone is now larger than that of the metacone. We refer to this change in cusp proportions as the ‘paracone/metacone shift’. Figure 2 illustrates this shift by plotting these two variables against one another. Specimens attributed to Australopithecus and Paranthropus and the pooled early Homo sample generally fall above the region of equal-sized cusps, indicating a relative metacone area that is larger than that of the paracone. In contrast, the vast majority of specimens from later Homo taxa fall below the region of equal-sized cusps, indicating the opposite relationship. Thus, there appears to be a fairly clear division within the genus Homo when the relative areas of the paracone and metacone are compared with one another.
Fig. 2.
Bivariate plot of the relative paracone area vs. the relative metacone area across the hominin sample. Specimens that fall within the shaded area between the solid lines are considered to show equal-sized cusps (see Methods). Specimens attributed to Australopithecus, Paranthropus and early Homo largely fall above the region of equal-sized cusps, indicating they combine a large relative metacone with a small relative paracone. In contrast, most later Homo taxa fall below this same region, indicating the opposite relationship. The positions of a few specimens which depart from this general pattern are indicated.
We scored each Homo, Australopithecus and Paranthropus specimen as having: (1) a larger relative paracone area, (2) a larger relative metacone area or (3) relative paracone and metacone areas that are approximately equal (see Methods). Within the pooled early hominin sample (i.e. Australopithecus and Paranthropus) 83% of the specimens show the primitive condition of a relative metacone area that is larger than the relative paracone area (Table 9). Only a single individual (TM 1601) shows the derived condition of a relative paracone area that is larger than the metacone, while 13% of the specimens show equal-sized cusps.
Table 9.
Relationship between the relative areas of the M1 paracone and metacone in fossil and living hominins
| Sample (n) | Relative metacone larger than relative paracone % (n) | Relative metacone smaller than relative paracone % (n) | Cusps equal in size * % (n) |
|---|---|---|---|
| SAFGRA (7) | 85.7 (6) | 0.0 (0) | 14.3 (1) |
| EAFGRA (3) | 66.7 (2)** | 0.0 (0) | 33.3 (1) |
| SAFROB (17) | 82.3 (14) | 5.9 (1) | 11.8 (2) |
| EAFROB (3) | 100.0 (3) | 0.0 (0) | 0.0 (0) |
| Pooled non-Homo (30) | 83.3 (25) | 3.3 (1) | 13.3 (4) |
| SAFHOM (4) | 25.0 (1) | 00.0 (0) | 75.0 (3) |
| EAFHOM (14) | 78.6 (11) | 21.4 (3) | 0.0 (0) |
| Pooled Early Homo (18) | 66.7 (12) | 16.7 (3) | 16.7 (3) |
| EAFHERG (1) | 0.0 (0) | 100.0 (1) | 0.0 (0) |
| ASIAHER (5) | 20.0 (1) | 80.0 (4) | 0.0 (0) |
| HOMANT (2) | 100.0 (2) | 0.0 (0) | 0.0 (0) |
| HOMHEID (4) | 0.0 (0) | 100.0 (4) | 0.0 (0) |
| NEAN (21) | 0.0 (0) | 100.0 (21) | 0.0 (0) |
| Pooled archaic Later Homo (33) | 9.1 (3) | 90.9 (30) | 0.0 (0) |
| MPHSAP (7) | 0.0 (0) | 85.7 (6) | 14.3 (1) |
| UPHSAP (15) | 6.7 (1) | 80.0 (12) | 13.3 (2) |
| Pooled fossil later Homo (55) | 7.3 (4) | 87.3 (48) | 5.4 (3) |
| HOMSAP (59) | 11.9 (7) | 76.3 (45) | 11.9 (7) |
| Pooled later Homo (114) | 9.6 (11) | 81.6 (93) | 8.8 (10) |
Sample labels follow Table 1.
Cusps were considered to be equal in size if their relative areas differed by ≤ 1.0 (see text for discussion).
Includes specimen LH 21 based on absolute cusp areas (see text for discussion).
Within the pooled early Homo sample, 67% of the specimens show the primitive condition. Derived cusp proportions are found in three East African individuals (KNM-ER 1813, OH 21 & OH 39) (Fig. 2), and equal-sized paracone and metacone are found in three of the southern African specimens (SE 255, SKX 268 & SKW 3114) (Table 9). In contrast, the pooled later Homo sample shows an overwhelming predominance (c. 82%) of the derived condition, while the primitive cusp proportions are still present in just less than 10% of the specimens and ~9% of the specimens have equal-sized cusps. Thus, the primitive cusp proportions can still occasionally be found in later Homo samples, including ca. 12% of modern humans. Among the later archaic Homo specimens, both H. antecessor and the H. erectus s. s. specimen Sangiran 4 retained the primitive condition (Fig. 2).
The effects of tooth size
To investigate whether the paracone/metacone shift is related to differences in overall crown area (TCBA) or the absolute size of any individual cusps, the relative sizes of the paracone and metacone were expressed as a ‘paracone/metacone index’ (relative paracone area × 100/relative metacone area). Lower values for this index indicate a relatively large metacone or a relatively small paracone and are generally associated with the primitive condition for the hominin M1.
A strong significant negative correlation (r = –0.85) was found only with the absolute size of the metacone, indicating that changes in the absolute size of this cusp can explain about 72% of the variation in the paracone/metacone index. None of the other individual absolute cusp areas, or the TCBA, showed significant correlations with the paracone/metacone index. This would suggest that higher values for this index (associated with the derived condition) are not related to overall tooth size, but appear mainly due to a reduction in the absolute size of the metacone across the genus Homo.
Discussion
The preceding analyses have demonstrated significant variation in M1 crown size and cusp proportions within the genus Homo.
Cusp proportions in early Homo – maintenance of a primitive pattern
In 83% of Australopithecus and Paranthropus specimens the relative area of the metacone exceeds that of the paracone, whereas the opposite configuration only occurs in a single P. robustus individual (TM 1601). Johanson et al. (1982) also noted that three of four Au. afarensis individuals (75%) possessed a metacone larger than the paracone; the fourth individual had subequal cusps. Moggi-Cecchi & Boccone (2007) showed that metacone > paracone is the predominant M1 pattern in their sample of southern African early hominins.
Despite a slight increase in the relative size of the paracone, this same size relationship (relative metacone > relative paracone) is found in two-thirds of the early Homo specimens in the present study. It is present in each of the three earliest (> 2.0 Ma) specimens attributed to the genus Homo (A.L. 666-1, Omo SH1-17 & Omo P933-1). Among younger (< 2.0 Ma) early Homo specimens, this primitive pattern is recognized in seven of nine Olduvai Gorge specimens, one from Koobi Fora (KNM-ER 1590), and one (SK 27) from Swartkrans. In contrast, in three early Homo individuals from East Africa (KNM-ER 1813, OH 21 and OH 39) the relative paracone area is larger than that of the metacone. The high prevalence of the relative metacone > relative paracone pattern in Australopithecus and Paranthropus suggests that teeth belonging to fossils assigned to early Homo preserve the likely primitive condition for the hominin clade.
The opposite pattern of the relative metacone area larger than that of the paracone (i.e. relative paracone > relative metacone) is seen in most later Homo taxa, and this shift is the most striking change in M1 cusp proportions within the genus Homo. These derived cusp proportions are found in 80% of Asian H. erectus s. s. specimens from Sangiran and Zhoukoudian as well as nearly all later members of the genus Homo. Teeth belonging to the H. heidelbergensis/H. neanderthalensis lineage show a 100% occurrence of this derived morphology, but the primitive condition is found in low frequencies in both Upper Paleolithic and contemporary H. sapiens.
Cusp proportions in H. ergaster – emergence of a derived pattern?
Compared to early Homo the M1 of the single H. ergaster specimen, KNM-WT 15 000, has a relatively larger paracone area and a relatively smaller hypocone area, whereas there is less difference in the relative areas of the protocone and metacone. Nevertheless, this specimen already shows the derived relative paracone > relative metacone condition (Fig. 2; Table 9). Although the shift in the relative sizes of the paracone and metacone apparently marks a reasonably clear division within the genus Homo, the Konso Gardula M1 (KGA11-350), dated to around 1.4 Mya and attributed to H. erectus (Suwa et al. 2007), has been described as having a metacone larger than the paracone (i.e. it possesses the primitive condition for Homo). Thus, it may be premature at this point to assume that H. ergaster shows a predominance of the derived condition of this trait. Alternatively, the presence of primitive cusp proportions in the isolated M1 from Konso Gardula (KGA11-350) may indicate that the closest affinities for this specimen lie with early Homo rather than H. ergaster. Neither of these specimens (KNM-WT 15 000 or KGA11-350) shows the relatively reduced metacone that characterizes later Homo taxa.
Cusp proportions in H. erectus s. s
In a description of the H. erectus s. s. teeth from Sangiran, Grine & Franzen (1994) reported the absolute cusp size order of the M1s. Four of the seven specimens were described as having the paracone larger than the metacone. Two additional specimens were said to show cusps that were approximately equal in size, while the size order in one specimen was not clear due to wear. Although measurements of the relative cusp areas in the Sangiran specimens were not presented by Grine & Franzen (1994), the absolute and relative cusp areas measured in these specimens for the present study generally agree with these observations. The one exception to this pattern is Sangiran 4 (see below), which was not considered by Grine & Franzen (1994). Thus, the derived cusp proportions (relative paracone > relative metacone) are present in four of five H. erectus s. s. specimens.
The relative sizes of the protocone and the paracone in H. erectus s. s. are similar to those observed in other later Homo groups (Table 8). In contrast, significant differences were found mainly in the relative area of the metacone, which is significantly smaller than in early Homo and significantly larger than in H. neanderthalensis. The hypocone is significantly larger than in contemporary H. sapiens, but no significant differences were found from either the Qafzeh or Upper Paleolithic H. sapiens samples for any of the relative cusp areas (Table 8). Only Sangiran 4 (see above) departs from the rest of the sample in resembling early Homo specimens, which show a relatively small paracone and relatively large metacone.
Early Homo cusp proportions in H. antecessor
Our results agree with those of Bermúdez de Castro et al. (1999) who suggested that H. antecessor resembles early Homo specimens in all its cusp proportions. This suggests that the evolutionary origins for H. antecessor may be traced from a population(s) that had not undergone the paracone/metacone shift that characterizes later Homo.
Metacone reduction and hypocone enlargement in H. heidelbergensis/H. neanderthalensis
Bailey (2004) has previously reported that the combination of a small relative metacone and large relative hypocone may be a derived feature of H. neanderthalensis, and the expanded sample in the present study confirms this pattern. The same morphology is also seen in H. heidelbergensis and this would be an additional character state of the H. heidelbergensis/H. neanderthalensis lineage (Tables 2 and 8). In fact, these specimens show the smallest relative metacone area and largest relative hypocone area of any of the hominin samples.
Protocone increase and hypocone reduction in H. sapiens
The fossil and contemporary H. sapiens samples have a larger relative protocone and a smaller relative hypocone than the other taxa in later Homo (Table 2), but they are not significantly different from each other (Table 8). While following the pattern of other H. sapiens in relative protocone size, the Qafzeh sample maintains a larger relative hypocone compared to later H. sapiens. In general, there appears to be a shift in emphasis toward the mesial M1 cusps with the emergence of our own species, a trend that was continued in Upper Paleolithic and contemporary populations.
Crown size, shape and cusp proportions
Early members of the genus Homo show a ca. 10–16% smaller M1 overall crown area compared with Australopithecus and Paranthropus, respectively (Table 2). Subsequent M1 crown reduction within the genus Homo apparently occurred in two main stages. The first stage (a reduction of ca. 17%) is associated with the emergence of more derived forms of the genus, including H. ergaster and H. erectus s. s. Thereafter came a period of little or no change in M1 crown area during most of the Pleistocene. The second stage (a reduction of ca. 10%) occurs after the appearance of our own species H. sapiens. This reduction is not seen in the Qafzeh sample, but in Europe it may plausibly be associated with the advent of Upper Paleolithic tool technology.
Crown reduction in early Homo is associated with a slight increase in the relative size of the paracone compared with Australopithecus and Paranthropus. Further crown reduction beginning with H. ergaster and H. erectus s. s. coincides with the appearance of the derived (relative paracone > relative metacone) condition for the paracone/metacone shift. In addition, crown reduction in Upper Paleolithic and contemporary H. sapiens is associated with a further reduction in the relative size of the hypocone and an increase in the relative size of the protocone. Thus, the major changes in the M1 cusp proportions in the genus Homo are broadly associated with dental reduction.
Within individual Homo groups, cusp size generally scales isometrically with crown size (TCBA). The correlations between the relative sizes of the individual cusps and the TCBA in the contemporary H. sapiens sample in the present study (0.01 < r < –0.23) were lower than those reported previously for the M1 in modern humans (Hills et al. 1983). An isometric relationship between crown size and cusp size was also generally found across the genus Homo based on group means, suggesting that as crown size reduces, the absolute sizes of the individual cusps also reduce. However, this reduction is less pronounced in the paracone. Thus, the phylogenetically oldest cusp in the upper molars also seems to be the most stable cusp in the M1. Regarding the relative areas of the individual cusps, a strong negative correlation was found only between the relative area of the paracone and TCBA (Table 6). Thus as crown size reduces, the relative contribution of the paracone to overall crown area increases. The other cusps reduce their area at a more rapid rate because their relationship to overall crown area is isometric as opposed to the negative allometry of the paracone.
Changes in the paracone/metacone index are not related to changes in either overall crown size (TCBA) or the absolute size of the paracone. Rather, a strong relationship (r = –0.85) was found only with the absolute size of the metacone. This suggests that the emergence of the derived condition (relative paracone > relative metacone) in the genus Homo is primarily related to an isometric reduction of the metacone. This metacone reduction occurs in the context of the relative stability in absolute paracone area as overall crown area decreases. This is because the relative size relationships of the paracone differ from those of the other cusps (see above).
The results for cusp proportions in the present study agree with those based on the shape of the crown outline. The external crown shape of the hominin M1 has recently been quantified using 3D geometric morphometrics (Gómez-Robles et al. 2007), and the results suggest that early Homo shares the presumably primitive crown shape with Australopithecus and Paranthropus, whereas the H. heidelbergensis/H. neanderthalensis M1 crown outline is distinctive. The present study has documented a relatively large hypocone in the H. heidelbergensis/H. neanderthalensis lineage, and this may contribute to their distinctive crown outline (Gómez-Robles et al. 2007; Souday, 2008). In addition, the reduction in the relative size of the metacone in the M1 in later Homo taxa seen in the present study is consistent with the suggestion that M1 crown size reduction in later Homo species involved the protocone/metacone axis (Gómez-Robles et al. 2007).
Possible taxonomic implications of M1 cusp proportions in the genus Homo
Despite considerable change in both the TCBA and absolute metacone area in the M2 and M3 in the hominin clade, nearly all taxa show a paracone that predominates over the metacone in both these molars (Macho & Moggi-Cecchi, 1992; Grine & Franzen, 1994; Kimbel et al. 1996; Bermúdez de Castro et al. 1999; Bailey, 2002; Schwartz & Tattersall, 2003; Trefný, 2005; Moggi-Cecchi & Boccone, 2007; Suwa et al. 2007). This suggests that the pattern of variation in the relative sizes of these two cusps seen in the M1 in the present study may have some taxonomic significance. Thus, although there is some variation within fossil Homo taxa, we suggest that the relative size of the M1 paracone and metacone may be a useful taxonomic tool within the genus Homo.
Specifically, the results are consistent with the suggestion that the early Homo taxa H. habilis and H. rudolfensis lack some of the features shared by more derived members of the genus Homo (Wood & Collard, 1999; Wood & Richmond, 2000; Bailey & Wood, 2007). The M1 cusp proportions in these early Homo specimens are more similar to those of early hominin taxa such as Australopithecus and Paranthropus than they are to H. erectus s. s. The derived M1 proportions seen in three early Homo individuals from East Africa, KNM-ER 1813, OH 21 and OH 39, may be interpreted as a manifestation of the polymorphism we have seen in other groups of hominin taxa, or it could be evidence that the taxonomic allocation of these specimens warrants revision.
Relevant to the latter interpretation, other researchers (Hublin, 1983; Schwartz & Tattersall, 2003) have suggested that KNM-ER 1813 may belong to H. erectus or H. ergaster. In addition, one of the main reasons provided by Tobias (1991) for assigning OH 39 (which consists of isolated teeth) to H. habilis, the small mesiodistal length of the I2, may not be a valid way of differentiating this taxon from later forms of Homo. Similarly, small I2 dimensions are also found in some later Homo specimens from Olduvai (OH 29) (Tobias, 1991) and Sangiran (Grine & Franzen, 1994). Finally, the OH 21 isolated specimen represents a surface find from deposits which were subsequently shown to be disturbed, so its provenience remains uncertain (Tobias, 1991).
At the same time, H. antecessor appears to have retained the primitive condition (i.e. metacone > paracone) for the genus Homo. The same can be said of the Sangiran 4 specimen from Java, and the primitive character of the dentition in these specimens has been highlighted previously by other researchers (Weidenreich, 1945; Rightmire, 1990; Bermúdez de Castro et al. 1999). The presence of a primitive feature in both Sangiran 4 and H. antecessor cannot necessarily be taken to indicate a close evolutionary relationship between them. However, the M1 cusp proportions in H. antecessor most likely represent a primitive retention in this taxon from their Plio-Pleistocene ancestors. The source area for this ancestral population can perhaps be found in Africa, but the lack of published information on the M1 cusp proportions in the Dmanisi hominins leaves this question currently unresolved.
Conclusions
The M1 crown areas of the earliest Homo specimens are smaller than those of the early hominin genera Australopithecus and Paranthropus, and this overall reduction is associated with a slight increase in the relative size of the paracone. Nevertheless, the same size relationship between the paracone and metacone (i.e. relative paracone > relative metacone) is found in the majority of early Homo specimens and the early hominin M1s sampled in this study. We hypothesize that this metacone > paracone 2D relative cusp area relationship is the primitive condition for the later part of the hominin clade. Subsequent changes in cusp proportions within the genus Homo appear to be broadly associated with two main stages in M1 crown size reduction.
All later Homo taxa, except for H. antecessor, show the derived condition of a relatively large paracone and relatively small metacone, and we have called this change in cusp proportions the paracone/metacone shift. Although there is some variation in this feature, all of the hominin groups in the present study showed a clear dominant condition, with some groups reaching 100% expression of one condition. Although the paracone/metacone shift is associated with the first stage in M1 crown size reduction within the genus Homo, it can be more clearly related to an isometric reduction of the metacone. This metacone reduction occurs in the context of relative stability in the paracone area as the overall size of the crown decreases. The presence of the derived cusp proportions in KNM-ER 1813, OH 21 and OH 39 differentiates these individuals from other fossils assigned to early Homo. At the same time, both H. antecessor and the H. erectus s. s. specimen Sangiran 4 show the primitive cusp proportions, and this is consistent with previous suggestions of a primitive dental anatomy in these specimens.
The enlarged H. neanderthalensis sample in the present study has confirmed previous suggestions (Bailey, 2004) that this group of hominins is characterized by a derived pattern of cusp proportions, showing a reduced metacone and enlarged hypocone. This same pattern is also seen in the small H. heidelbergensis sample, suggesting that this feature appears early in the Neandertal evolutionary lineage.
The early modern human sample from Qafzeh shows a slight increase in the relative protocone area, but maintains a large hypocone like that of the earlier taxa. Crown size in the Qafzeh sample is unchanged from that seen in earlier Homo taxa. The second stage in M1 crown size reduction only occurs during Upper Paleolithic times and is associated with a further reduction in the size of the hypocone. Modern human M1 cusp proportions, then, emerged very late in the human lineage.
The reasons behind these changes in cusp proportions in the genus Homo appear to be some combination of dental reduction and allometry, and the pattern of cusp proportions in different hominin taxa appears to have some taxonomic significance. Additional studies in larger and taxonomically more diverse samples and the consideration of other tooth classes may reveal further distinctions in cusp proportions in the hominin clade and offer new insights into the evolutionary process.
Acknowledgments
The authors thank the anonymous referees and associate editor for their constructive comments, which helped to improve the manuscript considerably. The authors also wish to thank the following individuals and institutions for allowing the study of fossils and fossil casts housed under their care: I. Tattersall, K. Mowbray, and G. Sawyer at the American Museum of Natural History, New York; P. Ungar at the University of Arkansas, Fayetteville; F. Grine at Stony Brook University; C. Stringer and R. Kruzinski at the Natural History Museum, London; G. Manzi at the University of Rome; G. Giacabini of the University of Turin; F. Mallegni of the University of Pisa; G. Koufos of the Aristotle University of Thessaloniki; Y. Coppens of the College of France, Paris; P. Tassy at the National Museum of Natural History, Paris; J. Leopold of the Museum of National Antiquities, St. Germainen-Laye; M. Tavaso and F. Marchal at the Laboratory of Historical Geology, Marseille; J-J. Cleyet-Merle and A. Morala at the National Museum of Prehistory, Les Eyzies; V. Merlin-Anglade and G. Marchesseau at the Museum of Perigord; E. Ladier at the Museum of Natural History, Montauban; R. Ziegler at the National Museum of Natural History, Stuttgart; S. Dusek at the Museum of Prehistory, Weimar; H-E. Joachim at the State Museum of the Rhine, Bonn; W. Menghin at the Museum of Prehistory, Berlin; the late N. Farsan of the University of Heidelberg; M. Teschler-Nicola, at the Natural History Museum, Vienna; R. Orban and P. Semal of the Royal Institute of Natural Sciences of Belgium, Brussels; J. Radovčić at the Croatian Natural History Museum, Zagreb; M. Paunovič of the Institute for Quaternary Geology and Paleontology, Zagreb; J. Svoboda, of the Institute of Archaeology – Paleolithic and Paleoethnology Research Center, Dolní Věstonice; M. Dockalova at the Moravian Museum, Brno; F. Schrenk at the Senckenberg Institute; F. Thackeray at the Transvaal Museum (Northern Flagship Institution); Y. Rak at Tel Aviv University; P. Mennecier at the Musée de l’Homme; H. de Lumley at the Institut de Paléontologie Humaine; H. Bonet at the Museo de Prehistoria in Valencia; C. Cacho at the Museo Arqueológico Nacional; E. Baquedano at the Museo Arqueológico Regional. The authors are especially grateful to F. Grine who provided data on several unpublished specimens.
R. Quam has been supported by a grant from the Fundación Duques de Soria/Fundación Atapuerca. A portion of this research was supported by the Ministerio de Ciencia y Tecnología of the Government of Spain, Project no. CGL2006-13532-C03-02. S. Bailey has been supported by grants from the National Science Foundation (BCS-0 002 481), LSB Leakey foundation, and the Philanthropic Educational Organization (PEO) and has also received funding for data collection from The George Washington University and the Max Planck Institute for Evolutionary Anthropology. B. Wood is supported by the George Washington University Professorship in Human Origins and GW's Academic Excellence Program.
Footnotes
Note: The single exception, LH 21, is an incomplete tooth for which crown area cannot be determined. However, the difference in absolute size between the paracone and metacone (2.7 mm2) is larger than that seen in any of the specimens deemed to have equal-sized cusps based on the above criteria. Thus, we have scored this specimen as showing a relatively large metacone area and a relatively small paracone area.
References
- Andrews P. An alternative interpretation of the characters used to define Homo erectus. Cour Forschinst Senckenberg. 1984;69:167–175. [Google Scholar]
- Antón S. Evolutionary significance of cranial variation in Asian Homo erectus. Am J Phys Anthropol. 2002;118:301–323. doi: 10.1002/ajpa.10091. [DOI] [PubMed] [Google Scholar]
- Antón S. Natural history of Homo erectus. Yrb Phys Anthropol. 2003;46:126–170. doi: 10.1002/ajpa.10399. [DOI] [PubMed] [Google Scholar]
- Antón S, Spoor F, Fellman C, Swisher C. Defining Homo erectus: size considered. In: Henke W, Tattersall I, editors. Handbook of Paleoanthropology, Vol. 3, Phylogeny of Hominids. Heidelberg: Springer; 2007. pp. 1655–1693. (eds. [Google Scholar]
- Arsuaga JL, Martínez I, Gracia A, Carretero JM, Carbonell E. Three new human skulls from the Sima de los Huesos site in Sierra de Atapuerca, Spain. Nature. 1993;362:534–537. doi: 10.1038/362534a0. [DOI] [PubMed] [Google Scholar]
- Arsuaga JL, Martínez I, Gracia A, Lorenzo C. The Sima de los Huesos crania (Sierra de Atapuerca, Spain). A comparative study. J Hum Evol. 1997;33:219–282. doi: 10.1006/jhev.1997.0133. [DOI] [PubMed] [Google Scholar]
- Arsuaga JL, Villaverde V, Quam R, et al. New Neandertal remains from Cova Negra (Valencia, Spain) J Hum Evol. 2007;52:31–58. doi: 10.1016/j.jhevol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Bailey S. Department of Anthropology. Tempe, AZ: Arizona State University; 2002. Neandertal dental morphology: implications for modern human origins. [Google Scholar]
- Bailey S. A morphometric analysis of maxillary molar crowns of Middle-Late Pleistocene hominins. J Hum Evol. 2004;47:183–198. doi: 10.1016/j.jhevol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Bailey S, Lynch J. Diagnostic differences in mandibular P4 shape between Neandertals and anatomically modern humans. Am J Phys Anthropol. 2005;126:268–277. doi: 10.1002/ajpa.20037. [DOI] [PubMed] [Google Scholar]
- Bailey S, Wood B. Trends in postcanine occlusal morphology within the hominin clade: The case of Paranthropus. In: Bailey S, Hublin J, editors. Dental Perspectives in Human Evolution. State-of-the-Art Research in Dental Paleoanthropology. Dordrecht: Springer; 2007. pp. 33–52. (eds), pp. [Google Scholar]
- Bailey S, Pilbrow V, Wood B. Interobserver error involved in independent attempts to measure cusp base areas of Pan M1s. J Anat. 2004;205:323–331. doi: 10.1111/j.0021-8782.2004.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez de Castro JM, Nicolás ME. Posterior dental size reduction in hominids: the Atapuerca evidence. Am J Phys Anthropol. 1995;96:335–356. doi: 10.1002/ajpa.1330960403. [DOI] [PubMed] [Google Scholar]
- Bermúdez de Castro JM, Rosas A, Nicolás ME. Dental remains from Atapuerca-TD6 (Gran Dolina site, Spain) J Hum Evol. 1999;37:523–566. doi: 10.1006/jhev.1999.0323. [DOI] [PubMed] [Google Scholar]
- Boccone S, Moggi-Cecchi J. Upper molar cusp dimensions of South African australopithecines. Hum Evol. 2006;21:65–70. [Google Scholar]
- Brown B. Comparative dental anatomy of African Homo erectus. Cour Forschsinst Senckenberg. 1994;171:175–184. [Google Scholar]
- Clarke R. A juvenile cranium and some adult teeth of early Homo from Swartkrans, Transvaal. S Afr J Sci. 1977;73:46–49. [Google Scholar]
- Clarke R, Howell F. Affinities of the Swartkrans 847 hominid cranium. Am J Phys Anthropol. 1972;37:319–325. doi: 10.1002/ajpa.1330370302. [DOI] [PubMed] [Google Scholar]
- Collard M, Wood B. Defining the genus Homo. In: Henke W, Tattersall I, editors. Handbook of Paleoanthropology, Vol. 3. Phylogeny of Hominids. Berlin: Springer; 2007. pp. 1575–1611. (eds. [Google Scholar]
- Coppens Y. The differences between Australopithecus and Homo; preliminary conclusions from the Omo research expedition's studies. In: Königsson L, editor. Current Argument on Early Man. Oxford: Pergamon Press; 1980. pp. 207–225. (ed), pp. [Google Scholar]
- Corruccini R, McHenry H. Cladometric analysis of Pliocene hominids. J Hum Evol. 1980;9:209–221. [Google Scholar]
- Dahlberg A. The changing dentition of man. J Am Dent Assoc. 1945;32:676–690. [Google Scholar]
- Gómez-Robles A, Martinón-Torres M, Bermúdez de Castro J, et al. A geometric morphometric analysis of hominin upper first molar shape. J Hum Evol. 2007;53:272–285. doi: 10.1016/j.jhevol.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Gómez-Robles A, Martinón-Torres M, Bermúdez de Castro J, Prado L, Sarmiento S, Arsuaga J. Geometric morphometric analysis of the crown morphology of the lower first premolar of hominins, with special attention to Pleistocene. Homo J Hum Evol. 2008;55:627–638. doi: 10.1016/j.jhevol.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Grine F. New hominid fossils from the Swartkrans formation (1979–86 excavations): craniodental specimens. Am J Phys Anthropol. 1989;79:409–449. doi: 10.1002/ajpa.1330790402. [DOI] [PubMed] [Google Scholar]
- Grine F. Description and preliminary analysis of new hominid craniodental fossils from the Swartkrans formation. In: Brain C, editor. Swartkrans. A Cave's Chronicle of Early Man. Pretoria: Transvaal Museum; 1993. pp. 75–116. (ed), pp. [Google Scholar]
- Grine F. Early Homo at Swartkrans, South Africa: a review of the evidence and an evaluation of recently proposed morphs. S Afr J Sci. 2005;101:43–52. [Google Scholar]
- Grine F, Franzen J. Fossil hominid teeth from the Sangiran Dome (Java, Indonesia) Cour Forschinst Senckenberg. 1994;171:75–103. [Google Scholar]
- Grine F, Strait D. New hominid fossil from Member 1 ‘Hanging Remnant’, Swartkrans Formation, South Africa. J Hum Evol. 1994;26:57–75. [Google Scholar]
- Grine F, Demes B, Jungers W, Cole T. Taxonomic affinity of the early Homo cranium from Swartkrans, South Africa. Am J Phys Anthropol. 1993;92:411–426. doi: 10.1002/ajpa.1330920402. [DOI] [PubMed] [Google Scholar]
- Guatelli-Steinberg D, Irish J. Brief communication: early hominin variability in first molar dental trait frequencies. Am J Phys Anthropol. 2005;128:477–484. doi: 10.1002/ajpa.20194. [DOI] [PubMed] [Google Scholar]
- Hills M, Graham S, Wood B. The allometry of relative cusp size in hominoid mandibular molars. Am J Phys Anthropol. 1983;62:311–316. doi: 10.1002/ajpa.1330620310. [DOI] [PubMed] [Google Scholar]
- Hlusko L. Identifying metameric variation in extant hominoid and fossil hominid mandibular molars. Am J Phys Anthropol. 2002;118:86–97. doi: 10.1002/ajpa.10051. [DOI] [PubMed] [Google Scholar]
- Hlusko L, Maas M, Mahaney M. Statistical genetics of molar cusp patterning in pedigreed baboons: Implications for primate dental development and evolution. J Exp Zool (Mol Dev Evol) 2004;302B:268–283. doi: 10.1002/jez.b.21. [DOI] [PubMed] [Google Scholar]
- Howell F, Haesaerts P, de Heinzelin J. Depositional environments, archaeological occurrences and hominids from Members E and F of the Shungura Formation (Omo basin, Ethiopia) J Hum Evol. 1987;16:665–700. [Google Scholar]
- Hublin J. Les superstructures occipitales chez les prédécesseurs d’Homo erectus en Afrique: quelques remarques sur l’origine du torus occipital transverse. Bull Mem Soc Anthropol Paris. 1983:303–312. Series 13 Vol. 10. [Google Scholar]
- Hublin JJ. Climatic changes, paleogeography, and the evolution of the Neandertals. In: Akazawa T, Aoki K, Bar-Yosef O, editors. Neandertals and Modern Humans in Western Asia. New York: Plenum Press; 1998. pp. 295–310. (eds), pp. [Google Scholar]
- Johanson D, White T, Coppens Y. Dental remains from the Hadar formation, Ethiopia: 1974–77 collections. Am J Phys Anthropol. 1982;57:545–603. [Google Scholar]
- Kimbel W, Johanson D, Rak Y. Systematic assessment of a maxilla of Homo from Hadar, Ethiopia. Am J Phys Anthropol. 1996;103:235–262. doi: 10.1002/(SICI)1096-8644(199706)103:2<235::AID-AJPA8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kraus B, Jordan R, Abrams L. Dental Anatomy and Occlusion. Baltimore: Williams & Wilkins Co; 1969. [Google Scholar]
- Lieberman D. Testing hypothesis about recent human evolution from skulls: integrating morphology, function, development and phylogeny. Curr Anthropol. 1995;36:159–197. [Google Scholar]
- Macho G, Moggi-Cecchi J. Reduction of maxillary molars in Homo sapiens sapiens: a different perspective. Am J Phys Anthropol. 1992;87:151–159. doi: 10.1002/ajpa.1330870203. [DOI] [PubMed] [Google Scholar]
- Martinón-Torres M, Bastir M, Bermúdez de Castro J, et al. Hominin lower second premolar morphology: evolutionary inferences through geometric morphometric analysis. J Hum Evol. 2006;50:523–533. doi: 10.1016/j.jhevol.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Moggi-Cecchi J, Boccone S. Maxillary molars cusp morphology of South African australopithecines. In: Bailey S, Hublin JJ, editors. Dental Perspectives on Human Evolution. State-of-the-Art Research in Dental Paleoanthropology. Dordrecht: Springer; 2007. pp. 53–64. (eds. [Google Scholar]
- Rightmire GP. The Evolution of Homo Erectus. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Rightmire GP. Evidence from facial morphology for similarity of Asian and African representatives of Homo erectus. Am J Phys Anthropol. 1998;106:61–85. doi: 10.1002/(SICI)1096-8644(199805)106:1<61::AID-AJPA5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Tattersall I. The human chin revisited: what is it and who has it? J Hum Evol. 2000;38:367–409. doi: 10.1006/jhev.1999.0339. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Tattersall I. The Human Fossil Record, Vol. 2. Craniodental Morphology of Genus Homo (Africa and Asia) New York: Wiley-Liss; 2003. [Google Scholar]
- Souday C. Paris: Muséum National d’Histoire Naturelle; Analyse morphométric des molaires déciduales et définitives dans le genre Homo: perspectives phylogénétiques et biogéographiques. [Google Scholar]
- Spoor F, Leakey M, Gathogo P, et al. Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya. Nature. 2007;448:688–691. doi: 10.1038/nature05986. [DOI] [PubMed] [Google Scholar]
- Suwa G, Wood B, White T. Further analysis of mandibular molar crown and cusp areas in Pliocene and Early Pleistocene hominids. Am J Phys Anthropol. 1994;93:407–426. doi: 10.1002/ajpa.1330930402. [DOI] [PubMed] [Google Scholar]
- Suwa G, White T, Howell F. Mandibular postcanine dentition from the Shungura Formation, Ethiopia: crown morphology, taxonomic allocations and Plio-Pleistocene hominid evolution. Am J Phys Anthropol. 1996;101:247–282. doi: 10.1002/(SICI)1096-8644(199610)101:2<247::AID-AJPA9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Suwa G, Asfaw B, Haile-Selassie Y, et al. Early Pleistocene Homo erectus fossils from Konso, southern Ethiopia. Anthropol Sci. 2007;115:133–151. [Google Scholar]
- Henke W. Tattersall I. Tattersall I. Handbook of Paleoanthropology. Berlin: Springer; 2007. Homo ergaster and its contemporaries; pp. 1633–1653. Vol. 3 (eds. [Google Scholar]
- Tillier AM. Les Enfants Mousteriens de Qafzeh: Interpretation Phylogenetique et Paleoauxologique. Paris: CNRS Editions; 1999. [Google Scholar]
- Tobias PV. The earliest Transvaal members of the genus Homo with another look at some problems of hominid taxonomy and systematics. Z Morphol Anthropol. 1978;69:225–265. [PubMed] [Google Scholar]
- Tobias PV. Olduvai Gorge, Vol. 4. The Skulls, Teeth and Endocasts of Homo Habilis. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Trefný P. Size reduction of the Le Moustier 1 molars – a 2D analysis. In: Ullrich H, editor. The Neandertal Adolescent le Moustier 1. New Aspects, New Results. Berlin: Staatliche Museen zu Berlin; 2005. pp. 187–196. ed. [Google Scholar]
- Vandermeersch B. Les Hommes Fossiles de Qafzeh (Israel) Paris: CNRS; 1981. [Google Scholar]
- Weidenreich F. Giant early man from Java and South China. Anthropol Papers Am Mus Nat Hist. 1945;40:1–134. [Google Scholar]
- White T. New fossil hominids from Laetoli, Tanzania. Am J Phys Anthrop. 1977;46:197–229. doi: 10.1002/ajpa.1330460203. [DOI] [PubMed] [Google Scholar]
- Wood B. Koobi Fora Research Project, Vol. 4. Hominid Cranial Remains. Oxford: Clarendon Press; 1991. [Google Scholar]
- Wood B. Taxonomy and evolutionary relationships of Homo erectus. Cour Forschinst Senckenberg. 1994;171:159–165. [Google Scholar]
- Wood B, Abbott S. Analysis of the dental morphology of Plio-Pleistocene hominids I. Mandibular molars: crown area measurements and morphological traits. J Anat. 1983;136:197–219. [PMC free article] [PubMed] [Google Scholar]
- Wood B, Collard M. The human genus. Science. 1999;284:65–71. doi: 10.1126/science.284.5411.65. [DOI] [PubMed] [Google Scholar]
- Wood B, Engleman C. Analysis of the dental morphology of Plio-Pleistocene hominids V. Maxillary postcanine tooth morphology. J Anat. 1988;161:1–35. [PMC free article] [PubMed] [Google Scholar]
- Wood B, Richmond B. Human evolution: taxonomy and paleobiology. J Anat. 2000;196:19–60. doi: 10.1046/j.1469-7580.2000.19710019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B, Uytterschaut H. Analysis of the dental morphology of Plio-Pleistocene hominids. III. Mandibular premolar crowns. J Anat. 1987;154:121–156. [PMC free article] [PubMed] [Google Scholar]
- Wood B, Abbott S, Graham S. Analysis of the dental morphology of Plio-Pleistocene hominids II. Mandibular molars – study of cusp areas, fissure patterns and cross sectional shape of the crown. J Anat. 1983;137:287–314. [PMC free article] [PubMed] [Google Scholar]
- Wood B, Abbott S, Uytterschaut H. Analysis of the dental morphology of Plio-Pleistocene hominids. IV. Mandibular postcanine root morphology. J Anat. 1988;156:107–139. [PMC free article] [PubMed] [Google Scholar]