Abstract
The petrosal anatomy and inner ear structure of Jurassic cladotherian mammals represent the ancestral morphological conditions (groundplan) from which modern therian mammals (marsupials and placentals) have evolved. We present the reconstruction of the petrosal and inner ear features of the Late Jurassic dryolestoid mammal Henkelotherium guimarotae from high-resolution computed tomography and three-dimensional imaging analysis. This study of Henkelotherium revealed a combination of derived and primitive features, including: cladotherian apomorphies, such as the promontorial sulcus for the internal carotid artery and reduced lateral trough; trechnotherian characters, such as an enclosed cochlear canaliculus for the perilymphatic duct, post-promontorial tympanic sinus and caudal tympanic process; in addition to plesiomorphic mammalian features, such as the cavum supracochleare and prootic canal. The inner ear of Henkelotherium shows a division between the utricle and saccule, a cochlear canal coiled through at least 270°, a distinctive primary bony lamina for the basilar membrane, and a secondary bony lamina. The development of the primary and secondary bony laminae in the cochlear canal is suggested here to be correlated with the concurrent coiling of the bony canal and membranous duct of the inner ear cochlea, apomorphies of the more inclusive cladotherian clade that also represent the ancestral morphotype of modern therian mammals. Because these features are crucial for high-frequency hearing in extant therian mammals, their early appearance in Late Jurassic cladotherians suggests a more ancient origination for high-frequency hearing in mammalian history than previously thought.
Keywords: bony labyrinth, cochlea, computed tomography, ear, evolution, Mammalia, petrosal bone
Introduction
The mammalian basicranial region, especially the petrosal bone and inner ear, underwent major anatomical transformation in their early evolutionary history. Petrosal characteristics are complex yet highly conserved morphologically and can be a useful source of phylogenetic information (see overviews by Kielan-Jaworowska et al. 2004; Rougier & Wible, 2006). Inner ear structures are also crucial for hearing adaptation of mammals (Allin & Hopson, 1992; Luo, 2007). The dryolestoid mammal Henkelotherium guimarotae (Krebs, 1991) is an extinct relative of modern therians, i.e. marsupials and placentals. Henkelotherium is a stem cladotherian (sensuMcKenna & Bell, 1997); it is more derived than the more plesiomorphous ‘symmetrodonts’ but more basal than prototribosphenidan and boreosphenidan mammals primarily by dental and post-cranial characters (Ji et al. 1999; Luo et al. 2002, 2007; Luo & Wible, 2005; Luo, 2007; Rougier et al. 2007). Because of its position in therian mammal phylogeny, the petrosal characteristics and inner ear structure of Henkelotherium can shed light on morphological evolution in advance of the origin of modern therian mammals.
Henkelotherium guimarotae is from the Upper Jurassic (Kimmeridgian) Guimarota Coal Mine in west-central Portugal (Krebs, 1991, 2000). This fossil site has yielded a wealth of vertebrate fossils (Martin & Krebs, 2000; Martin, 2001) and is an important locality for mammals of the Late Jurassic (Martin & Krebs, 2000; Kielan-Jaworowska et al. 2004). The Guimarota mammalian assemblage consists of docodonts, multituberculates and dryolestoidans (Dryolestidae and Paurodontidae), which are the basal members of the cladotherian clade. Collectively, these mammals are represented by about 800 dentaries and more than 7000 isolated teeth (Martin, 2001), and by some 20 compressed mammalian skulls and additional skull bones. Also recovered are a partial skeleton of the docodont Haldanodon exspectatus (Henkel & Krusat, 1980; Martin, 2005) and an almost complete skeleton of the paurodontid Henkelotherium guimarotae (Krebs, 1991, 2000).
The cranial structure of Henkelotherium has been difficult to study because its skull is fragmentary and compressed in preservation in coals. The partially preserved basicranium of Henkelotherium on the counterslab of the holotype specimen (Gui Mam 138/76b) was interpreted erroneously as part of the exoccipital bone in its original study (Krebs, 1991, 34, Abb. 2). High-resolution computed tomography (CT) of the counterpart of the Henkelotherium has now revealed a relatively well-preserved left petrosal bone that was partially covered by other bones and bone fragments.
As the petrosal is a dense and durable bone often preserved in fossils, this element has been well studied in a wide range of Mesozoic mammals, including basal metatherians (Wible, 1990; Meng & Fox, 1995; Marshall & Muizon, 1995; Rougier et al. 1998; Muizon, 1998; Ladevèze, 2004, 2007; Sánchez-Villagra et al. 2007), basal eutherians (Wible et al. 2001, 2004, in press; Ekdale et al. 2004), the prototribosphenidan Vincelestes (Rougier et al. 1992), the spalacotheroid Zhangheotherium (Hu et al. 1997), multituberculates (Kielan-Jaworowska et al. 1986; Miao, 1988; Wible & Hopson, 1995; Wible & Rougier, 2000), eutriconodonts (Crompton & Luo, 1993; Wible & Hopson, 1993; Rougier et al. 1996; Wang et al. 2001) and many mammaliaforms (Kermack et al. 1981; Lillegraven & Krusat, 1991; Crompton & Luo, 1993; Wible & Hopson, 1993; Lucas & Luo, 1993; Luo et al. 2001). However, it is more difficult to gain information on the structure of the inner ear of early mammals. Several studies have documented early mammalian inner ear structure in naturally exposed or broken petrosals (Lillegraven & Krusat, 1991; Lillegraven & Hahn, 1993; Rougier, 1993; Meng & Fox, 1995; Meng & Wyss, 1995; Fox & Meng, 1997) or by serial sections of fossils (Graybeal et al. 1989; Luo et al. 1995; Hurum, 1998; Luo, 2001). Although there have been some previous attempts to use CT in studying the inner ear of fossil mammals (e.g. Luo & Ketten, 1991), it is only in recent years that the application of high-resolution CT to studies of mammalian petrosals has resulted in much better documentation of the detailed inner ear structure (e.g. Spoor et al. 2002; Schmelzle et al. 2007; Ladevèze et al. 2008).
The CT scanning of the petrosal in the holotype specimen of Henkelotherium has revealed not only many characters of the petrosal but also the structure of the bony labyrinth of the inner ear, including the cochlea for hearing and the vestibule and semicircular canals for balance and equilibrium. These newly described features of Henkelotherium may be useful for phylogenetic studies of early mammals, inferring the evolution of hearing in early mammals and establishing the groundplan (Hennig, 1950, 1966) of the petrosal and inner ear for cladotherian mammals.
Materials and methods
The investigated specimen was the smaller counterpart slab (Fig. 1) of the holotype specimen of H. guimarotae (Guimarota Mammal Collection, Gui Mam 138/76b). This specimen is now permanently housed in the collection of the Museo Geológico (Lisbon, Portugal). Both the slab and counterslab of the holotype specimen were transferred from the original coal matrix to an artificial resin matrix that is translucent (Drescher, 2000). The specimens embedded in this way can be studied on both sides (observation of the embedded side through the transparent plastic) (Fig. 1). However, the petrosal is mostly covered and obscured by other skull fragments and the residual coal matrix, which was probably why it was not identified in the original study of this specimen (Krebs, 1991, Abb. 2).
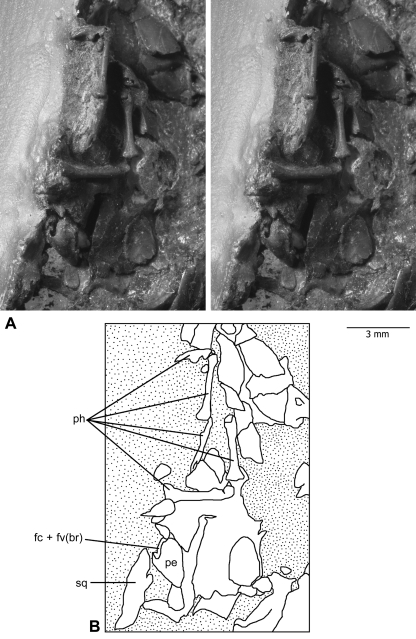
Fig. 1.
Left petrosal bone of Henkelotherium guimarotae(Gui Mam 138/76b), housed in the smaller slab (counterpart) of the type specimen. (A) Stereophotographs of the petrosal bone preserved among fragmentary skull elements and some manual phalanges. Most of the petrosal is in the resin embedding materials, not visible to the naked eye but can be visualized by computed tomography scanning. Stipple pattern indicates the coal matrix or embedding resin. (B) Explanatory drawing of (A). fc + fv(br), broken area of fenestra cochleae and fenestra vestibuli (possibly including the fossa for the stapedius muscle); pe, petrosal bone; ph, manual phalanges; sq, squamosal.
High-resolution CT was used to study the obscured features of the petrosal, including its internal structure. This allowed the study of structures hidden by overlying bone fragments or remaining coal matrix. The counterslab was scanned on the OMNI-X Universal HD600 Scanner (Center for Quantitative Imaging, Energy Institute, Pennsylvania State University, State College, PA, USA). The images had a 1024 pixel resolution of 0.015 × 0.015 × 0.02194 mm. Stacks of digital images were used to produce virtual reconstructions of the petrosal bone and the inner ear bony labyrinth by the manual segmentation function of the Amira 4.1® software. Linear measurements were taken directly from this endocast of the inner ear bony labyrinth with the Amira® software.
Anatomical terms used in the description generally follow terminology summarized by Wible & Hopson (1995), Wible et al. (2001), Kielan-Jaworowska et al. (2004) and Rougier & Wible (2006). Additional terms were added when necessary. Discussion of the systematic distribution and pattern of evolution of the observed features is based on phylogenetic relationships according to Kielan-Jaworowska et al. (2004), Luo & Wible (2005), Rougier et al. (2007), Luo (2007) and Luo et al. (2007).
Results
External morphology of petrosal bone
The petrosal bone is preserved together with other posterior skull bones that are fragmented and displaced. Also associated with these skull fragments are the phalanges of two manual digits (Fig. 1). The ventral (tympanic) aspect of the pars cochlearis, which encloses the saccule and cochlear canal, is naturally exposed and its features are clearly visible. The endocranial aspect of the pars cochlearis and pars canalicularis (housing the vestibule and semicircular canals) is covered by other skull bone fragments or the original coal matrix but can be viewed very clearly in the CT scans (Figs 2 3 4). The anterodorsal portion of the pars canalicularis is damaged and incomplete (Figs 3B 4A). However, the structure of the cavum epiptericum [the extradural intracranial space housing the trigeminal ganglion (Gaupp, 1908; Zeller, 1989)], which would be located in this region, is not known and neither is it possible to determine the several features around the cavum epiptericum. However, it is possible that the anterior lamina, which would contribute to the sidewall of the braincase, is preserved, although displaced from its normal anatomical position (Fig. 2A–D, alp?). The subarcuate fossa, which houses the paraflocculus of the cerebellum, is also fragmented. These features cannot be accurately described.
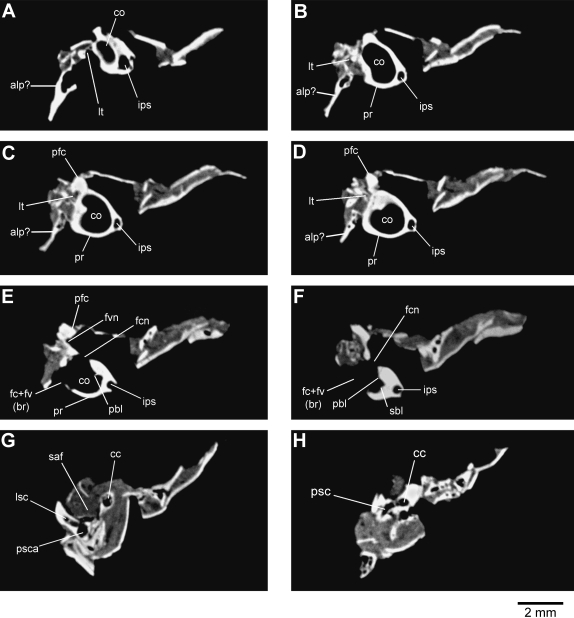
Fig. 2.
Representative computed tomography images of the left petrosal bone of Henkelotherium guimarotae (Gui Mam 138/76b). Series is from the anterior (apex of cochlea) to the posterior (posterior semicircular canal). (A) Image 054; (B) image 069; (C) image 078; (D) image 083; (E) image 096; (F) image 110; (G) image 169; (H) image 195. alp?, possible remnant of anterior lamina of petrosal; cc, crus commune; co, cochlear canal; fc + fv(br), broken area of fenestra cochleae and fenestra vestibuli (possibly including the fossa for the stapedius muscle); fcn, foramen for cochlear nerve; fvn, foramen for vestibular nerve; ips, inferior petrosal sinus; lsc, lateral semicircular canal; lt, lateral trough (incomplete); pbl, primary bony lamina; pfc, prefacial commissure; pr, promontorium; psc, posterior semicircular canal; psca, posterior semicircular canal ampulla; sbl, secondary bony lamina for basilar membrane; saf, subarcuate fossa (incomplete).
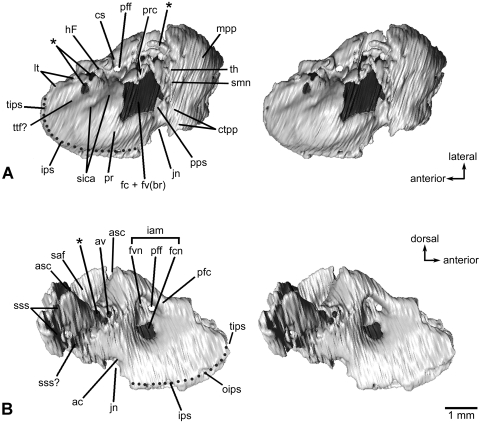
Fig. 3.
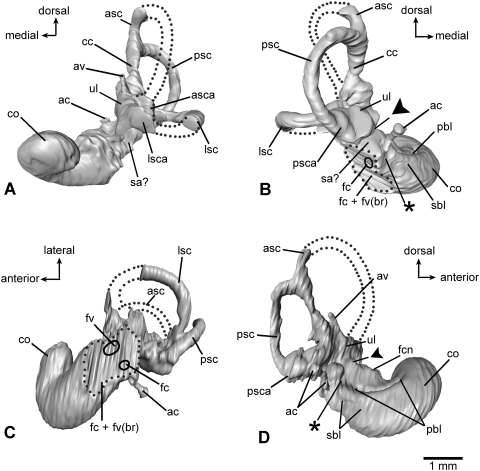
Left petrosal bone of Henkelotherium guimarotae(Gui Mam 138/76b). Virtual reconstruction from computed tomography scans. (A) Ventral (tympanic) view (stereo pair); (B) dorsomedial (endocranial) view (stereo pair). Dark grey pattern indicates the exposed inner ear bony labyrinth (including damaged area). Asterisks are opening(s) caused by damage and are not the original anatomical structures. Dotted line refers to the canal of the inferior petrosal sinus. ac, opening of aqueductus cochleae; asc, anterior semicircular canal (broken); av, opening of aqueductus vestibuli; cs, cavum supracochleare (housing the geniculate ganglion of the facial nerve, broken and incomplete); ctpp, caudal tympanic process of petrosal; fc + fv(br), broken areas of the fenestra cochleae and fenestra vestibuli (possibly including the fossa for the stapedius muscle); fcn, foramen for cochlear nerve (cranial nerve VIII); fvn, foramen for vestibular nerve; hF, hiatus Fallopii (opening for the greater superficial petrosal nerve of the facial nerve, broken and open); iam, internal acoustic meatus; ips, canal for inferior petrosal sinus; jn, jugular notch (incomplete); lt, lateral trough (incomplete); mpp, mastoid part of petrosal (mastoid exposure of the pars canalicularis); oips, opening for inferior petrosal sinus, pfc, prefacial commissure; pff, primary foramen for facial nerve (cranial nerve VII); pps, post-promontorial tympanic sinus; pr, promontorium; prc, prootic canal (broken and open); saf, subarcuate fossa (incomplete); sica, sulcus for internal carotid artery; smn, stylomastoid notch (exit for facial nerve); sss, sulcus for sigmoid sinus; sss?, possible sulcus for sigmoid sinus; th, attachment for tympanohyal; tips, tributary of inferior petrosal sinus; ttf?, possible tensor tympani fossa.
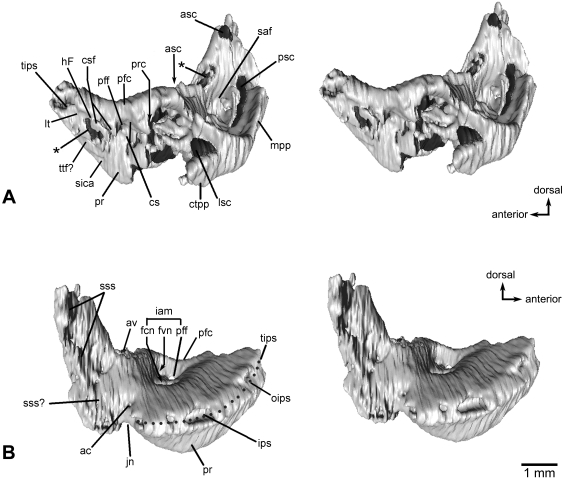
Fig. 4.
Left petrosal bone of Henkelotherium guimarotae(Gui Mam 138/76b). Virtual reconstruction based on computed tomography scans. (A) Lateral view (stereo pair); (B) medial view (stereo pair). The vestibular portion and lateral trough area are damaged and the rostrodorsal part is missing. Asterisks indicate openings caused by damage. ac, opening of aqueductus cochleae; asc, anterior semicircular canal (broken and open); av, opening of aqueductus vestibuli; cs, cavum supracochleare; csf, cavum supracochleare floor (remnant of the broken and incomplete cavum); ctpp, caudal tympanic process of petrosal; fcn, foramen for cochlear nerve; fvn, foramen for vestibular nerve; hF, hiatus Fallopii (opening for the greater superficial petrosal nerve of the facial nerve, open and broken); iam, internal acoustic meatus; ips, canal for inferior petrosal sinus; jn, jugular notch (incomplete); lsc, lateral semicircular canal (broken and incomplete); lt, lateral trough (broken); mpp, mastoid part of petrosal (mastoid exposure of the pars canalicularis); oips, opening of inferior petrosal sinus; pfc, prefacial commissure; pff, primary facial foramen; pr, promontorium; prc, prootic canal (broken and open); psc, posterior semicircular canal (broken and open); saf, subarcuate fossa (broken and incomplete); sica, sulcus for internal carotid artery; sss, sulcus for sigmoid sinus; tips, tributary of inferior petrosal sinus; sss?, possible sulcus for sigmoid sinus; ttf?, possible tensor tympani fossa.
Ventral aspect of petrosal bone
The ventral surface of the pars cochlearis forms a well-developed promontorium (Figs 2B–E 3A 4A,B). It has an oval outline and bulbous (externally convex) surface. The distance from the incomplete anterior border of the fenestra vestibuli (oval window) to the preserved apex (pole) of the promontorium is about 2.2 mm. The width of the promontorium from the primary facial foramen to the medial edge of the promontorium is about 1.8 mm.
The crista interfenestralis, which separates the fenestra vestibuli from the fenestra cochleae (round window) in an intact mammalian petrosal, is broken and damaged in the holotype of Henkelotherium. As a consequence, the fenestra vestibuli and fenestra cochleae are confluent, forming a large artefactual opening on the posterior surface of the promontorium about 1.9 mm long by 1.4 mm wide (Figs 2E,F 3A). This artefactual opening has two portions: an anterolateral part, the remnant of the fenestra vestibuli, and a more posteromedial part, the remnant of the fenestra cochleae. The exact shape and size of these two fenestrae are unknown.
The promontorium shows a shallow but very distinct longitudinal sulcus. It extends from the remnant anterior rim of the broken fenestra vestibuli towards the apex of the promontorium. This is interpreted as the sulcus for the internal carotid artery (Fig. 3A, sica). The lateral trough of the petrosal is represented by a depression adjacent to the anterolateral part of the promontorium. Between the lateral trough and the sulcus for the internal carotid artery is a small area that is probably the site of origin for the tensor tympani muscle (Fig. 3A, ttf?). The anterior part of the lateral trough is incomplete but its preserved portion suggests that the lateral trough is narrower and more reduced than its counterparts in ‘symmetrodonts’ such as Zhangheotherium (Kielan-Jaworowska et al. 2004) (Figs 2A–D 3A, lt). Within the preserved lateral trough depression are two irregular openings that are interpreted as damaged areas on the basis of the CT scans. The cavum supracochleare, a bony space that would contain the geniculate ganglion of the facial nerve, is positioned dorsolateral to the cochlear housing (Fig. 3A, cs). The cavum supracochleare is separated from the endocranial aspect of the cochlear housing by a well-developed prefacial (suprafacial) commissure (Figs 2C–E 3 4, pfc). Because the ventral and lateral floors of the cavum supracochleare are damaged, the cavum is broken open and the lateral aspect of the primary facial foramen is visible. In the specimen, the remnant of the bony floor of the broken cavum supracochleare (Fig. 4A, csf) is still visible. The hiatus Fallopii, the foramen for the greater petrosal nerve of the facial nerve, is broken although still identifiable. The hiatus Fallopii is represented by a small incisure near the anterior (albeit broken and incomplete) floor of the cavum supracochleare and by a sulcus leading anteriorly from this incisure (Figs 3A 4A, hF).
In intact petrosals, if the prootic canal, a venous channel for the prootic sinus (Rougier & Wible, 2006), is present, its endocranial aperture is anterolateral to the subarcuate fossa; the canal traverses the petrosal bone to its tympanic aperture, which is located posterior to the cavum supracochleare (if the cavum is present) and lateral to the stapedius fossa housing the stapedius muscle (Rougier et al. 1992, 1996; Wible et al. 1995, 2001; Hu et al. 1997; Luo et al. 2001). This area of the petrosal in Henkelotherium is damaged. Nonetheless, the course of the prootic sinus, which is preserved as a groove in the broken prootic canal (Fig. 4A, prc), is identified in the same area where this canal is positioned in all other Mesozoic mammals. A deep sulcus extends posteriorly from the area of the cavum supracochleare and the putative tympanic aperture of the prootic canal. This sulcus is interpreted as the shared pathway of the facial nerve and lateral head vein, into which the prootic sinus drains (Rougier & Wible, 2006). The stapedius fossa cannot be observed in Henkelotherium in the area where it is normally positioned in all mammals because of the large damaged area that also encompasses the missing crista interfenestralis and the broken fenestrae vestibuli and cochleae [illustrated by the large grey area posterior to the promontorium in Fig. 3, fc + fv(br)]. A shallow but clearly visible depression is located lateral to the jugular notch and posterior to the position of fenestra cochleae. It is interpreted as the post-promontorial tympanic sinus, a feature that is present in some multituberculates, the spalacotheroid Zhangheotherium, the prototribosphenidan Vincelestes, metatherians and eutherians (Wible, 1990; Rougier et al. 1992, 1998; Wible et al. 1995, 2001; Hu et al. 1997; Muizon, 1998; Wible & Rougier, 2000; Ladevèze, 2004, 2007).
Medial margin of the petrosal
Several features can be observed along the medial margin of the petrosal. The jugular notch or incisure (Fig 3, jn) is in the posteromedial corner of the pars cochlearis. In intact mammalian skulls, the notch forms the lateral border of the jugular foramen for cranial nerves IX, X and XI, and blood vessels (Evans, 1993; Wible, 2003). The external aperture of the cochlear canaliculus (aqueductus cochleae for the perilymphatic duct) is on the medial margin of the jugular notch. The opening connects externally to the subarachnoid space and internally to the scala tympani in the cochlear canal, near the saccular space in the inner ear bony labyrinth (Fig. 5A,B, sa? and ac). The medial margin of pars cochlearis has a prominent canal for the inferior petrosal sinus (Figs 2A–F 4B, ips). The inferior petrosal sinus connects the cavernous sinus and internal jugular vein in extant mammals (Rougier & Wible, 2006). In the virtual restoration from the CT scans, the inferior petrosal sinus canal can be seen because its path is locally broken and this helps to expose it as an intrapetrosal structure (Figs 3A,B 4B). The posterior opening of the canal appears to open to the external side of the petrosal/basioccipital suture at the jugular notch. Anteriorly, the canal of the inferior petrosal sinus opens at a large opening (Fig. 4B, oips). Anterodorsally, the canal for the inferior petrosal sinus is confluent with a second canal, which is probably for a tributary of the sinus (Fig. 4B, tips). Anterolaterally, this second canal opens into the anterior area of the supposed inferior opening of the cavum epiptericum. A small opening occurs at the rostral apex of the promontorium and is interpreted as a small tributary of the inferior petrosal sinus (Fig. 3A, tips). Inside the petrosal bone are two small vascular canals continuous with the inferior petrosal sinus canal. The anterior small vascular canal connects the distal (apical) portion of the cochlear duct with the inferior petrosal sinus canal. The posterior vascular canal is situated directly anterior to the cochlear canaliculus; it originates from inside the cochlear canal between the primary and secondary bony laminae (for the basilar membrane) and connects to the posterior part of the inferior petrosal sinus canal.
Fig. 5.
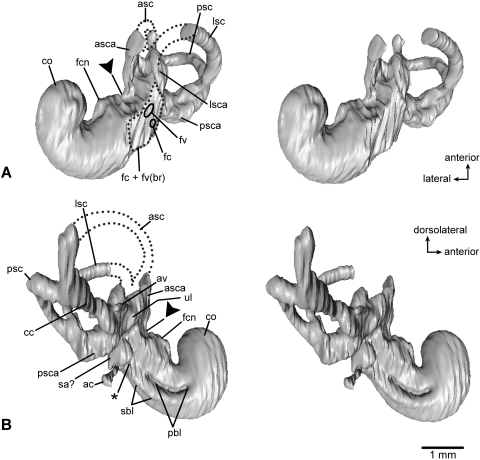
Virtual endocast of the inner ear bony labyrinth of Henkelotherium guimarotae type specimen (Gui Mam 138/76b), left side. Asterisks indicate the separation between the saccular space and the base of cochlear canal. Dark triangles indicate the separation between the utricle and saccule. (A) Anterior (rostral) view; (B) posterior view; (C) ventral view; (D) medial (endocranial) view. ac, aqueductus cochleae (endocast); asc, anterior semicircular canal (incomplete, broken segment indicated by dotted lines); asca, anterior semicircular canal ampulla; av, aqueductus vestibuli (endocast); cc, crus commune; co, cochlear duct (endocast); fc + fv(br), dashed outline indicating the broken area of fenestra cochleae, fenestra vestibuli and possibly the fossa for the stapedius muscle; fc, hypothetical position of fenestra cochleae (not preserved); fcn, foramen for cochlear nerve; fv, hypothetical position of fenestra vestibuli (not preserved); lsc, lateral semicircular canal (incomplete, broken segment indicated by dotted lines); lsca, lateral semicircular canal ampulla; pbl, basal portion of primary bony lamina for basilar membrane; psc, posterior semicircular canal; psca, posterior semicircular canal ampulla; sa?, possible saccular part of vestibule; sbl, secondary bony lamina for basilar membrane; ul, utricle.
Posterior aspect of the petrosal
The pars canalicularis forms an extensive exposure on the posterior aspect of the petrosal, the mastoid exposure (Figs 3A 4A, mpp). On the ventral edge of the mastoid exposure, a small and elevated area forms a process, which is identified as the attachment site or the base of the tympanohyal (Fig. 3A, th). There are two nutritive foramina on the posterior aspect of the mastoid exposure dorsal to the tympanohyal. The low, shallow margin medial to the tympanohyal is probably the stylomastoid notch (Fig. 3A, smn), marking the exit of the facial nerve from the tympanic cavity. A relatively massive and elevated area on the ventral edge of the mastoid exposure and posterior to the depression of the post-promontorial tympanic sinus represents the caudal tympanic process of the petrosal (Figs 3A 4A, ctpp).
Endocranial (mediodorsal) aspect of the petrosal
The endocranial surface of the pars cochlearis is slightly concave. The main feature is a prominent internal acoustic meatus. The large opening or depression of the internal acoustic meatus is subdivided by a low transverse crest into two parts (Figs 2E 3B 4B). The ventral opening is very large and represents the foramen acusticum inferius and serves for the cochlear nerve (of cranial nerve VIII) (Fig. 2E,F). The cribriform-like foramina (the tractus spiralis foraminosus) that are associated with the cochlear nerve's numerous minute entries into the cochlear labyrinth in extant therian mammals could not be observed. It is possible that the tractus spiralis foraminosus was not present in Henkelotherium and it is also possible that the petrosal area where cribriform-like foramina would be located was not ossified. These structures are very fragile in most extant mammals and therefore it is also possible that preservation was not sufficient to have the structures detected by the CT scans.
The cavity within the internal acoustic meatus dorsal to the transverse crest, the foramen acusticum superius, consists of two smaller openings (Figs 3B 4B). The posterior foramen opens into the vestibular part of the bony labyrinth as can be traced in the CT scan; this foramen is the conduit of the vestibular nerve (VIII). The anterior opening is the primary facial foramen for the passage of the facial nerve (VII) into the cavum supracochleare. The anterolateral border of the internal acoustic meatus is formed by the prefacial commissure. The commissure bulges out prominently (Figs 2C–E 3B 4, pfc).
Three more openings are identifiable behind the internal acoustic meatus. The large subarcuate fossa is only partly preserved. More than half of its rim is damaged as is the entire dorsolateral portion of the pars canalicularis (Figs 2G 3B 4A, saf). The rim of the subarcuate fossa is formed by the underlying anterior semicircular canal. Because of damage, the cross-section of the anterior semicircular canal is exposed (Fig. 4A, asc). The opening for the aqueductus vestibuli (for the endolymphatic duct) is positioned at the posteromedial border of the subarcuate fossa (Figs 3B 4B, av). As can be traced in the CT scans and virtual reconstruction, the aqueductus vestibuli is connected to the base of the crus commune of the anterior and posterior semicircular canals (Fig. 5A,B,D, av and cc). Along the broken medial edge of the pars canalicularis, the sulcus for the sigmoid sinus is represented by an elongate depression that is somewhat fractured (Figs 3B 4B, sss?). The ventromedial extension of the sigmoid sinus sulcus toward the jugular notch is recognizable but this is tentative because of the fragmented preservation (Figs 3B 4B, sss?). The sigmoid sinus sulcus is situated on the endocranial surface overlying the posterior portion of the lateral semicircular canal and the crus commune. Two small foramina are present in the curved depression of the sigmoid sinus sulcus and these openings lead to a pair of curved channels lateral to the posterior semicircular canal. These are interpreted to be nutritive foramina, which in general are morphologically variable.
Inner ear bony labyrinth structure
As in extant therians, three distinct portions of the bony labyrinth can be distinguished on the virtual endocast (Figs 5 6): the cochlear canal and some of its internal structure, the saccular and utricular parts of the vestibule, and the bony canals of the semicircular ducts. The cochlear canal is slightly curved in the basal (‘proximal’) part of the canal. Its apical (‘distal’) part is coiled through about 270° of arc to form about three-quarters of a complete turn around the entry point of the cochlear nerve into the cochlear canal (Figs2A–E 5 6, co). The estimated length of the cochlear canal is 2.7 mm. The transverse outline of the cochlear canal is nearly circular in the curved (‘uncoiled’) basal part and middle segment of the canal (Figs 2D 5). However, it is dorsoventrally compressed (‘oval-shaped’) in cross-section in the apical (distal) part of the canal. Accordingly, the apical part of the canal appears to be dorsoventrally compressed and is coiled in a nearly horizontal plane on the reconstructed endocast (Figs 2A 5A,C). The apical part of the cochlear canal was probably flattened by the post-mortem compression of the pars cochlearis as the petrosal shows some minor damage in this area (indicated by asterisks in Fig. 3A).
Fig. 6.
Virtual endocast of the inner ear bony labyrinth of Henkelotherium guimarotae type specimen (Gui Mam 138/76b), left side. Asterisk indicates the separation between the saccular space and the base of cochlear canal. Dark triangles indicate the separation between the utricle and saccule. (A) Ventrolateral view (stereo pair); (B) dorsomedial view (stereo pair). ac, aqueductus cochleae (endocast); asc, anterior semicircular canal (incomplete, broken segment indicated by dotted lines); asca, anterior semicircular canal ampulla; av, aqueductus vestibuli (endocast); cc, crus commune; co, cochlear duct (endocast); fc + fv(br), dashed outline indicating the broken area of fenestra cochleae, fenestra vestibuli and possibly the fossa for the stapedius muscle; fc, hypothetical position of fenestra cochleae (not preserved); fcn, foramen for cochlear nerve; fv, hypothetical position of fenestra vestibuli (not preserved); lsc, lateral semicircular canal (incomplete, broken segment indicated by dotted lines); lsca, lateral semicircular canal ampulla; pbl, primary bony lamina for basilar membrane; psc, posterior semicircular canal; psca, posterior semicircular canal ampulla; sa?, possible saccular part of vestibule; sbl, secondary bony lamina for basilar membrane; ul, utricle.
The surface of the cochlear canal endocast shows the base of the primary bony lamina of the basilar membrane (lamina spiralis ossea primaria) on the endocranial (axial) side of the cochlear canal (Figs 2E,F 5B,D 6B, pbl). We interpret that the basal portion (although not the entirety) of the primary lamina is preserved in this specimen of Henkelotherium (Gui Mam 138/76b). On the reconstructed virtual endocast of the cochlear canal, the base of the primary bony lamina is represented by a prominent groove that starts from near the entry point of the cochlear canaliculus and extends along the curved mid-section of the cochlear canal beyond the cochlear nerve entry into the canal. However, the primary bony lamina is not present in the coiled apical part of the canal (Figs 2A–D 5D 6B) and its groove on the endocast surface fails to reach the apical turn. The primary bony lamina is ventral to the large single foramen of the cochlear nerve (Figs 2E,F 5D 6, fcn).
The secondary bony lamina for the basilar membrane (lamina spiralis ossea secundaris) is also present in the basal part of the cochlear canal (Figs 2F 5B,D 6B, sbl). On the cochlear canal endocast, the secondary bony lamina starts at the fenestra cochleae, extends along the medial margin of the cochlear endocast (the radial wall of the cochlear canal) and eventually fades in the middle section of the cochlea. The secondary bony lamina is less developed in size than the primary lamina in both the cross-section of the canal and its extension along the length of the cochlear canal. Its impression is shallow and short but its presence is clear.
The lateral semicircular canal is oriented nearly horizontally in intact mammalian skulls and on the reconstructed endocast of the inner ear the proximal part of the cochlear canal forms a 140° angle to the plane of the lateral semicircular canal.
On the endocast and near the entry point of the cochlear canaliculus, a groove separates the base of the cochlea from the saccular space of the vestibule (Figs 5 6, asterisk). The putative ‘saccular space’ is clearly demarcated from the utricle (Figs 5 6, solid triangle). Because the subarcuate fossa is damaged, most of the anterior semicircular canal is missing. Nonetheless, the ampulla of the anterior semicircular canal and a short segment connected to the crus commune are preserved. The broken ends of the anterior semicircular canal are exposed on the petrosal (Figs 3B 4A, asc). The posterior two-thirds of the lateral semicircular canal and its ampulla are preserved (Figs 2G 5A–C, lsc, lsca). The height of the lateral semicircular canal is about 0.9 mm and the reconstructed width is approximately 1.1 mm. The posterior semicircular canal is complete and bent laterally. It has a height of 1.2 mm measured from the vestibulum to the inner wall of the semicircular canal and a width of 0.9 mm measured perpendicular to the height. The posterior ampulla is distinctive but broken (Figs 2G 5B,D 6B, psca). The posterior arm of the lateral semicircular canal is confluent with the inferior arm of the posterior semicircular canal to form the so-called second crus commune (see more discussion below). This area remains ambiguous because the posterior ampulla is present but it appears to be incomplete (Figs 2G 5B,D, psca). The crus commune, the confluent segment of the anterior and posterior semicircular canals, is well developed (Figs 2G,H 5A,B 6B, cc). Along the medial side of the crus commune a small, short canal for the aqueductus vestibuli is present and is connected to the base of the crus commune (Fig. 5A,D, av).
Discussion
Systematic distribution and evolution of petrosal characters
The majority of the petrosal characters seen in the holotype of Henkelotherium are also present in Vincelestes, albeit in slightly modified conditions. In fact, Henkelotherium more closely resembles the morphological condition observed in prototribosphenidans in general than in other more basal Mesozoic mammalian clades. Henkelotherium shows a more bulbous and oval-shaped promontorium similar to Vincelestes (Rougier et al. 1992) and the more derived metatherians and eutherians. This contrasts with the cylindrical (elongate, narrow and not inflated) promontorium in the Early Cretaceous spalacotheroid Zhangheotherium(Hu et al. 1997) and in triconodontids such as the Late Jurassic Priacodon (Rougier et al. 1996), the Early Cretaceous Cloverly Formation tricondontid (Crompton & Luo, 1993) and the British Early Cretaceous Trioracodon (Wible & Hopson, 1993). Henkelotherium has a distinct sulcus on the promontorium for the internal carotid artery, a feature also present in more derived Vincelestes and most basal eutherians (Rougier et al. 1992; McKenna et al. 2000; Wible et al. 2001, 2007, in press; Ekdale et al. 2004). This differs from eutriconodonts, most multituberculates, Zhangheotherium and metatherians, which lack the promontorial sulcus for the internal carotid artery (Miao, 1988; Wible, 1990; Wible & Hopson 1993, 1995; Rougier et al. 1996; Wible & Rougier, 2000; Ladevèze, 2004, 2007; Ladevèze et al. 2008; Horovitz et al. 2008).
The lateral trough is broad in cynodonts, tritylodontids, tritheledontids, brasilitheriids and a wide range of pre-mammalian mammaliaforms (Wible & Hopson, 1993, 1995; Luo et al. 1995, 2001; Rougier et al. 1996; Bonaparte et al. 2005). In eutriconodonts, the lateral trough is reduced in size and becomes narrow. The narrow lateral trough is partially contacted by the lateral flange in multituberculates (Wible & Hopson, 1995; Rougier et al. 1996; Wible & Rougier, 2000). In Vincelestes, this trough is further reduced (Rougier et al. 1992) and the preserved part of the lateral trough in Henkelotherium is similar to that of Vincelestes.
Although the cavum supracochleare floor is fractured and broken in Henkelotherium, the cavum is reconstructed tentatively as having a complete bony floor for the geniculate ganglion of the facial nerve, a derived feature of eutriconodonts, multituberculates, Vincelestes and most therians (Rougier et al. 1996, 1998). However, because the cavum epiptericum region is completely missing and the cavum supracochleare floor is broken in Henkelotherium, it is not clear if it would resemble the triconodontid and multituberculate condition in which the cava supracochleare and epiptericum are completely confluent with each other by the broadly open semilunar notch. However, it cannot be excluded that Henkelotherium resembles Vincelestes and derived therians such as Prokennalestes, in which the cavum supracochleare is enclosed by the petrosal but is interconnected with the cavum epiptericum through the fenestra semilunaris (Rougier et al. 1992; Wible et al. 2001).
The post-promontorial tympanic sinus and the caudal tympanic process in Henkelotherium (Fig. 3A, pps, ctpp) are also present in Zhangheotherium, Vincelestes and therians (Rougier et al. 1992; Hu et al. 1997; Wible & Rougier, 2000; Wible et al. 2001; Ekdale et al. 2004; Ladevèze, 2004, 2007). These are synapomorphies of trechnotherians (Zhangheotherium through Henkelotherium to therians) but are absent in most (but not all) multituberculates, eutriconodonts, the monotreme Ornithorhynchus and mammaliaforms such as Sinoconodon, Morganucodon and Haldanodon. Of other preserved characters on the petrosal of Henkelotherium, the presence of the prootic canal and the relatively shallow internal acoustic meatus are primitive features of mammaliaforms (Kermack et al. 1981; Lillegraven & Krusat, 1991; Wible & Hopson, 1993; Luo et al. 1995).
In Sinoconodon, Morganucodon, Priacodon, multituberculates and the monotreme Ornithorhynchus, there is an open groove (for the perilymphatic duct) on the petrosal from the jugular notch that enters the inner ear through an opening on the back of the promontorium, the perilymphatic foramen; a fenestra cochleae (round window) is lacking (Lillegraven & Hahn, 1993; Zeller, 1993; Luo, 1994; Rougier et al. 1996; Hurum, 1998). During phylogeny with the enlargement of the pars cochlearis and isolation of the inner ear from the middle ear cavity, a processus recessus has grown to enclose the perilymphatic duct and separates the fenestra cochleae from the perilymphatic foramen in therians (Zeller, 1985). Among living mammals, an enclosed perilymphatic canal, the cochlear canaliculus, occurs in the vast majority of therians and most probably convergently in the adult monotreme Tachyglossus (De Beer, 1937; Kuhn, 1971; Zeller, 1993). Among fossils, the cochlear canaliculus has been described only in Vincelestes and therians (Wible, 1990; Rougier et al. 1992; Wible et al. 2001; Ekdale et al. 2004; Ladevèze, 2004, 2007; Ladevèze et al. 2008; Schmelzle et al. 2007), and in an isolated petrosal that is referable to the ‘symmetrodont’ Gobiotheriodon (Wible et al. 1995). Because the cochlear canaliculus is also present in more derived taxa than ‘symmetrodonts’ including Henkelotherium, it probably represents an apomorphic trechnotherian character. The formation of the perilymphatic canal occurred earlier in phylogenetic evolution of therian mammals than the development of a cochlear canal coiled through at least 270°.
Implication for evolution of hearing in Mesozoic mammals
Inner ear structures underwent fundamental changes during the evolution from non-mammalian cynodonts to modern mammals (Allin & Hopson, 1992; Luo, 2001; Kielan-Jaworowska et al. 2004). The elongation of the bony cochlear canal is correlated with the development of a ventral eminence of the pars cochlearis, leading to the fully developed promontorium, as described for Sinoconodon, Morganucodon, Haldanodon, multituberculates and Zhangheotherium (Kermack & Musset, 1983; Graybeal et al. 1989; Lillegraven & Krusat, 1991; Luo & Ketten, 1991; Luo et al. 1995; Meng & Wyss, 1995; Hurum, 1998). Both the elongate cochlear canal and promontorium are regarded as apomorphic features of mammaliaforms (Lucas & Luo, 1993; Wible & Hopson, 1993; Luo, 1994; Rougier et al. 1996). In some pre-mammalian mammaliamorphs, such as the tritylodontid Yunnanodon, the cochlear canal has already formed (Luo, 2001). In most basal mammaliaforms, the cochlear canal is longer relative to skull length (Table 1 in Luo et al. 1995) than in pre-mammaliaform cynodonts. A straight, elongate cochlear canal is also present in eutriconodonts, such as Jeholodens, and in the spalacotheroid Zhangheotherium (Hu et al. 1997; Ji et al. 1999; Luo, unpublished data). In multituberculates, the cochlear canal can be straight (Luo & Ketten, 1991; Lillegraven & Hahn, 1993) or slightly curved (Meng & Wyss, 1995; Fox & Meng, 1997; Hurum, 1998).
Monotremes and therians have membranous cochlear ducts that are coiled, which distinguishes them from all living non-mammalian amniotes. In monotremes, the membranous cochlear duct is coiled (Ornithorhynchus anatinus) or distally hooked (Tachyglossus aculeatus) (Alexander, 1904; Fleischer, 1973; Zeller, 1989) but the bony cochlear canal is less curved than the membranous cochlear duct (Fig. 6 in Fox & Meng, 1997). There is not a close correlation between coiling of the membranous cochlear duct and coiling of the ossified cochlear canal. The coiled basilar membrane in monotremes is not supported by any bony lamina(e) inside the bony labyrinth. This observation has been made in embryonic sections (Zeller, 1989, 1993), in naturally broken adult petrosals with desiccated membranous labyrinth tissues inside (Luo & Ketten, 1991) and in a scanning electron microscope study of the broken pars cochlearis of adults (Fig. 7 in Fox & Meng, 1997). Although Fleischer (1973, 177, Abb. 78) speculated that Ornithorhynchus may have a short primary bony lamina, this has not been confirmed by the more recent studies that show no such structure in serial sections of late-stage embryos (Zeller, 1989) or in the exposed interior of the cochlear canal through dissection of the pars cochlearis (Fig. 7 in Fox & Meng, 1997).
In contrast, in extant marsupials and placentals, the soft-tissue cochlear duct of the membranous labyrinth and the cochlear canal of the bony labyrinth are coiled together. The coiled basilar membrane within the cochlea is supported by the primary bony lamina. The latest reviews of these characters present the consensus that the coiling of the membranous cochlear duct is not homologous between monotremes and extant therians, as indicated by these fundamental structural differences (e.g. Lewis et al. 1985; Zeller, 1989; Luo & Ketten, 1991; Hu et al. 1997; Fox & Meng, 1997; Luo et al. 2002). This view is also supported by recent phylogenies (e.g. Luo & Wible, 2005; Rougier et al. 2007; Luo, 2007; Luo et al. 2007). The primary bony lamina (or a bony base for the basilar membrane lamina) of Henkelotherium, as identified in this study (Figs 2E,F 5B,D 6B), is ventral to the entry point of the cochlear nerve, whereas the putative primary lamina, as identified for Ornithorhynchus by Fleischer (1973, Abb. 78), is dorsal to the entry point of the cochlear nerve. This is consistent with the broad difference in the bony supporting structure (or the lack thereof) for the membranous labyrinth cochlear duct between monotremes on the one hand and the cladotherians on the other.
Previously, Early Cretaceous Vincelestes was the most primitive-known stem taxon to extant therians to have a bony cochlear canal coiled through 270°. Vincelestes also has the secondary bony lamina on a naturally exposed cochlear canal endocast (Fig. 34 in Rougier, 1993), although it is unknown if Vincelestes has the primary bony lamina as the relevant part of the inner ear is not exposed or studied by CT scans. Late Cretaceous stem metatherians and eutherians are the most primitive-known therians to have the primary and secondary bony laminae in the cochlear canal (Meng & Fox, 1995). This CT study of the cochlear structure of Henkelotherium reveals a coiled cochlear canal with primary and secondary bony laminae for the basilar membrane. Therefore, a cochlear canal curved through 270° with primary and secondary bony laminae is part of the apomorphic groundplan for the inner ear of the Cladotheria, the clade defined by the common ancestor of dryolestoids (including Henkelotherium) and therians. This is a more ancient origin for cochlear coiling with bony laminae for the basilar membrane in therian evolution in a more inclusive clade than Protribosphenida (Vincelestes and therians).
The functional significance of the primary and secondary bony laminae for the basilar membrane has been studied in placental mammals. The distribution, size and structural form of the primary and secondary bony laminae and their relationship to the basilar membrane in the Organ of Corti for hearing are known in cetaceans and bats (Yamada & Yoshizaki, 1959; Pye, 1970; Wever et al. 1971; Fleischer, 1976a,b; Ramprashad et al. 1979; Ketten & Wartzok, 1990; Geisler & Luo, 1996; Luo & Marsh, 1996). A narrow width of the basilar membrane and a more rigid bony support for the basilar membrane by well-developed primary and secondary bony laminae in the basal part of the cochlear canal have been shown to be correlated with highly sensitive hearing in ultra-high-frequency sound for microchiropteran bats and odontocete whales (Wever et al. 1971; Fleischer, 1973, 1976b; Ramprashad et al. 1979). This provides a basis for understanding the functional implications of the newly discovered structures in Henkelotherium.
The secondary bony lamina is a derived feature because it is known in many (but not all) placental taxa and has a more restricted systematic distribution than the primary bony lamina. The latter structure is universally present in all extant therians and is now by the present study extended to be synapomorphic for the clade of extant therians plus Henkelotherium. In those living placentals that have the secondary bony lamina, the lamina is on the radial wall of the cochlear canal to reinforce the radial edge of the basilar membrane (Pye, 1970; Fleischer 1976b; Ramprashad et al. 1979; Ketten, 1992). With the exception of some primates, all of chiropterans and cetaceans, this structure is confirmed to be absent in many extant placentals, such as humans, manatees, camels and pigs (Bast & Anson, 1949; Fleischer, 1973; personal observation by authors on several taxa). Contrary to an earlier (and anecdotal) reference to this structure in some rodents (Reysenbach de Haan, 1957), most rodents that have been examined by histological sections lack this feature (Pye, 1977, pl. 1, 1979, pl. I). A weakly developed and short secondary lamina is reported by Fleischer (1973) for a range of placental mammals (including pangolins and xenarthrans) but not confirmed for some of these taxa by other studies using histological sections (Pye 1979, pls. II and III). Information on the secondary bony lamina is more limited on living marsupials than on living placentals. It is not seen in the histological sections of some didelphids (Pye, 1979) but appears to be present on the virtual endocast of the inner ear from CT scans of the didelphid Caluromys (Sánchez-Villagra & Schmelzle, 2007). It seems to be absent from the inner ear virtual endocasts of the fossil marsupials Necrolestes (Ladevèze et al. 2008) and Herpetotherium (Horovitz et al. 2008). In light of its scattered distribution, the secondary bony lamina is homoplastic among the diverse groups of living therians, possibly down to low taxonomic levels. It cannot be ruled out that this character can be variable among individuals of the same taxa and that some of the conflicting reports are due to sampling with different techniques. When present, the secondary bony lamina has its greatest width at the basal-most part in the basal cochlear turn and becomes diminishingly narrower toward the apex of the cochlea. Consequently, the width or other size-related features can vary with different locations along the basal cochlear canal turns. A full-scale assessment of the variability of this feature is beyond the scope of the present study.
Our CT scanning clearly demonstrates the presence of the secondary bony lamina in Henkelotherium. This is consistent with an earlier observation of this structure in Vincelestes (Rougier, 1993) and in the broken cochlear canal of some Cretaceous metatherian and eutherian petrosals, which appears to be representative of the character conditions (groundplan) of metatherians and eutherians as a whole (Meng & Fox, 1995; Fox & Meng, 1997). The consistent distribution of secondary bony lamina in the phylogenetically and successively nested taxa from Henkelotherium and Vincelestes to the representative metatherians and eutherians is in contrast to their conspicuous absence in many extant marsupials and placentals. For the systematic distribution of this feature in the above-mentioned clades, there can be two alternative hypotheses of the phylogenetic evolution of this character. (i) Because the secondary bony lamina is present in the basal members of the cladotherian clade for which this character has been investigated, it is parsimonious to hypothesize that the secondary lamina is an apomorphy of the cladotherian clade (Henkelotherium, Vincelestes through therians) and that its absence in the majority of extant therian mammals would present a secondary loss within this clade. (ii) Alternatively, the absence of the secondary bony lamina would be the ancestral condition of many extant therian mammals, shared by the pre-cladotherian ‘symmetrodonts.’ The secondary lamina is homoplastic and evolved independently in the basal cladotherians Henkelotherium, Vincelestes, some stem metatherians and some stem eutherians, and separately in chiropterans and in cetaceans within the placental group. The latter hypothesis is consistent with the fact that chiropterans with this lamina are nested within laurasiatherians, many of which lacked this feature ancestrally, and that cetaceans are nested within cetartiodactyls, many of which also lacked this feature ancestrally. Unless it is demonstrated by future CT scanning or histological studies of cochlear structure that the secondary lamina is present in more taxa of the basal-most eutherians and metatherians, we prefer the former hypothesis.
Regardless of the uncertainty in interpreting the pattern of phylogenetic distribution of the secondary bony lamina among the basal-most therians, there is clear insight into the hearing of Henkelotherium from the newly observed secondary bony lamina (in addition to the primary bony lamina). The presence of these structures suggests that the basilar membrane, whose dimension and structural support determine the sensitivity of hearing frequencies, had a more rigid structural support in Henkelotherium, at least more so than in the pre-cladotherian Mesozoic mammals lacking these structures. Presence of the secondary lamina would narrow the width of the basilar membrane and would provide more rigid structural support for the basilar membrane. Both characters are correlated with more sensitive hearing of high-frequency sounds [reviewed by Fleischer (1976b) and Ketten (1992)]. This can be hypothesized to indicate that Henkelotherium had more acute hearing for high-frequency sounds than other contemporary fossil mammals without such structures, everything else being equal for the relevant soft-tissue structures. The fossil evidence of the primary and secondary bony laminae in Henkelotherium has pushed back the first appearance of this improved capacity for high-frequency hearing from the Early Cretaceous to the Late Jurassic. Henkelotherium represents a transitional stage in the earliest evolution of the therian cochlear structure, according to our assumption that the presence of this secondary bony lamina is a synapomorphy of cladotherians.
Even though the pars vestibularis of the bony labyrinth of Henkelotherium is partly preserved, the morphology of the semicircular canals resembles the typical mammalian condition. As is the case in Henkelotherium, in the Oligocene metatherian Herpetotherium (Horovitz et al. 2008), several plesiomorphic extant marsupials (Isoodon, Monodelphis, Dasyurus and Caluromys) (Sánchez-Villagra & Schmelzle, 2007; Schmelzle et al. 2007) as well as early eutherians (Meng & Fox, 1995), certain extant carnivorans (Hyrtl, 1845; Gray, 1908) and rodents (personal observations), the posterior and lateral semicircular canals produce a secondary crus commune. Because the distribution of the secondary crus commune has not been mapped in other Mesozoic mammals, currently it is difficult to provide a phylogenetic interpretation of this character.
Acknowledgments
We thank T. R. Ryan (Department of Anthropology and Center for Quantitative Imaging, Pennsylvania State University) for producing the CT scans and G. Oleschinski (Steinmann-Institut für Geologie, Mineralogie und Paläontologie, Bonn) for preparing the photographs. This research has been supported by National Science Foundation (USA) Grants (DEB 0316558 and EF0629959), a Humboldt-Forschungspreis from Alexander von Humboldt-Stiftung (Germany) to Z.-X.L., National Science Foundation (USA) Grant (EF0629959) to J.R.W. and a Max Kade Foundation (New York) Fellowship to T.M. We would like to thank G. W. Rougier and an anonymous reviewer for their helpful comments that greatly improved the manuscript.
References
- Alexander G. Entwickelung und Bau des inneren Gehörorganes von Echidna aculeata. Denkschr Med-naturwiss Ges Jena. 1904;6:1–118. [Google Scholar]
- Allin EF, Hopson JA. Evolution of the auditory system in Synapsida (‘mammal-like reptiles’ and primitive mammals) as seen in the fossil record. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer-Verlag; 1992. pp. 587–614. (eds), pp. [Google Scholar]
- Bast TH, Anson BJ. The Temporal Bone and the Ear. Springfield, Illinois: Charles C. Thomas; 1949. [Google Scholar]
- Bonaparte JF, Martinelli AG, Schultz CL. New information on Brasilodon and Brasilitherium(Cynodontia, Probainognathia) from the Late Triassic, southern Brazil. Rev Brasil Paleontol. 2005;8:25–56. [Google Scholar]
- Crompton AW, Luo Z-X. The relationships of the Liassic mammals Sinoconodon, Morganucodon oehleri and Dinnetherium. In: Szalay FS, Novacek MJ, McKenna MC, editors. Mammal Phylogeny – Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials. New York: Springer-Verlag; 1993. pp. 30–44. (eds), pp. [Google Scholar]
- De Beer GR. The Development of the Vertebrate Skull. Oxford: Clarendon Press; 1937. [Google Scholar]
- Drescher E. Preparation of vertebrate fossils from the Guimarota mine. In. In: Martin T, Krebs B, editors. Guimarota – a Jurassic Ecosystem. München: Verlag Dr Friedrich Pfeil; 2000. pp. 137–142. (eds), pp. [Google Scholar]
- Ekdale EG, Archibald JD, Averianov AO. Petrosal bones of placental mammals from the Late Cretaceous of Uzbekistan. Acta Palaeontol Pol. 2004;49:161–176. [Google Scholar]
- Evans H. Miller's Anatomy of the Dog. Philadelphia: W. B. Saunders Company; 1993. [Google Scholar]
- Fleischer G. Studien am Skelett des Gehörorgans der Säugetiere, einschließlich des Menschen. Säugetierkd Mitt. 1973;21:131–239. [Google Scholar]
- Fleischer G. Hearing in extinct cetaceans as determined by cochlear structure. J Paleontol. 1976a;50:133–152. [Google Scholar]
- Fleischer G. On bony microstructures in the dolphin cochlea, related to hearing. Neues Jahrb Geol Paläontol Abh. 1976b;151:166–191. [Google Scholar]
- Fox RC, Meng J. An X-radiographic and SEM study of the osseous inner ear of multituberculates and monotremes (Mammalia): impressions for mammalian phylogeny and evolution of hearing. Zool J Linn Soc. 1997;121:249–291. [Google Scholar]
- Gaupp E. Zur Entwickelungsgeschichte und vergleichenden Morphologie des Schädels von Echidna aculeata var. typica. Denkschr Med-naturwiss Ges Jena. 1908;6:539–788. [Google Scholar]
- Geisler JH, Luo Z–X. Petrosal and inner ear structures of Herpetocetus, their implications on relationships and hearing function of archaic mysticetes. J Paleontol. 1996;70:1045–1066. [Google Scholar]
- Gray AA. The Labyrinth of Animals. London: J. and A. Churchill; 1908. [Google Scholar]
- Graybeal A, Rosowski JJ, Ketten DR, Crompton AW. Inner-ear structure in Morganucodon, an early Jurassic mammal. Zool J Linn Soc. 1989;96:107–117. [Google Scholar]
- Henkel S, Krusat G. Die Fossil-Lagerstätte in der Kohlengrube Guimarota (Portugal) und der erste Fund eines Docodontiden-Skelettes. Berl Geowiss Abh A. 1980;20:209–216. [Google Scholar]
- Hennig W. Grundzüge einer Theorie der phylogenetischen Systematik. Berlin: Deutscher Zentralverlag; 1950. [Google Scholar]
- Hennig W. Phylogenetic Systematics. Urbana: University of Illinois Press; 1966. [Google Scholar]
- Horovitz I, Ladevèze S, Argot C, et al. The anatomy of Herpetotherium cf. fugax Cope, 1873, a metatherian from the Oligocene of North America. Palaeontographica (A) 2008;284:109–141. [Google Scholar]
- Hu Y, Wang Y, Luo Z-X, Li C. A new symmetrodont mammal from China and its implications for mammalian evolution. Nature. 1997;390:137–142. doi: 10.1038/36505. [DOI] [PubMed] [Google Scholar]
- Hurum JH. The inner ear of two Late Cretaceous multituberculate mammals, and its implications for multituberculate hearing. J Mammal Evol. 1998;5:65–93. [Google Scholar]
- Hyrtl J. Vergleichend-anatomische Untersuchungen über das innere Gehörorgan des Menschen und der Säugethiere. Prague: Friedrich Ehrlich; 1845. [Google Scholar]
- Ji Q, Luo Z-X, Ji S-A. A Chinese triconodont mammal and mosaic evolution of mammalian skeleton. Nature. 1999;398:326–330. doi: 10.1038/18665. [DOI] [PubMed] [Google Scholar]
- Kermack KA, Musset F. The ear in mammal-like reptiles and early mammals. Acta Palaeontol Pol. 1983;28:147–158. [Google Scholar]
- Kermack KA, Mussett F, Rigney HW. The skull of Morganucodon. Zool J Linn Soc. 1981;71:1–158. [Google Scholar]
- Ketten DR. The marine mammal ear: specializations for aquatic audition and echolocation. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer-Verlag; 1992. pp. 717–750. (eds), pp. [Google Scholar]
- Ketten DR, Wartzok D. Three-dimensional reconstructions of the dolphin ear. In: Thomas J, Kastelein R), editors. Sensory Abilities of Cetaceans. New York: Plenum Press; 1990. pp. 81–105. (eds. [Google Scholar]
- Kielan-Jaworowska Z, Cifelli RL, Luo Z-X. Mammals from the Age of Dinosaurs. New York: Columbia University Press; 2004. [Google Scholar]
- Kielan-Jaworowska Z, Presley R, Poplin C. The cranial vascular system in taeniolabidoid multituberculate mammals. Phil Trans R Soc Lond. 1986;313:525–602. [Google Scholar]
- Krebs B. Das Skelett von Henkelotherium guimarotae gen. et sp. nov. (Eupantotheria, Mammalia) aus dem Oberen Jura von Portugal. Berl Geowiss Abh A. 1991;133:1–110. [Google Scholar]
- Krebs B. The henkelotheriids from Guimarota mine. In: Martin T, Krebs B, editors. Guimarota – a Jurassic Ecosystem. München: Verlag Dr Friedrich Pfeil; 2000. pp. 121–128. (eds), pp. [Google Scholar]
- Kuhn H-J. Die Entwicklung und Morphologie des Schädels von Tachyglossus aculeatus. Abh Senckenberg Naturforsch Ges. 1971;528:1–224. [Google Scholar]
- Ladevèze S. Metatherian petrosals from the late Paleocene of Itaboraí, Brazil, and their phylogenetic implications. J Vertebr Paleontol. 2004;24:202–213. [Google Scholar]
- Ladevèze S. Petrosal bones of metatherian mammals from the late Palaeocene of Itaboraí (Brazil), and a cladistic analysis of petrosal features in metatherians. Zool J Linn Soc. 2007;150:85–115. [Google Scholar]
- Ladevèze S, Asher RJ, Sánchez-Villagra MR. Petrosal anatomy in the fossil mammal Necrolestes: evidence for metatherian affinities and comparisons with the extant marsupials mole. J Anat. 2008;213:686–697. doi: 10.1111/j.1469-7580.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The Vertebrate Inner Ear. Boca Raton, FL: CRC Press; 1985. [Google Scholar]
- Lillegraven JA, Hahn G. Evolutionary analysis of the middle and inner ear of Late Jurassic multituberculates. J Mammal Evol. 1993;1:47–74. [Google Scholar]
- Lillegraven JA, Krusat G. Cranio-mandibular anatomy of Haldanodon expectatus(Docodonta; Mammalia) from the Late Jurassic of Portugal and its implications to the evolution of mammalian characters. Contrib Geol Univ Wyoming. 1991;28:39–138. [Google Scholar]
- Lucas SG, Luo Z-X. Adelobasileus from the Upper Triassic of western Texas: the oldest mammal. J Vertebr Paleontol. 1993;13:309–334. [Google Scholar]
- Luo Z-X. Sister-group relationships of mammals and transformations of diagnostic mammalian characters. In: Fraser NC, Sues H-D, editors. In the Shadow of the Dinosaurs – Early Mesozoic Tetrapods. Cambridge: Cambridge University Press; 1994. (eds), pp. [Google Scholar]
- Luo Z-X. The inner ear and its bony housing in tritylodontids and implications for evolution of the mammalian ear. Bull Mus Comp Zool. 2001;156:81–97. [Google Scholar]
- Luo Z-X. Transformation and diversification in early mammal evolution. Nature. 2007;450:1011–1019. doi: 10.1038/nature06277. [DOI] [PubMed] [Google Scholar]
- Luo Z-X, Chen P-J, Li G, Chen M. A new eutriconodont mammal and evolutionary development in early mammals. Nature. 2007;446:288–293. doi: 10.1038/nature05627. [DOI] [PubMed] [Google Scholar]
- Luo Z-X, Crompton AW, Lucas SG. Evolutionary origins of the mammalian promontorium and cochlea. J Vertebr Paleontol. 1995;15:113–121. [Google Scholar]
- Luo Z-X, Crompton AW, Sun A-L. A new mammaliaform from the Early Jurassic of China and evolution of mammalian characteristics. Science. 2001;292:1535–1540. doi: 10.1126/science.1058476. [DOI] [PubMed] [Google Scholar]
- Luo Z-X, Ketten DR. CT scanning and computerized reconstructions of the inner ear of multituberculate mammals. J Vertebr Paleontol. 1991;11:220–228. [Google Scholar]
- Luo Z-X, Kielan-Jaworowska Z, Cifelli RL. In quest for a phylogeny of Mesozoic mammals. Acta Palaeontol Pol. 2002;47:1–78. [Google Scholar]
- Luo Z-X, Marsh KK. Petrosal (periotic) and inner ear of a Pliocene kogiine whale (Kogiinae, Odontoceti): implications on relationships and hearing evolution of toothed whales. J Vertebr Paleontol. 1996;16:328–348. [Google Scholar]
- Luo Z-X, Wible JR. A new Late Jurassic digging mammal and early mammalian diversification. Science. 2005;308:103–107. doi: 10.1126/science.1108875. [DOI] [PubMed] [Google Scholar]
- Muizon C de. Marshall LG, Muizon C de. Part II: The skull. Pucadelphys andinus (Marsupialia, Mammalia) from the early Paleocene of Bolivia. 1995;165:21–90. Mém Mus Natt Hisl(ed. [Google Scholar]
- Martin T. Mammalian fauna of the Late Jurassic Guimarota ecosystem. A.P.A. Pub Especial. 2001;7:123–126. [Google Scholar]
- Martin T. Postcranial anatomy of Haldanodon exspectatus(Mammalia, Docodonta) from the Late Jurassic (Kimmeridgian) of Portugal and its bearing for mammalian evolution. Zool J Linn Soc. 2005;145:219–248. [Google Scholar]
- Martin T, Krebs B. Guimarota – a Jurassic Ecosystem. München: Verlag Dr Friedrich Pfeil; 2000. [Google Scholar]
- McKenna MC, Bell SK. Classification of Mammals above the Species Level. New York: Columbia University Press; 1997. [Google Scholar]
- McKenna MC, Kielan-Jaworowska Z, Meng J. Earliest eutherian mammal skull from the Late Cretaceous (Coniacian) of Uzbekistan. Acta Palaeontol Pol. 2000;45:1–54. [Google Scholar]
- Meng J, Fox RC. Osseous inner ear structures and hearing in early marsupials and placentals. Zool J Linn Soc. 1995;115:47–71. [Google Scholar]
- Meng J, Wyss A. Monotreme affinities and low-frequency hearing suggested by multituberculate ear. Nature. 1995;377:141–144. [Google Scholar]
- Miao D. Skull morphology of Lambdopsalis bulla(Mammalia, Multituberculata) Contrib Geol Univ Wyoming Spec Pap. 1988;4:1–104. [Google Scholar]
- Muizon C de. Mayulestes ferox, a borhyaenoid (Metatheria, Mammalia) from the early Palaeocene of Bolivia. Phylogenetic and palaeobiologic implications. Geodiversitas. 1998;20:19–142. [Google Scholar]
- Pye A. The structure of the cochlea in Chiroptera – a selection of Microchiroptera from Africa. J Zool (Lond) 1970;162:335–343. [Google Scholar]
- Pye A. The structure of the cochlea in some myomorph and caviomorph rodents. J Zool (Lond) 1977;182:309–321. [Google Scholar]
- Pye A. The structure of the cochlea in some mammals. J Zool (Lond) 1979;187:39–53. [Google Scholar]
- Ramprashad F, Money FE, Landolt JP, Laufer J. A morphometric study of the cochlea of the little brown bat (Myotis luciffugus. J Morphol. 1979;160:345–358. doi: 10.1002/jmor.1051600306. [DOI] [PubMed] [Google Scholar]
- Reysenbach de Haan FW. Hearing in whales. Acta Oto-Laryngol. 1957;134(Suppl):1–114. [PubMed] [Google Scholar]
- Rougier GW. Vincelestes neuquenianus Bonaparte (Mammalia, Theria) un primitivo mamífero del Cretácico Inferior de la Cuenca Neuquina. Buenos Aires: Universidad Nacional de Buenos Aires; 1993. Ph.D. dissertation. [Google Scholar]
- Rougier GW, Martinelli AG, Forasiepi AM, Novacek MJ. New Jurassic mammals from Patagonia, Argentina: a reappraisal of australosphenidan morphology and interrelationships. Am Mus Novit. 2007;3566:1–54. [Google Scholar]
- Rougier GW, Wible JR. Major changes in the mammalian ear region and basicranium. In: Carrano MT, Gaudin TJ, Blob RW, Wible JR, editors. Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds, and Reptiles. Chicago: University of Chicago Press; 2006. pp. 269–311. (eds), pp. [Google Scholar]
- Rougier GW, Wible JR, Hopson JA. Reconstruction of the cranial vessels in the Early Cretaceous mammal Vincelestes neuquenianus: implications for the evolution of the mammalian cranial vascular system. J Vertebr Paleontol. 1992;12:188–216. [Google Scholar]
- Rougier GW, Wible JR, Hopson JA. Basicranial anatomy of Priacodon fruitaensis(Triconodontidae, Mammalia) from the Late Jurassic of Colorado, and a reappraisal of mammaliaform interrelationships. Am Mus Novit. 1996;3183:1–38. [Google Scholar]
- Rougier GW, Wible JR, Novacek MJ. Implications of Deltatheridium specimens for early marsupial history. Nature. 1998;396:459–463. doi: 10.1038/24856. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Schmelzle T. Anatomy and development of the bony inner ear in the woolly opossum, Caluromys philander(Didelphimorphia, Marsupialia) Mastozool Neotrop. 2007;14:53–60. [Google Scholar]
- Sánchez-Villagra MR, Ladevèze S, Horovitz I, et al. Exceptionally preserved North American Paleogene metatherians: adaptations and discovery of a major gap in the opossum fossil record. Biol Lett. 2007;3:318–322. doi: 10.1098/rsbl.2007.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle T, Sánchez-Villagra MR, Maier W. Vestibular labyrinth diversity in diprotodontian marsupial mammals. Mammal Study. 2007;32:83–97. [Google Scholar]
- Spoor F, Bajpai S, Hussain ST, Kumar K, Thewissen JGM. Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature. 2002;417:163–166. doi: 10.1038/417163a. [DOI] [PubMed] [Google Scholar]
- Wang Y-Q, Hu Y-M, Meng J, Li C-K. An ossified Meckel's cartilage in two Cretaceous mammals and origin of the mammalian middle ear. Science. 2001;294:357–361. doi: 10.1126/science.1063830. [DOI] [PubMed] [Google Scholar]
- Wever EG, McCormick JG, Palin J, Ridgway SH. Cochlea of the dolphin Tursiops truncates: the basilar membrane. Proc Nat Acad Sci USA. 1971;68:2381–2385. doi: 10.1073/pnas.68.11.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible JR. Petrosals of Late Cretaceous marsupials from North America, and a cladistic analysis of the petrosal in therian mammals. J Vertebr Paleontol. 1990;10:183–205. [Google Scholar]
- Wible JR. On the cranial osteology of the short-tailed opossum Monodelphis brevicaudata(Didelphidae, Marsupialia) Ann Carnegie Mus. 2003;72:137–202. [Google Scholar]
- Wible JR, Hopson JA. Basicranial evidence for early mammal phylogeny. In: Szalay FS, Novacek MJ, McKenna MC, editors. Mammal Phylogeny – Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials. New York: Springer-Verlag; 1993. pp. 45–62. (eds), pp. [Google Scholar]
- Wible JR, Hopson JA. Homologies of the prootic canal in mammals and non-mammalian cynodonts. J Vertebr Paleontol. 1995;15:331–356. [Google Scholar]
- Wible JR, Novacek MJ, Rougier GW. New data on the skull and dentition of the Mongolian Cretaceous eutherian mammal Zalambdalestes. Bull Am Mus Nat Hist. 2004;281:1–144. [Google Scholar]
- Wible JR, Rougier GW. Cranial anatomy of Kryptobaatar dashzevegi(Multituberculata, Mammalia) from the Mongolian Late Cretaceous, and its bearing on the evolution of mammalian characters. Bull Am Mus Nat Hist. 2000;247:1–124. [Google Scholar]
- Wible JR, Rougier GW, Novacek MJ, Asher RJ. Cretaceous eutherians and Laurasian origin for placental mammals near the K-T boundary. Nature. 2007;477:1003–1006. doi: 10.1038/nature05854. [DOI] [PubMed] [Google Scholar]
- Wible JR, Rougier GW, Novacek MJ, Asher RJ. The eutherian mammal Maelestes gobiensis from the Late Cretaceous of Mongolia and the phylogeny of Cretaceous Eutheria. Bull Am Mus Nat Hist. in press. [Google Scholar]
- Wible JR, Rougier GW, Novacek MJ, McKenna MC. Earliest eutherian ear region: a petrosal referred to Prokennalestes from the Early Cretaceous of Mongolia. Am Mus Novit. 2001;3322:1–44. [Google Scholar]
- Wible JR, Rougier GW, Novacek MJ, McKenna MC, Dashzeveg D. A mammalian petrosal from the Early Cretaceous of Mongolia: implications for the evolution of the ear region and mammaliamorph interrelationships. Am Mus Novit. 1995;3149:1–19. [Google Scholar]
- Yamada M, Yoshizaki F. Osseous labyrinth of Cetacea. Sci Rep Whale Res Inst (Tokyo) 1959;14:291–304. [Google Scholar]
- Zeller U. The morphogenesis of the Fenestra rotunda in mammals. Fortschr Zool. 1985;30:153–157. [Google Scholar]
- Zeller U. Die Entwicklung und Morphologie des Schädels von Ornithorhynchus anatinus(Mammalia: Prototheria: Monotremata) Abh Senckenberg Naturforsch Ges. 1989;545:1–188. [Google Scholar]
- Zeller U. Ontogenetic evidence for cranial homologies in monotremes and therians, with special reference to Ornithorhynchus. In: Szalay FS, Novacek MJ, McKenna MC, editors. Mammal Phylogeny – Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials. New York: Springer-Verlag; 1993. pp. 95–107. (eds), pp. [Google Scholar]