Abstract
Evidence regarding the components of the renin–angiotensin (Ang) system suggests that this system plays an important role in male reproduction. However, there are few data available in the literature on the effects of Ang-(1–7) on the male reproductive system. The present study investigated the effects of the genetic deletion and chronic blockage of Ang-(1–7) receptor Mas on spermatogenesis and male fertility. The localization of Mas in mouse and rat testes was determined by binding assays and immunofluorescence, whereas the testis structure and spermatogenic process were morphologically and stereologically analysed by light microscopy. Ang-(1–7) binding and immunofluorescence revealed the presence of Mas in the testes of mice and rats. Although the total numbers of Sertoli and Leydig cells per testis and Leydig cell size were similar in both wild-type and Mas-deficient mice, Mas−/– animals exhibited a significant reduction in testis weight and a greater volume of apoptotic cells, giant cells and vacuoles in the seminiferous epithelium. In both mice and rats, an increased number of apoptotic cells were found during meiosis. Due to disturbed spermatogenesis, daily sperm production was markedly reduced in Mas−/– mice. Moreover, chronic infusion of A-779 [an Ang-(1–7) antagonist] in rats significantly increased the total number of apoptotic cells and primary spermatocytes in particular stages of spermatogenesis. Taken together, these findings strongly suggest that Ang-(1–7) receptor Mas plays an important role in the regulation of spermatogenesis.
Keywords: A-779, angiotensin-(1–7), angiotensin II, apoptosis, renin–angiotensin system, spermatogenesis, stereology, testis
Introduction
In recent years, the classical concept of the renin–angiotensin system (RAS) as an endocrine regulator of the renal and cardiovascular function has undergone a change (for a review, see Santos et al. 2005; Ferrario et al. 2005). The identification of several RAS components, such as prorenin, renin, angiotensinogen, angiotensin (Ang) I, Ang II, Ang-converting enzyme (ACE) and ACE2, in the testis and epididymis of humans and mammals (Langford et al. 1993; Speth et al. 1999; Donoghue et al. 2000; Leung & Sernia, 2003; Coates, 2003; Fuchs et al. 2005; Deguchi et al. 2007) strongly indicates the existence of a local RAS involved in male reproduction.
The ACE2 is a human homologue of ACE and is highly expressed in the heart, kidney and testis of humans and mammals (Donoghue et al. 2000; Tipnis et al. 2000). Douglas et al. (2004) found that ACE2 expression in the testis is restricted to Leydig cells in rats and Leydig and Sertoli cells in humans. These authors also suggested a role for ACE2 in the control of testicular function, possibly regulating steroidogenesis or some other Leydig cell function (Douglas et al. 2004). ACE2 is the main Ang-(1–7)-forming enzyme and can produce Ang-(1–7) from Ang II (Vickers et al. 2002) or, less efficiently, through the hydrolysis of Ang I to Ang-(1–9) (Donoghue et al. 2000; Burrell et al. 2004), with the subsequent generation of Ang-(1–7) through ACE and neutral endopeptidase hydrolysis (Rice et al. 2004).
Recently, Ang-(1–7) has been identified as an endogenous ligand for the G protein-coupled receptor Mas (Santos et al. 2003). This cell surface receptor is highly expressed in the brain, heart, kidney, endothelium and testis (reviewed in Alenina et al. 2008). Like the ACE2 expression pattern, Mas mRNA in the testis is located in Leydig and Sertoli cells, being much more pronounced in Leydig cells (Alenina et al. 2002). Moreover, a recent study in Mas-knockout mice found that Mas deletion affects the expression of enzymes involved in the biosynthesis of testosterone in Leydig cells (steroidogenic acute regulatory protein and 3β-hydroxysteroid dehydrogenase 1 and 6), suggesting a possible role for Mas in the regulation of the androgen metabolism in the male reproductive system (Xu et al. 2007a). Apparently, however, Mas-knockout mice on a mixed genetic background exhibit normal fertility (Walther et al. 1998) and additional studies are required to elucidate the fine mechanisms related to these findings and other possible effects of Ang-(1–7) in the male reproductive tract.
Regarding fertility, the literature suggests that Ang is related to epidydimis and sperm function. Transgenic mice lacking both the somatic and testicular forms of the ACE exhibit reduced fertility, whereas males lacking solely the somatic form are fertile. This suggests that infertility reflects the loss of sperm rather than epidydimal function (see review in Leung & Sernia, 2003).
An accurate quantitative evaluation of testis morphology and spermatogenesis, including the evaluation of the stages of the seminiferous epithelium cycle (SEC), can provide answers to important questions regarding testis structure and function and correlations with physiological and biochemical findings (Berndtson, 1977; Wing & Christensen, 1982; França & Russell, 1998). Another very important parameter in the evaluation of testis function is the determination of Sertoli cell efficiency, which is the best indicator of spermatogenic efficiency (daily sperm production per gram of testis) (Leal & Franca, 2006; Almeida et al. 2006).
The present study was carried out to investigate the effects of the genetic deletion of Mas on the spermatogenetic process, using molecular, morphological and stereological approaches. The effect of the chronic blockage of Mas on testis function, particularly spermatogenesis, was also investigated.
Materials and methods
Animals
Wild-type (Mas+/+) and Mas-knockout (Mas−/–) mice on the C57BL/6 genetic background (Xu et al. 2007a) (70 days old) were obtained from the transgenic animal facilities of the Hypertension Laboratory, Federal University of Minas Gerais, Brazil. Male Wistar rats weighing 250–300 g (90 days old) were obtained from Cebio – Institute of Biological Sciences, Federal University of Minas Gerais, Brazil. All animals were euthanized with pentobarbital during the perfusion procedure used for fixation of the testis. All experimental procedures were performed in compliance with the guidelines for the ethical treatment of animals established by our institution (Institutional Animal Welfare Committee – Federal University of Minas Gerais), which are in accordance with standard international norms.
In-vitro fluorescent-labelled angiotensin-(1–7) binding in Mas+/+ and Mas−/– mice
Testes (n =5 for each group) were snap-frozen in cooled isopentane (–32 °C) and the sections were serially cut (6µm) starting from the central area of the testis, mounted on gelatin-coated slides and dried at 4°C prior to the assay. The sections were pre-incubated in the assay buffer [10−2molL−1 Na-phosphate buffer, pH 7.4, 1.2 × 10−1mol L−1 NaCl, 5 × 10−3mol L−1 MgCl2, 0.2% bovine serum albumin (BSA), 0.005% bacitracin] for 30 min. The experiment was performed using assay buffer containing 2.5 × 10−5mol L−1 phenylmethylsulphonyl fluoride, 6 × 10−10mol L−1 1,10-phenanthroline and 2 × 10−9mol L−1 rhodamine-labelled-Ang-(1–7) in the presence (non-specific binding) or absence (total binding) of Ang-(1–7) (10−6mol L−1) for 1 h at 22–24 °C. Images were captured using a conventional fluorescence microscope and relative fluorescence measurements were performed using Adobe Photoshop 7.0 software. Specific binding was determined by the difference between the fluorescence intensity of total binding and non-specific binding measurements.
Immunofluorescence of Mas in Wistar rat testis
To demonstrate the presence of Mas in rat testis and provide some insights regarding the localization of Mas in this organ, immunofluorescence was performed using testes from Wistar rats (n =2 from different rats). The primary antibody used was a polyclonal Mas antibody (1:100 in 1% BSA) raised in Mas−/– mice produced in the Department of Physiology and Biophysics at the Federal University of Minas Gerais (Becker et al. 2007). The secondary antibody used was an Alexa 594 goat anti-mouse (Molecular Probes, USA) diluted 1:200 in 1% BSA. Cryostat-cut sections (10 µm thick) of rat testis pre-fixed in 4% paraformaldehyde and frozen at –80°C in Optimal Cutting Temperature (OCT) embedding medium were rehydrated in 2x10−2mol L−1 phosphate-buffered saline (PBS) for 1 h. Sections were subsequently incubated with a permeating buffer containing 0.2% Triton in PBS for 30 min, followed by a blocking step of 30 min with 3% BSA in PBS. Primary antibody incubation was performed at 4 °C overnight. Sections were washed three times (5 min each) in PBS and then incubated with the secondary antibody at room temperature (22–23 C) for 90 min. Subsequently, these sections were washed three times (5 min each) in PBS and mounted on subbed slides with 90% glycerol in 5 × 10−2 mol L−1 Tris-HCl, pH 7.4. To validate the staining procedure, testis sections were incubated with the secondary antibody alone (antibody control). Immunostaining was detected using a Zeiss LSM 510 Meta Confocal microscope equipped with an oil-immersion objective lens (×63).
Chronic infusion of A-779
Osmotic minipumps (ALZET, model 2001) containing either A-779 (2 µg h−1, 14 days, n = 5) or vehicle (0.9% NaCl, 1 µL h−1, 14 days, n = 6) were implanted subcutaneously in the dorsal region of young adult male Wistar rats under tribromoethanol anaesthesia (2.5%, 1 mL 100−1 g body weight). After 14 days of treatment, tissues were collected and used for further analysis as described below.
Tissue preparation
Five Mas+/+ and five Mas−/– adult male mice and 11 Wistar rats were used for testis stereology. Animals were perfused through the left ventricle first with 0.9% saline and then with 4% buffered glutaraldehyde for 25–30 min by gravity-fed perfusion. Prior to surgery, all animals received an i.p. injection of heparin (125 IU kg−1) and pentobarbital (50 mg kg−1). After fixation, the testis, epididymides, prostate gland, seminal vesicles and coagulating gland were removed and weighed. Testis tissue fragments were embedded in plastic (glycol methacrylate) and routinely prepared. Sections of 4 µm thickness were stained with blue toluidine for qualitative and quantitative analyses by light microscopy.
Testis stereology, cell counts and cell numbers
The tubular diameter and the height of the seminiferous epithelium in mice were measured at 200× magnification using an ocular micrometer calibrated with a stage micrometer. Thirty tubular profiles that were round or nearly round were chosen randomly and measured for each animal. Epithelium height was determined in the same tubules used to determine tubular diameter. The volume densities of various testicular tissue components were determined by light microscopy, using a 441-intersection grid placed under the ocular of the light microscope. Fifteen randomly chosen fields (6615 points) were scored for each animal at 400× magnification. The points were classified as follows: seminiferous tubule, comprising tunica propria, epithelium and lumen; Leydig cell; blood and lymphatic vessels; and connective tissue. The presence of apoptotic germ cells, multinucleated giant cells and vacuoles was also determined in the seminiferous epithelium. The volume of each testis component was determined as the product of the volume density and testis volume. For subsequent morphometric calculations, the specific gravity of the testis tissue was considered to be 1.0 (Leal & Franca, 2006; Almeida et al. 2006). In order to obtain the testis parenchyma volume, the testis capsule (3.5%) volume was excluded from the total testis volume. The total length (m) of seminiferous tubule was obtained by dividing the seminiferous tubule volume by the squared radius of the tubule × π (Johnson & Neaves, 1981).
All germ cell nuclei and Sertoli cell nucleoli present in Stage VII of the cycle were counted in 10 round or nearly round seminiferous tubule cross-sections (chosen at random) for each animal. These counts were corrected for section thickness and nucleus or nucleolus diameter, based on the procedure described by Abercrombie (1946) and modified by Amann (1962). For this purpose, 10 diameters of nuclei or nucleoli were measured for each cell type analysed per animal. Cell ratios were obtained from the corrected counts obtained in Stage VII. The total number of Sertoli cells was determined from the corrected counts of Sertoli cell nucleoli per seminiferous tubule cross-section and the total length of seminiferous tubules, based on the procedure described by Hochereau-de Reviers & Lincoln (1978). Daily sperm production (DSP) per testis and per gram of testis (spermatogenic efficiency) were obtained based on the formula developed by França (1992): DSP = total number of Sertoli cells per testis × ratio of round spermatids to Sertoli cells in Stage VII × Stage VII relative frequency (%)/Stage VII duration (days).
The individual volume of Leydig cells was obtained from nucleus volume and the proportion between nucleus and cytoplasm. As most Leydig cell nuclei in mice are spherical, the nucleus volume was obtained from the mean nuclear diameter. For this purpose, the diameter of 30 round nuclei with evident nucleoli was measured for each animal. The Leydig cell nucleus volume was expressed in µm3 and obtained by the formula (4/3)πR3, in which R = nuclear diameter/2. To calculate the proportion between nucleus and cytoplasm, a 441-intersection grid was placed over the sectioned material at 400× magnification. One thousand points over Leydig cells were counted for each animal. The number of Leydig cells per testis was estimated from the individual Leydig cell volume and volume occupied by Leydig cells in the testis parenchyma.
Statistical analysis
All data are shown as the mean ± S.E.M. Statistical analysis was performed using the unpaired Student's t-test and the STATISTICA for Windows program (StatSoft, Inc., Tulsa, OK). The level of significance was set at P < 0.05.
Results
In-vitro fluorescent-labelled angiotensin-(1–7) binding in Mas+/+ and Mas−/– mice
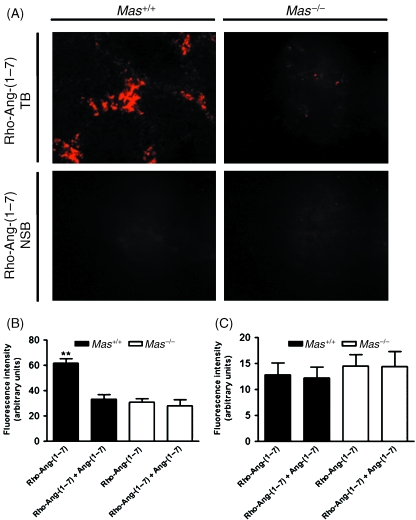
Fluorescent-labelled Ang-(1–7) binding experiments revealed the presence of specific Ang-(1–7) binding in testis sections from Mas+/+ mice, whereas such binding was completely absent in Mas−/– mice (Fig. 1). Moreover, specific Ang-(1–7) binding was prominent in the intertubular compartment, probably in Leydig cells (Fig. 1B), whereas binding was not observed in the tubular compartment (Fig. 1C). Figure 1A displays the total binding in Mas+/+ testis, which exhibited a punctuated staining pattern, as would be expected for a G protein-coupled receptor (Santos et al. 2003). Conversely, the specific binding of rhodamine-labelled Ang-(1–7) to testis sections from Mas−/– mice was essentially abolished, as evaluated by quantitative fluorescence analysis. These findings were also observed in 125I-Ang-(1–7) autoradiography experiments (data not shown).
Fig. 1.
In-vitro fluorescent-labelled angiotensin-(1–7) binding in Mas+/+ and Mas−/– mouse testis. (A) Representative photomicrographs showing total binding (TB) of rhodamine-labelled angiotensin-(1–7) [Rho-Ang-(1–7)] and non-specific binding (NSB) of Rho-Ang-(1–7) in Mas+/+ and Mas−/– mouse testis. (B) Quantitative image analysis of Rho-Ang-(1–7) binding in Mas+/+ and Mas−/– mice in the intertubular compartment. (C) Quantitative image analysis of Rho-Ang-(1–7) binding in Mas+/+ and Mas−/– mice in the tubular compartment (**P < 0.0001, unpaired Student's t-test).
Testis biometry, stereology, cell counts and cell numbers
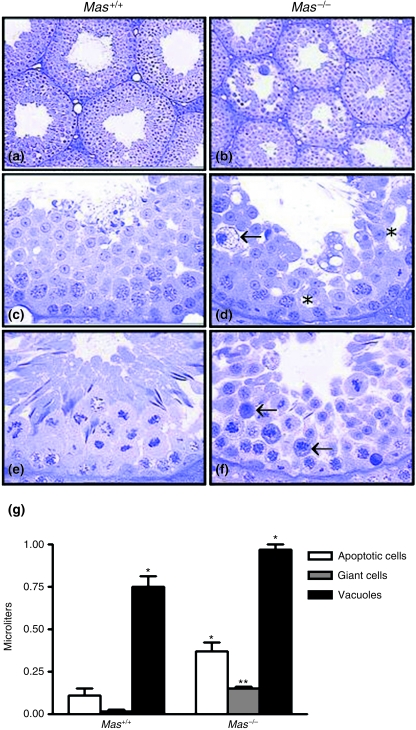
Table 1 displays the data on testis biometry, stereology, cell counts and cell numbers. The mean body weight and epididymis weight in Mas+/+ and Mas−/– mice were similar. However, the testis weight and gonadosomatic index values were approximately one-third lower (P < 0.05) in Mas−/– mice, whereas the seminal vesicle plus coagulating gland weight was significantly greater in these animals. No significant difference was observed for ventral prostate gland weight. The tubular diameter was nearly 20% smaller (P < 0.05) in Mas−/–. Except for the tunica propria, Leydig cells and lymphatic space, which achieved similar values (P > 0.05) in both groups, several other testicular components, such as the seminiferous epithelium, tubular lumen and blood vessels, had their volume significantly (P < 0.05) reduced in Mas−/– animals. Moreover, the volumes occupied by apoptotic cells, giant cells and vacuoles in the seminiferous epithelium were much greater (3.5-fold, 11-fold and 1.3-fold, respectively, P < 0.05) in Mas−/– mice (Fig. 2). Most apoptotic cells were found during the meiotic prophase, particularly in meiotic divisions, whereas giant cells were composed mainly of round spermatids present in several stages of the SEC. These findings were confirmed by a significantly lower (P < 0.05) Sertoli cell efficiency (–53%; round spermatids per Sertoli cell), meiotic index (–35%; number of round spermatids formed from each pachytene spermatocyte) and overall rate of spermatogenesis (–56%; number of spermatids per type A spermatogonia) counted in SEC Stage VII. However, it should be mentioned that the number of Sertoli cell nucleoli, type A spermatogonia and preleptotene spermatocytes per tubular cross-section evaluated in Stage VII were similar (P > 0.05) in Mas+/+ and Mas−/– animals. Likewise, the total numbers of Sertoli and Leydig cells per testis as well as individual Leydig cell volumes were similar in both groups. The daily sperm production per testis and per gram of testis was noticeably reduced in Mas−/– mice (∼55% and ∼30%, respectively), probably due to the disturbed meiosis and spermiogenesis. Despite this finding, the Mas−/– mice remained fertile.
Table 1.
Biometrical and stereological parameters in Mas+/+ and Mas−/– mice (mean ± S.E.M.)
| Parameter | Mas+/+ (n = 5) | Mas−/– (n = 5) |
|---|---|---|
| Body weight (g) | 21.3 ± 0.5 | 22.8 ± 0.5 |
| Testis weight (mg) | 83.9 ± 4.5 | 56.4 ± 3.2* |
| Gonadosomatic index (%) | 0.8 ± 0.03 | 0.5 ± 0.02* |
| Epididymis weight (mg) | 36.4 ± 1.5 | 32.4 ± 2.7 |
| SV + CG weight (mg) | 90.4 ± 6.3 | 120.9 ± 1.8* |
| Ventral prostate gland weight (mg) | 34.9 ± 2.9 | 47.8 ± 7.8 |
| Tubular diameter (µm) | 212 ± 3.9 | 176 ± 5.2* |
| Tunica propria (µL) | 1.9 ± 0.2 | 1.6 ± 0.1 |
| Leydig cells (µL) | 2.4 ± 0.3 | 2.8 ± 0.2 |
| Lymphatic space (µL) | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Seminiferous epithelium (µL) | 64.7 ± 3.5 | 43.9 ± 2.9* |
| Tubular lumen (µL) | 9.9 ± 1.1 | 4.7 ± 0.3* |
| Blood vessels (µL) | 1.6 ± 0.1 | 1.0 ± 0.2* |
| Sertoli cell efficiency | 10.1 ± 0.4 | 4.7 ± 0.6* |
| Meiotic index | 2.6 ± 0.04 | 1.7 ± 0.07* |
| Leydig cell volume (µm3) | 919 ± 31 | 897 ± 45 |
| Overall rate of spermatogenesis (round spermatids:type A spermatogonia) | 68.7 ± 6.0 | 30.2 ± 2.7* |
| Number of Sertoli cells nucleolus/tubule cross-section | 6.4 ± 0.2 | 6.9 ± 0.2 |
| Number of Sertoli cells per testis (×106) | 3.5 ± 0.2 | 3.6 ± 0.2 |
| Number of Leydig cells per testis (×106) | 2.6 ± 0.4 | 3.1 ± 0.1 |
| Type A spermatogonia/tubule cross-section | 1.0 ± 0.1 | 1.1 ± 0.1 |
| Preleptotene spermatocytes/tubule cross-section | 26.1 ± 0.7 | 28.2 ± 0.8 |
| Daily sperm production per testis (×106) | 4.1 ± 0.3 | 1.9 ± 0.3* |
| Daily sperm production per gram of testis (×106) | 50 ± 2.5 | 35 ± 3.7* |
P < 0.05, Student's t-test. SV + CG, seminal vesicle + coagulating gland; gonadosomatic index, paired testes weight/body weight.
Fig. 2.
Cross-sections of seminiferous tubules in Mas+/+ (a, c and e) and Mas−/– (b, d and f) mice. Spermatogenesis is disturbed in the Mas−/– mice (b), leading to the presence of giant cells (arrow, d), vacuoles (*, d) and a large number of apoptotic cells during meiotic divisions (arrows, f). (g) Quantification of cellular volume for different cell types in Mas+/+ and Mas−/– mice. **P < 0.0001; *P < 0.01 Mas−/– vs. Mas+/+.
Immunofluorescence of Mas in Wistar rat testis
Mas was present mainly in Leydig cells of the testis of Wistar rats, as demonstrated by immunofluorescence analysis (Fig. 3).
Fig. 3.
Mas immunofluorescence in Wistar rat testis showing strong staining in the intertubular compartment (interstitial cells). Arrows, intertubular compartment; ST, seminiferous tubules.
Chronic infusion of A-779
To further assess the role of Mas on the spermatogenic process, Wistar rats were chronically treated with A-779 [a specific Ang-(1–7) receptor Mas antagonist]. The mean body and testis weight and the gonadosomatic index were similar in vehicle-infused and chronically A-779-infused rats (Table 2). However, considering all SEC stages, A-779 infusion doubled (P < 0.05) the total number of apoptotic germ cells in the seminiferous epithelium. The loss of germ cells was significantly higher (P < 0.05) in Stages I, X, XII and XIII of the rat SEC (Table 2). These cells were mostly primary spermatocytes in early to middle phases. There were apoptotic cells in the seminiferous epithelium displaying the characteristics of Sertoli cells. Moreover, no giant germ cells or apoptosis in other somatic cells in the testis (such as peritubular myoid, Leydig and endothelial cells) were observed.
Table 2.
Biometrical and stereological parameters in vehicle-and A-779-infused Wistar rats (mean ± S.E.M.)
| Parameter | Vehicle-infused (n = 6) | A-779-infused (n = 5) |
|---|---|---|
| Body weight (g) | 304 ± 15 | 312 ± 22 |
| Testis weight (g) | 1.5 ± 0.04 | 1.6 ± 0.10 |
| Gonadosomatic index (%) | 1.0 ± 0.03 | 1.1 ± 0.07 |
| Apoptosis: | ||
| Total apoptosis | 0.2 ± 0.03 | 0.4 ± 0.05* |
| Stage I | 0.19 ± 0.004 | 0.45 ± 0.1* |
| Stage II–III | 0.01 ± 0.007 | 0.12 ± 0.1 |
| Stage IV | 0.04 ± 0.002 | 0.15 ± 0.1 |
| Stage V | 0.009 ± 0.009 | 0.003 ± 0.02 |
| Stage VI | 0.009 ± 0.009 | 0.002 ± 0.002 |
| Stage VII | 0.03 ± 0.02 | 0.04 ± 0.09 |
| Stage VIII | 0 ± 0 | 0.006 ± 0.006 |
| Stage IX | 0.01 ± 0.009 | 0.03 ± 0.03 |
| Stage X | 0.03 ± 0.02 | 0.25 ± 0.07* |
| Stage XI | 0.46 ± 0.24 | 0.5 ± 0.12 |
| Stage XII | 0.45 ± 0.19 | 1.32 ± 0.36* |
| Stage XIII | 0.34 ± 0.1 | 0.91 ± 0.17* |
| Stage XIV | 0.97 ± 0.32 | 1.57 ± 0.42 |
Gonadosomatic index, paired testes weight/body weight; total apoptosis, data related at all stages of seminiferous epithelium cycle.
<0.05 Student's t-test
Discussion
The recent identification and characterization of novel components of the RAS in the male reproductive tract, including the Ang-(1–7)-forming enzyme ACE2 (Donoghue et al. 2000; Tipnis et al. 2000; Vickers et al. 2002; Douglas et al. 2004) and the Ang-(1–7) receptor Mas (Metzger et al. 1995; Alenina et al. 2002), have raised important questions related to the role of the ACE2–Ang-(1–7)–Mas receptor axis (Simões e Silva et al. 2006) in male reproductive function.
In the present study, fluorescent-labelled binding experiments were performed with Ang-(1–7) in testis sections of wild-type and Mas-deficient mice in order to further evaluate the role of Mas in Ang-(1–7) binding and its function in the male reproductive system. Qualitative fluorescent-labelled binding exhibited specific Ang-(1–7) binding in the testis sections of Mas+/+ mice. However, Ang-(1–7) binding was absent in Mas−/– mouse testes, suggesting that Mas may be a functional Ang-(1–7) receptor in this organ. Corroborating data on the expression of Mas mRNA in Leydig cells (Alenina et al. 2002), specific binding of fluorescent-labelled Ang-(1–7) in testis occurred mainly in the intertubular compartment (probably in Leydig cells), suggesting a possible role of the Ang-(1–7) receptor Mas in male reproductive function. These findings may be correlated with the lower Sertoli efficiency, meiotic index, overall rate of spermatogenesis and daily sperm production observed in Mas−/– mice. However, it should be mentioned that the numbers of Sertoli cell nucleoli, type A spermatogonia and preleptotene spermatocytes per tubular cross-section were very similar in Mas+/+ and Mas−/– animals in Stage VII, suggesting that the spermatogonial phase of spermatogenesis (measured as the coefficient of efficiency of spermatogonial mitosis) was not compromised in mice with Ang-(1–7) receptor deficiency.
To determine the action of Ang-(1–7) on the male reproductive physiology of other mammalian species, immunofluorescence was performed on Wistar rat testes for Mas localization and the effect of chronic blockage of Mas on the spermatogenic process was evaluated. As observed for mice, Mas was located mainly in the intertubular compartment of the rat testis. Immunofluorescence labelling was also detected in some cells located in the seminiferous epithelium of Wistar rats. However, we were not able to precisely identify the cell types labelled or in which SEC stage they were present. Nevertheless, the results obtained with chronic Mas blockage revealed that apoptosis was significantly increased in treated rats, particularly in primary spermatocytes present in late and early SEC stages.
Although cellular and molecular mechanisms remain poorly understood, androgens are considered crucial for the control of spermatogenesis. The androgen receptors in the testis are located exclusively in the somatic cells of this organ, particularly in Sertoli, Leydig, peritubular myoid and endothelial cells (for reviews see Sharpe, 1994; McLachlan et al. 2002; Verhoeven et al. 2007). In a very elegant investigation, De Gendt et al. (2004) demonstrated that knockout mice for the androgen receptor exclusively in Sertoli cells (SCARKO) had spermatogenic blockage in late meiosis and spermatogenesis was apparently normal up to early spermatocytes. Xu et al. (2007b) found that male mice lacking the androgen receptor in Leydig cells exhibited infertility with abnormal spermatogenesis and spermatogenic arrest predominately in the round spermatid stage. Likewise, a recent study (Xu et al. 2007a) found that gene expression for crucial enzymes and factors for androgen (testosterone) production, such as steroidogenic acute regulatory protein, 3β-hydroxysteroid dehydrogenase and growth factor erv1, were significantly downregulated in Ang-(1–7) receptor Mas-deficient mice. Although we found that Mas was important for spermatogenesis in both mice and rats [in which apoptosis (particularly during meiosis) was a common finding], a specific role for Ang-(1–7) in the physiology of germ cells is difficult to infer based on the methodological approaches utilized in the present study. Nevertheless, based on previous results we could hypothesize or speculate that Ang-(1–7) may be an important peptide for the regulation of testis function. However, the possible mechanisms involved in the regulation of spermatogenesis remain to be investigated, along with the determination of whether Ang-(1–7) plays a direct role in the physiology of germ cells or whether it is indirectly involved via Sertoli cells, as found in SCARKO mice (Verhoeven et al. 2007).
Unfortunately, we were unable to measure plasma testosterone levels in the present investigation. However, based on the weights of androgen-dependent organs in mice, such as the epidydimis, ventral prostate and seminal vesicle plus coagulating glands, it appears that androgen levels were unaltered. Moreover, we cannot exclude the possibility that the reproductive phenotype found in Mas-deficient mice was induced by changes in intratesticular hormone levels, such as testosterone concentration. However, this is unlikely, as it has been suggested that up to a two-thirds decrease in intratesticular testosterone levels does not cause any disturbances in the testis (Sharpe, 1994). Thus, together with the results showing that the total number of Sertoli and Leydig cells per testis and individual Leydig cell volume were similar in both wild-type and Mas-deficient mice, these findings strongly suggest that gonadotropins (luteinizing hormone and follicle-stimulating hormone) as well as other hormones and growth factors related to Sertoli and Leydig cell proliferation were probably unaltered in the Mas−/– mice.
One could argue that the reproductive phenotype observed in Mas-deficient mice may be due to decreases in testicular blood flow. In fact, it has been shown that Mas−/– mice exhibit pronounced changes in the distribution of the regional blood flow. A significant decrease in the vascular conductance followed by a decrease in blood flow in the testis of Mas−/– mice has been found using fluorescent microspheres (Botelho-Santos et al. 2008). Thus, it is possible that decreases in testicular blood flow or perfusion may induce functional disturbances in the avascular tubular compartment.
In conclusion, the results of the present investigation demonstrate that the Ang-(1–7) receptor Mas may play an important role in the regulation of spermatogenesis. Additional studies are being developed to elucidate the fine mechanisms related to these findings and other possible effects of Ang-(1–7) in the male reproductive tract.
Acknowledgments
This work was supported by the Brazilian agencies FAPEMIG, CAPES and CNPq-PRONEX. The authors would like to thank the Centro de Microscopia (CEMEL) of the Institute of Biological Sciences, Federal University of Minas Gerais, for the Zeiss confocal microscope that was used in some analyses. Technical help from Adriano Moreira was also highly appreciated.
References
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:238–248. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Alenina N, Baranova T, Smirnow E, et al. Cell type-specific expression of the Mas proto-oncogene in testis. J Histol Cytol. 2002;50:691–696. doi: 10.1177/002215540205000510. [DOI] [PubMed] [Google Scholar]
- Alenina N, Xu P, Rentzsch B, Patkin EL, Bader M. Genetically altered animal models for Mas and angiotensin-(1–7) Exp Physiol. 2008;93:528–537. doi: 10.1113/expphysiol.2007.040345. [DOI] [PubMed] [Google Scholar]
- Almeida FFL, Leal MC, Franca LR. Testis morphometry, duration of spermatogenesis, and spermatogenic efficiency in the wild boar (Sus scrofa scrofa. Biol Reprod. 2006;75:792–799. doi: 10.1095/biolreprod.106.053835. [DOI] [PubMed] [Google Scholar]
- Amann RP. Reproductive capacity of dairy bull. III. The effect of ejaculation frequency, unilateral vasectomy and age on spermatogenesis. Am J Anat. 1962;110:49–67. doi: 10.1002/aja.1001100106. [DOI] [PubMed] [Google Scholar]
- Becker LK, Etelvino GM, Walther T, Santos RAS, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol. 2007;293:H1416–H1424. doi: 10.1152/ajpheart.00141.2007. [DOI] [PubMed] [Google Scholar]
- Berndtson WE. Methods for quantifying mammalian spermatogenesis: a review. J Anim Sci. 1977;44:818–833. doi: 10.2527/jas1977.445818x. [DOI] [PubMed] [Google Scholar]
- Botelho-Santos GA, Bader M, Santos RAS. Altered regional blood flow distribution in Mas-deficient mice. Hypertension. 2008;52:e34–e131. doi: 10.1177/1753944712461164. [DOI] [PubMed] [Google Scholar]
- Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metabol. 2004;15:166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates D. The angiotensin converting enzyme (ACE) Int J Biochem Cell Biol. 2003;35:769–773. doi: 10.1016/s1357-2725(02)00309-6. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PTK, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Nat Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi E, Tani T, Watanabe H, Yamada S, Kondoh G. Dipeptidase-inactivated tACE action in vivo: selective inhibition of sperm-zona pellucida binding in the mouse. Biol Reprod. 2007;77:794–802. doi: 10.1095/biolreprod.107.060004. [DOI] [PubMed] [Google Scholar]
- Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Douglas GC, O’Bryan MK, Hedger MP, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França LR. Proceedings of the 12th International Congress on Animal Reproduction and Artificial Insemination. vol. 4. pp. The Hague: The ICAR; 1992. Daily sperm production in Piau boars estimated from Sertoli cell population and Sertoli cell index; pp. 1716–1718. [Google Scholar]
- Martínez F, Regadera J. França LR, Russell LD. Male Reproduction. A Multidisciplinary Overview. Madrid, Spain: Churchill Livingstone; 1998. The testis of domestic animals; pp. 197–219. [Google Scholar]
- Fuchs S, Frenzel K, Hubert C, et al. Male fertility is dependent on dipeptidase activity of testis ACE. Nature Med. 2005;11:1140–1142. doi: 10.1038/nm1105-1140. [DOI] [PubMed] [Google Scholar]
- Hochereau-de Reviers MT, Lincoln GA. Seasonal variation in the histology of the testis of the red deer, Cervus elephus. J Reprod Fertil Suppl. 1978;54:209–213. doi: 10.1530/jrf.0.0540209. [DOI] [PubMed] [Google Scholar]
- Johnson L, Neaves WB. Age-related changes in the Leydig cell population, seminiferous tubules and sperm production in stallions. Biol Reprod. 1981;24:703–712. doi: 10.1095/biolreprod24.3.703. [DOI] [PubMed] [Google Scholar]
- Langford KG, Zhou Y, Russell LD, Wilcox JN, Bernstein KE. Regulated expression of testis angiotensin-converting enzyme during spermatogenesis in mice. Biol Reprod. 1993;48:1210–1218. doi: 10.1095/biolreprod48.6.1210. [DOI] [PubMed] [Google Scholar]
- Leal MC, Franca LR. The seminiferous epithelium cycle length in the black tufted-ear marmoset (Callithrix penicillata) is similar to humans. Biol Reprod. 2006;74:616–624. doi: 10.1095/biolreprod.105.048074. [DOI] [PubMed] [Google Scholar]
- Leung OS, Sernia C. The renin-angiotensin system and male reproduction: new functions for old hormones. J Mol Endocrinol. 2003;30(3):263–270. doi: 10.1677/jme.0.0300263. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, O'Donnell L, Meachen SJ, et al. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- Metzger R, Bader M, Ludwig T, Berberich C, Bunnemann B, Ganten D. Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett. 1995;357:27–32. doi: 10.1016/0014-5793(94)01292-9. [DOI] [PubMed] [Google Scholar]
- Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RAS, Ferreira AJ, Pinheiro SV, Sampaio WO, Touyz R, Campagnole-Santos MJ. Angiotensin-(1–7) and its receptor as a potential targets for new cardiovascular drugs. Expert Opin Investig Drugs. 2005;14:1019–1031. doi: 10.1517/13543784.14.8.1019. [DOI] [PubMed] [Google Scholar]
- Santos RAS, Simões e Silva AC, Maric C, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Nat Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven; 1994. pp. 1363–2434. (eds), pp. [Google Scholar]
- Simões e Silva AC, Pinheiro SV, Pereira RM, Ferreira AJ, Santos RAS. The therapeutic potential of Angiotensin-(1–7) as a novel Renin-Angiotensin System mediator. Mini Rev Med Chem. 2006;6:603–609. doi: 10.2174/138955706776876203. [DOI] [PubMed] [Google Scholar]
- Speth RC, Daubert DL, Grove KL. Angiotensin II: a reproductive hormone too? Regul Pept. 1999;79:25–40. doi: 10.1016/s0167-0115(98)00141-4. [DOI] [PubMed] [Google Scholar]
- Tipnis SR, Hoover NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Verhoeven G, Denolet E, Swinnen JV, et al. The role of androgens in the control of spermatogenesis: lessons from transgenic models involving a Sertoli cell-selective knockout of the androgen receptor. Anim Reprod. 2007;4:3–14. [Google Scholar]
- Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Walther T, Balschun D, Voigt JP, et al. Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J Biol Chem. 1998;273:11867–11873. doi: 10.1074/jbc.273.19.11867. [DOI] [PubMed] [Google Scholar]
- Wing TY, Christensen AK. Morphometric studies on rat seminiferous tubules. Am J Anat. 1982;165:13–25. doi: 10.1002/aja.1001650103. [DOI] [PubMed] [Google Scholar]
- Xu P, Santos RAS, Bader M, Alenina N. Alterations in gene expression in the testis of angiotensin-(1–7)-receptor Mas-deficient mice. Regul Pept. 2007a;138:51–55. doi: 10.1016/j.regpep.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Xu Q, Lin HY, Yeh SD, et al. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine. 2007b;32:96–106. doi: 10.1007/s12020-007-9015-0. [DOI] [PubMed] [Google Scholar]