Abstract
Human mesenchymal stem cells (hMSC) are a heterogeneous cell population, which is reflected in varying morphological and biological properties. Three subpopulations with intrinsic characteristics can be distinguished: small rapidly self-renewing cells, spindle-shaped cells and large, flattened cells. Unfortunately, it has neither been possible to morphologically define these distinct cells consistently, nor to relate them to specific surface marker features. Here, the primary hMSC subpopulations of three donors are clearly defined by maximum cell diameter and area. Furthermore, these cells were stained for the putative hMSC surface markers CD105, CD90 as well as CD73, and evaluated by three-colour flow cytometry and simultaneous multicolour immunocytochemistry. Interestingly, cell cultures with a high rate of triple-positive hMSC featured a higher content of rapidly self-renewing cells. On the other hand, a higher fraction of flattened cells correlated with a loss of one or more hMSC surface markers. The expression of CD73 showed the highest heterogeneity. Immunocytochemistry further confirmed that flattened cells mainly lack CD73 expression, whereas rapidly self-renewing cells were steadily positive for all three hMSC markers. In the literature, hMSC properties are especially conceded to rapidly self-renewing cells, whereas flattened cells have been suggested to represent early stages of lineage-specific progenitors. We reveal that among the recently suggested surface markers, CD73 is the most sensitive, as it seems to be down-regulated in the early stages of differentiation. Our morphological and immunocytochemical characterization of hMSC subpopulations indicates the yield of early multipotent hMSC and thereby provides a quality control approach for hMSC culturing.
Keywords: characterization, flattened cells, heterogeneity, mesenchymal stem cells, morphology, rapidly self-renewing cells, spindle-shaped cells, subpopulation
Introduction
The utilization and manipulation of human mesenchymal stem cells (hMSC) has been one of the most promising approaches in tissue engineering and regenerative medicine for the last decade (Pittenger et al. 1999; Bianco et al. 2001; Vieyra et al. 2005; Docheva et al. 2007; Phinney & Prockop, 2007). Although there have been outstanding break-throughs even in vivo applications of hMSC (Pountos et al. 2006; Giordano et al. 2007), a generally accepted definition concerning the designation, origin and differentiation capacity of the heterogeneous cell population of hMSC is still missing (Delorme et al. 2006). Therefore the International Society of Cellular Therapy (ISCT) recently recommended three minimal criteria for defining multipotent stromal cells (Dominici et al. 2006). Among others, hMSC have to express a certain panel of surface markers, namely CD105, CD90 and CD73. At the same time, hMSC have to be negative for specific haematological markers. According to most definitions, the existence of a common multipotent early progenitor cell, the mesenchymal stem cell, has been assumed (Pittenger et al. 1999; Bianco et al. 2001; Delorme et al. 2006; Chamberlain et al. 2007). However, the heterogeneous nature of hMSC populations is immediately apparent upon examination of the individual cell morphologies. Hence, a series of studies from the Prockop group (DiGirolamo et al. 1999; Colter et al. 2000, 2001; Sekiya et al. 2002; Smith et al. 2004) investigated different subpopulations. They showed that hMSC can be assigned to at least three morphological subpopulations: rapidly self-renewing cells (RS cells); elongated, fibroblastic-like, spindle-shaped cells (SS cells as indicated here) and slowly replicating, large, cuboidal or flattened cells (FC). Furthermore, distinct subpopulations could be associated with intrinsic qualities. Small RS cells manifested the highest multipotentiality. Wider, spindle-shaped cells showed the greatest potential to differentiate into cartilage (Sekiya et al. 2002). The larger FC were referred to as more mature hMSC that, in part, had differentiated into osteoblastic precursors (Colter et al. 2000, 2001). These observations suggest that the heterogeneity of hMSC cultures is due to different mesenchymal progenitors and that RS cells constitute the earliest precursors (Smith et al. 2004).
Our group has already been engaged in several studies on the immunophenotypical, morphological and biomechanical characteristics of hMSC and their subpopulations (Pautke et al. 2004; Schieker et al. 2004, 2007; Docheva et al. 2008b). Morphological properties depend on cytoskeleton organization, cell adhesion and activated pathways. Therefore, cell shape can be considered to be an indicator for cell fate and differentiation (Settleman,2004). Unfortunately, it has not been possible to relate specific surface marker features to these morphological impressively described subpopulations. Furthermore, general morphological criteria to define the hMSC subgroups have not been established.
Therefore the first goal of the present study was to define clearly the morphometric parameters area and maximum diameter for the three hMSC subpopulations RS, SS and FC. As the distinct subpopulations have not been comprehensively characterized in terms of their surface marker profile, our second goal was to correlate the hMSC subpopulations with the expression pattern of the three hMSC surface markers CD73, CD90 and CD105. These insights facilitate a better understanding of the hMSC subgroups and can provide an easily applicable quality control tool for primary hMSC culturing, increasing effectiveness and standardization in the emerging field of cell-based therapy and regenerative medicine.
Materials and methods
Cells and cell culture
HMSC of three different donors (donor XI, XII and XIV) were purchased from Lonza Corporation (USA). Donor XI, XII and XIV were 19, 18 and 25 years, respectively. Donor XII was male, whereas the other two donors were female. Primary isolation and expansion, as well as testing for differentiation capacity into the osteogenic, chondrogenic and adipogenic lineage, were performed by the company. In addition, the three-lineage plasticity of each donor was verified by our group using von Kossa staining upon osteogenic differentiation, oil red O staining upon adipogenic differentiation and safranin O as well as collagen II staining upon chondrogenic differentiation (data not shown). Furthermore, positive and negative hMSC markers were assessed by flow cytometry (positive for CD29, CD105 and CD166; negative for CD14, CD34 and CD45) by the company. As a homogeneous control population we used a clonally expanded hMSC line (SCP-1), in which we over-expressed hTERT by lentiviral gene transfer. SCP-1 cells show an extended lifespan and preserved hMSC characteristics. Details have been published elsewhere (Böcker et al. 2008). HMSC were cultured after the 3rd passage in MEM Alpha GlutaMAX™ culture media (Invitrogen, Germany) supplemented with 10% FBS (Sigma-Aldrich, Germany) in a humidified incubator at 5% CO2 and 37 °C and seeded in a density of 500 cells cm−2. After cells had reached adequate cell numbers, cultures were washed twice with PBS and trypsinized using trypsin (0.5 g L−1) with EDTA-4Na (0.2 g L−1) dissolved in PBS (Gibco, Germany). Trypsinization was performed in a humidified incubator at 5% CO2 and 37 °C until all cells were detached. Thereafter, morphological evaluation was performed and the cells subsequently underwent FACS analysis and immunocytochemistry. During all experiments hMSC were used between the 4th and 7th passage; a confluency of 50% was never exceeded to prevent differentiation (Colter et al. 2000). SCP-1 cells were assessed in the 102nd and 103rd passage. All experiments were performed in three independent experimental repeats. From the 2nd to 3rd experimental repeat, cells were passaged once more during culturing.
Flow cytometry
Flow cytometric analyses were performed on a FACSCalibur flow cytometer (BD Bioscience, Germany). HMSC were trypsinized and washed twice with PBS. A total number of 1 × 105 hMSC were used for each run. To evaluate the hMSC marker profile, cells were incubated in 100 µL of PBS with 3 µL of CD105-FITC (MCA1557F, AbD Serotec, Germany), CD73-PE and CD90-PE-Cy5 (555597 and 550257, BD Pharmingen, Germany) or appropriate isotype controls (AbD Serotec and BD Pharmingen, Germany) for 20 min at room temperature. Antibody concentration was 0.1 mg mL−1. Cells were washed twice with PBS and finally diluted in 200 µL of PBS. The expression of each surface marker was assessed by the mean fluorescence in FL1, FL2 and FL3, respectively. FlowJoV.8 software (Tree Star Inc., Switzerland) was used for the final evaluation. The percentage of cells positive for a particular antigen was determined by subtracting the percentage of cells stained non-specifically with isotype control antibodies.
Microscopy and morphological analysis
Phase-contrast and fluorescent photomicrographs were taken using an Axiocam MR camera mounted on an Axioskop 2 microscope and analysed with AxioVision Software (Zeiss, Germany). According to the secondary antibodies, standard filters sets were used for blue, green and red spectra (filter sets #01, #09 and #15, respectively; Zeiss). Morphological analyses were performed using phase-contrast microscopy and ImageJ software as previously published (Abramoff et al. 2004; Collins,2007). The software included a special plug-in ‘measure and label’ enabling objective calculation of each cell's area and the Feret's diameter after manually marking its surroundings. Feret's diameter is the measured distance between parallel lines at a tangent to an object's profile and perpendicular to the ocular scale. Therefore, Feret's diameter is the greatest distance possible between any two points along the boundary of a region of interest, analogous to the maximum diameter. Area and maximum diameter of 100 SCP-1 cells and 100 primary hMSC of each donor were individually determined. In total, experiments were repeated three times independently. Data was processed with SigmaPlot version 8 (Systat Software GmbH, Germany) and visualized on a dot plot graph showing area vs. maximum diameter.
Immunocytochemistry
HMSC were seeded in a density of 500 cells cm−2 on sterile glass slides and grown in MEM Alpha GlutaMAX™ culture media (Invitrogen, Germany) supplemented with 10% FBS (Sigma-Aldrich, Germany). Fixation with 4% paraformaldehyde for 10 min followed after 72 h of cultivation. Subsequently, slides were divided into separate fields with a hydrophobic pen (DAKO, Germany) allowing different staining procedures on exactly the same cell population. Blocking was performed with 2% BSA in PBS for 1 h following incubation with Image-iT™ FX signal enhancer (Molecular Probes, Germany) for 20 min. Primary antibodies for CD73 (41-0200, Invitrogen), CD90 (sc-42837, Santa Cruz, USA), and CD105 (DNL-15445, Dianova, Germany) were raised in different species and incubated for 30 min in a concentration of 50 µg mL−1, 10 µg mL−1 and 20 µg mL−1, respectively. Simultaneous multicolour labelling was permitted by appropriate secondary antibodies from the same species conjugated with different fluorochromes, namely AlexaFluor488 for CD105 (A21206, Invitrogen, Germany) AMCA for CD90 and Rhodamine Red-X for CD73 (705-155-147 and 715-295-151, Dianova, Germany) in a concentration of 10 µg mL−1, 14 µg mL−1 and 14 µg mL−1, respectively. Details on antibodies have been published elsewhere (Schieker et al. 2004, 2007). Negative controls were carried out by omitting the primary antibody. As control for the labelling profile, an additional single-colour staining was performed on the same slide. In every independent experimental run, 90 cells of every donor were analysed. Of these, equal numbers of RS, SS and FC cells were randomly picked by phase-contrast microscopy.
Results
Flow cytometry
To evaluate the hMSC surface marker profile, we simultaneously stained against CD105, CD90 and CD73 and performed three-colour flow cytometry. Results represent mean values ± SD of three independent experiments (Table 1). All three markers were positive in 99.3% (± 0.5%) of the SCP-1 cells, with highly reproducible results of triple-positive cells. Assessing the data of each single channel revealed homogeneous expression of all three markers. The mean expression of CD105, CD90 and CD73 was 99.3%, 99.8% and 99.7%, respectively. All three experimental repeats showed consistent results with the lowest standard deviation compared to the donor cells.
Table 1.
Evaluation of the hMSC surface marker profile using simultaneous multicolour flow cytometry
| CD 105 | CD 90 | CD 73 | Triple+ | |
|---|---|---|---|---|
| SCP-1 | 99.4 | 99.8 | 99.6 | 99.4 |
| 98.9 | 99.5 | 99.5 | 98.7 | |
| 99.7 | 100 | 100 | 99.7* | |
| Mean | 99.3 | 99.8 | 99.7 | 99.3 |
| SD | 0.4 | 0.3 | 0.3 | 0.5 |
| Donor XI | 99.2 | 98.5 | 99.9 | 98.4 |
| 96.6 | 93.8 | 86.8 | 81.6 | |
| 98.9 | 99.1 | 66.9 | 65.8* | |
| Mean | 98.2 | 97.1 | 84.5 | 81.9 |
| SD | 1.4 | 2.9 | 16.6 | 16.3 |
| Donor XII | 98.5 | 94.2 | 99.4 | 94.1 |
| 99.7 | 92.7 | 97.5 | 91.6 | |
| 99.6 | 98.6 | 71.3 | 70.5* | |
| Mean | 99.3 | 95.2 | 89.4 | 85.4 |
| SD | 0.7 | 3.1 | 15.7 | 13.0 |
| Donor XIV | 99.6 | 96.5 | 99.8 | 96.4 |
| 99.4 | 99.9 | 96.7 | 96.7 | |
| 99 | 96.9 | 74.8 | 73.1* | |
| Mean | 99.3 | 97.8 | 90.4 | 88.7 |
| SD | 0.3 | 1.9 | 13.6 | 13.5 |
Numbers represent percentages of positive cells. Mean = statistical average of results from three independent experiments.
Cells were passaged once more compared to the other experiments.
The primary cultured cells of donors XI, XII and XIV showed fewer triple-positive cells. HMSC were positive for CD105, CD90 and CD73 in 81.9%, 85.4% and 88.7% of the above donors. The single repeats showed a high variance of 65.8–98.4% in regard to the triple-positive cells. Evaluating the data of each single channel revealed homogeneous expression of CD105 and CD90 between the different donors. The expression of CD105 was rather constant, between 96.6% and 99.7%. CD90 showed a more variable yet high expression ranging from 92.7% to 99.9%. In contrast, the expression of CD73 revealed a notable higher variability. Interestingly, only 84.5%, 89.4% and 90.4% of the cells of donors XI, XII and XIV were positive for CD73, respectively. In particular, the cells passaged once more during culturing (indicated in Table 1 by *) showed a remarkable lower expression of CD73 in 66.9%, 71.3% and 74.8% for donors XI, XII and XIV, respectively.
Morphology
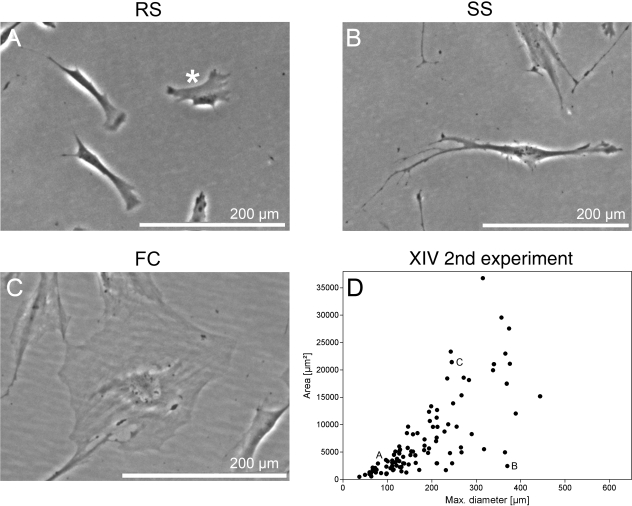
In general, three main morphological cell shapes could be observed. RS cells revealed mostly a triangular or star-like cell shape with few accentuated phase-dense membrane regions (Fig. 1A). Cells indicated as SS cells were elongated and spindle-shaped (Fig. 1B). FC showed a large, cuboidal or flattened pattern (Fig. 1C). To further characterize these hMSC subgroup morphologies, we evaluated 100 cells from each donor and the SCP-1 line in terms of maximum cell diameter and area. Results were presented on a dot plot graph showing diameter vs. area (Fig. 1D).
Fig. 1.
Exemplary cell culture appearance of the three main hMSC subpopulations. Phase-contrast images of hMSC culture containing three distinct subpopulations: (A) small, triangular or star-shaped RS cells with accentuated membrane regions; (B) elongated, fibroblastic-like, spindle-shaped cells, indicated SS cells; (C) large, flattened cells with prominent nucleus, named FC. (D) Dot plot graph presents morphometric results as maximum cell diameter vs. area exemplarily for one donor at one experiment. The cells of A–C represent the labelled dots shown on the graph.
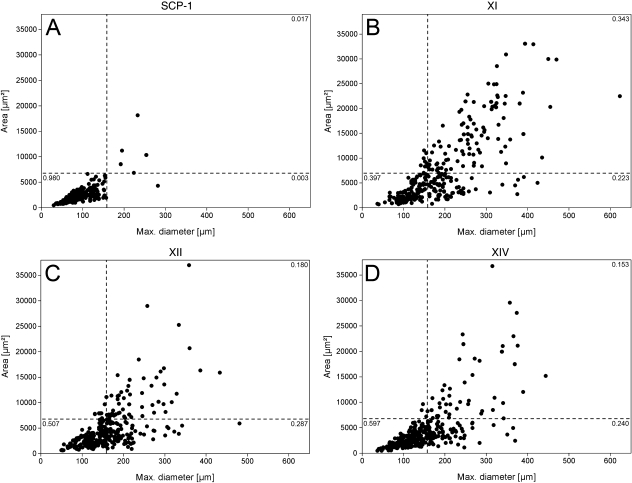
Given the fact that SCP-1 cells represent a homogeneous and rapidly self-renewing immortalized hMSC population (Böcker et al. 2008), the SCP-1 cluster was based to set the gates between RS, SS and FC cells (Fig. 2A). Accordingly, RS cells were defined as shorter than 157 µm and smaller than 6617 µm2. SS cells had a greater maximum diameter than RS cells, and FC possessed both a larger area, greater than 6617 µm2, and a larger maximum diameter, longer than 157 µm.
Fig. 2.
Morphological analysis of primary hMSC and SCP-1 cell line. Dot plot graphs represent maximum cell diameter and area. (A) The SCP-1 cluster was based to set the gates between RS, SS and FC cells. Accordingly, RS cells were defined as < 157 µm and < 6617 µm2. SS cells excelled RS cells in maximum diameter, whereas FC were greater than both, with an area > 6617 µm2 and a maximum diameter > 157 µm. With respect to SCP-1 cells, 98% fulfilled RS cell criteria, whereas the donor-derived cells varied in their distribution pattern. (B) Donor XI revealed the lowest fraction of RS cells and the highest amount of FC. (D) In contrast, donor XIV featured the highest percentage of RS cells and the lowest fraction of FC.
SCP-1 cells showed a homogeneous morphologic pattern with 98% fulfilling RS cell criteria, whereas only 0.3% and 1.7% possessed SS cell and FC shapes, respectively (Fig. 2A). In contrast, hMSC from donor XI (Fig. 2B) featured the most heterogeneous morphologic pattern with the lowest amount of RS cells. Only 39.7% of the cells were configured RS cells, compared to 50.7% and 59.7%, respectively, for donors XII (Fig. 2C) and XIV (Fig. 2D). On the other hand, donor XI possessed the largest fraction of FC, covering 34.3% of the cells, compared to donors XII and XIV, with 18% and 15.3%, respectively. A similar proportion of SS cells was found in all donors; donor XI possessed 22.3% SS cells, donor XII 28.7%, and donor XIV 24% SS cells. Of the total number of 1200 analysed cells, only 22 cells did not integrate with our morphological classification.
Immunohistochemistry
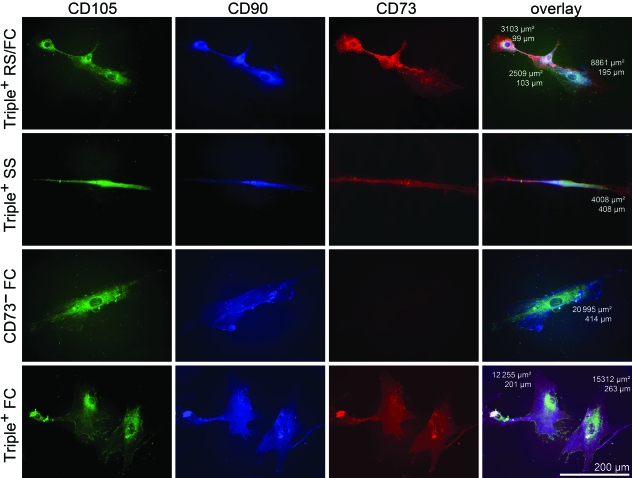
After categorization of the hMSC subpopulations by morphological criteria, hMSC were analysed on single cell level in regard to their surface marker expression. Results of cells missing one or more surface markers are presented by subgroups as the percentage of total number of negative cells (Table 2). We could exclude cross-reaction and co-localization by comparing immunofluorescence single and multicolour staining patterns. CD90 and CD73 were located homogeneously on the cell membrane with perinuclear accentuation. CD105 showed a more granular staining pattern, but appeared equally intense in membrane regions (Fig. 3).
Table 2.
Immunocytological subgroup analysis of cells missing one or more surface marker according to their morphological classification
| n | RS (%) | SS (%) | FC (%) | |
|---|---|---|---|---|
| Total number | 53 | 2 (3.8) | 13 (24.5) | 38 (71.7) |
| Single negative | 42 | 2 (3.8) | 11 (20.8) | 29 (54.7) |
| CD73 | 26 | 1 (1.4) | 6 (11.3) | 19 (35.9) |
| CD90 | 11 | – | 5 (9.4) | 6 (11.3) |
| CD105 | 5 | 1 (1.4) | – | 4 (4.4) |
| Double negative | 11 | – | 2 (3.8) | 9 (17) |
| CD73/CD90 | 11 | – | 2 (3.8) | 9 (17) |
| CD73/CD105 | – | – | – | – |
| CD90/CD105 | – | – | – | – |
| Triple negative | – | – | – | – |
Values in brackets represent percentages of the total number of 53 negative cells.
Fig. 3.
Phenotypical characterizations of hMSC subpopulations using multicolour immunofluorescence. Indirect simultaneous multicolour immunofluorescence was performed for surface markers CD105, CD90 and CD73, visualized with the fluorochromes Alexa Fluor488, AMCA and Rhodamine Red-X, respectively. The first three columns show corresponding single colour channels, and the last column represents a digital overlay of three fluorochromes and includes the morphometric measurements area and maximum diameter of the presented cells. Triple+ = cells positive for CD105, CD90 and CD73. Scale bar is representative for every panel.
Of 270 analysed cells, 53 cells (19.6%) did not stain for any of the three surface markers. In particular, 42 were negative for a single marker and 11 cells lacked the expression of two markers.
Further detailed subgroup analysis of the above-mentioned 53 cells revealed only two RS cells (3.8%) that either did not express CD73 or CD105. A fraction of 24.5% consisted of SS cells. Thereof 20.8% were single-marker negative, with CD73 and CD90 expression absent in almost equal parts (11.3% and 9.4%, respectively). Cells were double-marker negative in 3.8%, missing the combination of CD73 and CD90. More than 70% of the cells that failed the hMSC expression profile were FC. Here cells missed a single marker in 54.7%. CD73 was not expressed in 35.9% of the cells, whereas CD90 and CD105 were absent in 11.3% and 4.4%, respectively. Again, the combination of the two missing markers was CD73 and CD90 in 17%.
Furthermore, the immunocytological analysis revealed 217 cells triple-positive for CD105, CD90 and CD73. This majority of cells consisted in 40.5% of RS, in 35.5% of SS and in 24.0% of FC.
Discussion
One of the major difficulties in hMSC research is the alternating heterogeneity of cultured cells, especially in regard to the comparability of different studies and the standardization of therapeutic protocols in the field of regenerative medicine (Bianco et al. 2001; Delorme et al. 2006, 2008; Dominici et al. 2006; Docheva et al. 2008a). Therefore, the aim of the present study was to clearly define the three subpopulations RS, SS and FC in hMSC cultures. The used morphometric parameters area and maximum diameter are easily accessible by phase-contrast microscopy, allowing quick evaluation of the composition of the primary cell culture in terms of their subpopulation. However, a cell is a three-dimensional object and the cell volume should be also considered defining the cellular shape. To accurately measure the cell volume, more sophisticated microscopy, such as atomic force microscopy (AFM), is necessary. Therefore, our study focuses on the two-dimensional parameters area and maximum diameter.
According to the ISCT proposal, simultaneous expression of CD105, CD90 and CD73 (> 95%) phenotypically defines hMSC. The second aim of the study was therefore to correlate the pattern of these hMSC surface markers with the defined three subpopulations. Some later studies further introduced CD271 and GD2, neural antigens, as promising MSC-specific markers (Bühring et al. 2007; Martinez et al. 2007). But as CD105, CD90 and CD73 still represent the best validated and described hMSC surface markers, we performed simultaneous multicolour FACS analysis of these marker antigens and evaluated their expression pattern on three primary hMSC populations and one immortalized hMSC line.
Within the assessed donor cells, CD73 expression revealed the highest inconsistency and furthermore seemed to be down-regulated over culture time (Table 1). These findings are well in line with previous observations (Conget & Minguell, 1999). In contrast, the SCP-1 line showed a constantly high expression of CD73 and thus represented a positive control in the following experiments.
CD73 is a widely expressed, membrane-bound glycoprotein with both ecto-5′-nucleotidase activity and signal transduction capability. Initially, the monoclonal antibodies SH3 and SH4 recognized hMSC specific antigens (Haynesworth et al. 1992), which later were identified as distinct epitopes of CD73 (Barry et al. 2001). At sites of injury and hypoxia, CD73 metabolizes extracellular AMP to adenosine, which subsequently activates adenosine receptors maintaining endothelial and epithelial barrier functions. Thus, adenosine accumulation is suggested to protect various tissues during oxygen supply imbalance (Synnestvedt et al. 2002). Furthermore, adenosine appears to play an important role in tumour growth and metastasis as it promotes invasion, migration and adhesion (Cho et al. 2006; Wang et al. 2008). Even though the expression of CD73 is a mandatory condition to define hMSC, its exact role on these cells remains elusive. In regard to its function in adhesion, invasion and tissue protection it might have an impact on hMSC homing processes. In terms of increasing the stem cell potential of hMSC cultures, previous studies have shown that a CD45−,CD14−,CD73+ selected subset of cells features an increased ability to generate colony-forming units-fibroblast (CFU-F) (Boiret et al. 2005).
The surface protein CD105, also known as endoglin or SH2, is part of the homodimeric TGF β receptor complex, involved in migration and proliferation processes (Haynesworth et al. 1992; Barry et al. 1999).
CD90 or Thy-1 is a surface glycoprotein, expressed on the cytoplasmic membrane of various cells (Ades et al. 1980; Crawford & Barton, 1986). CD90 can trigger a variety of cellular functions, such as proliferation, differentiation and apoptosis, but its exact function remains unknown (Gunter et al. 1984; Tentori et al. 1988; Hueber et al. 1994).
As CD105 and CD90 showed consistently high expression (Table 1), we consider CD73 to be the most sensitive marker to describe hMSC. The consequence of varying CD73 expression was that cell culture populations erratically fulfilled hMSC criteria. The loss of CD73 in hMSC culture might be due to either a down-regulation of CD 73 on actual progenitor cells or a beginning of differentiation and therefore changing fractions of subpopulations towards cells without CD73 expression. Unfortunately, no superior surface marker has been identified for the distinct subpopulations (Sekiya et al. 2002; Smith et al. 2004; Docheva et al. 2007).
Nevertheless, morphological differences of subpopulations in hMSC cultures have been reported. Apart from inconsistency in terms of classification and terminology, current opinion is that morphological heterogeneity represents different stages of cell differentiation rather than the existence of distinct cell types or subtypes (DiGirolamo et al. 1999; Colter et al. 2000, 2001; Prockop et al. 2001; Sekiya et al. 2002; Smith et al. 2004; Docheva et al. 2008b). Therefore, we further intended to correlate the morphological subgroups with phenotype changes and decreasing hMSC marker expression. The ability to isolate the subset of hMSC that possesses the most extensive replication and differentiation potential would be of the highest importance for applications in the field of regenerative medicine. Former studies mainly distinguished small, rapidly self-renewing progenitors and more mature cells (Colter et al. 2000, 2001; Prockop et al. 2001). A few studies went even further and sub-divided the more mature hMSC morphologically into spindle-shaped (Muraglia et al. 2000) and large, flattened cells (Docheva et al. 2008b). However, many ambiguities and inconsistencies in terms of morphological subgroup criteria were raised. To date there is no reasonable morphological classification for hMSC subpopulations, as most criteria are only based on FACS analyses (Colter et al. 2000; Smith et al. 2004). Only Sekiya et al. (2002) applied a morphological classification for primary hMSC within the first 10 days of cell culture. The study introduced a rather vague classification system subdividing the cells within early hMSC culture into thin spindle-shaped cells (RS-1A), wider spindle-shaped cells (RS-1B) and still-wider spindle-shaped cells (RS-1C). Therefore the parameters used were the area and the maximal widths perpendicular to the long axis. However, the distinctions remained descriptive rather than defining clear criteria for the specification of different subpopulations (Sekiya et al. 2002). In our approach to a clear categorization based on adherent cell culture morphology, the SCP-1 cell line served as a reference. Their morphological analysis revealed a homogeneous cluster including 98% of the cells. As those clonally expanded hMSC unrestrictedly fulfil the required antigen phenotype in more than 99% (Böcker et al. 2008), their homogeneous morphology was set as archetype to define limits in relation to area and maximum diameter (Fig. 1). Correlating our observations with their increased ability of self replication, they appear closest to the claimed RS cell properties (Colter et al. 2000, 2001; Prockop et al. 2001; Smith et al. 2004). Cells exceeding area and length were defined as FC, whereas cells only excelling maximum diameter were indicated as SS cells. Comparing phenotypical and morphological results revealed a clear correlation between CD73 expression and the distribution of subpopulations. The percentage of RS cells was positively correlated with the percentage of cells positive for all three hMSC surface markers. On the other hand, the percentage of FC was inversely correlated with the percentage of cells positive for CD105, CD90 and CD73 (Table 1, Fig. 2). To validate these findings, further investigations using simultaneous multicolour immunocytochemistry on the single cell level and correlating subgroup assessment were carried out. These investigations revealed that FC are especially likely to down-regulate CD73, whereas its expression is consistent in RS cells (Table 2, Fig. 3). Considering the earlier mentioned minimal criteria, FC are no mesenchymal stem cells in the strict sense, suggesting that primary hMSC in culture are prone to differentiate. Accordingly, FC represented the largest fraction of cells missing the expression of two postulated hMSC markers. Furthermore, recent studies reported a down-regulation of CD73 in hMSC after adipogenic and osteogenic induction (Delorme et al. 2008). This is well in line with our previous observations that FC exhibit an increased alkaline phosphatase activity, an attribute of osteogenic differentiation. In these studies, FC show neither increasing rates of apoptosis nor signs of cell senescence (Colter et al. 2001; Docheva et al. 2008b). Taken together, there is increasing evidence that the subpopulation of FC represents a very early osteogenic progenitor with the subsequent loss of stem cell characteristics. Hence, a profound knowledge about subpopulations in hMSC culturing is of major importance. Our morphometry-based criteria define hMSC subpopulations and thereby provide a reasonable tool for the assessment of primary hMSC cultures. The combination of morphological and immunocytochemical characterization of the distinct hMSC subpopulations indicates the yield of early multipotent hMSC in consensus with current hMSC definitions and can thereby help to increase the effectiveness and standardization of tissue engineering approaches.
Acknowledgments
Florian Haasters and Wolf Christian Prall were supported by the Friedrich-Baur-Foundation, LMU Munich (project 0018/2008) and the Faculty of Medicine, LMU Munich (FöFoLe, project 565), respectively. Denitsa Docheva acknowledges the support of the Bavarian Research Foundation (grant DPA-31/05), and the Friedrich-Baur-Foundation, LMU Munich (project 0030/2007). This study was further supported by grants from LMU excellent research professorship, the excellence cluster CIPSM 114, the SFB-TR 36 (Stefan Endres) and from the Else-Kröner Fresenius Foundation, from the German Research Foundation: DFG En 169/7-2 and the Graduiertenkolleg 1202 (Stefan Endres, Carole Bourquin).
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Ades EW, Zwerner RK, Acton RT, Balch CM. Isolation and partial characterization of the human homologue of Thy-1. J Exp Med. 1980;151:400–406. doi: 10.1084/jem.151.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton R, Murphy M, Haynesworth S, Zaia J. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun. 2001;289:519–524. doi: 10.1006/bbrc.2001.6013. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Böcker W, Yin Z, Drosse I, et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J Cell Mol Med. 2008;12:1347–1359. doi: 10.1111/j.1582-4934.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiret N, Rapatel C, Veyrat-Masson R, et al. Characterization of nonexpanded mesenchymal progenitor cells from normal adult human bone marrow. Exp Hematol. 2005;33:219–225. doi: 10.1016/j.exphem.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Cho SY, Polster J, Engles JM, Hilton J, Abraham EH, Wahl RL. In vitro evaluation of adenosine 5’-monophosphate as an imaging agent of tumor metabolism. J Nucl Med. 2006;47:837–845. [PubMed] [Google Scholar]
- Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Crawford JM, Barton RW. Thy-1 glycoprotein: structure, distribution, and ontogeny. Lab Invest. 1986;54:122–135. [PubMed] [Google Scholar]
- Delorme B, Chateauvieux S, Charbord P. The concept of mesenchymal stem cells. Regen Med. 2006;1:497–509. doi: 10.2217/17460751.1.4.497. [DOI] [PubMed] [Google Scholar]
- Delorme B, Ringe J, Gallay N, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- DiGirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- Docheva D, Haasters F, Schieker M. Mesenchymal stem cells and their cell surface receptors. Curr Rheum Rev. 2008a;4:155–160. [Google Scholar]
- Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva D, Padula D, Popov C, Mutschler W, Clausen-Schaumann H, Schieker M. Researching into the cellular shape, Volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J Cell Mol Med. 2008b;12:537–552. doi: 10.1111/j.1582-4934.2007.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- Gunter KC, Malek TR, Shevach EM. T cell-activating properties of an anti-Thy-1 monoclonal antibody. Possible analogy to OKT3/Leu-4. J Exp Med. 1984;159:716–730. doi: 10.1084/jem.159.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- Hueber AO, Raposo G, Pierres M, He HT. Thy-1 triggers mouse thymocyte apoptosis through a bcl-2-resistant mechanism. J Exp Med. 1994;179:785–796. doi: 10.1084/jem.179.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(7):1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- Pautke C, Schieker M, Tischer T, et al. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004;24:3743–3748. [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pountos I, Jones E, Tzioupis C, McGonagle D, Giannoudis PV. Growing bone and cartilage. The role of mesenchymal stem cells. J Bone Joint Surg Br. 2006;88:421–426. doi: 10.1302/0301-620X.88B4.17060. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3:393–396. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- Schieker M, Pautke C, Haasters F, et al. Human mesenchymal stem cells at the single-cell level: simultaneous seven-colour immunofluorescence. J Anat. 2007;210:592–599. doi: 10.1111/j.1469-7580.2007.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieker M, Pautke C, Reitz K, et al. The use of four-colour immunofluorescence techniques to identify mesenchymal stem cells. J Anat. 2004;204:133–139. doi: 10.1111/j.1469-7580.2004.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Settleman J. Tension precedes commitment – even for a stem cell. Mol Cell. 2004;14:148–150. doi: 10.1016/s1097-2765(04)00207-2. [DOI] [PubMed] [Google Scholar]
- Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–831. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5’-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentori L, Pardoll DM, Zuniga JC, et al. Proliferation and production of IL-2 and B cell stimulatory factor 1/IL-4 in early fetal thymocytes by activation through Thy-1 and CD3. J Immunol. 1988;140:1089–1094. [PubMed] [Google Scholar]
- Vieyra DS, Jackson KA, Goodell MA. Plasticity and tissue regenerative potential of bone marrow-derived cells. Stem Cell Rev. 2005;1:65–69. doi: 10.1385/SCR:1:1:065. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhou X, Zhou T, et al. Ecto-5’-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–372. doi: 10.1007/s00432-007-0292-z. [DOI] [PubMed] [Google Scholar]