SUMMARY
Examination of embryonic myogenesis of two distinct, but functionally related, skeletal muscle dystrophy mutants (mdx and cav-3−/−) establishes for the first time that key elements of the pathology of Duchenne muscular dystrophy (DMD) and limb-girdle muscular dystrophy type 1C (LGMD-1c) originate in the disruption of the embryonic cardiac and skeletal muscle patterning processes. Disruption of myogenesis occurs earlier in mdx mutants, which lack a functional form of dystrophin, than in cav-3−/− mutants, which lack the Cav3 gene that encodes the protein caveolin-3; this finding is consistent with the milder phenotype of LGMD-1c, a condition caused by mutations in Cav3, and the earlier [embryonic day (E)9.5] expression of dystrophin. Myogenesis is severely disrupted in mdx embryos, which display developmental delays; myotube morphology and displacement defects; and aberrant stem cell behaviour. In addition, the caveolin-3 protein is elevated in mdx embryos. Both cav-3−/− and mdx mutants (from E15.5 and E11.5, respectively) exhibit hyperproliferation and apoptosis of Myf5-positive embryonic myoblasts; attrition of Pax7-positive myoblasts in situ; and depletion of total Pax7 protein in late gestation. Furthermore, both cav-3−/− and mdx mutants have cardiac defects. In cav-3−/− mutants, there is a more restricted phenotype comprising hypaxial muscle defects, an excess of malformed hypertrophic myotubes, a twofold increase in myonuclei, and reduced fast myosin heavy chain (FMyHC) content. Several mdx mutant embryo pathologies, including myotube hypotrophy, reduced myotube numbers and increased FMyHC, have reciprocity with cav-3−/− mutants. In double mutant (mdxcav-3+/−) embryos that are deficient in dystrophin (mdx) and heterozygous for caveolin-3 (cav-3+/−), whereby caveolin-3 is reduced to 50% of wild-type (WT) levels, these phenotypes are severely exacerbated: intercostal muscle fibre density is reduced by 71%, and Pax7-positive cells are depleted entirely from the lower limbs and severely attenuated elsewhere; these data suggest a compensatory rather than a contributory role for the elevated caveolin-3 levels that are found in mdx embryos. These data establish a key role for dystrophin in early muscle formation and demonstrate that caveolin-3 and dystrophin are essential for correct fibre-type specification and emergent stem cell function. These data plug a significant gap in the natural history of muscular dystrophy and will be invaluable in establishing an earlier diagnosis for DMD/LGMD and in designing earlier treatment protocols, leading to better clinical outcome for these patients.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is the severest of a family of debilitating congenital diseases associated with disruption of function of the trans-sarcolemmal dystrophin-glycoprotein complex (DGC). Classic DMD, which affects the entire skeletal musculature, arises from loss of an essential intracellular component of the DGC, dystrophin (Hoffman et al., 1987). The condition is characterised by early (~3 years age) and progressive disruption of skeletal muscle function, which leads to extensive muscle damage and causes early death (usually in the 20s). Death commonly results from complications arising from failure of the respiratory muscles, however, 95% of patients with DMD develop a cardiomyopathy, which is the primary cause of death in 10–30% of cases (Cox and Kunkel, 1997). The mdx mouse, a model of DMD that is deficient in dystrophin owing to a point mutation in the Dmd gene, exhibits many of the postnatal features of DMD, including cardiomyopathy; skeletal muscular dystrophy associated with bouts of fibre regeneration; fibrosis; hyperproliferation; and apoptosis of skeletal muscle myoblasts (Harper et al., 2002; Smith et al., 1995). The phenotype is less severe than human DMD, although the life span of mdx mice is curtailed with respect to wild-type (WT) mice (Chamberlain et al., 2007). Loss of caveolin-3 results in a milder form of muscular dystrophy (MD), known as limb-girdle muscular dystrophy type 1C (LGMD-1c), in which the affected muscle groups are predominantly the limb girdle and heart; caveolin-3-deficient mice (cav-3−/−) lack the Cav3 gene and exhibit LGMD, T-tubule defects, cardiomyopathy and skeletal muscle apoptosis (Hagiwara et al., 2000; Minetti et al., 2002).
Embryonic skeletal muscles originate from the dermamyotomal region of the somites (Cossu et al., 1996; Hollway and Currie, 2003). In the chick embryo, all regions of the dermamyotome appear to contribute myogenic precursors to this process in a time-dependent manner (Gros et al., 2004). Dystrophin is first expressed in embryonic somites from embryonic day (E)9.5, and could, thus, have a function in early myogenesis (Huang et al., 2000; Ilsley et al., 2002). Under the control of inducing factors that are secreted by surrounding tissues, the myotome produces cells that become the (Myf5 positive) stem cell populations, which then generate (from E10.5) the epaxial (deep muscles of the back) and (from E11.5) the hypaxial (limb, abdominal, diaphragm) muscles (reviewed by Hollway and Currie, 2003).
In mammalian embryonic skeletal muscle differentiation, there are thought to be two myogeneic ‘waves’ that generate the hypaxial lineage, producing a scaffold of primary myotubes and, subsequently, large numbers of secondary myotubes, which comprise the bulk of newly formed muscle (Cossu et al., 1996). Secondary myotubes are a morphologically distinct subset of myotubes, which are longer and thinner than primary myotubes, and that form in clusters around a single, larger primary myotube (Cho et al., 1994).
Myotube differentiation is dependent on the presence of functional muscle stem cell populations. Myf5 is expressed in the somite at E8.5 making it the earliest of the myogenic regulatory factors (MRF); in addition, it is the only MRF that is found before muscle differentiation and that persists in all embryonic muscle groups throughout both primary and secondary myogenesis (Cossu et al., 1996). Myf5 is therefore a good marker for the embryonic myoblast population. Lineage analysis suggests that a majority of satellite cells derive from the somite (Armand et al., 1983). Extensive evidence now links the Pax7-positive cell population, in particular, as being essential to the correct functioning of the adult skeletal muscle stem cell (the ‘satellite’ cell) population and particularly to its repair function (reviewed by Zammit et al., 2006). Pax7 is a transcription factor, a repressor of myogenesis, and plays important roles in the maintenance and specification of the adult satellite cell population; it is expressed in undifferentiated muscle stem cells that emerge from the somite from E11.5 (Merrick et al., 2007; Relaix et al., 2006; Seale et al., 2000).
The specificity of function of individual skeletal muscle groups depends on the appropriate localisation of fast myosin isoforms to different myotubes; a process initiated during the late stages of gestation (Merrick et al., 2007). In mammalian embryos, developmental (embryonic and neonatal) myosin isoforms are co-expressed in newly formed secondary myotubes with adult fast myosin isoforms (Cho et al., 1994). In later stages, developmental myosin isoforms are downregulated and replaced by adult myosin heavy chain (MyHC) isoforms in a muscle-specific pattern, a process that is not completed until several weeks after birth (Agbulut et al., 2003; Merrick et al., 2007). The embryonic heart expresses two myosin isoforms (cardiac myosin α and slow/cardiac myosin β) in a temporally regulated manner. Mutation of β-cardiac myosin is implicated in cardiomyopathy (Geisterfer-Lowrance et al., 1990).
Dystrophin associates with the β-subunit of dystroglycan on the intracellular side of the sarcolemma and is an essential component of the DGC, a multifunctional protein complex, which links the extracellular matrix to the actin cytoskeleton in skeletal muscle myotubes (Ervasti and Campbell, 1993). In postnatal muscle, dystrophin deficiency results in complete breakdown of the DGC and the secondary downregulation of a majority of the DGC proteins (Ohlendieck et al., 1993). Caveolin-3 localises to both skeletal muscle caveolae and the DGC where, by means of a specific WW domain, it binds to the same PPXY motif in the β-dystroglycan c-terminus that recognises and binds to dystrophin, thus blocking the interaction between dystrophin and β-dystroglycan (Jung et al., 1995; Sotgia et al., 2000).
The competitive interaction between caveolin-3 and dystrophin for the β-dystroglycan binding site may be crucial for some aspects of muscle development, but this has not been studied extensively. All three genes are expressed early in development in the chick, mouse, zebrafish and Xenopus laevis embryos (Biederer et al., 2000; Houzelstein et al., 1992; Nixon et al., 2005; Razani et al., 2002; Shin et al., 2003; Anderson et al., 2007). In zebrafish, loss of any of these proteins has been shown to disrupt myogenesis and cause gross muscle abnormalities, whereas in the mouse, dystroglycan deficiency leads to early embryonic lethality (Bassett et al., 2003; Nixon et al., 2005; Parsons et al., 2002; Williamson et al., 1997).
In this study, we examine the impact of the loss of caveolin-3 and dystrophin on skeletal muscle development in order to establish a functional role for these two proteins during myogenesis.
RESULTS
Branching fibre misalignment and malformed myotubes characterise the embryonic phenotype of MD
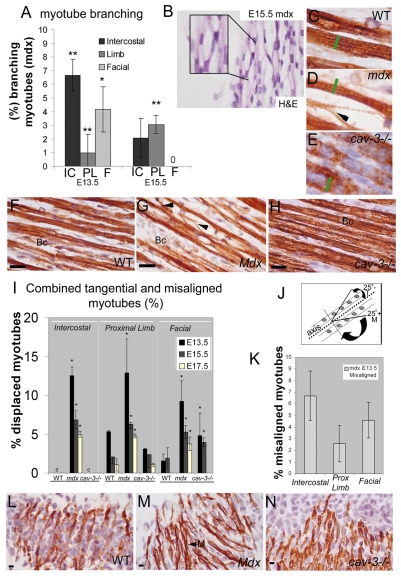
WT, cav-3−/− and mdx mice were immunostained with a pan-myosin antibody (MF20) to reveal muscle fibre architecture (Fig. 1). Branching and fibre splitting is found in mdx epaxial, hypaxial and facial muscles from E13.5 to E17.5, and shows a dynamic temporal and spatial pattern that is consistent with it being an early event associated with myotube formation (Fig. 1A–C,G). In matched muscle groups, at matched stages, mdx myotubes are hypotrophic, whereas cav-3−/− myotubes are hypertrophic, and myotube width varies more in dystrophic embryos with respect to WT embryos (Fig. 1C–E, green bars). Maximum morphological defects occur earlier in mdx intercostals (E13.5) than proximal limb muscles (E15.5), which is consistent with the later development of the proximal limb muscles (Fig. 1A). There is no fibre splitting in cav-3−/− and WT embryonic myotubes (Fig. 1A,C,E,F,H). Myotube alignment is also disrupted early in mdx myogenesis (Fig. 1I). At E13.5, up to an eighth of mdx myotubes are displaced from the median fibre alignment in the proximal limb, intercostal and facial muscles (Fig. 1I). This is statistically different from E13.5 WT and cav-3−/− muscle, in which fewer than 5% of myotubes were displaced. When myotubes were divided into those which deviate by more than 25° from the median (misalignment) and those that are displaced by less than 25° (tangential fibres), a significant proportion of the displaced mdx myotubes in individual muscles were misaligned (Fig. 1K), the rest being tangential, whereas all displaced myotubes in WT or cav-3−/−muscles were tangentially displaced. In the intercostal muscles of E13.5 mdx embryos, 7–9% of all myotubes (i.e. more than half of the total displaced myotubes) were misaligned. This was also true of the other stages examined (data not shown). The proportion of displaced myotubes declines with gestational age and is muscle group dependent (Fig. 1I). At E13.5, 12% of myotubes in mdx intercostal muscles were aligned incorrectly, this declines with gestational stage but we found misalignment and tangential displacement of myotubes in mdx intercostal muscles at all gestational stages (E13.5–E17.5, Fig. 1I,M). At the same stages, WT and cav-3−/− intercostal myotubes were aligned correctly to the median and there were no deviating myotubes in WT or cav-3−/− intercostals, suggesting that the alignment mechanism in these muscles is regulated tightly and disrupted severely in mdx mutants (Fig. 1I–N).
Fig. 1.
Branching and displacement defects in mdx embryonic myotubes. (A) Skeletal myotube branching in mdx myotubes. IC, intercostal; PL, proximal limb; F, facial muscle groups. The zero indicates that no abnormalities were detected. (B) Myotube branching in E15.5 mdx biceps as shown by haematoxylin and eosin (H&E) staining. The insert shows an enlarged section. (C–H) MF20 immunostaining of proximal muscle in WT myotubes (C,F), mdx hypotrophic myotubes (D,G), and cav-3−/− hypertrophic myotubes (E,H). The green bars indicate the width of a WT myotube and the black arrowheads point to myotube splitting in mdx myotubes. (F–H) Variable myotube diameters in mdx (G) and cav-3−/− (H) muscle fibres compared with in WT (F) muscle fibres. Bc, biceps. (I) Displaced myotubes (combined tangential and misaligned myotubes) as a proportion of the total counted. The zeros indicate that no fibres were displaced. (J) Displaced myotube scoring strategy: tangential myotubes (T) were classed as <25° from the median, misaligned myotubes (M) were >25° from the median. (K) Proportion of misaligned mdx myotubes at E13.5. (L–N) MF20 immunostaining of intercostal muscles at E13.5. Misaligned myotubes (M) were observed in mdx muscle (M, black arrow), but no myotube displacment was seen in WT (L) or cav-3−/− (N) muscles. Error bars indicate s.d. Student’s t-test: *P<0.01, **P<0.001 when compared with WT value. Bars, 20 μm.
Morphological defects in the dystrophic embryonic heart
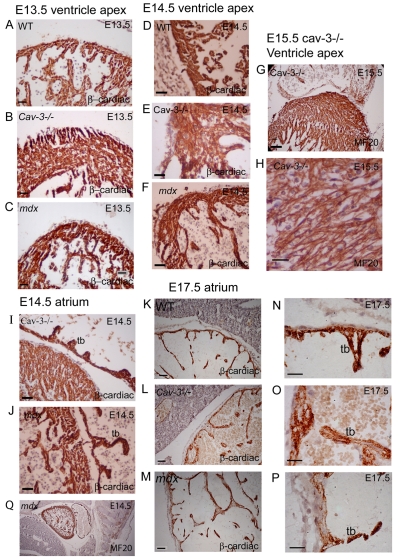
Cardiomyopathy is a significant clinical consequence of both LGMD (1C) and DMD. We established the morphology and myosin localisation pattern of dystrophic and WT embryonic hearts using MF20 and the cardiac β-myosin specific antibody N2.261 (Fig. 2). Consistent with the literature, skeletal fast myosin isoforms (FMyHC) could not be detected in embryonic hearts between E13.5 and E17.5 (My32 antibody, data not shown). Cardiac β-myosin is present in WT and dystrophic ventricular and atrial myocytes between E13.5 and E17.5 (Fig. 2A–F,I–P). Both mutants exhibit ventricular wall thickening (Fig. 2A–F) and cardiac myocyte disorganisation, which is substantially worse in cav-3−/− mutants, where myocytes form in a criss-crossed fashion, overlapping one another (Fig. 2E–H). Atrial trabecular formation is also impaired in dystrophic embryos; the trabeculae in E14.5 cav-3−/− embryos are short and stubby, whereas in mdx embryos they are long and hook shaped (Fig. 2I–J). At E17.5, distension or separation of the myocardial and endocardial cell layers of dystrophic atria occurs in the mutant embryos, and loss of cardiac β-myosin is also observed (Fig. 2K–P); this is in contrast to WT atria, where staining is uniform and the two layers are adjoined tightly (Fig. 2K,N). There are distinct differences between the two dystrophic hearts; in cav-3−/− mutants at E17.5, we observed patchiness and a reduction in staining in the myocardial wall, which was most evident in the trabeculae (Fig. 2O). In the mdx mutants, the loss of staining was extensive in the intertrabecular regions of the myocardium, but largely retained in the tips of trabeculae (Fig. 2P).
Fig. 2.
Disrupted development of the heart in dystrophic mice. (A–F,I–N) Mid-section hearts immunostained for β-cardiac myosin heavy chain. At E13.5, cav-3−/− (B) and mdx (C) hearts show mild and extensive ventricular wall hypertrophy, respectively. At E14.5, the ventricle apex structure is disorganised in cav-3−/− hearts (E) and less well developed in mdx hearts (F) when compared with WT hearts (D). (G,H) An MF20-stained E15.5 cav-3−/− heart showing disorganisation of the ventricular wall (G) and cardiac myocytes that criss-cross each other (H). (I,J) E14.5 atrial trabeculae (tb) are short and stubby in cav-3−/− mutants (I) and hook shaped in mdx mutants (J). (K–P) At E17.5, attenuated N2.261 labelling and distension of cell layers was visible in cav-3−/− trabeculae (L,O) and in the mdx atrial wall (M,P), compared with in WT atria (K,N). (Q) Low-magnification image of an MF20-labelled mdx heart to illustrate the orientation and matching of hearts that were sectioned sagitally through the most central portion of the heart. Bars, 20 μm.
Myonuclei misplacement and fusion abnormalities
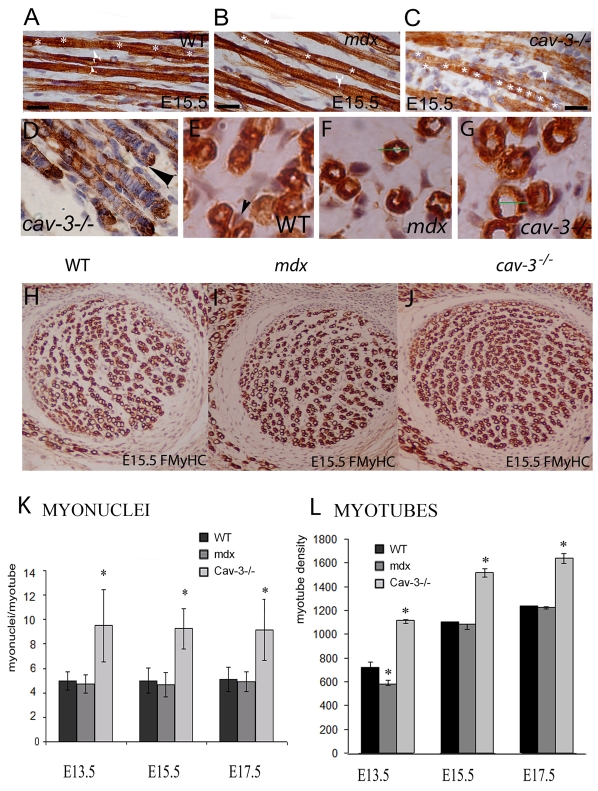
In E15.5 WT embryos, myonuclei are spaced evenly along the length of the myotube and, except for in newly formed myotubes, are arranged evenly and helically around the edge (Fig. 3A, white asterisks). In mdx embryos, myonuclei are more frequently located centrally and slightly further apart than in WT embryos (Fig. 3B). By contrast, cav-3−/− myonuclei are found closer together and exhibit severely disrupted myonuclei spacing and nuclei ‘bunching’, which is particularly evident at the ends of myotubes (Fig. 3C–D). Transverse sections demonstrate the presence of peripheral and central nuclei in WT muscle at this stage (E15.5) and show that only central nuclei are present in mdx and cav-3−/− muscles (Fig. 3E–G). To quantify these phenotypes, we scored total myonuclei and myofibres over a fixed area, to establish that cav-3−/− myotubes contained twice as many myonuclei as those of mdx or WT myotubes; this difference is statistically significant at E13.5, E15.5 and E17.5 (Fig. 3K) (P<0.05). There is also a substantial excess of myotubes in cav-3−/− mutants at these stages (P<0.05) and a smaller, but significant, reduction (P<0.05) in myotube number in mdx mutants (Fig. 3H–J,L). The disproportion in myotube numbers in cav-3−/− and mdx embryos lessens between E13.5 and E17.5, but is statistically different to WT embryos at all stages.
Fig. 3.
Size, shape and number of myotubes are altered in dystrophic mice. (A–C) Arrangement of myonuclei in E15.5 proximal muscle: (A) WT, helical with even spacing; (B) mdx, central location; (C) cav-3−/−, close central location with irregular spacing. White asterisks indicate myonuclei spacing in one myotube; white arrows point to myonuclei. In the WT proximal muscle (A), the white arrows indicate peripheral nuclei in two separate myotubes. Peripheral nuclei are not evident in mdx and cav-3−/− muscles (B,C). (D) Bunching of myonuclei at myotube ends in cav-3−/− muscle. (E–G) Transverse sections of E15.5 WT (E), mdx (F) and cav-3−/− (G) lower proximal limb myotubes showing that peripheral myonuclei (arrow) are associated with WT, but not with dystrophic embryo, myotubes at this stage. The hypotrophy of mdx and hypertrophy of cav-3−/− myotubes can also be seen; the green lines in F and G represent the WT myotube diameter. (H–J) Lower magnification images of the lower proximal limb regions of WT (H), mdx (I) and cav-3−/− (J) embryos showing the reduced and increased muscle fibre densities in mdx and cav-3−/− embryos, respectively, when compared with WT fibres in matched embryo sections. (K) Myonuclei numbers are doubled in cav-3−/− myotubes (E13.5–E17.5) when compared with WT myotubes; *P<0.05. There was a slight, but not statistically significant, reduction in the number of mdx myonuclei (E13.5–E17.5) when compared with WT myonuclei. The mean myonuclei content of myotubes was constant between E13.5 and E17.5. (F) Myotube density was increased in cav-3−/−mutants (E13.5–E17.5) and decreased in mdx mutants (E13.5) when compared with WT embryos. Myotubes were counted over a fixed, matched muscle area for each strain and stage; *P<0.05. Bars, 20 μm.
Hyperproliferation and apoptosis in cultured embryonic muscle stem cells
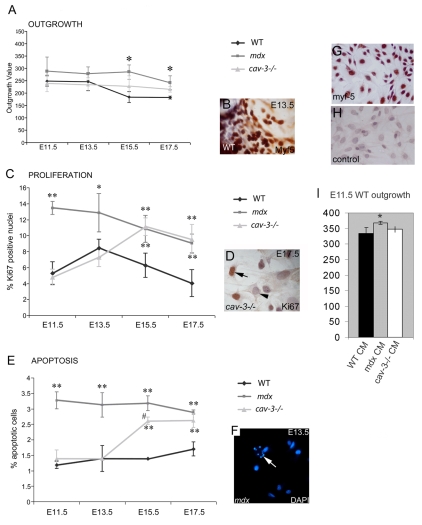
To characterise the embryonic muscle stem cell population we used dystrophic and WT (E11.5–E17.5) embryonic myoblast cultures (Smith and Merrick, 2008). The somite origin of these cells was established using Myf5 staining (Fig. 4). mdx and cav-3−/− explants both have outgrowth rates that deviate from WT explants (Fig. 4A–B). Compared with WT embryos, outgrowth of Myf5-positive cells is significantly greater at all stages (E11.5–E17.5) in mdx embryos and significantly greater from E15.5-E17.5 in cav-3−/− embryos. In earlier stages (E11.5–E13.5), the cav-3−/− outgrowth rate is indistinguishable from that of WT embryos, suggesting that defects occur later in this mutant. Between E11.5 and E17.5, myoblast proliferation (determined by Ki67 immunoreactivity) and apoptosis (shown by DAPI staining) were both significantly elevated in mdx mutants, whereas in cav-3−/− mutants, apoptosis and proliferation rates were equal to those of WT embryos until E15.5 when there was a sharp elevation in both parameters to levels approaching those of mdx embryos (Fig. 4C,E). In the mdx mutants, proliferation (and apoptosis) of myoblasts declined from E13.3 to E17.5, although it remained significantly higher than WT levels; however, the proliferation and apoptotic rates of cav-3−/− myoblasts increased until E15.5 (Fig. 4C–F). At E17.5, the proliferative rate of cav-3−/−cultures declined slightly, in line with mdx levels, whereas the apoptotic rate is maintained at the level of E15.5 cav-3−/− myoblasts (Fig. 4E). The mdx explant-derived soluble factors were shown to increase outgrowth of E11.5 WT explants (Fig. 4I).
Fig. 4.
Dystrophic, embryonic Myf5-positive myoblasts are hyperproliferative and prone to apoptosis. (A) The outgrowth rate of embryonic myoblasts from muscle explant cultures is increased both in mdx mutants from E11.5 and in cav-3−/− mutants at E15.5 and E17.5 when compared with WT explants cultured in parallel. (B) A Myf5-immunostained explant. (C) Hyperproliferation of embryonic myoblasts in mdx mutants from E11.5 and in cav-3−/− mutants from E15.5, as determined by Ki67-positive immunoreactivity (D). (E) Elevated apoptosis from E11.5 in mdx embryos and from E15.5 in cav-3−/− embryos, as shown by DAPI staining (F); the arrow in F points to an apoptotic cell. *P<0.05 compared with WT; **P<0.01 compared with WT; #P<0.05 when comparing mdx with cav-3−/−. (G,H) E15.5 primary cultured WT embryonic myoblasts with Myf5 staining (G) and a second antibody control (H). (I) The outgrowth rate of E11.5 WT explants increased (*P<0.05) in E11.5 mdx explant-conditioned medium (CM), but not in cav-3−/− or WT CM. Error bars indicate s.d.
The Pax7 skeletal muscle stem cell population is attenuated and disorganised in dystrophic embryos
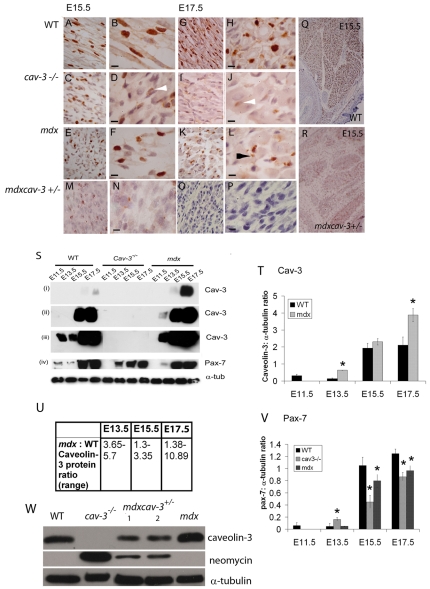
At E17.5, the WT Pax7-positive stem cell population is interspersed more sparsely between skeletal muscle myotubes, although levels of Pax7 protein continue to increase with muscle size (Merrick et al., 2007) (Fig. 5A,B,G,H,M,O). In WT embryos, the Pax7 staining intensity is uniform and constant with gestational age (Fig. 5A,B,G,H,Q; lower proximal limb). Between E15.5 and E17.5, both cav-3−/− and mdx embryos undergo attrition of their Pax7-positive cell population throughout their musculature so that, by E17.5, there is a significant reduction of Pax7 staining and Pax7 protein in both mutants (Fig. 5C–L,M,P; shown for lower proximal limb). This is particularly evident in cav-3−/− proximal hind limb muscles, where Pax7-positive cells are almost absent at E17.5 and those that remain stain very weakly (Fig. 5J, white arrowhead). At E15.5 (cav-3−/− and mdx) and E17.5 (mdx), Pax7-positive cell fragments are found in dystrophic muscles (Fig. 5L, indicated by a black arrowhead), suggesting that these cells may be undergoing apoptosis. Mutant mdxcav-3+/− embryos, which are deficient in dystrophin (mdx) and heterozygous for caveolin-3 (cav-3+/−), have a much more severe phenotype in the proximal limb where Pax7-positive cells are very sparsely present at E15.5 (Fig. 5M,N,R); furthermore, Pax7-positive cells are almost entirely absent in the lower proximal limb of these embryos at E17.5 (Fig. 5O–P) and are very much attenuated throughout the musculature (see Fig. 7).
Fig. 5.
Depletion of Pax7-positive myoblasts in embryonic dystrophic muscles. Pax 7 immunostaining in WT, mdx, cav-3−/− and mdxcav-3+/− lower proximal limbs at E15.5 (A–F,M,N) and E17.5 (G–L,O,P) at low magnification (A,C,E,G,I,K,M,O) and high magnification (B,D,F,H,J,L,N,P). Bars, 10 μm. (Q,R) Whole-region views of WT (Q) and mdxcav-3+/− (R) lower proximal limbs at E15.5 showing the massive reduction in Pax7-immunoreactive cells in the double mutant (mdxcav-3+/−). Putative apoptotic (fragmented) Pax7-positive nuclei are visible in mdxcav-3+/−, cav-3−/− and mdx mutant limbs, but not WT limbs, at E15.5 and in mdx limbs (L) at E17.5 (black arrowhead). Attenuation of Pax7 staining, as indicated by white arrowheads, was seen in cav-3−/− (C,D) and mdxcav-3+/− (M,N) limbs at E15.5 and in cav-3−/− limbs at E17.5 (I–J). At E17.5, mdxcav-3+/− lower proximal limb muscles are devoid of Pax7-positive cells (O,P). (S) Whole-embryo immunoblotting for caveolin-3 (blots i–iii) and Pax7 (blot iv); exposure times: 10 seconds (i), 1 minute (ii), 4 minutes (iii,iv). Pax7 protein levels are reduced in E15.5 and E17.5 dystrophic embryos. At E11.5, following a 4-minute exposure, Pax7 is detected only in WT embryos. (T) Densitometric analysis of the caveolin-3:α-tubulin ratio confirms the increased caveolin-3 levels in mdx mutant embryos. *P<0.05; mean±s.d. of two separate experiments. (U) The estimated fold increase of caveolin-3 in mdx embryos (over WT embryos), following separate analysis of three embryonic stages (four experiments per stage). P=0.05 for all stages. (V) Densitometric analysis of the Pax7:α-tubulin ratio demonstrating the reduction of Pax7 protein in cav-3−/− and mdx embryos (E15.5–E17.5). E13.5 cav-3−/−embryos contain significantly more Pax7 than WT embryos. *P<0.05; mean±s.d. of two separate experiments. (W) Immunoblot demonstrating that caveolin-3 protein is reduced in E15.5 mdxcav-3+/− embryos [by 50% with respect to WT embryos, based on densitometric analysis of the caveolin-3: α-tubulin ratio in WT embryos (track 1) compared with mdxcav-3+/− embryos (tracks 3 and 4)] and significantly reduced when compared with E15.5 mdx embryo siblings, in which caveolin-3 levels are increased when compared with WT embryos. Neomycin immunostaining confirms the presence of the Cav3 knockout transgene in cav-3−/− and mdxcav-3+/− embryos. The reduced neomycin staining seen in mdxcav-3+/−embryos compared with in cav-3−/− embryos reflects their heterozygosity for the Cav3 knockout transgene.
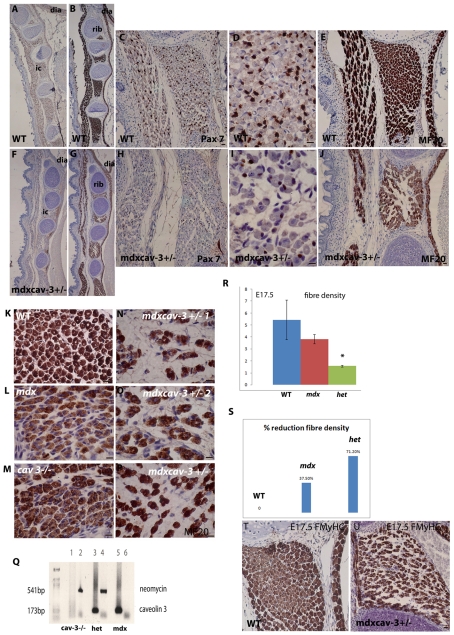
Fig. 7.
Substantial muscle fibre loss accompanied by depletion of Pax7-positive myoblasts in double mutant embryos. WT mice (A–E) and mdx mice that are heterozygous for caveolin-3 (mdxcav-3+/−) (F–J), immunostained with MF20 (B,E,G,J) or Pax7 (A,C,D,F,H,I) at E17.5 to illustrate the extensive loss of Pax7 myoblasts and muscle fibre density in the muscles of late gestation embryos. (K–P) Higher magnification images of MF20-labelled intercostal muscle sections (matched fourth intercostal for each embryo) showing the difference in myotube density between WT (K), mdx (L), cav-3−/− (M) and three different mdxcav-3+/− embryos (N–P) at E17.5. (R) Fibre density assessed over a fixed grid area and (S) percentage reduction in fibre density in WT, mdx and mdxcav-3+/− (het) embryonic intercostal muscle at E17.5. WT (T) and mdxcav-3+/− (U) E17.5 intercostal muscle, labelled with My32 antibody, showing that almost all mdxcav-3+/− fibres are strongly FMyHC positive; by contrast, FMyHC is undergoing downregulation in many WT intercostal fibres. Bars, 20 μm
Whole-embryo immunoblotting demonstrates an increase in caveolin-3 protein in WT (and mdx) embryos at E15.5–E17.5 compared with at E11.5 and E13.5, and confirms its absence in cav-3−/− embryos (Fig. 5M–N). In mdx mutants, caveolin-3 is not detected at E11.5 (Fig. 5M,N) but there is a several fold increase in caveolin-3 content at E13.5–E17.5 compared with in WT embryos, as measured by densitometry on four separate gel runs (P=0.05 for all three stages) (Fig. 5O). Pax7 content increases in WT embryos during gestation but is reduced substantially in cav-3−/− and mdx mutant embryos at E15.5–E17.5, with the reduction being greater in cav-3−/− embryos than mdx embryos at both stages, suggesting that caveolin-3 may regulate Pax7-positive myoblast survival in late gestation. At E15.5, there is a reduction in the intensity of the Pax7 bands in mdx and cav-3−/− dystrophic embryos by 15% and 60%, respectively (Fig. 5N,P). At E13.5, Pax7 is elevated slightly in cav-3−/− embryos and reduced in mdx embryos; this may relate to the increase and decrease in myotube number that is found in cav-3−/−and mdx embryos, respectively, at this stage (see Fig. 3), and suggests that developmental timing may be disrupted in these embryos.
Mislocalisation of fast myosin isoforms in dystrophic embryos follows a reciprocal pattern in dystrophin- and caveolin-3-deficient embryonic muscles
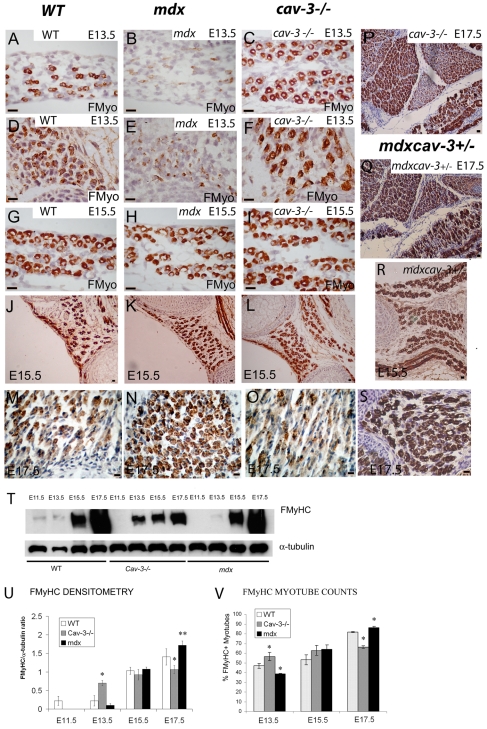
In WT embryos, FMyHC is present in small numbers of secondary myotubes by as early as E11.5, and by E15.5 it is localised strongly to around 45% of secondary myotubes across a wide range of muscle groups. Around 80% of myotubes contain FMyHC at E17.5, but staining intensity is heterogeneous and weak in many myotubes (Merrick et al., 2007). This dynamic and muscle-specific pattern of fast myosin localisation is disrupted in cav-3−/− and mdx mutant embryos, which show defects in: (1) the developmental timing of FMyHC staining, (2) the total number of fast-myosin-positive myotubes present and (3) the intensity of fast myosin staining (Fig. 6; supplementary material Fig. S1). At E13.5, FMyHC is elevated above WT levels in some cav-3−/− muscles (notably the respiratory muscles) but is attenuated substantially in all mdx muscles and in cav-3−/− proximal muscles (Fig. 6A–F).
Fig. 6.
Fast myosin fibre-type specification is disrupted in dystrophic embryos. (A–F) FMyHC immunostaining in the diaphragm (A–C) and intercostals (D–F) at E13.5. WT (A,D), mdx (B,E) and cav-3−/− (C,F) sections showing reduced FMyHC in mdx embryos (B,E) and increased FMyHC in the respiratory muscles of cav-3−/− embryos (C,F). (G–L) FMyHC immunostaining in the diaphragm (G–I) and intercostals (J–L) of WT (G,J), mdx (H,K) and cav-3−/− (I,L) embryos at E15.5. An increase in FMyHC staining was observed in mdx embryos (N) and a decrease in FMyHC staining was observed in cav-3−/− embryos (O) when compared with FMyHC staining in WT proximal limb (M) at E17.5. (P–S) FMyHC is also increased in mdxcav-3+/− embryos compared with in WT embryos at E17.5 (Q,S) (proximal limb shown) and E15.5 (R) (intercostal and diaphragm shown). Furthermore, comparison of a cav-3−/− embryo (P) with an mdxcav-3+/−embryo (Q) at E17.5 establishes that dystrophin deficiency suppresses the downregulation of FMyHC that is seen in cav-3−/− and WT embryos. (T) Immunoblotting of WT, cav-3−/− and mdx embryos shows that, with respect to WT embryos, FMyHC is delayed in both cav-3−/− and mdx embryos until E13.5. In the later stages, FMyHC is attenuated in cav-3−/− embryos and overproduced in mdx embryos (T). (U) FMyHC densitometry. The FMyHC:α-tubulin ratio demonstrates a statistically significant increase and reduction in FMyHC content in mdx and cav-3−/− embryos, respectively, at E17.5. At E13.5, there is a significant excess of FMyHC in cav-3−/− embryos; mean±s.d. of two separate experiments. (V) FMyHC-positive myotubes expressed as a proportion of the total counted in WT, mdx and cav-3−/− embryos (at E13.5, E15.5 and E17.5). Error bars indicate s.d. *P<0.05, **P< 0.01. Bars, 20 μm.
The appearance of FMyHC-positive myotubes is delayed in mdx embryos; at E13.5, there are very small numbers only of FMyHC-positive myotubes throughout the musculature of mdx embryos, compared with in WT or cav-3−/− embryos at the same stage (respiratory muscles Fig. 6A–F) [proximal muscles show a similar phenotype (data not shown)]. This finding is supported by immunoblotting where there is a statistically significant reduction in the intensity of the mdx E13.5 band (Fig. 6T,U) and by myotube counts (Fig. 6V), which demonstrate a statistically significant deficiency of FMyHC-positive myotubes in E13.5 mdx embryos (P<0.05). A total of six mdx and four WT E13.5 embryos were used to establish these data. In mdx embryos, the staining intensity and proportion of FMyHC-positive myotubes increases by developmental stage so that, from E15.5 to E17.5, there is a substantial excess of My32 staining in dystrophin-deficient myotubes compared with in WT myotubes, and a progressive increase in the staining intensity of FMyHC bands in mdx whole-embryo immunoblots (Fig. 6G–O,T). Densitometry confirmed this conclusion by revealing an increase in FMyHC content in mdx embryos and a decrease in FMyHC content in cav-3−/− embryos; these results were statistically significant at P<0.001 and P<0.05, respectively (Fig. 6U). Quantification of matched, FMyHC-immunostained muscle sections from WT and dystrophic embryos established that the proportion of FMyHC-positive myotubes is perturbed in cav-3−/− and mdx embryos (Fig. 6V). mdx embryos that were heterozygous for the Cav3 gene (mdxcav-3+/−) also failed to downregulate FMyHC and show increased FMyHC staining throughout the musculature at E15.5 (Fig. 6R) and E17.5 (Fig. 6Q,S), even in muscles such as the intercostals where myotubes are depleted severely (Fig. 7T–U).
Extensive intercostal muscle fibre loss and Pax7 depletion in mdxcav-3+/− mutant embryos
To establish whether upregulated caveolin-3 was likely to ameliorate, or contribute to, the mdx/DMD phenotype, we generated double mutant embryos that were deficient in dystrophin (mdx) and heterozygous for the caveolin-3 null mutation (cav-3+/−). At E15.5, these mdxcav-3+/− embryos have a 50% reduction of caveolin-3 compared with WT embryos (Fig. 5). Immunostaining with MF20 and Pax7 establishes that, at E17.5, mdxcav-3+/−embryos have a more severe phenotype that, in addition to loss of hind limb Pax7 myoblasts (see Fig. 5), also includes a severe depletion of their intercostal muscle fibres, which is accompanied by significant attenuation of Pax7-positive intercostal myoblasts (Fig. 7A–J). Even at low magnification, the fibre-loss phenotype can be identified by the presence of gaps (‘white space’) between the fibres of mdxcav-3+/− intercostals (Fig. 7A,D,F,I), which are packed together densely in WT intercostal muscle fibres. At higher magnification (Fig. 7K–P), it is clear that this phenotype is a result of there being far fewer fibre clusters in mdxcav-3+/− intercostals compared with in WT, mdx or cav-3+/− intercostal muscles. This phenotype is very consistent; three different mdxcav-3+/− embryos are shown (Fig. 7N–P). In the mdx embryos, the intercostal fibres also appear to be distributed more sparsely than in WT embryos, suggesting a milder form of this phenotype. Quantitation of fibre density was achieved by counting intercostal fibre number over a fixed grid area and established that both mdx and het (mdxcav-3+/−) embryos have reduced fibre densities; this reduction was statistically significantly in the het mutant (P<0.05) (Fig. 7R). When expressed as a proportion of WT intercostal myotubes, the mdxcav-3+/− mutant embryos have a massive depletion (71.2%) of their intercostal myotubes by E17.5 (Fig. 7S) (based on the mean of counts from three different mdxcav-3+/− embryos at E17.5), whereas mdx embryos have a depletion of a third (37.5%). The majority of these fibres express FMyHC in both mutants (Fig. 7U).
DISCUSSION
In this study, we demonstrate the embryonic phenotype of mdx and cav-3−/− dystrophic mice and show that the embryonic phenotype is significantly more severe in mice that are mutant for both proteins (mdxcav-3+/−). We establish crucial roles for dystrophin and caveolin-3 in murine embryogenesis, and establish that the pathologies that define the adult dystrophic phenotype and clinical picture of DMD and LGMD-1c originate in the failure of embryonic muscle differentiation. The key findings of this analysis are summarised in Table 1.
Table 1.
A summary of the phenotypic differences between dystrophic and wild type embryos
| Phenotype compared with WT embryo | mdx | cav-3−/− | mdxcav-3+/− | |
|---|---|---|---|---|
| Morphometry | Myotube size | Hypotrophy | Hypertrophy | Hypotrophy |
| Total myotube number/muscle | Reduced | Increased | Reduced | |
| Intercostal fibre density (E17.5) | 37.5% reduction | ND | 71.2% reduction (P<0.05) | |
| Fibre density (limb) | Decreased | Increased (P<0.05) | ND | |
| High number of centrally located myonuclei (E17.5) | Yes | No | Yes | |
| Myotube misalignment | Yes (P<0.01) | No | Yes | |
| Branching and fibre splitting | Yes (P<0.001) | No | ND | |
| Increased myonuclei | No | Yes (P<0.05) | ND | |
| Myonuclei displacement (bunching) | No | Yes (P<0.05) | ND | |
| Cardiac left ventricular wall thickening | Yes (minimal) | Yes | ND | |
| Cardiac atrium-trabecular defects | Yes, short and stubby | Yes, long and hooked | ND | |
| Stem cell behaviour | Proliferation | Hyperproliferation (from E11.5) (P<0.01) | Hyperproliferation (from E15.5) (P<0.01) | ND |
| Apoptosis | Elevated from E11.5(P<0.01) | Elevated from E15.5(P<0.01) | ND | |
| Pax7-positive cell loss | Yes (+) | Yes (++) | Yes (+++) | |
| Total Pax7 protein (WB) | 15% reduction (E15.5) (P<0.05) | 60% reduction (E15.5) (P<0.05) | ND | |
| Fast myosin mislocalisation | Developmental delay | Yes | No | Yes |
| Increased FMyHC-positive myotubes (%) | Yes | No (reduced) | Yes | |
| Total FMyHC protein (WB) | Increase (P<0.001) | Decrease (P<0.05) | ND | |
| Caveolin-3 | Total caveolin-3 protein | 1.3–10-fold increase (P<0.05) | Absent | Reduced (50%) at E15.5 |
| Developmental delay | Yes (E13.5) | Not applicable | ND | |
Summary of the phenotypic differences between dystrophic mdx, cav-3−/− and mdxcav-3+/− embryos compared with WT embryos. The evidence is sub-divided under the key headings: morphometry, stem cell behaviour and fast myosin mislocalisation, which together establish a significant phenotypic difference between dystrophic and WT embryos, and demonstrate the embryonic basis for the Duchenne (DMD) and limb girdle (LGMD) forms of muscular dystrophy. A fourth category demonstrates the disruption of embryonic caveolin-3 protein. Phenotypic analyses that includes double mutant embryos (mdxcav-3+/−) are shown in bold, as are probability values for parameters with a statistical significance of at least P<0.05.
Early muscle patterning is disrupted in dystrophin-deficient embryos
The growth behaviour of Myf5-positive mdx embryonic myoblasts is disrupted from E11.5, and mdx Myf5-positive myoblasts are hyperproliferative and apoptotic. Myf5 marks the embryonic myoblast population; it is expressed in myotome (E8.5) before myotube differentiation is initiated and is found in all muscle groups throughout myogenesis (Hadchouel et al., 2003; Ott et al., 1991). Dystrophin is expressed at a crucial stage in myotome differentiation (E9.5), one day later than Myf5 expression at E8.5, and 24 hours before the appearance of the first fully differentiated myotomal (epaxial) myotubes and the first expression of myosin heavy chain (MyHC) (E10.5) (Houzelstein et al., 1992; Ott et al., 1991; Schofield et al., 1993). Early hypaxial and secondary myogenesis are severely disrupted and delayed in mdx embryos, as shown by the late appearance of the FMyHC, Pax7 and caveolin-3 proteins and by the incomplete formation and disorganisation of mdx musculature at between E11.5 and E13.5 (Figs 1, 6; supplementary material Fig. S1). These data suggest that dystrophin has an essential role in myotome differentiation, which is disrupted in mdx embryos with severe consequences for patterning and function of the entire musculature.
Also at E10.5, Myf5-positive myoblasts migrate from the myotome to initiate hypaxial muscle formation (Cusella-De Angelis et al., 1992). Myoblasts migrate under the control of Hox genes (Pax3 and Lbx) and differentiate in response to growth factors (Wnt1 and Shh) secreted by adjacent tissues (Gross et al., 2000; Hadchouel et al., 2003). The cues that trigger embryonic myoblast migration are not known. In other tissues, stem cell migration is regulated by guidance cues from originating and target tissues; this process is essential for the correct patterning of embryonic structures and can be disrupted easily (Lehmann, 2001). It is probable, therefore, that E10.5–E11.5 epaxial myotubes provide signalling cues that trigger migration of Myf5-positive myoblasts from the myotome, and initiate hypaxial and secondary myogenesis. In the absence of dystrophin, these cues are absent and both the migration and initiation processes are impaired. The aberrant behaviour of E11.5 WT explants cultured in E11.5 mdx CM (Fig. 4I) supports the view that the myotome releases secreted factors that modify the behaviour of the embryonic myoblast population at E11.5. Disruption of early myogenesis in mdx embryos suggests these early role(s) for dystrophin are distinct from those of utrophin. This conclusion is consistent with published mRNA in situ patterns for dystrophin and utrophin, which differ in the early embryonic stages (Houzelstein et al., 1992; Schofield et al., 1993). In the later stages of gestation (see below), there appears to be a ‘catch-up process’ in mdx myogenesis, which could be mediated by the subsequent overproduction of caveolin-3 in these embryos or by compensatory expression of another protein, for example a-utrophin. a-utrophin is upregulated in postnatal, dystrophin-deficient muscles and may take over some functions of dystrophin in mdx embryos (Weir et al., 2004). The increased severity of the double mutant phenotype (mdxcav-3+/−) in late gestation indicates that there may be a compensatory effect of caveolin-3 in late gestation mdx embryos.
Caveolin-3 is regulated by muscle regulatory factors (MRFs), activated during myotube differentiation, and expressed later than dystrophin in the E11.5 myotome (Biederer et al., 2000). This is consistent with our detection of caveolin-3 at E11.5 in WT embryos. MRFs also regulate Pax7, which also emerges at E11.5 (Merrick et al., 2007). In cav-3−/− embryos, the early muscle patterning process appears intact, Myf5-positive myoblast behaviour is not disrupted, and the localisation and timing of hypaxial muscle formation is comparable to that seen in WT embryos. Caveolin-3 does not, therefore, seem to be required for these early stages of myogenesis. In mdx embryos, the appearance of both caveolin-3 and Pax7 is delayed; this could be as a consequence of the developmental delay in myogenesis in these embryos or, more specifically, a downstream result of the failure to activate dystrophin.
Hypaxial musculature and secondary myogenesis
Interpretation of the role of dystrophin in later embryonic events is complicated by the upregulation of caveolin-3 in mdx embryos from E13.5. In postnatal tissues, overexpression of caveolin-3 causes dystrophin downregulation and a DMD-like phenotype (Galbiati et al., 2000). Loss of dystrophin causes breakdown of the DGC and suppression of dystrophin-associated proteins (Ohlendieck et al., 1993; Vaghy et al., 1998). However, the muscles of DMD patients and adult mdx embryos have a 1.5–2.4-fold excess and a 2–3-fold excess, respectively, of caveolin-3 (Vaghy et al., 1998). In E13.5–E17.5 mdx embryos, we found, on average, a 3–5-fold excess of caveolin-3 (depending on the embryonic stage), suggesting that disruption of caveolin-3 expression is an early and significant consequence of dystrophin deficiency. Embryonic mRNA expression patterns of dystrophin, but not utrophin, overlap those of dystroglycan in WT embryos and this pattern is unchanged in mdx embryos (Houzelstein et al., 1992; Schofield et al., 1995). Therefore, dystrophin may negatively regulate caveolin-3 by competitive binding to β-dystroglycan (Ilsley et al., 2002).
In zebrafish embryos, caveolin-3 mutants have cytoskeletal and fusion defects, and dystrophin mutants have unstable muscle attachments, suggesting a role for caveolin-3 in the placement and orientation of embryonic myotubes (Bassett et al., 2003; Nixon et al., 2005). Myogenesis is disrupted in cav3−/− and dmd zebrafish mutants at E13.5; the resulting phenotypes suggest that murine caveolin-3 and dystrophin have similar roles to their zebrafish counterparts. In E13.5 mdx embryos, we found myotube displacement, splitting and branching. Myotube orientation defects are not found in caveolin-3-deficient mutants. Instead, embryonic cav-3−/− myonuclei are bunched at the myotube ends, indicative of the T-tubule defect that has been reported postnatally for cav-3−/−mice (Minetti et al., 2002). Excessive cav-3−/− myotube production was also found between E13.5 and E17.5, in addition to a twofold increase in myonuclei content and hypertrophy. These data match those of an in vitro study that used cultured cav-3−/− myoblasts and found a reciprocal phenotype (i.e. reduced myotube number, fewer myonuclei and hypertrophy) in caveolin-3-overexpressing myoblasts, suggesting that caveolin-3 suppresses myoblast fusion (Volonte et al., 2003). E13.5 mdx embryos partially reproduce the overexpression phenotype because they have hypotrophic myotubes and reduced myotube numbers, although the number of myonuclei is not affected. However, although caveolin-3 levels remain elevated in later stages (E15.5–E17.5), mdx myotube numbers are comparable to those of WT embryos. This suggests that caveolin-3 may regulate myotube size, but that the E13.5 myotube deficit is the result of delayed hypaxial myogenesis in mdx embryos (see section above), rather than an excess of caveolin-3.
Reciprocal disruption of fast fibre specification in cav-3−/− and mdx embryos
FMyHC-positive myotubes appear first in WT epaxial muscles at around E11.5 and herald the start of secondary myogenesis (Merrick et al., 2007). Fibre-type switching begins at E15.5, when some myotubes switch between slow and fast myosin expression, and the process of establishing adult fibre-type ratios begins (Cho et al., 1994; Merrick et al., 2007). FMyHC-positive myotube differentiation is significantly perturbed in mdx embryos (see sections above) and fast fibre-type specification is disrupted in both mdx and cav-3−/− embryos (Fig. 6). Between E15.5 and E17.5, there is a reciprocal phenotype where mdx embryos have a significant excess of the FMyHC protein and a higher proportion of FMyHC-positive myotubes compared with WT embryos; there is substantial reduction of FMyHC in cav-3−/− mutants. The overproduction of FMyHC in mdx embryos could result from a ‘catch-up process’ in E13.5 mdx muscles, facilitated by a compensatory increase in caveolin-3. However, the loss of FMYHC-positive myotubes in cav-3−/− mutants at E15.5–E17.5 suggests that caveolin-3 is also required for fast fibre specification, and that overproduction of caveolin-3 in mdx embryos is pathogenic for this phenotype and causes an overproduction of FMyHC fibres in E15.5–E17.5 mdx mutants. Postnatal fast muscle fibres degenerate preferentially in DMD and in mdx mice, with severe consequences for muscle function (Webster et al., 1988). In mutants that are heterozygous for caveolin-3, but entirely deficient for dystrophin (mdxcav-3+/−), FMyHC-positive fibres are still over-represented at E17.5 compared with in WT embryos, suggesting that it is the balanced relationship between dystrophin and caveolin-3 that is crucial for correct fast fibre proportion rather than caveolin-3 levels alone.
In adult tissues, caveolin-3 and dystrophin interact directly by competitive binding to β-dystroglycan in the DGC (Sotgia et al., 2000). These data establish distinct roles for dystrophin and caveolin-3 in myogenesis, but suggest that both proteins and a functional DGC may be required for fibre-type specification.
Loss of Pax7 myoblasts
At E15.5–E17.5, cav-3−/− and mdx embryos exhibit Pax7-positive myoblast attrition and a significant depletion of Pax7 protein. Loss of Pax7 occurs rapidly in cav-3−/− mutants at E15.5, a time point when caveolin-3 is strongly upregulated in WT embryos, and the loss is greater than in E15.5 mdx embryos. Caveolin-3 can elicit survival signalling in muscle, as can Pax7. Our data suggest a direct role for caveolin-3 in the rapid loss of Pax7-positive myoblasts at E15.5 in cav-3−/− mutants, and suggest that the attrition of Pax7-positive myoblasts could be partially compensated for in mdx muscles by their increased caveolin-3 levels (Relaix et al., 2006; Smythe et al., 2003). This conclusion is supported by the finding that, in mdxcav-3+/− mutants, Pax7 is depleted substantially more than in either single mutant, such that, by E17.5, Pax7 cells are entirely absent in mdxcav-3+/− lower proximal limb muscles and are very much reduced in other muscles. Pax7-positive myoblasts are crucial for normal postnatal satellite cell emergence (Relaix et al., 2006; Seale et al., 2000). In the absence of Pax7 (Pax7−/− mice), satellite cells are reduced in number and apoptosis is elevated. However, owing to the presence of Pax3-positive myoblasts, satellite cells are not lost entirely, and there appear to be sufficient satellite cells to establish and sustain (postnatal) juvenile muscle development in these mice, although regeneration is impaired in adults (Oustanina et al., 2004; Relaix et al., 2006; Seale et al., 2000). Our findings that E17.5 mdxcav-3+/− embryonic intercostal muscles are severely depleted of myotubes, as well as of Pax7-positive cells, suggests that Pax7 may also play an important role in embryonic myogenesis. The findings suggest that dystrophic embryos have a reduced capacity for generating muscle fibres from late gestation, which may be carried over to postnatal life. Dystrophic muscle regeneration is known to be abnormal, being associated with myoblast apoptosis, hyperproliferation, irregular fibre size and progressive fibrotic deposition. Both mdx and cav-3−/− muscles experience bouts of degeneration and regeneration in the early postnatal weeks, which are associated with elevated levels of apoptosis (Hagiwara et al., 2000; Smith et al., 1995). In mdx mutants, there is progressive failure of muscle regeneration from 4 months of age, which, in mdx mice but not DMD patients, is ameliorated by a concomitant reduction of degenerative processes that may, in part, be mediated by upregulation of utrophin (Reimann et al., 2000; Roig et al., 2004; Roma et al., 2004). Regeneration has not been studied in detail in adult cav-3−/− mice. Our data suggest that the regenerative machinery of dystrophic mutants is abnormal at birth, because these mutants are deficient in Pax7; this early loss could underlie the progressive impairment of the regenerative process in adulthood. The significant loss of muscle fibre density in the respiratory muscles of E17.5 mdxcav-3+/− embryos, together with a massive loss of Pax7-positive cells, further strengthens this conclusion and suggests that increased caveolin-3 levels play an important compensatory role in the degenerative phenotype seen in mdx mice (and potentially in the early stages of DMD).
Correlation between dystrophic mouse phenotypes and the clinical pathology of MD
DMD and LGMD-1c are early onset, progressive skeletal muscle diseases of children affecting cardiac and skeletal muscle function and muscle stability (Hoffman et al., 1987; Minetti et al., 2002). In DMD, there is widespread, progressive malfunction of the entire musculature, abnormal caveolin-3 expression, myoblast apoptosis, cardiomyopathy, and regeneration defects (Cox and Kunkel, 1997; Smith et al., 1995; Vaghy et al., 1998).
Patients with LGMD-1c exhibit similar, but restricted, myopathic changes that particularly affect the muscles of the limb, diaphragm and heart (Galbiati et al., 2001; Hagiwara et al., 2000; Smythe et al., 2003). In most respects, the mdx and cav-3−/− postnatal phenotypes are sufficiently similar to the clinical pathologies of DMD and LGMD to be used widely as disease models (Chamberlain et al., 2007; Chan et al., 2007; Galbiati et al., 2001; Hagiwara et al., 2000; Roig et al., 2004; Vaghy et al., 1998). The embryonic phenotypes of mdx and cav-3−/− mutants strengthen the validity of these mice as models for LGMD and DMD, respectively; provide new insight into the mechanisms underlying MD and the mode of function of dystrophin and caveolin-3; and suggest new approaches to dissecting the differences between the human and murine forms of the disease. We identify important developmental stages, myotome differentiation (E11.5) and secondary myogenesis (E13.5), at which dystrophin and caveolin-3, respectively, play key roles. In addition, we reveal two pathologies in late gestation, fast fibre specification and attrition of Pax7 myoblasts, that provide insight into the mechanism underlying MD pathology, and that suggest routes for therapeutic intervention and earlier diagnosis of MD.
Cardiomyopathy, particularly left ventricular failure, is a significant clinical consequence of DMD and many other MDs, and an established pathology of cav-3−/−, mdx and caveolin-3 overexpressing mice (Aravamudan et al., 2003; Cox and Kunkel, 1997; Hayashi et al., 2004; Quinlan et al., 2004; Woodman et al., 2002; Yue et al., 2003). Dystrophin and caveolin-3 are expressed in the mouse embryonic heart at E9.5 and E10.5, respectively (Biederer et al., 2000; Houzelstein et al., 1992). At E13.5, mdx mice have moderate thickening of the ventricular apex and atrial trabecular defects. In cav-3−/− mutants, there is a severe thickening of the ventricle, as well as atrial defects and a progressively worsening disruption of myocyte organisation that is characteristic of cardiomyopathy and consistent with the early-onset postnatal cardiomyopathy seen in caveolin-3-deficient mice at 3–4 months of age. These data suggest that caveolin-3 is required for normal heart development and has role(s) in cardiomyopathy. β-cardiac myosin is mislocalised in mdx and cav-3−/− embryos at E17.5, and trabeculae are abnormal. β-cardiac myosin defects underlie some familiar cardiomyopathies; therefore, the disrupted expression of β-cardiac myosin in dystrophic hearts is significant (Cuda et al., 1993). Atrial defects have not previously been reported for either mouse. The finding in mdx mice suggests a developmental origin for a recent report of hypertrabeculation in a 28-year-old DMD patient (Finsterer et al., 2005). Although postnatal mdx hearts are reported to have WT levels of β-cardiac myosin and increased levels of utrophin, which may compensate for the dystrophin deficiency, these mice have a progressive cardiomyopathy (Quinlan et al., 2004). Localisation of β-cardiac myosin has not yet been established postnatally; its disrupted atrial localisation could, therefore, persist into the adult or may be lost in the perinatal or juvenile period (Wilding et al., 2005).
Hyperproliferation and elevated muscle cell apoptosis are well-established, widespread features of postnatal MD muscle pathology, which characterise both mouse and human forms of mdx/DMD and cav-3−/−/LGMD-1c, as well as most other MD types (Baghdiguian et al., 1999; Smith et al., 1995; Smith et al., 2000; Smythe et al., 2003). Dystrophin, dystroglycan and caveolin-3 have roles in survival signalling (Glass, 2005; Smythe et al., 2003). In mdx muscles, high levels of myoblast apoptosis and myoblast hyperproliferation are established from 1 week postbirth to adulthood (Smith, 1996; Smith et al., 1995; Spencer et al., 1997), and, in this study, during embryonic stages from E11.5 to E17.5. These data suggest that disrupted stem cell behaviour and apoptosis are an early consequence of loss of dystrophin and an important contributor to the pathology of DMD. Myoblast apoptosis arises later in cav-3−/− mice, at E15.5, suggesting that this phenotype may be an accurate predictor of severity of disease.
New insights: significance for MD
These data offer a new perspective on the aetiology of MD; establish embryonic roles for dystrophin and caveolin-3 that progress our understanding of myogenesis; and suggest that Pax7 myoblast replacement, and therapeutic strategies that seek to modify fibre-type specification, might be productive in the early treatment of MD. Earlier diagnosis and therapeutic intervention are likely to improve the quality of life of MD patients.
METHODS
Mouse models
WT (C57BL/10) and isogenic mdx and cav-3−/− mouse strains were used. cav-3−/− dystrophic mice on a C57BL/10 background were from Yoshito Hagiwara (Tokyo) (Hagiwara et al., 2000). mdx and C57BL/10 mice were generated in-house (Merrick et al., 2007). Double mutant mice (mdxcav-3+/−) were null for dystrophin (mdx) and heterozygous for caveolin-3 (cav-3+/−), and were generated by intercrossing mdx and cav-3−/− mice using a strategy described previously to generate dystrophin-deficient mutants that are heterozygous for an Igf2 transgene (mdxIgf-2+/−) (Smith et al., 2000). Genotyping was achieved by PCR for neomycin (to detect the Cav3 knockout transgene) and caveolin-3 (expressed by WT, mdx and cav-3+/− mice, but not by cav-3−/− mice) (Fig. 7). The data shown are derived from two separate litters, each of E15.5 and E17.5 embryos, containing approximately a 50:50 ratio of mdx and mdxcav-3+/− embryos. Litter size (7–9 embryos per litter) was comparable to those obtained from WT and mdx mice.
Preparation of mouse embryos
Staged WT, cav-3−/−, mdx and mdxcav-3+/− embryos were fixed and processed for paraffin wax embedding, as described previously (Smith and Merrick, 2008). The morning of plug detection was estimated as E0.5. All sections were sagittal (5 μM). Plane and depth of cut were established at the midline of the embryo (bisection point) and by using Kaufman’s atlas of embryology (Kaufman, 1995). Matched WT, cav-3−/−, mdx and mdxcav-3+/− sections from at least three separate embryos per strain were used for all analyses.
Immunohistochemistry
Immunostaining and conditions for pan-myosin (MF20), FMyHC (My32) and Pax7 antibodies have been described previously (Merrick et al., 2007). An extended peroxidase blocking step was included in all staining runs. Secondary antibody controls showed no staining. β-cardiac myosin was detected using the N2.261 antibody (1:1000; DHSB, Iowa City). FMyHC staining was quantified, as shown previously, by counting the proportion of FMyHC-positive myotubes over a fixed area, and analysed using the Student’s t-test and analysis of variance (ANOVA) (Merrick et al., 2007). At least 3000 myotubes were counted for each data point.
Analysis of myotube morphology and quantitation in MF20-stained sections
Sagittally cut, MF20-stained WT, cav-3−/− and mdx embryos were matched for stage, plane and angle of section. To avoid artefacts, we counted only splits and branches that were entirely within the plane of cut; this was carefully controlled between sections. This analysis may underestimate splitting/branching events. Sagittal sections from E13.5, E15.5 and E17.5 embryos were matched carefully against a standard published mouse atlas (Kaufman, 1995), using the midline point of the embryo (to facilitate matching), to determine the location of morphological features. Sections were counted blind, by two separate observers, for both misalignment and branching phenotypes, and were subject to statistical analysis. For each mutant and WT embryo scored, branching was scored over a fixed area using a grid graticule in the same longitudinally presenting muscles in the intercostal, upper and lower limb, and facial muscle regions. Scoring of misaligned fibres was achieved by orienting the direction of the muscle fibres in longitudinal sections to a grid graticule and scoring a fixed area for fibres that deviated by more than a 25 degree angle. We are confident our data are a reliable indicator of splitting/branching: when two experimenters counted slides, the second being unaware of the strain, splitting/branching was only identified in mdx mutants and was statistically significant. For myotube counts, we did not attempt to establish the ends of every myotube (an impossible task in sectioned embryos), but instead counted carefully matched sections of proximal, distal, intercostal and deep back muscles in three different embryos per strain over a fixed area (total myotubes counted per strain: 2000–3000). This enabled us to estimate the proportion of myonuclei to myotubes and the average number of myotubes per section. Data was analysed using the Student’s t-test and ANOVA.
Embryo explants
E11.5–E17.5 embryos were dissected to isolate areas that were rich in skeletal muscle cells. The head, spinal cord and internal organs were removed from all embryos. In older embryos (E15.5–E17.5), the skin and cartilage/bone were also removed. Muscle-rich tissues were microdissected into microexplants and cultured in microwells (Smith and Merrick, 2008; Smith and Schofield, 1994). WT, mdx or cav-3−/− E11.5 explant-conditioned medium (CM) was removed from confluent cultures, filtered (with a 0.2 μm Acrodisc syringe filter; VWR International, UK), replenished with standard media supplements (20% FCS and 2 mM glutamine), and added to fresh E11.5 WT explants before being cultured for 18 days (Smith and Schofield, 1997). 180 explants from three embryos were analysed for each of the three strains included in this study. Data analysis was by ANOVA.
Outgrowth analysis
Outgrowth is a reliable and highly reproducible measure of the growth rate of skeletal muscle explants (Smith and Schofield, 1994). Explants were cultured for 3 weeks and scored according to the level of confluence of cells in each well. Myf5 immunostaining (rabbit anti-Myf5 C-20, 1:5000; Santa Cruz Biotechnology) was used to establish the muscle origin of E11.5–E17.5 WT, mdx and cav-3−/− explant cultures; more than 85% of cells were Myf5 positive (Smith and Merrick, 2008). The secondary antibody controls that were performed with each section always stained negatively. Myf5 C-20 is used extensively to establish myogenicity (Frock et al., 2006; Lindon et al., 1998).
Measurement of apoptosis and proliferation
Confluent explant cultures with myoblast morphological features were subcultured with dispase (Smith and Schofield, 1994) and plated onto coverslips (at 5×103 cells/cm2) for 6 hours before fixation. Apoptotic nuclei cells were stained with 10 μg/ml of DAPI for 3 minutes (Smith et al., 1995). Proliferative cells were immunostained with Ki67 (rabbit anti-Ki67, 1:1000; Novocastra Laboratories, UK), as discussed above. For antigen retrieval (using a pressure cooker), coverslips were first firmly attached onto glass slides using standard paper clips. Three separate experiments were performed in duplicate for each strain (WT, cav-3−/−, mdx).
Isolation of protein and Immunoblotting
Protein was extracted from embryos directly into a glass homogeniser (1–5 ml; VWR International) containing RIPA buffer. Immunoblotting was carried out using standard protocols and detected by ECL (enhanced chemiluminescence) (Pierce Endogen Hyclone). The antibodies used were: fast myosin (My32, 1:1000; Sigma), α-tubulin (1:1000; Sigma), Pax7 (1:1000; Developmental Studies Hybridoma Bank, Iowa City, IA), caveolin-3 (1:1000; Santa Cruz Biotechnology) and goat anti-mouse IgG-HRP (1:2000; Santa Cruz Biotechnology). Protein concentration was determined using an ELISA form of the Bradford assay (Merrick et al., 2007).
Supplementary Material
Acknowledgments
We thank the Wellcome Trust (VS/05/BIR/A8) for funding L.K.J.S. and the BBSRC for their support of D.M. and D.L. The Royal Society (RSRG19484), Muscular Dystrophy Campaign (RA2/592/2) and SPARKS (02BHM04) also supported this work. We thank Yoshito Hagiwara for supplying cav-3−/− mice. Deposited in PMC for release after 6 months. This article is freely accessible online from the date of publication.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
J.S. wrote the paper and designed the study; she also contributed to the photography, interpretation and analysis of data and to the construction of all figures. D.M. contributed data and analysis to Figs 1, 3–6. L.K.J.S. contributed data and analysis to Figs 2, 5, 6. Both D.M. and L.K.J.S. contributed to the writing of the manuscript. D.L. contributed data to Figs 5, 6, 7.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.001008/-/DC1
REFERENCES
- Agbulut O, Noirez P, Beaumont F, Butler-Browne G. (2003). Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell 95, 399–406 [DOI] [PubMed] [Google Scholar]
- Anderson C, Winder SJ, Borycki AG. (2007). Dystroglycan protein distribution coincides with basement membranes and muscle differentiation during mouse embryogenesis. Dev Dyn. 236, 2627–2635 [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Volonte D, Ramani R, Gursoy E, Lisanti MP, London B, Galbiati F. (2003). Transgenic overexpression of caveolin-3 in the heart induces a cardiomyopathic phenotype. Hum Mol Genet. 12, 2777–2788 [DOI] [PubMed] [Google Scholar]
- Armand O, Boutineau AM, Mauger A, Pautou MP, Kieny M. (1983). Origin of satellite cells in avian skeletal muscles. Arch Anat Microsc Morphol Exp. 72, 163–181 [PubMed] [Google Scholar]
- Baghdiguian S, Martin M, Richard I, Pons F, Astier C, Bourg N, Hay RT, Chemaly R, Halaby G, Loiselet J, et al. (1999). Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A. Nat Med. 5, 503–511 [DOI] [PubMed] [Google Scholar]
- Bassett DI, Bryson-Richardson RJ, Daggett DF, Gautier P, Keenan DG, Currie PD. (2003). Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development 130, 5851–5860 [DOI] [PubMed] [Google Scholar]
- Biederer CH, Ries SJ, Moser M, Florio M, Israel MA, McCormick F, Buettner R. (2000). The basic helix-loop-helix transcription factors myogenin and Id2 mediate specific induction of caveolin-3 gene expression during embryonic development. J Biol Chem. 275, 26245–26251 [DOI] [PubMed] [Google Scholar]
- Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. (2007). Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 21, 2195–2204 [DOI] [PubMed] [Google Scholar]
- Chan S, Head S, Morley J. (2007). Branched fibers in dystrophic mdx muscle are associated with a loss of force following lengthening contractions. Am J Physiol Cell Physiol. 293, C985–C992 [DOI] [PubMed] [Google Scholar]
- Cho M, Hughes SM, Karsch-Mizrachi I, Travis M, Leinwand LA, Blau HM. (1994). Fast myosin heavy chains expressed in secondary mammalian muscle fibers at the time of their inception. J Cell Sci. 107, 2361–2371 [DOI] [PubMed] [Google Scholar]
- Cossu G, Tajbakhsh S, Buckingham M. (1996). How is myogenesis initiated in the embryo? Trends Genet. 12, 218–223 [DOI] [PubMed] [Google Scholar]
- Cox GF, Kunkel LM. (1997). Dystrophies and heart disease. Curr Opin Cardiol. 12, 329–343 [PubMed] [Google Scholar]
- Cuda G, Fananapazir L, Zhu WS, Sellers JR, Epstein ND. (1993). Skeletal muscle expression and abnormal function of beta-myosin in hypertrophic cardiomyopathy. J Clin Invest. 91, 2861–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusella-De Angelis MG, Lyons G, Sonnino C, De Angelis L, Vivarelli E, Farmer K, Wright WE, Molinaro M, Bouche M, Buckingham M, et al. (1992). MyoD, myogenin independent differentiation of primordial myoblasts in mouse somites. J Cell Biol. 116, 1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti J, Campbell K. (1993). A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 122, 809–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J, Gelpi E, Stollberger C. (2005). Left ventricular hypertrabeculation/noncompaction as a cardiac manifestation of Duchenne muscular dystrophy under non-invasive positive-pressure ventilation. Acta Cardiol. 60, 445–448 [DOI] [PubMed] [Google Scholar]
- Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. (2006). Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 20, 486–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M, Bermudez J, Pedemonte M, Weidenheim KM, Pestell RG, et al. (2000). Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci USA 97, 9689–9694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Engleman J, Volonte D, Zhang X, Minetti C, Li M, Hou H, Kneitz B, Edelmann W, Lisanti M. (2001). Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and T-tubule abnormalities. J Biol Chem. 276, 21425–21433 [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG. (1990). A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 62, 999–1006 [DOI] [PubMed] [Google Scholar]
- Glass D. (2005). A signalling role for dystrophin: inhibiting skeletal muscle atrophy pathways. Cancer Cell 5, 351–352 [DOI] [PubMed] [Google Scholar]
- Gros J, Scaal M, Marcelle C. (2004). A two-step mechanism for myotome formation in chick. Dev Cell 6, 875–882 [DOI] [PubMed] [Google Scholar]
- Gross MK, Moran-Rivard L, Velasquez T, Nakatsu MN, Jagla K, Goulding M. (2000). Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development 127, 413–424 [DOI] [PubMed] [Google Scholar]
- Hadchouel J, Carvajal JJ, Daubas P, Bajard L, Chang T, Rocancourt D, Cox D, Summerbell D, Tajbakhsh S, Rigby PW, et al. (2003). Analysis of a key regulatory region upstream of the Myf5 gene reveals multiple phases of myogenesis, orchestrated at each site by a combination of elements dispersed throughout the locus. Development 130, 3415–3426 [DOI] [PubMed] [Google Scholar]
- Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T. (2000). Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 9, 3047–3054 [DOI] [PubMed] [Google Scholar]
- Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, Harper HA, Robinson AS, Engelhardt JF, Brooks SV, et al. (2002). Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 8, 253–261 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Arimura T, Ueda K, Shibata H, Hohda S, Takahashi M, Hori H, Koga Y, Oka N, Imaizumi T, et al. (2004). Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 313, 178–184 [DOI] [PubMed] [Google Scholar]
- Hoffman E, Brown R, Kunkel L. (1987). Dystrophin: the protein product of the Duchenne Muscular Dystrophy locus. Cell 51, 919–928 [DOI] [PubMed] [Google Scholar]
- Hollway GE, Currie PD. (2003). Myotome meanderings: cellular morphogenesis and the making of muscle. EMBO Rep. 4, 855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzelstein D, Lyons GE, Chamberlain J, Buckingham ME. (1992). Localization of dystrophin gene transcripts during mouse embryogenesis. J Cell Biol. 119, 811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Poy F, Zhang R, Joachimiak A, Sudol M, Eck M. (2000). Structure of a WW domain-containing fragment of dystrophin complexed with beta -dystroglycan. Nat Struct Biol. 7, 634–638 [DOI] [PubMed] [Google Scholar]
- Ilsley JL, Sudol M, Winder SJ. (2002). The WW domain: linking cell signalling to the membrane cytoskeleton. Cell Signal. 14, 183–189 [DOI] [PubMed] [Google Scholar]
- Jung D, Yang B, Meyer J, Chamberlain J, Campbell K. (1995). Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem. 270, 27305–27310 [DOI] [PubMed] [Google Scholar]
- Kaufman M. (1995. The Atlas of Mouse Development. 2nd edn London: Academic Press [Google Scholar]
- Lehmann R. (2001). Cell migration in invertebrates: clues from border and distal tip cells. Curr Opin Genet Dev. 11, 457–463 [DOI] [PubMed] [Google Scholar]
- Lindon C, Montarras D, Pinset C. (1998). Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J Cell Biol. 140, 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D, Ting T, Stadler L, Smith J. (2007). A role for Insulin-like growth factor 2 in specification of the fast skeletal muscle fibre. BMC Dev Biol. 7, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C, Bado M, Broda P, Sotgia F, Bruno C, Galbiati F, Volonte D, Lucania G, Pavan A, Bonilla E, et al. (2002). Impairment of caveolae formation and T-system disorganization in human muscular dystrophy with caveolin-3 deficiency. Am J Pathol. 160, 265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ, Wegner J, Ferguson C, Mery PF, Hancock JF, Currie PD, Key B, Westerfield M, Parton RG. (2005). Zebrafish as a model for caveolin-associated muscle disease; caveolin-3 is required for myofibril organization and muscle cell patterning. Hum Mol Genet. 14, 1727–1743 [DOI] [PubMed] [Google Scholar]
- Ohlendieck K, Matsumura K, Ionasescu VV, Towbin JA, Bosch EP, Weinstein SL, Sernett SW, Campbell KP. (1993). Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology 43, 795–800 [DOI] [PubMed] [Google Scholar]
- Ott MO, Bober E, Lyons G, Arnold H, Buckingham M. (1991). Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development 111, 1097–1107 [DOI] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. (2004). Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 23, 3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Campos I, Hirst EM, Stemple DL. (2002). Removal of dystroglycan causes severe muscular dystrophy in zebrafish embryos. Development 129, 3505–3512 [DOI] [PubMed] [Google Scholar]
- Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS. (2004). Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 14, 491–496 [DOI] [PubMed] [Google Scholar]
- Razani B, Park DS, Miyanaga Y, Ghatpande A, Cohen J, Wang XB, Scherer PE, Evans T, Lisanti MP. (2002). Molecular cloning and developmental expression of the caveolin gene family in the amphibian Xenopus laevis. Biochemistry 41, 7914–7924 [DOI] [PubMed] [Google Scholar]
- Reimann J, Irintchev A, Wernig A. (2000). Regenerative capacity and the number of satellite cells in soleus muscles of normal and mdx mice. Neuromuscul Disord. 10, 276–282 [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. (2006). Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 172, 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig M, Roma J, Fargas A, Munell F. (2004). Longitudinal pathologic study of the gastrocnemius muscle group in mdx mice. Acta Neuropathol. 107, 27–34 [DOI] [PubMed] [Google Scholar]
- Roma J, Munell F, Fargas A, Roig M. (2004). Evolution of pathological changes in the gastrocnemius of the mdx mice correlate with utrophin and beta-dystroglycan expression. Acta Neuropathol. 108, 443–452 [DOI] [PubMed] [Google Scholar]
- Schofield J, Houzelstein D, Davies K, Buckingham M, Edwards YH. (1993). Expression of the dystrophin-related protein (utrophin) gene during mouse embryogenesis. Dev Dyn. 198, 254–264 [DOI] [PubMed] [Google Scholar]
- Schofield JN, Gorecki DC, Blake DJ, Davies K, Edwards YH. (1995). Dystroglycan mRNA expression during normal and mdx mouse embryogenesis: a comparison with utrophin and the apo-dystrophins. Dev Dyn. 204, 178–185 [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786 [DOI] [PubMed] [Google Scholar]
- Shin DH, Kim JS, Kwon BS, Lee KS, Kim JW, Kim MH, Cho SS, Lee WJ. (2003). Caveolin-3 expression during early chicken development. Brain Res Dev Brain Res. 141, 83–89 [DOI] [PubMed] [Google Scholar]
- Smith J. (1996). Muscle Growth factors, ubiquitin and apoptosis in dystrophic muscle: apoptosis declines with age in the mdx mouse. Basic Appl Myol. 6, 279–284 [Google Scholar]
- Smith J, Schofield PN. (1994). The effects of fibroblast growth factors in long-term primary culture of dystrophic (mdx) mouse muscle myoblasts. Exp Cell Res. 210, 86–93 [DOI] [PubMed] [Google Scholar]
- Smith J, Schofield PN. (1997). Stable integration of an mdx skeletal muscle cell line into dystrophic (mdx) skeletal muscle: evidence for stem cell status. Cell Growth Differ. 8, 927–934 [PubMed] [Google Scholar]
- Smith J, Merrick D. (2008. Embryonic Skeletal Muscle Micro-Explant Culture and Isolation of Skeletal Muscle Stem Cells. Totowa, NJ: Humana Press; [DOI] [PubMed] [Google Scholar]
- Smith J, Fowke G, Schofield P. (1995). Programmed cell death in dystrophic (mdx) muscle is inhibited by IGF-II. Cell Death Differ. 2, 243–251 [PubMed] [Google Scholar]
- Smith J, Goldsmith C, Ward A, LeDieu R. (2000). IGF-II ameliorates the dystrophic phenotype and coordinately down-regulates programmed cell death. Cell Death Differ. 7, 1109–1118 [DOI] [PubMed] [Google Scholar]
- Smythe GM, Eby JC, Disatnik MH, Rando TA. (2003). A caveolin-3 mutant that causes limb girdle muscular dystrophy type 1C disrupts Src localization and activity and induces apoptosis in skeletal myotubes. J Cell Sci. 116, 4739–4749 [DOI] [PubMed] [Google Scholar]
- Sotgia F, Lee J, Das K, Bedford M, Petrucci T, Macioce P, Sargiacomo M, Dagna Bricarelli F, Minetti C, Sudol M, et al. (2000). Caveolin-3 directly interacts with the C-terminal tail of β-dystroglycan. J Biol Chem. 275, 38048–38058 [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Walsh CM, Dorshkind KA, Rodriguez EM, Tidball JG. (1997). Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J Clin Invest. 99, 2745–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghy P, Frang J, Wu W, Vaghy L. (1998). Increased caveolin-3 levels in mdx mouse muscles. FEBS Lett. 431, 125–127 [DOI] [PubMed] [Google Scholar]
- Volonte D, Peoples AJ, Galbiati F. (2003). Modulation of myoblast fusion by caveolin-3 in dystrophic skeletal muscle cells: implications for Duchenne muscular dystrophy and limb-girdle muscular dystrophy-1C. Mol Biol Cell 14, 4075–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C, Silberstein L, Hays AP, Blau HM. (1988). Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52, 503–513 [DOI] [PubMed] [Google Scholar]
- Weir AP, Morgan JE, Davies KE. (2004). A-utrophin up-regulation in mdx skeletal muscle is independent of regeneration. Neuromuscul Disord. 14, 19–23 [DOI] [PubMed] [Google Scholar]
- Wilding J, Jürgen E, Schneider A, Sang E, Davies K, Neubauer S, Clarke K. (2005). Dystrophin- and MLP-deficient mouse hearts: marked differences in morphology and function, but similar accumulation of cytoskeletal proteins. FASEB J. 19, 79–81 [DOI] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. (1997). Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet. 6, 831–841 [DOI] [PubMed] [Google Scholar]
- Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, et al. (2002). Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 277, 38988–38997 [DOI] [PubMed] [Google Scholar]
- Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D. (2003). Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation 108, 1626–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. (2006). The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 54, 1177–1191 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.