Abstract
Objectives:
Determine the utility of optical coherence tomography (OCT) to detect clinical and subclinical remote optic neuritis (ON), its relationship to clinical characteristics of ON and visual function, and whether the retinal nerve fiber layer (RNFL) thickness functions as a surrogate marker of global disease severity.
Methods:
Cross-sectional study of 65 subjects with at least 1 clinical ON episode at least 6 months prior. Measures included clinical characteristics, visual acuity (VA), contrast sensitivity (CS), OCT, and visual evoked potentials (VEP).
Results:
Ninety-six clinically affected optic nerves were studied. The sensitivity of OCT RNFL after ON was 60%, decreasing further with mild onset and good recovery. VEP sensitivity was superior at 81% (p = 0.002). Subclinical ON in the unaffected eye was present in 32%. VEP identified 75% of all subclinically affected eyes, and OCT identified <20%. RNFL thickness demonstrated linear correlations with VA (r = 0.65) and CS (r = 0.72) but was unable to distinguish visual categories <20/50. RNFL was thinner with severe onset and disease recurrence but was unaffected by IV glucocorticoids. OCT measurements were not related to overall disability, ethnicity, sex, or age at onset. The greatest predictor for RNFL in the unaffected eye was the RNFL in the fellow affected eye.
Conclusions:
Visual evoked potentials (VEP) remains the preferred test for detecting clinical and subclinical optic neuritis. Optical coherence tomography (OCT) measures were unrelated to disability and demographic features predicting a worse prognosis in multiple sclerosis. OCT may provide complementary information to VEP in select cases, and remains a valuable research tool for studying optic nerve disease in populations.
GLOSSARY
- ANOVA
= analysis of variance;
- CIS
= clinically isolated syndrome;
- CS
= contrast sensitivity;
- EDSS
= Expanded Disability Status Score;
- logMAR
= logarithm of the minimum angle of resolution;
- MS
= multiple sclerosis;
- MSSS
= Multiple Sclerosis Severity Score;
- NCRR
= National Center for Research Resources;
- NMO
= neuromyelitis optica;
- NS
= not significant;
- OCT
= optical coherence tomography;
- ON
= optic neuritis;
- RNFL
= retinal nerve fiber layer;
- VA
= visual acuity;
- VEP
= visual evoked potentials.
Optical coherence tomography (OCT) uses infrared light reflected from retinal subsurfaces to measure the retinal nerve fiber layer (RNFL) thickness via optical interferometry.1–3 Optic nerve axonal count and RNFL thickness have been correlated by histopathology.4 Optic neuritis (ON) has been an established etiology of optic nerve injury that contributes to a reduced RNFL after 6 months.5 Optic atrophy and RNFL thinning have been noted in many patients with multiple sclerosis (MS).6–8
OCT has been established as a sensitive technique for detecting optic nerve injury within a population. It may serve as an objective measure of axonal injury after ON, and may reflect subclinical disease or progressive axonal attrition in the clinically unaffected eye.9,10 The degree of RNFL thinning correlates with decreases in visual function, overall disability, and brain atrophy.11–17
The main objective of this study was to explore the utility of OCT in clinical practice, as it might be used for the individual patient. We hypothesized that OCT would provide objective confirmation after a clinical episode of ON and could reveal subclinical optic nerve involvement for confirmation of CNS dissemination. RNFL thickness may provide useful information about the disease process and function of the optic nerve as it relates to visual outcome categories, recurrent episodes, the severity of vision loss during the acute phase of ON, and the use of IV glucocorticoids. A thinned RNFL might reflect the severity of disease affecting the entire CNS as assessed by overall disability and demographic factors associated with prognosis.
METHODS
This study was approved by the local Human Research Protection Office/Institutional Review Board, and all subjects provided informed consent. Subjects were aged 18–65 years and had a clinical history of ON in at least one eye, 6 months or more before enrollment. Those with other ocular pathologies were excluded. Subjects were preferentially recruited to have had ON with severe onset (≤20/200) and poor recovery (<20/40) to evaluate OCT throughout the range of visual function.
Visual acuity (VA) was measured by a Snellen 20-foot wall chart. Contrast sensitivity (CS) was measured by a Pelli–Robson chart at 1 meter (Metropia Ltd., Cambridge, UK). Best vision was obtained with prescription glasses and pinhole occluder. OCT measurements of fast RNFL thickness were obtained by a trained technician on a Zeiss Stratus OCT III with version 4.0 software, using a signal strength of ≥5. In eyes with poor visual function, OCT was obtained by external fixation of the “good eye” as the technician ensured scan quality. Normal RNLF thickness for adults is 100.1 ± 11.6 μm (n = 328).18 The RNFL thickness cutoff was based on published reference values, which comprise the built-in Zeiss Stratus OCT database, using 2 SD below the normal mean, stratified by age. Thus, average RNFL thickness cutoffs for defining abnormal were 84.3 μm for ages 18–29 years, 83.9 μm for 30–39 years, 75.5 μm for 40–49 years, 74.0 μm for 50–59 years, and 75.3 μm for 60–65 years. The established reference for the temporal RNFL quadrant thickness is 69.0 ± 12.7 μm,18 and a cutoff of 44 μm was defined as abnormal. Visual evoked potential (VEP) P100 latencies (normal mean 98.95 msec; upper limit, also defined by 2 SD, was 112.9 msec) were read blinded. If the waveform was unobtainable because of poor vision, the value of 170 msec was used, representing the most prolonged obtainable waveform for this machine. The nadir VA during the acute phase of the ON was determined from chart review when available. If unavailable, the onset nadir VA was classified as severe if the subject “could not recognize a spouse or loved-one at conversation distance in front of them.”
The McNemar test was used to compare whether dichotomous proportions were equivalent. Analysis of variance (ANOVA) was used for comparisons of RNFL across multiple outcome categories, along with a post hoc t test for intergroup comparisons. Linear regression modeling was used to determine predictors of RNFL in the unaffected eye. To create clustered box plots, visual acuity was categorized based on the Ranges of Vision Loss by the International Council of Ophthalmology (normal, ≥0.80; mild, 0.67 to 0.3; moderate, 0.2 to 0.125; severe, <0.125 to 0.05; profound, <0.05).19 Analyses of disability included Spearman correlations for scales and ANOVA for categories of disease.
RESULTS
Subject characteristics.
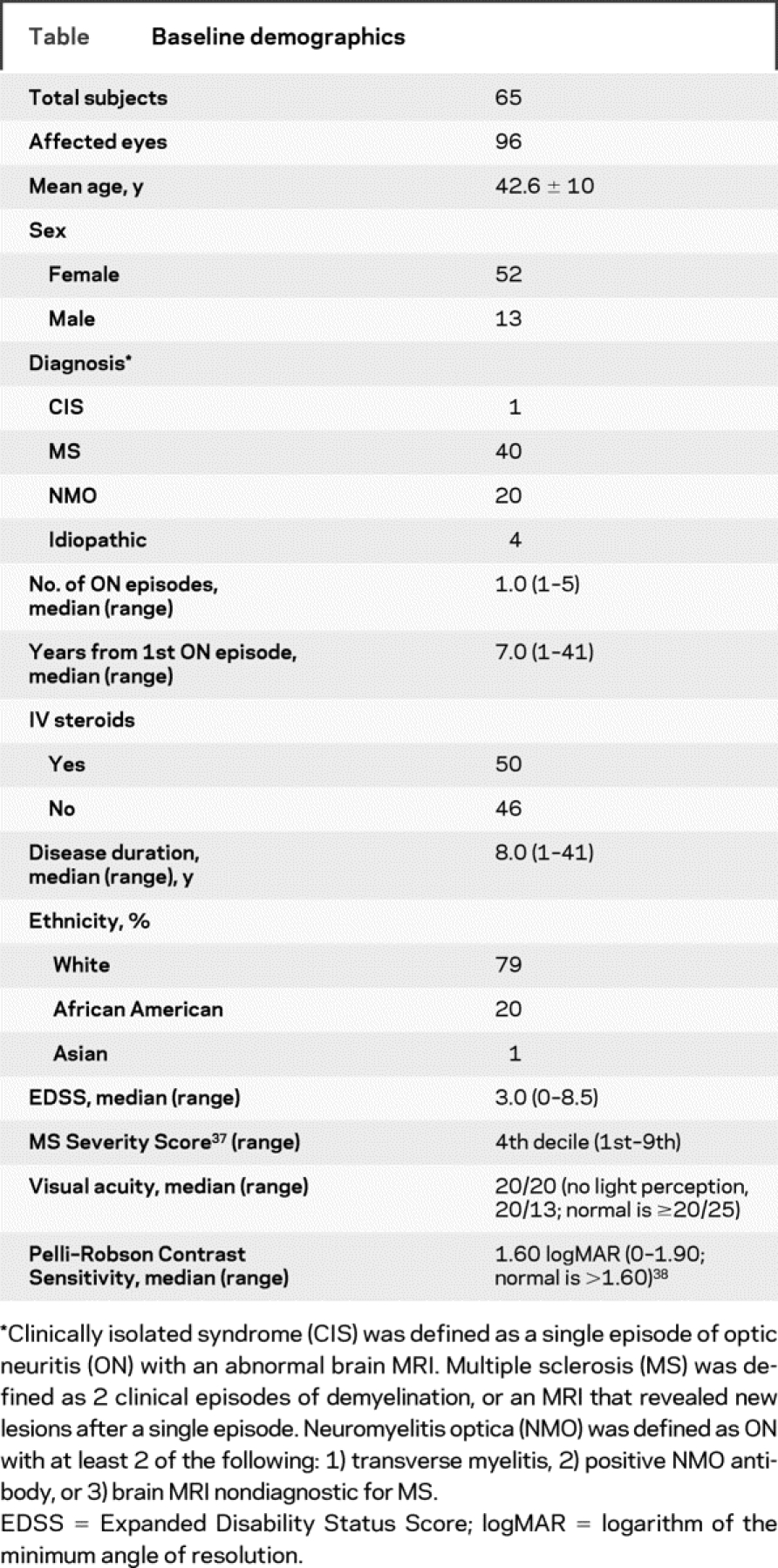
Sixty-five subjects provided 96 eyes clinically affected by ON. Baseline demographics are summarized in the table.
Table Baseline demographics
OCT had moderate sensitivity to detect optic nerve changes within the individual clinically affected eye but was inferior to VEPs.
After at least 1 episode of clinical ON (n = 96), 60% had abnormal average RNFL thinning, 60% had an abnormal temporal quadrant RNFL, and 81% had an abnormal VEP. VEP was the superior test when compared with the average RNFL thickness (McNemar test, p = 0.002). Of those who recovered to an acuity ≥20/25 (n = 55), 35% had an abnormal average RNFL thickness, 42% had an abnormal temporal field RNFL thickness, and 72% had an abnormal VEP. Again, VEP performed better for this subgroup with good recovery (p = 0.002).
After an episode of mild or moderate ON (n = 33), defined by the vision nadir during the acute setting, 27% had an abnormal RNFL thickness, 39% had an abnormal temporal RNFL thickness, and 68% had an abnormal VEP. VEP was superior to OCT for those with mild to moderate ON at onset (p = 0.007). For those with a mild to moderate nadir and recovery to ≥20/25 (n = 25), VEP was favored over OCT (p = 0.006). In this subgroup, 16% had an abnormal average RNFL thickness, 28% had an abnormal temporal RNFL thickness, and 65% had an abnormal VEP.
After an episode of severe ON (n = 66), 77% had an abnormal average RNFL thickness, 70% had an abnormal temporal RNFL thickness, and 88% had an abnormal VEP. After severe ON, OCT and VEP perform similarly (p = 0.14). For those with a severe nadir who recovered to ≥20/25 (n = 30), 50% were abnormal by average RNFL thickness, 53% were abnormal by temporal RNFL thickness, and 85% were abnormal by VEP (p = 0.14).
OCT detected fewer optic nerve changes in the clinically unaffected eye in comparison with VEPs.
To identify subclinical ON in the unaffected eye, the following scenarios were used: 1) an abnormal VEP P100, 2) an abnormal average RNFL thickness, or 3) CS >2 SD from the mean (≤1.60 logarithm of the minimum angle of resolution). Of 34 unaffected eyes, 6% had an abnormal RNFL, 24% had an abnormal VEP, and 12% had abnormal CS. Using the 3 combined definitions for subclinical disease, 32% were abnormal. Of the unaffected eyes with an abnormal VEP or OCT, 86% were identified by VEP alone, and 14% were identified by OCT alone. Of the subclinically affected eyes with an abnormal OCT or CS, 100% were identified with CS, and 0% were identified by OCT. Defining subclinical ON by an abnormal VEP and CS, 72% were detected by VEP alone, 14% were detected by CS alone, and 14% overlapped.
Neither OCT nor VEP discriminated among clinically defined categories of visual loss.
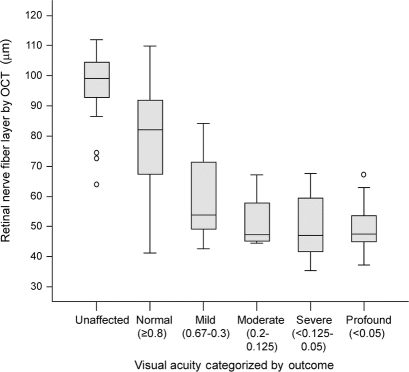
A thinning RNFL demonstrated a strong linear correlation with VA (Spearman rho = 0.65, p < 0.001). Additionally, RNFL thinning displayed a strong linear trend after categorizing VA severity as suggested by the International Council of Ophthalmology into normal (≥20/25), mild (20/30 to 20/60), moderate (20/70 to 20/100), severe (20/200 to 20/400), and profound (≤20/800) (figure 1; ANOVA, p < 0.001). However, comparing individual categories revealed a floor effect for RNFL measures, with an inability to differentiate between mild, moderate, severe, and profound categories. OCT discriminated between categorizations of VA for unaffected (n = 31, 97.1 ± 11.3 μm) vs normal recovery (n = 58, 79.9 ± 17.3 μm) (p < 0.001) and normal vs mild recovery (n = 12, 60.0 ± 13.9 μm) (p < 0.001). Conversely, OCT could not discriminate between mild vs moderate recovery (n = 4, 51.5 ± 10.5 μm) (p = not significant [NS]), moderate vs severe recovery (n = 8, 49.9 ± 11.7 μm) (p = NS), or severe vs profound recovery (n = 14, 49.6 ± 8.5 μm) (p = NS).
Figure 1 Box plot of retinal nerve fiber layer thickness categorized by visual acuity outcome
Optical coherence tomography (OCT) is able to distinguish unaffected from normal and normal from mild, but not mild from moderate, severe, or profound. Note that the median value is approximately 50 μm, starting from the mild category through the profound.
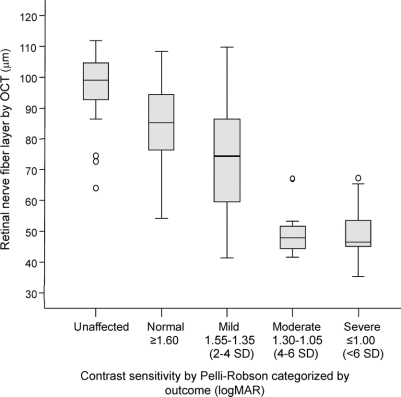
Similarly, RNFL thickness demonstrated a strong linear correlation with CS (rho = 0.72, p < 0.001) and a strong linear trend after categorizing CS based on standard deviations from the normal values (figure 2; p < 0.001). The RNFL was able to discriminate CS of unaffected (n = 29, 96.8 ± 11.6 μm) vs normal recovery (n = 39, 84.3 ± 13.5 μm) (p < 0.001) and normal vs mild recovery (n = 26, 74.6 ± 18.3 μm) (p < 0.001), but not mild vs moderate (n = 12, 50.1 ± 8.6 μm) (p = NS) or moderate vs severe recovery (n = 21, 48.6 ± 8.7 μm) (p = NS).
Figure 2 Box plot of retinal nerve fiber layer thickness categorized by contrast sensitivity outcome
Optical coherence tomography (OCT) is able to distinguish unaffected from normal and normal from mild, but not mild from moderate or severe. LogMAR = logarithm of the minimum angle of resolution.
VEP P100 also demonstrated a linear correlation with VA (rho = −0.67, p < 0.001) but was also unable to discriminate between all individual categories of VA loss. Hence, distinction between VA categories was noted between unaffected (n = 22, 106.7 ± 9.3 msec) vs normal (n = 49, 127.6 ± 21.3 msec) (p < 0.001), but not normal vs mild (n = 11, 138.0 ± 22.3 msec) (p = NS), mild vs moderate (n = 3, 144 ± 23.6 msec) (p = NS), moderate vs severe (n = 4, 170 ± 0 msec) (p = NS), or severe vs profound (n = 13, 170 ± 0 msec) (p = NS). Likewise, VEP P100 was strongly correlated with CS (Spearman rho = −0.69, p < 0.001), but was only able to distinguish CS categories of unaffected (n = 20, 105.9 ± 9.2 msec) vs normal (n = 32, 123.0 ± 17.1) (p < 0.001) and moderate (n = 9, 141.4 ± 20.9 msec) vs severe (n = 18, 170.0 ± 0 msec) (p < 0.001). For CS, VEP did not distinguish normal vs mild (n = 23, 132.7 ± 24.2) (p = NS) and mild vs moderate (p = NS).
Severe acute ON is associated with a thinner RNFL remotely.
After a single attack of ON, the severity of VA nadir was a predictor of population mean RNFL thickness after 6 months (ANOVA, p = 0.02). Although OCT could not distinguish mild onset (n = 13, 82.5 ± 19.1 μm) from moderate (n = 14, 78.0 ± 19.6 μm), it did distinguish the moderate from the severe group (n = 37, 66.4 ± 20.0 μm) (p = 0.03) and mild from severe (p = 0.02). Even among those who recovered to a “normal” VA of ≥20/25, the RNFL at 6 months remained thinner for the severe onset group vs mild or moderate onset groups (ANOVA, p = 0.02).
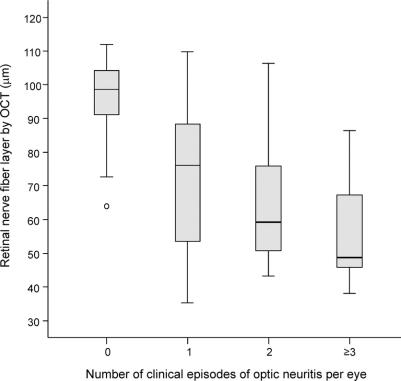
Recurrent clinical episodes of ON were also associated with a linear trend in thinning mean RNFL (figure 3; ANOVA, p < 0.001).
Figure 3 Box plot of retinal nerve fiber layer changes with repeated episodes of optic neuritis
Discrete recurrence of optic nerve inflammation was associated with a decremental decline in the retinal nerve fiber layer. OCT = optical coherence tomography.
RNFL thickness was not altered by the use of glucocorticoids.
Treatment with IV glucocorticoids, consisting of at least 3 days of methylprednisolone at 1,000 mg or an equivalent IV preparation of dexamethasone, did not seem to impact the mean RNFL for those eyes with a single episode of ON (figure 4). Even after stratifying for the nadir VA at onset, no treatment effect on RNFL was noted for the subgroup of mild to moderate nadir (n = 17, 79.8 ± 18.1 μm for untreated vs n = 9, 81.5 ± 21.6 μm for treated) (p = 0.8) or for the severe nadir group (n = 17, 65.7 ± 16.5 μm for untreated vs n = 18, 68.0 ± 23.9 μm for treated) (p = 0.7).
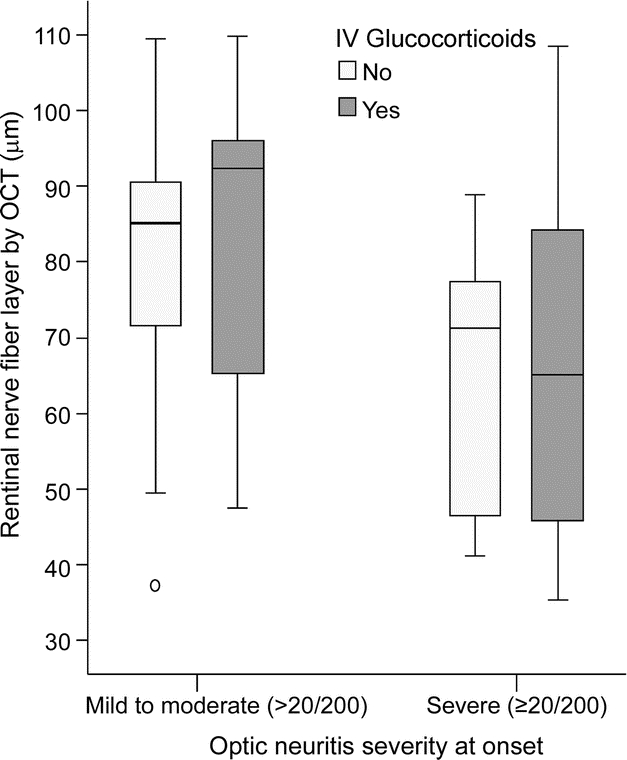
Figure 4 Box plot comparing retinal nerve fiber layer thickness categorized by use of IV glucocorticoids
The nadir acuity during the acute episode was stratified to prevent a bias toward a preferential use of IV steroids for those with severe clinical disease. Only first episodes of optic neuritis were analyzed. There was no difference in retinal nerve fiber layer thickness with glucocorticoids for the mild/moderate or severe groups. OCT = optical coherence tomography.
RNFL thickness was not related to overall disability in MS.
In the subjects with MS within this cohort, no correlations of RNFL measurements to Expanded Disability Status Score (EDSS) or Multiple Sclerosis Severity Score (MSSS) were observed. All permutations of analyzing a single eye within each individual were examined, including all left eyes or all right eyes (n = 46), the clinically affected eyes on either side (right, n = 33; left, n = 32), or the unilateral unaffected eyes (n = 28). Likewise, no significant relationship of RNFL to clinical severity was observed when subjects were divided into 3 or 4 EDSS categories consisting of mild (0–3.0), moderate (3.5–5.5), and severe (6.0–9.5) or consisting of normal (0–0.5), mild (1.0–3.0), moderate (3.5–5.5), and severe (6.0–9.5).
OCT measurements were not related to ethnicity, sex, or age at ON onset.
No significant difference was observed using OCT between Caucasian (n = 72) and African American (n = 19) eyes with at least a single episode of ON, whether taken as a group or stratified according to visual function. Likewise, no significant difference was seen between women (n = 73) and men (n = 20). Finally, the age at which ON occurred, or whether it occurred as the initial event or after the clinical onset of MS bore no relationship to OCT measures.
RNFL thickness in the unaffected eye was predicted by the fellow affected eye.
A linear regression model was created to explore predictors of RNFL measures in the unaffected eye. RNFL thickness in the affected fellow eye was the single greatest predictor, explaining 20% of the variability (r = 0.44, p = 0.01). Other predictors of a decreased RNFL thickness in the unaffected eye included increased time from ON in the fellow affected eye and having a diagnosis of MS. Together, these 3 predictors explained 32% of the variability in the unaffected RNFL (p = 0.02). Age, sex, ethnicity, EDSS, and MSSS were not predictive of RNFL thickness in the unaffected eye.
DISCUSSION
OCT is a quick and noninvasive test that may reveal important clinical information for individual care. Potential uses include detection of clinical and subclinical ON, assessment of risk of future visual disability, and use as a surrogate marker of axonal involvement in the entire CNS. There is a need to understand the utility and limitations of OCT in clinical practice.
VEP is the current test of choice for confirming clinical and subclinical ON. After a clinical episode of ON, the sensitivity of a prolonged VEP P100 latency is reported to be 60% to 80%.20 In this cohort, after confirmed clinical ON, VEP was abnormal in 81%, OCT was abnormal in 60%, and either was abnormal in 83%. Of those abnormal with either test, OCT detected 7% not identified by VEP, and VEP found 28% undetected by OCT. In addition, the sensitivity of OCT decreased when the visual nadir was not severe (27%) or if there was good recovery (35%). Although the maculopapular bundle is preferentially affected in ON, focusing on the temporal quadrant did not increase the sensitivity. In contrast to OCT, VEP maintained a relatively high sensitivity in cases with mild to moderate visual nadir (68%) or after good recovery (72%). In clinical situations, VEP seems to have an advantage, particularly in detecting past mild ON.
A diagnosis of MS or neuromyelitis optica requires dissemination within the CNS, and OCT may help to identify subclinical optic nerve abnormalities. In demyelinating diseases, VEP detected injury in 24% of clinically unaffected optic nerves, similar to the reported value of 35%.20 OCT was less sensitive in our study and detected only 14% of all subclinically affected nerves missed by VEP. Neither OCT nor VEP is specific for ON or MS, and should be used as complementary tests for diagnosis. OCT is not uniform among people, because a 2-μm RNFL loss occurs with each decade, and the RNFL is thinner with increased orbit axial length and reduced optic disk area.18
Severe acute ON has a high probability for good recovery.21 In this study, those with severe early visual loss were at risk for greater nerve injury, even with recovery back to “normal” vision. Despite preserved central acuity, self-reported visual dysfunction in established MS can be high, leading to a decreased quality of life.22–24 Several severe and recurrent ON episodes, even with recovery, may lead to a reduced compensatory reserve.
Unfortunately, the early use of IV glucocorticoids did not improve eventual RNFL thickness. The fate for demyelination and axonal injury seems to be set at onset of ON with limited treatment options to change the course. Thus, the best current strategy for reducing future episodes of ON and visual disability seems to be through preventative treatment with disease-modifying therapies.25–27
Although OCT is a sensitive tool for differentiating populations with and without ON, it does not differentiate well among visual recovery groups. RNFL quickly reaches maximal atrophy, even with relative preservation of central vision. With vision <20/50, the RNFL thickness approaches a bottom value, suggesting that some RNFL structural elements, such as glial tissue, may not be susceptible to thinning.28,29 All subjects with vision ≤20/100 were associated with an RNFL measurement under 70 μm, with a median of approximately 50 μm.
In contrast to published reports,11,16,17 we did not observe a correlation of RNFL thickness with EDSS and MSSS. Prior episodes of optic neuritis may have obscured any correlations. Demographic variables of unfavorable prognosis in MS, including male sex, older age onset, and African American ethnicity, were not associated with a thinner RNFL.30,31
A reduced RNFL in the clinically unaffected optic nerve may be due to inflammation in the chiasm or optic tract of the clinically affected fellow eye, subclinical discrete episodes of ON, or accumulating axonal loss distinct from inflammatory episodes. In support of the first mechanism, clinical ON in the fellow eye was the single best predictor. Duration of time from ON onset in the fellow eye was the second best predictor, supporting the second and third mechanisms. The third mechanism would predict axonal loss throughout the CNS and a worse EDSS. However, a significant relationship of RNFL thickness with overall disability was not observed.
A limitation of this study was the omission of perimetry, because the World Health Organization incorporates visual fields within the definition of visual impairment. Although ON will predominantly affect central vision, we may have misclassified some subjects as having good vision based on central acuity, whereas peripheral fields might show significant visual impairment. Also, perimetry may be a useful tool for diagnosis in select individuals, although unlikely to be more sensitive than OCT or VEP.15,32 Although VEP P100 amplitude correlated with RNFL thickness (r = 0.43, p = 0.01),33–35 the amplitude ratio was not used as diagnostic criteria because of many subjects having bilateral disease and because of a low sensitivity in detecting ON by using unilateral amplitude (reference 8.3 ± 3.3 mV).
Only 2 of our original hypotheses proved true, that severe onset ON and repeated episodes predict a lower mean RNFL thickness. It was disappointing that IV glucocorticoids provided no discernible benefit on RNFL outcome, consistent with no statistically significant clinical benefit of IV glucocorticoids in ON.36 OCT at a single time point seems to have a limited role in detecting optic nerve injury within individuals. This finding should not preclude longitudinal OCT studies, because progressive RNFL thinning may be more predictive of disability. Despite limitations in the clinical setting, OCT may be a relevant surrogate endpoint in trials of neuroprotective agents and remains a valuable research tool.
DISCLOSURE
R.T.N. is a participant in clinical trials for Fampridine SR by Acorda Therapeutics; has received consulting fees and speaking honoraria from Bayer Healthcare, Biogen Idec, Elan Pharmaceuticals, and Teva Neurosciences; and receives research support from the NIH and National Multiple Sclerosis Society. N.T.T. reports no disclosures. J.X. reports no disclosures. J.B.S. has received speaking honoraria from Pfizer Pharmaceuticals. E.C.K. has received speaking honoraria from Teva Neurosciences. S.-K.S. reports no disclosures. A.H.C. has received research funding from the NIH, National Multiple Sclerosis Society USA, and Consortium of Multiple Sclerosis Centers; clinical trial funding from Acorda Therapeutics, Bayer Healthcare, BioMS, and Teva Neuroscience; and speaking honoraria and consulting fees from Bayer Healthcare, Biogen Idec, Genentech, Inc., Teva Neurosciences, Serono, and Pfizer.
Address correspondence and reprint requests to Dr. Robert T. Naismith, Neurology, Box 8111, 660 S. Euclid Ave., St. Louis, MO 63110 naismithr@neuro.wustl.edu
NIH funding included K23NS052430-01A1 (R.T.N.), K12RR02324902 (R.T.N.), UL1RR024992 (E.C.K.), K24 RR017100 (A.H.C.), and CA1012 (A.H.C.). National Multiple Sclerosis Society funding included FG1782A1 (J.X.), CA1012 (A.H.C., S.-K.S.), and RG 3670 (S.-K.S.). A.H.C. was supported in part by the Manny and Rosalyn Rosenthal–Dr. John L. Trotter Chair in Neuroimmunology. American Academy of Neurology Foundation Clinical Research Training Fellowship (E.C.K.).
This publication was made possible by grant UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Disclosure: Author disclosures are provided at the end of the article.
Received December 18, 2008. Accepted in final form April 1, 2009.
REFERENCES
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991;254:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toth CA, Narayan DG, Boppart ST, et al. A comparison of retinal morphology viewed by optical coherence tomography and by light microscopy. Arch Ophthalmol 1997;115:1425–1428. [DOI] [PubMed] [Google Scholar]
- 3.Chen TC, Cense B, Miller JW, et al. Histologic correlation of in vivo optical coherence tomography images of the human retina. Am J Ophthalmol 2006;141:1165–1168. [DOI] [PubMed] [Google Scholar]
- 4.Yucel YH, Gypta N, Kalichman MW, et al. Relationship of optic disc topography to optic nerve fiber number in glaucoma. Arch Opthalmol 1998;116:493–497. [DOI] [PubMed] [Google Scholar]
- 5.Costello F, Hodge W, Pan Y, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler 2008;14:893–905. [DOI] [PubMed] [Google Scholar]
- 6.Kerrison JB, Flynn T, Green R. Retinal pathologic changes in multiple sclerosis. Retina 1994;14:445–451. [DOI] [PubMed] [Google Scholar]
- 7.Elbol P, Work K. Retinal nerve fiber layer in multiple sclerosis. Acta Ophthalmol 1990;68:481–486. [DOI] [PubMed] [Google Scholar]
- 8.Frisen LF, Boyt WF. Insidious atrophy of retinal nerve fibers in multiple sclerosis. Arch Opthalmol 1974;92:91–97. [DOI] [PubMed] [Google Scholar]
- 9.Naismith RT, Tutlam NT, Xu J, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology 2009;72:1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naismith RT, Xu J, Tutlam NT, et al. Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology 2009;72:589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006;113:324–332. [DOI] [PubMed] [Google Scholar]
- 12.Steel DH, Waldock A. Measurement of the retinal nerve fibre layer with the scanning laser polarimetry in patients with previous demyelinating optic neuritis. J Neurol Neurosurg Psychiatry 1998;64:505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 2007;69:2085–2092. [DOI] [PubMed] [Google Scholar]
- 14.Pueyo V, Martin J, Fernandez J, et al. Axonal loss in the retinal nerve fiber layer in patients with multiple sclerosis. Mult Scler 2008;14:609–614. [DOI] [PubMed] [Google Scholar]
- 15.Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 2006;59:963–969. [DOI] [PubMed] [Google Scholar]
- 16.Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 2007;69:1603–1609. [DOI] [PubMed] [Google Scholar]
- 17.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia–Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 2007;68:1488–1494. [DOI] [PubMed] [Google Scholar]
- 18.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology 2007;114:1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resolution Adopted by the International Council of Ophthalmology, Sydney, Australia, April 20, 2002. Available at: www.icoph.org/pdf/visualstanres.pdf. Accessed May 7, 2009.
- 20.Fredrikson JL, Petrera J. Serial visual evoked potentials in 90 untreated patients with acute optic neuritis. Surv Ophthalmol 1999;44(suppl 1):S54–S62. [DOI] [PubMed] [Google Scholar]
- 21.Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med 1991;326:581–588. [DOI] [PubMed] [Google Scholar]
- 22.Balcer LJ, Baier ML, Kunkle AM, et al. Self-reported visual dysfunction in multiple sclerosis: results from the 25-item national eye institute visual function questionnaire. Mult Scler 2000;6:382–385. [DOI] [PubMed] [Google Scholar]
- 23.Ma SL, Shea JA, Galetta SL, et al. Self-reported visual dysfunction in multiple sclerosis: new data from the VFQ-25 and development of an MS-specific vision questionnaire. Am J Ophthalmol 2002;133:686. [DOI] [PubMed] [Google Scholar]
- 24.Rudick RA, Miller D, Clough JD, Gragg LA, Farmer RG. Quality of life in multiple sclerosis. Comparisons with inflammatory bowel disease and rheumatoid arthritis. Arch Neurol 1992;49:1237–1242. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000;343:898–904. [DOI] [PubMed] [Google Scholar]
- 26.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 2001;357:1576–1582. [DOI] [PubMed] [Google Scholar]
- 27.Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006;67:1242–1249. [DOI] [PubMed] [Google Scholar]
- 28.Ogden TE. Nerve fiber layer of the primate retina: thickness and glial content. Vision Res 1983;23:581–587. [DOI] [PubMed] [Google Scholar]
- 29.Chan CK, Miller NR. Peripapillary nerve fiber layer thickness measured by optical coherence tomography in patients with no light perception from long-standing nonglaucomatous optic neuropathies. J Neuroophthalmol 2007;27:176–179. [DOI] [PubMed] [Google Scholar]
- 30.Weinshenker BG, Rice GPA, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study, 3: multivariate analysis of predictive factors and models of outcome. Brain 1991;114:1045–1056. [DOI] [PubMed] [Google Scholar]
- 31.Naismith RT, Trinkaus K, Cross AH. Phenotype and prognosis in African-Americans with multiple sclerosis: a retrospective chart review. Mult Scler 2006;12:775–781. [DOI] [PubMed] [Google Scholar]
- 32.Brusa A, Jones SJ, Plant GT. Long term remyelination after optic neuritis: a 2-year visual evoked potential and psychophysical serial study. Brain 2001;124:468–479. [DOI] [PubMed] [Google Scholar]
- 33.Klistorner A, Arvind H, Nguyen T, et al. Axonal loss and myelin in early ON loss in postacute optic neuritis Ann Neurol 2008;64:325–331. [DOI] [PubMed] [Google Scholar]
- 34.Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer loss and visual dysfunction in optic neuritis. Ann Neurol 2005;58:383–391. [DOI] [PubMed] [Google Scholar]
- 35.Trip SA, Schlottmann PG, Jones SJ, et al. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage 2006;31:286–293. [DOI] [PubMed] [Google Scholar]
- 36.Vedula A, Brodney-Folse S, Gal R, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database System Rev 2007;(1):CD001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roxburgh RHSR, Seaman SR, Masterman T, et al. Multiple sclerosis severity score. Neurology 2005;64:1144–1151. [DOI] [PubMed] [Google Scholar]
- 38.Mantyjarvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg 2001;27:261–266. [DOI] [PubMed] [Google Scholar]