Abstract
Osteoporosis is an important systemic disorder, affecting mainly Caucasian women, with a diverse and multifactorial etiology. A large variety of animal species, including rodents, rabbits, dogs, and primates, have been used as animal models in osteoporosis research. Among these, the laboratory rat is the preferred animal for most researchers. Its skeleton has been studied extensively, and although there are several limitations to its similarity to the human condition, these can be overcome through detailed knowledge of its specific traits or with certain techniques. The rat has been used in many experimental protocols leading to bone loss, including hormonal interventions (ovariectomy, orchidectomy, hypophysectomy, parathyroidectomy), immobilization, and dietary manipulations. The aim of the current review is not only to present the ovariectomized rat and its advantages as an appropriate model for the research of osteoporosis, but also to provide information about the most relevant age and bone site selection according to the goals of each experimental protocol. In addition, several methods of bone mass evaluation are assessed, such as biochemical markers, densitometry, histomorphometry, and bone mechanical testing, that are used for monitoring and evaluation of this animal model in preventive or therapeutic strategies for osteoporosis.
Abbreviations: BMD, bone mineral density; DEXA, dual-energy X-ray absorptiometry; μCT, microcomputerized tomography; pQCT, peripheral quantitative computerized tomography
Osteoporosis is a multifactorial skeletal disease, characterized by reduction in bone mass and disruption of the microarchitectural structure of bone tissue, resulting in loss of mechanical strength and increased risk of fracture.2 The disorder can be localized or involve the entire skeleton. Generalized osteoporosis can be primary (postmenopausal and senile) or secondary. In the European Union, osteoporosis is a leading cause of mortality and morbidity in the elderly and a key factor in the high cost of medical care.34
Although osteoporosis usually makes its appearance late in life, and age is a major risk factor, its roots can be tracked back into adolescence. Particularly during periods of rapid bone growth, dietary calcium levels are of high importance.34 Other factors that contribute to the pathogenesis of osteoporosis are lifestyle and genetic and hormonal attributes.13,71 Reduced physical activity increases the rate of bone loss, and muscle contraction is the prevailing source of skeletal loading. Regarding hormonal factors, women, especially in the decade after menopause, can show a severe reduction of bone mass, thus explaining the high incidence of osteoporotic fractures in women compared with men.34
The multiple factors implicated in osteoporosis, its obscure pathogenesis, the dramatic decline in quality of life, high incidence of the disorder (especially in postmenopausal women), financial cost, and high mortality, make the need for further experimentation in animal models imperative. Experimental research can improve our understanding of pathogenesis and of the activity of pharmaceutical agents in the prevention or treatment of the disease. Although many aspects of the disorder have been revealed, others remain unclear, including the mechanisms involved in calcium homeostasis in the extracellular space and its effect on bone physiology and disease65 and the cell and molecular pathways triggered after mechanical loading to orchestrate bone renewal.53 Current research is focused on new therapeutic possibilities targeting the osteolytic enzymes of the osteoclast and the mechanisms activating bone progenitor cells and those controlling apoptosis as new potential treatments.63,64
Many therapeutic advances in the management of osteoporosis were studied first in diverse animal models and then entered clinical practice.31,67,69 All of these models should fulfill similar basic criteria: they must comply with national and local ethical and legislative considerations, be accessible to experimental centers, be easy and safe to handle, have a low cost of acquisition, require little maintenance, reliably reproduce the disease and the biological material to be examined should be readily available. Laboratory rats meet most of these criteria. In addition, the availability of detailed knowledge of the rat skeleton and protocols for rapid induction of osteopenia, have increased this model's popularity. Here we review the advantages and limitations of the use of the laboratory rat in osteoporosis research.
Strengths and limitations of rat models of osteoporosis
Bone modeling and remodeling.
In the selection of the most adequate animal model for the study of a disease, the reliable reproduction of the disorder is of paramount importance. One of the first questions to be addressed in osteoporosis research is whether the prevailing activity in the rat skeleton is modeling or remodeling. In the adult human skeleton, bone formation is coupled to bone resorption, temporally and locally, following the sequence of activation–resorption–formation. This process, which is called remodeling, has characteristic morphologic features that can be recognized histomorphometrically.10,15,17,55
In contrast to remodeling, modeling is the formation and resorption of bone in a specific site; these 2 processes occur over long periods of time and independently of each other. In modeling, the cycles of activity are activation–formation and activation–resorption. If the site is cancellous bone, the term minimodeling is used. The morphologic features of modeling differ from those seen in remodeling, and these features can be differentiated histomorphometrically.10,17,55
In the past, many scientists held that in the rat skeleton, the prevailing activity is modeling and consequently the rat was not an appropriate model for osteoporosis. The rat skeleton, however, shows a gradual transition from modeling to remodeling that is related to age progression in both cancellous and cortical bone.13 In the cancellous bone of the lumbar vertebrae, this transition is evident from the age of 3 mo, whereas in the proximal tibial metaphysis this transition takes place from 6 until 9 mo of age.29 In addition, in the endocortical bone of the lumbar vertebrae and the proximal tibial metaphysis, this gradual transition happens from the age of 3 to 6 mo and 9 to 12 mo, respectively (Table 1)31 The prevailing activity in cancellous and cortical bone in the lumbar vertebral body and proximal tibial metaphysis in the rat skeleton, after the age of 12 mo, is remodeling.14,31 The modeling-to-remodeling transition is associated with reduction of longitudinal bone growth to very low rates. To conduct research on new potential modalities for osteoporosis in rats, the bone site and age of the animal must be such that remodeling is the predominant activity.
Table 1.
| Cancellous | Endocortical | |
| Proximal tibial metaphysis | 6 to 9 mo | 9 to 12 mo |
| Lumbar vertebra | 3 mo | 3 to 6 mo |
Bone site-specific characteristics.
Another parameter to take into account is that, in rats, some bones retain their ability for longitudinal growth throughout most of their life. In male rats, the epiphyses of many long bones remain open past 30 mo. In female rats, bone growth in the proximal tibial and distal tibial epiphysis stops at the age of 15 and 3 mo respectively, whereas the same process for the lumbar vertebrae lasts 21 mo.31 After the age of 10 mo, the bone growth rate for the proximal tibial epiphysis is less than 3 μm/day and stops after the age of 15 mo. 14,31 If experimentation starts around 10 mo of age, which marks the peak bone mass age for the rat, the longitudinal bone growth adjacent to the epiphyseal plate of the tibia will be less than 1 mm. Densitometric, tomographic, or histomorphometric measurements should not be made adjacent to the epiphyseal growth plate of the proximal tibia, where the prevailing activity is modeling. Measurements should be conducted 1 mm distal to the growth plate where the prevailing activity is remodeling. In this way, measurements of normal bone growth are avoided (Table 2).
Table 2.
Distance from the epiphyseal growth plate to avoid the primary spongiosa, time of epiphyseal closure, and the earliest stastistically significant bone loss after ovariectomy or immobilization in the skeletally mature female rat.
| Distance from the epiphyseal growth plate | Epiphyseal closure (mo) | Earliest detection of significant bone loss after ovariectomy (d) | Earliest detection of significant bone loss after immobilization (d) | |
| Femoral neck | — | — | 30 | — |
| Femoral diaphysis | — | — | — | 21 |
| Proximal tibial metaphysis | 1 mm | 15 | 14 | 14–30 |
| Distal tibial metaphysis | Immediately below the subchondral bone | 3 | none | 14–30 |
| Tibial diaphysis | — | — | — | 42 |
| Lumbar vertebra | 0.5 mm | 21 | 60 | — |
—, not applicable
In the lumbar vertebrae, a distance of 0.5 mm from the epiphyseal growth plate is sufficient to avoid the primary spongiosa.14 For the distal tibia, where the growth plates close at the age of 3 mo, the measurement can involve the whole spongiosa and should start immediately below the subchondral bone.31 The continuous growth of specific sites of the rat skeleton prompts the mandatory use of a control group to differentiate the gain or loss of bone mass attributed to age.
Limitations.
A potential drawback to the use of rat models for osteoporosis is the lack of Haversian remodeling in the rat skeleton. In humans, increased Haversian remodeling is the main cause of cortical porosity, but rats lack a well-developed Haversian remodeling system. In the rat skeleton, cortical bone gain occurs in the periosteum, and cortical bone is lost at the endosteum.67 Larger animal models such as rabbits,6 dogs, and primates31 are considered more appropriate for the study of Haversian remodeling. However, the species-specific traits of osteoporosis in dogs (inappropriate model for postmenopausal osteoporosis, high cost of maintenance, ethical dilemmas) and primates (high cost of acquisition and maintenance, reduced availability in experimental centers, ethical dilemmas) limit their use in osteoporosis research. In all animal models, the bulk of bone loss is focused on the endosteal surface. However, ovariectomy of skeletally mature rats leads to a condition similar to menopause, in that the surgery leads to cancellous and endocortical bone loss by increasing the overall rate of bone remodeling and by altering the balance between bone formation and bone resorption, such that resorption predominates at selected skeletal sites.31 Losses of endocortical as well as cancellous bone are the primary causes of postmenopausal osteoporosis, whereas intracortical remodeling-induced bone loss in the Haversian system plays a minor role.29 Given the difficulties with other animal models of osteoporosis, the lack of the Haversian remodeling in the rat skeleton is a shortcoming that often can be accommodated.69
Rat osteopenia due to age, ovariectomy (in the female rat), and immobilization bears a strong resemblance to human osteopenia, both in its anatomical features as well as in the transitional and steady states of the bone dynamics. The main attributes of human osteoporosis are spontaneous and low-impact fractures, neither of which has ever been reproduced in any animal model.18 Nevertheless, taking into account the definition of the World Health Organization, which states that the osteoporosis is present when bone mineral density (BMD) is more than 2.5 standard deviations below the young adult reference mean, with or without the presence of any fracture,33 the nonfractured osteopenic rat can be considered an appropriate model for osteoporosis research.
Rat models of osteoporosis
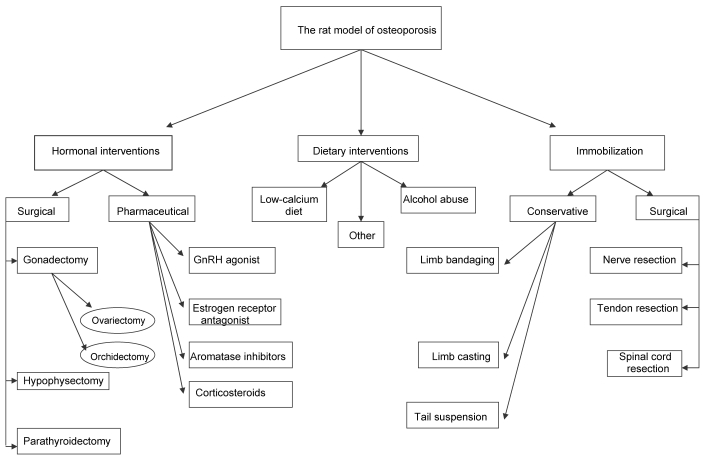
Several experimental interventions are used to induce osteopenia and osteoporosis in the rat (Figure 1). The rate of loss of bone mass in male and female rats is highly dependent on the method used to induce osteoporosis and the site evaluated and whether this loss concerns cancellous or cortical bone.31
Figure 1.
Algorhythm for the selection of experimental interventions to induce osteopenia or osteoporosis in the laboratory rat.
All experimental osteoporosis protocols can be implemented in skeletally immature or mature rats.60 Although rats reach sexual maturity at the age of 2.5 mo, their skeleton is considered mature after the age of 10 mo.31 If skeletally immature rats are involved, then a low peak bone mass is achieved, a fact that is considered to be a high risk factor for human osteoporotic fractures. This trait is why the skeletally immature rat is an appropriate animal model in the research of endocrine, nutritional and environmental factors, all of which can influence peak bone mass. The skeletally mature rat is an appropriate animal model for the research of postmenopausal and immobilization osteoporosis.69
Postmenopausal osteoporosis.
The ovariectomized rat model is most commonly used in research on postmenopausal osteoporosis. After ovariectomy, bone resorption exceeds bone formation initially, causing bone loss. Soon thereafter, bone remodeling reaches a steady state, where resorption and formation are balanced. Statistically significant bone loss is seen in the proximal tibial metaphysis after 14 d,72,74 in the lumbar vertebral body after 60 d,73 and in the femoral neck after 30 d.43 In addition, the time needed for the proximal tibial metaphysis to reach steady state is 90 d,72,74 compared with 270 d for the lumbar vertebral body and femoral neck.43,73 In contrast, ovariectomy does not induce bone mass loss in the epiphyses of long bones, the distal tibial metaphysis, or caudal vertebrae.40,42,45,50
In cortical bone, enlargement of the marrow cavity is an indirect measure of bone loss. This enlargement in the diaphysis of long bones is due to increased endosteal bone resorption70 and periosteal bone apposition.49 Endosteal resorption and the simultaneous periosteal bone formation result in a very slow rate of cortical bone loss.38 Analysis of the inner half area of the shaft in cortical bones is a very sensitive index, because the bulk of bone loss occurs at this site. The earliest changes in the cortical bone width and the marrow cavity of the femoral and tibial shaft are noticed between 90 and 120 d after ovariectomy,11,37,75 whereas cortical bone requires to 180 d or longer after surgery to achieve steady state.31 Researchers must bear in mind that age-related decreases in cortical BMD in intact female rats start in the lumbar vertebrae at the age of 15 mo and in the proximal tibial metaphysis at the age of 12 mo.19
Alternative methods to surgical ovariectomy leading to osteoporosis are the administration of pharmaceutical agents, such as a gonadotropin releasing hormone agonist,25 estrogen receptor antagonist,20 and aromatase inhibitor, that is reversible after their withdrawal.23 These pharmaceutical agents, which are used in humans for the endocrine treatment of endometriosis or breast cancer, are associated with accelerated bone loss.69 The administration of gonadotropin releasing hormone agonists creates a hypogonadotrophic–hypogonadal model, whereas estrogen receptor antagonists and aromatase inhibitors produce a hypergonadotrophic–hypogonadal model of osteoporosis. Experimentation on the rat as a model of glucocorticoid-induced osteoporosis has yielded inconsistent results. Some studies were unable to detect bone loss in mature rats,60 but other reports correlate glucocorticoid administration with bone loss.52 Compelling evidence showing that the rat is capable of accurately replicating the glucocorticoid-induced bone loss noted in adult humans is unavailable.31,57,60 Other hormonal interventions resulting in osteopenia, including hypophysectomy,30 orchidectomy,28 and parathyroidectomy,4 also are well studied in the rat skeleton.
Immobilization osteoporosis.
Another method for inducing osteoporosis in rats is through immobilization. There are several methods of immobilization, which can be either surgical, such as nerve,77 tendon66 and spinal cord resection,54 or conservative, such as casting,62 suspension,51 and limb bandaging.3 Because of the regional acceleratory phenomenon, the rate of bone loss is more rapid after surgical methods than immobilization.16 In the immobilization model, the bulk of bone loss occurs in the hind limbs, because they are the sites of greatest mechanical loading, but in general, the rate of bone loss is faster in cancellous than in cortical bone. This difference can partly be attributed to the surface to volume ratio, which is increased in cancellous bone.31 The earliest statistically significant bone loss in the proximal and distal tibial metaphysis for this model is seen at 14 to 30 d after initiation of immobilization, whereas the time needed to achieve steady state for the these sites is 126 and 45 d respectively.41 One of the advantages of this method is that bone changes also occur in the distal tibial metaphysis of rats, the architecture of which is similar to that in human adults. Furthermore, because of its low bone turnover rate, this site is suitable for the research of anabolic agents in the prevention and treatment of osteoporosis.27
In the immobilization model, in contrast to the ovariectomized model, periosteal bone formation ceases in cortical bone, but endosteal resorption, leading to slow bone loss, continues. Changes in the tibial diaphysis in regard to marrow cavity enlargement and reduction of cortical bone width first become statistically significant approximately 42 days after immobilization, whereas in the femoral diaphysis, bone cavity enlargement is apparent by day 21. Cortical bone loss can reach 10% at 26 wk after immobilization.3,40
Combining ovariectomy and immobilization pairs the advantages of both methods and can markedly reduce the time when bone mass loss becomes apparent, especially for cortical bone.9,54
Other experimental methods inducing osteoporosis in rats.
The rat has been used to understand the pathogenesis and severity of bone mass loss after alcohol abuse.57,68 Osteopenia has also been studied after administration of low-calcium diet to immature rats.59 The effects of the calcium:phosphorus ratio in food as well as dietary magnesium supplementation also have been investigated in the rat model of ovariectomy-induced osteoporosis.35,39
From preclinical studies to clinical practice
Several therapeutic options have been investigated in the ovariectomized rat model, some of which have already been translated into clinical practice. Estrogen administration prevented osteopenia and decreased bone turnover in the ovariectomized rat model, a finding consistent with the skeletal effects of estrogen therapy in postmenopausal women.72 Selective estrogen receptor modulators such as raloxifene had beneficial effects on bone mineral density, without significant adverse uterine effects.5 Calcitonin administration promoted osteoblastic activity, thus augmenting bone mineral density.36 Treatment with bisphosphonates, such as zoledronic acid, increased bone structure and mechanical strength of bones of ovariectomized adult rats.26 Parathyroid hormone has been found in promoting bone strength in this animal model.61 Finally, the administration of strontium ranelate improved bone strength by improving bone mass and microarchitecture.47
Methods of evaluating osteopenia in rats
The methods used in the evaluation of bone mass, architecture, and metabolism in the rat skeleton are, generally speaking, the same as those used for humans. The need for further investigation in this field has led to the introduction of methods for rats that are more specialized and perhaps more invasive than those used for humans.
Noninvasive methods.
Biochemical markers.
Measurements of calcium, phosphorus, and magnesium in blood and urine can be obtained from animal models. Biochemical markers of bone turnover include peptides originating from osteoblasts or osteoclasts and organic compounds released during the synthesis and resorption of bone matrix.44 Markers may be measured by using a variety of methods, including liquid chromatography, radioimmunoassay, immunoradiometric assay, luminescence immunoassay and enzyme-linked immunosorbent assay.44,58 Markers of bone formation (for example alkaline phosphatase, osteocalcin) need to be distinguished from those of bone resorption (for example pyridinoline, tartrate-resistant acid phosphatase, urinary type I collagen crosslinked N-telopeptides); all markers of bone turnover represent bone metabolism changes in the whole skeleton.58 Proteins and peptides vary in structure between species. If these markers are measured by immunoassay, the antibody must recognize the specific protein or peptide structure of the species being analyzed.44
The availability of specialized biochemical markers in animal models is limited, compared with those used in humans. Although human reagents for detecting biochemical markers can be modified for use in animal models, loss of specificity and sensitivity result. Other disadvantages further limit the value of using biochemical markers in animals.69 Specifically, these compounds do not provide information regarding bone mass and strength, they reflect changes in the whole skeleton, and they fail to identify whether these changes stem from cortical or cancellous bone. Because of these limitations, biochemical markers of bone turnover should be used with caution and should always supplement other assays that directly evaluate bone mass and bone turnover.69 In humans, combining biochemical markers with densitometry significantly increases the specificity of prediction for future fractures without adversely affecting sensitivity.21
Densitometry.
Bone densitometry is often used in animal models to evaluate BMD and bone mineral content.9 Dual-energy X-ray absorptiometry (DEXA) with small animal software can be used to measure both total and regional bone density in animals as small as mice. However, the evaluation of BMD can be challenging in growing animals.69 Apparent changes in BMD as measured by DEXA in growing animals is a reflection of growth and size and not changes in true mineral density.46 However, BMD is not the only determinant of bone strength. BMD measurement has been estimated to account for 60% to 70% of bone strength variation; other parameters, including dimensions, architecture, and bone quality, also contribute to bone strength. This situation has led to the introduction of new diagnostic techniques, which can provide additional information regarding determinants of bone strength.1
Peripheral quantitative computerized tomography (pQCT) is a powerful imaging technique that captures a 3-dimensional image of fixed thickness.24 Advantages of pQCT over DEXA include the ability to analyze cancellous and cortical bone separately, applicability to growing animals, and earlier detection of changes in bone mass, even in rodents as small as mice.8 Under normal experimental conditions, pQCT is unable to detect structural or morphologic changes at the level of an individual trabeculum in small animals. Therefore, the information gained from pQCT is complementary to histomorphometry. The accuracy of the pQCT method is 92% to 98%, with a coefficient of variation of 0.1% to 0.2%.22
Microcomputerized tomography (μCT) provides images of high resolution. The method is capable of 3-dimensional study, and data can be obtained from an area of bone as small as an individual trabeculum. In addition, μCT can evaluate parameters that correlate to bone connectivity and elasticity. A 3-dimensional digital model of trabecular bone has been developed, and histomorphometric parameters, including trabecular thickness and separation, can be calculated from it.24 Comparison of 2-dimensional histomorphometry and 3-dimensional μCT shows the superiority of μCT in revealing early changes in bone architecture.32 Another intriguing feature of the method is that μCT can detect local bone density changes after acute administration of heavy metals. If this approach becomes feasible, μCT could provide 3-dimensional data in a way similar to fluorochrome-labeling microscopy.70 Originally μCT was developed for ex vivo use. Animals were euthanized at predefined intervals to assess bone changes during the experimental period. This methodology demanded large numbers of animals to overcome intersubject variability to obtain statistically significant results. The recent arrival of in vivo μCT provides the ability to monitor microarchitectural changes in individual animals.7,23
Magnetic resonance microscopy is a noninvasive, nonionizing radiation technology. This imaging system can assess the fat and water of the bone marrow; as a consequence it presents trabecular bone as a negative image. Magnetic resonance microscopy can be applied in vitro and in vivo and is very promising for evaluation of trabecular architecture.24
Invasive methods.
Histomorphometry.
Histomorphometry provides a 2-dimensional study of bone mass and architecture at a very high resolution compared with those of the aforementioned imagining techniques. Histomorphometry accurately evaluates bone architecture and indices of bone fragility independently of bone mass.10 Parameters measured by histomorphometry include the numbers of osteoblasts, osteoclasts, osteocytes, and active osteoblasts relative to bone perimeter; trabecular thickness, number, and separation; and so on.48 The use of fluorochrome labeling is especially valuable in the evaluation of bone changes. Histomorphometry, however, is not free of limitations. The histologic assessment of an area does not represent changes in the whole skeleton. Furthermore, by definition, only a very small sampling area can be assessed and then normalized to a tissue sampling area. This method provides credibility when the sampling area is comparable in all groups.9,56 Although many histomorphometric parameters are expressed in volume, users must bear in mind that the data have been evaluated in 2-dimensional sections. Nevertheless, histomorphometric data provide a documented assessment of a bone's stereologic organization.10 The combination of BMD and histomorphometric modalities can explain 90% of variability in bone strength.12
Mechanical strength evaluation.
The realization that increases in bone mass do not always reflect fracture reduction in humans and the infeasibility of observing low-impact fractures in animal models have made ex vivo mechanical testing imperative.31 Three-point bending, 4-point bending, and torsion testing are used frequently to assess bone mechanical strength.70 However, these tests can only be done in diaphyses of long bones, in sites where osteoporotic fractures are rare. New tests have been developed to assess the vertebral (compression testing) and femoral head (cantilever testing) mechanical strength, sites where osteoporotic fractures are common in humans.61,76
Conclusions
The high incidence, long-term implications, high mortality, financial burden and dramatically decreased quality of life indicate the severity of osteoporosis in humans. The need to better understand the multifactorial nature of this disorder and to develop new preventive and therapeutic methods makes the use of animal models of osteopenia necessary. The similarities in pathophysiologic responses between the human and rat skeleton, combined with the husbandry and financial advantages, have made the rat a valuable model in osteoporosis research. However, researchers’ selection of a model should be based on scientific criteria and not ease of use, and investigators should always bear in mind that experimentation should adhere to the 3Rs of ethical animal use: replacement, reduction, and refinement.
References
- 1.Ammann P, Rizzoli R. 2003. Bone strength and its determinants. Osteoporos Int 14:S13–S18 [DOI] [PubMed] [Google Scholar]
- 2.Anonymous 2000. Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 17:1–45 [PubMed] [Google Scholar]
- 3.Bagi CM, Mecham M, Weis J, Miller SC. 1993. Comparative morphometric changes in rat cortical bone following ovariectomy and/or immobilization. Bone 14:877–883 [DOI] [PubMed] [Google Scholar]
- 4.Berdud I, Martin-Malo A, Almaden Y, Aljama P, Rodriguez M, Felsenfeld A. 1998. The PTH-calcium relationship during a range of infused PTH doses in the parathyroidectomized rat. Calcif Tissue Int 62:457–461 [DOI] [PubMed] [Google Scholar]
- 5.Black LJ, Sato M, Rowley ER, Magee DE, Williams DL, Cullinan GJ, Bendele R, Kauffman RF, Bensch WR. 1994. Raloxifene prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest 93:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnarens F, Einhorn TA. 1984. Production of a standard closed fracture in laboratory animal bone. J Orthop Res 2:97–101 [DOI] [PubMed] [Google Scholar]
- 7.Boyd SK, Moser S, Kuhn M, Klinck RJ, Krauze PL, Muller R, Gasser JA. 2006. Evaluation of three-dimensional image registration methodologies for in vivo microcomputed tomography. Ann Biomed Eng 34:1587–1599 [DOI] [PubMed] [Google Scholar]
- 8.Breen SA, Loveday BE, Millest AJ, Waterton JC. 1998. Stimulation and inhibition of bone formation: use of peripheral quantitative computed tomography in the mouse in vivo. Lab Anim 32:467–476 [DOI] [PubMed] [Google Scholar]
- 9.Cavolina JM, Evans GL, Harris SA, Zhang M, Westerlind KC, Turner RT. 1997. The effects of orbital spaceflight on bone histomorphometry and messenger ribonucleic acid levels for bone matrix proteins and skeletal signaling peptides in ovariectomized growing rats. Endocrinology 138:1567–1576 [DOI] [PubMed] [Google Scholar]
- 10.Dalle Carbonare L, Valenti MT, Bertoldo F, Zanatta M, Zenari S, Realdi G, Lo Cascio V, Giannini S. 2005. Bone microarchitecture evaluated by histomorphometry. Micron 36:609–616 [DOI] [PubMed] [Google Scholar]
- 11.Danielsen CC, Mosekilde L, Svenstrup B. 1993. Cortical bone mass, composition, and mechanical properties in female rats in relation to age, long-term ovariectomy, and estrogen substitution. Calcif Tissue Int 52:26–33 [DOI] [PubMed] [Google Scholar]
- 12.Dempster DW. 2003. Bone microarchitecture and strength. Osteoporos Int 14:S54–S56 [DOI] [PubMed] [Google Scholar]
- 13.Dennison E, Cole Z, Cooper C. 2005. Diagnosis and epidemiology of osteoporosis. Curr Opin Rheumatol 17:456–461 [DOI] [PubMed] [Google Scholar]
- 14.Erben RG. 1996. Trabecular and endocortical bone surfaces in the rat: modeling or remodeling? Anat Rec 246:39–46 [DOI] [PubMed] [Google Scholar]
- 15.Frost HM. 1977. A method analysis of trabecular bone dynamics. : Meunier PJ, editor Bone histomorphometry. Second international workshop on bone morphometry. Lyon: University of Claude Bernard; p 455–475 [Google Scholar]
- 16.Frost HM. 1983. The regional acceleratory phenomenon. A review. Henry Ford Hosp Med J 31:3–9 [PubMed] [Google Scholar]
- 17.Frost HM. 1986. Intermediary organization of the skeleton. Boca Raton (FL): CRC Press [Google Scholar]
- 18.Frost HM, Jee WSS. 1992. On the rat model of human osteopenias and osteoporosis. Bone Miner 18:227–236 [DOI] [PubMed] [Google Scholar]
- 19.Fukuda S, Iida H. 2004. Age-related changes in bone mineral density, cross-sectional area, and the strength of long bones in the hind limbs and the first lumbar vertebra in female Wistar rats. J Vet Med Sci 66:755–760 [DOI] [PubMed] [Google Scholar]
- 20.Gallagher A, Chambers TJ, Tobias JH. 1993. The estrogen antagonist ICI 182,780 reduces cancellous bone volume in female rats. Endocrinology 133:2787–2791 [DOI] [PubMed] [Google Scholar]
- 21.Garnero P, Dargent-Molina P, Hans D, Schott AM, Breart G, Meunier PJ, Delmas PD. 1998. Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurements for the prediction of hip fracture in elderly women? The EPIDOS prospective study. Osteoporos Int 8:563–569 [DOI] [PubMed] [Google Scholar]
- 22.Gasser JA. 1995. Assessing bone quantity by pQCT. Bone 17:145S–154S [DOI] [PubMed] [Google Scholar]
- 23.Gasser JA, Green JR, Shen V, Ingold P, Rebman A, Bhatnagar AS, Evans DB. 2006. A single intravenous administration of zoledronic acid prevents the bone loss and mechanical compromise induced by aromatase inhibition in rats. Bone 39:787–795 [DOI] [PubMed] [Google Scholar]
- 24.Genant HK, Jiang Y. 2006. Advanced imaging assessment of bone quality. Ann N Y Acad Sci 1068:410–428 [DOI] [PubMed] [Google Scholar]
- 25.Goulding A, Gold E. 1989. A new way to induce oestrogen-deficiency osteopenia in the rat: Comparison of the effect of surgical ovariectomy and administration of the LHRH agonist buserelin on bone resorption and composition. J Endocrinol 121:293–298 [DOI] [PubMed] [Google Scholar]
- 26.Hornby SB, Evans GP, Hornby SL, Pataki A, Glatt M, Green JR. 2003. Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int 72:519–527 [DOI] [PubMed] [Google Scholar]
- 27.Ijiri K, Ma YF, Jee WSS, Akamine T, Liang X. 1995. Adaptation of nongrowing former epiphyses and metaphyseal bones to aging and immobilization in the rat. Bone 17(4 Suppl):207S–212S [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto J, Takeda T, Ischimura S. 2004. Differential effect of short-term etidronate treatment on three cancellous bone sites in orchidectomized adult rats. Keio J Med 53:12–17 [DOI] [PubMed] [Google Scholar]
- 29.Iwaniec UT, Turner RT. 2008. Animal models of osteoporosis. : Marcus R, Feldman D, Nelson DA, Rosen CJ, Osteoporosis, 3rd ed Amsterdam: Elsevier; p 985–1110 [Google Scholar]
- 30.Iwamoto J, Takeda T, Sato Y, Yeh JK. 2007. Effects of vitamin K2 and growth hormone on the long bones in hypophysectomized young rats: a bone histomorphometry study. J Bone Miner Metab 25:46–53 [DOI] [PubMed] [Google Scholar]
- 31.Jee WSS, Yao W. 2001. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 1:193–207 [PubMed] [Google Scholar]
- 32.Jiang Y, Zhao J, Liao EY, Dai RC, Wu XP, Genant HK. 2005. Application of μCT-assessment of 3D bone microstructure in preclinical and clinical studies. J Bone Miner Metab 23:122–131 [DOI] [PubMed] [Google Scholar]
- 33.Kanis JA. 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int 4:368–381 [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson P, Oden A, Zethraeus N, Pfleger B, Khaltaev N. 2005. Assessment of fracture risk. Osteoporos Int 16:581–589 [DOI] [PubMed] [Google Scholar]
- 35.Katsumata SI, Matsuzaki H, Uehara M, Suzuki K. 2005. Effect of dietary magnesium supplementation on bone loss in rats fed a high phosphorus diet. Magnes Res 18:91–96 [PubMed] [Google Scholar]
- 36.Kavuncu V, Sahin S, Baydas G, Ilhan N, Ozercan I, Yasar A, Pekkutucu I, Ilhan N, Ozercan R. 2003. A comparison of estrogen and two different doses of calcitonin in ovariectomized rats. Yonsei Med J 44:508–516 [DOI] [PubMed] [Google Scholar]
- 37.Ke HZ, Jee WSS, Zeng QQ, Li M, Lin BY. 1993. Prostaglandin E2 increased rat cortical bone mass when administered immediately following ovariectomy. Bone Miner 21:189–201 [DOI] [PubMed] [Google Scholar]
- 38.Kimmel DB, Wronski TJ. 1990. Nondestructive measurement of bone mineral in femurs from ovariectomized rats. Calcif Tissue Int 46:101–110 [DOI] [PubMed] [Google Scholar]
- 39.Koshihara M, Masuyama R, Uehara M, Suzuki K. 2004. Effect of dietary calcium: phosphorus ratio on bone mineralization and intestinal calcium absorption in ovariectomized rats. Biofactors 22:39–42 [DOI] [PubMed] [Google Scholar]
- 40.Li XJ, Jee WS. 1991. Adaptation of diaphyseal structure to aging and decreased mechanical loading in the adult rat: a densinometric and histomorpohometric study. Anat Rec 229:291–297 [DOI] [PubMed] [Google Scholar]
- 41.Li XJ, Jee WSS, Chow SY, Woodbury DM. 1990. Adaptation of cancellous bone to aging and immobilization in the rat: a single photon absorptiometry and histomorphometry study. Anat Rec 227:12–24 [DOI] [PubMed] [Google Scholar]
- 42.Li M, Shen Y, Qi H, Wronski TJ. 1996. Comparison study of skeletal response to estrogen depletion at red and yellow marrow sites in rats. Anat Rec 245:472–480 [DOI] [PubMed] [Google Scholar]
- 43.Li M, Shen Y, Wronski TJ. 1997. Time course of femoral neck osteopenia in ovariectomizd rats. Bone 20:55–61 [DOI] [PubMed] [Google Scholar]
- 44.Loeb WF. 1999. The rat. : Loeb WF, Quimby FW, The clinical chemistry of laboratory animals, 2nd ed Ann Arbor: Edwards Brothers; p 33–45 [Google Scholar]
- 45.Ma YF, Ke HZ, Jee WSS. 1994. Prostagladin E2 adds bone to a cancellous bone site with a growth plate and low bone turnover in ovariectomized rats. Bone 15:137–146 [DOI] [PubMed] [Google Scholar]
- 46.Manolagas SC, Kousteni S, Jilka RL. 2002. Sex steroids and bone. Recent Prog Horm Res 57:385–409 [DOI] [PubMed] [Google Scholar]
- 47.Marie P, Ammann P, Shen V, Bain S, Robin B, Dupin-Roger I. 2003. Evidence that strontium ranelate increases bone quality in rats by improving bone strength and architecture. Bone 32:S80 [Google Scholar]
- 48.Meunier PJ. 1988. Assessment of bone turnover by histomorphometry in osteoporosis. : Riggs BL, Melton LJ, Osteoporosis: etiology, diagnosis, and management. New York: Raven Press; p 317–332 [Google Scholar]
- 49.Miller SC, Bowman BM, Miller MA, Bagi CM. 1991. Calcium absorption and osseous organ-, tissue-, and envelops-specific changes following ovariectomy in rats. Bone 12:439–446 [DOI] [PubMed] [Google Scholar]
- 50.Miyakoshi N, Sato K, Tsuchida T, Tamura Y, Kudo T. 1999. Histomorphometric evaluation of the effects of ovariectomy on bone turnover in rat caudal vertebrae. Calcif Tissue Int 64:318–324 [DOI] [PubMed] [Google Scholar]
- 51.Morey ER. 1979. Spaceflight and bone turnover: correlation with a new rat model of weightlessness. Bioscience 29:168–172 [Google Scholar]
- 52.Nitta T, Fukushima T, Nakamuta H, Koida M. 1999. Glucocorticoid-induced secondary osteopenia in female rats: a time course study as compared with ovariectomy-induced osteopenia and response to salmon calcitonin. Jpn J Pharmacol 79:379–386 [DOI] [PubMed] [Google Scholar]
- 53.Noble SB, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, Skerry TM, Lanyon LE. 2003. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol 284:C934–C943 [DOI] [PubMed] [Google Scholar]
- 54.Okumura H, Yamamuro T, Kassai R, Hirashi T, Tada K, Nishii Y. 1987. Effect of 1 alpha-hydroxyvitamin D3 on osteoporosis induced by immobilization combined with ovariectomy in rats. Bone 8:351–355 [DOI] [PubMed] [Google Scholar]
- 55.Parfitt AM. 1984. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int 36:S37–S45 [DOI] [PubMed] [Google Scholar]
- 56.Rosen HN, Tollin S, Ballena R, Middlebrooks VL, Beamer WG, Donohue LR, Rosen C, Turner A, Holick M, Greenspan SL. 1995. Differentiating between ovariectomized rats and controls using measurements of trabecular bone density: a comparison among DEXA, histomorphometry, and peripheral quantative computerized tomography. Calcif Tissue Int 57:35–39 [DOI] [PubMed] [Google Scholar]
- 57.Sampson HW, Perks N, Champney TJ, Defee B. 1996. Alcohol consumption inhibits bone growth and development in young actively growing rats. Alcohol Clin Exp Res 20:1375–1384 [DOI] [PubMed] [Google Scholar]
- 58.Seibel MJ. 2000. Molecular markers of bone turnover: biochemical, technical, and analytical aspects. Osteoporos Int 11(Suppl 6):S18–S29 [DOI] [PubMed] [Google Scholar]
- 59.Seto H, Aoki K, Kasugai S, Ohya K. 1999. Trabecular bone turnover, bone marrow cell development, and gene expression of bone matrix proteins after low calcium feeding in rats. Bone 25:687–695 [DOI] [PubMed] [Google Scholar]
- 60.Shen V, Birchman R, Liang XG, Wu DD, Lindsay R, Dempster DW. 1997. Prednisolone alone, or in combination with estrogen or dietary calcium deficiency or immobilization, inhibits bone formation but does not induce bone loss in mature rats. Bone 21:345–351 [DOI] [PubMed] [Google Scholar]
- 61.Sogaard CH, Wronski TJ, McOsker JE, Mosekilde L. 1994. The positive effect of parathyroid hormone on femoral neck bone strength in ovariectomized rats is more pronounced than that of estrogen or bisphosphonates. Endocrinology 134:650–657 [DOI] [PubMed] [Google Scholar]
- 62.Steinberg ME, Trueta J. 1981. Effects of activity on bone growth and development in the rat. Clin Orthop Relat Res 156:52–60 [PubMed] [Google Scholar]
- 63.Stoch SA, Wagner JA. 2008. Cathepsin K inhibitors: a novel target for the osteoporosis therapy. Clin Pharmacol Ther 83:172–176 [DOI] [PubMed] [Google Scholar]
- 64.Taketa T, Sakai A, Tanaka S, Nakai K, Menuki K, Yamane H, Tanaka K, Nakamura T. 2008. Selective cyclooxygenase-2 inhibitor prevents reduction of trabecular bone mass in collagen-induced arthritic mice in association with suppression of RANKL/OPG ratio and IL6 mRNA expression in synovial tissues but not in bone marrow cells. J Bone Miner Metab 26:143–151 [DOI] [PubMed] [Google Scholar]
- 65.Talmage DW, Talmage RV. 2007. Calcium homeostasis: how bone solubility relates to all aspects of bone physiology. J Musculoskelet Neuronal Interact 7:108–112 [PubMed] [Google Scholar]
- 66.Thompson DD, Rodan GA. 1988. Indomethacin inhibition of tenotomy-induced bone resorption in rats. J Bone Miner Res 3:409–414 [DOI] [PubMed] [Google Scholar]
- 67.Turner AS. 2001. Animal models of osteoporosis—necessity and limitations. Eur Cell Mater 1:66–81 [DOI] [PubMed] [Google Scholar]
- 68.Turner RT, Aloia RC, Segel LD, Hannon KS, Bell NH. 1988. Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcohol Clin Exp Res 12:159–162 [DOI] [PubMed] [Google Scholar]
- 69.Turner RT, Lotinun S, Hefferan T, Evans GL, Zhang M, Sibonga JD. 2001. Animal models for osteoporosis. Rev Endocr Metab Disord 2:117–127 [DOI] [PubMed] [Google Scholar]
- 70.Turner RT, Vandersteenhooven JJ, Bell NH. 1987. The effects of ovariectomy and 17β estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res 2:115–122 [DOI] [PubMed] [Google Scholar]
- 71.Walker-Bone K, Walter G, Cooper C. 2002. Recent developments in the epidemiology of osteoporosis. Curr Opin Rheumatol 14:411–415 [DOI] [PubMed] [Google Scholar]
- 72.Wronski TJ, Cintron M, Dann LM. 1988. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int 43:179–183 [DOI] [PubMed] [Google Scholar]
- 73.Wronski TJ, Dann LM, Horner SL. 1990. Time course of vertebral osteopenia in ovariectomized rats. Bone 10:295–301 [DOI] [PubMed] [Google Scholar]
- 74.Wronski TJ, Dann LM, Scott KS, Cintron LM. 1989. Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int 45:360–366 [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto N, Jee WSS, Ma YF. 1995. Bone histomorphometric changes in the femoral neck of aging and ovariectomized rats. Anat Rec 243:175–185 [DOI] [PubMed] [Google Scholar]
- 76.Yao W, Hadi T, Jiang Y, Lotz J, Wronski TJ, Lane NE. 2005. Basic fibroblast growth factor improves trabecular bone connectivity and bone strength in the lumbar vertebral body of osteopenic rats. Osteoporos Int 16:1939–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng QQ, Jee WSS, Bigornia AE, King JG, D’ Souza SM, Li XJ, Ma YF, Wechter WJ. 1996. Time responses of cancellous and cortical bones to schiatic neurectomy in growing female rats. Bone 19:13–21 [DOI] [PubMed] [Google Scholar]