Abstract
Responses of platelets from diabetic and diabetic–hyperlipidemic pigs were studied. Pigs were made diabetic with single dose of alloxan, which acts by selectively destroying insulin-producing pancreatic β cells thus inducing type 1 diabetes. Pigs were kept for 1 or 12 wk, during which thrombin-induced aggregation was monitored in washed platelets. The platelets showed increased sensitivity to aggregation within 1 wk of induction of diabetes. Hyperlipidemia alone for 12 wk did not increase platelet hypersensitivity, but hyperlipidemia together with diabetes significantly increased thrombin-induced platelet aggregation. Because this hypersensitivity occurred in washed platelets, this characteristic appears to be independent of any contribution by plasma factors or other blood cells. The hypersensitivity of platelets from diabetic pigs correlated with decreased activity of mitogen-activated protein kinase. These studies offer the first evidence that platelet hyperactivity occurs during the early stages (within a week) of induction of diabetes in pigs and before manifestation of other cardiovascular problems.
Abbreviations: MAPK, mitogen-activated protein kinase
Both hyperlipidemia and diabetes are risk factors for cardiovascular disease. In diabetic patients, platelets show increased sensitivity to arachidonic acid, ADP, thrombin, and platelet activating factor.3,20,24 Numerous biochemical and signaling changes occur in the responses of these diabetic platelets, including hypersensitivity to agonists; increased production of thromboxane A2 and arachidonic acid; increases in adhesiveness, secretion (for example, von Willebrand factor), PAI1 release, P selectin; altered glycoprotein or integrin receptors; and altered metabolism. Changes in cell signaling include increased phosphoinositide turnover, calcium mobilization, and activation of protein kinase C and myosin light-chain kinase and decreases in cGMP responses.24 Hyperglycemia may play a role in this altered response. However, exposure of human platelets in platelet-rich plasma (devoid of erythrocytes) to high glucose concentrations (20 to 100 mM) for as long as 2 d did not cause hypersensitivity and therefore suggests the importance of endogenous factors in this phenomenon in vivo.8 Other studies, in which whole blood (containing erythrocytes) was incubated with higher concentrations of glucose, showed that increases in osmolarity increased platelet reactivity under study conditions.13,22
One of the main difficulties in studies involving platelets from diabetic humans is the difficulty of obtaining samples that are well controlled for other variables (for example, hypertension, medications). No current animal models of diabetes that reflect pathophysiologic changes similar to those of humans are sufficiently large to provide adequate quantities of platelets to study.10 In this context, development of the pig as a diabetic animal model is quite encouraging.18 For example, alloxan-treated pigs fed a high fat–high cholesterol diet show alterations in cardiovascular parameters quite similar to those of diabetic humans, including increased plasma low-density:high-density lipoprotein ratio, triglyceride concentration, glycated plasma protein levels, and early cardiovascular disease, including increased vasoconstriction, decreased vasodilation, and arterial fatty streaks.4 Underlying cellular mechanisms of vascular disease include intracellular Ca2+ dysregulation and increased tyrosine phosphorylation.15,26 Hyperlipidemic diabetic pigs therefore are emerging as a viable animal model for a variety of basic and clinically relevant research issues.7,18,21 In the present study, we used platelets from this pig model to study their thrombin–induced aggregation profile, with the goals of identifying how soon after induction of diabetes changes in the responses of platelets are seen and of examining the role of hyperlipidemia alone in this effect. Because kinase regulation is altered in the diabetic swine model, we also determined the association of platelet aggregation with mitogen-activated protein kinase (MAPK) levels.
Materials and Methods
Materials.
Platelets were isolated from freshly drawn blood from pigs as described later. All chemicals and reagents were purchased from Sigma Chemical Company (St Louis, MO) and were of the highest analytical grade available.
Diabetic pig model and blood collection.
Pigs weighing about 50 kg were used. For inducing diabetes, pigs were injected with single dose of alloxan (150 mg/kg) dissolved in 0.9% saline by means of a catheter placed in an ear vein. Control animals were injected with an equal volume of sterile saline. We used domestic male pigs (Sus scrofa domestica) treated with alloxan for a 1-wk study. In another series, we assessed male Sinclair miniature swine4,18 that were or were not made diabetic and were fed normal pig chow (controls) or a high fat–high cholesterol diet for 12 wk. The details of this pig model, including the high fat–high cholesterol feeding regimen, have been published.4 In this model, plasma total cholesterol increases about 5-fold and plasma triglycerides about 3-fold.4 Anesthesia was induced by intramuscular injection of atropine (0.05 mg/kg), ketamine (20 mg/kg), and xylazine (2 mg/kg). The level of anesthesia was maintained with isoflurane gas (maximum, 4%). Animals were kept in a biosecure environment and cared for by designated personnel. At the end of the experiment, each pig was anesthetized, the chest was opened to achieve euthanasia, and blood (60 to 70 ml) was collected into a syringe containing acid–citrate–dextrose (1 volume acid–citrate–dextrose to 5 volumes blood) through cardiac puncture by trained personnel. Blood samples were used immediately for platelet experiments.
The protocol for the use of pig as a model was approved by the University of Missouri Institutional Animal Care and Use Committee, which follows the NIH guidelines for the humane use of animals. All animal procedures complied with those approved by the American Veterinary Medical Association Panel on Euthanasia.
Platelet isolation and activation.
Washed platelets from pig blood were isolated essentially as for rabbit platelets.19 Pig blood was centrifuged at 200 × g for 15 min at 24 °C. The supernatant containing platelet-rich plasma was gently layered on 2 ml Histopaque 1077 (Sigma) in 15-ml centrifuge tubes and was centrifuged at 830 × g for 20 min. The white band at the interface, consisting of platelets, was collected carefully into a tube containing 10 ml Tyrode–gelatin–EGTA buffer (0.1 mM EGTA, pH 6.5). The platelet suspension was mixed gently and centrifuged again at 830 × g for 15 min. The supernatant was discarded and the pellet resuspended in Tyrode–gelatin–EGTA buffer and washed by centrifugation as described. The platelet pellet was resuspended to a concentration of 1 × 109 platelets per milliliter in Tyrode–gelatin buffer containing Ca2+ (2 mM Ca2+, pH 7.1) and allowed to equilibrate for 30 to 45 min at 37 °C prior to stimulation by various doses of thrombin. Aggregation of platelets was monitored using an aggregometer (Chronolog, Havertown, PA) attached to a chart recorder.
Determination of MAP kinase activity.
Mitogen-activated protein kinase was monitored in cytosolic fractions obtained from platelets essentially as described earlier12 and assayed by using established methods.1,12 Briefly, cytosolic fractions were incubated with myelin basic protein in the presence of γ32P, and the incorporation of radioactivity into myelin basic protein was determined. Values were calculated as disintegrations per min of radioactivity incorporated per milligram of cytosolic protein.
All data are expressed as mean ± 1 SD as calculated by using statistics software (GraphPad Prism, GraphPad, La Jolla, CA), and differences with P values of less than 0.05 were considered significant.
Results
We conducted 2 series of pig platelet experiments. In 1 experiment, pigs were made hyperglycemic and thus diabetic for 1 wk but remained normolipidemic because they consumed standard pig chow.4 In the 12-wk experiment, pigs were fed high fat–high cholesterol diet; a diet control group was included. Plasma glucose levels were 55 ± 1 mg/dl for the nondiabetic pigs on standard chow, 65 ± 8 mg/dl in the nondiabetic animals on high-fat diet, and 304 ± 61 mg/dl for the diabetic pigs on the high-fat diet. Because washed platelets were used to avoid any contribution from other plasma components or factors, the results (by definition) represent altered platelet responses.
Thrombin responses of pig platelets after 1 wk of diabetes.
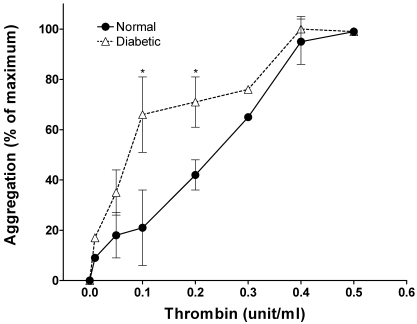
In the initial phase of these investigations, we monitored the thrombin-dose–related aggregation of platelets from pigs that were made diabetic with alloxan and were hyperglycemic but normolipidemic for 1 wk. Remarkably, even this brief period of diabetes rendered platelets hypersensitive to thrombin (Figure 1). At 0.1 unit thrombin, control platelets showed less than 10% maximal aggregation compared with 50% for samples from diabetic pigs. The 50% effective concentration of thrombin for diabetic platelets was 0.1 U/ml compared with 0.25 U/ml for control platelets.
Figure 1.
Thrombin response of pig platelets after 1 wk of diabetes. Blood was collected from pigs treated with a single dose of alloxan (see Methods) and rendered diabetic for 1 wk. The aggregation response of washed platelets to various doses of thrombin was monitored. Data are presented as mean ± 1 SD (n = 4) of percentage of maximal aggregation. An asterisk indicates significant difference (P < 0.05) compared with the control value.
Thrombin-induced aggregation of pig platelets after 12 wk of diabetes and hyperlipidemia.
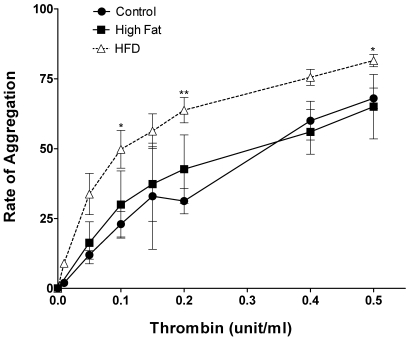
In another series of experiments we monitored platelet aggregation in 3 groups of pigs, that is control, high fat/cholesterol-fed (hyperlipidemic), and diabetic + hyperlipidemic. In these experiments the rate of aggregation was determined. The aggregation profiles (Figure 2) indicated that diabetic + hyperlipidemic pig platelets were hypersensitive to thrombin induced aggregation as compared with control. At 0.1 unit thrombin, the diabetic + hyperlipidemic platelets showed 2 to 3 times higher rate of aggregation. There was a slight tendency for hyperlipidemic platelets (from nondiabetic pigs) to exhibit increased aggregation, but this did not reach statistical significance (Figure 2). As mentioned above in the 3 groups of pigs, the serum glucose levels indicated severe hyperglycemia in diabetic + hyperlipidemic pigs only.
Figure 2.
Dose-dependent thrombin-induced aggregation of platelets from pigs that were diabetic and hyperlipidemic for 12 wk. Washed platelets from 3 groups of pigs (nondiabetic and normal diet [control], n = 5; nondiabetic and high fat–high cholesterol diet [High Fat], n = 3; diabetic and high fat–high cholesterol diet [HFD], n = 4) were treated with the indicated concentrations of thrombin. The data are presented as mean ± 1 SD of the rate of aggregation of platelets. An asterisk indicates significant difference (P < 0.05) compared with the control value.
MAPK levels in pig platelets after 12 wk of diabetes and hyperlipidemia.
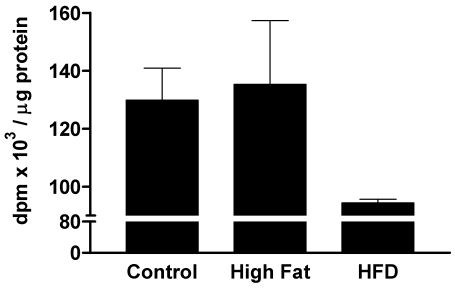
An inverse relationship between MAPK levels and platelet aggregation responses has been suggested.11,12 This suggestion was based on observations that increases in the basal MAPK concentration of human platelets reduced platelet aggregation and that a decrease in MAPK increased platelet sensitivity. If this relationship holds true, diabetic platelets can be predicted to have lower MAPK levels than do control platelets. We tested this hypothesis and found that platelets from the control and high fat–high cholesterol groups had similar levels of basal MAPK, but the MAPK levels of platelets from diabetic and hyperlipidemic animals were decreased by about 30% (Figure 3). This finding supports the conclusion that the decreased basal MAPK levels in platelets from diabetic subjects correlate with platelet hyperactivity.
Figure 3.
MAPK activity in platelets from pigs that were diabetic and hyperlipidemic for 12 wk. Groups of pigs were the same as those described for Figure 2. MAPK activity (mean ± 1 SD; n = 3 to 5) is presented as the number of disintegrations per min (dpm) · µg−1 protein.
Discussion
Thrombin acts on platelets via protease-activated receptors 1 and 4, both of which are coupled to G proteins (for example, G12/13, Gq).9,14 Which of these thrombin receptors is modulated in diabetes and results in platelet hypersensitivity is unknown currently. Similarly, the mechanism underlying the increased sensitivity and reduced basal platelet MAPK levels requires further investigation.
Taken together, our results indicate that pigs are a viable animal model for studying platelet responses in diabetes. Platelets from animals have been used in several studies.5,17,23 One of the advantages of using platelets from animals is that various parameters, including dietary intake, can be better controlled than for platelets from humans. Further, the opportunity for sequential resampling means that platelets from animals can be monitored for the progression of pathophysiologic abnormalities. In this context, rats and rabbits have been used widely as diabetic models for platelet studies.5,17,23 Issues regarding in vivo versus in vitro differences in platelet hyperactivity in diabetes and the influence of plasma factors in the hyperactivity of platelets from diabetic subjects17 remain to be investigated fully in various animal models. Platelets from sheep and swine have also been used in cardiovascular research.6 Increased aggregation of swine platelets exposed to thrombin is consistent with data from human diabetics, and in this and other features diabetes in pigs mimics the human disease. In addition, the size of pigs (approximately 30 to 80 kg for sexually mature miniature swine) is highly conducive to studies of platelets because of the large volumes of blood (approximately 100 ml) that can be drawn weekly by using vascular access ports.7,16 These methods enable studies of the full time-course of increased platelet aggregation relative to the metabolic and cardiovascular disease status of the pig.16 Therefore, in addition to the widespread use of rodent models for diabetes research,10 swine have distinct advantages.21,25 In particular, whether patients receiving drug-eluting coronary artery stents have an increased risk of thrombosis27 currently is under debate. The mechanisms underlying this potential risk can only be investigated by using swine as the preclinical model.2
Compared with those of controls, the aggregation responses of platelets to thrombin were higher even only 1 wk after alloxan treatment. Diabetic patients without vascular complications display enhanced basal platelet activation and decreased antioxidant status.23 Therefore, platelet hypersensitivity is apparent quite early in the disease process and before the manifestation of any other cardiovascular problem. Similarly, platelets from chronically diabetic hyperlipidemic pigs (12 wk) were hypersensitive to aggregation. Because we used washed platelets, any contribution by other plasma factors or blood cells to hypersensitivity is unlikely. This notion supports the view that, in diabetes, intrinsic alterations in platelets render them hypersensitive8 and, therefore, increased osmolarity due to hyperglycemia may not be the only underlying mechanism. Although hyperlipidemia alone did not render platelets hypersensitive to thrombin, it may contribute to their increased sensitivity in diabetic subjects. The data presented here also support the hypothesis that MAPK levels are inversely related to platelet aggregation responses. Therefore, the decreased basal MAPK level likely contributes to the hypersensitivity of platelets from diabetic patients.
References
- 1.Ahn NG, Weil JE, Chan CP, Krebs EG. 1990. Identification of multiple EGF-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem 265:11487–11494 [PubMed] [Google Scholar]
- 2.Carter AJ, Bailey L, Devries J, Hubbard B. 2000. The effects of uncontrolled hyperglycemia on thrombosis and formation of neointima after coronary stent placement in a novel diabetic porcine model of restenosis. Coron Artery Dis 11:473–479 [DOI] [PubMed] [Google Scholar]
- 3.Colwell JA, Nesto RW. 2003. The platelet in diabetes. Diabetes Care 26:2181–2188 [DOI] [PubMed] [Google Scholar]
- 4.Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. 1999. Dyslipidemia and vascular dysfunction in diabetic pig fed an atherogenic diet. Arterioscler Thromb Vasc Biol 19:2981–2992 [DOI] [PubMed] [Google Scholar]
- 5.Eldor A, Merin S, Bar-On H. 1978. The effect of streptozotocin diabetes on platelet function in rats. Thromb Res 13:703–714 [DOI] [PubMed] [Google Scholar]
- 6.Goodman SL. 1999. Sheep, pig, and human platelet–material interactions with model cardiovascular materials. J Biomed Mater Res 45:240–250 [DOI] [PubMed] [Google Scholar]
- 7.Henderson KK, Mokelke EA, Turk JR, Rector RS, Laughlin MH, Sturek M. 2003. Maintaining vascular access port patency and asepsis in Yucatan miniature swine. Contemp Top Lab Anim Sci 42:28–32 [PubMed] [Google Scholar]
- 8.Ho H, Shukla SD, Klachko DM. 1998. In vitro effect of elevated glucose on human platelet aggregation. EndoTrends 5:5–7 [Google Scholar]
- 9.Holinstat M, Voss B, Bilodeau ML, McLaughlin JN, Cleator J, Hamm HE. 2006. PAR4, but not PAR1, signals human nplatelet aggregation via calcium mobilization and synergistic P2Y12 receptor activation. J Biol Chem 281:26665–26674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, Rabadán-Diehl C, Goldberg IJ. 2007. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res 100:1415–1427 [DOI] [PubMed] [Google Scholar]
- 11.Kansra SV, Reddy MA, Weng Y, Shukla SD. 1999. Activation of human platelet MAP Kinase by genistein. Pharmacol Res 39:21–31 [DOI] [PubMed] [Google Scholar]
- 12.Kansra SV, Shukla SD. 2000. Reverse relationship between MAP kinase and human platelet aggregation. Clin Exp Hypertens 22:145–154 [DOI] [PubMed] [Google Scholar]
- 13.Keating FK, Sobel BE, Schneider DJ. 2003. Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am J Cardiol 92:1362–1365 [DOI] [PubMed] [Google Scholar]
- 14.Leger AJ, Covic L, Kuliopulos A. 2006. Protease-activated receptors in cardiovascular diseases. Circulation 114:1070–1077 [DOI] [PubMed] [Google Scholar]
- 15.Lee DL, Wamhoff BR, Katwa LC, Reddy HK, Voelker DJ, Dixon JL, Sturek M. 2003. Increased endothelin-induced Ca2+ signaling, tyrosine phosphorylation, and coronary artery disease in diabetic dyslipidemic swine are prevented by atorvastatin. J Pharmacol Exp Ther 306:132–140 [DOI] [PubMed] [Google Scholar]
- 16.Otis CR, Wamhoff BR, Sturek M. 2003. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med 53:53–64 [PubMed] [Google Scholar]
- 17.Paul W, Queen LR, Page CP, Ferro A. 2007. Increased platelet aggregation in vivo in the Zucker diabetic fatty rat: differences from the streptozotocin diabetic rat. Br J Pharmacol 150:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips RW, Panepinto LM. 1986. Swine as a model for human diabetes. : Tumbelson ME, editor Swine in biomedical research, vol 1. New York: Plenum Press: p 549–560 [Google Scholar]
- 19.Shukla SD, Hanahan DJ. 1984. Acetylglycerylether phosphorylcholine (platelet activating factor) induced stimulation of rabbit platelets: correlation between phosphatidic acid level, 45Ca2+ uptake and [3H] serotonin secretion. Arch Biochem Biophys 232:458–466 [DOI] [PubMed] [Google Scholar]
- 20.Shukla SD, Paul A, Klachko D. 1992. Hypersensitivity of diabetic human platelets to platelet activating factor. Thromb Res 66:239–246 [DOI] [PubMed] [Google Scholar]
- 21.Sturek M, Alloosh M, Wenzel J, Byrd JP, Edwards JM, Lloyd PG, Tune JD, March KL, Miller MA, Mokelke EA, Brisbin IL., Jr 2007. Ossabaw Island miniature swine: cardiometabolic syndrome assessment. : Swindle MM, editor Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques, 2nd ed Boca Raton (FL): CRC Press; p 397–402 [Google Scholar]
- 22.Sudic D, Razmara M, Forslund M, Ji Q, Hjemdahl P, Li N. 2006. High glucose levels enhance platelet activation: involvement of multiple mechanisms. Br J Haematol 133:315–322 [DOI] [PubMed] [Google Scholar]
- 23.Vericel E, Januel C, Carreras M, Moulin P, Lagarde M. 2004. Diabetic patients without vascular complications display enhanced basal platelet activation and decreased antioxidant status. Diabetes 53:1046–1051 [DOI] [PubMed] [Google Scholar]
- 24.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. 2001. Platelet dysfunction in type 2 diabetes. Diabetes Care 24:1476–1485 [DOI] [PubMed] [Google Scholar]
- 25.Winocour PD. 1992. Platelet abnormalities in diabetes mellitus. Diabetes 41 Suppl 2:26–31 [DOI] [PubMed] [Google Scholar]
- 26.Witczak CA, Wamhoff BR, Sturek M. 2006. Exercise training prevents Ca2+ dysregulation in coronary smooth muscle from diabetic dyslipidemic Yucatan swine. J Appl Physiol 101:752–762 [DOI] [PubMed] [Google Scholar]
- 27.Zahn R, Hamm CW, Schneider S, Zeymer U, Richardt G, Kelm M, Levenson B, Bonzel T, Tebbe U, Sabin G. 2006. Predictors of death or myocardial infarction during follow-up after coronary stenting with the sirolimus-eluting stent. Results from the prospective multicenter German Cypher Stent Registry. Am Heart J 152:1146–1152 [DOI] [PubMed] [Google Scholar]