Abstract
The present study investigates the transfer of aversively conditioned respondent elicitation through equivalence classes, using skin conductance as the measure of conditioning. The first experiment is an attempt to replicate Experiment 1 in Dougher, Augustson, Markham, Greenway, and Wulfert (1994), with different temporal parameters in the aversive conditioning procedure employed. Match-to-sample procedures were used to teach 17 participants two 4-member equivalence classes. Then, one member of one class was paired with electric shock and one member of the other class was presented without shock. The remaining stimuli from each class were presented in transfer tests. Unlike the findings in the original study, transfer of conditioning was not achieved. In Experiment 2, similar procedures were used with 30 participants, although several modifications were introduced (formation of five-member classes, direct conditioning with several elements of each class, random sequences of stimulus presentation in transfer tests, reversal in aversive conditioning contingencies). More than 80% of participants who had shown differential conditioning also showed the transfer of function effect. Moreover, this effect was replicated within subjects for 3 participants. This is the first demonstration of the transfer of aversive respondent elicitation through stimulus equivalence classes with the presentation of transfer test trials in random order. The latter prevents the possibility that transfer effects are an artefact of transfer test presentation order.
Keywords: transfer and transformation of function, aversive conditioning, derived stimulus relations, stimulus equivalence, fear, skin conductance, humans
Transfer or transformation of functions is referred to as the indirect acquisition or change of a behavioral function by one or several stimuli in an equivalence class or other relational network, after a novel function has been directly trained to a different stimulus or subset of stimuli in that class or network1 (Dougher & Markham, 1994, 1996; Dymond & Rehfeldt, 2000; Hayes, Barnes-Holmes, & Roche, 2001). The work by Dougher, Augustson, Markham, Greenway, and Wulfert (1994, Experiment 1) illustrates this phenomenon very clearly. Participants were trained on a series of interrelated conditional discrimination tasks that led to the emergence of two 4-member equivalence classes (A1–B1–C1–D1 and A2–B2–C2–D2). A mild electric shock applied to each participant's forearm then served as an unconditional stimulus (UCS) that followed presentations of B1 (i.e., aversive respondent conditioning). Stimulus B2 was also presented, but in the absence of the UCS. Conditioned emotional responses to B1 and B2 were measured as skin conductance responses (SCRs) and tonic changes in skin conductance level (SCL). Participants were then presented with some of the remaining members of each equivalence class to test for a transfer of eliciting function. Depending on the measure under consideration, 5 (as measured with SCRs) or 6 (as measured with tonic SCL changes) of the 8 participants showed evidence of respondent conditioning and a transfer of respondent function (e.g. SCRs to both C1 and D1 were higher than SCRs to both C2 and D2).
Transformation of functions, derived stimulus relations, and more generally, derived relational responding, are presumed to be key processes for a behavior-analytic account of language, cognition, and symbolic and novel behavior (e.g. Hayes, Barnes-Holmes, & Roche, 2001; Hayes & Hayes, 1989; Sidman, 1994). Recent approaches have emphasized the role that processes of transfer and transformation of functions may play in the etiology and maintenance of anxiety disorders (Forsyth, 2000; Forsyth & Eifert, 1996; Forsyth, Eifert, & Barrios, 2006; Friman, Hayes, & Wilson, 1998; Hayes, 2004; Hayes, Strohsal, & Wilson, 1999; Wilson & Blackledge, 2000). Specifically, the transformation of aversively controlled respondents and avoidance-evoking functions seems critical in this regard. These processes would serve to account, among other issues, for anxiety (i.e., covarying public and private responses like increased autonomic arousal and reports of fear) and avoidance responses that do not result from direct aversive conditioning. This issue, the indirect acquisition of fear, is one that has long been used as a basis for criticizing traditional behavioral models of anxiety based on Pavlovian conditioning and avoidance (e.g. Field, 2006; Rachman, 1977, 1991).
However, in spite of recent conceptual interest in the role of transformation of function processes for a behavioral analysis of anxiety and anxiety disorders, the fact is that there is a paucity of basic experimental research directly relevant to this issue. Although there are published reports concerning transformation of a wide diversity of behavioral functions (for a review see Dymond & Rehfeldt, 2000), research on the transformation of aversive respondents and avoidance-evoking functions is still scarce, and some published reports are not exempt from problems, as will be shown later. There are a few published reports on the transformation of avoidance evocation (Augustson & Dougher, 1997; Dymond, Roche, Forsyth, Whelan, & Rhoden, 2007, 2008), on the transfer of aversive respondent elicitation and extinction (Augustson, Dougher, & Markham, 2000; Dougher et al., 1994; Markham, Dougher, & Augustson, 2002), on the transfer of self-reported arousal functions (Smyth, Barnes-Holmes, & Forsyth, 2006), and on the transformation of aversive respondent elicitation in accordance with comparative size relations (Dougher, Hamilton, Fink, & Harrington, 2007).
The clearest evidence to date of the transfer of aversively controlled respondents is the above-mentioned work by Dougher et al. (1994), which consisted of two experiments, the first one dealing with the transfer of respondent elicitation, and the second one with the transfer of respondent extinction. Indeed, the former is the only published evidence to date of the transfer of aversive respondent elicitation with single stimuli. (There are another two studies by the same group on this phenomenon: Augustson et al., 2000; Markham et al., 2002; however, they used compound stimuli, a feature that complicates the interpretation of their results.) Given the alleged importance of transfer of aversive respondent elicitation as a key process for a behavior-analytic account of human emotional behavior (e.g., Forsyth, 2000; Forsyth et al., 2006; Friman et al. 1998), replication and extension of the pioneering work by Dougher et al. (1994, Experiment 1) become all the more relevant, especially upon careful consideration of specific features of the original experiment.
First, and most importantly, there is one feature of the experiment that can be deemed critical, as it can put into question the validity of its results. In brief, after the acquisition of differential conditioning with B1 as CS+ and B2 as CS−, two of the remaining stimuli in each class were presented (one at a time) as transfer probe trials according to any one of four specific sequences, all of which began with the presentation of C2 or D2 (the CS− related elements). This was done because pilot work had shown that the presentation of C1 or D1 (the CS+ related elements) as the first probe (i.e., in extinction) resulted in the emergence of strong respondents to C2 and D2 in subsequent probes, as if contingencies had shifted (see Dougher et al., 1994, p. 336). That is, transfer effects (i.e. larger SCRs to C1 and D1 than to C2 or D2) were observed only in specific conditions of stimulus presentation sequencing during transfer tests (i.e. CS− related elements first). Provided that there was no control condition, it could be argued that the observed results might be due to an artefact. A similar constraint in the presentation order of probes has been used in a study on human sensory preconditioning (see Vansteenwegen, Crombez, Baeyens, Hermans, & Eelen, 2000), as it seems to facilitate responses to the CS+ related elements and to prevent responses to the CS− related elements. Other researchers investigating the transfer of respondents, in this case with appetitive sexual stimuli (see Roche & Barnes, 1997), have used a partial CS–UCS contingency to prevent the shift-in-contingencies effect resulting from the presentation of probe trials in extinction and as a means to have more than a single transfer test trial available with any stimulus.
Apart from this critical issue (nonrandom sequences of probe trials), some parameters in the conditioning task and the form of response quantification used by Dougher et al. (1994) make it difficult to compare their results with the existing literature on human aversive conditioning. Obviously this is far less important in methodological terms. However it is an issue relevant to some extent if we want transfer of function research to have an impact on researchers from different theoretical perspectives dealing with the same phenomena (e.g. conditioning models of fear and anxiety).
Dougher et al. (1994, Experiment 1) employed an aversive differential delay conditioning procedure with skin conductance (SC) as the dependent variable. This procedure has been widely used in research on human autonomic aversive conditioning (e.g. Lipp, 2006; Öhman, Hamm, & Hugdahl, 2000) and it has the advantage that it allows for the observation of within-subject conditioning effects (Prokasy, 1977; Rescorla, 1967). However, the CS–UCS intervals (20–40 s) and intertrial intervals (ITIs) (90–150 s) employed by Dougher et al. were unusually long, probably due to the fact that a concurrent operant task was superimposed on their respondent conditioning procedure. A more usual parameter in this sort of research is CS duration between 5 and 10 s (see Lipp, 2006; Lovibond, 1992). The utilization of the latter would facilitate the comparison of transfer of function results with the general findings on human aversive conditioning and other forms of learning transfer that are akin to transfer of function, like sensory preconditioning (see Vansteenwegen et al., 2000; White & Davey, 1989) and higher order conditioning (Davey & Arulampalam, 1982).
Response quantification in the Dougher et al. (1994) study was performed measuring both SCL (measured as tonic changes against a constant preconditioning baseline) and SCRs (phasic changes in skin conductance level) in probe trials in extinction after CS offset (i.e. during the time interval where the UCS should have been presented: UCS-omission responses). A more usual practice currently in delay conditioning procedures is to measure anticipatory SCRs (during the CS–UCS interval, prior to the presentation of the UCS) with shorter CS duration intervals and ITIs (Lipp, 2006; Öhman et al., 2000). The measurement of anticipatory SCRs allows for the observation of conditioning acquisition across trials, without the need to present probe trials in extinction (as the actual SCRs measured always take place prior to UCS presentation, and are not contaminated by unconditioned responses). Accordingly, it also eliminates the need for probe trials in extinction during transfer tests, as it will be explained in detail below, when we introduce Experiment 2. Besides, anxiety is usually conceived as anticipatory in nature (Barlow, 2002), thus anticipatory arousal responses are presumed to be a more externally valid index of conditioning for the study of anxiety (Lipp, 2006). Although some researchers (e.g. Lovibond, 1992) have advocated the use of long CS–UCS and intertrial intervals, and for the measurement of both tonic changes in SCL and SCRs, as a potential alternative to the “current standard procedure in which CS duration is relatively short (5–10 s) and fixed, and conditioning is assessed by phasic electrodermal responses” (Lovibond, p. 621), they have actually relied on anticipatory measures of conditioning, not on UCS omission responses. This is due to the fact that the latter can be elicited by the omission of innocuous stimuli, and that their magnitude is often considered small and unreliable (Lovibond, p. 622; Siddle, 1985).
The present article describes two experiments that attempted to extend on the work by Dougher et al. (1994) and to overcome the methodological limitations already mentioned. The first one attempted to replicate Dougher et al.'s Experiment 1, with shorter CS-duration intervals and ITIs and with the measurement of anticipatory SCRs. Another procedural difference was that no concurrent operant task was superimposed on the aversive conditioning procedure, because Dougher et al. (p. 333) had already shown that such a task was not useful in obtaining a supplementary index of conditioning (i.e. conditioned suppression). The second experiment included no probe trials in extinction, with direct conditioning of two elements instead of one in each class, with the presentation of stimuli in random order in transfer tests, and a within-subject reversal in the aversive conditioning contingencies.
EXPERIMENT 1
Method
Participants
Participants were 17 undergraduates from different courses at Universidad de Almería (age range 18–25 years; 10 female, 7 male). All participants were recruited through in-class announcements, and none of them had any previous experience in psychological experiments. At the beginning of the experiment participants read and signed a statement of informed consent highlighting that the experiment involved the administration of mild electric shocks, and that participants were free to discontinue participation at any time. Upon termination of the experiment, all participants were debriefed and received a canteen voucher (exchangeable for breakfast or a snack) as a compensation for participation.
Setting, Apparatus, and Stimuli
The experiment was conducted in a laboratory consisting of two adjacent rooms (an experimental room and an observation room) of equal size (4 m by 3 m). The experimental room was dimly lit by a 40-W bulb and it was equipped with a table, a comfortable armchair, a two-way mirror for participant observation, an HP nx9010 laptop computer with a 381-mm (15-inch) color screen, and several modules for the recording of physiological responses. The computer was programmed (with VisualBasic® 6.0) to present visual stimuli and to coordinate the presentation of electric shocks, as well as to record participants' responses on some tasks. The observation room contained the remaining parts of the technical equipment for physiological response recording, as well as a desktop computer (Apple Macintosh PowerPC 5500) that served to store and analyze physiological response data from the physiological recorder.
A computerized physiological recording system (BIOPAC Instruments MP100W with a GSR100C module) was used to measure and record skin conductance (SC) through nonpolarizable Ag/AgCl finger electrodes attached to the palmar side of the distal phalanx of the first and third fingers of the participant's nondominant hand. The electrodes had a round contact area (6 mm in diameter) inside a polyurethane mould, forming a cavity 1.6 mm deep that was filled with isotonic (0.5% NaCl) electrode gel (Biopac Gel101). The system delivered a constant voltage (0.5 V) current that allowed for the continuous measurement of tonic skin conductance level (DC) (i.e. constant voltage technique of exosomatic recording; see Dawson, Schell, & Filion, 1990). Analog–digital conversion was performed at a rate of 50 Hz. For the administration of aversive stimulation, an isolated square-wave stimulator (Laffayette 82415-IS) was used. It delivered constant voltage electric shocks (300 ms in duration) through two disposable adhesive round electrodes (15 mm diameter and 40 mm apart) attached to the inner surface of the participant's nondominant arm. The laptop computer that presented the experimental tasks was connected to the psychophysiological recorder and the stimulator through a custom-made parallel-port cable that delivered the electric pulses that served as stimulus markers for the physiological records and that triggered the presentation of shocks.
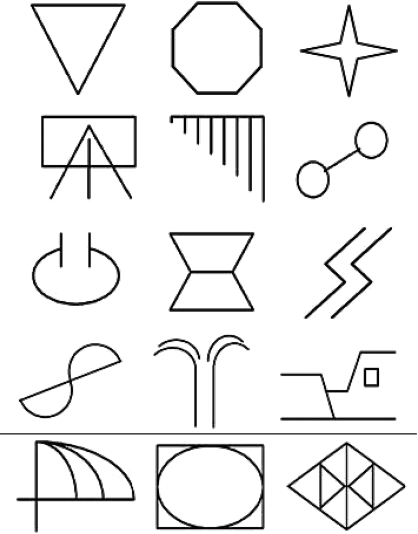
The visual stimuli used in both experiments were black shapes framed in a square white background, presented on a general black background. The size of the stimuli was 8 × 8 cm for the equivalence class formation tasks, and 10 × 10 cm for the aversive conditioning task. Specifically, 12 abstract shapes (adapted from Dougher et al., 1994, p. 333) were used for Experiment 1 (see Figure 1). For each participant, the shapes were randomly distributed into three 4-member groups by the computer. The stimuli in each group were designated with alphanumerical labels (e.g. A1, B2, C3, etc.) for procedural purposes. The participants were unaware of these labels.
Fig 1.
Abstract shapes used as arbitrary stimuli for the formation of equivalence classes in Experiments 1 and 2. The 12 shapes above the horizontal line were used in Experiment 1. All 15 shapes were used in Experiment 2.
Procedure
All the procedures in this and the following experiment were reviewed and approved by the Ethics Board of Research with Human Participants at Universidad de Almería. The experiment comprised four different phases (see Figure 2). The first one consisted of the formation (training and evaluation) of two 4-member equivalence classes. The second one consisted of an aversive differential conditioning procedure, where an element of the first class served as a CS+, while an element of the second class served as a CS−. Electric shocks of moderate intensity were used as unconditional stimulation (UCS). The third phase consisted of the assessment of transfer of respondent elicitation to the other elements of each class. The fourth phase consisted of a retest of the derived relations previously established during Phase 1.
Fig 2.
Schematic depiction of the phases in Experiment 1.
All phases were conducted in one experimental session that lasted between 60 and 120 min, except in the case of participants who failed equivalence class formation, who attended a second session the next day. Participants were run individually. All of the instructions participants were presented with at the different phases of this and the following experiment were in Spanish language. The instructions included here are a translation into English of the original ones.
Upon reporting to the laboratory, participants were interviewed and presented with an explanation of the general procedures that would follow. The experimenter placed special emphasis on the fact that participation was absolutely voluntary, and that participants were free to abandon the experiment at any time. He also told them that participation entailed receiving the administration of several shocks of moderate intensity, the level of which would be selected by the participants themselves, so that the sensation produced by the shock was definitely unpleasant, but not painful. After that, participants read and signed a statement of informed consent that, in addition to the points already mentioned by the experimenter, explicitly highlighted that participants suffering from any sort of cardiovascular disease, epilepsy, or any serious illness should not take part in the study. Then they were conducted to the experimental room and seated in the armchair facing the laptop computer.
Phase 1: Formation of equivalence classes
During this phase, participants underwent training in six different conditional discriminations (A1B1, A1C1, A1D1, A2B2, A2C2, and A2D2) through a simultaneous one-to-many matching-to-sample procedure (Sidman & Tailby, 1982) with three comparisons. After the establishment of these six relations, they underwent the assessment of derived symmetry and transitivity relations, in order to determine whether two equivalence classes had been established (Class 1: A1–B1–C1–D1; Class 2: A2–B2–C2–D2). One set of abstract shapes (A3, B3, C3, and D3) served as incorrect comparisons in conditional discrimination training and testing. No specific relations were trained nor tested for this set of stimuli. This was done because the interest was in teaching two equivalence classes, not three, but it was also important to have three comparisons in each trial, in order to control for responding by exclusion (see Carrigan & Sidman, 1992). Besides, this procedure is a direct replication of that in Dougher et al. (1994).
Both during training and test trials, a sample (e.g. A1) appeared in the center of the upper third of the screen. One second later, three comparisons (e.g. B1, B2 and B3) appeared in line in the horizontal lower third of the screen. On each trial, each comparison's position (left, center, or right) was randomly assigned. Participants responded by selecting one of the three comparisons with a mouse-click, after which all the stimuli were removed from the screen. During training trials, correct responses were followed by the word “BIEN” (i.e. good) in capital letters and white color, centered on the screen. Incorrect responses were followed by the word “MAL” (i.e. wrong) in the same format. Feedback remained on the screen for 2 s, after which the screen went blank for 1.5 s (i.e., the ITI). During test trials, no feedback was presented and thus responses were followed by the ITI only. The following instructions were presented on the screen at the start of this phase:
“In this part of the experiment you will see four shapes on the screen in each trial, one in the middle of the top of the screen, and the other three at the bottom: one on the right, one on the left, and one in the middle. The task consists of selecting the correct shape out of those three in the lower part of the screen, by clicking on it with the mouse. During the first part of the task, the computer will give you feedback on each response, indicating whether it is correct (BIEN) or wrong (MAL). Later in the task, the computer will not give feedback about your responses. However, there will be correct and incorrect responses, and you should do your best to have as many correct responses as possible.
At first the task will be easy, and you may even notice that it is not necessary to pay a lot of attention. However, task difficulty will increase gradually, and in order to select the correct shapes during the last part of the task, you must have previously performed the task correctly during the initial parts. So, it is important that you pay a lot of attention from the beginning of the task. Everything you learn during this part of the experiment will be important for later phases. If you have any doubts, please ask the experimenter. Click here to start.”
The six directly trained relations were presented in six trial blocks (one trial per relation). Each trial consisted of the presentation of the sample and its corresponding group of comparisons. Within each block, the presentation order of trials was randomized. Blocks were presented continuously until the participant achieved a mastery criterion of 46 correct responses out of eight consecutive complete blocks. After that, participants underwent a test of symmetry relations, preceded by the following instructions:
“Now you will keep performing the task. This time the computer will not give you any feedback about your responses, but there are still correct and incorrect responses. You have to get as many correct responses as possible. Click here to continue.”
The six tested symmetry relations (B1A1, B2A2, C1A1, C2A2, D1A1, and D2A2) were continuously presented in six-trial blocks (one trial per relation in random order within each block) until participants reached the mastery criterion (46 correct responses out of eight consecutive blocks), up to a maximum of five 8-block sets (i.e. 240 trials). In case the criterion was not achieved, participants were scheduled for retraining the next day.
Upon criterion achievement participants underwent a final combined test of symmetry and equivalence relations, preceded by the same instructions as for the symmetry test. In addition to the six symmetry relations, this test comprised 12 equivalence relations (B1C1, B2C2, B1D1, B2D2, C1D1, C2D2, C1B1, C2B2, D1C1, D2C2, D1B1, and D2B2) and both types of relation were presented continuously in 18-trial blocks (one trial per relation in random order) until achievement of the mastery criterion (103 correct responses out of six consecutive blocks, with a maximum of two errors for each particular relation), up to a maximum of five 6-block sets (i.e. 540 trials). In case the criterion was not achieved, participants were scheduled for retraining the next day. After that, participants passed to the next phase of the experiment.
Phase 2: Aversive conditioning
This phase began with a shock work-up procedure for the selection of voltage that would be used as unconditional stimulation during the conditioning procedure. First, the experimenter cleaned with ethanol (96% v/v) the participant's relevant skin areas and attached the stimulation and recording electrodes (as described in the Setting, Apparatus, and Stimuli section). Then he left the room, started the recording, asked the participant to breathe deeply, and checked if this produced a visible increase in SCL, which happened in all cases. The selection of shock voltage level was then performed as follows: starting with 20 V, the experimenter gradually increased the voltage of each subsequent shock in 20-V steps, until the participant reported that the shock was unpleasant enough or that it was painful. In the latter case, the experimenter kept delivering increasingly less intense shocks (in 10-V steps) until the participant reported the shock to be unpleasant, but not painful. The minimum and maximum voltages selected by any participant were 40 V and 100 V, respectively. Actually, the latter was the maximum voltage that the stimulator could deliver. The stimulator did not allow for the control of current intensity administered to each participant. When constant voltage is supplied, intensity depends on each individual's electrodermal resistance and hence it is subject to some uncontrollable variation. In any case, what can be assured is that each participant received a constant voltage shock that s/he subjectively reported as definitely unpleasant, though not painful (see Dougher et al., 2007, p. 183). After shock voltage selection, the following instructions were presented on the screen:
“During this phase of the experiment you will be presented with some shapes on the screen, one at a time. It is important that you pay attention to the screen and to the shapes appearing on it. On some occasions, you will receive shocks of the same magnitude you have just selected. Initially the computer screen will remain blank for a few minutes, just in order to have your physiological activity at a steady level. After this interval, shapes will start to appear on the screen. During this phase you don't have to use the mouse. All you have to do is to pay attention to the screen, and remain seated and quiet. It is important that you try not to move, cough, sneeze, laugh, etc., as all of these actions can interfere with the recordings, which are very sensitive. If you have any doubts, please ask the experimenter. Otherwise, press the space bar to continue.”
Once the participant confirmed that s/he had understood the instructions by stating them in her own words, the experimenter went into the observation room where he could control the recording of SCRs. There was a 5-min baseline period during which the screen remained blank. Then, the presentation of stimuli began.
The experimental task consisted of an aversive differential delay conditioning procedure, where B1 served as CS+ and B2 served as CS−. Both CSs were individually presented, centered in the computer screen, with a fixed duration of 8 s. The offset of B1 was always simultaneous to the presentation of electric shock (UCS onset). B2 was directly followed by the ITI, which had a randomly assigned variable duration of between 25 and 35 s. In sum, each CS was presented six times in quasirandom order, with the constraint that the same stimulus could not appear consecutively more than twice.
Phase 3: Transfer of function tests
This phase was presented as part of the same experimental task. Right after the acquisition of conditioning was finished the following instructions were presented on the screen:
“Now you will keep performing the same task. As you have seen, all you have to do is look at the screen with attention and keep quiet and still. During the task, on some occasions you will receive electric shocks of the same magnitude as the previous ones. Again, you will see shapes appearing on the screen, one at a time. This time you will see some other shapes apart from the two you've just seen. Initially the screen will remain blank for a few minutes. After that the shapes will start to appear on the screen. Remember that it is important that you try to be as still and quiet as possible. Press the space bar to begin.”
After that, there was a 5-min baseline period (blank screen), and then stimulus presentation began with the same CS duration and ITI parameters as employed previously. All the elements of each class were presented, with the exception of A1 and A2, as the responses to these two elements could be interpreted in terms of higher-order conditioning, rather than in terms of transfer of functions (see Dymond & Rehfeldt, 2000). First, B1 was presented, paired with shock, followed by the presentation of B2 (these two presentations served as reminders of the aversive conditioning contingencies established in the previous phase). Next, C1, D1, C2, and D2 were presented, one at a time, according to one of the four sequences presented in Table 1. None of them was paired with shock. As can be seen in Table 1, in all cases the first transfer probe trial took place with either C2 or D2, as in the study by Dougher et al. (1994, p. 336). Finally, B1 was presented in extinction, as a final probe of conditioning with this stimulus (the CS+). After that, all electrodes and leads were removed, and the participant was told to remain seated for the last phase of the experiment.
Table 1.
Sequences of trial presentation during Phase 3, Experiment 1. The stimuli were presented according to one of these four sequences. In each case, the stimuli were presented in the same order they have in each row, from left to right. (+) paired with shock; (−) presented without shock.
| Conditioning reminder | Transfer probes | Conditioning probe |
| B1 (+), B2 (−) | C2 (−), C1 (−), D2 (−), D1 (−) | B1 (−) |
| B1 (+), B2 (−) | C2 (−), D1 (−), D2 (−), C1 (−) | B1 (−) |
| B1 (+), B2 (−) | D2 (−), D1 (−), C2 (−), C1 (−) | B1 (−) |
| B1 (+), B2 (−) | D2 (−), C1 (−), C2 (−), D1 (−) | B1 (−) |
Phase 4: Retest of equivalence classes
Participants underwent a new assessment of the previously established derived relations. The task consisted of six 18-trial blocks (i.e. 108 trials), one trial in each block for each symmetry and equivalence relation in random order, with no feedback.
Response Quantification
Two types of SCR were measured: anticipatory SCRs in all trials and post-CS SCRs (UCS omission responses) in probe trials in extinction. This article just reports anticipatory SCR data2. Response amplitude was the parameter selected for quantification according to the following criteria: for each visual stimulus presented during the acquisition of conditioning and transfer tests, the largest increase in SCL (measured in µSiemens [µS]) was calculated during the visual stimulus duration interval (8 s). This variation was measured from the point of response onset, whenever this point took place at least 0.5 s after CS onset, to the highest SCL value within the 8-s interval. As noted in the literature (e.g. Dawson et al., 1990), it is possible to observe the occurrence of several consecutive or overlapped responses during this interval. In this case, the quantified response (i.e. SCR) was always the largest increase in SCL, from onset of the first response to the quantifiable maximum of the last response within the CS-duration interval. SCL decreases or SCL increases whose onset point was prior to 0.5 s after visual stimulus onset were quantified as zero.
Results
Equivalence Class Formation
The 17 participants showed the formation of two 4-member equivalence classes. Table 2 shows, for each participant, the number of trials to criterion and the number of correct responses and errors in the last group of trial blocks in each stage. For each stage, the mastery criterion refers to a specific number of correct responses out of a certain number of consecutive blocks. With “last group of trial blocks” we are referring to those final consecutive blocks that are the reference against which criterion achievement can be determined.
Table 2.
Individual results for equivalence class formation and retest. The numbers between parentheses indicate correct responses.
| Trained
relations |
Symmetry tests |
Symmetry/equivalence
tests |
Re-test (Phase
4) |
|||||
| Trials to criterion | % correct | Trials to criterion | % correct | Trials to criterion | % correct | Correct responses | % correct | |
| S1 | 120 (92) | 100% (48/48) | 48 (47) | 97.9% | 108 | 100% | ||
| S2 | 72 (61) | 100% (48/48) | 48 (47) | 97.9% | 108 (104) | 96.3% | 108 (104) | 96.3% |
| S3 | 162 (124) | 95.8% (46/48) | 48 (46) | 95.8% | 108 | 100% | 108 (107) | 99.1% |
| S4 | 66 (55) | 95.8% (46/48) | 48 (46) | 95.8% | 108 (105) | 97.2% | 108 (107) | 99.1% |
| S5 | 96 (70) | 95.8% (46/48) | 48 | 100% | 108 (105) | 97.2% | 108 (107) | 99.1% |
| S6 | 162 (107) | 97.9% (47/48) | 48 (46) | 95.8% | 108 (105) | 97.2% | 108 (107) | 99.1% |
| S7 | 72 (54) | 95.8% (46/48) | 48 | 100% | 108 | 100% | 108 (103) | 95.37% |
| S8 | 96 (72) | 95.8% (46/48) | 48 (46) | 95.8% | 144 (135) | 99.1% (107/108) | 108 (107) | 99.1% |
| S9 | 96 (74) | 97.9% (47/48) | 48 | 100% | 108 (107) | 99.1% | 108 (105) | 97.2% |
| S10 | 96 (75) | 100% (48/48) | 48 | 100% | 108 | 100% | 108 | 100% |
| S11 | 180 (100) | 95.8% (46/48) | 48 | 100% | 108 (107) | 99.1% | ||
| S12 | 132 (89) | 95.8% (46/48) | 48 | 97.9% | 108 (105) | 97.2% | 108 | 100% |
| S13 | 90 (73) | 95.8% (46/48) | 48 | 100% | 108 (105) | 97.2% | 108 (106) | 98.1% |
| S14 | 156 (99) | 95.8% (46/48) | 48 | 100% | 108 (107) | 99.1% | ||
| S15 | 132 (95) | 97.9% (47/48) | 48 (47) | 97.9% | 108 (106) | 98.1% | 108 | 100% |
| S16 | 180 (91) | 95.8% (46/48) | 48 | 100% | 108 (107) | 99.1% | 108 (107) | 99.1% |
| S17 | 72 (61) | 100% (48/48) | 48 | 100% | 108 (107) | 99.1% | 108 | 100% |
There were considerable differences in conditional discrimination training performance (trials to criterion), from 66 trials for S4, to 180 for S11 and S16. All participants passed symmetry tests after the first 48 trials. Except for S8, who took 144 trials, all participants achieved the criterion in the combined symmetry/equivalence test after the first 108 trials. The maximum number of errors for this test was four (participant S2), with a maximum of two errors for a specific relation.
Aversive Conditioning and Transfer of Functions (Anticipatory SCRs)
Data from 2 participants (S1 and S14) had to be discarded, as their physiological records were lost due to technical problems (thus, the sample was reduced to 15 participants).
The criterion to determine if conditioning had been acquired for each participant was: larger SCRs to B1 (CS+) than to B2 (CS−) in more than half of the conditioning trials (i.e., four out of seven trials), with an average difference of at least 0.05 µS between B1 and B2. This criterion is an adaptation of that used by Vansteenwegen et al. (2000) in their study on sensory preconditioning. The criterion to determine whether transfer of functions had been obtained was that both SCRs to the elements of Class 1 (C1 and D1) should be larger than both SCRs to the elements of Class 2 (C2 and D2).
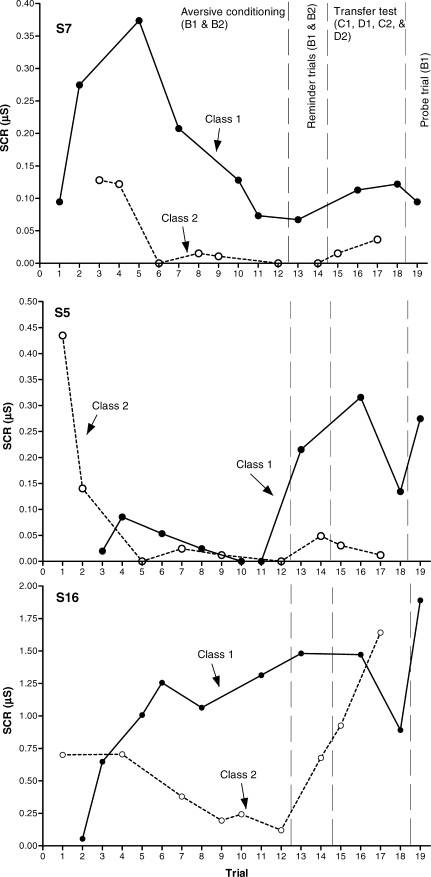
Figure 3 includes three graphs, each one depicting the anticipatory SCRs to each stimulus during Phases 2 and 3 for one representative participant. The upper graph presents the results for participant S7, who showed a good level of differential conditioning and a transfer of function effect. The medium graph presents the results for S5, who showed a transfer-like effect, but who did not acquire differential conditioning previously. The lower graph presents the results for S16, who showed a good level of differential conditioning, but who failed to show transfer of functions. Each participant's results regarding conditioning acquisition and transfer of functions are presented in Table 3. Twelve out of the 15 participants that completed the experiment reached the differential conditioning criterion (all but S5, S11, and S12) before undergoing the transfer tests. Only 3 (S7, S13, and S15) out of these 12 participants (25%) reached the transfer criterion, that is, they showed larger anticipatory SCRs to both C1 and D1 than to C2 and D2. Participant S5 showed a response pattern that could qualify as a transfer effect; however this participant did not reach the conditioning criterion. This represents 33% of the subjects who did not show a good conditioning effect, which is somehow indicative of the low power of the transfer effect observed (i.e. the response pattern that qualifies as transfer was equally likely whether previous conditioning had been established or not). The working hypothesis is that once the equivalence relations are established, given appropriate contextual cues, the direct acquisition of aversive properties by one element of the class should result in the derived emergence of aversive functions for the other elements of the class. According to this, the results for S5 cannot have the same consideration as those of the other participants who achieved the transfer criterion. If B1 did not acquire a respondent function through direct conditioning, no transfer of function effects should be expected for this participant.
Fig 3.
Experiment 1. Aversive conditioning and transfer of function results (anticipatory SCR) for S7 (upper graph), S5 (middle graph), and S16 (lower graph). Trials 1–12 pertain to Phase 2 (conditioning acquisition, B1 and B2). Trials 13–19 pertain to Phase 3 (13–14, conditioning reminders with B1 and B2; 15–18, transfer probe trials with C1, D1, C2, and D2; 19, final probe trial with B1).
Table 3.
Individual SCR results for conditioning and transfer tests. Column B1 > B2 includes the number of trials with larger SCRs to CS+ than to CS−; column dif. (µS) indicates the average difference between SCRs to CS+ and SCRs to CS−; SCR columns (C1, D1, C2 and D2) comprise the raw SCR for each element during transfer probe tests; columns designated criterion indicate achievement of conditioning or transfer criteria (criterion achievement highlighted in grey).
| Transfer of function
trials |
||||||||
| Conditioning
acquisition |
SCRs
(µS) |
criterion | ||||||
| B1 > B2 | dif.(µS) | criterion | C1 | D1 | C2 | D2 | ||
| S7 | 6 | 0.13 | YES | 0.11 | 0.12 | 0.04 | 0.02 | YES |
| S13 | 7 | 0.12 | YES | 0.07 | 0.11 | 0.05 | 0.00 | YES |
| S15 | 7 | 0.18 | YES | 0.10 | 0.11 | 0.02 | 0.06 | YES |
| S2 | 7 | 0.13 | YES | 0.00 | 0.24 | 0.12 | 0.03 | NO |
| S3 | 6 | 0.13 | YES | 0.03 | 0.04 | 0.05 | 0.02 | NO |
| S4 | 6 | 0.23 | YES | 0.28 | 1.11 | 0.96 | 0.14 | NO |
| S6 | 6 | 0.15 | YES | 0.07 | 0.03 | 0.07 | 0.00 | NO |
| S8 | 6 | 0.13 | YES | 0.07 | 0.06 | 0.07 | 0.02 | NO |
| S9 | 6 | 0.79 | YES | 1.02 | 0.69 | 0.48 | 1.22 | NO |
| S10 | 6 | 0.61 | YES | 1.60 | 0.63 | 1.21 | 0.39 | NO |
| S16 | 5 | 0.54 | YES | 0.89 | 1.47 | 0.93 | 1.64 | NO |
| S17 | 6 | 0.06 | YES | 0.03 | 0.08 | 0.06 | 0.00 | NO |
| S5 | 3 | −0.04 | NO | 0.32 | 0.13 | 0.03 | 0.01 | YES |
| S11 | 4 | 0.03 | NO | 0.00 | 0.07 | 0.00 | 0.00 | NO |
| S12 | 3 | 0.01 | NO | 0.00 | 0.00 | 0.00 | 0.00 | NO |
Retest of Equivalence Classes
With the exception of participant S11, who had to abandon the experimental session, all participants underwent the retest of the derived relations established in Phase 1. In all cases, performance in the symmetry/equivalence test maintained the high level achieved in the first phase, with percentages of correct responses always above 95% (see Table 2).
Discussion
The results show that the transfer of respondent elicitation effect obtained by Dougher et al. (1994) was not replicated with temporal parameters of conditioning like those commonly used in research on human aversive conditioning (e.g. Lipp, 2006). The proportion of participants that reached the transfer criterion was very low (not far from the chance level of 16.67%) whether that proportion is taken only from those who showed conditioning or out of the total sample. The lack of this effect cannot be attributed to a weak conditioning procedure, as most participants (12 out of 15) showed a clear conditioning effect according to a strict criterion. This criterion did not only require an averaged minimum difference between responses to the CS+ and the CS− (as in Vansteenwegen et al., 2000), but also a minimum number of trials where each SCR to the CS+ was larger than the corresponding SCR to the CS−.
The absence of transfer effects cannot be attributed to a problem in the maintenance of equivalence relations either. As can be observed from the results in the equivalence retest, all the participants showed the maintenance of derived relations. However, the fact that derived relations were present in the same context where they were trained and tested (i.e., the MTS procedure) does not necessarily entail that they were relevant during the transfer probe trials. For these, it could be argued that the arranged contingencies conflicted with previous contingencies according to which members of Class 1 (B1) reliably predicted the presentation of shock. The fact that transfer probe trials were presented in extinction made sure that when each participant was presented with the second transfer probe for each class, they already had experience with conflicting conditioning contingencies; that is, after repeated pairing of B1 with shock and B2 with absence of shock, the fact that either C1 or D1 were presented in extinction conflicted with the functional equivalence that would be expected from the previously established equivalence relations among the stimuli in each class. This may have had the effect of establishing that under the conditioning-transfer context the previously established relations were not relevant for the prediction of shock presentation.
The problem of the potential contaminating effects of probe trials in extinction is not new, and previous research in the area has had to deal with it, either with the use of nonrandom constrained sequences (Dougher et al., 1994; Vansteenwegen et al., 2000) or with the use of a partial CS–UCS contingency during acquisition (Roche & Barnes, 1997; Vansteenwegen et al., 2000). Although the former procedure has been used here, it has not been effective.
All in all, this experiment failed to replicate the results of Dougher et al. (1994). Further research was necessary to obtain a clear demonstration of the transfer of aversively controlled respondent functions. The next experiment was designed to overcome the limitations in Experiment 1.
EXPERIMENT 2
According to the previous discussion, the variable that in our view best accounted for the failure to observe transfer effects in the previous experiment was the presentation of transfer probe trials in extinction. Accordingly, the present experiment was designed so that transfer tests were presented in a way that allowed for the maintenance of the contingencies established during differential conditioning acquisition. This maintenance of conditioning contingencies during transfer tests may seem an obstacle for the observation of transfer effects. However, it is important to note that when measuring anticipatory SCRs with a delay conditioning procedure (i.e. SCRs are measured during the CS–UCS interval, prior to UCS presentation), the responses occurring during the first conditioning trial with any transfer stimulus take place before the first actual pairing of that specific stimulus with the UCS. Accordingly, these responses to the first presentation of a visual stimulus cannot be attributed to a history of direct conditioning, but to a different variable (e.g. novelty, orientation, stimulus generalization, transfer of functions). The use of a differential conditioning procedure would control for possible contaminating variables like novelty or orientation, and the use of randomly assigned physically similar stimuli in each class would control for possible stimulus generalization effects. According to this, the first presentation of any transfer stimulus would serve as a transfer test, even though this very stimulus is actually paired with the UCS on that presentation (the anticipatory SCRs are measured before the pairing actually takes place). If transfer is assessed only upon the first presentation of each transfer stimulus, then it does not matter whether that stimulus is paired with shock or not. This arrangement allows the participants' experience during the transfer tests to be consistent with the previously established equivalence relations and conditioning contingencies. It also allows for the presentation of transfer trials in random order, provided that there would be no extinction for CS+ related stimuli during those trials. This would prevent possible artefacts due to sequences with a constrained order of presentation of the stimuli in each class. The option of using alternative procedures like a partial reinforcement CS–UCS contingency was discarded on the basis of pilot work.
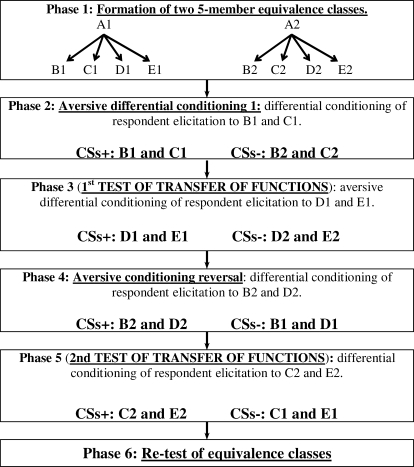
In addition, in order to further facilitate the transfer effects, conditioning was directly established with two members of each class before each transfer test. That is, two members of Class 1 served as CSs+ and two members of Class 2 served as CSs−. This modification required an increase in the size of equivalence classes, from four to five members, in order to still have two members of each class available for transfer tests. Finally, all of these variations allowed for the implementation of a reversal design aimed to see if the transfer effect could be replicated within-subject.
Method
Participants
Thirty undergraduate and graduate students recruited through in-class announcements at Universidad de Almería took part in the experiment (age range: 18–29; 14 female, 16 male). They were interviewed and the experimenter explained the general experimental procedures to them. Next, they had to read and sign the same statement of informed consent as in Experiment 1. None of them had any previous experience in psychological experiments. Upon termination of the experiment all participants were debriefed and received a voucher for the University canteen.
Setting, Apparatus, and Stimuli
The experiment was carried out in the same experimental context and with the same apparatus and materials as Experiment 1. Apart from the 12 abstract shapes used in Experiment 1, another 3 shapes of the same sort were used as visual stimuli (Figure 1). For each participant the 15 shapes were randomly distributed into three groups of five elements. Each shape was designated with an alphanumerical label. Participants were unaware of these labels.
Procedure
The experiment included six different phases (see Figure 4). The first one consisted of the formation (training and testing) of two 5-member equivalence classes. Phases 2 to 5 each consisted of an aversive differential conditioning procedure, where two elements of one class served as CSs+ and two elements of the other class served as CSs−, with electric shocks as the UCS. Phases 2 and 3 had Class 1 elements as CSs+, and Class 2 elements as CSs−. The first block of trials in Phase 3 served as the first transfer of function test. Phases 4 and 5 involved a reversal in the conditioning contingencies previously established, now with members of Class 2 as CSs+ and members of Class 1 as CSs−. The first block of trials in Phase 5 served as the second transfer of function test. The sixth phase was a retest of symmetry and equivalence relations.
Fig 4.
Schematic depiction of the phases of Experiment 2.
Almost all of the particulars regarding participant reception, explanation of procedures, signature of informed consent, and general preparation and procedure of the experiment (i.e. participant preparation, electrode placement, instructions, etc.) were as in Experiment 1. Accordingly, for each phase only the details that were different will be described.
Phase 1: Formation of equivalence classes
The procedure is an adaptation of that in Experiment 1 to the training and testing of five-member classes. Training and test trials were carried out identically to Experiment 1, with the same parameters, consequences for performance, and instructions.
Participants were trained in eight conditional discriminations: A1B1, A2B2, A1C1, A2C2, A1D1, A2D2, A1E1, and A2E2. After that, they underwent the assessment of symmetry and equivalence relations. During training, participants were continuously presented with eight-trial blocks (one trial per relation in random order) until achievement of the mastery criterion (61 correct responses out of eight consecutive blocks, with a maximum of two errors per specific relation). Then they underwent the assessment of eight symmetry relations (B1A1, B2A2, C1A1, C2A2, D1A1, D2A2, E1A1, and E2A2), presented continuously in eight-trial blocks (one trial per relation, in random order within each block) without feedback until achievement of the same performance criterion as in training, up to a maximum of five 8-block sets (i.e. 320 trials). In case the criterion was not achieved, participants were scheduled for retraining the next day. Finally, they underwent the combined assessment of symmetry and equivalence relations, which, apart from the eight symmetry relations, included the following 24 equivalence relations: B1C1, B2C2, B1D1, B2D2, B1E1, B2E2, C1B1, C2B2, C1D1, C2D2, C1E1, C2E2, D1B1, D2B2, D1C1, D2C2, D1E1, D2E2, E1B1, E2B2, E1C1, E2C2, E1D1, and E2D2. Relations were presented continuously in 32-trial blocks (one trial per relation, eight symmetry and 24 equivalence trials, in random order without feedback) until criterion achievement (153 correct responses out of five consecutive 32-trial blocks, with a maximum of two errors per specific relation), up to a maximum of five 5-block sets (i.e. 800 trials). In case the criterion was not achieved, participants were scheduled for retraining the next day.
Phase 2: Aversive conditioning with B1, C1, B2, and C2
This phase began with a shock work-up procedure (i.e. voltage selection) identical to that in Experiment 1. Although they will be described separately, Phases 2, 3, 4, and 5 were presented consecutively as part of the same experimental task, without breaks or stops amongst phases. The aversive conditioning procedure was identical to the one employed in the previous experiment (i.e. 8-s delay aversive differential conditioning, with UCS onset simultaneous with CS+ offset). The only difference, both here and in the next phases, was the fact that there were two CSs+ and two CSs−. Each CS+ was always followed by the presentation of a shock (UCS), while each CS− was always followed immediately by the ITI.
Once the participants were prepared for psychophysiological recording and the shock voltage had been selected, they were presented with the same instructions as in the aversive conditioning acquisition phase in Experiment 1 (Phase 2). After a 5-min baseline period, during which the screen remained blank, the presentation of visual stimuli took place as follows: B1, C1, B2, and C2 were presented in four-trial blocks (one trial per stimulus), in random order within each block; a total of three blocks was presented, that is, 12 trials, 3 per stimulus; B1 and C1 served as CSs+, while B2 and C2 served as CSs−.
Phase 3: Aversive conditioning with D1, E1, D2, and E2 (1stTRANSFER OF FUNCTION TEST)
This phase was almost identical to the previous one, with the only difference that the stimuli employed were D1 and E1 (CSs+) and D2 and E2 (CSs−). Stimuli were presented in four-trial blocks in random order within each block. The first four-trial block served as the first transfer test. As already stated, the anticipatory SCRs during these four trials cannot be attributed to a direct conditioning history, provided that this was the first time that each of these stimuli was presented in the conditioning task. Therefore, none of them had served as a CS+ or CS− yet.
Phase 4: Aversive conditioning with B1, D1, B2, and D2 (CONTINGENCY REVERSAL)
So far, participants had undergone the acquisition of aversive conditioning with four elements of each class. B1, C1, D1, and E1 had served as CSs+ for three trials each (B1 and C1 during Phase 2, and D1 and E1 during Phase 3), whereas B2, C2, D2, and E2 had each served as CSs− for three trials (B2 and C2 during Phase 2, and D2 and E2 during Phase 3). In this phase there was a contingency reversal, so that B2 and D2 served as CSs+, while B1 and D1 served as CSs−. As in previous phases, all four stimuli were presented in three 4-trial blocks (one trial per stimulus in random order within each block).
Phase 5: Aversive conditioning with C1, E1, C2 and E2 (2ndTRANSFER OF FUNCTION TEST)
This phase was almost identical to the previous one, with the only difference that the stimuli employed were C2 and E2 (CSs+) and C1 and E1 (CSs−). Stimuli were presented in four-trial blocks in random order within each block. Following the same rationale presented in Phase 3 (first transfer test), the first block in this phase served as a transfer test after the contingency reversal. This block was the first presentation of each of these four stimuli (C1, E1, C2, and E2) with the new contingency arrangement. So far, each of these stimuli had had a direct conditioning history that was the opposite of the reversed contingency arrangement.
Phase 6: Retest of equivalence classes
After finishing the aversive conditioning task (comprising Phases 2, 3, 4, and 5) subjects underwent a retest of symmetry and equivalence relations. The procedure consisted of the presentation of five 32-trial blocks, one trial per relation (8 symmetry and 24 equivalence relations) in random order within each block.
Response Quantification
Response quantification was carried out as in the previous experiment for anticipatory SCRs.
Results
Formation of Equivalence Classes
The 30 participants that took part in the experiment showed the formation of two 5-member equivalence classes. Table 4 shows, for each participant, the number of trials to criterion for each stage, as well as the number of correct responses and errors in the last group of trial blocks for each stage. Participants showed great variability in length of conditional discrimination training, from 96 trials in the case of S3, S9, S12, S23, and S30, to 208 trials in the case of S11. Most participants passed the symmetry test after the first 64 trials. S29 needed 280 trials to reach criterion in the symmetry test, while participants S7, S8, S22, and S30 had to be retrained in a second experimental session (the next day), as they did not reach the performance criterion in the symmetry test after more than 320 trials. Combined symmetry and equivalence tests were completed after the first 160 trials by all participants but 2. S4 took 192 trials and S19 took 224 trials to finish this test.
Table 4.
Individual results for equivalence class formation and retest. Numbers between parentheses indicate correct responses.
| Training |
Symmetry tests |
Symmetry/equivalence
tests |
Retest (Phase
6) |
|||||
| Trials to criterion | % correct | Trials to criterion | % correct | Trials to criterion | % correct | Correct responses | % correct | |
| S1 | 104 (79) | 95.3% (61/64) | 64 | 100% | 160 | 100% | 159 | 99.4% |
| S2 | 152 (113) | 96.9% (62/64) | 64 | 100% | 160 | 100% | 159 | 99.4% |
| S3 | 96 (78) | 96.9% (62/64) | 64 | 100% | 160 | 100% | 11 | 6.9% |
| S4 | 112 (97) | 96.9% (62/64) | 64 (63) | 98.4% | 192 (185) | 100% (160/160) | 159 | 99.4% |
| S5 | 112 (89) | 95.3% (61/64) | 64 | 100% | 160 | 100% | 160 | 100% |
| S6 | 136 (97) | 95.3% (61/64) | 64 | 100% | 160 (159) | 99.4% | ||
| S7 | 136 (97) | 95.3% (61/64) | >320 | |||||
| 64 (61) | 95.3% (61/64) | 64 | 100% | 160 | 100% | 160 | 100% | |
| S8 | 112 (95) | 96.9% (62/64) | >320 | |||||
| 88 (82) | 98.4% (63/64) | 64 | 100% | 160 | 100% | |||
| S9 | 96 (76) | 98.4% (63/64) | 64 | 100% | 160 (156) | 97.5% | 160 | 100% |
| S10 | 184 (124) | 95.3% (61/64) | 64 | 100% | 160 (159) | 99.4% | 160 | 100% |
| S11 | 208 (161) | 96.9% (62/64) | 64 (62) | 96.9% | 160 (158) | 98.8% | 155 | 96.9% |
| S12 | 96 (82) | 96.9% (62/64) | 64 | 100% | 160 (159) | 99.4% | 160 | 100% |
| S13 | 176 (132) | 96.9% (62/64) | 64 | 100% | 160 (159) | 99.4% | ||
| S14 | 128 (94) | 96.9% (62/64) | 64 (63) | 98.4% | 160 | 100% | 160 | 100% |
| S15 | 144 (104) | 98.4% (63/64) | 64 | 100% | 160 (159) | 99.4% | 160 | 100% |
| S16 | 128 (100) | 95.3% (61/64) | 64 (62) | 96.9% | 160 (157) | 98.1% | 156 | 97.5% |
| S17 | 120 (100) | 96.9% (62/64) | 64 (63) | 98.4% | 160 (158) | 98.8% | 160 | 100% |
| S18 | 112 (87) | 96.9% (62/64) | 64 | 100% | 160 (159) | 99.4% | 160 | 100% |
| S19 | 184 (139) | 95.3% (61/64) | 64 | 100% | 224 (204) | 98.8% (158/160) | 160 | 100% |
| S20 | 152 (96) | 96.9% (62/64) | 64 (63) | 98.4% | 160 (155) | 96.9% | 148 | 92.5% |
| S21 | 104 (89) | 95.3% (61/64) | 64 | 100% | 160 (159) | 99.4% | ||
| S22 | 120 (95) | 96.9% (62/64) | >320 | |||||
| 80 (71) | 98.4% (63/64) | 64 | 100% | 160 | 100% | 159 | 99.4% | |
| S23 | 96 (83) | 96.9% (62/64) | 64 | 100% | 160 (156) | 97.5% | 153 | 95.6% |
| S24 | 136 (95) | 95.3% (61/64) | 64 | 100% | 160 | 100% | 160 | 100% |
| S25 | 192 (141) | 96.9% (62/64) | 64 | 100% | 160 (153) | 95.6% | 156 | 97.5% |
| S26 | 136 (101) | 95.3% (61/64) | 64 | 100% | 160 | 100% | 160 | 100% |
| S27 | 152 (100) | 95.3% (61/64) | 64 (62) | 96.9% | 160 | 100% | 159 | 99.4% |
| S28 | 136 (103) | 95.3% (61/64) | 64 | 100% | 160 | 100% | 159 | 99.4% |
| S29 | 136 (98) | 95.3% (61/64) | 280 (251) | 95.3% (61/64) | 160 | 100% | ||
| S30 | 96 (74) | 95.3% (61/64) | >320 | |||||
| 64 | 100% | 64 | 100% | 160 | 100% | 160 | 100% | |
Aversive Conditioning and Transfer of Functions
Data from some participants were discarded in this phase for different reasons. The records for S6, S8, S13, and S21 were lost (their data files were overwritten by mistake). For S19 the experimental session was not programmed correctly, and this participant just underwent Phases 2 and 3 (but not the contingency reversal); accordingly, only the data regarding Phases 2 and 3 are presented for this participant. There was a failure in the power supply while S7 was undergoing the third trial of the second transfer test; the task terminated in that moment (there was a blackout), so the data for this test are not complete and only the data regarding Phases 2 and 3 (that were correctly stored) are presented here; after the resumption of power supply, this participant directly underwent the equivalence retest. For S24 there was a technical problem with the shocker and this participant did not receive any shocks after the start of Phase 4 (contingency reversal); thus, only the data regarding Phases 2 and 3 will be presented. Accordingly, the final sample is left as follows: for Phases 2 and 3, N = 26 (all participants but S6, S8, S13, and S21); for Phases 4 and 5, N = 23 (the former 26 participants minus S7, S19, and S24).
The criteria established to determine whether conditioning or transfer had been obtained were as in Experiment 1. In each transfer test, participants had to show larger SCRs to the CS+ related elements (D1 and E1 in the first transfer test, and C2 and E2 in the second transfer test). During the conditioning acquisition phases (Phases 2 and 4) participants had to show larger SCRs to the CSs+ (B1 and C1 in Phase 2, and B2 and D2 in Phase 4) than to the CSs− in more than half of the acquisition trials (i.e., in at least 4/6), with a minimum average difference of 0.05 µS between both classes.
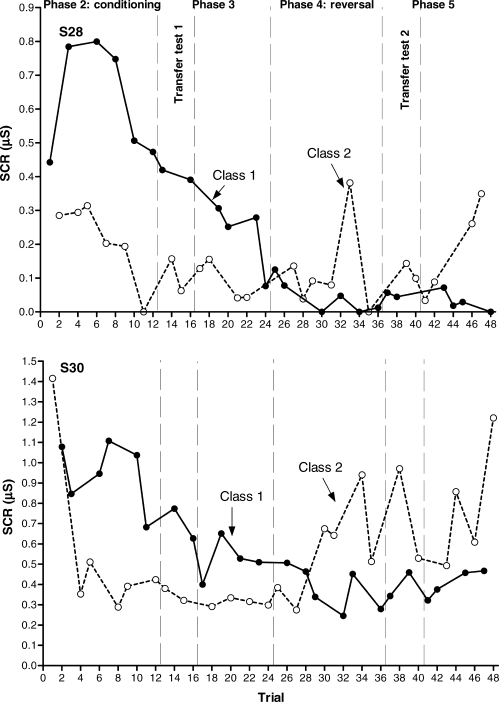
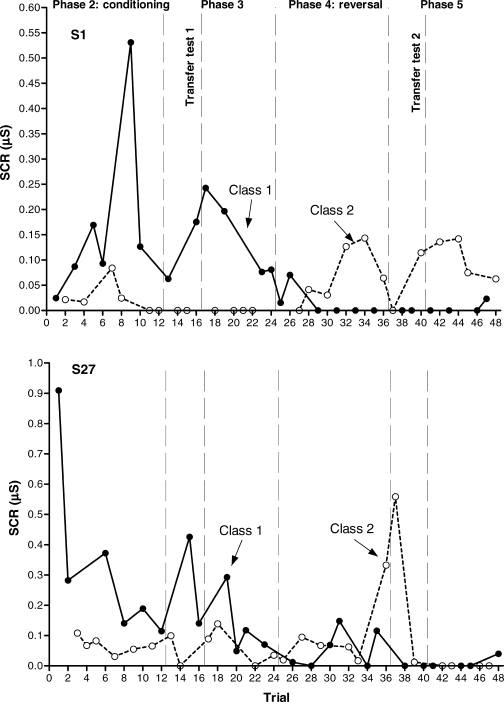
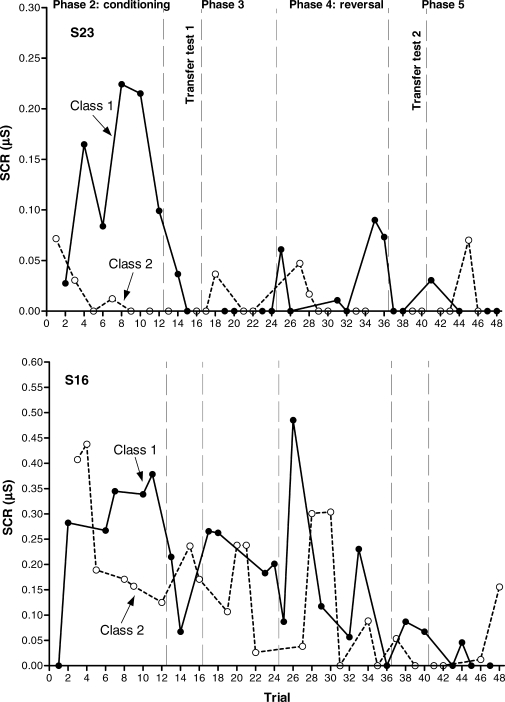
Figure 5 presents the results for S28 and S30. These participants showed differential conditioning and transfer of functions before and after the contingency reversal, and they represent the ideal response pattern of a within-subject replication of the transfer of function effect. Figure 6 presents the results for S1 and S27. S1 showed differential conditioning both in Phases 2 (first conditioning acquisition) and 4 (contingency reversal), and transfer of functions only in the first transfer test (Phase 3, before the reversal). S27 showed differential conditioning and transfer of functions before the contingency reversal (Phases 2 and 3), but neither reversed conditioning nor transfer after the reversal (Phases 4 and 5). Figure 7 presents the results for S23 and S16. Before the reversal, S23 showed differential conditioning (Phase 2) but not transfer of functions (Phase 3); after the reversal, neither conditioning nor transfer were observed. S16 did not show conditioning or transfer either before or after the reversal.
Fig 5.
Experiment 2. Aversive conditioning and transfer of function results (anticipatory SCRs) for S28 (upper graph) and S30 (lower graph). Transfer tests are the first four-trial blocks in Phases 3 and 5, respectively.
Fig 6.
Experiment 2. Aversive conditioning and transfer of function results (anticipatory SCRs) for S1 (upper graph) and S27 (lower graph). Transfer tests are the first four-trial blocks in Phases 3 and 5, respectively.
Fig 7.
Experiment 2. Aversive conditioning and transfer of function results (anticipatory SCRs) for S23 (upper graph) and S16 (lower graph). Transfer tests are the first four-trial blocks in Phases 3 and 5, respectively.
Table 5 presents each participant's results regarding conditioning acquisition and transfer of functions. In the table, participants appear in order from top to bottom, depending on whether they reached the conditioning and transfer criteria. The grey areas indicate those cases where the corresponding criterion (either for conditioning or transfer) was reached. The order column refers to the presentation order of stimuli in transfer tests, according to class membership (note that it was randomized). This is relevant to see whether certain sequences (e.g. the presentation of members from Class 2 prior to members of Class 1) might have facilitated or interfered with transfer of function effects.
Table 5.
SCR aversive conditioning and transfer of functions for Experiment 2. Column B1C1 > B2C2 indicates the number of trials where SCRs to B1 or C1 (CSs+) were larger than the corresponding responses to B2 or C2 (the CSs−) in Phase 2. Column B2D2 > B1D1 indicates the number of trials where SCRs to B2 and D2 (CSs+) were larger than the corresponding SCRs to B1 and D1 (CSs−) in Phase 4. The columns designated dif. (μS) indicate the average difference in μS between SCRs to the CSs+ and CSs− in Phases 2 and 4. Columns designated Cr. indicate the achievement of conditioning or transfer criteria in each phase. The columns designated Order indicate the presentation order of stimuli in transfer tests (according to class membership). The four columns below each transfer test present the raw SCR data for each stimulus in that test.
| Phase 2 Conditioning
1 |
Phase 3 Transfer
1 |
||||||||
| B1C1 > B2C2 | dif. (μS) | Cr. | Class 1 D1 or E1 | Class 2 D2 or E2 | Order | Cr. | |||
| S2 | 4 | 0.26 | Y | 0.14 | 0.10 | 0.06 | 0.08 | 1,2,2,1 | Y |
| S28 | 6 | 0.41 | Y | 0.42 | 0.39 | 0.16 | 0.06 | 1,2,2,1 | Y |
| S30 | 5 | 0.39 | Y | 0.77 | 0.63 | 0.38 | 0.32 | 2,1,2,1 | Y |
| S1 | 6 | 0.15 | Y | 0.06 | 0.18 | 0.00 | 0.00 | 1,2,2,1 | Y |
| S15 | 6 | 0.15 | Y | 0.05 | 0.10 | 0.03 | 0.00 | 2,1,1,2 | Y |
| S3 | 6 | 0.08 | Y | 0.15 | 0.08 | 0.04 | 0.04 | 2,2,1,1 | Y |
| S14 | 4 | 0.05 | Y | 0.05 | 0.05 | 0.01 | 0.02 | 2,2,1,1 | Y |
| S17 | 5 | 0.12 | Y | 0.10 | 0.06 | 0.02 | 0.00 | 2,1,2,1 | Y |
| S5 | 5 | 0.09 | Y | 0.08 | 0.06 | 0.02 | 0.02 | 2,1,1,2 | Y |
| S27 | 6 | 0.27 | Y | 0.43 | 0.14 | 0.10 | 0.00 | 2,2,1,1 | Y |
| S22 | 4 | 0.10 | Y | 0.17 | 0.17 | 0.00 | 0.00 | 1,2,2,1 | Y |
| S29 | 5 | 0.09 | Y | 0.07 | 0.10 | 0.00 | 0.01 | 1,1,2,2 | Y |
| S19 | 4 | 0.05 | Y | 0.12 | 0.08 | 0.00 | 0.00 | 2,1,1,2 | Y |
| S24 | 4 | 0.06 | Y | 0.06 | 0.03 | 0.00 | 0.00 | 2,2,1,1 | Y |
| S9 | 4 | 0.09 | Y | 0.21 | 0.14 | 0.16 | 0.23 | 2,2,1,1 | N |
| S4 | 5 | 0.16 | Y | 0.28 | 0.16 | 0.55 | 0.26 | 2,2,1,1 | N |
| S23 | 5 | 0.12 | Y | 0.04 | 0.00 | 0.00 | 0.00 | 2,1,1,2 | N |
| S18 | 2 | −0.01 | N | 0.05 | 0.04 | 0.01 | 0.02 | 2,2,1,1 | Y |
| S20 | 3 | 0.03 | N | 0.14 | 0.16 | 0.00 | 0.04 | 1,2,2,1 | Y |
| S12 | 2 | −0.02 | N | 0.00 | 0.02 | 0.00 | 0.01 | 1,1,2,2 | N |
| S10 | 1 | −0.01 | N | 0.00 | 0.00 | 0.00 | 0.00 | 2,1,1,2 | N |
| S11 | 1 | −0.08 | N | 0.00 | 0.05 | 0.00 | 0.03 | 2,1,2,1 | N |
| S16 | 4 | 0.02 | N | 0.22 | 0.07 | 0.24 | 0.17 | 1,1,2,2 | N |
| S25 | 0 | −0.10 | N | 0.04 | 0.00 | 0.06 | 0.00 | 2,2,1,1 | N |
| S26 | 3 | 0.01 | N | 0.00 | 0.08 | 0.00 | 0.00 | 1,2,1,2 | N |
| S7 | 4 | 0.03 | N | 0.12 | 0.02 | 0.04 | 0.04 | 2,2,1,1 | N |
| Phase 4 (reversal)
Conditioning 2 |
Phase 5 Transfer
2 |
|||||||
| B2D2 > B1D1 | dif. (μS) | Cr. | Class 1 C1 or E1 | Class 2 C2 or E2 | Order | Cr. | ||
| 6 | 0.11 | Y | 0.18 | 0.02 | 0.39 | 0.20 | 1,2,2,1 | Y |
| 4 | 0.08 | Y | 0.06 | 0.04 | 0.14 | 0.10 | 1,1,2,2 | Y |
| 4 | 0.19 | Y | 0.34 | 0.46 | 0.97 | 0.53 | 1,2,1,2 | Y |
| 4 | 0.05 | Y | 0.00 | 0.00 | 0.00 | 0.11 | 2,1,1,2 | N |
| 6 | 0.16 | Y | 0.02 | 0.02 | 0.08 | 0.02 | 1,2,2,1 | N |
| 3 | 0.01 | N | 0.00 | 0.02 | 0.26 | 0.05 | 1,2,1,2 | Y |
| 2 | −0.00 | N | 0.00 | 0.00 | 0.02 | 0.03 | 2,1,2,1 | Y |
| 3 | −0.00 | N | 0.00 | 0.00 | 0.04 | 0.02 | 1,2,1,2 | Y |
| 5 | 0.04 | N | 0.02 | 0.01 | 0.01 | 0.02 | 2,1,1,2 | N |
| 4 | 0.04 | N | 0.00 | 0.00 | 0.56 | 0.00 | 2,1,2,1 | N |
| 2 | 0.08 | N | 0.02 | 0.41 | 0.07 | 0.05 | 1,2,1,2 | N |
| 3 | −0.16 | N | 0.03 | 0.04 | 0.03 | 0.11 | 1,1,2,2 | N |
| 4 | 0.04 | N | 0.12 | 0.08 | 0.09 | 0.00 | 1,2,2,1 | N |
| 1 | −0.09 | N | 0.26 | 0.22 | 0.30 | 0.24 | 1,2,1,2 | N |
| 1 | −0.03 | N | 0.00 | 0.00 | 0.00 | 0.00 | 1,1,2,2 | N |
| 3 | 0.01 | N | 0.00 | 0.00 | 0.02 | 0.03 | 2,1,2,1 | Y |
| 2 | 0.01 | N | 0.00 | 0.00 | 0.26 | 0.17 | 2,1,2,1 | Y |
| 5 | 0.02 | N | 0.00 | 0.01 | 0.03 | 0.02 | 1,2,1,2 | Y |
| 3 | 0.04 | N | 0.00 | 0.02 | 0.03 | 0.00 | 1,1,2,2 | N |
| 1 | −0.05 | N | 0.00 | 0.00 | 0.03 | 0.00 | 1,2,2,1 | N |
| 1 | −0.04 | N | 0.09 | 0.07 | 0.05 | 0.00 | 2,1,2,1 | N |
| 4 | 0.02 | N | 0.00 | 0.00 | 0.00 | 0.00 | 1,1,2,2 | N |
| 0 | 0.00 | N | 0.00 | 0.00 | 0.00 | 0.00 | 1,2,1,2 | N |
As can be seen, 17 out of 26 participants (S2 to S23) reached the conditioning criterion in the first conditioning phase (65% of the sample). Fourteen of those 17 (82%) showed the transfer effect in the first test. Besides, 2 participants (S18 and S20) reached criterion in the first transfer test without having previously reached the conditioning criterion (22% of those who did not show conditioning). Considering that the odds of obtaining a transfer-like effect by chance are 1/6 (16.67%), these 2 participants cannot be considered in the same way as those who had previously shown good conditioning. For the latter, the achievement of the transfer criterion clearly points to a transfer of function effect, as shown by a significant difference between the observed proportion and the chance proportion, χ2(1) = 12.159, p = .0005. For the former, however, the observed proportion of participants that achieved the transfer criterion did not differ from chance, χ2(1) = .089; p = .7657.
Only 5 out of 23 participants (22% of the sample) reached the conditioning criterion in Phase 4, according to the contingency reversal. Three out of those 5 showed the transfer effect (S2, S28, and S30). Moreover, these 3 participants showed conditioning and transfer in all phases, which means that the within-subject replication of the transfer effect was observed for only a reduced proportion of the total sample (3/23: 13%), but for a substantial proportion of the participants who reached the conditioning criterion in Phases 2 and 4 (3/5: 60%).
There were 6 participants (S3, S14, S17, S18, S20, and S12) who reached criterion in the second transfer test without having previously reached the conditioning criterion in Phase 4. That is, 6 out of the 18 participants (33%) who did not show reversed conditioning in Phase 4 reached the performance criterion during the second transfer test. If we consider the total sample, we find that 9 out of 23 (39%) reached the performance criterion in the second transfer test. It is important to point out that 8 of these had previously reached the criterion in the first transfer test (89%). This datum includes both participants who had shown good conditioning levels and participants who had not. We think this result merits further discussion (see below).
Five out of 17 participants who achieved the conditioning criterion during Phase 2 had a Class 1 element (either D1 or E1) as the first transfer test trial, while the remaining 12 had an element of Class 2 (either D2 or E2). For the former, 100% (5/5) showed the transfer of function effect, while for the latter, 75% (9/12) did. That is, the transfer of function effect in the first transfer test did not depend on the order of presentation of each class's elements. In the second test it is complicated to draw conclusions about the influence of the order of stimulus presentation, given that only 5 participants showed reversed conditioning in Phase 4. Four of those (S2, S15, S28, and S30) underwent the transfer test with an element of Class 1 as the first test trial, and 3 of them showed the transfer of function effect. The only participant (S1) that began the test with an element of Class 2 did not show the transfer effect.
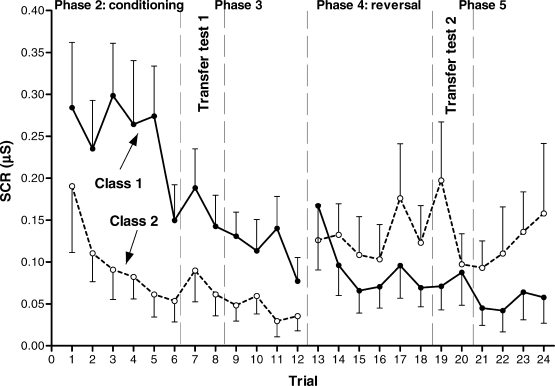
Finally, because only representative individual graphs have been presented, to give an idea of the general results for most participants, Figure 8 presents a graphic depiction of the averaged SCRs in each trial for all participants who reached the conditioning criterion in the first conditioning acquisition phase (Phase 2). Unlike the individual graphs presented above, where each trial refers to a single stimulus (either a member of Class 1 or Class 2) in the exact order they were presented, in this graph each trial refers to the corresponding presentation of a stimulus from each class (as usual in group graphs of differential conditioning). That is, trial one refers to the first presentation of a member from Class 1 and the first presentation of a member from Class 2, trial 2 to the second, and so on. The graph appears divided into two separate halves: the first for Phases 2 (first conditioning acquisition) and 3 (first transfer test); the second for Phases 4 (conditioning reversal) and 5 (second transfer test). This is because 2 participants (S19 and S24) who completed Phases 2 and 3 did not undergo Phases 4 and 5 (see Table 5). Two separate Greenhouse-Geisser adjusted 3 × 2 repeated measures ANOVAs (trial × stimulus class) were performed (in order to compare conditioning and transfer both before and after the contingency reversal). The first ANOVA compared the last conditioning trial in Phase 2 (trial 6, see Figure 8) with the transfer test trials from Phase 3 (trials 7 and 8 in Figure 8). There were significant differences for stimulus class, F(1, 16) = 12.703, p = .003; η2 = .443, but not for trial, F(2, 32) (ε = 0.709) = 3.770, p = .052 or the trial × stimulus-class interaction, F < 1. The second ANOVA compared the last conditioning trial in Phase 4 (trial 18, see Figure 8) with the transfer test trials from Phase 5 (trials 19 and 20 in Figure 8). Again, there were significant differences for stimulus class, F(1, 14) = 5.524, p = .034; η2 = .283, but not for trial or for the interaction (both ps > .120). According to this, SCRs during transfer trials significantly differed between stimulus classes in both tests (larger for Class 1 in the first test, and larger for Class 2 in the second test). Besides, they were of a similar magnitude to the corresponding directly conditioned SCRs in the last conditioning trial of each acquisition phase (Phases 2 and 4).
Fig 8.
Experiment 2. Aversive conditioning and transfer of function results. Averaged anticipatory SCRs (error bars show SEM) for all participants reaching the conditioning criterion in Phase 2. Transfer tests are trials 7 and 8 (Phase 3) and trials 19 and 20 (Phase 5). Participants S19 and S24 did not undergo Phases 4 and 5 (see Table 5). Accordingly, for Phases 2 and 3, N = 17, and for Phases 4 and 5, N = 15.
Retest of Equivalence Classes
Except for S29, who had to leave the experimental session before starting the retest, all participants underwent the equivalence retest. With the exception of S3 and S20, all the other participants showed near perfect (>95% correct responses) performance (see Table 4).
Discussion
The results in the present experiment show a clear effect of transfer of aversively controlled respondent elicitation functions. In summary, before the contingency reversal more than 80% of participants who showed conditioning also showed transfer of functions. After the reversal, 60% of participants showing a good differential conditioning effect also demonstrated transfer of functions (although this is a percentage of a very reduced sample); in all cases, they were participants who had already shown a good level of conditioning and transfer before the reversal (i.e. they showed a within-subject replication of the transfer effect). These results did not seem to depend on class membership of the first stimulus presented in the transfer tests.
This effect is observed for anticipatory SCRs, and it is the first empirical evidence of transfer of aversive respondent functions with the temporal parameters more usually employed in psychophysiological research in human autonomic conditioning (e.g. Lipp, 2006; Öhman et al., 2000). The number of between-subject replications of this effect is higher than has been usual in this and related areas of behavior analytic research (e.g. Augustson & Dougher, 1997; Augustson et al., 2000; Dougher et al., 1994; Dougher et al., 2007; Dymond et al., 2007; Markham et al., 2002; Roche & Barnes, 1997). In addition, an important methodological improvement in comparison with previous studies (e.g. Dougher et al., 1994; Dougher et al, 2007) is the fact that these results were obtained with random sequences of stimulus presentation during transfer tests. Accordingly, these outcomes cannot be explained in terms of a methodological artefact resulting from the presentation order of stimuli in transfer test trials.
The transfer effect before the contingency reversal was clearly dependent on the previous acquisition of differential conditioning. This is consistent with the working hypothesis of the experiment and with the general view on transfer and transformation of functions (e.g. Dougher & Markham, 1996). After the reversal, the proportion of participants who achieved the conditioning criterion was much reduced, which complicates the interpretation of results. The proportion of participants who showed the transfer effect out of those showing conditioning was still high (60%: 3/5), all of them showing a complete within-subject replication of the transfer effect. Although this represents only a small portion of the total sample, this finding is important in confirming the effectiveness of the experimental manipulations implemented in Experiment 2. The presentation of aversive conditioning contingencies to all class members in the task, together with the fact that conditioning was established for two class members, instead of one, before transfer tests, seem to have served to facilitate the transfer effect. Both manipulations are consistent with the idea that in order to facilitate transfer, the experimental task should clearly indicate that the aversive conditioning contingencies are applicable to all class members. However, provided that both manipulations were introduced concurrently it is not possible to know which of them was more important in this regard. Further research should try to isolate the relative contribution of each of these experimental manipulations to the achievement of transfer of function effects. Besides, other manipulations might be tested, such as multiple exemplar training in direct aversive conditioning with all class members for several stimulus sets, and subsequent tests with a novel stimulus set after direct conditioning with only a limited subset of its elements.
After the contingency reversal almost 40% of participants showed a transfer-like pattern, regardless of their previous achievement of the conditioning criterion. Almost 90% of them had already achieved the performance criterion in the first transfer test. It is questionable to presume that in all of these cases the achievement of the transfer criterion in the second test reflects a transfer of function effect. However, it is a very high percentage out of those who reached the criterion in the second test. According to this result, reaching the transfer criterion in the first test seems a better predictor of reaching the transfer criterion in the second test than the level of differential conditioning achieved in the second conditioning phase. This is difficult to interpret, because if the achievement of the transfer criterion is indicative of a transfer effect, the aversive function must have been directly acquired previously in order to transfer. However, discarding these results as purely a chance effect may be unwise, considering that the probability of achieving the transfer criterion in both tests at chance is very low (1/36). A possible explanation for these results may be that the amount of differential conditioning trials in the reversal conditioning phase (Phase 4) was too short for the observation of a clear counterconditioning effect, but that these trials were sufficient to produce function covariation (e.g., Markham & Markham, 2002) and thus a derived transfer effect in the second transfer test. Note that the second test took place after a reversal of the conditioning contingencies, so that participants had experience with both classes not only in terms of the equivalence relations established among them, but also in the sense that the elements in each class were functionally equivalent by virtue of a commonly trained respondent function during Phases 2 and 3 (see Goldiamond, 1966; Markham & Markham, 2002). Research has shown that stimulus classes can be established through training in a common function for different stimuli (e.g. Markham & Markham, 2002). Thus, when a generalized pattern of functional equivalence has emerged, contingency reversals may initially not affect all members overtly, but show up emergently for class members that have not yet been exposed to the new contingency arrangement. Accordingly, when a participant was presented with the second transfer test in the current study, he or she already had experience with a reversal in contingencies for two class members. This effect would be similar to that observed when pigeons are exposed to successive contingency reversals (e.g. Vaughan, 1988). Of course, the foregoing is rather speculative, and further research is needed. An alternative hypothesis is that a general habituation effect for most participants in the study made it difficult to observe a clear differentiation of responding to each class during and after the reversal. This is consistent with the fact that some participants achieved the transfer criterion in the second test with very low amplitude SCRs (S14, S17, S18, S12). A combination of both effects is also a possible explanation.
GENERAL DISCUSSION
The results provide clear evidence of transfer of function effects with aversively controlled respondents. These findings are relevant in regard to several research issues, both from basic and clinical points of view. First, the findings from the second experiment confirm and extend the results obtained in previous studies, where transfer of aversively established respondent functions was observed (Dougher et al., 1994). The resemblance between the parameters employed here and those commonly used in the literature on human autonomic conditioning may facilitate consideration of transfer of function effects by human conditioning researchers with different theoretical points of view (e.g., see the volume by Craske, Hermans, & Vansteenwegen, 2006).
Second, these findings constitute an important empirical support to recent behavior-analytic approaches to the study of anxiety and, in general, of human emotional responding and psychopathology. In recent times, it has been suggested from a behavior-analytic perspective that verbal processes (derived relational responding) may explain fear and avoidance that occur in the absence of a direct conditioning history (e.g., Blackledge, 2004; Eifert & Forsyth, 2005; Forsyth, 2000; Forsyth & Eifert, 1996; Forsyth et al., 2006; Friman et al, 1998; Hayes, 2004). The transfer effects observed in this study constitute evidence of such verbal processes by which individuals can come to fear stimuli that have never been paired with aversive experiences. According to our results, fear may be triggered by a multitude of apparently safe situations by verbal–relational means.
Finally, we will point out some caveats pertaining to the results presented here and will sketch some future research lines that may stem from this work. First, both experiments used mildly aversive stimulation. Even though all participants presumably felt the shocks as disturbing enough, the experiments took place in a safe context, with very brief aversive stimuli, most of them clearly predictable on the basis of prior experience during the experiment. As such, the conditioned and derived fear responses observed in the experiments are not much like clinically relevant fears or other emotionally relevant situations in everyday life. Further research in this area should attempt to extend on these findings by replicating them with different experimental measures of fear and anxiety. For instance, Lissek et al. (2005) have suggested that conditioning preparations with the startle reflex (see Lang, Bradley, & Cuthbert, 1990) as a dependent variable show higher differences in conditioning between clinically anxious patients and normals. Perhaps research on transfer of emotional functions with this sort of measure would allow for exploring transfer of function processes with clinical and subclinical populations.
Second, individual differences may have prevented the observation of clearer effects with autonomic responses. Unlike other studies (e.g. Dougher et al., 1994, 2007; Levis & Smith, 1987), participants were never screened for autonomic responsiveness before each experiment. If participants had been selected on the basis of their score in an autonomic sensitivity test (like the balloon-burst test; see Levis & Smith) prior to conditioning, less variable results might have been obtained. For instance, it would be interesting to see whether “high SC responders” (see Levis & Smith) would more easily reach the criterion for conditioning after the reversal, thus allowing a higher number of within-subject replications of the transfer of function effect.
Third, these findings have been obtained for a specific index of autonomic arousal (electrodermal activity), which is customarily taken as a relevant measure for the study of fear and anxiety (e.g. Lipp, 2006). The extent to which they can be considered clinically relevant might be arguable, especially if we consider that from a behavior-analytic point of view the key element to the analysis of anxiety and its disorders is avoidance responding (e.g. Hayes, 1976). Nevertheless, in our view these results complement the existing findings on the transformation of avoidance evoking functions (Augustson & Dougher, 1997; Dymond et al., 2007) and, to the extent that we grant any role to respondent conditioning in the analysis of emotions (e.g. Skinner, 1953), they are of obvious relevance.
All in all, we hope that these findings become a relevant step in a fruitful research line exploring the transformation of clinically relevant functions, allowing other researchers to advance along this path. We also hope that they contribute to a better understanding of fear and anxiety from a behavior-analytic perspective.
Acknowledgments
This study was done in partial fulfilment of the requirements for a Ph.D. degree granted to Miguel Rodríguez-Valverde by the University of Almería, while he was funded by a research grant from the Spanish Ministry of Education and Science (Beca FPU-MEC, ref. AP2002-0868). Parts of it were presented at the Association for Behavior Analysis Annual Convention, Chicago, May 2005.
Footnotes
It has been argued that “transformation of stimulus functions” is a more generic term than “transfer”, as it can be applied to situations when changes in stimulus functions involve relations other than equivalence (Barnes-Holmes, Hayes, Dymond, & O'Hora, 2001). When equivalence relations are involved both terms can be used interchangeably. We will adopt this practice in the present article.
In order to shorten the present report we have decided not to include UCS-omission SCR data. The results with this measure (according to the use of Dougher et al.'s criteria for conditioning and transfer; op. cit., pp. 337–338) are very similar to those obtained with anticipatory SCRs, and provided that the latter are the only SCRs measured in Experiment 2, we have decided to favour their inclusion here over the former. The results regarding UCS-omission responses are available from the first author on request.
REFERENCES
- Augustson E.M, Dougher M.J. The transfer of avoidance evoking functions through stimulus equivalence classes. Journal of Behavior Therapy and Experimental Psychiatry. 1997;28:181–197. doi: 10.1016/s0005-7916(97)00008-6. [DOI] [PubMed] [Google Scholar]
- Augustson E.M, Dougher M.J, Markham M.R. Emergence of conditional stimulus relations and transfer of respondent eliciting functions among compound stimuli. The Psychological Record. 2000;50:745–770. [Google Scholar]
- Barlow D.H. Anxiety and its disorders: The nature and treatment of anxiety and panic (2nd ed.) New York: Guilford Press; 2002. [Google Scholar]
- Barnes-Holmes D, Hayes S.C, Dymond S, O'Hora D. Multiple stimulus relations and the transformation of stimulus functions. In: Hayes S.C, Barnes-Holmes D, Roche B, editors. Relational Frame Theory: A post-Skinnerian approach to human language and cognition. New York: Kluwer; 2001. pp. 51–72. In. [Google Scholar]
- Blackledge J.T. Functional contextual processes in posttraumatic stress. International Journal of Psychology and Psychological Therapy. 2004;4:443–467. [Google Scholar]
- Carrigan P.F, Sidman M. Conditional discrimination and equivalence relations: A theoretical analysis of control by negative stimuli. Journal of the Experimental Analysis of Behavior. 1992;58:183–204. doi: 10.1901/jeab.1992.58-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske M.G, Hermans D, Vansteenwegen D, editors. Fear and Learning. From basic processes to clinical implications. Washington D.C: American Psychological Association; 2006. [Google Scholar]
- Davey G.C.L, Arulampalam T. Second-order “fear” conditioning in humans. Persistence of CR2 following extincion of CR1. Behaviour Research & Therapy. 1982;20:391–396. doi: 10.1016/0005-7967(82)90099-7. [DOI] [PubMed] [Google Scholar]
- Dawson M.E, Schell A.M, Filion D.L. The electrodermal system. In: Cacioppo J.T, Tassinary L.G, editors. Principles of psychophysiology. New York: Cambridge University Press; 1990. pp. 295–324. In. [Google Scholar]
- Dougher M.J, Augustson E, Markham M.R, Greenway D.E, Wulfert E. The transfer of respondent eliciting and extinction functions through stimulus equivalence classes. Journal of the Experimental Analysis of Behavior. 1994;62:331–351. doi: 10.1901/jeab.1994.62-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougher M.J, Hamilton D.A, Fink B.C, Harrington J. Transformation of the discriminative and eliciting functions of generalized relational stimuli. Journal of the Experimental Analysis of Behavior. 2007;88:179–197. doi: 10.1901/jeab.2007.45-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougher M.J, Markham M.R. Stimulus equivalence, functional equivalence and the transfer of function. In: Hayes S.C, Hayes L.J, Sato M, Ono K, editors. Behavior analysis of language and cognition. Reno, NV: Context Press; 1994. In. [Google Scholar]
- Dougher M.J, Markham M.R. Stimulus classes and the untrained acquisition of stimulus functions. In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. Amsterdam: North Holland; 1996. pp. 71–90. In. [Google Scholar]
- Dymond S, Rehfeldt R.A. Understanding complex behavior: The transformation of stimulus functions. The Behavior Analyst. 2000;23:239–254. doi: 10.1007/BF03392013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Roche B, Forsyth J.P, Whelan R, Rhoden J. Transformation of avoidance response functions in accordance with same and opposite relational frames. Journal of the Experimental Analysis of Behavior. 2007;88:249–262. doi: 10.1901/jeab.2007.22-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Roche B, Forsyth J.P, Whelan R, Rhoden J. Derived avoidance learning: transformation of avoidance response functions in accordance with same and opposite relational frames. The Psychological Record. 2008;58:269–286. doi: 10.1901/jeab.2007.22-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifert G.H, Forsyth J.P. Acceptance and Commitment Therapy for anxiety disorders: A practitioner's treatment guide to using mindfulness, acceptance, and value based behavior change strategies. Oakland, CA: New Harbinger; 2005. [Google Scholar]
- Field A.P. Is conditioning a useful framework for understanding the development and treatment of phobias. Clinical Psychology Review. 2006;26:857–875. doi: 10.1016/j.cpr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Forsyth J.P. A process-oriented approach to the etiology, maintenance, and treatment of anxiety disorders. In: Dougher M.J, editor. Clinical behavior analysis. Reno NV: Context Press; 2000. pp. 153–180. In. [Google Scholar]
- Forsyth J.P, Eifert G.H. The language of feeling and the feeling of anxiety: contributions of the behaviorisms toward understanding the function-altering effects of language. The Psychological Record. 1996;46:607–649. [Google Scholar]
- Forsyth J.P, Eifert G.H, Barrios V. Fear conditioning in an emotion regulation context: A fresh perspective on the origins of anxiety disorders. In: Craske M.G, Hermans D, Vansteenwegen D, editors. Fear and learning: Basic science to clinical application. Washington, DC: American Psychological Association; 2006. pp. 133–153. In. [Google Scholar]
- Friman P.C, Hayes S.C, Wilson K.G. Why behavior analysts should study emotion: The example of anxiety. Journal of Applied Behavior Analysis. 1998;31:137–156. doi: 10.1901/jaba.1998.31-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldiamond I. Perception, language, and conceptualization rules. In: Kleinmuntz B, editor. Problem solving: Research, method, and theory. New York: Wiley; 1966. pp. 183–224. In. [Google Scholar]
- Hayes S.C. The role of approach contingencies in phobic behavior. Behavior Therapy. 1976;7:28–36. [Google Scholar]
- Hayes S.C. Acceptance and Commitment Therapy, Relational Frame Theory, and the third wave of behavioral and cognitive therapies. Behavior Therapy. 2004;35:639–665. doi: 10.1016/j.beth.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Hayes S.C, Barnes-Holmes D, Roche B. Relational Frame Theory: a post-Skinnerian account of human language and cognition. New York: Kluwer; 2001. [DOI] [PubMed] [Google Scholar]
- Hayes S.C, Hayes L.J. The verbal action of the listener as a basis for rule-governance. In: Hayes S.C, editor. Rule-governed behavior: Cognition, contingencies, and instructional control. New York: Plenum Press; 1989. In. [Google Scholar]
- Hayes S.C, Strohsal K.D, Wilson K.G. Acceptance and Commitment Therapy. An experiential approach to behavior change. New York: Guilford; 1999. [Google Scholar]
- Lang P.J, Bradley M.M, Cuthbert B.N. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Levis D.J, Smith J.E. Getting individual differences in autonomic conditioning to work for you instead of against you: Determining the dominant psychological stress channel on the basis of a biological stress test. Psychophysiology. 1987;24:346–352. doi: 10.1111/j.1469-8986.1987.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Lipp O.V. Human fear learning: contemporary procedures and measurement. In: Craske M.G, Hermans D, Vansteenwegen D, editors. Fear and learning: Basic science to clinical application. Washington, DC: American Psychological Association; 2006. pp. 37–52. In. [Google Scholar]
- Lissek S, Powers A.S, McClure E.B, Phelps E.A, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lovibond P.F. Tonic and phasic electrodermal measures of human aversive conditioning with long duration stimuli. Psychophysiology. 1992;29:621–632. doi: 10.1111/j.1469-8986.1992.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Markham M.R, Dougher M.J, Augustson E.M. Transfer of operant discrimination and respondent elicitation via emergent relations of compound stimuli. The Psychological Record. 2002;52:325–350. [Google Scholar]
- Markham R.G, Markham M.R. On the role of covarying functions in stimulus class formation and transfer of function. Journal of the Experimental Analysis of Behavior. 2002;78:509–525. doi: 10.1901/jeab.2002.78-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Hamm A, Hugdahl K. Cognition and the autonomic nervous system. Orienting, anticipation and conditioning. In: Cacioppo J.T, Tassinary L.G, Berntson G.G, editors. Handbook of psychophysiology (2nd ed.) New York: Cambridge University Press; 2000. pp. 533–575. In. [Google Scholar]
- Prokasy W.F. First-interval skin conductance responses: conditioned or orienting responses. Psychophysiology. 1977;14:360–367. doi: 10.1111/j.1469-8986.1977.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Rachman S.J. The conditioning theory of fear acquisition: a critical examination. Behaviour Research and Therapy. 1977;15:375–387. doi: 10.1016/0005-7967(77)90041-9. [DOI] [PubMed] [Google Scholar]
- Rachman S.J. Neo-conditioning and the classical theory of fear acquisition. Clinical Psychology Review. 1991;11:155–173. [Google Scholar]
- Rescorla R.A. Pavlovian conditioning and its proper control procedures. Psychological Review. 1967;74:71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- Roche B, Barnes D. A transformation of respondently conditioned stimulus function in accordance with arbitrarily applicable relations. Journal of the Experimental Analysis of Behavior. 1997;67:275–301. doi: 10.1901/jeab.1997.67-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle D.A.T. Effects of stimulus omission and stimulus change on dishabituation of the skin conductance response. Journal of Experimental Psychology: Learning, Memory and Cognition. 1985;11:206–216. doi: 10.1037//0278-7393.11.2.206. [DOI] [PubMed] [Google Scholar]
- Sidman M. Equivalence relations and behavior: A research story. Boston, MA: Authors Cooperative; 1994. [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination versus matching to sample: An expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner B.F. Science and human behavior. New York: McMillan; 1953. [Google Scholar]
- Smyth S, Barnes-Holmes D, Forsyth J.P. A derived transfer of simple discrimination and self-reported arousal functions in spider fearful and non-spider-fearful participants. Journal of the Experimental Analysis of Behavior. 2006;85:223–246. doi: 10.1901/jeab.2006.02-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteenwegen D, Crombez G, Baeyens F, Hermans D, Eelen P. Pre-extinction of sensory preconditioned electrodermal activity. Quarterly Journal of Experimental Psychology. 2000;53B:359–371. doi: 10.1080/713932734. [DOI] [PubMed] [Google Scholar]
- Vaughan W., Jr Formation of equivalence sets in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:36–42. [Google Scholar]
- White K, Davey G.C.L. Sensory preconditioning and UCS inflation in human “fear” conditioning. Behaviour Research and Therapy. 1989;27:161–166. doi: 10.1016/0005-7967(89)90074-0. [DOI] [PubMed] [Google Scholar]
- Wilson K.G, Blackledge J.T. Recent developments in the behavioral analysis of language: Making sense of clinical phenomena. In: Dougher M.J, editor. Clinical behavior analysis. Reno NV: Context Press; 2000. pp. 27–46. In. [Google Scholar]