Abstract
Acquisition and maintenance of touch-screen responding was examined in naïve cynomolgus monkeys (Macaca fascicularis) under automaintenance and classical conditioning arrangements. In the first condition of Experiment 1, we compared acquisition of screen touching to a randomly positioned stimulus (a gray square) that was either stationary or moving under automaintenance (i.e., banana pellet delivery followed an 8-s stimulus presentation or immediately upon a stimulus touch). For all subjects stimulus touching occurred within the first session and increased to at least 50% of trials by the end of four sessions (320 trials). In the subsequent condition, stimulus touching further increased under a similar procedure in which pellets were only delivered if a stimulus touch occurred (fixed ratio 1 with 8-s limited hold). In Experiment 2, 6 naive subjects were initially exposed to a classical conditioning procedure (8-s stimulus preceded pellet delivery). Despite the absence of a programmed response contingency, all subjects touched the stimulus within the first session and responded on about 50% or more of trials by the second session. Responding was also sensitive to negative, neutral, and positive response contingencies introduced in subsequent conditions. Similar to other species, monkeys engaged in stimulus-directed behavior when stimulus presentations were paired with food delivery. However, stimulus-directed behavior quickly conformed to response contingencies upon subsequent introduction. Video recordings of sessions showed topographies of stimulus-directed behavior that resembled food acquisition and consumption.
Keywords: response acquisition, autoshaping, automaintenance, negative automaintenance, touch screen, monkey
A classical or respondent conditioning procedure arranges for a stimulus (conditional stimulus, CS) to reliably precede the occurrence of some biologically significant event (unconditional stimulus, US). CS-directed responding has been engendered and maintained by stimulus-pairing (SP) procedures in a wide variety of species, despite the absence of a programmed response contingency (Balsam, Drew, & Yang, 2002; Buzsaki, 1982; Colwill, Absher, & Roberts, 1988a, 1988b; Tomie, 1976). Autoshaping procedures superimpose response contingencies on behavior elicited by SP (e.g., responses produce immediate food) and have often been employed as a means of training operant behavior in naive animals (Brown & Jenkins, 1968; Downing & Neuringer, 1976; Gamzu & Schwartz, 1973; Gamzu & Williams, 1973; Gibbon, Baldock, Locurto, Gold, & Terrace, 1977; Hursh, Navarick, & Fantino, 1974; Locurto, Terrace, & Gibbon, 1976; Newlin & LoLordo, 1976; Papachristos & Gallistel, 2006; Poling & Poling, 1978; Rachlin, 1969). Consistent with findings from SP experiments, the topography of CS-directed responding in autoshaping often resembles the behavior elicited by the US (e.g., drinking and eating; Eldridge & Pear, 1987; Jenkins & Moore, 1973; Timberlake, 1983). However, in addition to CS-directed behavior (e.g., “sign tracking”), prior research has also shown that rats may engage in “goal-tracking” (e.g., approaching the food cup) during the CS (Cleland & Davey, 1983; Silva, Silva, & Pear, 1992). US-directed behavior during CS presentations may suggest interactions of operant and classical conditioning. Thus, measures of CS- and US-directed behavior in SP provide an important metric of performance. To characterize the relative contributions of classical and operant contingencies inherent in autoshaping, recent experiments have contrasted performance under autoshaping (more recently described as positive automaintenance, or PAM, procedures) to performance maintained under procedures arranging for food delivery dependent upon the absence of a response (negative automaintenance, or NAM, procedures). Experiments examining responding under SP, PAM, and NAM aid in characterizing the interactions of operant and classical conditioning processes (Allan & Matthews, 1991; Belke & Garland, 2007; Davey, Oakley, & Cleland, 1981; Killeen, 2003; Sanabria, Sitomer, & Killeen, 2006).
Response topographies elicited by CS presentation often correspond to the US in classical conditioning experiments and serve as evidence of classical conditioning processes (Staddon & Simmelhag, 1971). In a classical conditioning arrangement using rats, Peterson, Ackil, Frommer, and Hearst (1972) found distinct lever-directed response topographies depending on whether lever extension was paired with food or electrical brain stimulation (EBS). Rats would lick or gnaw the lever when it was paired with food, whereas pairing with EBS resulted in lever-directed sniffing and exploratory behavior. Similarly, Jenkins and Moore (1973) found that pigeons developed distinct response topographies depending on whether food or water was delivered following key-light presentations. When key-light illumination was followed by grain presentation, the topography of key pecking was similar to food consummatory behavior, characterized by an open-beak peck, whereas closed-beak key pecks were more common with water as the US. Under both SP and PAM, response topography was a function of the particular US.
Evidence for classical conditioning processes in PAM also comes from findings of response maintenance under NAM. In a now seminal study, Williams and Williams (1969) found that pigeon key pecking originally produced under PAM was maintained when NAM was implemented. The authors reasoned that if behavior under this procedure was maintained by adventitious consequences between key pecking and food delivery then changing the contingency to one in which responding prevented reinforcement (an omission contingency) should result in response cessation. Indeed, responding continued to occur, albeit at a lower rate, in spite of the omission contingency between responding and food delivery. These results thus suggest sensitivity to both respondent and operant conditioning: respondent control was demonstrated by the persistence of behavior under conditions in which responses resulted in the prevention of food delivery, whereas operant control was shown by reductions in the frequency of key pecking.
Interestingly, research examining both criterion and subcriterion responding under NAM has found that decreases in criterion responding are accompanied by unchanged or increased sub-criterion responding (Barrera, 1974; Davey, Oakley, & Cleland, 1981). For example, Davey et al. used touch-sensitive response levers to investigate the effects of NAM on response force and frequency in rats. Following response acquisition under PAM, the effects of NAM were examined with respect to both subcriterion (< .078 N) and criterion (> .078 N) responses. Although criterion lever presses decreased under NAM, the frequency of lever touches remained relatively constant. This mirrors the finding that pigeons engage in off-key pecking proximal to the CS, rather than cease pecking, under NAM (Barrera, 1974). These findings suggest that CS–US pairings generate a broad response class of stimulus-directed behavior that is sensitive to subsequent selection by operant contingencies. In the case of NAM, only members falling outside the negative response contingency (subcriterion responses) are preserved.
In contrast to other species, relatively few experiments have investigated monkeys under PAM, SP, or NAM. The available literature has highlighted several points of contact and disconnect between monkeys and other species, in some cases raising the possibility of species differences in performance (Gamzu & Schwam, 1974; Itakura, Fushimi, & Asano, 1992; Schwam & Gamzu, 1975). Sidman and Fletcher (1968), in a systematic replication of Brown and Jenkins (1968), showed that a PAM procedure rapidly produced key-pressing with rhesus monkeys. Their experiment extended the range of species under which PAM had been demonstrated; however, subjects were not exposed to NAM or SP, thereby limiting comparisons to other experiments. Gamzu and Schwam found that the responding of squirrel monkeys under PAM, NAM, and SP differed from that reported for other species responding under similar arrangements. In their study, response acquisition under PAM was slow and response frequency decreased markedly or ceased entirely under SP and NAM. Further, monkeys were observed engaging in highly variable behavior as food delivery approached, in contrast to the US-specific response topographies reported with pigeons and rats. Itakura et al. found that Japanese monkeys (Macaca fuscata fuscata) rapidly acquired a key-pressing response under a PAM procedure; however, responding ceased under NAM, again failing to replicate the results observed with rats and pigeons (Jenkins & Moore, 1973; Timberlake, 1983; Williams & Williams, 1969). Examination of monkey performance under NAM and SP adds points of comparison between monkeys and other species while also refining an automated touch screen response acquisition procedure.
Experiment 1 of the present article systematically replicated studies by Brown and Jenkins (1968) and Sidman and Fletcher (1968), arranging PAM with either a moving or stationary stimulus across groups of subjects. Experiment 1 was designed primarily to assess the utility of a PAM procedure to produce touch-screen responding in naive monkeys and served as training for later placement on a delayed match-to-sample procedure. Subjects were exposed initially to PAM, followed by a fixed-ratio (FR) 1 with 8-s limited hold. In light of research suggesting that moving stimuli enhance learning with monkeys, a moving stimulus was used with some subjects (Washburn, 1993; Washburn, Hopkins, & Rumbaugh, 1989). As discussed by Timberlake (1983), moving stimuli may elicit response sequences in predatory animals that could differentially affect the outcome of respondent conditioning. Although cynomolgus monkeys are primarily frugivorous, when fruit is scarce they predate a variety of animals including insects, frogs, and crabs (Cawthon-Lang, 2006; Son, 2003); thus a moving stimulus may result in faster conditioning.
In Experiment 2, naive subjects were exposed to SP, NAM, and then returned to SP using a stationary stimulus. If responding is primarily under operant control, then variations in response force or location generated under NAM should remain following a return to SP, as there is no specific change in contingency or rate of food delivery that would require criterion responding to return to its previous levels. However, if responding is largely a function of CS–US pairings, then similar performances would be expected under SP before and after a history with NAM. Thus, comparisons of criterion and subcriterion responding under SP, before and after exposure to NAM, allow for an assessment of the relative contributions of CS–US and response–consequence relations in controlling stimulus-directed behavior.
The last two conditions of Experiment 2 arranged for a conjunctive FR 1 fixed-time (FT) 8-s schedule and a FR 1 with an 8-s limited hold (8 s in which to emit one response), respectively. The conjunctive schedule allowed an assessment of responding when food delivery was contingent on a response, but not necessarily temporally contiguous with food delivery (Kennan & Leslie, 1984). The FR 1 with 8-s limited hold was intended to establish high response probabilities in all subjects as a final condition prior to placement on a match-to-sample procedure. To examine the relation between response topography and food presentation with monkeys, we occasionally videotaped sessions (see Author Note) for 3 of 6 animals in Experiment 2. Due to equipment constraints we were only able to video record sessions occasionally, but the video footage we captured illustrated consistent forms of responding.
In summary, the present experiments were designed to replicate and extend the results of Sidman and Fletcher (1968) and Gamzu and Schwam (1974) with a new species (cynomolgus monkeys) using novel apparatus (touch-screen monitors). The objectives of the current study were to evaluate use of a PAM procedure to train large numbers of monkeys rapidly to touch a stimulus presented in a random location on a touch screen, to examine whether the use of a moving stimulus produces more rapid response acquisition than a stationary stimulus under PAM contingencies, to replicate and extend prior findings of monkeys responding under SP and NAM procedures while recording changes in on- and off-stimulus response frequency, and to develop a behavioral assay that rapidly and simultaneously captures respondent and operant conditioning processes.
METHOD
Subjects
Eighteen experimentally naïve male cynomolgus monkeys (Macaca fascicularis) maintained at approximately 95% of free-feeding weight (range 2.8 to 4.0 kg) served as subjects. Fresh water and toys were continuously available in home cages. All subjects were obtained from a commercial vendor and were approximately 3 to 4 years old based on vendor report and morphological data. Due to apparatus destruction resulting in power failure and early termination of experimental contingencies, one session for both subjects M3 and M5 was not completed and was excluded from analysis. For 2 subjects in the moving-stimulus group in Experiment 1, the apparatus was damaged on successive days in a manner that disrupted feeder operation while stimulus presentations continued, resulting in a number of extinction trials. This experience confounded interpretations of response acquisition under PAM and both subjects were thus excluded from analysis (however both subjects readily acquired stimulus touching). In Experiment 2, sessions were occasionally terminated early due to power failure. Sessions which were not completed were excluded from analysis. Exclusions are denoted in figures by unconnected symbols. Further mishaps were prevented by installing Plexiglas protective coverings around the perimeter of each panel.
Apparatus
Subjects were tested using an aluminum intelligence panel affixed to the front of the home cage during each session. Unhindered access to the panel was achieved by securing the cage door in the open position. Each panel consisted of a touch screen monitor (38-cm flat panel LCD, Model 1547L, ELO®, Inc.), a food cup equipped with a white 12-V LED and clear acrylic door attached to a microswitch (requiring 1.7 N of force to operate), a pellet dispenser that could deliver a 190-mg Bio-Serv banana-flavored precision pellet, and a notebook computer (Dell Latitude® D620 running Windows XP®), which controlled experimental events and collected data via a custom-written Visual Basic® 6.0 computer program. A screen touch of greater than approximately 0.69 N of force was counted as a response. Pellet dispenser operation and detection of hopper door switch closures were managed by a USB relay I/O interface (Ontrak Control Systems®, ADU208) connected to the computer.
Procedure
Hopper training
All subjects were initially exposed to a 30-min session that began with the sequential delivery of 30 banana pellets, one every second. This session provided subjects with experience retrieving pellets from the recessed hopper through the acrylic door. During hopper training the touch screen was off and inoperative.
General procedure
Sessions began following a 5-min presession delay and were composed of 80 trials. Each trial began with a random intertrial interval (ITI) averaging 60 s (10 to 110 s), during which the touch screen was blank. Upon termination of the ITI, a 4.5-cm gray square appeared for 8 s in a random location on the touch screen and its termination was immediately followed by pellet delivery unless otherwise specified. Each pellet delivery was accompanied by a 0.25-s white screen flash and illumination of the hopper. Conditions were in effect for a fixed number of sessions. In Experiment 1, conditions remained in effect for a minimum of 3 sessions and until all subjects were touching the stimulus on greater than 50% of trials. In Experiment 2, conditions were run for a minimum of 6 sessions. The third and fourth conditions were extended to 12 and 11 sessions, respectively. Table 1 presents the order of conditions and number of sessions per condition.
Table 1.
Order of conditions and number of sessions per condition
| Number of Sessions | |
| Experiment 1 | |
| Positive Automaintenance (PAM) | 4 |
| FR 1 with 8-s limited hold | 3 |
| Experiment 2 | |
| Stimulus Pairing | 6 |
| Negative Automaintenance (NAM) | 6 |
| Stimulus Pairing | 12 |
| Conjunctive FR 1 FT 8 s | 11 |
| FR 1 with 8-s limited hold | 6 |
Experiment 1
In Experiment 1, two groups of naive monkeys were exposed to identical procedures throughout with the exception that for one group (N = 7), after the stimulus appeared in a random location on the screen it moved at a rate of approximately 3.3 cm/s in a randomly selected horizontal or vertical direction, while for the other group it remained stationary (N = 5). In the first condition, the contingencies were arranged according to the general procedure outlined above (PAM). If no stimulus touches occurred within a given trial a pellet was delivered following the 8-s presentation. However, upon occurrence of a stimulus touch the stimulus terminated and a pellet was immediately delivered (often described as an “autoshaping procedure”). The second condition was identical to the first except that pellets were only delivered if a stimulus touch occurred (FR 1 with 8-s limited hold).
Experiment 2
To approximate more closely the stimulus arrangements used in prior research, only stationary stimuli were used in Experiment 2. Six naive monkeys were exposed to a series of conditions across which a contingency between touching and pellet delivery was gradually introduced. In Condition 1, there were no programmed response contingencies; pellet delivery always followed the 8-s stimulus presentation regardless of responding (SP). Condition 2 arranged an omission contingency such that, following stimulus termination after 8 s, a pellet was delivered only if a stimulus touch had not occurred (NAM). Condition 3 replicated Condition 1. In Condition 4, a conjunctive FR 1 FT 8-s schedule was arranged such that a stimulus touch was required for pellet delivery at the end of the 8-s stimulus presentation. Condition 5 replicated Condition 2 of Experiment 1; a pellet was delivered immediately if a stimulus touch occurred (FR 1 with 8-s limited hold).
RESULTS
Experiment 1
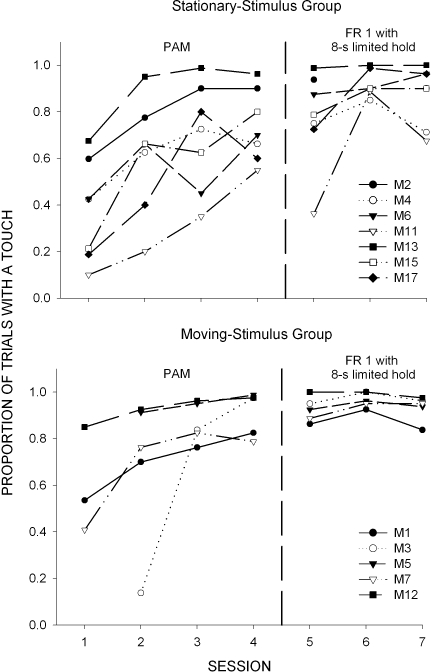
Figure 1 shows, for each subject, the proportion of stimulus presentations during which at least one stimulus touch occurred plotted as a function of session and condition. Condition changes are denoted by vertical dashed lines. As shown in Figure 1, subjects touched the stimulus on multiple occasions by the end of Session 1, and across subsequent sessions, the proportion of stimulus presentations with a touch increased in all cases. A trend toward less variability and higher proportions of touching was observed in the moving-stimulus group. The average proportion of trials with a touch equaled .91 (range of .79 to .99) in the moving-stimulus condition and equaled .74 (range of .55 to .96) in the stationary-stimulus condition. Transition to the FR 1 with 8-s limited hold schedule generally produced slight increases in these proportions in both groups of subjects. (Subsequently, all subjects were successfully transitioned to match-to-sample training procedures with a FR 1 schedule operating on the sample and comparison stimuli.)
Fig 1.
Proportion of stimulus presentations during which at least one stimulus touch occurred in Experiment 1 plotted as a function of session and conditions. Vertical dashed line separates the positive automaintenance (PAM) condition from the subsequent FR1 with 8-s limited hold.
Experiment 2
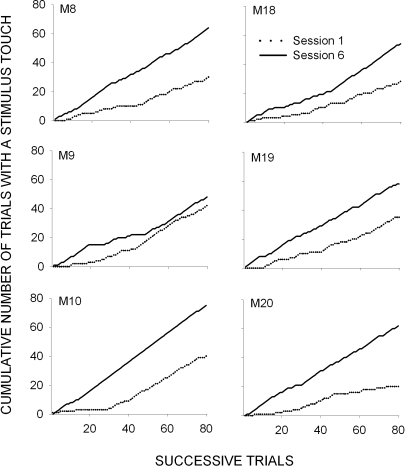
To characterize the time course of response acquisition, we calculated the cumulative number of trials with at least one touch for the first and last session of the first condition and plotted the results in Figure 2. The first stimulus touch occurred within 10 stimulus-food pairings for all 6 subjects, and the slopes were generally steeper for the last half of Session 1 than for the first half. By the sixth session, a relatively constant response probability was observed throughout the session, and, for all subjects, the total number of touches was higher than that in Session 1.
Fig 2.
Cumulative number of trials with at least one stimulus touch plotted as a function of the first (dashed lines) and last (solid lines) sessions of the first stimulus-pairing condition in Experiment 2.
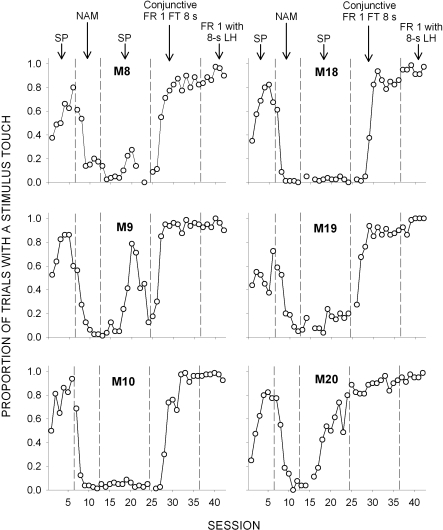
Figure 3 plots, for each subject, the proportion of stimulus presentations during which at least one stimulus touch occurred as a function of session and condition. All 6 subjects touched the stimulus within the first session of the SP procedure arranged in Condition 1. The touch probabilities were comparable to those observed in the first condition of Experiment 1 and ranged from .25 to .53. The second condition of Experiment 2 introduced the NAM procedure, and the results are shown following the first vertical dashed line in Figure 3. The proportion of trials with a stimulus touch decreased markedly as a function of experience for all subjects to a value of less than .2 by the third session and remained low throughout the remaining sessions of the condition. Following NAM, subjects were once again exposed to SP contingencies, replicating the first condition. Interestingly, experience under NAM produced a lasting effect for 5 out of 6 subjects upon a return to SP, with only M20 recovering previous levels of responding, even though this condition remained in effect for nearly 1000 trials. Partial recovery of responding was observed for M9 and M19 when the omission contingency was removed.
Fig 3.
Proportion of stimulus presentations during which at least one stimulus touch occurred in Experiment 2 plotted as a function of session and conditions. Vertical dashed lines denote condition changes from stimulus pairing (SP), negative automaintanence (NAM), conjunctive Fixed-Ratio 1 Fixed-Time 8 s, and Fixed Ratio 1 with an 8-s limited hold.
Introduction of the positive operant contingency in the fourth condition (a Conjunctive FR 1 FT 8-s schedule) produced and maintained a high proportion of trials with a stimulus touch, with all subjects responding on about 80% of all trials by the fifth session of the condition. In the fifth condition, response–reinforcer contiguity was augmented by allowing each stimulus touch to produce immediate food presentation. The proportion of trials with a stimulus touch increased slightly or remained high and approximated the maximum possible.
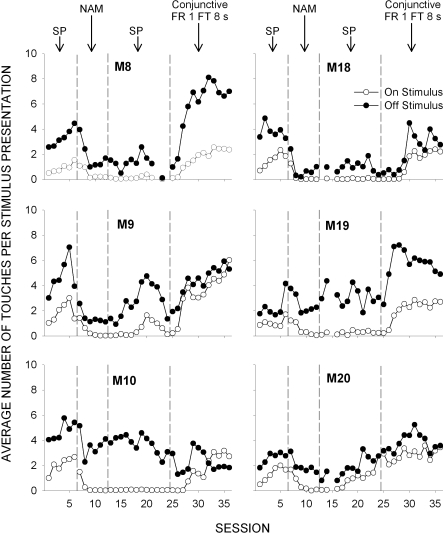
The use of a touch screen allowed for the direct recording of touch response location anywhere on the screen surface. Given that the stimulus was 4.5 cm, the off-stimulus area of the touch screen was over 32 times greater. Thus, random responding to the screen would result in 32 times more off-stimulus than on-stimulus touches. Figure 4 plots the average number of on-stimulus and off-stimulus touches that occurred during 8-s stimulus presentations as a function of session and condition (the fifth condition is not shown because the stimulus duration varied as a function of subject responding and was often much less than the nominal 8 s). In the first condition, the number of off-stimulus touches exceeded the number of on-stimulus touches for all subjects. Introduction of the NAM condition reduced on-stimulus touches to near 0 for all subjects, but off-stimulus touches, although reduced, persisted. In the third condition, the SP procedure was replicated and on-stimulus touching recovered in only one subject (M20). Both on-stimulus and off-stimulus touching remained virtually unchanged. In the fourth condition, the number of on-stimulus touches increased for all subjects to a level exceeding those observed in the first condition. For 4 monkeys (M8, M9, M18, and M19), these increases in on-stimulus touching were accompanied by sizable increases in off-stimulus touching. M10 was the only case in which on-stimulus touches increased while off-stimulus touches decreased.
Fig 4.
Average number of on-stimulus (open circles) and off-stimulus (closed circles) touches that occurred during 8-s stimulus presentations for all sessions of the first four conditions of Experiment 2 (stimulus pairing [SP], negative automaintanence [NAM], conjunctive Fixed-Ratio 1 Fixed-Time 8 s).
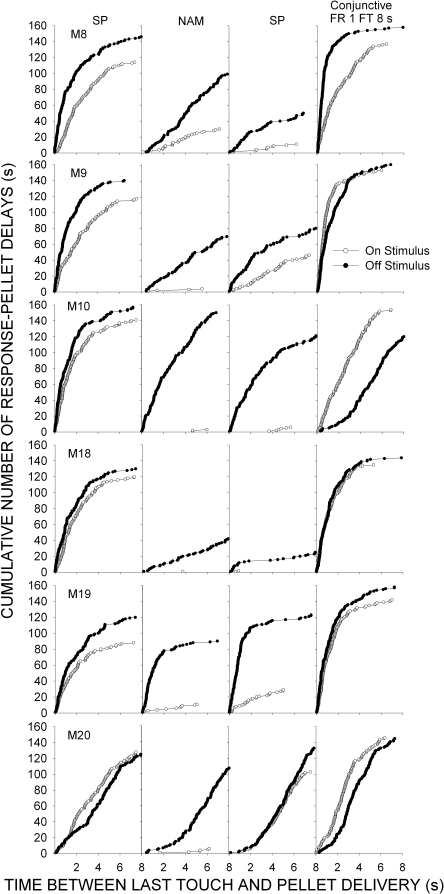
Figure 5 plots, for each subject, the cumulative number of on- and off-stimulus touches preceding pellet delivery as a function of the obtained response–pellet delay, collapsed across the last two sessions (160 trials) of each condition. Although on-stimulus touches under NAM prevented pellet delivery, the obtained delay between touches and the 8-s stimulus offset are plotted to show the obtained contiguity between a touch and pellet omission. Under the initial exposure to SP, the majority of response-pellet delays were 4 s or less for both on- and off-stimulus touches. For 5 of 6 monkeys, the obtained response–pellet delays for off-stimulus touches were shorter than those for on-stimulus touches. The introduction of NAM substantially reduced the number of trials in which an on-stimulus touch occurred to almost zero for 5 of 6 subjects. Off-stimulus touches were reduced to a lesser extent, and a considerable rightward shift in the obtained response–pellet delay distribution was observed for all monkeys except M19. Under the second exposure to SP, recovery of on-stimulus touching was observed for only 2 subjects (M9 and M20), and in both cases the off-stimulus touching distribution concomitantly shifted toward shorter obtained response–pellet delays. The introduction of the conjunctive FR 1 FT 8-s schedule produced a high number of on- and off-stimulus touches, both with short obtained response–pellet delays.
Fig 5.
Cumulative number of on- and off-stimulus touches during 8-s stimulus presentations (preceding pellet delivery) as a function of the obtained response–pellet delay, collapsed across the last two sessions (160 trials) of the first four conditions from Experiment 2 (stimulus pairing [SP], negative automaintenance [NAM], conjunctive Fixed-Ratio 1 Fixed-Time 8 s).
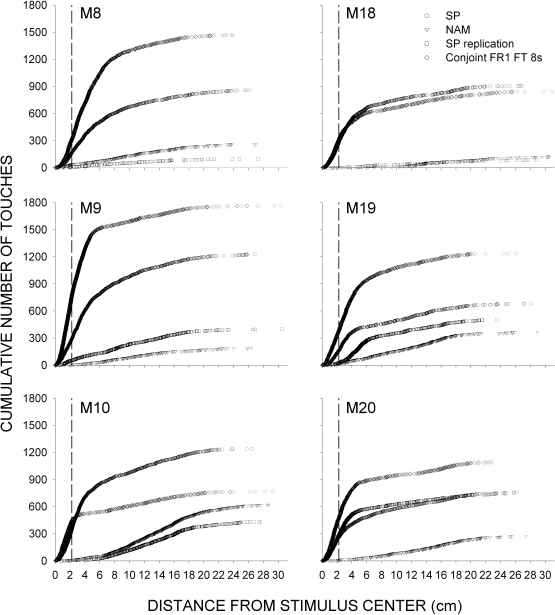
Figure 6 plots the cumulative number of screen touches occurring during 8-s stimulus presentations as a function of distance from the center of the stimulus for each subject, collapsed across the last two sessions of each condition. The conditions are denoted by different symbols. Touches to the left of the dashed vertical line fell within the 4.5-cm square stimulus. For all subjects, under the initial exposure to SP and under the conjunctive FR 1 FT 8-s schedule, most responses occurred less than 6 cm from the center of the stimulus and a number of these fell within the stimulus (i.e., on-stimulus responses). Exposure to NAM greatly reduced responses occurring on or near the stimulus. Reintroduction of SP recovered stimulus touching for only M20. However, upon introduction of the conjunctive schedule, stimulus touches increased to levels at or above those observed under the first SP condition, often accompanied by a sizable number of off-stimulus touches. Recall that the number of off-stimulus touches typically exceeded the number of on-stimulus touches during stimulus presentations in most conditions; however, off-stimulus touches occurred with a frequency that was far less than would be expected by chance alone (the off-stimulus area was much larger than the on-stimulus area) with the majority occurring proximal to the stimulus.
Fig 6.
Cumulative number of screen touches occurring during 8-s stimulus presentations as a function of distance from the stimulus center. Data are collapsed across the last two sessions of the first four conditions from Experiment 2, with each condition denoted by different symbols (stimulus pairing [SP], negative automaintenance [NAM], conjunctive Fixed-Ratio 1 Fixed-Time 8 s). Data points left of the vertical dashed lines denote on-stimulus touches.
DISCUSSION
All monkeys acquired and maintained stimulus-directed screen touching under PAM in Experiment 1 and engaged in stimulus touching on greater than 50% of trials after four sessions, replicating the findings of Sidman and Fletcher (1968) and Itakura et al. (1992). These results extend response acquisition using PAM (autoshaping) to a new species (cynomolgus macaques) and stimulus type (moving versus stationary touch-screen stimuli). The moving-stimulus condition produced slightly higher and less variable response acquisition. The positive-response contingency in the second condition of Experiment 1 (FR 1 with 8-s limited hold) maintained comparably high levels of stimulus touching for all subjects.
The small increase in speed of acquisition of stimulus touching under the moving stimulus when compared to the stationary stimulus aligns with research investigating stimulus movement (Washburn, 1993; Washburn et al., 1989). Washburn (1993) found that with rhesus monkeys use of moving stimuli increased accuracy on a series of discrimination tasks. It was suggested that use of moving stimuli increased the difficulty of the tasks, requiring greater attention to the contingencies, and thereby increasing performance. Although the present results are limited in that several subjects were excluded due to apparatus issues in Experiment 1, in light of the topographical features of responding (discussed below) an alternative interpretation is provided by behavior-systems theory (Timberlake, 1993). This interpretation suggests that the moving stimulus may have made greater contact with the food acquisition behavior system in this species, thereby enhancing acquisition.
Despite the absence of a response contingency under the initial condition of Experiment 2, SP readily produced and maintained touch-screen responding in naive monkeys, replicating previous research with rats and pigeons (Atnip, 1977; Davey et al., 1981; Woodruff & Williams, 1976). Introduction of NAM greatly reduced on-stimulus touching while producing relatively small decreases in off-stimulus touching. Off-stimulus touches under SP occurred most frequently at a distance of less than 6 cm away from the center of the stimulus. Visual observation suggested that these touches were largely postural components of a response chain of orienting toward and/or contacting the stimulus. Subsequent exposure to SP following NAM revealed a robust history effect: on- and near-stimulus touching remained suppressed in 4 of 6 subjects. Upon introduction of a positive response contingency in the fourth condition, stimulus touches once again occurred on a high proportion of presentations, an effect that was maintained after introduction of an FR 1 with 8-s limited hold in the final condition of Experiment 2. The results of Experiment 2 replicate prior findings with other species in demonstrating response acquisition and maintenance under SP while also revealing a NAM history effect.
Both on- and off-stimulus touches decreased in frequency under NAM. On-stimulus touches ceased almost entirely (as shown in Figure 4) while off-stimulus touches decreased proportionally less. These data are consistent with the results of Davey et al. (1981) in suggesting that CS-directed behavior engendered by SP can be modified by operant contingencies, rather than simply decreasing the probability of CS-directed behavior under NAM. Exposure to NAM decreased the frequency of only certain topographical response variants, with responses falling outside the NAM contingency decreasing relatively less. Davey et al. found that exposure to NAM altered the force, but not frequency, of lever touches. In the present study, NAM decreased responding overall, but differentially decreased on- and off-stimulus touching, and shifted response location away from the stimulus. However, interpretations of these data must acknowledge that some control conditions were absent, including a condition in which pellets are delivered in the absence of, or with uncorrelated, stimulus presentations. It is possible that simply presenting pellets on a time-based schedule (with or without a stimulus change) would produce screen touches comparable in frequency to the off-stimulus touches observed under NAM.
The present results also suggest a possible reinterpretation of differences in performance between monkeys and other species under SP and NAM reported by Gamzu and Schwam (1974). In their study, subjects had a prior history with a number of contingencies, including NAM, prior to SP. As in the present study, experience of this sort may alter responding under subsequent conditions in which CS-directed responding has no programmed consequence. However, the authors did report that they observed subjects orienting and approaching the response key upon illumination under NAM. This observation further corresponds to the present experiment: exposure to NAM may directly select against stimulus-touching while failing to select against subcriterion response-class members.
Analysis of hopper entries before and after CS presentations aligns with other results in suggesting joint control by both classical and operant processes. Silva et al. (1992) suggest that the probability of goal-tracking is a function of the distance between the goal and the CS. According to this account, the probability of goal tracking increases as the cost of sign tracking increases. For example, travel (time or distance) between CS and US should increase US-directed and decrease CS-directed behavior through operant selection via response cost. If CS-directed behavior increased the latency to contact the US, a similar operant selection against CS-directed behavior would be expected. In the present study, the US was presented directly below the touch screen, thereby allowing CS-directed behavior without large increases in the delay to US contact. In the present study, CS-directed behavior predominated. Even under NAM, when stimulus touches prevented food delivery and were reduced in frequency, goal tracking was unaltered. This suggests that goal- and sign-tracking responses (unlike on- and off-stimulus screen touches) are not members of the same response class. Thus, on- and off-stimulus touches during the CS were both components of CS-directed behavior, whereas hopper entries were under control of US delivery.
As noted earlier, several experiments with pigeons and rats have found a correspondence between CS- and US-directed behavior engendered by response-independent reinforcement schedules (with and without SP). These findings have been argued to provide evidence of continued respondent processes in operant conditioning (Buzsaki, 1982; Staddon & Simmelhag, 1971; Timberlake & Lucas, 1985). Although far fewer experiments have investigated monkeys exposed to PAM, NAM, or SP, existing reports have noted a lack of correspondence between the conditioned response, a key or lever press, and the consummatory response (Gamzu & Schwam, 1974; Sidman & Fletcher, 1968). One possible explanation of this difference, discussed by Gamzu and Schwam, is that while pigeons and rats generally acquire and consume food with their mouths, monkeys consume food by licking and chewing, responses that are topographically distinct from food-acquisition responses (touching and grasping). A second possibility is that apparatus differences rather than species differences account for the differing results with monkeys and other animals. Cook, Geller, Zhang, and Gowda (2004) found that a touch-screen preparation with rats produced stimulus-directed responding and conditional discriminations more quickly than the use of response levers. In the present experiments, response acquisition may have been enhanced by use of a touch screen. Traditional monkey response manipulanda (keys or levers) could potentially limit response topography to hand movements. The use of touch screen technology and video recording allowed for a cursory evaluation of both possibilities.
To examine response topographies, selected sessions were video recorded (see Author Note) from the third and fourth conditions of Experiment 2 for 3 monkeys. Figure 7 shows pictures of response topographies involved in stimulus touches for each of 3 subjects. Two out of the 3 monkeys examined had response topographies that involved licking or biting motions on or toward the stimulus—a finding consistent with strong respondent control of CS touching. However, mouth contact occurred as part of a sequence that also involved hand contact (swatting or pressing) on or near the stimulus. This finding suggests that off-stimulus responses were mostly postural responses that co-occurred with stimulus-directed behavior (e.g., bracing one or both hands against the screen while licking the stimulus). The video supplement for this article (see Author Note) shows representative trials from recorded sessions of M10, M18, and M19. This video shows that M10 primarily engaged in stimulus-directed biting or licking, M18 contacted the stimulus with one or both hands, and M19 contacted the stimulus with hands first and then with mouth. We observed consistent topographies of responding within subjects but variability between subjects. However, although response topographies between subjects varied, they were generally composed of topographies that resembled food-acquisition and/or food-consumption responses. This finding corresponds well with the predictions of behavior-systems theory (Timberlake, 1993).
Fig 7.
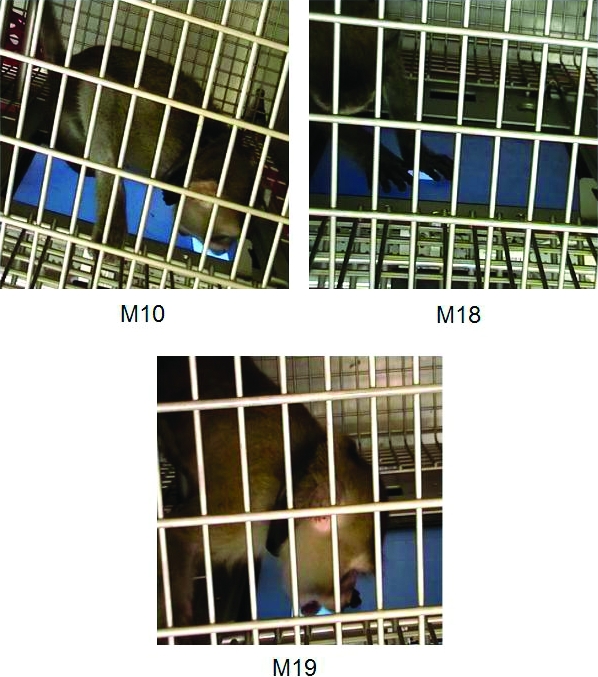
Pictures of representative stimulus–touch response topographies for each of 3 monkeys from Experiment 2.
The data from the two experiments presented here support the interpretation that both respondent and operant processes maintained a continued influence on responding. Acquisition of responding under SP and analysis of response topographies suggest the operation of respondent processes whereas responding under NAM and PAM show control by operant conditioning. From the perspective of behavioral-systems theory the present results can be viewed in the context of an evolved food acquisition and consumption system (Timberlake, 1993; Woodruff & Williams, 1976). According to this interpretation, acquisition of stimulus touching under SP in the present procedure occurred in the context of species-typical food acquisition behavior, setting the initial conditions on which PAM and NAM operated. The topographical features of responding shown in the video (see Author Note) support this interpretation. To the extent that such responding was not selected against by the operant contingencies under PAM, they persisted. When such topographical variants were selected against (on-stimulus touches under NAM), the probability of such variants was reduced. Thus, operant contingencies operate in the context of a behavior system, altering the probability of specified classes of responses in the background of respondent behavior inherent in the system. Operant contingencies further refine these requirements and, to the extent that particular forms of responding confer no benefit, topographies corresponding to the relevant behavior system predominate.
In summary, the present results demonstrate the utility of PAM and SP procedures to produce stimulus-directed responding using a novel preparation (touch screen) and species (cynomolgus macaques) and generally support the concept of species continuity between monkeys and other species regarding performance under such procedures. In contrast to prior work, we found that SP maintained responding with monkeys and stimulus-directed behavior was similar in topography to food acquisition and consumption. Divergence of the present results and those reported by Gamzu and Schwam (1974) may reflect differences in apparatus and history rather than species. Touch screen technology allows for manipulation of size, position, color, and movement of a stimulus, allowing for assessments of the contribution of species-specific stimulus sensitivities in setting the initial conditions on which response contingencies exert their effects.
Acknowledgments
Video clip showing examples of response topographies for 3 subjects from Experiment 2 will be available in the supplemental section of this article available at PubMedCentral.
The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army or the Department of Defense.
The experimental protocol was approved by the Animal Care and Use Committee at the United States Army Medical Research Institute of Chemical Defense and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, Publication No. 85-23, 1996), and the Animal Welfare Act of 1966 (P.L. 89-544), as amended.
This research was supported by the Defense Threat Reduction Agency – Joint Science and Technology Office, Medical S&T Division.
REFERENCES
- Allan R.W, Matthews T.J. “Turning back the clock” on serial-stimulus sign tracking. Journal of the Experimental Analysis of Behavior. 1991;56:427–443. doi: 10.1901/jeab.1991.56-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atnip G.W. Stimulus- and response-reinforcer contingencies in autoshaping, operant, classical, and omission training procedures in rats. Journal of the Experimental Analysis of Behavior. 1977;28:56–69. doi: 10.1901/jeab.1977.28-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam P.D, Drew M.R, Yang C. Timing at the start of associative learning. Learning and Motivation. 2002;33:141–155. [Google Scholar]
- Barrera F.J. Centrifugal selection of signal-directed pecking. Journal of the Experimental Analysis of Behavior. 1974;22:341–355. doi: 10.1901/jeab.1974.22-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke T.W, Garland T. A brief opportunity to run does not function as a reinforcer for mice selected for high daily wheel-running rates. Journal of the Experimental Analysis of Behavior. 2007;88:199–213. doi: 10.1901/jeab.2007.62-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P.L, Jenkins H.M. Auto-shaping of the pigeon's key-peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. The “where is it?” reflex: Autoshaping the orienting response. Journal of the Experimental Analysis of Behavior. 1982;37:461–484. doi: 10.1901/jeab.1982.37-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon-Lang K.A.Primate Factsheets: Long-tailed macaque (Macaca fascicularis) Taxonomy, Morphology, & Ecology. 2006. < http://pin.primate.wisc.edu/factsheets/entry/long-tailed_macaque>.
- Cleland G.G, Davey G.C.L. Autoshaping in the rat: The effects of localizable visual and auditory signals for food. Journal of the Experimental Analysis of Behavior. 1983;40:47–56. doi: 10.1901/jeab.1983.40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill R.M, Absher R.A, Roberts M.L. Context-US learning in Aplysia californica. The Journal of Neuroscience. 1988a;8((12)):4434–4439. doi: 10.1523/JNEUROSCI.08-12-04434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill R.M, Absher R.A, Roberts M.L. Conditional discrimination learning in Aplysia californica. The Journal of Neuroscience. 1988b;8((12)):4440–4444. doi: 10.1523/JNEUROSCI.08-12-04440.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R.G, Geller A.I, Zhang G, Gowda R. Touchscreen-enhanced visual learning in rats. Behavior Research Methods, Instruments, & Computers. 2004;36:101–106. doi: 10.3758/bf03195555. [DOI] [PubMed] [Google Scholar]
- Davey G.C.L, Oakley D, Cleland G.G. Autoshaping in the rat: Effects of omission on the form of the response. Journal of the Experimental Analysis of Behavior. 1981;36:75–91. doi: 10.1901/jeab.1981.36-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing K, Neuringer A. Autoshaping as a function of prior food presentations. Journal of the Experimental Analysis of Behavior. 1976;26:463–469. doi: 10.1901/jeab.1976.26-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge G.D, Pear J.J. Topographical variations in behavior during autoshaping, automaintenance, and omission training. Journal of the Experimental Analysis of Behavior. 1987;47:319–333. doi: 10.1901/jeab.1987.47-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamzu E, Schwam E. Autoshaping and automaintenance of a key-press response in squirrel monkeys. Journal of the Experimental Analysis of Behavior. 1974;21:361–371. doi: 10.1901/jeab.1974.21-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamzu E, Schwartz B. The maintenance of key pecking by stimulus-contingent and response-independent food presentation. Journal of the Experimental Analysis of Behavior. 1973;19:65–72. doi: 10.1901/jeab.1973.19-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamzu E.R, Williams D.R. Associative factors underlying the pigeon's key pecking in auto-shaping procedures. Journal of the Experimental Analysis of Behavior. 1973;19:225–232. doi: 10.1901/jeab.1973.19-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J, Baldock M.D, Locurto C, Gold L, Terrace H.S. Trial and intertrial durations in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:264–284. [Google Scholar]
- Hursh S.R, Navarick D.J, Fantino E. “Automaintenance”: The role of reinforcement. Journal of the Experimental Analysis of Behavior. 1974;21:117–124. doi: 10.1901/jeab.1974.21-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura S, Fushimi T, Asano T. Autoshaping in Japanese monkeys (Macaca Fuscata) Bulletin of Oita Prefectural College of Arts and Culture. 1992;30:131–137. [Google Scholar]
- Jenkins H.M, Moore B.R. The form of the auto-shaped response with food or water reinforcers. Journal of the Experimental Analysis of Behavior. 1973;20:163–181. doi: 10.1901/jeab.1973.20-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennan M, Leslie J.C. Separating response dependency and response-reinforcer contiguity within a recycling conjunctive schedule. Journal of the Experimental Analysis of Behavior. 1984;41:203–210. doi: 10.1901/jeab.1984.41-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen P.R. Complex dynamic processes in sign tracking with an omission contingency (negative automaintenance) Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:49–61. doi: 10.1037/0097-7403.29.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locurto C, Terrace H.S, Gibbon J. Autoshaping, random control, and omission training in the rat. Journal of the Experimental Analysis of Behavior. 1976;26:451–462. doi: 10.1901/jeab.1976.26-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin R.J, LoLordo V.M. A comparison of pecking generated by serial, delay, and trace autoshaping procedures. Journal of the Experimental Analysis of Behavior. 1976;25:227–241. doi: 10.1901/jeab.1976.25-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristos E.B, Gallistel C.R. Autoshaped head poking in the mouse: A quantitative analysis of the learning curve. Journal of the Experimental Analysis of Behavior. 2006;85:293–308. doi: 10.1901/jeab.2006.71-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G.B, Ackil J.E, Frommer G.P, Hearst E.S. Conditioned approach and contact behavior toward signals for food or brain-stimulation reinforcement. Science. 1972;117:1009–1011. doi: 10.1126/science.177.4053.1009. [DOI] [PubMed] [Google Scholar]
- Poling A, Poling T. Automaintenance in guinea pigs: Effects of feeding regimen and omission training. Journal of the Experimental Analysis of Behavior. 1978;30:37–46. doi: 10.1901/jeab.1978.30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H. Autoshaping of key pecking in pigeons with negative reinforcement. Journal of the Experimental Analysis of Behavior. 1969;12:521–531. doi: 10.1901/jeab.1969.12-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria F, Sitomer M.T, Killeen P.R. Negative automaintenance omission training is effective. Journal of the Experimental Analysis of Behavior. 2006;86:1–10. doi: 10.1901/jeab.2006.36-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwam E, Gamzu E. Constraints on autoshaping in the squirrel monkey: Stimulus and response factors. Bulletin of the Psychonomic Society. 1975;5:369–372. [Google Scholar]
- Sidman M, Fletcher G. A demonstration of auto-shaping with monkeys. Journal of the Experimental analysis of Behavior. 1968;11:307–309. doi: 10.1901/jeab.1968.11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva F.J, Silva K.M, Pear J.J. Sign- versus goal-tracking: Effects of conditioned-stimulus-to-unconditioned-stimulus distance. Journal of the Experimental Analysis of Behavior. 1992;57:17–31. doi: 10.1901/jeab.1992.57-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son V.D. Diet of Macaca fasicicularis in a mangrove forest, Vietnam. Laboratory Primate Newsletter. 2003;42:1–5. [Google Scholar]
- Staddon J.E.R, Simmelhag V.L. The “superstition” experiment: A reexamination of its implications for the principles of adaptive behavior. Psychological Review. 1971;78:3–43. [Google Scholar]
- Timberlake W. Rats' responses to a moving object related to food or water: A behavior-systems analysis. Animal Learning and Behavior. 1983;11:309–320. [Google Scholar]
- Timberlake W. Behavior systems and reinforcement: An integrative approach. Journal of the Experimental Analysis of Behavior. 1993;60:105–128. doi: 10.1901/jeab.1993.60-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W, Lucas G.A. The basis of superstitious behavior: Chance contingency, stimulus substitution, or appetitive behavior. Journal of the Experimental Analysis of Behavior. 1985;44:279–299. doi: 10.1901/jeab.1985.44-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A. Retardation of autoshaping: Control by contextual stimuli. Science. 1976;192:1244–1246. doi: 10.1126/science.192.4245.1244. [DOI] [PubMed] [Google Scholar]
- Washburn D.A. The stimulus movement effect: Allocation of attention or artifact. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:380–390. doi: 10.1037//0097-7403.19.4.380. [DOI] [PubMed] [Google Scholar]
- Washburn D.A, Hopkins W.D, Rumbaugh D.M. Video-task assessment of learning and memory in macaques (Macaca mulatta): Effects of stimulus movement on performance. Journal of Experimental Psychology: Animal behavior processes. 1989;15:393–400. [PubMed] [Google Scholar]
- Williams D.R, Williams H. Auto-maintenance in the pigeon: Sustained pecking despite contingent non-reinforcement. Journal of the Experimental Analysis of Behavior. 1969;12:511–520. doi: 10.1901/jeab.1969.12-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff G, Williams D.R. The associative relation underlying autoshaping in the pigeon. Journal of the Experimental Analysis of Behavior. 1976;26:1–13. doi: 10.1901/jeab.1976.26-1. [DOI] [PMC free article] [PubMed] [Google Scholar]