Abstract
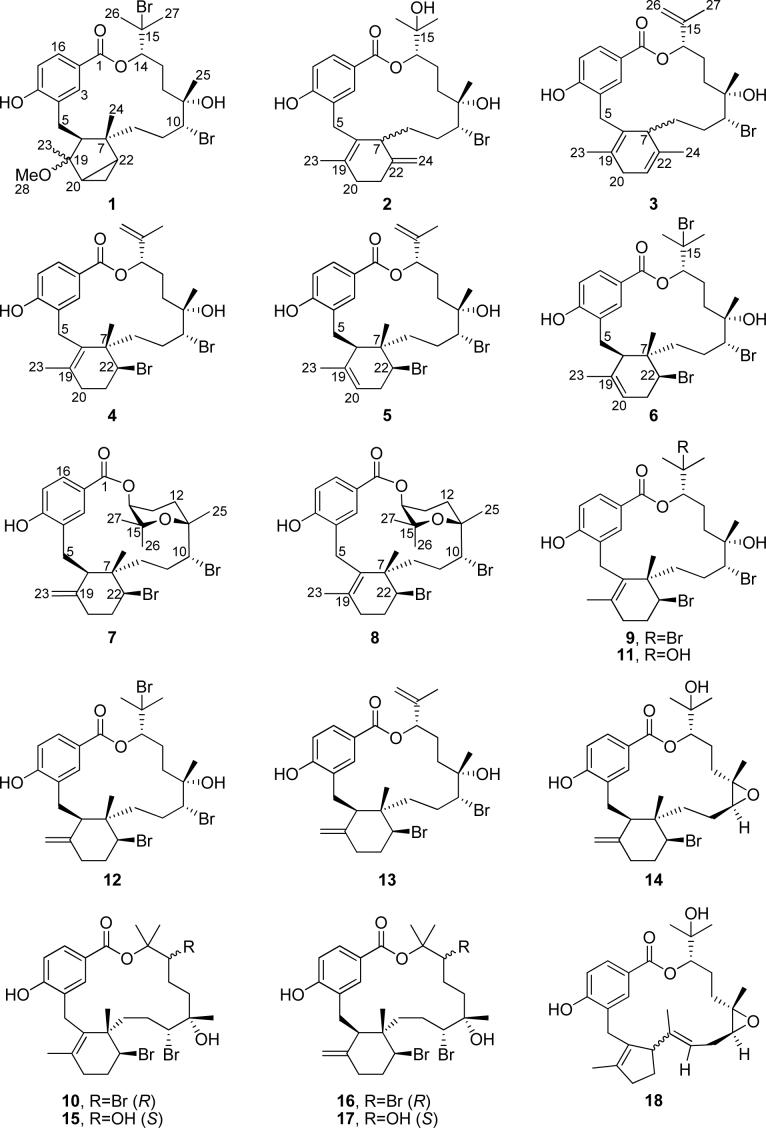
Bromophycolides J-Q (1-8) were isolated from extracts of the Fijian red alga Callophycus serratus and identified with 1D and 2D NMR spectroscopy and mass spectral analyses. These diterpene-benzoate macrolides represent two novel carbon skeletons and add to the ten previously reported bromophycolides (9-18) from this alga. Among these 18 bromophycolides, several exhibited activities in the low micromolar range against the human malaria parasite Plasmodium falciparum.
Introduction
Over 500 million cases of malaria are reported annually, causing one- to three-million deaths worldwide.1 Although several antimalarial drugs are currently on the market, resistance to these treatments is on the rise, thus providing an increasing need for novel antimalarial medicines.2 As a recognized source of pharmacologically-active natural products,3,4 marine organisms such as macroalgae may offer unique natural products for the development of novel treatments for malaria and other infectious diseases.
From the Fijian red alga Callophycus serratus, we previously reported the discovery of ten bromophycolides, unusual C27 diterpene-benzoate macrolides.5,6 Exploration of additional C. serratus collections then led to discovery of ten novel callophycoic acids and callophycols, C27 diterpene-benzoic acids and C26 diterpene-phenols.7 Herein, we report identification of eight additional macrolides, bromophycolides J-Q (1-8), representing two novel carbon skeletons, two isomeric macrolides possessing a tetrahydropyran ring, two regioisomers of the known bromophycolide E (13), and a regioisomer of bromophycolide A (9), and adding further evidence that this red alga is an abundant source of chemically diverse and biologically active natural products.
Results and Discussion
Following the isolation and identification of ten bromophycolides from Callophycus serratus,5,6 LCMS evaluation of extracts from a Yanuca (Fiji) collection of this red macroalga suggested the presence of additional bromophycolide-like metabolites. Reversed- and normal-phase HPLC yielded eight novel metabolites, bromophycolides J-Q (1-8), in quantities sufficient for structure elucidation.
A molecular formula of C28H40O5Br2 was established for bromophycolide J (1), based on a mass spectral parent ion at m/z 613.1160, supported by a dibrominated isotopic splitting pattern. Inspection of 1H, 13C, HSQC, HMBC, and COSY NMR spectral data for 1 revealed a 4-hydroxybenzoyl group common to all bromophycolides (Table 1, Supporting Information).5,6 Comparison of spectral data for 1 with bromophycolide A (9) supported a bromine-substituted isopropyl group at the diterpene head and established diterpene-aryl connectivity identical to that of 9.6
Table 1.
13C and 1H NMR spectral data for bromophycolides J-Q (1-8) (500 MHz; in CDCl3).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ 13C | δ 1H (JH,H) |
δ 13C | δ 1H (JH,H) |
δ 13C | δ 1H (JH,H) |
δ 13C | δ 1H (JH,H) |
δ 13C | δ 1H (JH,H) |
δ 13C | δ 1H (JH,H) |
δ 13C | δ 1H (JH,H) |
δ 13C | δ 1H (JH,H) |
|
| 1 | 165.6 | - | 167.6 | - | 165.9 | - | 165.3 | - | 164.7 | - | 165.5 | - | 164.9 | - | 165.0 | - |
| 2 | 121.2 | - | 122.8 | - | 122.9 | - | 122.5 | - | 123.0 | - | 123.0 | - | 123.1 | - | 122.9 | - |
| 3 | 130.1 | 8.11 brs | 133.3 | 7.67 d (1.7) |
132.3 | 7.67 d (1.9) |
130.6 | 7.85 brs | 131.4 | 7.82 s | 129.5 | 7.89 s | 133.9 | 8.06 d (1.6) |
129.8 | 7.64 s |
| 4 | 128.3 | - | 125.4 | - | 124.6 | - | 126.4 | - | 129.5 | - | 128.4 | - | 128.7 | - | 128.2 | - |
| 5 | 27.1 | 2.37 d (15) 2.69 m |
29.4 | 3.29 d (15) 3.64 d (15) |
29.7 | 3.22 d (15), 3.46 d (15) |
28.5 | 3.24 d (8.8) 3.57 d (18) |
27.3 | 2.60 d (15), 2.83 dd (15, 9.0) |
25.8 | 2.82 m, 2.98 d (13) |
25.5 | 2.61 d (15), 3.17 dd (15, 8.0) |
28.3 | 3.15 d (17), 3.65 d (17) |
| 6 | 45.5 | 2.59 m | 138.6 | - | 132.6 | - | 130.8 | - | 47.8 | 2.67 m | 43.7 | 2.84 m | 51.1 | 2.37 m | 131.0 | - |
| 7 | 45.8 | - | 50.6 | 2.62 d (8.7) |
49.0 | 3.41 m | 43.4 | - | 41.3 | - | 40.9 | - | 44.7 | - | 43.9 | - |
| 8 | 42.7 | 1.21 m 2.62 m | 30.0 | 1.45 m 1.98 m | 27.1 | 1.53 m, 1.97 m | 37.6 | 1.58 m 1.92 m | 37.2 | 1.58 m, 2.18 m | 36.6 | 1.23 m, 2.08 m | 37.4 | 1.71 m, 2.13 m | 37.7 | 1.58 m, 1.80 m |
| 9 | 31.3 | 1.37 m 1.75 m | 31.9 | 1.44 m 2.20 m | 30.7 | 2.44 m, 2.50 m | 28.8 | 1.93 m 2.07 m | 26.0 | 2.20 m, 2.39 m | 28.5 | 1.98 m, 2.00 m | 25.2 | 2.14 m, 2.58 m | 27.2 | 2.05 m, 2.28 m |
| 10 | 70.5 | 3.99 d (11) |
72.0 | 3.96 m | 67.8 | 3.97 dd (11, 3) |
71.5 | 3.82 d (8.8) |
66.4 | 4.20 d (11) |
70.0 | 3.79 d (11) |
64.7 | 4.43 dd (11, 2.5) |
63.1 | 4.46 dd (12, 2.5) |
| 11 | 72.5 | - | 73.3 | - | 73.0 | - | 73.4 | - | 74.4 | - | 73.0 | - | 76.0 | - | 76.1 | - |
| 12 | 35.4 | 1.50 m 1.58 m | 32.6 | 1.24 m 1.46 m | 31.6 | 1.67 m, 1.74 m | 33.3 | 1.52 m 1.77 m | 32.2 | 1.86 m 2.02 m | 33.8 | 1.53 m, 1.98 m | 26.5 | 1.72 m, 2.35 m | 25.8 | 1.69 m, 2.20 m |
| 13 | 26.4 | 2.11 m 2.65 m | 23.6 | 1.83 m 1.94 m | 25.8 | 1.73 m, 1.86 m | 28.6 | 1.79 m 1.83 m | 27.0 | 1.70 m 2.05 m | 26.4 | 2.13 m, 2.47 m | 21.8 | 1.88 m, 2.30 m | 21.9 | 1.80 m, 2.24 m |
| 14 | 80.5 | 4.88 d (11) |
81.5 | 4.97 dd (2.7, 12) |
74.9 | 5.52 brs | 76.3 | 5.36 d (6.8) |
76.0 | 5.23 brs | 79.2 | 5.00 dd (9.4, 3.4) |
70.6 | 5.08 dd (6.6, 1.7) |
70.9 | 4.94 d (7.0) |
| 15 | 66.2 | - | 72.1 | - | 140.7 | - | 142.6 | - | 140.9 | - | 66.5 | - | 74.4 | - | 73.6 | - |
| 16 | 129.6 | 7.81 d (8.0) |
129.5 | 7.74 dd (1.8, 8.4) |
129.2 | 7.73 dd (8.4, 1.9) |
129.7 | 7.82 d (8.3) |
129.3 | 7.75 d (7.9) |
129.3 | 7.74 d (8.3) |
128.6 | 7.70 dd (8.3, 1.9) |
129.0 | 7.77 d (8.5) |
| 17 | 115.5 | 6.78 d (8.2) |
115.9 | 6.81 d (8.4) |
115.6 | 6.78 d (8.3) |
115.2 | 6.80 d (8.3) |
115.9 | 6.76 d (8.3) |
115.7 | 6.81 d (8.3) |
115.8 | 6.73 d (8.3) |
115.0 | 6.80 d (8.5) |
| 18 | 157.8 | - | 159.1 | - | 158.0 | - | 157.5 | - | 156.6 | - | 156.5 | - | 156.9 | - | 157.1 | - |
| 19 | 89.8 | - | 132.7 | - | 136.8 | - | 132.6 | - | 137.2 | - | 135.8 | - | 145.7 | - | 132.9 | - |
| 20 | 28.0 | 1.55 m | 36.7 | 2.24 m 2.37 m | 37.8 | 2.30 m, 2.42 m | 32.4 | 2.13 m 2.26 m | 120.3 | 5.19 m | 119.4 | 5.33 brs | 37.5 | 1.97 m, 2.34 m | 32.6 | 2.05 m, 2.20 m |
| 21 | 8.5 | 0.31 m 0.42 dd (5.8, 10) |
36.0 | 1.95 m 2.17 m | 122.5 | 4.81 t (6.5) |
29.9 | 2.25 m 2.36 m | 34.4 | 2.53 m 2.59 m | 34.0 | 2.65 m | 34.8 | 2.08 m, 2.22 m | 30.7 | 2.20 m, 2.28 m |
| 22 | 31.9 | 1.14 m | 150.3 | - | 138.8 | - | 61.3 | 4.49 dm (9.5) |
59.8 | 4.28 dd (11, 5.3) |
60.2 | 4.24 dd (6.8, 6.2) |
62.0 | 4.33 dd (12, 4.1) |
62.1 | 4.57 d (10) |
| 23 | 19.8 | 1.38 s | 14.1 | 1.91 s | 14.0 | 1.89 s | 20.8 | 1.41 s | 20.9 | 1.50 s | 22.0 | 1.65 s | 110.5 | 4.89 s, 5.30 s | 20.7 | 1.34 s |
| 24 | 20.0 | 0.55 s | 108.6 | 4.46 s 4.66 s | 18.2 | 1.38 s | 26.0 | 1.27 s | 17.1 | 1.06 s | 18.8 | 0.89 s | 17.5 | 0.99 s | 27.8 | 1.34 s |
| 25 | 29.0 | 1.17 s | 26.1 | 1.26 s | 27.5 | 1.30 s | 30.2 | 1.26 s | 28.0 | 1.40 s | 29.3 | 1.32 s | 27.0 | 1.47 s | 27.5 | 1.42 s |
| 26 | 31.2 | 1.80 s | 25.6 | 1.31 s | 111.5 | 4.98 s, 5.07 s | 111.3 | 4.92 s 4.99 s | 111.0 | 4.84 s 4.95 s | 30.2 | 1.81 s | 26.3 | 1.36 s | 26.6 | 1.34 s |
| 27 | 30.5 | 1.78 s | 26.8 | 1.31 s | 19.5 | 1.79 s | 19.2 | 1.80 s | 19.7 | 1.79 s | 31.5 | 1.83 s | 27.8 | 1.09 s | 27.6 | 1.15 s |
| 28 | 49.9 | 3.33 s | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| OH | - | 6.05 brs | - | 5.48 brs | - | 5.31 s | - | 5.49 brs | - | 5.33 brs | - | 5.21 brs | - | 5.68 brs | - | 5.28 brs |
br=broad; s=singlet; d=doublet; dd=doublet of doublets; t=triplet; m=multiplet
Further comparison of NMR spectral data for 1 and 9 revealed substantial differences between these two natural products only in the vicinity of the carbocyclic terpene ring.6 For 1, HMBC correlations from Me-23 (δ 1.38) to C-6 (δ 45.5), C-19 (δ 89.8), and C-20 (δ 28.0) established C-6—C-19—C-20 connectivity. An HMBC correlation from OMe-28 (δ 3.33) to C-19 established quaternary C-19 as the site of attachment for OMe-28 and Me-23. HMBC correlations from Me-24 (δ 0.55) to C-6, C-7 (δ 45.8), and C-22 (δ 31.9) established connectivity between these carbons. COSY correlations between H-22 (δ 1.14) and both H-21 protons (δ 0.31, 0.42), between H-20 (δ 1.55) and both H-21 protons, and between H-22 and H-20, as well as the shielded chemical shift observed for methylene C-21 (13C δ 8.5) prompted assignment of a cyclopropyl moiety comprised of C-20, C-21, and C-22. HMBC and COSY correlations established connection between this ring system and the benzoate system via C-5, analogous to previously identified metabolites.5,6
Stereochemical assignments for 1 were facilitated by comparison of 1H-1H scalar couplings and NOE correlations with 9.6 Observation of predicted scalar couplings and NOE correlations for 1 (Table 1, Supporting Information) prompted assignment of 10R, 11S, 14S stereochemistry as for 9, whose absolute configuration was previously established by X-ray crystallography.6 Given a proposed, common biogenesis and an observed NOE between H-5b (δ 2.69) and Me-24, it seemed highly probable that a 7R configuration would also be shared between 1 and 9. NOE correlations between H-6 (δ 2.59) and H-20, but not between H-6 and Me-24, established a 6S stereocenter. This assignment matched absolute configurations reported for all bromophycolides bearing a stereocenter at this site (e.g., bromophycolide D (12)).5 Due to difficulties assigning stereochemistry of 5-membered rings from NOE data, the configurations of C-19, C-20, and C-22 were not assigned at this time.
Bromophycolide K (2) was assigned a molecular formula of C27H37O5Br from the parent ion observed at m/z 519.1767 ([M - H]-). Comparison of 1H, 13C, HSQC, HMBC, and COSY NMR spectral data with known bromophycolides confirmed a 15-membered macrolide framework analogous to 1 and 9 (Supporting Information).5,6 For 2, a hydroxy substituent was assigned at C-15 (δ 72.1) on the basis of 13C NMR chemical shift precedents.5,6 As with 1, HMBC and COSY correlations suggested that 2 diverged from other bromophycolides within the terpene carbocyclic moiety. Within this group, observation of HMBC correlations from Me-23 (δ 1.91) to C-6 (δ 138.6), C-19 (δ 132.7), and C-20 (δ 36.7) and from H-5a (δ 3.29) to C-7 (δ 50.6) established the tetrasubstituted olefin. COSY correlations from both H-20 protons (δ 2.24, 2.37) to both H-21 protons (δ 1.95, 2.17) and HMBC correlations from both H-24 protons (δ 4.46, 4.66) to C-7 and C-21 (δ 36.0) closed the six-membered ring containing exoand endocyclic double bonds.
High resolution mass spectral data indicated that bromophycolide L (3) differed from 2 by a loss of one H2O molecule, displaying an [M - H]- m/z of 501.1677, appropriate for a molecular formula of C27H35O4Br. HMBC correlations from Me-27 (δ 1.79) to C-14 (δ 74.9), C-15 (δ 140.7), and C-26 (δ 111.5) suggested an isopropenyl diterpene head identical with that of bromophycolide E (13) (Table 1).5 Likewise, HMBC correlations from both H-26 vinyl protons (δ 4.98, 5.07) to C-14, C-15, and C-27 (δ 19.5) confirmed this connectivity. Evaluation of 1H, COSY, and HMBC NMR spectral data of 3 to that of 2 indicated an additional difference within the terpene carbocyclic system. HMBC correlations from Me-24 (δ 1.38) to C-7 (δ 49.0), C-21 (δ 122.5), and C-22 (δ 138.8) suggested that the rearranged terpene skeleton was present as in 2; however, the unsaturation was determined to be endocyclic at Δ21,22 through COSY correlations of olefinic H-21 (δ 4.81) with H-20b (δ 2.42) and a weak long range COSY correlation between H-21 and Me-24 (Supporting Information).
For 3, similar NOEs were observed as for bromophycolide E (13), suggesting a 10R, 11S, 14S configuration (Supporting Information).5 NOEs were present between H-7 (δ 3.41) and H-20b, located 1,4 relative to each other across their six-membered ring, thus suggesting a pseudo-boat conformation of this ring. The lack of stereocenters near C-7 prevented stereochemical assignment at this position in either 2 or 3, given that an R or S configuration would be expected to result in NOEs between the axial protons H-7 and H-20b.
Bromophycolide M (4) exhibited a molecular formula of C27H36O4Br2 ([M - H]- m/z 581.0906), isomeric to 13.5 A combination of 1D and 2D NMR spectral data for 4 supported assignment of a carbon skeleton and most functionalities identical to that of 13. For 4, HMBC correlations from Me-23 (δ 1.41) to fully substituted olefinic carbons C-6 (δ 130.8) and C-19 (δ 132.6) as well as to C-20 (δ 32.4) suggested regioisomerization of the carbon-carbon double bond relative to 13. Finally, 7S, 10R, 11S, 14S, 22S stereochemistry was proposed for 4, based on comparison of NOE correlations with those of 9 and 13 (Supporting Information).
The mass spectrum of bromophycolide N (5), with [M - H]- m/z of 581.0907, suggested yet another regioisomer of 13, with a molecular formula of C27H36O4Br2. Comparison of 1H, COSY, and HMBC NMR spectral data of 5 with that of 4 and 13 suggested a difference in the cyclohexenyl double bond. HMBC correlations observed from Me-23 (δ 1.50) to C-6 (δ 47.8), C-19 (δ 137.2), and C-20 (δ 120.3), along with COSY correlations between both H-5 protons (δ 2.60, 2.83) and H-6 (δ 2.67), supported the Δ19,20 assignment. Because similar NOEs were observed for 5 as for 4 and 13, 6R, 7S, 10R, 11S, 14S, 22S stereochemistry was proposed for 5 (Supporting Information).
Bromophycolide O (6) exhibited an [M - H]- m/z of 661.0182 with a tribrominated isotopic pattern, appropriate for a molecular formula of C27H37O4Br3 as seen with bromophycolides A (9), B (10), and D (12). Inspection of 1D and 2D NMR spectral data of 6 suggested yet another 15-membered macrocyclic skeleton. As with 5, regioisomerization of the cyclohexenyl double bond to Δ19,20 was supported by observation of HMBC correlations from Me-23 (δ 1.65) to C-6 (δ 43.7), C-19 (δ 135.8), and C-20 (δ 119.4), as well as COSY correlations between H-5a (δ 2.82) and H-6 (δ 2.84) and a long-range COSY correlation between Me-23 and H-20 (δ 5.33). Similar NOEs were observed for 6 as with 9 and 12, thus a 6R, 7S, 10R, 11S, 14S, 22S stereochemistry was inferred for 6 (Supporting Information).
Bromophycolide P (7) also displayed the same mass spectral parent ion as 4, 5 and 13, with an [M - H]- m/z ion of 581.0865, appropriate for a molecular formula of C27H36O4Br2. Comparison of spectral data of 7 with other known bromophycolides supported another 15-membered macrocycle. HMBC correlations from both H-23 protons (δ 4.89, 5.30) to C-6 (δ 51.5) and C-20 (δ 37.5), as well as correlations from both H-5 protons (δ 2.61, 3.17) to C-6 and C-19 (δ 145.7), indicated the presence of an exo-methylene group (C-23, δ 110.5). Interestingly, carbon chemical shifts of 7 significantly differed from other known C. serratus natural products at C-12 (δ 26.5) and C-13 (δ 21.8). Having assigned all olefinic carbons and protons, one ring remained unaccounted for in 7 based on the index of hydrogen deficiency; thus a third six-membered ring was assigned via an ether linkage between C-11 (δ 76.0) and C-15 (δ 74.4), which accounted for the differences seen in the carbon chemical shifts at C-12 and C-13. Moreover, the phenolic hydroxyl proton (δ 5.68) was observed in the 1H NMR spectrum, providing evidence that the ether linkage did not involve this position (C-18, δ 156.9). However, another possibility was that rather than an ether linkage, both C-11 and C-15 were hydroxylated, and the ESIMS ion observed at m/z 581.0865 resulted from dehydration at C-11 or C-15 during ionization. In order to test this hypothesis, 7 was acetylated, and then subjected to 1H NMR spectroscopy (data not shown). Only one acetyl group was observed in the 1H NMR spectrum, rejecting the notion of a polyhydroxylated natural product and confirming the presence of a single free, phenolic hydroxyl group along with the tetrahydropyran ring in 7.
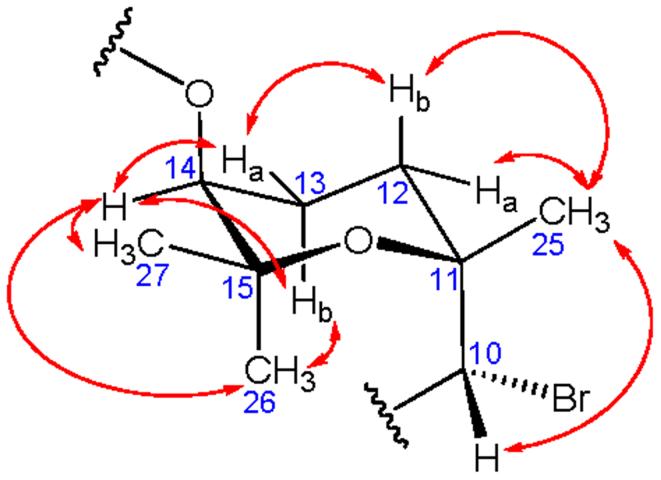
Retention of the 6R, 7S, 10R, 22S configuration of 7 was proposed by comparison of NOEs for 7 with other bromophycolides possessing the C-19-C-23 unsaturation (e.g. 12-14, 16-17, Figure 1, Supporting Information)5 and by inferring a common biosynthetic origin. NOEs observed between H-14 (δ 5.08) and both H-13s (δ 1.88, 2.30), Me-26 (δ 1.36), and Me-27 (δ 1.09) suggested an equatorial position for H-14 within the tetrahydropyran ring of 7 (Figure 2, Supporting Information). Furthermore, 1,3-diaxial NOE correlations were observed between H-13b and Me-26. NOEs were also seen between equatorial Me-25 (δ 1.47) and both H-12 protons (δ 1.72, 2.35). Collectively, these data supported a configuration of 11R, 14S for 7.
Figure 1.
Novel bromophycolides J-Q (1-8) and previously reported bromophycolides A-I (9-17) and debromophycolide A (18) from Callophycus serratus.
Figure 2.
Key NOE correlations (double-headed arrows) observed for bromophycolide P (7) established the stereochemistry around the tetrahydropyran ring as 10R, 11R, 14S.
High resolution mass spectral data of bromophycolide Q (8), [M - H]- m/z 581.0869, suggested a molecular formula of C27H36O4Br2, as for 4-5, and 7. The 1H NMR spectral data for 8 were identical to that of 7, except for the loss of the exomethylene signals and the presence of one additional methyl group. HMBC correlations from H-5a (δ 3.15) to olefinic carbons C-6 (δ 131.0) and C-19 (δ 132.9), as well as from Me-23 (δ 1.34) to C-6, C-19 and C-20 (δ 32.6) suggested regioisomerization of the double bond in 8 relative to 7. A configuration of 7S, 10R, 11R, 14S, 22S stereochemistry was proposed for 8 based on similar NOEs observed for 7 and 8 (Supporting Information).
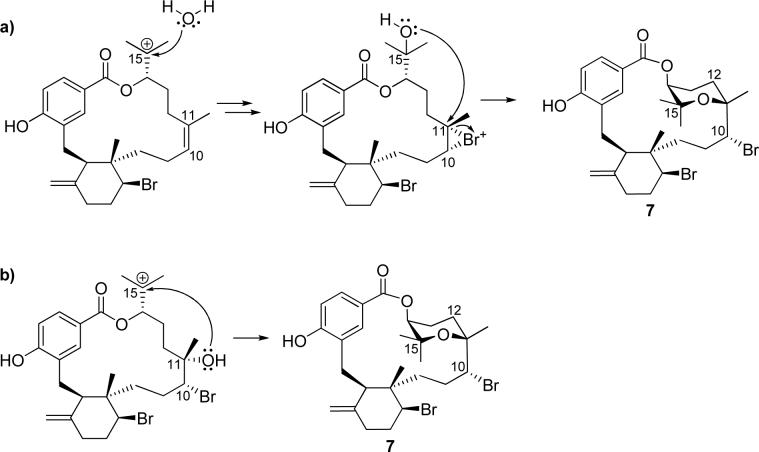
Together, bromophycolides J-Q (1-8) represent two novel carbon skeletons, two unique tetrahydropyran-containing bromophycolides, plus two regioisomers of previously reported bromophycolide E (13) and a regioisomer of bromophycolide A (9). Among the 28 known natural products from C. serratus, bromophycolide J (1) is unique as the only methoxy-substituted metabolite as well as the only bromophycolide bearing a bicyclo[3.1.0] hexane ring in a new carbon skeleton. However, the bicyclo[3.1.0] hexane ring in 1 could have arisen as an artifact from methanolysis of 6, initiated by cleavage of the C-22—Br bond followed by a ring closure of the bicyclo[3.1.0] hexane by homoallylic substitution of bromine by methanol. Bromophycolides K (2) and L (3) represent a second novel carbon skeleton, differing from known bromophycolide structural motifs by a proposed biosynthetic 1,2-methyl shift. Both methyl and hydride shifts are common in terpene biosynthesis;8 however, 2 and 3 represent the first bromophycolides exhibiting a rearranged carbon skeleton. Bromophycolides P (7) and Q (8) are also structurally distinct from the other natural products of this class, each with a tetrahydropyran ring within the macrocycle that significantly increases the hydrophobicity and conformational rigidity of the molecule. All of these structural features, including stereochemistry, may be accounted for with biosynthetic mechanisms that incorporate the same bromonium intermediate previously suggested for five- and six-membered ring cyclizations in bromophycolides.6 The tetrahydropyran ring in 7-8 could arise from attack of nucleophilic C-15 hydroxyl on an electrophilic bromonium ion intermediate at C-10-C-11 (Scheme 1a). Another possible biosynthetic route could involve bromohydrin formation at C-10-C-11, as in other bromophycolides, followed by attack of the C-11 hydroxyl onto a C-15 carbocation (Scheme 1b). The structural novelty observed among the diterpene systems within these 28 natural products suggests a high biosynthetic flexibility within this group.
Scheme 1.
Possible biosynthetic pathways of the tetrahydropyran ring formation in bromophycolide P (7); a) nucleophilic attack of C-15 hydroxyl on bromonium intermediate at C-10-C-11; b) nucleophilic attack of C-11 hydroxyl on C-15 carbocation.
Bromophycolides J-Q (1-8) exhibited low micromolar activities against the most common and deadly human malaria parasite, Plasmodium falciparum (malaria tropica), prompting evaluation of antimalarial activities for previously reported bromophycolides A-I (9-17) and debromophycolide A (18, Table 2). Bromophycolides A (9), D (12), E (13), H (16), and M (4), representing both 15- and 16-membered lactone frameworks, exhibited potent antimalarial activity with IC50's of 0.3-0.9 μM, suggesting that neither mode of lactonization confers an inherent bioactivity advantage. Furthermore, a macrolide motif appears to be essential for antimalarial activity, considering that non-macrocyclic callophycoic acids and callophycols also isolated from C. serratus were less active against P. falciparum.7 Current natural product-derived antimalarial drugs include the artemisinines and quinines, of terpene and alkaloid biogenesis, respectively.9 Artemisinines are the most active and rapid acting antimalarial agents known today (IC50 values <7 nM)9,10 and are also cytotoxic to certain types of human cancer cells.11 While multiple treatment options are available, many malaria strains have evolved drug resistance over the past half-century; also, prophylactic drugs remain obscure, thus making the need for new treatments an immediate concern.9,12 The 15- and 16-membered bromophycolide frameworks represent a new scaffold from which novel and potent antimalarial drugs could be developed.
Table 2.
Antimalarial activities of bromophycolides J-Q (1-8) and previously reported bromophycolides A-I (9-17) and debromophycolide A (18).
| compound | antimalarial IC50 (μM) |
|---|---|
| 1 | 2.7 |
| 2 | 44 |
| 3 | 9.8 |
| 4 | 0.5 |
| 5 | 1.4 |
| 6 | 1.4 |
| 7 | 2.9 |
| 8 | 1.4 |
| 9 | 0.9 |
| 10 | 4.8 |
| 11 | 56 |
| 12 | 0.3 |
| 13 | 0.8 |
| 14 | 18 |
| 15 | 14 |
| 16 | 0.9 |
| 17 | 2.5 |
| 18 | >100 |
Of the newly discovered compounds, bromophycolides P (7) and Q (8) exhibited the most potent antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF, Table 3), suggesting that the conformational rigidity and/or hydrophobicity conferred by the tetrahydropyran system contributes to antibacterial activity. While all tested bromophycolides exhibited moderate antineoplastic activity, only 5 displayed some cell line selectivity, with an IC50 of 1.5 μM against the breast tumor cell line DU4475, the most sensitive cancer tested (Table 3). Interestingly, while 5 demonstrated cancer cell line selectivity, its regioisomer 4 was quite active against all cancer cell lines tested (IC50's 2.1-7.2 μM). Bromophycolide Q (8) was the most potent C. serratus natural product evaluated (mean anticancer IC50 value of 2.0 μM), but showed little cell line selectivity.
Table 3.
Pharmacological activities of bromophycolides J-Q (1-8).
| antibacterial activity (μM) |
anticancer activity (μM) |
antifungal activity |
|||||
|---|---|---|---|---|---|---|---|
| cmpd. | MRSA IC50 | VREF IC50 | antitubercular MIC | meana | DU4475b | cell line selectivity (IC50 max/IC50 min) | IC50 (μM)c |
| 1 | 80 | 66 | 94 | 10 | 3.3 | 10 | NT |
| 2 | NT | NT | NT | 31 | 18 | 3.6 | NT |
| 3 | 8.2 | 26 | 49 | NT | NT | - | 46 |
| 4 | 6.7 | 21 | >100 | 3.1 | 2.1 | 3.5 | >90 |
| 5 | 7.2 | 56 | >50 | 8.6 | 1.5 | 15 | 44 |
| 6 | 8.9 | 18 | NT | 9.7 | 7.3 | 3.5 | >75 |
| 7 | 1.4 | 13 | 48 | 7.9 | 21 | 4.5 | 45 |
| 8 | 1.8 | 5.8 | 22 | 2.0 | 2.0 | 5.5 | >90 |
Mean of 12 cancer cell lines (see Experimental section for details)
breast tumor cell line
Using amphotericin-resistant Candida albicans
NT indicates not tested due to insufficient material
The current study expands the number of bioactive bromophycolide metabolites to 28 and represents two additional novel carbon skeletons, thus suggesting that unexplored red algal families could be an untapped resource of biologically active and interesting natural products.
Experimental Section
Biological material
Callophycus serratus (Harvey ex Kutzing 1957) (family Solieriaceae, order Gigartinales, class Rhodophyceae, phylum Rhodophyta) was collected from Yanuca in the Fiji Islands (18° 23' 57” S, 177° 57' 59” E). Samples were frozen at - 20° C until extraction. Voucher specimens were identified by comparison with previously described morophological traits,13 preserved in aqueous formalin, and deposited at the University of the South Pacific in Suva, Fiji and at Georgia Institute of Technology as ICBG-G-0004, ICBG-G-0005, ICBG-G-0021, and ICBG-G-0049.
Isolation
Frozen Callophycus serratus was extracted successively with water, methanol, and methanol/dichloromethane (1:1 and 1:2). Extracts were combined, reduced in vacuo, and subjected to liquid partitioning between methanol/water (9:1) and petroleum ether. The methanol/water ratio of the aqueous fraction was then adjusted to 3:2 and this fraction partitioned against chloroform. The chloroform fraction was subjected to multiple rounds of reversed-phase C18 HPLC (using Agilent Zorbax SB-C18, 5 μm, 9.4 × 250 mm or Alltech Alltima C18, 5 μm, 10 × 250 mm) with a gradient of acetonitrile/water and methanol/water mobile phases, followed by normal phase silica HPLC (using Agilent RX-SIL columns, 5 μm, 9.4 × 250 mm) with isocratic hexanes/ethyl acetate (82:18) to yield bromophycolides J-Q (1-8). All NMR spectra were collected in CDCl3 and referenced to residual CHCl3 (δ 7.24 and 77.0 ppm for 1H and 13C, respectively).
Pharmacological Assays
All pharmacological assays were performed as previously described.5-7 Briefly, antimalarial activity was determined with a SYBR Green based parasite proliferation assay, adapted from Smilkstein14 and Bennett.15 Plasmodium falciparum parasites (3D7 strain MR4/ATCC, Manassas, VA) were cultured in human O+ erythrocytes as previously described.16
Antibacterial assays were performed using methicillin-resistant Staphylococcus aureus (MRSA, ATCC 10537) and vancoymcin-resistant Enterococcus faecium (VREF, ATCC 12952) as test pathogens. Vancomycin and chloramphenicol were used as positive controls for MRSA and VREF, respectively, and DMSO was used as negative control. The optical density was measured at 600 nm using a microplate reader, and the IC50 of each compound was calculated using the dose concentration at 50% inhibition on a sigmoidal dose response curve generated using GraphPad Prism version 4.00 for Windows, GraphPad software, San Diego, CA, USA.
Amphotericin B-resistant Candida albicans (ATCC 90873) was used in the antifungal assays. A mixed nystatin/amphotericin B solution was used as a positive control, and DMSO was used as a negative control. The optical density was then measured at 600 nm using a microplate reader and the IC50 was calculated for each in the same method as the antibacterial assays.
Antitubercular activity was assessed against Mycobacterium tuberculosis strain H37Rv (ATCC 27294) using the microplate alamar blue assay (MABA) as described previously.17 Compounds 1 and 4 were tested at a maximum concentration of 100 μM, and 3, 5, 7-8 were tested at a maximum concentration of 50 μM.
Bromophycolides J (1), K (2) and M-Q (4-8) were evaluated against a panel of 12 tumor cell lines including breast, colon, lung, prostate, and ovarian cancer cells. Specific cell lines were: BT-549, DU4475, MDA-MD-468, PC-3, SHP-77, LNCaP-FGC, HCT116, MDA-MB-231, A2780/DDP-S, Du145, CCRF-CEM, and A549. In vitro cytotoxicity was tested with the (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxylmethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt) MTS dye conversion assay as described previously.18
Bromophycolide J (1)
white amorphous solid (1.0 mg; 0.023 % plant dry mass); [α]23D +35 (c 0.057 g/100 mL, MeOH); UV (MeOH) λmax (log ε) 265 (3.78) nm; 1H NMR (CDCl3, 500 MHz) and 13C/DEPT NMR (CDCl3, 125 MHz) data, Table 1; NOE, COSY, HMBC NMR data, Supporting Information; HRESIMS [M - H]- m/z 613.1160 (calcd for C28H39O5Br2, 613.1164).
Bromophycolide K (2)
white amorphous solid (0.8 mg; 0.018 % plant dry mass); [α]23D +22 (c 0.046 g/100 mL, MeOH); UV (MeOH) λmax (log ε) 264 (3.54) nm; 1H NMR (CDCl3, 500 MHz) and 13C/DEPT NMR (CDCl3, 125 MHz) data, Table 1; NOE, COSY, HMBC NMR data, Supporting Information; HRESIMS [M - H]- m/z 519.1767 (calcd for C27H36O5Br, 519.1746).
Bromophycolide L (3)
white amorphous solid (0.3 mg, 0.007% plant dry mass); [α]24D +70 (c 0.033 g/100 mL, MeOH); UV (MeOH) λmax (log ε) 262 (3.72) nm; 1H NMR (CDCl3, 500 MHz) and C NMR (CDCl3, 125 MHz) data, Table 1; NOE, COSY, and HMBC NMR data, Supporting Information; HRESIMS [M - H]- m/z 501.1677 (calcd for C27H34O4Br, 501.1640).
Bromophycolide M (4)
white amorphous solid (1.8 mg; 0.041 % plant dry mass); [α]23D +68 (c 0.10 g/100 mL, MeOH); UV (MeOH) λmax (log ε) 262 (3.66) nm; 1H NMR (CDCl3, 500 MHz) and 13C/DEPT NMR (CDCl3, 125 MHz) data, Table 1; NOE, COSY, HMBC NMR data, Supporting Information; HRESIMS [M - H]- m/z 581.0906 (calcd for C27H35O4Br2, 581.0902).
Bromophycolide N (5)
white amorphous solid (1.0 mg, 0.023% plant dry mass); [α]24D +101 (c 0.033 g/100 mL, MeOH); UV (MeOH) λmax (log ε) 260 (3.42) nm; 1H NMR (CDCl3, 500 MHz) and 13C/DEPT NMR (CDCl3, 125 MHz) data, Table 1; NOE, COSY, and HMBC NMR data, Supporting Information; HRESIMS [M - H]- m/z 581.0907 (calcd for C27H35O4Br2, 581.0902).
Bromophycolide O (6)
white amorphous solid (0.5 mg, 0.012% plant dry mass); [α]24D +88 (c 0.011 g/100 mL, MeOH); UV (MeOH) λmax (log ε) 260 (3.54) nm; 1H NMR (CDCl, 500 MHz) and 13C NMR (CDCl3, 125 MHz) data, Table 1; NOE, COSY, and HMBC NMR data, Supporting Information; HRESIMS [M - H]- m/z 661.0182 (calcd for C27H36O4Br3, 661.0169).
Bromophycolide P (7)
white amorphous solid (4.0 mg, 0.092% plant dry mass); [α]24D +120 (c 0.05 g/100 mL, MeOH); UV (MeOH) λmax (log ε) 260 (4.02) nm; 1H NMR (CDCl, 500 MHz) and 13C NMR (CDCl3, 125 MHz) data, Table 1; NOE, COSY, and HMBC NMR data, Supporting Information; HRESIMS [M - H]- m/z 581.0865 (calcd for C27H35O4Br2, 581.0902).
Bromophycolide Q (8)
white amorphous solid (1.0 mg, 0.023% plant dry mass); [α]24D +102 (c 0.03 g/100 mL, MeOH); UV (MeOH) λmax (log ε) 260 (3.83) nm; 1H NMR (CDCl, 500 MHz) and 13C NMR (CDCl3, 125 MHz) data, Table 1; NOE, COSY, and HMBC NMR data, Supporting Information; HRESIMS [M - H]- m/z 581.0869 (calcd for C27H35O4Br2, 581.0902).
Supplementary Material
Acknowledgements
This research was supported by the U.S. National Institutes of Health's International Cooperative Biodiversity Groups program (grant No. U01 TW007401). The authors thank the Government of Fiji for permission to perform research in their territorial waters and for permission to export samples. We especially thank the Roto Tui Serua and the people of Yanuca Island for facilitating this work. We thank A. Chequer and C. Kicklighter for field assistance; M. Sharma, K. Feussner, and A. Wang for assistance with extractions and separations; B. Smith for insightful experimental comments; T. Davenport and S. Engel for antimicrobial assays; M.C. Sullards and D. Bostwick for mass spectral analyses; L. Gelbaum for NMR assistance; and A. Bommarius and T. Rogers for use of their spectropolarimeter.
Footnotes
Supporting Information Available. 2D NMR data tables (COSY, HMBC, ROESY/NOESY), 1H, 13C, 1H-1H COSY, and ROESY NMR spectral data for 1-8 are available free of charge via the Internet at http://pubs.acs.org.
References and Footnotes
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyde JE. FEBS J. 2007;274:4688–4698. doi: 10.1111/j.1742-4658.2007.05999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faulkner DJ. Nat. Prod. Rep. 2000;17:1–6. doi: 10.1039/a909113k. [DOI] [PubMed] [Google Scholar]
- 4.Blunt JW, Copp BR, Hu WP, Munro MHG, Northcote PT, Prinsep MR. Nat. Prod. Rep. 2008;25:35–94. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- 5.Kubanek J, Prusak AC, Snell TW, Giese RA, Fairchild CR, Aalbersberg W, Hay ME. J. Nat. Prod. 2006;69:731–735. doi: 10.1021/np050463o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubanek J, Prusak AC, Snell TW, Giese RA, Hardcastle KI, Fairchild CR, Aalbersberg W, Raventos-Suarez C, Hay ME. Org. Lett. 2005;7:5261–5264. doi: 10.1021/ol052121f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane AL, Stout EP, Hay ME, Prusak AC, Hardcastle K, Fairchild CR, Franzblau SG, Le Roch K, Prudhomme J, Aalbersberg W, Kubanek J. J. Org. Chem. 2007;72:7343–7351. doi: 10.1021/jo071210y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbert RB, editor. The Biosynthesis of Secondary Metabolites. 2nd ed. Chapman & Hall; London: 1989. [Google Scholar]
- 9.Schlitzer M. Arch. Pharm. Chem. Life Sci. 2008;341:149–163. doi: 10.1002/ardp.200700184. [DOI] [PubMed] [Google Scholar]
- 10.Basco LK, Le Bras J. Am. J. Trop. Med. Hyg. 1993;49:301–307. doi: 10.4269/ajtmh.1993.49.301. [DOI] [PubMed] [Google Scholar]
- 11.Singh NP, Lai HC. Anticancer Res. 2004;24:2277–2280. [PubMed] [Google Scholar]
- 12.Schiltzer M. ChemMedChem. 2007;2:944–986. doi: 10.1002/cmdc.200600240. [DOI] [PubMed] [Google Scholar]
- 13.Littler DS, M.M. L. South Pacific Reef Plants. Offshore Graphics, Inc.; Washington, D.C.: 2003. [Google Scholar]
- 14.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Antimicrob. Agents Ch. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. Antimicrob. Agents Ch. 2004;48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trager W, Jensen JB. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 17.Falzari K, Zhu Z, Pan D, Liu H, Hongmanee P, Franzblau SG. Antimicrob. Agents Ch. 2005;49:1447–1454. doi: 10.1128/AAC.49.4.1447-1454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee FYF, Borzilleri R, Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, Vite GD, Rose WC, Kramer RA. Clin. Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.