Abstract
Brain injury in premature infants is of enormous public health importance because of the large number of such infants who survive with serious neurodevelopmental disability, including major cognitive deficits and motor disability. This type of brain injury is generally thought to consist primarily of periventricular leukomalacia (PVL), a distinctive form of cerebral white matter injury. Important new work shows that PVL is frequently accompanied by neuronal/axonal disease, affecting the cerebral white matter, thalamus, basal ganglia, cerebral cortex, brain stem, and cerebellum. This constellation of PVL and neuronal/axonal disease is sufficiently distinctive to be termed “encephalopathy of prematurity”. The thesis of this Review is that the encephalopathy of prematurity is a complex amalgam of primary destructive disease and secondary maturational and trophic disturbances. This Review integrates the fascinating confluence of new insights into both brain injury and brain development during the human premature period.
Introduction
The enormity of the problem of encephalopathy in premature infants relates in substantial part to the large number of affected infants. Every year in the USA, approximately 63000 infants are born with a very low birthweight (VLBW; ≤1500 g).1 This group represents 1·5% of all livebirths, a proportion that has increased gradually over the past decade. The importance of encephalopathy in this large group is indicated by the subsequent occurrence of cognitive, behavioural, attentional, or socialisation deficits in 25–50%, and of major motor deficits (eg, cerebral palsy) in 5–10%.2–8 Cognitive deficits without major motor deficits are by far the dominant neurodevelopmental sequelae in infants with VLBW. Particular note should be made of the increasingly important contribution to this burden of disability by the most premature infants. Because of sharply increased survival (50–70%) in recent years, these extremely premature infants comprise a substantial proportion of infants with VLBW in many centres. Disability in this subset exceeds 50% in most studies.8–12
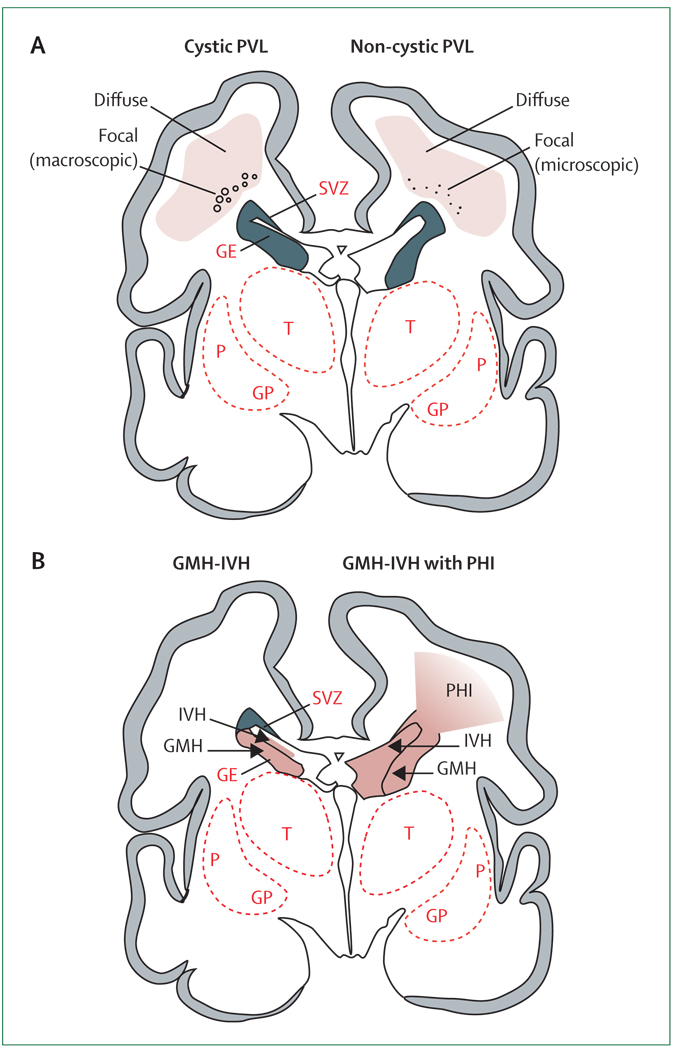
The neuropathological correlates of this encephalopathy include various lesions, most notably periventricular leukomalacia (PVL; figure 1), and accompanying neuronal/axonal deficits that involve the cerebral white matter, thalamus, basal ganglia, cerebral cortex, brainstem, and cerebellum. Severe germinal matrix haemorrhage–intraventricular haemorrhage (GMH-IVH), particularly with periventricular haemorrhagic infarction (PHI; figure 1), is an important, albeit quantitatively less common, lesion in premature infants. Imaging studies indicate that 50% or more of infants with VLBW show findings consistent with PVL and apparent neuronal/axonal disease, whereas severe GMH-IVH with PHI occurs in only approximately 5%.8 (Importantly, the occurrence of PHI can rise to as much as 20–30% in infants below 750 g.) Thus, the emphasis of this Review is on PVL and neuronal/axonal disease, because quantitatively, this constellation seems to account for most of the brain injury and the resulting neurological sequelae. The term “encephalopathy of prematurity” is proposed for this combination. However, the emerging role for severe GMH-IVH with PHI, especially in the smallest infants, is discussed briefly.
Figure 1. Cystic and non-cystic periventricular leukomalacia (PVL) and germinal matrix haemorrhage–intraventricular haemorrhage (GMH-IVH) and GMH-IVH with periventricular haemorrhagic infarction (PHI).
Coronal sections from the brain of a 28-week-old premature infant. The dorsal cerebral subventricular zone (SVZ), the ventral germinative epithelium of the ganglionic eminence (GE), thalamus (T), and putamen (P)/globus pallidus (GP) are shown. (A) The focal necrotic lesions in cystic PVL (small circles) are macroscopic in size and evolve to cysts. The focal necrotic lesions in non-cystic PVL (black dots) are microscopic in size and evolve to glial scars. The diffuse component of both cystic and non-cystic PVL (pink) is characterised by the cellular changes, as described in the text. (B) Haemorrhage (red) into the GE results in GMH, which could burst through the ependyma to cause an IVH (left). When the GHM-IVH is large, PHI might result (right).
The pathogenesis of PVL has been reviewed in detail elsewhere,8,13 and will not be discussed here. The main initiating pathogenetic mechanisms are ischaemia and inflammation, the latter often due to maternal intrauterine infection or postnatal sepsis. These two upstream mechanisms often co-exist and can potentiate each other. The main downstream mechanisms are excitotoxicity and free-radical attack. Various maturation-dependent factors, including a propensity for premature infants to experience episodes of cerebral ischaemia and infection or inflammation, and an intrinsic susceptibility to excitotoxicity and free-radical accumulation, converge to accentuate vulnerability. The cellular targets of these pathogenetic mechanisms are discussed below.
The thesis of this Review is that the encephalopathy of prematurity is a complex amalgam of primary destructive disease and secondary maturational and trophic disturbances. Recent delineation of the extraordinary array of rapidly developing neurobiological processes that occur at 20–40 weeks of gestation in the human brain provides new insights into the bases for the likely maturational/trophic disturbances. I will first review the neuropathology of the encephalopathy of the premature infant, then describe the brain developmental events that occur in the premature period, and finally discuss the likely interrelations of destructive and developmental mechanisms in the genesis of the encephalopathy.
Neuropathology
The main neuropathological processes in the premature infant—PVL and neuronal/axonal disease—have been defined in recent years both in vivo by MRI and post mortem by advanced histological and immunocytochemical techniques. The neuropathology of severe GMH-IVH with PHI, a venous infarction, has been well delineated by conventional histological approaches and by cranial ultrasonography and is described in standard sources.8
PVL
PVL refers to injury to cerebral white matter, classically with two components: focal and diffuse (figure 1).8 The focal component consists of localised necrosis deep in periventricular white matter, with loss of all cellular elements. These necroses can be macroscopic in size (several millimetres or more) and evolve over several weeks to multiple cystic lesions, readily visualised by cranial ultrasonography and known as “cystic PVL” (figure 1). In modern neonatal intensive care units, this severe lesion is observed in less than 5% of infants with VLBW and therefore accounts for a small minority of PVL.14–18 Much more commonly, focal necroses are microscopic in size and evolve over several weeks to glial scars that are not readily seen by neuroimaging. This form of PVL, which accounts for the vast majority of cases, is termed “non-cystic PVL” (figure 1).8
The second component of PVL, which is more diffusely apparent in cerebral white matter, is characterised by marked astrogliosis and microgliosis, and initially by a decrease in premyelinating oligodendrocytes (pre-OLs).19–21 Subsequently, the decrease in cells of the oligodendroglial lineage is counteracted by an increase in oligodendroglial progenitors.22 This response of oligodendroglial progenitors to injury in the developing brain has been shown in several animal models.23,24 However, in PVL, these cells, which often lack processes, seem not to have the capacity for full differentiation to mature myelin-producing cells, and hypomyelination with ventriculomegaly is the later sequela.22,25–33 The cause of the apparent disturbance of pre-OL maturation is currently unknown, but the failure of the regenerating oligodendroglial progenitors to mature has been well documented in a neonatal animal model of PVL.34 In this animal model, these progenitors are exquisitely vulnerable to a subsequent hypoxic–ischaemic insult, a common feature in premature infants. Although precise imaging and anatomical correlations are lacking, the correlates of the diffuse component of PVL on MRI in the neonatal period seem to include diffuse signal abnormalities and disturbances in diffusion parameters.2,15,17,18,35–41
Neuronal/axonal disease
Neuronal/axonal disease is a previously under-recognised accompaniment of PVL. The regions of involvement include the cerebral white matter (axons and subplate neurons), thalamus, basal ganglia, cerebral cortex, brainstem, and cerebellum (figure 2). The neuronal/axonal involvement has been delineated, particularly in vivo, by volumetric MRI analyses, which show in infants with VLBW a decreased volume of neuronal structures such as the thalamus, basal ganglia, cerebral cortex, and cerebellum, as early as term-equivalent age, as well as later in childhood, adolescence, and adulthood. Diffusion tensor MRI studies have similarly suggested the possibility of axonal disturbance at these various maturational times. Recent neuropathological studies have provided further insight into the neuronal/axonal involvement.
Figure 2. Main neuronal/axonal structures affected in premature infants with periventricular leukomalacia.
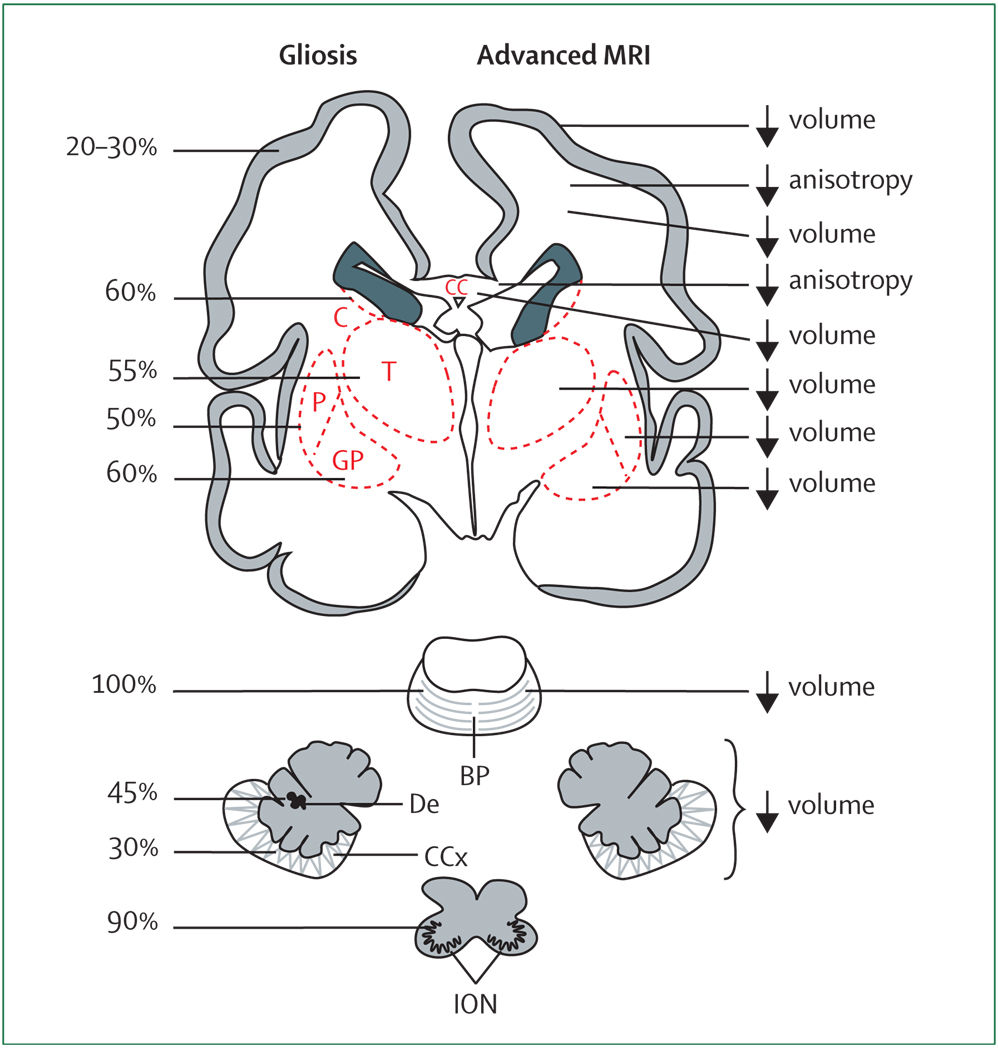
Coronal sections of the cerebrum, pons, cerebellum, and medulla (inferior olivary nuclei) are shown. The frequency of gliosis by neuropathological study and the major abnormalities detected by advanced MRI (volumetric and diffusion-based MRI) are shown. See text for details. BP=basis pontis. C=caudate. CC=corpus callosum. CCx=cerebellar cortex. De=dentate. GP=globus pallidus. ION=inferior olivary nuclei. P=putamen. T=thalamus.
Cerebral white matter: axons
Cerebral white matter axons (ie, projection, commissural, and association fibres) are in a phase of rapid growth during the premature period, the peak period of vulnerability for PVL. Earlier neuropathological evidence for axonal injury in PVL was derived from studies of the necrotic foci and, expectedly, included findings of axonal spheroids and positive immunocytochemical staining for beta-amyloid precursor protein, both indicators of overt axonal damage.21,42–47 Of particular interest, a recent report used the apoptotic marker fractin to show that widespread axonal degeneration is present in the diffuse component of PVL, separate from the focal necroses.48 Although the latter finding does not allow distinction of a primary destructive lesion from a secondary disturbance, the observation suggests that axonal abnormality with PVL is more pervasive than previously thought.
Axonal disturbance is also suggested by MRI studies of premature infants, especially by diffusion tensor MRI (figure 2).33,39,49–59 These reports show that the normal rapid increase in relative anisotropy in various fibre tracts of premature infants is blunted, especially (although not exclusively) in association with the MRI appearance of non-cystic PVL. The normal increase in this measure of preferred directionality of diffusion parallel to the fibre tract is likely to relate to increased axonal size or density, or axonal microstructural changes.60 However, ensheathment of axons by pre-OLs, also an active process in the premature brain, might be involved in the increase in anisotropy.49,56,60–63 Another potential indicator of cerebral axonal disturbance in premature infants, especially those with PVL, is the subsequent impairment of growth of the corpus callosum, as shown by MRI.64–70
Cerebral white matter: subplate neurons
The major neuronal type in cerebral white matter is the subplate neuron. This transient population of neurons reaches a maximum during the peak period for the occurrence of PVL in the premature infant and is central to both cortical and thalamic development. Subplate neurons contain excitatory amino acid receptors (both NMDA and calcium-permeable [GLUR2-deficient] AMPA receptors),71 and have been shown in a developing animal model to be selectively vulnerable to hypoxia–ischaemia.72
Because hypoxia–ischaemia and excitotoxicity are important in the pathogenesis of PVL, and because PVL is associated with volumetric deficits of the cerebral cortex and thalamus, it is reasonable to suggest the occurrence of concomitant injury to subplate neurons. Initial work does show increased apoptosis (activated caspase 3 expression) in the subplate of premature infants with PVL versus those without PVL.21 However, more detailed studies are needed.
Thalamus
Thalamic neurons are commonly affected in premature infants, especially those with PVL. The most detailed neuropathological analysis of 41 premature infants from a modern neonatal intensive care unit showed that, of supratentorial structures, neuronal loss (40%) and gliosis (60%) were most common in the thalamus (figure 2).73 Neuronal loss was absent in those without PVL. In a later, more detailed study of the thalamus in 22 cases of PVL, thalamic pathology, consisting of neuronal loss, gliosis, and axonal abnormality (fractin expression), was noted in 60%.74 The mediodorsal and reticular nuclei were especially involved, which is of particular relevance to the neurological sequelae in premature infants.
The neuropathological findings are consistent with the finding of diminished volume of the thalamus (often measured with basal ganglia) by MRI studies of premature infants at term-equivalent age and later in childhood and adolescence (figure 2).16,18,25–29,75–78 For those studies that assessed the presence of PVL by MRI, the thalamic volumetric deficit was found to be particularly characteristic of (although not always confined to) infants with imaging features of white matter injury.16,18,25 The MRI abnormalities correlated with subsequent cognitive deficits.18,25,77 The mechanism that underlies the neuronal loss and gliosis observed neuropathologically and the volumetric deficits observed by MRI is not clear from these studies. The findings could indicate direct injury or a maturational/trophic disturbance, or both.
Basal ganglia
Basal ganglia neurons are affected only slightly less commonly than are thalamic neurons, again mainly in infants with PVL (figure 2). Thus, in the recent aforementioned post-mortem study of 41 premature infants, neuronal loss was observed in the caudate and putamen in approximately 15% of infants with PVL and in none of the infants without PVL.73 In infants with PVL, gliosis occurred in these basal ganglia nuclei in 50–60%. As for the thalamus, the findings do not allow distinction of a primary destructive lesion from a secondary disturbance.
MRI volumetric studies of living premature infants at term-equivalent age or older show diminished basal ganglia volumes (figure 2).16,26,28,29,76–79 Of the studies done with systematic MRI in the neonatal period to identify non-cystic PVL, a clear relation of the deep nuclear deficits with the presence of white matter injury has been apparent.16
Cerebral cortex
Neurons of the cerebral cortex are affected less than those of the thalamus and basal ganglia (figure 2). Earlier work showed that cortical neuronal injury might accompany particularly severe forms of cystic PVL.43,80–82 The neuropathological study of the modern, less severe, noncystic PVL showed that neuronal loss or gliosis, or both, could be detected in several cortical regions in 13–30% of PVL cases, but only rarely in non-PVL controls.73 Whether the cortical neuronal loss and gliosis indicated a primary destructive effect or a secondary maturational/trophic effect or both could not be determined.
MRI studies of living infants with VLBW also indicate a disturbance of the cerebral cortex, especially in the presence of PVL (figure 2). Decreased volume of the cerebral cortex in premature infants with non-cystic PVL has been documented as early as term-equivalent age.16,83,84 Volumetric deficits occurred in multiple cortical regions, especially parieto-occipital cortex, which overlies the region of white matter most susceptible to PVL. Premature infants studied later in childhood, adolescence, and adulthood show persisting cerebral cortical volumetric deficits.26–29,32,79 The most pronounced decreases generally occur in parieto-occipital, sensorimotor, premotor, temporal, and hippocampal cortices.26,27,29,77,78,85,86 These cortical neuronal deficits correlate with a wide variety of cognitive deficits observed at follow-up.26,32,77–79,84,86
Cerebellum (and brainstem relay nuclei)
Cerebellar abnormality is particularly characteristic of premature infants with VLBW. The aforementioned neuropathological analysis of 41 premature infants identified neuronal loss in the dentate nucleus and the cerebellar cortex in 25–30% of the infants (figure 2).73 In the cerebellar relay nuclei, basis pontis, and inferior olive, neuronal loss was found in 15–20%. However, gliosis was more common, identified in the cerebellar cortex and dentate in 30–45%, and in the pons and olive in 90–100% (figure 2). The abnormalities were generally more likely to occur in the presence of PVL than in non-PVL, but in non-PVL cases, gliosis in the pons and olive did occur in 80–90%. Of note, PVL involving cerebellar white matter was unusual, occurring in only 8% of the infants. More detailed neuropathological study of the cytological characteristics of the cerebellar abnormality is needed.
MRI studies have been particularly valuable in the identification of cerebellar disease in premature infants. Cerebellar involvement has consisted most often of bilateral, generally symmetric, decreases in cerebellar hemispheric volumes at term-equivalent age or later in childhood or adolescence (figure 2).26,87–99 A strong correlation with supratentorial lesions, particularly PVL, but also haemorrhagic lesions (eg, PHI), has been documented.93–97 In two MRI studies that analysed pontine size, both pontine diameter and cerebellar volume were reduced in premature infants with PVL (figure 2).92,98 In unilateral cerebral lesions, decreased volume of the contralateral cerebellar hemisphere has been greater than the decrease in the ipsilateral cerebellar hemisphere, consistent with an element of crossed cerebellar diaschisis.93
Clinico-pathological correlations
Definition of specific clinico-pathological correlations in premature infants has been difficult, mainly because of a relative paucity of (1) detailed high-resolution, regional neuroimaging, (2) careful correlative neuropsychological studies, and (3) co-occurrence of white matter and neuronal/axonal disease. However, some important general conclusions seem warranted. With regard to PVL, cystic PVL probably accounts for the small group of infants who show spastic diplegia.8 Non-cystic PVL correlates with the cognitive deficits observed later, usually in the absence of major motor deficits.18,41 However, the full spectrum of cognitive, attentional, behavioural, and socialisation deficits is likely to relate in major part to neuronal/axonal disease. Such deficits include impairments in overall intelligence, object working memory, various executive functions, impulse control, and some characteristics of autistic spectrum disorders.2,3,7,77,78,85,87,100–102 Initial correlations with deficits in volumetric development of the cerebral cortex, thalamus, basal ganglia, and cerebellum have been made. The correlations are consistent with studies in older children that concern the roles not only of the cerebral cortex, but also of the dorsomedial and reticular nuclei of the thalamus, the basal ganglia, and the cerebellum in this cognitive spectrum.103–107
Brain development during the premature period
The neuropathology of brain injury in the premature infant as described above occurs against a background of multiple active developmental events that take place at 24–40 weeks of gestation and involve pre-OLs, microglia, axons, subplate neurons, the proliferative cerebral dorsal subventricular zone (SVZ) and ventral germinative epithelium of the ganglionic eminence (GE), thalamus, cortex, and cerebellum. Because of the very active and complex characteristics of these events, they are likely to be vulnerable to exogenous and endogenous insults, such as ischaemia, inflammation, excitotoxicity, and free-radical attack. The possibilities of vulnerability to certain drugs, hormones, undernutrition, or other facets of neonatal intensive care deserve further study. This concept of enhanced vulnerability of rapidly developing events during brain maturation was postulated and corroborated by the classic studies of the effects of infantile undernutrition by Dobbing and colleagues nearly 40 years ago.108,109
pre-OLs
pre-OLs, which have been shown to be a key cellular target in PVL, are in a phase of active development during weeks 24–40 of gestation.8,110–114 The four sequential stages of oligodendroglial maturation include the oligodendroglial progenitor, the pre-oligodendrocyte (or late oligodendroglial progenitor; positive for monoclonal antibody O4), the immature oligodendrocyte (positive for monoclonal antibodies O4 and O1), and the mature myelin-producing oligodendrocyte (positive for myelin basic protein). Pre-oligodendrocytes and the immature oligodendrocytes are referred here together as pre-OLs. These differentiating forms (especially the O4/O1-positive immature oligodendrocytes) ensheath axons in preparation for full differentiation to myelin-producing oligodendrocytes (figure 3). Mature, myelin-basic-protein-expressing and ultimately myelin-producing oligodendrocytes do not become abundant in cerebral white matter until after term.
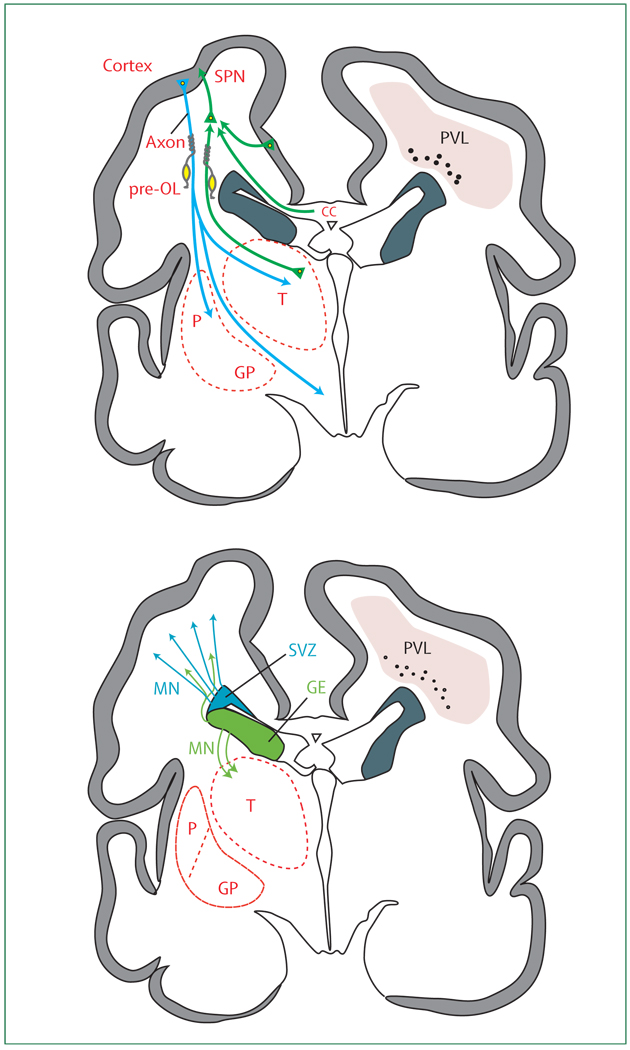
Figure 3. Cerebrum in coronal section at 28 weeks’ gestation showing critical events in cortical development.
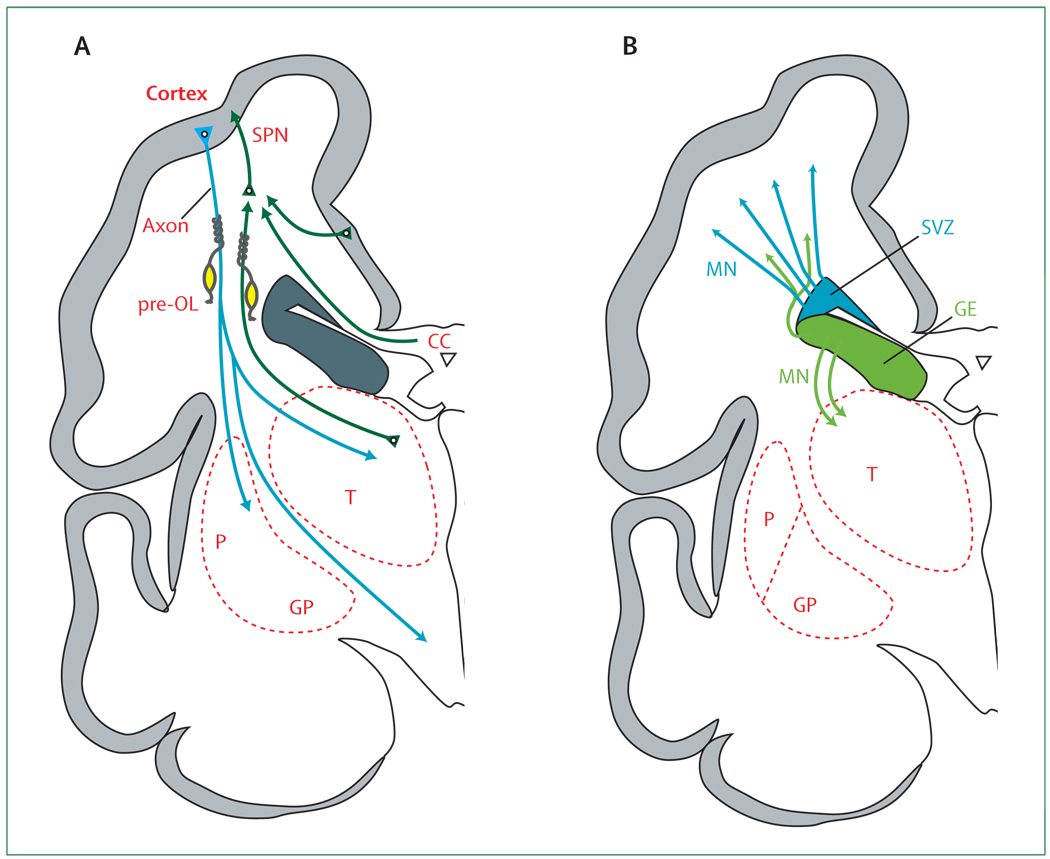
(A) The axons (green) emanate from the thalamus (T; projection fibres), corpus callosum (CC; commissural fibres), and cortex (association fibres), which synapse initially on subplate neurons (SPNs). SPNs send axons to the cortex and promote cortical development before the thalamo-cortical and cortico-cortical fibres enter the cortex. From the cortex, axons (blue) descend to the thalamus, basal ganglia, and corticospinal (and corticopontine) tracts. Premyelinating oligodendrocytes (pre-OLs; yellow) enstheath axons before full differentiation to mature myelin-producing oligodendrocytes. (B) The proliferation and migration of GABAergic interneurons from the subventricular zone (SVZ) and ventral germinative epithelium of the ganglionic eminence (GE) are shown. Neurons from the SVZ (blue) migrate radially to the cortex and from the GE (green), tangentially and then radially, to the cortex. The migrating stream of interneurons from the GE to the dorsal thalamus is also shown. GP=globus pallidus. MN=migrating neurons. P=putamen.
During the peak period of PVL, the O4-positive late oligodendroglial progenitors predominate in cerebral white matter and at 28 weeks account for 90% of the total oligodendroglial population.114 At 28–40 weeks of gestation, the O4-positive cells begin differentiation to O1-positive immature oligodendrocytes, which account for approximately 30% of the total oligodendrocyte population during the later premature period and about 50% by term. These two early differentiating forms show maturation-dependent characteristics that render them especially vulnerable to injurious insults, such as ischaemia and inflammation, which lead to excitotoxicity and generation of free radicals. These pre-OL characteristics include enhanced vulnerability to the following factors: (1) reactive oxygen and nitrogen species, because of impaired antioxidant defences; (2) excitotoxicity, because of exuberant expression of calcium-permeable glutamate receptors (ie, GLUR2-deficient AMPA receptors, preferentially on cell bodies, and NMDA receptors, preferentially on cell processes), and enhanced expression of the main glutamate transporter, which can become a source of injurious glutamate; and (3) cytokine injury, because of both expression of the interferon-gamma receptor on the pre-OL in the context of pronounced availability of interferon gamma in the abundant astrocytes of PVL, and sensitivity to injury by tumour necrosis factor, which is secreted by the abundant activated microglia.8,13,19,20,71,115–125
Microglia
Microglia have key roles during brain development, involving apoptosis, vascularisation, axonal development, and myelination.126–129 Accordingly, these cells become prominent in the forebrain at 16–22 weeks of gestation,130–133 and reach a peak abundance in cerebral white matter in the third trimester.133 Microglia seem to be key effectors of cellular injury with both ischaemia and inflammation.8 These cells generate free radicals, secrete injurious cytokines, and enhance excitotoxicity.117–121,131,134–136 Because microglia are particularly abundant in normal cerebral white matter, they are in the right place at the right time in large numbers to lead to injury to white matter constituents (ie, pre-OLs), but also to axons and subplate neurons.133 Not surprisingly, many activated microglia are present diffusely in cerebral white matter in association with preOL injury in PVL.19
Axons
Axonal development is remarkably exuberant in the developing cerebrum over the last trimester of gestation and in the early postnatal period (figure 3). Haynes and colleauges126 used an immunocytochemical approach, with staining for growth-associated protein-43, which is expressed on growing axons, to show pronounced expression in the cerebral white matter to the region of the subplate at 20 weeks, to the subplate and cortex at 27 weeks, and abundantly within the cortex at 37 weeks. These striking findings are consistent with detailed studies by Kostovic and coworkers137–140 of the axonal connections to and from the subplate during the last half of gestation (panel 1).
Subplate neurons
This crucial transient population of neurons reaches its peak size and maximum developmental impact at 24–32 weeks of gestation, the peak period for occurrence of PVL (figure 3).137–141 These neurons are largely glutamatergic, originating in the dorsal telencephalic ventricular zone, and fewer are GABAergic, originating in the ventral telencephalic GE. Development of the subplate is inter-twined closely with the cerebral cortex, subcortical structures (especially thalamus), and axons (projection, commissural, and association; panel 1). The crucial main roles of subplate neurons are to serve as sites of synaptic contacts for so-called “waiting” thalamocortical and commissural/association cortico-cortical afferents before differentiation of the cortical plate, to serve as a functional link between these waiting afferents and their cortical targets, to provide axonal guidance into the cerebral cortex for the ascending afferents, to facilitate cerebral cortical organisation and synaptic development, and to provide pioneering axonal guidance for projections from the cortex to subcortical targets (eg, the thalamus).8,137,142–149
SVZ (and cortical GABAergic neurons)
The SVZ is derived mainly from radial progenitors (radial glial cells) and consists of so-called intermediate progenitors (figure 3, panel 2).141 Although some studies had suggested that the SVZ gives rise primarily to glia, recent studies show that early SVZ progenitors are largely neurogenic.141,150–153 These early intermediate precursors produce neurons, particularly for deeper cortical layers. These cells reach the cortex by radial migration before the premature period. Moreover, the previous notion that the SVZ is gliogenic after the early phases of neuronal proliferation is not accurate for the more complex brains of primates.141 Indeed, after the 20th gestational week and extending into at least weeks 25–27, the SVZ actively generates neurons, mainly GABAergic interneurons for the upper cortical layers, the hallmark of the human cortex. Bystron and colleagues thus concluded that the SVZ becomes the main source of cortical neurons in the expanded human cerebrum.141 These later arriving neurons are generated largely (65%) from the dorsal telencephalic SVZ and migrate radially, although approximately 35% are generated from the ventral GE and migrate first tangentially, parallel to the cortical plate, to the region of the dorsal SVZ, from which they migrate radially to the cortex (figure 3).141,152,154 The origin of 65% of cortical GABAergic interneurons from the dorsal telencephalic SVZ is characteristic of the human brain (unlike the rodent brain), and seems to be critical for the development of the expanded upper cortical layers. When the generation of GABAergic neurons in the SVZ ceases after 27 weeks is unknown, but the SVZ per se is clearly a prominent structure during the entire premature period.155
Thalamus
The thalamus receives its initial neurons early in the second trimester from the diencephalic ventricular zone.141 However, recent data show that there is a second, later wave of neurons that are generated in the ventral telencephalic GE and migrate to the dorsal thalamus (figure 3).141,154,156 These neurons are mainly GABAergic and migrate by homotypic–neurophilic interactions. In the primate brain, approximately 30% of the neurons in every thalamic nucleus are GABAergic.157,158 This population of telencephalon-derived dorsal thalamic neurons are unique to the human brain, and might lead to a specific increase in the population of GABAergic neurons in the large association nuclei (ie, the mediodorsal and pulvinar nuclei).141 As mentioned previously, the mediodorsal nucleus in particular shows neuronal loss and gliosis in premature infants with PVL. In human beings, these unique telencephalon-derived neurons are probably linked to the expansion of the thalamic association nuclei, which are in turn anatomically related to the enlargement of association cortices involved in multiple higher cognitive functions.156 The timing of this critical later development of the thalamus is not entirely known, but probably occurs during a long period from 15 weeks to approximately 34 weeks of gestation.141,156
Panel 1: Development of human subplate and cerebral axons
<20 weeks
Subplate layer apparent at approximately 10 weeks
Invasion of subplate by thalamic afferents (waiting afferents)
Increase in size of subplate from 10 to 20 weeks
20–24 weeks
Thalamic afferents abundant in subplate, with glutamatergic and GABAergic synapses on subplate neurons
Axons (projection, commissural, and association) grow actively, especially in periventricular regions
24–32 weeks
Thalamocortical afferents enter cortex
Callosal (commissural) and association (cortico-cortical) axons enter subplate
Subplate reaches maximum size (4–5 times thicker than cortical plate at 27–30 weeks)
32–36 weeks
Callosal and cortico-cortical fibres enter cortex
Subplate layer gradually decreases
Data based primarily on studies of Kostovic and co-workers.137–140
Panel 2: Human SVZ—recent concepts
SVZ previously thought to appear late in gestation and to produce mainly glia
SVZ in human beings appears early in gestation and early SVZ is mainly neurogenic
Early SVZ progenitors give rise to neurons of deeper cortical layers
Later SVZ progenitors give rise to neurons (especially GABAergic interneurons) of the expanded upper cortical layers, a hallmark of human cortex
SVZ is still proliferating at 25–27 weeks’ gestation (and probably later)
Summary from Bystron and co-workers.141 SVZ=subventricular zone.
Cerebral cortex
Most, but not all, of the neurons of the cerebral cortex have migrated from the proliferative dorsal telencephalic ventricular/subventricular zones before 24 weeks of gestation.8,141 Subsequent events include development of regional, laminar, and cytological complexity. During weeks 24–32 of gestation, synapses become apparent in the deep cortical plate as thalamocortical axons exit the subplate and enter the cortex (panel 1).139 The permanent sensory-driven circuitry of specific cortical areas also begins to evolve at this time.139,140 Parallel acceleration of dendritic differentiation becomes prominent. Indeed, this dendritic development and the extensive elaboration in the cortex of afferent axonal terminals from thalamic, associative, and commissural fibres that enter the cortex after synapsing on subplate neurons leads to the striking four-times increase in cerebral cortical volume documented from 28 to 40 weeks’ post-conceptional age by volumetric MRI.137,159,160 Thus, this critical phase of cortical development occurs simultaneously with the premature period.
A crucial feature of this development is the disproportionate increase in thickness of upper cortical layers.141 This thickening results because of later-arriving GABAergic interneurons from the dorsal SVZ and the ventral GE, as described above (figure 3). The time of termination of this process of GABAergic cortical neuronal proliferation and migration is unknown, but probably extends well into the third trimester, as noted above with regard to the SVZ.141,152 In association with the expansion of the superficial cortical layers, the increase in cortical surface area and rapid gyral development documented by MRI become apparent.8
Cerebellum
The cerebellum develops especially rapidly in the last half of human gestation. This fundamental finding was emphasised particularly by the work of Dobbing and co-workers.109,161 Volumetric MRI study of premature infants has documented an approximately three-times increase in cerebellar volume from 28 to 40 weeks’ gestation.95 Indeed, the rate of overall growth during this period exceeds that of the cerebral cortex, and both neuronal proliferation and migration are prominent (figure 4).162,163
Figure 4. The developing cerebellar cortex at four major time periods from 9 weeks’ gestation to 7 weeks into the postnatal period.
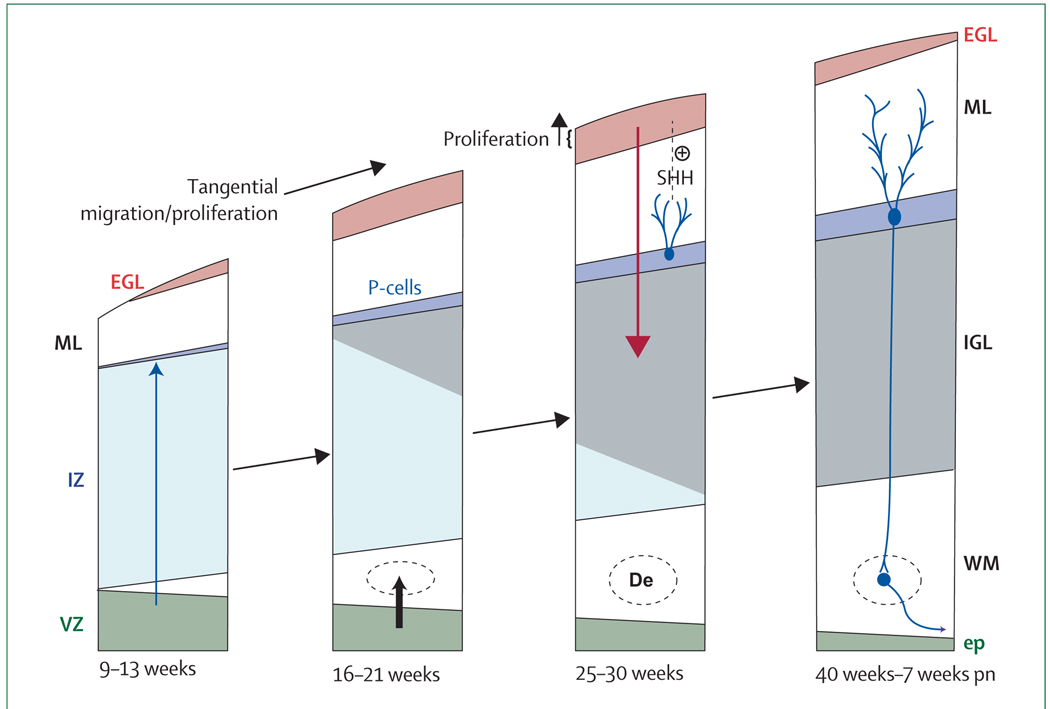
The two proliferative zones are the ventricular zone (VZ) and the external granule cell layer (EGL) derived from the rhombic lip. The VZ gives rise to the Purkinje cells and the deep nuclei (dentate nucleus [De]). The granule precursor cells of the EGL migrate over the surface of the cerebellum. Proliferation in the EGL is activated by sonic hedgehog homologue (SHH), secreted by Purkinje cells (P-cells). The proliferating cells are concentrated in the outer half of the EGL. When post-mitotic, these cells migrate radially inwards (guided by Bergman glia [not shown]) to form the internal granule cell layer (IGL). Note the markedly active proliferation and migration of the granule precursor cells of the EGL during the premature period. ep=ependyma. ML=molecular layer. IZ=intermediate zone. pn=postnatal. WM=white matter. Adapted from ten-Donkelaar et al, 162 with permission from Springer.
Major developmental events in the period before 24 weeks of gestation include establishment of the two proliferative zones, the ventrally located ventricular zone and the more dorsal rhombic lip.162,164 Analogous to the ventral and dorsal telencephalic proliferative zones, the former gives rise to GABAergic neurons and the latter to glutamatergic neurons. The GABAergic neurons originating in the ventricular zone migrate radially to form the roof nuclei, the Purkinje-cell layer, and the molecular layer. Of note, the late-migrating GABAergic neurons, destined to be basket and satellite cells of the molecular layer, also proliferate in cerebellar white matter during their migration, probably well into the premature period.164 The glutamatergic neurons originating in the rhombic lip largely migrate tangentially along the cerebellar surface to form the granule precursor cells of the external granule cell layer (figure 4; some rhombic lip neurons also migrate to the roof nuclei and to the pontine and olivary nuclei).
By 25 weeks, a prominent external granule cell layer is apparent (figure 4).162,163 The layer contains two discrete zones: inner and outer. The outer zone, contiguous with the subarachnoid space and cerebrospinal fluid (CSF), actively proliferates during the premature period, whereas the inner zone contains post-mitotic cells that will migrate inwards to form the internal granule cell layer. These neurons migrate radially along the processes of the Bergmann glia of the Purkinje-cell layer. The proliferative activity of the outer zone of the external granule cell layer is under the control of sonic hedgehog homologue, which is secreted by Purkinje cells.164 These events are remarkably active during the entire premature period. After 40 weeks, the external granule cell layer becomes less prominent, neuronal differentiation is active, and axonal outflow from the roof nuclei develops rapidly. Thus, the most dramatic events during the 25–40-week period occur at the surface of the cerebellum, especially the establishment of the external granule-cell layer by tangential migration and enlargement of this cell layer by proliferation, culminating finally in the inward (radial) migration of these neurons to form the densely packed internal granule cell layer (figure 4).
Combination of destructive and developmental disturbances
The ultimate degree of brain abnormality in survivors of premature birth is likely to depend on a combination of destructive and impaired trophic/maturational mechanisms. The relative importance of these two mechanisms and the nature and extent of their interactions are central issues. The trophic/maturational mechanisms include cell–cell interactions that can involve intercellular trophic support, retrograde effects, and anterograde effects (eg, Wallerian degeneration, trans-synaptic degeneration), among others.
For the discussion of these processes, I will focus on PVL and its accompanying neuronal/axonal abnormalities (ie, the encephalopathy of prematurity). The emphasis will first be on the supratentorial abnormalities. The cerebellar disturbance, an important component of the encephalopathy and also most common with PVL, is then discussed.
PVL and neuronal/axonal disease: encephalopathy of prematurity
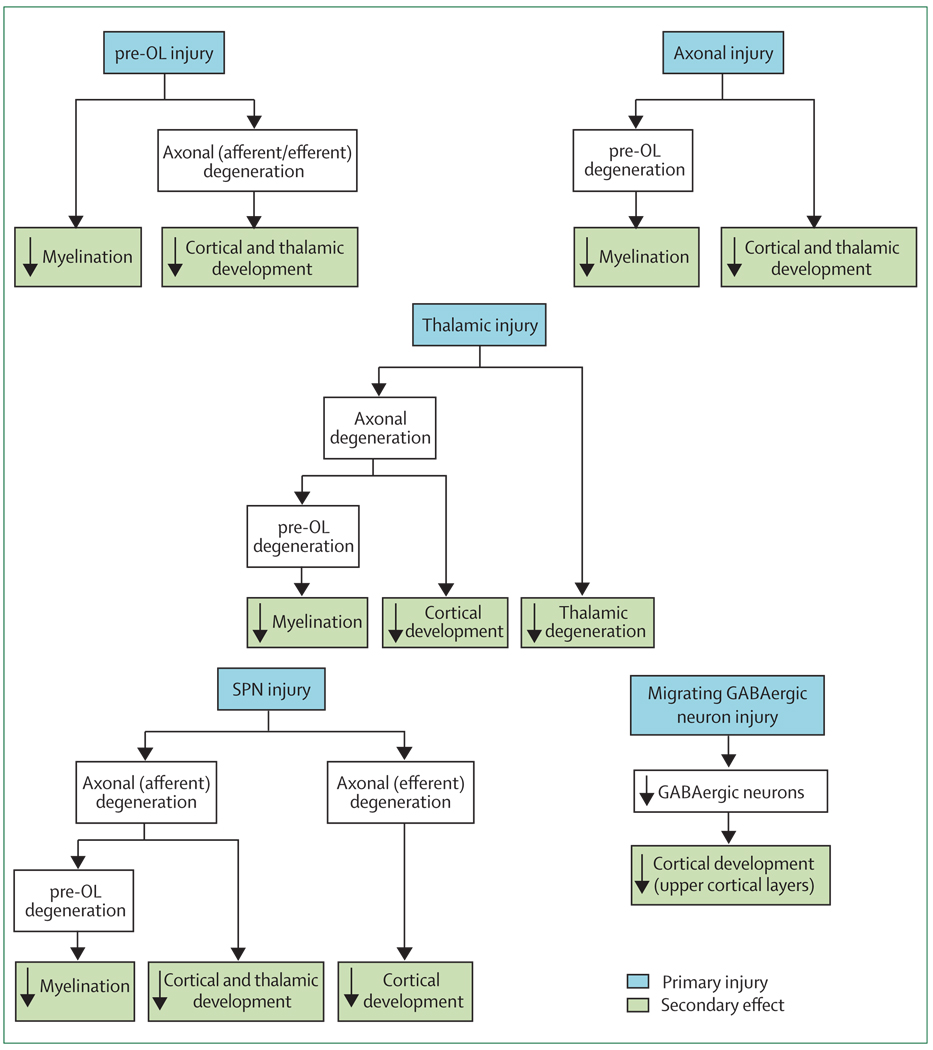
In PVL, the primary event is most likely to be a destructive process (injury) and the subsequent trophic/maturational (ie, developmental) disturbances are secondary. The following discussion will focus on the quantitatively more prominent diffuse component of PVL. The focal com ponent of modern PVL consists of microscopic areas of necrosis. These areas of necrosis involve all cellular elements, and thus focal loss of pre-OLs, axons, and perhaps late-migrating interneurons are to be expected. The consequences will be similar to, but quantitatively less than, the wider cellular effects of diffuse PVL. On the basis of available data, five potential scenarios concerning the primary and secondary events in supratentorial structures in diffuse PVL seem most likely (figure 5). Of these, the first is best supported by available data. However, because rigorous studies in human infants of the other four scenarios are relatively sparse, it is quite possible that all scenarios are operative and that the degree to which one or the other predominates in a given infant varies substantially.
Figure 5. Potential sequences of events leading to major brain sequelae observed with periventricular leukomalacia.
Potential events are hypomyelination, and impaired cortical and thalamic development (eg, seen on advanced MRI analysis by decreased volume). For each sequence, the initiating primary injury is shown, and the subsequent secondary effects are postulated to occur because of maturational/trophic disturbances, as described in the text. Pre-OL=premyelinating oligodendrocyte. SPN=subplate neuron.
pre-OL injury
The pre-OL seems to be the main cellular target in the diffuse component of PVL (figure 5 and figure 6). This vulnerability of pre-OLs has been shown not only in human PVL,19–21 but also in many excellent animal models.8,20,165–168 Injury of pre-OLs consists of cell loss or process loss (with intact soma).22 The cell loss mainly relates to activation of calcium-permeable AMPA receptors on the cell soma,71,169–179 and the process loss, with intact soma, to activation of NMDA receptors on pre-OL processes.8,124,180–183 The ultimate result of either event would be a deficit of mature oligodendroglia, and a consequential impairment of myelination, the hallmark of PVL (figure 5).
Figure 6. Anatomical relationships between the major developmental events and the topography of non-cystic periventricular leukomalacia (PVL).
For purposes of clarity, the developmental events are separated. CC=corpus callosum. GE=ganglionic eminence. GP=globus pallidus. MN=migrating neurons. P=putamen. Pre-OL=premyelinating oligodendrocytes. SPN=subplate neurons. SVZ=subventricular zone. T=thalamus.
However, pre-OL injury could also lead to failure of axonal development and ultimately axonal degeneration. The critical trophic role of oligodendrodrocytes for axonal development, survival, and function is well established in experimental models.62,184–194 The remarkable exuberance of axonal growth during the premature period suggests a particular need for trophic support at this time. Impaired axonal development and axonal degeneration would be consistent with diffusion-based MRI studies of cerebral white matter in premature infants that show abnormalities consistent with axonal deficiency.33,39,49–55 The consequences of axonal deficiency would be diminished cerebral cortical and thalamic/basal ganglia volumes secondary to retrograde and anterograde (trans-synaptic) effects (ie, projection fibres to and from the cortex, the thalamus, and the basal ganglia; figure 5 and figure 6).
Axonal injury
Axonal injury has been recognised for many years to be a feature of the focal necrotic component of PVL. Perhaps more important quantitatively, axonal degeneration, detected by the apoptotic marker fractin, has been recently found to be a feature of the diffuse component of human PVL.48 Axonal injury has been described in experimental models of hypoxic–ischaemic injury analogous to PVL.195–198 Whether the axonal degeneration observed in diffuse PVL is a primary injury or a secondary effect remains unclear. However, if primary axonal injury did occur, the expected results would be hypomyelination (via failure of axonal–oligodendroglial interactions) and decreased cortical and thalamic/basal ganglia volumes (figure 5 and figure 6). The active axonal development in cerebral white matter in premature infants could make these fibres particularly vulnerable.
Thalamic injury
The recent observations that neuronal loss and gliosis are more common in the thalamus than in other brain regions in human PVL is consistent with either primary injury or secondary anterograde and retrograde trophic effects.73,74 If primary neuronal injury occurs, the secondary effects would involve white matter axons, with subsequent hypomyelination and impaired development of cerebral cortex and thalamus/basal ganglia (figure 5 and figure 6). To date, no experimental studies have investigated the possibility of primary injury. However, human neuropathological data are of particular interest.
Subplate neuronal injury
The pivotal role of subplate neurons in the development of the cerebral cortex and deep nuclei in the human premature brain suggests that injury to these key transient cells could have far-reaching secondary trophic/maturational effects. As noted earlier, initial data show increased apoptosis in the subplate of infants with PVL.21 If this were a primary destructive event, secondary retrograde effects on afferent white matter axons and their originating neurons in the cerebral cortex and thalamus and anterograde efferent effects on developing cerebral cortical neurons could be substantial (figure 5 and figure 6). These suggestions are supported by abundant experimental data.8,137,142–149 The axonal degeneration would be accompanied by subsequent hypomyelination, as discussed for pre-OLs and axonal injury. In a neonatal rat model of hypoxic–ischaemic injury and PVL, selective subplate neuronal death was identified.72
SVZ and late-migrating neurons
Although it is possible that the dorsal telencephalic SVZ is affected in PVL, supporting data in human infants are lacking. Experimental studies suggest that progenitors in the dorsal telencephalic SVZ are vulnerable to major hypoxia–ischaemia,199 but in models of selective white matter injury similar to PVL, the SVZ responds by generating oligodendroglial progenitors after the insult.23,24
Although it seems unlikely that the SVZ is injured in PVL, one report suggests that late-migrating GABAergic neurons are affected (figure 5 and figure 6).21 This small neuropathological study of human premature infants with PVL showed a blunting of the normal increase in GABAergic neurons in the cerebral white matter after 28 weeks postconception and an overall diminution of white matter GABAergic neurons.21 Whether this phenomenon indicates decreased generation in the SVZ or injury during the neurons’ late migration is unknown (figure 5 and figure 6), although the latter seems to be more likely. Because these GABAergic interneurons contribute particularly to the thickness of upper cortical layers, a blunting or diminution of this migration could have important structural and functional consequences.
Cerebellum
The prominent disturbance in cerebellar growth in premature infants, which occurs particularly in association with PVL, is another vivid example in the human brain of a rapidly developing process that shows vulnerability. This vulnerability of the cerebellum during its phase of rapid growth was documented several decades ago in experimental models of undernutrition, x-irradiation, and glucocorticoid exposure.108,109,200,201 Diminished DNA content was the main outcome. More recent work has shown a similar vulnerability of the cerebellum in fetal sheep and neonatal rats subjected to hypoxia–ischaemia.202,203 Apoptosis of neuronal elements was the main outcome.
The fundamental nature of the cerebellar disturbance in premature infants is unclear. Except for the minority of infants who have cerebellar haemorrhage, overt tissue destruction is absent. A strong relation of the cerebellar growth failure with supratentorial white matter lesions, especially PVL, suggests that trophic interactions between the cerebrum and cerebellum might be operative. The excitatory interaction between the cerebellum and cerebral cortex via corticopontine tracts and then pontocerebellar connections might be crucial for cerebellar development.93 Of note, the brainstem cerebellar relay nuclei (pontine and inferior olivary nuclei) show gliosis in 90–100% of premature infants with PVL.73 In addition, trophic interactions between the cerebellum and cerebrum, presumably via cerebello–rubro–thalamo–cortical connect ions, can also be shown in premature infants.93 Because of the prominent thalamic disease in infants with PVL, negative retrograde effects on cerebellar growth might occur.
The occurrence of marked cerebellar growth failure in premature infants has been almost completely confined to infants of less than 32 weeks’ gestation and most commonly of 24–28 weeks’ gestation. The most striking developmental event in the cerebellum at this time, the proliferation and inward radial migration of external granule cells (figure 4), is centred on the surface of the cerebellum. Indeed, the outermost portion of the external granule layer contains the proliferating cells. Exposure of these cells to noxious compounds in the CSF (eg, free radicals,204 blood products [non-haem iron, haemosiderin],94,205–207 and proinflammatory cytokines208) could be deleterious.
Thus, the negative effects on cerebellar growth in the small premature infant could relate to direct effects on the cerebellum and to effects on cerebellar connections. Concerning the latter, the negative effects could relate both to loss of positive afferent effects from the cerebrum and brainstem cerebellar relay nuclei, and to development of negative retrograde effects from loss of efferent connections to the thalamus and cerebrum. The direct effects could be multiple, but a disturbance in the proliferation of granule precursor cells in the external granule-cell layer seems most likely.
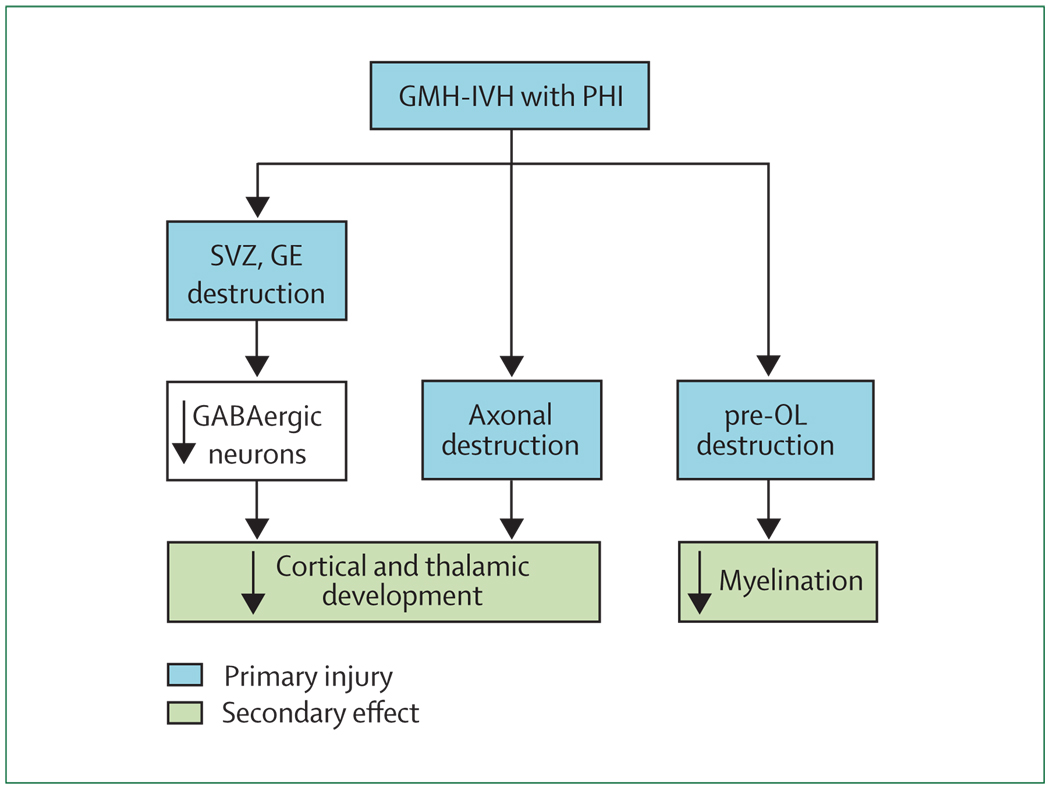
GMH-IVH with PHI
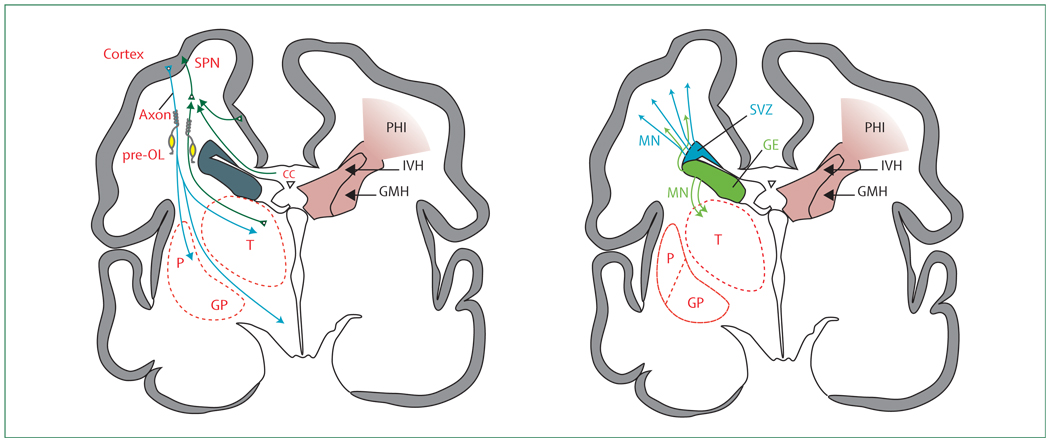
This severe form of GMH-IVH, associated with PHI, is unilateral or grossly asymmetric and accounts for most of the neurological disability related to the entire spectrum of GMH-IVH.8 Although this type of lesion occurs in only 4–5% of all premature infants with VLBW, the incidence in the most immature infants is markedly higher at 20–30% in those born at 24–26 weeks’ gestation or below 750 g birthweight.14,209,210 With rapidly increasing survival for these immature infants, the problem of GMH-IVH with PHI has increased markedly.8 The haemorrhage originates and destroys the germinal matrix, referred to earlier as the ventral telencephalic GE (figure 7 and figure 8). The associated venous infarction (the PHI) destroys the dorsal telencephalic SVZ and the overlying cerebral white matter, including preOLs and axons (figure 7 and figure 8). The consequences of this constellation are a combination of primary destructive and secondary maturational disturbances. The destruction of cerebral white matter axons and pre-OLs results in a large area of tissue loss (ie, a porencephalic cyst). Thalamocortical fibres are inter rupted,55 and overlying cortical development is impaired.81 Other effects are likely to include disturbance of the late GABAergic neuronal proliferation and migration from the SVZ and the GE to upper layers of the cerebral cortex and from the GE to the thalamus (figure 7 and figure 8). Some neuropathological data in human infants support the former contention.211
Figure 7. Likely sequence of events leading to the major neuropathological sequelae observed with germinal matrix haemorrhage (GMH)–intraventricular haemorrhage (IVH) with periventricular haemorrhagic infarction (PHI).
The primary destructive events involve the GMH-IVH with PHI and the associated destruction of premyelinating oligodendrocytes (pre-OLs) and axons. The secondary consequence of the former is hypomyelination, and that of the latter is impaired thalamic and cortical development. In addition, because of destruction of the dorsal subventricular zone (SVZ) and ventral germinative epithelium of the ganglionic eminence (GE), impaired proliferation and late migration of GABAergic interneurons to upper cortical layers and the thalamus could contribute to defective cortical and thalamic development.
Figure 8. The anatomical relationships between the major developmental events and the topography of germinal matrix haemorrhage (GMH)–intraventricular haemorrhage (IVH) with periventricular haemorrhagic infarction (PHI).
For purposes of clarity, the developmental events are separated. CC=corpus callosum. GE=ganglionic eminence. GP=globus pallidus. MN=migrating neurons. P=putamen. Pre-OL=premyelinating oligodendrocytes. SPN=subplate neurons. SVZ=subventricular zone. T=thalamus.
Conclusions
Brain abnormality in the premature infant is unlikely to consist of a straightforward addition of destructive non-haemorrhagic and haemorrhagic lesions, such as PVL and, less commonly, GMH-IVH with PHI. Recent insights into the full spectrum of the encephalopathy of prematurity and into the remarkable series of developmental events that occur in the brain during this period indicate a complex amalgam of destructive and developmental mechanisms. Although further clarification of this amalgam is needed, the general principle that in the premature period brain abnormality involves destructive and developmental mechanisms seems established.
Search strategy and selection criteria
References for this Review were obtained from personal reprint files, supplemented by PubMed searches, with varying search periods (from 1980 to November, 2008). PubMed searches were initiated with all the topical areas covered in the Review. The full list of search terms is available from the author on request and included "periventricular leukomalacia", "cerebral white matter injury", "subplate neurons", "cerebral cortex", "axonal development", "oligodendroglial development", "neuronal development", "glial–neuronal interactions", "glial–axonal interactions", "GABAergic neurons", and "subventricular zone" ("prematurity" and "human brain" were frequent modifiers). The bibliographies of the most recent articles were also screened to find other previously unidentified articles. Only English language articles were included.
Footnotes
Conflicts of interest
I have no conflicts of interest.
References
- 1.Martin JA, Kung HC, Mathews TJ, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 2.Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- 3.Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Hum Dev. 2007;83:247–254. doi: 10.1016/j.earlhumdev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Platt MJ, Cans C, Johnson A, et al. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet. 2007;369:43–50. doi: 10.1016/S0140-6736(07)60030-0. [DOI] [PubMed] [Google Scholar]
- 5.Larroque B, Ancel PY, Marret S, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–820. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- 6.Kobaly K, Schluchter M, Minich N, et al. Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics. 2008;121:73–81. doi: 10.1542/peds.2007-1444. [DOI] [PubMed] [Google Scholar]
- 7.Allin M, Walshe M, Fern A, et al. Cognitive maturation in preterm and term born adolescents. J Neurol Neurosurg Psychiatr. 2008;79:381–386. doi: 10.1136/jnnp.2006.110858. [DOI] [PubMed] [Google Scholar]
- 8.Volpe JJ. Neurology of the newborn. 5th edn. Philadelphia: Elsevier; 2008. [Google Scholar]
- 9.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 10.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child. 2005;90:F134–F140. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marlow N, Hennessy EM, Bracewell MA, Wolke D. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- 12.Wolke D, Samara M, Bracewell M, Marlow N. Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. J Pediatr. 2008;152:256–262. doi: 10.1016/j.jpeds.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larroque B, Marret S, Ancel PY, et al. White matter damage and intraventricular hemorrhage in very preterm infants: the EPIPAGE study. J Pediatr. 2003;143:477–483. doi: 10.1067/S0022-3476(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 15.Miller SP, Cozzio CC, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003;24:1661–1669. [PMC free article] [PubMed] [Google Scholar]
- 16.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 17.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 18.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 19.Haynes RL, Folkerth RD, Keefe R, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes is accompanied by microglial activation in periventricular leukomalacia in the human premature infant. J Neuropath Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 20.Back SA, Luo NL, Mallinson RA, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F(2)-isoprostanes. Ann Neurol. 2005;58:108–120. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 21.Robinson S, Li Q, Dechant A, Cohen ML. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billiards SS, Haynes RL, Folkerth RD, et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18:153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z, Covey MV, Bitel CL, Ni L, Jonakait GM, Levison SW. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;61:199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- 24.Sizonenko SV, Camm EJ, Dayer A, Kiss JZ. Glial responses to neonatal hypoxic-ischemic injury in the rat cerebral cortex. Int J Dev Neurosci. 2008;26:37–45. doi: 10.1016/j.ijdevneu.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 26.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 27.Nosarti C, Al-Asady MHS, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 28.Reiss AL, Kesler SR, Vohr B, et al. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr. 2004;145:242–249. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Kesler SR, Ment LR, Vohr B, et al. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31:318–325. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allin M, Henderson M, Suckling J, et al. Effects of very low birthweight on brain structure in adulthood. Dev Med Child Neurol. 2004;46:46–53. doi: 10.1017/s0012162204000088. [DOI] [PubMed] [Google Scholar]
- 31.Fearon P, O’Connell P, Frangou S, et al. Brain volumes in adult survivors of very low birth weight: a sibling-controlled study. Pediatrics. 2004;114:367–371. doi: 10.1542/peds.114.2.367. [DOI] [PubMed] [Google Scholar]
- 32.Lodygensky GA, Rademaker KJ, Zimine S, et al. Structural and functional brain developmental after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116:1–7. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- 33.Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. NeuroImage. 2006;32:1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- 34.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maalouf EF, Duggan PJ, Rutherford MA, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr. 1999;135:351–357. doi: 10.1016/s0022-3476(99)70133-2. [DOI] [PubMed] [Google Scholar]
- 36.Inder TE, Anderson NJ, Spencer C, Wells SJ, Volpe J. White matter injury in the premature infant: a comparison between serial cranial ultrasound and MRI at term. AJNR Am J Neuroradiol. 2003;24:805–809. [PMC free article] [PubMed] [Google Scholar]
- 37.Debillon T, Guyen SN, Muet A, Quere MP, Moussaly F, Roze JC. Limitations of ultrasonography for diagnosing white matter damage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F275–F279. doi: 10.1136/fn.88.4.F275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inder TE, Wells SJ, Mogridge N, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant—a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 39.Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112:1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Volpe JJ. Cerebral white matter injury of the premature infant—more common than you think. Pediatrics. 2003;112:176–179. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 41.Dyet LE, Kennea NL, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 42.Bell JE, Becher JC, Wyatt B, Keeling JW, McIntosh N. Brain damage and axonal injury in a Scottish cohort of neonatal deaths. Brain. 2005;128:1070–1081. doi: 10.1093/brain/awh436. [DOI] [PubMed] [Google Scholar]
- 43.Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 44.Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 45.Arai Y, Deguchi K, Mizuguchi M, Takashima S. Expression of β-amyloid precursor protein in axons of periventricular leukomalacia brains. Pediatr Neurol. 1995;13:161–163. doi: 10.1016/0887-8994(95)00149-a. [DOI] [PubMed] [Google Scholar]
- 46.Meng SZ, Arai Y, Deguchi K, Takashima S. Early detection of axonal and neuronal lesions in prenatal-onset periventricular leukomalacia. Brain Dev. 1997;19:480–484. doi: 10.1016/s0387-7604(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 47.Deguchi K, Oguchi K, Matsuura N, Armstrong DD, Takashima S. Periventricular leukomalacia: relation to gestational age and axonal injury. Pediatr Neurol. 1999;20:370–374. doi: 10.1016/s0887-8994(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 48.Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008;63:656–661. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huppi PS, Maier SE, Peled S, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Skranes J, Vangberg TR, Kulseng S, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 51.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imag. 2002;16:621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 52.Huppi PS, Murphy B, Maier SE, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 53.Martinussen M, Fischl B, Larsson HB, et al. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain. 2005;128:2588–2596. doi: 10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- 54.Anjari M, Srinivasan L, Allsop JM, et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 55.Counsell SJ, Dyet LE, Larkman DJ, et al. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. NeuroImage. 2007;34:896–904. doi: 10.1016/j.neuroimage.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 56.Counsell S, Shen Y, Boardman JP, et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on MRI at term equivalent age. Pediatrics. 2006;117:376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- 57.Bassi L, Ricci D, Volzone A, et al. Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain. 2008;131:573–582. doi: 10.1093/brain/awm327. [DOI] [PubMed] [Google Scholar]
- 58.Drobyshevsky A, Bregman J, Storey P, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29:289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- 59.Counsell SJ, Edwards AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008 doi: 10.1093/brain/awn268. published online Oct 24. [DOI] [PubMed] [Google Scholar]
- 60.Provenzale JM, Liang L, DeLong D, White LE. Diffusion tensor imaging assessment of brain white matter maturation during the first postnatal year. AJR Am J Roentgenol. 2007;189:476–486. doi: 10.2214/AJR.07.2132. [DOI] [PubMed] [Google Scholar]
- 61.Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J. Identification of “premyelination” by diffusion-weighted MRI. J Comput Assist Tomogr. 1995;19:28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kroenke CD, Bretthorst GL, Inder TE, Neil JJ. Diffusion MR imaging characteristics of the developing primate brain. NeuroImage. 2005;25:1205–1213. doi: 10.1016/j.neuroimage.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 64.Anderson NG, Laurent I, Woodward LJ, Inder TE. Detection of impaired growth of the corpus callosum in premature infants. Pediatrics. 2006;118:951–960. doi: 10.1542/peds.2006-0553. [DOI] [PubMed] [Google Scholar]
- 65.Caldu X, Narberhaus A, Junque C, et al. Corpus callosum size and neuropsychologic impairment in adolescents who were born preterm. J Child Neurol. 2006;21:406–410. doi: 10.1177/08830738060210050801. [DOI] [PubMed] [Google Scholar]
- 66.Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127:2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- 67.Anderson NG, Laurent I, Cook N, Woodward L, Inder TE. Growth rate of corpus callosum in very premature infants. AJNR Am J Neuroradiol. 2005;26:2685–2690. [PMC free article] [PubMed] [Google Scholar]
- 68.Davatzikos C, Barzl A, Lawrie T, Hoon AH, Melhem ER. Correlation of corpus callosal morphometry with cognitive and motor function in periventricular leukomalacia. Neuropediatrics. 2003;34:247–252. doi: 10.1055/s-2003-43259. [DOI] [PubMed] [Google Scholar]
- 69.Allin M, Nosarti C, Narberhaus A, et al. Growth of the corpus callosum in adolescents born preterm. Arch Pediatr Adolesc Med. 2007;161:1183–1189. doi: 10.1001/archpedi.161.12.1183. [DOI] [PubMed] [Google Scholar]
- 70.Narberhaus A, Segarra D, Caldu X, et al. Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J Child Neurol. 2007;22:761–765. doi: 10.1177/0883073807304006. [DOI] [PubMed] [Google Scholar]
- 71.Talos DM, Follett PL, Folkerth RD, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxiaischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pierson CR, Folkerth RD, Billiards SS, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ligam P, Haynes RL, Folkerth RD, et al. Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e3181998baf. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boardman JP, Counsell SJ, Rueckert D, et al. Abnormal deep grey matter development following preterm birth detected using deformation based morphometry. NeuroImage. 2006;32:70–78. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 76.Lin Y, Okumura A, Hayakawa F, Kato T, Kuno K, Watanabe K. Quantitative evaluation of thalami and basal ganglia in infants with periventricular leukomalacia. Dev Med Child Neurol. 2001;43:481–485. doi: 10.1017/s0012162201000883. [DOI] [PubMed] [Google Scholar]
- 77.Nosarti C, Giouroukou E, Healy E, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2007;131:205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 78.Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–520. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abernethy LJ, Cooke RW, Foulder Hughes L. Caudate and hippocampal volumes, intelligence, and motor impairment in 7-year-old children who were born preterm. Pediatr Res. 2004;55:884–893. doi: 10.1203/01.PDR.0000117843.21534.49. [DOI] [PubMed] [Google Scholar]
- 80.Armstrong DL, Sauls CD, Goddard Finegold J. Neuropathologic findings in short-term survivors of intraventricular hemorrhage. Am J Dis Child. 1987;141:617–621. doi: 10.1001/archpedi.1987.04460060035027. [DOI] [PubMed] [Google Scholar]
- 81.Marin Padilla M. Developmental neuropathology and impact of perinatal brain damage. II. White matter lesions of the neocortex. J Neuropathol Exp Neurol. 1997;56:219–235. doi: 10.1097/00005072-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Kinney HC, Armstrong DL. Perinatal neuropathology. In: Graham DI, Lantos PE, editors. Greenfield’s neuropathology. 7th edn. London: Arnold Publishers; 2002. pp. 519–606. [Google Scholar]
- 83.Inder TE, Huppi PS, Warfield S, et al. Periventricular white matter injury in the premature infant is associated with a reduction in cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 84.Peterson BS, Anderson AW, Ehrenkranz RA, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 85.Thompson DK, Wood SJ, Doyle LW, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63:642–651. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- 86.Isaacs E, Lucas A, Chong WK, et al. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47:713–720. doi: 10.1203/00006450-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 87.Allin M, Matsumoto H, Santhouse AM, et al. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 2001;124:60–66. doi: 10.1093/brain/124.1.60. [DOI] [PubMed] [Google Scholar]
- 88.Mercuri E, He J, Curati WL, Dubowitz LM, Cowan FM, Bydder GM. Cerebellar infarction and atrophy in infants and children with a history of premature birth. Pediatr Radiol. 1997;27:139–143. doi: 10.1007/s002470050085. [DOI] [PubMed] [Google Scholar]
- 89.Johnsen SD, Tarby TJ, Lewis KS, Bird R, Prenger E. Cerebellar infarction: an unrecognized complication of very low birthweight. J Child Neurol. 2002;17:320–324. doi: 10.1177/088307380201700502. [DOI] [PubMed] [Google Scholar]
- 90.Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20:60–64. doi: 10.1177/08830738050200011001. [DOI] [PubMed] [Google Scholar]
- 91.Bodensteiner JB, Johnsen SD. Cerebellar injury in the extremely premature infant: newly recognized but relatively common outcome. J Child Neurol. 2005;20:139–142. doi: 10.1177/08830738050200021101. [DOI] [PubMed] [Google Scholar]
- 92.Argyropoulou MI, Xydis V, Drougia A, et al. MRI measurements of the pons and cerebellum in children born preterm; associations with the severity of periventricular leukomalacia and perinatal risk factors. Neuroradiology. 2003;45:730–734. doi: 10.1007/s00234-003-1067-0. [DOI] [PubMed] [Google Scholar]
- 93.Limperopoulos C, Soul JS, Haidar H, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116:844–850. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- 94.Messerschmidt A, Brugger PC, Boltshauser E, et al. Disruption of cerebellar development: potential complication of extreme prematurity. AJNR Am J Neuroradiol. 2005;26:1659–1667. [PMC free article] [PubMed] [Google Scholar]
- 95.Limperopoulos C, Soul JS, Gauvreau K, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115:688–695. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 96.Srinivasan L, Allsop J, Counsell SJ, Boardman JP, Edwards AD, Rutherford M. Smaller cerebellar volumes in very preterm infants at term-equivalent age are associated with the presence of supratentorial lesions. AJNR Am J Neuroradiol. 2006;27:573–579. [PMC free article] [PubMed] [Google Scholar]
- 97.Shah DK, Anderson PJ, Carlin JB, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res. 2006;60:97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida S, Hayakawa K, Yamamoto A, Kanda T, Yamori Y. Pontine hypoplasia in children with periventricular leukomalacia. AJNR Am J Neuroradiol. 2008;29:425–430. doi: 10.3174/ajnr.A0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parker J, Mitchell A, Kalpakidou A, et al. Cerebellar growth and behavioural and neuropsychological outcome in preterm adolescents. Brain. 2008;131:1344–1351. doi: 10.1093/brain/awn062. [DOI] [PubMed] [Google Scholar]
- 100.Bohm B, Smedler AC, Forssberg H. Impulse control, working memory and other executive functions in preterm children when starting school. Acta Paediatr. 2004;93:1363–1371. doi: 10.1080/08035250410021379. [DOI] [PubMed] [Google Scholar]
- 101.Limperopoulos C, Bassan H, Gauvreau K, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 102.Limperopoulos C, Bassan H, Sullivan NR, et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics. 2008;121:758–765. doi: 10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mesulan MM. Principles of behavioral and cognitive neurology. Oxford: Oxford University Press; 2000. [Google Scholar]
- 104.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatr Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 105.Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- 106.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 107.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 108.Dobbing J, Hopewell JW, Lynch A, Sands J. Vulnerability of developing brain. I. Some lasting effects of x-irradiation. Exp Neurol. 1970;28:442–449. doi: 10.1016/0014-4886(70)90181-0. [DOI] [PubMed] [Google Scholar]
- 109.Dobbing J. The later growth of the brain and its vulnerability. Pediatrics. 1974;53:2–6. [PubMed] [Google Scholar]
- 110.Rivkin MJ, Flax J, Mozel R, Osthanondh R, Volpe JJ, Villa-Komaroff L. Oligodendroglial development in human fetal cerebrum. Ann Neurol. 1995;38:92–101. doi: 10.1002/ana.410380116. [DOI] [PubMed] [Google Scholar]
- 111.Back SA, Volpe JJ. Cellular and molecular pathogenesis of periventricular white matter injury. Ment Retard Dev Disabil Res Rev. 1997;3:96–107. [Google Scholar]
- 112.Kinney HC, Back SA. Human oligodendroglial development: relationship to periventricular leukomalacia. Semin Pediatr Neurol. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 113.Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol. 2002;61:197–211. doi: 10.1093/jnen/61.2.197. [DOI] [PubMed] [Google Scholar]
- 114.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Folkerth RD, Keefe RJ, Haynes RL, Trachtenberg FL, Volpe JJ, Kinney HC. Interferon-gamma expression in periventricular leukomalacia in the human brain. Brain Pathol. 2004;14:265–274. doi: 10.1111/j.1750-3639.2004.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Folkerth RD, Haynes RL, Borenstein NS, et al. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 2004;63:990–999. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- 117.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buntinx M, Moreels M, Vandenabeele F, et al. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res. 2004;76:834–845. doi: 10.1002/jnr.20118. [DOI] [PubMed] [Google Scholar]
- 119.Agresti C, D’Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumour necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. doi: 10.1111/j.1460-9568.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 120.Andrews T, Zhang P, Bhat NR. TNFα potentiates IFNγ-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 121.Pang Y, Cai ZW, Rhodes PG. Effect of tumor necrosis factor-alpha on developing optic nerve oligodendrocytes in culture. J Neurosci Res. 2005;80:226–234. doi: 10.1002/jnr.20450. [DOI] [PubMed] [Google Scholar]
- 122.Back SA, Gan X, Li Y, Rosenberg PR, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeSilva TM, Kinney HC, Borenstein NS, et al. The glutamate transporter is transiently expressed in developing human cerebral white matter. J Comp Neurol. 2007;501:879–890. doi: 10.1002/cne.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karadottir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manning SM, Talos DM, Zhou C, et al. NMDA receptor blockade with memantine attenuates white matter deficits in a rat model of periventricular leukomalacia. J Neurosci. 2008;28:6670–6678. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haynes RL, Borenstein NS, DeSilva TM, et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- 127.Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur J Neurosci. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 128.Rezaie P, Male D. Differentiation, ramification and distribution of microglia within the central nervous system examined. Neuroembryology. 2002;1:29–43. [Google Scholar]
- 129.Hamilton SP, Rome LH. Stimulation of in vitro myelin synthesis by microglia. Glia. 1994;11:326–335. doi: 10.1002/glia.440110405. [DOI] [PubMed] [Google Scholar]
- 130.Rezaie P, Dean A, Male D, Ulfig N. Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex. 2005;15:938–949. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- 131.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 132.Monier A, Evrard P, Gressens P, Verney C. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol. 2006;499:565–582. doi: 10.1002/cne.21123. [DOI] [PubMed] [Google Scholar]
- 133.Billiards SS, Haynes RL, Folkerth RD, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- 134.Xie Z, Wei M, Morgan TE, et al. Peroxynitrite mediates neurotoxicity of amyloid β-peptide1–42 - and lipopolysaccharide-activated microglia. J Neurosci. 2002;22:3484–3492. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lehnardt S, Massillon L, Follet P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Larouche A, Roy M, Kadhim H, Tsanaclis AM, Fortin D, Sebire G. Neuronal injuries induced by perinatal hypoxic-ischemic insults are potentiated by prenatal exposure to lipopolysaccharide: animal model for perinatally acquired encephalopathy. Dev Neurosci. 2005;27:134–142. doi: 10.1159/000085985. [DOI] [PubMed] [Google Scholar]
- 137.Kostovic I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- 138.Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 139.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 140.Kostovic I, Judas M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31:1157–1168. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 141.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 142.Ghosh A, Shatz CJ. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development. 1993;117:1031–1047. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- 143.McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- 144.Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- 145.Ghosh A, Shatz CJ. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 1992;255:1441–1443. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- 146.Volpe JJ. Subplate neurons—missing link in brain injury of the premature infant? Pediatrics. 1996;97:112–113. [PubMed] [Google Scholar]
- 147.Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- 148.Kanold PO. Transient microcircuits formed by subplate neurons and their role in functional development of thalamocortical connections. NeuroReport. 2004;15:2149–2153. doi: 10.1097/00001756-200410050-00001. [DOI] [PubMed] [Google Scholar]
- 149.Bystron I, Molnar Z, Otellin V, Blakemore C. Tangential networks of precocious neurons and early axonal outgrowth in the embryonic human forebrain. J Neurosci. 2005;25:2781–2792. doi: 10.1523/JNEUROSCI.4770-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zecevic N, Chen YH, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brazel CY, Romanko MJ, Rothstein RP, Levison SW. Roles of the mammalian subventricular zone in brain development. Prog Neurobiol. 2003;69:49–69. doi: 10.1016/s0301-0082(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 152.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 153.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- 154.Tan SS. Developmental neurobiology: cortical liars. Nature. 2002;417:605–606. doi: 10.1038/417605a. [DOI] [PubMed] [Google Scholar]
- 155.Bayer SA, Altman J. Atlas of human central nervous system development. London: CRC Press; 2004. The human brain during the third trimester. [Google Scholar]
- 156.Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci. 2001;4:931–936. doi: 10.1038/nn0901-931. [DOI] [PubMed] [Google Scholar]
- 157.Montero VM. The interneuronal nature of GABAergic neurons in the lateral geniculate nucleus of the rhesus monkey: a combined HRP and GABA-immunocytochemical study. Exp Brain Res. 1986;64:615–622. doi: 10.1007/BF00340502. [DOI] [PubMed] [Google Scholar]
- 158.Montero VM, Zempel J. The proportion and size of GABA-immunoreactive neurons in the magnocellular and parvocellular layers of the lateral geniculate nucleus of the rhesus monkey. Exp Brain Res. 1986;62:215–223. doi: 10.1007/BF00237420. [DOI] [PubMed] [Google Scholar]
- 159.Huppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 160.Kapellou O, Counsell SJ, Kennea NL, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.ten-Donkelaar HJ, Lammens M, Wesseling P, Thijssen HOM, Renier WO. Development and developmental disorders of the human cerebellum. J Neurol. 2003;250:1025–1036. doi: 10.1007/s00415-003-0199-9. [DOI] [PubMed] [Google Scholar]
- 163.ten-Donkelaar HJ, Lammens M, Hori A. Clinical neuroembryology: development and developmental disorders of the human central nervous system. Heidelberg: Springer-Verlag; 2006. [Google Scholar]
- 164.Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- 165.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–579. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- 168.Duncan JR, Cock ML, Scheerlinck J-PY, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52:941–949. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 169.Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci USA. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yoshioka A, Bacskai B, Pleasure D. Pathophysiology of oligodendroglial excitotoxicity. J Neurosci Res. 1996;46:427–438. doi: 10.1002/(SICI)1097-4547(19961115)46:4<427::AID-JNR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 171.Matute C, Sánchez-Gómez MV, Martínez-Millán L, Miledi R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci USA. 1997;94:8830–8835. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 173.Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- 174.Sanchez-Gomez MV, Matute C. AMPA and kainate receptors each mediate excitotoxicity in oligodendroglial cultures. Neurobiol Dis. 1999;6:475–485. doi: 10.1006/nbdi.1999.0264. [DOI] [PubMed] [Google Scholar]
- 175.Itoh T, Beesley J, Itoh A, et al. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002;81:390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- 176.Rosenberg PA, Dai W, Gan XD, et al. Mature myelin basic protein expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res. 2003;71:237–245. doi: 10.1002/jnr.10472. [DOI] [PubMed] [Google Scholar]
- 177.Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci. 2003;23:9519–9528. doi: 10.1523/JNEUROSCI.23-29-09519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Deng W, Wang H, Rosenberg PA, Volpe JJ, Jensen FE. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci USA. 2004;101:7751–7756. doi: 10.1073/pnas.0307850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deng W, Yue Q, Rosenberg PA, Volpe JJ, Jensen FE. Oligodendrocyte excitotoxicity determined by local glutamate accumulation and mitochondrial function. J Neurochem. 2006;96:213–222. doi: 10.1111/j.1471-4159.2006.03861.x. [DOI] [PubMed] [Google Scholar]
- 180.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 181.Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Micu I, Jiang Q, Coderre E, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 183.Matute C. Oligodendrocyte NMDA receptors: a novel therapeutic target. Trends Mol Med. 2006;12:289–292. doi: 10.1016/j.molmed.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 184.Bjartmar C, Yin X, Trapp BD. Axonal pathology in myelin disorders. J Neurocytol. 1999;28:383–395. doi: 10.1023/a:1007010205037. [DOI] [PubMed] [Google Scholar]
- 185.Biffger K, Bartsch S, Montag D, Aguzzi A, Schachner M, Bartsch U. Severe hypomyelination of the murine CNS in the absence of myelin-associated glycoprotein and fyn tyrosine kinase. J Neurosci. 2000;20:7430–7437. doi: 10.1523/JNEUROSCI.20-19-07430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gotow T, Leterrier JF, Ohsawa Y, et al. Abnormal expression of neurofilament proteins in dysmyelinating axons located in the central nervous system of jimpy mutant mice. Eur J Neurosci. 1999;11:3893–3803. doi: 10.1046/j.1460-9568.1999.00820.x. [DOI] [PubMed] [Google Scholar]
- 187.Lappe-Siefke C, Goebbels S, Gravel M, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 188.Rasband MN, Tayler R, Kaga Y, et al. CNP is required for maintenance of axon–glia interactions at nodes of Ranvier in the CNS. Glia. 2005;50:86–90. doi: 10.1002/glia.20165. [DOI] [PubMed] [Google Scholar]
- 189.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- 190.Roy K, Murtie JC, El-Khodor BF, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nakazawa T, Nakazawa C, Matsubara A, et al. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dai X, Lercher LD, Clinton PM, et al. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23:5846–5853. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Du YZ, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 195.Sizonenko SV, Sirimanne E, Mayall Y, Gluckman PD, Inder TE, Williams CE. Selective cortical alteration after hypoxic-ischemic injury in the very immature rat brain. Pediatr Res. 2003;54:263–269. doi: 10.1203/01.PDR.0000072517.01207.87. [DOI] [PubMed] [Google Scholar]
- 196.McCarran WJ, Goldberg MP. White matter axon vulnerability to AMPA/kainate receptor-mediated ischemic injury is developmentally regulated. J Neurosci. 2007;27:4220–4229. doi: 10.1523/JNEUROSCI.5542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tekkok SB, Goldberg MP. AMPA/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wakita H, Tomimoto H, Akiguchi I, et al. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 2002;924:63–70. doi: 10.1016/s0006-8993(01)03223-1. [DOI] [PubMed] [Google Scholar]
- 199.Romanko MJ, Rothstein RP, Levison SW. Neural stem cells in the subventricular zone are resilient to hypoxia/ischemia whereas progenitors are vulnerable. J Cereb Blood Flow Metabol. 2004;24:814–825. doi: 10.1097/01.WCB.0000123906.17746.00. [DOI] [PubMed] [Google Scholar]
- 200.Chase HP, Lindsley WF, Jr, O’Brien D. Undernutrition and cerebellar development. Nature. 1969;221:554–555. doi: 10.1038/221554a0. [DOI] [PubMed] [Google Scholar]
- 201.Howard E. Reductions in size and total DNA of cerebrum and cerebellum in adult mice after corticosterone treatment in infancy. Exp Neurol. 1968;22:191–208. doi: 10.1016/0014-4886(68)90051-4. [DOI] [PubMed] [Google Scholar]
- 202.Hutton LC, Yan E, Yawno T, Castillo-Melendez M, Hirst JJ, Walker DW. Injury of the developing cerebellum: a brief review of the effects of endotoxin and asphyxial challenges in the late gestation sheep fetus. Cerebellum. 2007 doi: 10.1007/s12311-014-0602-3. published online May 3. [DOI] [PubMed] [Google Scholar]
- 203.Joashi UC, Greenwood K, Taylor DL, et al. Poly(ADP ribose) polymerase cleavage precedes neuronal death in the hippocampus and cerebellum following injury to the developing rat forebrain. Eur J Neurosci. 1999;11:91–100. doi: 10.1046/j.1460-9568.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- 204.Inder TE, Mocatta T, Darlow B, Spencer C, Volpe JJ. Elevated free radical products in the cerebrospinal fluid of VLBW infants with cerebral white matter injury. Pediatr Res. 2002;52:213–218. doi: 10.1203/00006450-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 205.Savman K, Nilsson UA, Blennow M, Kjellmer I, Whitelaw A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilation. Pediatr Res. 2001;49:208–212. doi: 10.1203/00006450-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 206.Yakubu MA, Leffer CW. 5-hydroxtryptamine-induced vasoconstriction after cerebral hematoma in piglets. Pediatr Res. 1997;41:317–320. doi: 10.1203/00006450-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 207.Yakubu MA, Shibata M, Leffer CW. Subarachnoid hematoma attenuates vasodilation and potentiates vasoconstriction induced by vasoactive agents in newborn pigs. Pediatr Res. 1994;36:589–594. doi: 10.1203/00006450-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 208.Juliet PA, Mao X, Del Bigio MR. Proinflammatory cytokine production by cultured neonatal rat microglia after exposure to blood products. Brain Res. 2008;1210:1230–1239. doi: 10.1016/j.brainres.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 209.Murphy BP, Inder TE, Rooks V, et al. Posthemorrhagic ventricular dilatation in the premature infant—natural history and predictors of outcome. Arch Dis Child. 2002;87:F37–F41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bassan H, Feldman HA, Limperopoulos C, et al. Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol. 2006;35:85–92. doi: 10.1016/j.pediatrneurol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 211.Marin-Padilla M. Developmental neuropathology and impact of perinatal brain damage. I. Hemorrhagic lesions of neocortex. J Neuropathol Exp Neurol. 1996;55:758–773. doi: 10.1097/00005072-199607000-00002. [DOI] [PubMed] [Google Scholar]