Abstract
Clinical trials of a combination therapy of an inhaled corticosteroid, fluticasone propionate (FP), with a long-acting β2-agonist, salmeterol (Sal), have demonstrated a greater improvement in lung function and in quality of life measures after the combination compared with either component of alone. In a subanalysis of the data of the TRISTAN study, Sal/FP reduced exacerbation rates in COPD patients with a baseline FEV1<50% of predicted. A combination therapy of budesonide and formoterol improved quality of life and FEV1, and reduced exacerbations better than either component alone. In studies of FP or of Sal/FP in COPD, there was a reduction in all-cause mortality by 25% relative to placebo. Sal/FP has anti-inflammatory effects in COPD airways. FP inhibits markers of systemic inflammation, and it is not known whether Sal/FP has an advantage over FP alone. While long-acting β2-agonists such as Sal can be recommended for treatment of moderate COPD, addition of inhaled steroid therapy such as FP should be considered in more severe disease.
Keywords: fluticasone, salmeterol, COPD
Introduction
COPD is a disease of the airways and lungs characterized by a chronic inflammatory process, in which patients develop a progressive loss of lung function (eg, a fall in FEV1) and symptoms of breathlessness sometimes associated with chronic sputum production, leading to a reduction in quality of life measures. There is a wide spectrum of disease severity ranging from asymptomatic patients to those with severe capacity disease, recurrent exacerbations, and a higher risk of death. The categorization of COPD severity has been described in the Global Initiative for Chronic Obstructive Lung Disease (GOLD), based essentially on the level of airflow obstruction measured with FEV1 and on the presence of symptoms. It consists of at-risk (Stage 0, normal lung function with chronic productive cough), mild (Stage 1: FEV1/FVC<70%; FEV1>80% of predicted), moderate (Stage II: 50%<FEV1<80% of predicted with shortness of breath on exertion), severe (Stage III: 30%<FEV1<50% of predicted with increasing shortness of breath and repeated exacerbations), and very severe (Stage IV: <30% of predicted or chronic respiratory failure) (see http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=996). In most cases, cigarette smoking is directly linked to the development of COPD, although at-risk factors may also be involved such as environmental air pollution and respiratory infections. A diagnosis of COPD needs to be considered in current or ex-smokers who present with cough, sputum production, and shortness of breath, associated with spirometric evidence of irreversible airflow obstruction.
COPD is a more complex disease than just an affliction of the airways and lungs. It is a multi-system disease that includes systemic inflammation, oxidative stress, skeletal muscle dysfunction, and cardiovascular disease, among others. Other scores have been developed in an attempt to encompass the multi-system aspects of COPD. One such score is the BODE index that includes assessment of four factors in the disease: body mass index (B), airflow obstruction (O), dyspnea (D), and exercise capacity (E). This index has been particularly useful in predicting survival, and was shown to be better than spirometric measures (Celli et al 2004). Therefore, in terms of beneficial effects of anti-COPD therapies, it is not only necessary to determine their effects on lung function or respiratory symptoms but also on other components of this multisystem disorder.
Treatments for COPD
Treatments for COPD have remained purely on a symptomatic basis: relief of symptoms of breathlessness through reducing airflow obstruction, relying mostly on the use of inhaled bronchodilator therapy including β2-adrenergic agonists and anticholinergics. The recent introduction of long-acting β2-agonists (LABA) and long-acting anticholinergics (LAA) has led to an improvement in the management of patients with COPD, allowing for more sustained bronchodilation and symptom relief. The utility of inhaled corticosteroid therapy in COPD, in contrast to its well-established use in asthma, remains somewhat controversial. Large studies of inhaled corticosteroids (ICS) in COPD indicate that it has no effect in modifying the rate of decline in lung function (FEV1) in 3-year-long studies (Bourbeau et al 1998; Pauwels et al 1999; Vestbo et al 1999; Burge et al 2000; Lung Health Study Research Group 2000). There was a small improvement, however, on post-bronchodilator FEV1 in two of the studies (Pauwels et al 1999; Burge et al 2000). However, fluticasone propionate can reduce exacerbation rates (Burge et al 2000). The lack of effect of corticosteroids in COPD (on yearly decline) could reflect (a) a relative resistance to corticosteroids, as demonstrated in this disease with alveolar macrophages or (b) an inflammatory component that is unique in severe COPD that is not inhibited by corticosteroids, or inability to enhance a natural anti-inflammatory pathway or (c) the chronic bronchial and pulmonary inflammation is at a stage that is irreversible.
Combination therapy
This review will examine the effect of a salmeterol/fluticasone propionate (Sal/FP) combination therapy in COPD. To do so, it is important first to review the effects of each of these components separately on COPD, and to determine whether the combination has additive or synergistic effects. The role of the LABA, Sal, (Appleton et al 2000; Jarvis and Markham 2001) and that of the ICS, FP (Sin et al 2003; Donohue and Ohar 2004), in the treatment of COPD has already been reviewed.
Clinical trials of Sal/FP in COPD
Mahler et al (2002) reported that in 691 patients with COPD who received either the combination of FP (500 μg twice daily) and Sal (50 μg twice daily), or FP alone, or Sal alone, or placebo twice daily via the multi-dose drug powder inhaler Diskus® device (GlaxoSmithKline Inc., Research Triangle Park, NC, USA) for 24 weeks, there was a significantly greater increase in pre-dose FEV1 at the endpoint after combination (156 mL) compared with Sal (107 mL, p=0.012), with FP (109 mL; p=0.038) and placebo (−4 mL, p<0.0001). A greater increase in 2-hour post-dose FEV1 at the endpoint was observed after treatment with combination (261 mL) compared with FP (138 mL, p<0.001) and placebo (28 mL, p<0.001), but not with Sal (221 mL). There were greater improvements in the Transition Dyspnea Index (TDI), a measure of the degree of breathlessness, with combination (2.1) compared with FP (1.3, p=0.033), Sal (0.9, p<0.001), and placebo (0.4, p<0.0001). Treatment with combination resulted in a clinically important increase from baseline in overall Chronic Respiratory Disease Questionnaire (CRDQ) score that was significantly greater compared with that of placebo and FP, but to that of Sal (Mahler et al 2002)
Hanania et al (2003) reported a similarly designed 24-week study of 732 patients with nearly similar results regarding the pre- and post-dose FEV1, TDI, CRDQ, and PEFR, using a lower dose of FP of 250 μg twice daily with Sal 50 μg twice daily. They also reported that the effects on airway function were observed within 24 hours after start of therapy. In the TRISTAN study (Trial of Inhaled Steroids and Long-acting β2-Agonists), the effects of Sal and FP alone or in combination on peak expiratory flows and breathlessness were seen within the first 2 days and for FEV1 and PEF, the maximum response appeared within 2 weeks (Vestbo et al 2005). A small study suggested that Sal/FP improved FEV1 in mild-moderate more than in severe COPD patients (Terzano et al 2005).
Make et al (2005) conducted a shorter 8-week, multicentre, randomized, double-blind, double-dummy, parallel-group study of 361 subjects with moderate-to-severe COPD to compare Sal/FP 50/250 μg twice daily with the combined bronchodilator ipratropium and salbutamol 36/206 μg twice daily (Make et al 2005). They found that combination resulted in greater improvements in morning pre-dose FEV1, morning PEF, and 6-hour area-under-the-curve (AUC) FEV1 (all p<0.001), TDI score (p=0.026), overall daytime symptom score (p=0.024), percentage of symptom-free nights (p=0.010), night time awakenings due to respiratory symptoms (p=0.002), sleep symptom score (p=0.003), and percentage of days and nights without rescue salbutamol use compared with the bronchodilator combination (p=0.021 and p<0.001, respectively). There was greater bronchodilatation measured at the 8th week of treatment. Thus, compared with day 1, the FEV1 AUC at week 8 increased by 0.38 litre-hour with combination therapy and decreased by 0.18 litre-hour with bronchodilator combination (p<0.001 between groups) (Make et al 2005) Short-term treatment with inhaled Sal/FP resulted in greater control of lung function and symptoms than combined ipratropium bromide/salbutamol bronchodilator therapy, in patients with COPD (Donohue et al 2004).
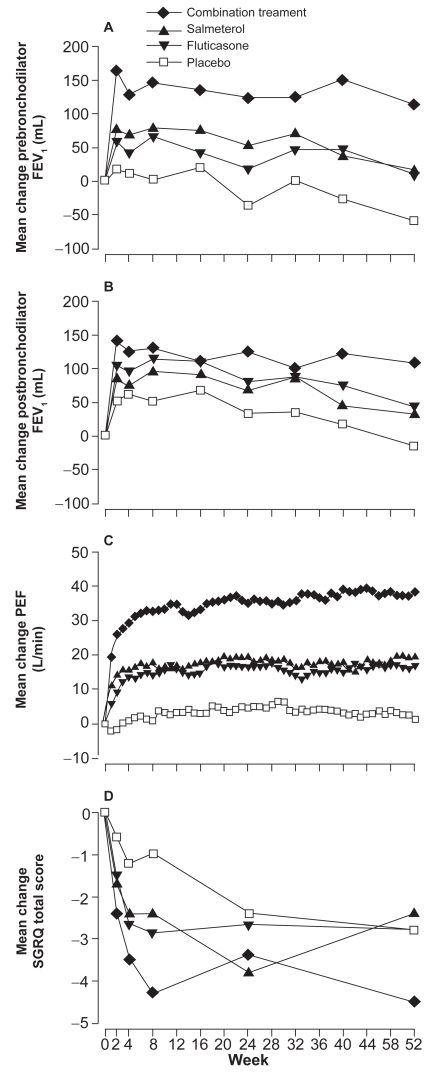
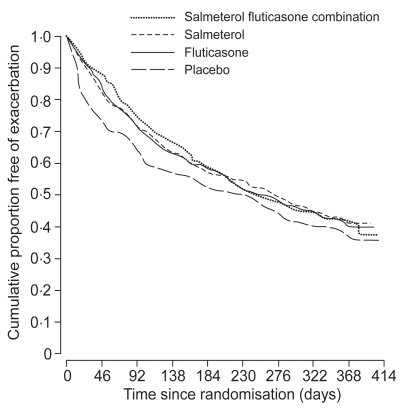
The TRISTAN study was a double-blind, parallel-group, placebo-controlled study involving 1465 patients with COPD comparing the effects of either 50 μg Sal twice daily, 500 μg FP twice daily, 50 μg Sal and 500 μg FP twice daily, or placebo for 12 months, all administered via the Diskus inhaler (Calverley et al 2003a). All active treatments improved lung function, symptoms, and health status and reduced use of rescue medication and frequency of exacerbations. Combination therapy was better than FP alone or Sal alone in terms of pre-treatment and post-bronchodilator FEV1 (the primary outcome measurement) and PEF but not in terms of exacerbation rates (Figure 1). Compared with placebo, all active treatments significantly reduced the number of exacerbations per patient per year and the number of exacerbations that needed treatment with oral corticosteroids. The rate of exacerbations fell by 25% in the combination group, by 20% in the Sal group, and by 19% in the FP group, compared with placebo (Figure 2). On subanalysis of the data, the treatment effect was more pronounced in patients with a baseline FEV1 <50% of predicted. The reduction in acute episodes of exacerbations requiring oral corticosteroids was reduced by 39% in the combination group, 29% in the Sal group, and 34% in FP group compared with placebo, with no statistical differences between the treatments.
Figure 1.
Mean changes from baseline of prebronchodilator FEV1 (A), postbronchodilator FEV1 (B), daily peak expiratoiry flows (C), and St Georges’ health status scores (D) in the TRISTAN study. Reprinted from Calverley P, Pauwels R, Vestbo J, et al. 2003a. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet, 361:449–56. Coypright © 2003 with permission from Elsevier.
Figure 2.
Cumulative risk of acute exacerbations in the TRISTAN study. There were no differences between the three active treatment groups. Reprinted from Calverley P, Pauwels R, Vestbo J, et al. 2003a. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet, 361:449–56. Coypright © 2003 with permission from Elsevier.
In a study of only 18 patients with COPD observed over 52 weeks, a marked decrease in exacerbations (from 3.5±0.8 to 1.2±0.8 exacerbations per year) was reported when patients previously treated with theophylline were switched to a Sal/FP inhaler, but not when switched to Sal or placebo. This was associated with an improvement of FEV1 of 7.3% (Dal Negro et al 2003). Which of the two components of combination therapy is important has been studied in studies in which either component has been withdrawn. Withdrawing the FP component of Sal/FP after a 3-month period of treatment led to an acute and persistent reduction in lung function and dyspnea, and an increase in mild exacerbations and % of disturbed nights (Wouters et al 2005). These results are somewhat similar to those of the COPE study in which patients were using only FP: withdrawal of the FP led to worsening of symptoms (van der Valk et al 2002). Re-analysis of the TRISTAN data indicated that gender did not influence the response to Sal/FP in COPD (Vestbo et al 2004).
Combination of budesonide and formoterol in COPD
There are two published studies of the combination of budesonide and formoterol inhaler delivered from the multidose dry powder inhaler device (Turbohaler®) in COPD. Szafranski et al (2003) found a small nonstatistical improvement in FEV1 with budesonide/formoterol (160/4.5 μg delivered dose; two inhlations twice daily) over formoterol alone, while significantly better than budesonide alone (p<0.001); however, the combination therapy was better than either one alone for morning or evening PEFR. The combination decreased all symptoms scores and use of reliever β2-agonist significantly vs placebo and budesonide, but not vs formoterol alone. The combination reduced the mean number of severe exacerbations per patient per year by 24% vs placebo, and 23% vs formoterol, with a significant difference vs budesonide.
In a second 12-month study of the combination of budesonide and formoterol, in which the patients were first treated with prednisolone 30 mg/day and formoterol for 2 weeks prior to randomization, Calverley et al (2003b), using a similar dose of budesonide/formoterol as Szafranski et al (2003), report that the combination prolonged the time to first exacerbation and maintained higher FEV1 better than budesonide or formoterol alone. The combination reduced the risk of having an exacerbation by 22.7, 29.5, and 28.5% vs budesonide, formoterol, and placebo respectively. Budesonide and formoterol alone did not affect exacerbation rate vs placebo. The combination also improved quality of life scores measured on the St Georges Respiratory Questionnaire (SGRQ) better than either alone, with an improvement of greater than 5.5 units compared to placebo.
There is only one small study of 16 patients with COPD comparing the combination of Sal/FP with budesonide/formoterol using a crossover design (Cazzola et al 2003). No difference in FEV1 response was observed, and efficacy and safety were deemed to be equivalent, but there has been no study on exacerbation rates. Such a study is too small to detect any small differences.
Systematic meta-analysis reviews
Systematic review of combination therapy with Sal/FP and budesonide/formoterol has been reported by Sin et al (2003) (3 studies, 2951 patients) and by Nannini et al (2004) (6 studies, 4118 patients). Nannini et al (2004) concluded that combination therapy was better than placebo, with clinically important improvement in lung function, mean exacerbation rate, and quality of life scores. For lung function, there were significant differences of combination therapy vs ICS alone, with Sal/FP better than Sal alone, but with budesonide/formoterol equal to formoterol alone. Analysis of all studies showed that there were less frequent exacerbations with combination therapy than with placebo or Sal, but not when compared with the FP alone. Nannini et al found that in comparison with long-acting β2-agonists, there was no significant difference between budesonide and formoterol, and FP and Sal. However, compared with their constituent long-acting β2-agonist, there was a significant reduction in exacerbation rate for the budesonide/formoterol combination but not for the Sal/FP combination. The rate-ratio of exacerbation rates for Sal/FP compared with Sal was 0.93 (0.81–1.08, 95% confidence interval), and for the combination of budesonide and formoterol compared with formoterol alone 0.76 (0.64–0.90). In COPD patients with a history of one or more exacerbations in the previous year, the difference of 24% could translate to one exacerbation less every 4 years.
A recent report calculated the number of patients needed to treat (NNT) to avoid one exacerbation requiring medical intervention in one year with budesonide and formoterol vs formoterol alone to be 2.38 in the Szafranski study (Szafranski et al 2003) and 2.13 in the Calverley study (Calverley et al 2003b), with corresponding values vs placebo being 2.22 and 2.38 respectively (Halpin 2005). In the same paper, an NNT value of 3.03 was obtained for the TRISTAN study for the prevention of exacerbations by the Sal/FP combination vs placebo.
Effect on survival
Although inhaled corticosteroids do not have an effect on the rate of decline of FEV1, observational studies of COPD databases have suggested that ICS either alone or in combination with long-acting β2-agonists may reduce mortality rates in COPD (Sin and Tu 2001; Soriano et al 2002, 2003). Another study did not show this advantage when data are analyzed according to an according-to-treat approach and the survival benefit of the previous studies was attributed to bias from unaccounted immortal time in the cohort design and analysis (Suissa 2004). The effects of Sal/FP or of budesonide/formoterol on mortality are unclear, but in an earlier meta-analysis of placebo-controlled studies, the relative risk of combination therapy vs placebo was found to be 0.52 (0.20–1.34) (Alsaeedi et al 2002). In a more recent meta-analysis from pooled data from 7 randomized trials involving 5058 patients taking ICS with or without LABA, active treatment reduced all-cause mortality by 25% relative to placebo during a mean follow-up of 26 months (Sin et al 2005). The effect of combination therapy and of its components alone on survival rate is being investigated in the TORCH trial (Towards a Revolution in COPD Health) (Vestbo 2004).
Safety and tolerability of Sal/FP
In all the studies with Sal/FP lasting for 8–48 weeks of therapy, the combination therapy was well tolerated. There were no differences between groups in the adverse event reports. In all the studies, there was greater incidence of candidiasis in the FP only or the combined Sal/FP treated groups compared with placebo and Sal groups. In the one year TRISTAN study, the frequency of candidiasis in the FP group was 7%, and in the combination group 8% compared with 2% in both placebo and Sal groups (Donohue and Ohar 2004). Morning cortisols decreased during FP and during combination therapy, while they increased in the placebo and Sal groups, after 52 weeks of treatment, but these changes were small and levels remained within the normal reference values. There was no difference in skin bruising between the groups.
The long-term side-effects of inhaled corticosteroids in COPD are unclear and further work is needed, since the population of COPD is generally an elderly population in which risk of side-effects may be greater than in a younger population exposed to similar doses of inhaled corticosteroids. In addition, these patients who are at the severe end of the disease may also have a greater risk of steroid side-effects, as they are more likely to be exposed to courses of oral corticosteroid therapy for treatment of exacerbations.
The dose-response of FP in the Sal/FP combination in COPD is unclear. From the studies of Make et al (2005), Hanania et al (2003), and Mahler et al (2002), it appears that there is little difference in effect on symptom response between 500 μg and 1000 μg per day dosage, but there is only one study on the effect of Sal/FP on exacerbations using 1000 μg daily dose (Calverley et al 2003a). The dose-response effect on reduction of exacerbations needs to be clarified.
Effect of Sal/FP on pulmonary and systemic inflammation
The effect of Sal/FP on airway inflammation measured in bronchial biopsies has been reported recently (Barnes et al 2006). Compared with placebo treatment, combination therapy reduced CD8+T cells by a median treatment difference of 118 cells/mm2 (36% reduction; p=0.02) from baseline, and CD4+ T cells by 45 cells/mm2 (40% reduction, p=0.017), but the ratio of CD8/CD4 was unchanged. There was a small decrease in neutrophil counts in the Sal/FP-treated group. Prebronchodilator FEV1 improved by 173 mL after Sal/FP (p<0.001), and exacerbation rate was reduced by 33% compared with a 16% reduction after placebo (p=0.025). In addition, there was a significant reduction in the percentage of (but not total) neutrophils in induced sputum of −53% in the Sal/FP therapy group compared with placebo. These results indicate that Sal/FP may possess anti-inflammatory effects in COPD, although the contribution of FP alone to these effects is unknown. In addition, because there was no comparative group treated with either FP or Sal alone, it is not possible to answer the question as to whether there was an additive effect of these two classes of drugs when administered together on inflammation. Were the clinical benefits of Sal/FP related to an anti-inflammatory effect rather than a “bronchodilator” effect? Post hoc evaluation of correlations between lung function (change in FEV1 as percentage of predicted normal and change in FVC) and primary and secondary biopsy parameters showed no convincing or significant correlations in the Barnes et al (2006) study. While these data on inflammatory cells in bronchial biopsies from the proximal airways are important, it is more pertinent to obtain information on the pathophysiological changes related to the small airways, where the airflow obstruction is the most prominent.
In a previous biopsy study, inhaled FP was shown to reduce the ratio of CD4 to CD8 T cells in the epithelium of patients with COPD, with a reduction in epithelial mast cells (Hattotuwa et al 2002); in another paper, these investigators also described a reduction in mucosal mast cell numbers (Gizycki et al 2002). In a study of induced sputum, the number of neutrophils was significantly lowered after FP (Confalonieri et al 1998), although another study did not report any significant changes (Culpitt et al 1999).
Further support for an effect of ICS in inhibiting inflammation of COPD comes from a study of serum CRP levels which are elevated in COPD. Withdrawal of ICS from patients with COPD established on ICS led to an increase in serum CRP levels, while 2 weeks of inhaled FP decreased serum CRP and IL-6 levels by 50% and 26% respectively (Sin et al 2004). A large study, the Advair Biomarkers in COPD (ABC study), will address the question of whether the effects of FP alone or in combination with Sal reduce systemic inflammation and improve health status in patients with COPD (Sin et al 2006). This study may provide direct evidence for the importance of systemic inflammation in determining the severity of symptoms and deterioration in health status in COPD. Whether the effects of Sal/FP on systemic inflammation are secondary to a systemic effect of inhaled FP or whether the control of systemic inflammation is the result of an effect on local lung inflammation may be important to determine.
Molecular basis for the actions of Sal/FP
The molecular basis for the superiority of combined LABA and ICS on the control of symptoms and lung function improvement compared with the effect of either alone in COPD has been reviewed recently (Johnson 2005). The major effects could result from interactions between ICS and LABA such as (i) the increase in β2-receptor synthesis induced by corticosteroids, (ii) the priming of glucocorticoid receptor for steroid-dependent activation by LABA (Eickelberg et al 1999), or (iii) the enhancement of glucocorticoid receptor nuclear translocation via the activation of the transcription factor CEBP-α (Roth et al 2002). However, more work is needed to establish whether these mechanisms actually occur in patients but there has been clear advances in our understanding of corticosteroid insensitivity over the recent years (Ito et al 2006). It is also possible that β2-agonists may restore to some extent the glucocorticoid insensitivity found in COPD resulting from excessive oxidative stress (Adcock and Chung 2002).
Clinical recommendation and conclusion
Bronchodilators remain central to the symptomatic treatment of COPD as they increase exercise capacity with or without significant changes in FEV1, and improve health status. Patients with mild to moderate COPD may progress from short-acting bronchodilators (β2-agonist, or anticholinergics, or a combination of the two) to long-acting bronchodilators (LABA or LAA) if they do not respond to the former. ICS may be added to bronchodilator therapy for the more severe COPD patient particularly for those with an FEV1 of <50% predicted at stage III and stage IV, and those with frequent exacerbations. ICS with long-acting β-agonists such as Sal/FP is more effective particularly for control of symptoms and improving health status, and should be reserved for the severe COPD patient, and for patients at stage III and stage IV, when bronchodilators alone have not been successful (Table 1).
Table 1.
Therapy at each stage of COPD
| Table 8 - Therapy at Each Stage of COPD | |||||
|---|---|---|---|---|---|
| Old | 0: At Risk | I: Mild | II: Moderate | III: Severe | |
| IIA | IIB | ||||
| New | 0: At Risk | I: Mild | II: Moderate | III: Severe | IV: Very Severe |
| Characteristics |
|
|
|
|
|
| Avoidance of risk factor(s); influenza vaccination | |||||
| Add short-acting bronchodilator when needed | |||||
| Add regular treatment with one or more long-acting bronchodilators Add rehabilitation | |||||
| Add inhaled glucocorticosteroids if repeated exacerbations | |||||
| Add long-term oxygen if chronic respiratory failure Consider surgical treatments | |||||
From: Global strategy for diagnosis, management, and prevention of COPD [online]. URL: http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=996.
Further work is needed in order to place more precisely the role of combination therapy such as Sal/FP in the management of COPD. The issue of the dose–response relationship, particularly in relation to the dose of FP, is important to resolve because the population of COPD patients may be more prone to the potential systemic side-effects of FP, particularly as the high daily dose of 1000 μg of FP has been most frequently studied. It is also possible that the symptoms or quality of life measures may respond at a different dose schedule than the rate of exacerbations. While the current recommendations have been based mostly on the results of clinical trials, the potential beneficial effects of combination therapy on all-cause mortality may lead to changes in these recommendations. Similarly, confirmation of the potential effects of combination therapy on lung and systemic inflammation may also lead to modifications to these recommendations. More importantly, understanding of the mechanisms by which FP interacts with Sal may provide ideas as to the development of improved combination therapies for greater efficacy. Once-daily combination therapy in COPD using longer-acting corticosteroids and β2-adrenergic agonists may be the next step in this direction.
Footnotes
Disclosures
KFC has received honoraria for participating in Advisory Board meetings for GSK, Astra-Zeneca, Novartis, Altana, and Celgene, and research grants from GSK, Novartis, and Scios.
References
- Adcock IM, Chung KF. Why are corticosteroids ineffective in COPD? Curr Opin Investig Drugs. 2002;3:58–60. [PubMed] [Google Scholar]
- Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med. 2002;113:59–65. doi: 10.1016/s0002-9343(02)01143-9. [DOI] [PubMed] [Google Scholar]
- Appleton S, Smith B, Veale A, et al. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2000;2:CD001104. doi: 10.1002/14651858.CD001104. [DOI] [PubMed] [Google Scholar]
- Barnes NC, Qiu Y-S, Pavord I, et al. Anti-inflammatory effects of sameterol/fluticasone propionate in chronic obstructive lung disease. Amer J Respir Crit Care Med 2006. 2006;173:736–743. doi: 10.1164/rccm.200508-1321OC. [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53:477–82. doi: 10.1136/thx.53.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. Br Med J. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003a;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003b;22:912–19. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Santus P, Di Marco F, et al. Bronchodilator effect of an inhaled combination therapy with salmeterol + fluticasone and formoterol + budesonide in patients with COPD. Respir Med. 2003;97:453–7. doi: 10.1053/rmed.2002.1455. [DOI] [PubMed] [Google Scholar]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- Confalonieri M, Mainardi E, Della PR, et al. Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary disease [see comments] Thorax. 1998;53:583–5. doi: 10.1136/thx.53.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt SV, Maziak W, Loukidis S, et al. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1635–9. doi: 10.1164/ajrccm.160.5.9811058. [DOI] [PubMed] [Google Scholar]
- Dal Negro RW, Pomari C, Tognella S, et al. Salmeterol and fluticasone 50 microg/250 microg bid in combination provides a better long-term control than salmeterol 50 microg bid alone and placebo in COPD patients already treated with theophylline. Pulm Pharmacol Ther. 2003;16:241–6. doi: 10.1016/s1094-5539(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Kalberg C, Emmett A, et al. A short-term comparison of fluticasone propionate/salmeterol with ipratropium bromide/albuterol for the treatment of COPD. Treat Respir Med. 2004;3:173–81. doi: 10.2165/00151829-200403030-00005. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Ohar JA. Effects of corticosteroids on lung function in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:152–60. doi: 10.1513/pats.200402-003MS. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Roth M, Lorx R, et al. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999;274:1005–10. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- Gizycki MJ, Hattotuwa KL, Barnes N, et al. Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissue. Thorax. 2002;57:799–803. doi: 10.1136/thorax.57.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin DM. Evaluating the effectiveness of combination therapy to prevent COPD exacerbations: the value of NNT analysis. Int J Clin Pract. 2005;59:1187–94. doi: 10.1111/j.1368-5031.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Darken P, Horstman D, et al. The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest. 2003;124:834–43. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- Hattotuwa KL, Gizycki MJ, Ansari TW, et al. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med. 2002;165:1592–6. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- Ito K, Chung KF, Adcock IM. Update on glucocorticoid actions and resistance. J Allergy Clin Immunol. 2006;117:522–543. doi: 10.1016/j.jaci.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Jarvis B, Markham A. Inhaled salmeterol: a review of its efficacy in chronic obstructive pulmonary disease. Drugs Aging. 2001;18:441–72. doi: 10.2165/00002512-200118060-00006. [DOI] [PubMed] [Google Scholar]
- Johnson M. Corticosteroids: potential beta2-agonist and anticholinergic interactions in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:320–5. doi: 10.1513/pats.200504-040SR. [DOI] [PubMed] [Google Scholar]
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Wire P, Horstman D, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1084–91. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- Make B, Hanania NA, ZuWallack R, et al. The efficacy and safety of inhaled fluticasone propionate/salmeterol and ipratropium/albuterol for the treatment of chronic obstructive pulmonary disease: an eight-week, multicenter, randomized, double-blind, double-dummy, parallel-group study. Clin Ther. 2005;27:531–42. doi: 10.1016/j.clinthera.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Nannini L, Cates CJ, Lasserson TJ, et al. Combined corticosteroid and long acting beta-agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;3:CD003794. doi: 10.1002/14651858.CD003794.pub2. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Lofdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease [see comments] N Engl J Med. 1999;340:1948–53. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- Roth M, Johnson PR, Rudiger JJ, et al. Interaction between glucocorticoids and beta2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360:1293–9. doi: 10.1016/S0140-6736(02)11319-5. [DOI] [PubMed] [Google Scholar]
- Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:760–5. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- Sin DD, Man P, Marciniuk DD, et al. Can Inhaled fluticasone alone or in combination with salmeterol reduce systemic inflammation in chronic obstructive pulmonary disease? Study protocol for a randomized controlled trial [clinicaltrials.gov NCT00120978] BMC Pulm Med. 2006;6:3. doi: 10.1186/1471-2466-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin DD, McAlister FA, Man SF, et al. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA. 2003;290:2301–12. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:580–4. doi: 10.1164/ajrccm.164.4.2009033. [DOI] [PubMed] [Google Scholar]
- Sin DD, Wu L, Anderson JA, et al. Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax. 2005;60:992–7. doi: 10.1136/thx.2005.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano JB, Kiri VA, Pride NB, et al. Inhaled corticosteroids with/without long-acting beta-agonists reduce the risk of rehospitalization and death in COPD patients. Am J Respir Med. 2003;2:67–74. doi: 10.1007/BF03256640. [DOI] [PubMed] [Google Scholar]
- Soriano JB, Vestbo J, Pride NB, et al. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J. 2002;20:819–25. doi: 10.1183/09031936.02.00301302. [DOI] [PubMed] [Google Scholar]
- Suissa S. Inhaled steroids and mortality in COPD: bias from unaccounted immortal time. Eur Respir J. 2004;23:391–5. doi: 10.1183/09031936.04.00062504. [DOI] [PubMed] [Google Scholar]
- Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- Terzano C, Petroianni A, Ricci A, et al. Combination therapy in COPD: different response of COPD stages and predictivity of functional parameters. Eur Rev Med Pharmacol Sci. 2005;9:209–15. [PubMed] [Google Scholar]
- van der Valk P, Monninkhof E, van der Palen J, et al. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med. 2002;166:1358–63. doi: 10.1164/rccm.200206-512OC. [DOI] [PubMed] [Google Scholar]
- Vestbo J. The TORCH (towards a revolution in COPD health) survival study protocol. Eur Respir J. 2004;24:206–10. doi: 10.1183/09031936.04.00120603. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Pauwels R, Anderson JA, et al. Early onset of effect of salmeterol and fluticasone propionate in chronic obstructive pulmonary disease. Thorax. 2005;60:301–4. doi: 10.1136/thx.2004.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestbo J, Sorensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353:1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Soriano JB, Anderson JA, et al. Gender does not influence the response to the combination of salmeterol and fluticasone propionate in COPD. Respir Med. 2004;98:1045–50. doi: 10.1016/j.rmed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Wouters EF, Postma DS, Fokkens B, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005;60:480–7. doi: 10.1136/thx.2004.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]