Abstract
Events of the past decade have stimulated development of new drug formulations and delivery devices that have improved the efficiency, ease of use, and environmental impact of inhaled drug therapy. Respimat® Soft Mist™ Inhaler is a novel, multidose, propellant-free, hand-held, liquid inhaler that represents a new category of inhaler devices. The aerosol cloud generated by Respimat contains a higher fraction of fine particles than most pressurized metered dose inhalers (pMDIs) and dry powder inhalers (DPIs), and the aerosol spray exits the inhaler more slowly and for a longer duration than with pMDIs. This translates into higher lung drug deposition and lower oropharyngeal deposition, making it possible to give lower nominal doses of delivered drugs without lowering efficacy. In clinical trials in patients with COPD, bronchodilator drugs delivered from Respimat were equally effective at half of the dose delivered from a pMDI. In one study of inhaler preference, Respimat was preferred over the pMDI by patients with COPD and other obstructive lung diseases. Respimat is a valuable addition to the range of inhaler devices available to the patient with COPD.
Keywords: Respimat, COPD, inhaler, bronchodilator
Introduction
For the past 50 years, the pressurized metered-dose inhaler (pMDI) and the nebulizer have been the primary means of delivering inhaled drugs to patients with asthma and COPD. These devices can be used effectively, but are inefficient and may be difficult or cumbersome to use. Some of the limitations of these standard inhaler devices are accentuated in patients with COPD, especially if they are elderly or have severe disease. Standard pMDIs at best deposit 10%–15% of the delivered dose in the lungs and most of the inhaled dose is deposited in the oropharynx. To perform optimally, pMDIs require coordination of actuation with inhalation as well as a slow inspiratory flow rate and breath-holding (Wolff and Niven 1994). Poor coordination of pMDI actuation with inhalation has been shown to result in lower lung deposition of drug and reduced lung function response (Newman et al 2001). The pMDI breathing maneuver requires education and repeated instruction, and can be problematic for older patients with COPD. Addition of a spacer or holding chamber can decrease oropharyngeal drug deposition and improve lung delivery, but makes the system less portable. Handling of a pMDI can also be more difficult if the patient has arthritis of the hands. While jet nebulizers may be popular with some patients with COPD, they are time-consuming, very inefficient, and require cleaning. For routine use of treatment for ambulatory patients, standard jet nebulizers are not portable and have not been proven to offer additional efficacy in asthma or COPD patients (Dolovich et al 2005). Other issues that may be prominent in an elderly COPD population are the confusion that arises with prescription of multiple inhalers with different instructions for use and also the expense of newer inhaled drug–device combinations.
Events of the past decade have stimulated development of novel drug formulations and delivery devices that have improved the efficiency, ease of use, and environmental impact of inhaled drug therapy. Because of deleterious effects of chlorofluorocarbon (CFC) propellants on the ozone layer, the Montreal Protocol was developed by the United Nations in 1987, banning substances that deplete the ozone layer (Leach 1995). This ban phased out the use of CFCs by 1996, although pharmaceutical companies had exemptions. Germany is the first country to complete the phasing out of CFCs. Further developed countries will follow in the next few years. While the contribution of CFC inhaler propellants has a minute environmental impact, this ban has had a large effect on subsequent development of inhaler technology. Development of novel inhaler devices has focused on three areas: pMDIs with hydrofluoroalkane (HFA) propellants, dry powder inhalers (DPIs), and liquid multidose spray devices that do not require propellants. There are advantages and drawbacks to all three types of drug–device systems. HFA-pMDIs generate aerosols that exit at lower velocity and may contain smaller particles. This may improve lung deposition, but these devices still require good coordination and slow inhalation. DPIs require high inspiratory flow rates and may not work effectively in a patient with severe COPD. DPIs also are associated with high oropharyngeal deposition (Fink 2000) and the drug powder can be sensitive to moisture. Respimat® Soft Mist™ Inhaler (Boehringer Ingelheim, Ingelheim, Germany) is a novel, multidose, propellant-free, hand-held liquid inhaler that represents a new category of inhaler devices. “Soft Mist” is used to describe the mechanism of aerosol generation and the qualities of the aerosol cloud. The aerosol plume generated by Respimat travels much slower and lasts much longer than aerosol clouds from other devices, properties that improve delivery and make this device a valuable addition to inhalers available for inhalation therapy to COPD patients.
Description and design of device
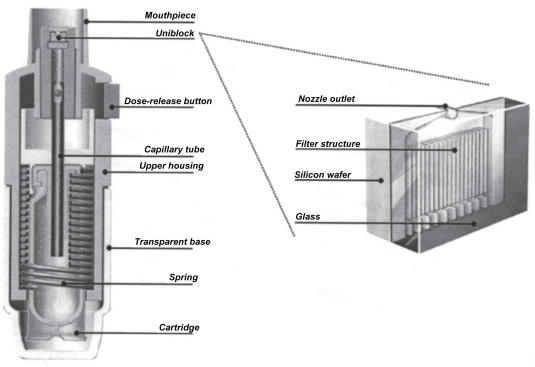
Respimat is a hand-held inhaler with a hinged cap that is similar in size to pMDIs and some DPIs (Figure 1). Figure 2 shows a schematic of the device (Dalby et al 2004). Medication is stored as a solution in the drug cartridge, an aluminum cylinder containing a double-walled, plastic, collapsible bag that contracts as the solution is used. The solution may be formulated with either ethanol or water, with benzalkonium chloride and ethylene diamine tetra-acetic acid (EDTA) added as preservatives. The amount of preservatives in each puff is extremely low: approximately 0.44 μg for benzalkonium chloride and 2.2 μg for EDTA. Tests on used cartridges have shown that patient use does not cause bacterial contamination of the solution in the cartridge. The first marketed product delivers 120 actuations and has a dose indicator, which is a color-coded gauge marked in increments of 30 doses. This indicator gives an estimate of doses remaining but does not count individual doses. After 120 actuations have been delivered, a locking mechanism prevents further use by preventing twisting of the base so that no further doses can be actuated. Respimat does not require a spacer, a battery, or outside power source. Twisting the base of the device 180 degrees compresses a spring and provides mechanical power to aerosolize the dose of drug and also transfers a metered dose of drug (usually 10–15 μL) from the drug cartridge through a capillary tube to the pump cylinder. When the dose-release button is pushed, the energy from the compressed spring forces the drug through a nozzle system called the “uniblock” (Figure 2). The uniblock consists of a silicone wafer bonded to a glass plate and measures approximately 2 x 2.5 mm. Channels are etched into the silicon wafer using a technique derived from microchip technology and these channels feed into the nozzle outlet.
Figure 1.
Respimat® Soft Mist Inhaler™. Reprinted from Dalby R, Spallek M, Voshaar T. 2004. A review of the development of Respimat® Soft Mist Inhaler™. Int J Pharm, 283:1–9. Copyright © 2004, with permission from Elsevier.
Figure 2.
Schematic of Respimat® Soft Mist Inhaler™ showing the details of the uniblock. Reprinted from Dalby R, Spallek M, Voshaar T. 2004. A review of the development of Respimat® Soft Mist Inhaler™. Int J Pharm, 283:1–9. Copyright © 2004, with permission from Elsevier.
Production of inhalation mist
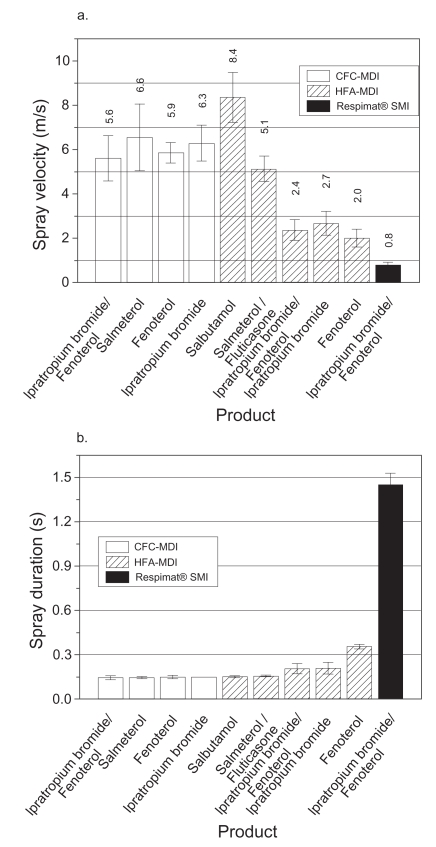
Actuation of the Respimat dose-release button utilizes the mechanical energy from the spring to force the metered drug solution through the channels in the uniblock, producing two fine jets of liquid at the outlet that converge at a predetermined angle to form the aerosol cloud (Spallek et al 2002). This cloud contains an aerosol with a fine-particle fraction (particles smaller than 5.8 μm) at least twice as high as most pMDIs and DPIs, which would allow a higher proportion of the emitted dose to be delivered to the lungs and less to the oropharynx. The fine particle fraction is higher for ethanolic formulations than for aqueous formulations, with a mass median aerodynamic diameter of 2.0±0.4 μm for aqueous solutions and 1.0±0.3 μm for ethanolic solutions (Zierenberg 1999). The fine-particle fraction is approximately 66% for an aqueous drug solution and 81% for an ethanolic solution. The “soft mist” moves more slowly and has a more prolonged duration than the aerosol cloud from a pMDI. Hochrainer et al (2005) have compared Respimat aerosol velocity and spray duration with that of CFC-pMDIs and HFA-pMDIs via video-recording (Figure 3). The velocity of the aerosol from Respimat was between one-sixth and one-tenth of that from the CFC-pMDIs and two of the HFA-pMDIs and approximately one-third of the velocity of the “slower” HFA-pMDIs. The mean velocity of the aerosol cloud measured at a 10 cm distance from the nozzle was 0.8 m/s for Respimat and 2.0–8.4 m/s for pMDIs, while the mean duration was 1.5 s and 0.15–0.36 s, respectively. The combination of smaller particle size, lower velocity, and longer duration of the aerosol cloud implies that there would be improved coordination of inhalation with actuation, higher lung deposition, and lower oropharyngeal deposition compared with pMDIs.
Figure 3.
(a) Mean aerosol spray velocities at 10 cm from nozzle for Respimat® and various CFC- and HFA-pMDIs. (b) Mean spray duration for the devices. Reprinted from Hochrainer D, Hölz H, Kreher C, et al. 2005. Comparison of the aerosol velocity and spray duration of Respimat® Soft Mist Inhaler™ and pressurized metered dose inhalers. J Aerosol Med, 18:273–82. Copyright © 2005, with permission from Mary Ann Liebert, Inc.
Abbreviations: CFC, chlorofluorocarbon; HFA, hydrofluoroalkane; pMDI, pressurized metered dose inhaler; SMI, Soft Mist™ inhaler.
After receiving a new Respimat inhaler, a patient must insert the cartridge by removing the transparent base and pushing the cartridge into the inhaler until it clicks into place. The dose is loaded by holding the inhaler in an upright position and turning the base 180 degrees until it clicks. The inhaler requires priming prior to first use by actuating until an aerosol cloud is visible and then completing three more actuations. Spray content uniformity measurements show priming actuations necessary to achieve 100% of target volume and also show spray uniformity over 120 doses without “tail-off” effect (Spalleck 2002). Priming with actuation of one dose is recommended after a week of no use, and full priming is recommended after 21 days of no use. Instructions for inhalation direct the patient to breathe out slowly and deeply and close lips around the end of the mouthpiece while holding the inhaler in a horizontal position. The dose-release button should be pushed while the patient takes a slow, deep breath in through their mouth and a 10-second breath-hold is recommended. The dose can be administered to the patient with the inhaler held in any position.
Mist deposition in lungs
Improved lung deposition of drug aerosols from the Respimat has been demonstrated by several studies using radiolabeled drug particles and gamma scintigraphy (Newman et al 1996, 1998; Steed et al 1997). This group of studies in healthy nonsmoking volunteers used either fenoterol, which was formulated in an aqueous medium, or flunisolide, formulated in 96% ethanol. In one study with flunisolide, the mean whole-lung deposition from Respimat (40% of the metered dose) was significantly higher than from the pMDI (15%) or pMDI plus spacer (28%) (Newman et al 1996). A follow-up study using flunisolide and a final prototype Respimat showed significantly more lung deposition with Respimat compared with pMDI plus spacer (45 vs 26%) (Newman et al 1998). In this same study, mean whole-lung deposition of fenoterol was significantly greater when delivered by Respimat (39%) than via pMDI (11%) or pMDI plus spacer (10%). In both of these studies in normal subjects, the oropharyngeal deposition was significantly lower with Respimat than with pMDI (approximately 40% vs 70%), but not as low as with pMDI plus spacer.
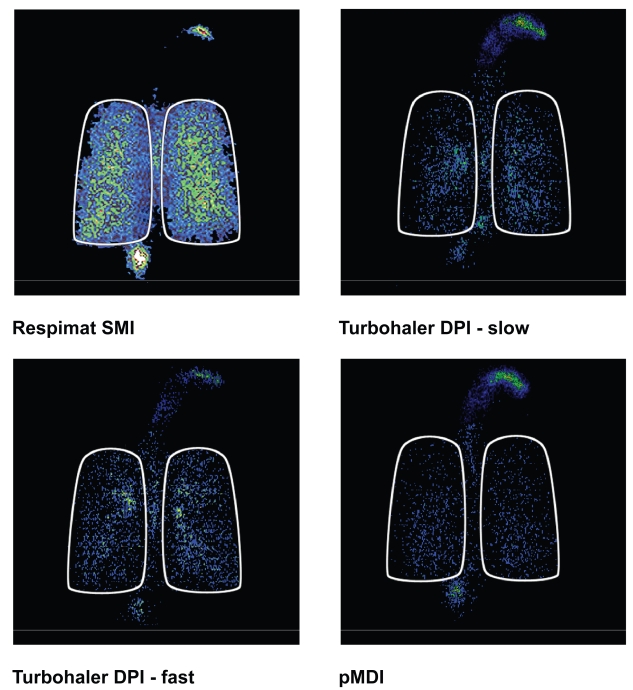
A more recent investigation has compared lung deposition of inhaled steroids in mild to moderate asthmatic subjects using Respimat, Turbuhaler, and a pMDI (Pitcairn et al 2005). Respimat contained budesonide solution, the Turbuhaler contained budesonide dry powder, and the pMDI contained beclomethasone dipropionate; the Turbuhaler inhalations were performed at either 60 or 30L/m. Results for deposition amounts are shown in Table 1 and also Figure 4. Mean whole-lung deposition of drug from Respimat was significantly greater than that from Turbuhaler DPI at fast or slow inhaled flow rates, or from the pMDI. The deposition pattern within the lungs was more peripheral for the Respimat than for the Turbuhaler. These data in normal and asthmatic subjects would predict that lower doses of drugs via the Respimat would provide equal efficacy to higher doses delivered via the pMDI or Turbuhaler.
Table 1.
Mean percentage dose of steroid deposited (and range) for different devices in asthmatic subjects (modified from Pitcairn et al 2005)
| Respimat® | Turbuhaler® (fast flow) | Turbuhaler® (slow flow) | pMDI | |
|---|---|---|---|---|
| Lungs (%) | 52 (46–57) | 29 (24–33) | 18 (14–22) | 9 (6–12) |
| Oropharynx (%) | 19 (15–24) | 49 (44–55) | 41 (35–46) | 82 (78–86) |
| Device (%) | 18 (14–22) | 21 (17–26) | 40 (35–46) | 9 (6–12) |
Figure 4.
Typical scintigraphic images for Respimat®, Turbuhaler® DPI at slow and fast inhaled flow rates, and CFC-pMDI. Reprinted from Pitcairn G, Reader S, Pavia D, et al. 2005. Deposition of corticosteroid aerosol in the human lung by Respimat® Soft Mist™ Inhaler compared with deposition by metered dose inhaler or by Turbuhaler® dry powder inhaler. J Aerosol Med, 18:264–72. Copyright © 2005, with permission from Mary Ann Liebert, Inc.
Abbreviations: CFC, chlorofluorocarbon; DPI, dry powder inhaler; pMDI, pressurized metered dose inhaler; SMI, Soft Mist™ inhaler.
Effectiveness and safety of device
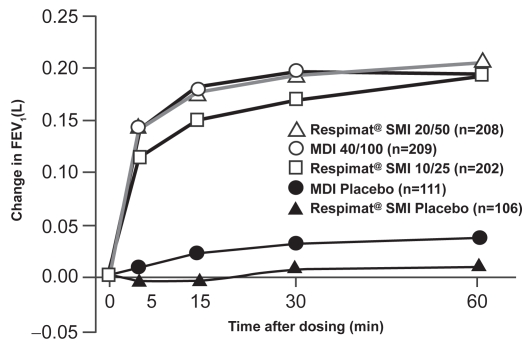
Currently, the Respimat is marketed with a combination of ipratropium bromide (IB) and fenoterol hydrobromide (FEN) (Berodual® Respimat) and was launched in Germany in January 2004. Five clinical studies have been performed with Berodual Respimat in patients with COPD and in adults and children with asthma (reviewed in Kässner et al 2004). A pivotal Phase III, multicenter, parallel-group study of 892 patients with moderate to severe COPD compared IB/FEN via Respimat with a CFC-pMDI (Kilfeather et al 2004). Patients were randomized to one of five treatment arms: Respimat containing IB 10 μg/FEN 25 μg, Respimat containing IB 20 μg/FEN50 μg, pMDI containing IB 20 μg/ FEN50 μg, Respimat placebo, or pMDI placebo four times daily for 12 weeks. The primary efficacy endpoint was the average change from pre-dose in FEV1 over the first hour after dose administration on day 85 of treatment and the study was powered for non-inferiority. The bronchodilator response to IB/FEN on day 85 showed that the 20/50 μg dose via Respimat was not inferior to the 40/100 μg dose via CFC-pMDI (Figure 5).
Figure 5.
Change in FEV1 from pre-dose value in first 60 minutes after dosing on day 85 for IB/FEN delivered by Respimat® (two doses) and by pMDI compared with placebo devices in COPD patients. Reprinted from Kilfeather SA, Ponitz HH, Beck E, et al. 2004. Improved delivery of ipratropium bromide/fenoterol from Respimat® Soft Mist™ Inhaler in patients with COPD. Respir Med, 98:387–97. Copyright © 2004, with permission from Elsevier.
Abbreviations: MDI, metered dose inhaler; SMI, Soft Mist™ inhaler.
Four trials of IB/FEN via Respimat have been conducted in patients with asthma (thee in adults and on in children). Two of these were proof-of-concept, Phase II, dose-ranging and cumulative dose studies in adults with asthma (Kunkel et al 2000; Goldberg et al 2001). In the dose-ranging study (Goldberg et al 2001), five different doses of IB/FEN (from 5/12.5 μg to 80/200 μg) were used and showed a log-linear dose response for average increase in FEV1 up to 6 hours. Responses to IB/FEN doses of 5/12.5 and 10/25 μg administered via Respimat were closest or slightly superior to that for the IB/FEN dose of 40/100 μg delivered with CFC-pMDI. Therapeutic equivalence could not be demonstrated statistically in this study due to higher-than-expected variability. The cumulative dose study in adults with asthma (Kunkel et al 2000) concluded that IB/FEN delivered by Respimat was as effective as the CFC-pMDI at half the cumulative dose. Two Phase III studies were done comparing IB/FEN via Respimat and CFC-pMDI in adults and children with asthma (Vincken et al 2004; von Berg et al 2004). These studies concluded that delivery by Respimat instead of a CFC-pMDI allows a two- to four-fold reduction in dosage of IB/FEN while achieving similar efficacy. These trials in COPD and asthmatic subjects provide evidence in favor of the deposition experiment predictions that IB/FEN delivered by Respimat can be given at a reduced dosage (one-fourth to one-half) compared with that given by CFC-pMDI without any loss of efficacy.
The only other clinical trial published to date using Respimat in COPD patients was a cumulative dose study of ipratropium bromide compared with delivery via pMDI (Iacono et al 2000). This was a three-period cross-over study in 36 patients in which two dosages of IB by Respimat were used (10 and 20 μg per puff) compared with 20 μg per puff via CFC-pMDI. The study found greater bronchodilation from half the cumulative dose of IB by Respimat compared with the pMDI. There have been two Phase II published comparative trials using FEN, given alone to asthmatic subjects via Respimat and CFC-pMDI (van Noord et al 2000; Vincken et al 2003), and again showing the ability to use reduced doses with the Respimat. There have also been clinical trials conducted examining clinical efficacy and safety of tiotropium bromide delivered by Respimat in COPD subjects, but these have not been published.
Safety analysis of the five clinical trials using IB/ FEN in the Respimat showed an adverse event profile similar to the CFC-pMDI. In one of the Phase II studies (Kunkel et al 2000), the 320/800 μg dose given with Respimat was associated with a slightly higher incidence of headache, nervousness, and tremor than the same dose by pMDI. This may have been due to slightly increased β2-adrenergic stimulation and cholinergic blockade with the greater drug delivery to the lungs by the Respimat. In the Phase III studies, the adverse-event profiles of the 10/25 and 20/50 μg doses of IB/FEN via Respimat were comparable with that of the 40/100 μg dose via CFC-pMDI, and the frequency of adverse events thought to be treatment-related was low across all groups. Because of the use of the preservatives benzalkonium chloride and EDTA in bronchodilator preparations in the Respimat, there have been questions about the possibility of paradoxical bronchoconstriction with Respimat. This has been analyzed in both the Phase II and Phase III data sets for IB/FEN and either drug alone, and incidence of paradoxical bronchoconstriction was not found to be increased compared with CFC-pMDI (Koehler et al 2004; Hodder et al 2005). For the COPD and asthma patients in this analysis, there were no observed episodes of bronchospasm following administration of active drug or placebo via the Respimat. The incidence of asymptomatic falls in FEV1 >15% from baseline after Respimat use was 0%–2.8% in the active treatment group and 0%–7.6% in the placebo group and was no different than that measured after CFC p-MDI.
Patient-focused perspectives such as correct inhaler use, patient satisfaction–acceptability, compliance, and uptake
Many patients lack coordination for the split-second timing required between beginning a slow inhalation and activation of a pMDI (McFadden 1995; Fink and Rubin 2005). Asynchronous actuation can greatly reduce the amount of medication inhaled from a pMDI (Wilkes et al 2001) and can also reduce clinical effect of the inhaled drug (McFadden 1995; Newman et al 2001). One study showed that incidence of critical errors in pMDI use were significantly higher in COPD patients (26%) compared with asthma patients (13%) (Melani et al 2004). Problems with hand–breath coordination can be addressed by use of breath-actuated pMDIs, pMDIs plus spacer devices, DPIs, or nebulizers (Newman 2005). Newer HFA-pMDIs create aerosols that exit at a lower velocity than CFC-pMDIs and may have a smaller particle size, both factors which may help overcome problems with poor coordination. Like the pMDI, Respimat is also a press and breathe device and requires some coordination of actuation and inhalation. Respimat demonstrates lower aerosol velocity and longer spray duration that either CFC or HFA pMDIs, which should allow patients to coordinate actuation and breathing more easily. The deposition data show that Respimat deposits significantly more drug in the lungs of asthmatic patients than the Turbuhaler DPI or a CFC-pMDI in the setting of optimal inhalation technique. To date, no studies have been published that examine drug deposition from the Respimat in subjects with COPD or assess deposition and efficacy of Respimat in patients with poor inhalation technique.
In a clinical trial comparing IB/FEN via Respimat or an HFA-pMDI in 245 patients with COPD, asthma or mixed disease, correct assembly, and inhaler technique were assessed after training at the beginning of each treatment period (Schurmann et al 2005). Patients were given up to five attempts to demonstrate satisfactory technique and were scored on seven different device handling and breathing tasks. At the beginning of each treatment period, 96.4% could demonstrate satisfactory inhaler technique with Respimat by the final attempt, compared with 98% of patients with HFA-pMDI. The proportion of patients achieving a score of 7 at the first attempt was higher for HFA-pMDI (80%) than for Respimat (48%), perhaps due to the novelty of the Respimat device. While patients required more training for good technique with Respimat, patients retained good technique with Respimat after 7 weeks (97%), compared with 94% in th HFA-pMDI group. Most patients found the Respimat easy to assemble and only one patient was unable to assemble the device.
With the multitude of inhaler devices now available, there has been increased interest in the assessment of patient preference and satisfaction, as preference for a particular medication or inhaler device may be associated with improved adherence with therapeutic regimens. Boehringer Ingelheim developed and validated a patient satisfaction and preference questionnaire for inhalation devices to better assess satisfaction with the Respimat compared with other devices (Kozma et al 2005). The Patient Satisfaction and Preference Questionnaire (PASAPQ) was developed using published papers, focus groups, and expert opinion. The final questionnaire contains 14 satisfaction items, including a global satisfaction question, as well as one preference question and one question on willingness to use the device in the future. The satisfaction items were grouped into two domains: performance (seven items) and convenience (six items). Of all device satisfaction instruments that have been used in clinical trials, only the PASAPQ has a published validation and determination of minimally important difference, which is very important for discriminating the degree of difference that is clinically significant.
The PASAPQ was used in a study specifically designed to examine preference for and satisfaction with inhaler devices in patients with COPD, asthma, or mixed disease in a crossover study of IB/FEN delivered via Respimat vs HFA-pMDI (Schürmann 2005). The questionnaire was administered after each 7-week treatment period and of the 201 out of 224 subjects expressing a preference, 81% preferred Respimat. Of the 44 patients who had concomitant diagnoses that might affect inhaler handling, such as eye problems or arthritis, 89% preferred Respimat. This preference was not affected by type of lung disease or age. Patients were more willing to continue to use Respimat and mean ratings for 13 of 15 items in the satisfaction questionnaire were significantly higher for Respimat. There were no differences between the inhalers for efficacy measures such as peak expiratory flow, rescue inhaler use, and symptom scores. Taking device preference and satisfaction into account when choosing an inhaler device may be associated with improved clinical outcomes, but this has not been proven to date.
Conclusions – role in therapy
Respimat represents a novel approach to the delivery of inhaled drugs and overcomes some of the limitations of pMDIs, DPIs, and nebulizers. It is portable, propellant-free, uses mechanical energy for actuation, and does not require cumbersome spacers or holding chambers. Unlike some DPIs, optimal aerosol generation does not depend on high inspiratory flow rates. Respimat produces an aerosol with a greater fine-particle fraction than most pMDIs, DPIs, and nebulizers and the aerosol spray produced exits the inhaler more slowly and lasts for a longer time. This translates into higher lung drug deposition and lower oropharyngeal deposition than the pMDI and also Turbuhaler DPI. For some drug formulations, therapeutic ratio could be improved with Respimat, by offering higher lung deposition with lower oropharyngeal deposition and lower nominal dosing. It is also possible that drug delivery and efficacy will be improved with Respimat in those patients who have difficulties in actuating and coordinating inhalation when using a pMDI, but the device still requires some degree of hand–breath synchronization. In clinical trials in patients with COPD, bronchodilator drugs delivered from Respimat were equally effective at half of the dose delivered from a pMDI. In one study of inhaler preference, Respimat was preferred over the pMDI by patients with COPD and other obstructive lung diseases. It is not clear, however, if preference for the device will lead to improved adherence and clinical outcomes.
Currently, Respimat is available in Germany containing a combination of ipratropium bromide and fenoterol. As availability increases, usage of Respimat will likely be affected by available drugs and also by cost. Respimat is a novel and valuable addition to the range of inhaler devices available to the patient with COPD. It overcomes the challenge of hand–breath coordination that may be a problem for patients when using a pMDI and does not require generation of high inspiratory flow rates required for some DPIs. Respimat also makes it possible to give lower nominal doses of delivered drugs without decreasing efficacy. A continuing challenge in this population of patients, however, is the expense and confusion engendered by use of multiple inhaler devices.
Footnotes
Disclosures
Dr Anderson is a member of the Respimat Scientific Advisory Board for Boehringer Ingelheim and has served as a Principal Investigator and Speaker for Boehringer Ingelheim.
References
- Dalby R, Spallek M, Voshaar T. A review of the development of Respimat® Soft Mist Inhaler™. Int J Pharm. 2004;283:1–9. doi: 10.1016/j.ijpharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines. Chest. 2005;127:335–71. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- Fink JB. Metered-dose inhalers, dry powder inhalers, and transitions. Resp Care. 2000;45:623–35. [PubMed] [Google Scholar]
- Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50:1360–74. [PubMed] [Google Scholar]
- Goldberg J, Freund E, Beckers B, et al. Improved delivery of fenoterol plus ipratropium bromide using Respimat® compared with a conventional metered dose inhaler. Eur Respir J. 2001;17:225–32. doi: 10.1183/09031936.01.17202250. [DOI] [PubMed] [Google Scholar]
- Hochrainer D, Hölz H, Kreher C, et al. Comparison of the aerosol velocity and spray duration of Respimat® Soft Mist Inhaler™ and pressurized metered dose inhalers. J Aerosol Med. 2005;18:273–82. doi: 10.1089/jam.2005.18.273. [DOI] [PubMed] [Google Scholar]
- Hodder R, Pavia D, Dewberry H, et al. Low incidence of paradoxical bronchoconstriction in asthma and COPD patients during chronic use of Respimat® Soft Mist™ inhaler. Respir Med. 2005;99:1087–95. doi: 10.1016/j.rmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Iacono P, Velicitat P, Guemas E, et al. Improved delivery of ipratropium bromide using Respimat® (a new soft mist inhaler) compared with a conventional metered dose inhaler: cumulative dose response study in patients with COPD. Respir Med. 2000;94:490–7. doi: 10.1053/rmed.1999.0770. [DOI] [PubMed] [Google Scholar]
- Kässner F, Hodder R, Bateman ED. A review of ipratripium bromide/ fenoterol hydrobromide (Berodual®) delivered via Respimat® Soft Mist Inhaler™ in patients with asthma and chronic obstructive pulmonary disease. Drugs. 2004;64:1671–82. doi: 10.2165/00003495-200464150-00005. [DOI] [PubMed] [Google Scholar]
- Kilfeather SA, Ponitz HH, Beck E, et al. Improved delivery of ipratropium bromide/fenoterol from Respimat® Soft Mist™ Inhaler in patients with COPD. Respir Med. 2004;98:387–97. doi: 10.1016/j.rmed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Koehler D, Pavia D, Dewberry H, et al. Low incidence of paradoxical bronchoconstriction with bronchodilator drugs administered by Respimat® Soft Mist™ Inhaler; results of phase II single-dose crossover studies. Respiration. 2004;71:469–76. doi: 10.1159/000080631. [DOI] [PubMed] [Google Scholar]
- Kozma CM, Slaton TL, Monz BU, et al. Development and validation of a patient satisfaction and preference questionnaire for inhalation devices. Treat Respir Med. 2005;4:41–52. doi: 10.2165/00151829-200504010-00005. [DOI] [PubMed] [Google Scholar]
- Kunkel G, Magnussen H, Bergmann KC, et al. Respimat® (a new soft mist inhaler) delivering fenoterol plus ipratropium bromide provides equivalent bronchodilation at half the cumulative dose compared with a conventional metered dose inhaler in asthmatic patients. Respiration. 2000;67:306–14. doi: 10.1159/000029515. [DOI] [PubMed] [Google Scholar]
- Leach CL. Approaches and challenges to use freon propellant replacements. Aerosol Sci Technol. 1995;22:328–34. [Google Scholar]
- McFadden ER. Improper patient techniques with metered dose inhalers: clinical consequences and solutions to misuse. J Allergy Clin Immunol. 1995;96:278–83. doi: 10.1016/s0091-6749(95)70206-7. [DOI] [PubMed] [Google Scholar]
- Melani AS, Zanchetta D, Barbato N, et al. Inhalation technique and variables associated with misuse of conventional metered-dose inhalers and newer dry powder inhalers in experienced adults. Ann Allergy Asthma Immunol. 2004;93:439–46. doi: 10.1016/s1081-1206(10)61410-x. [DOI] [PubMed] [Google Scholar]
- Newman SP. Inhaler treatment options in COPD. Eur Respir Rev. 2005;14:102–3. [Google Scholar]
- Newman SP, Brown J, Steed SP, et al. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines. Chest. 1998;113:957–63. doi: 10.1378/chest.113.4.957. [DOI] [PubMed] [Google Scholar]
- Newman SP, Steed KP, Reader SJ, et al. Efficient delivery to the lungs of flunisolide aerosol from a new portable hand-held multidose nebulizer. J Pharm Sci. 1996;85:960–4. doi: 10.1021/js950522q. [DOI] [PubMed] [Google Scholar]
- Newman SP, Weisz AWB, Talaee N, et al. Improvement of drug delivery with a breath actuated pressurized aerosol for patients with poor inhaler technique. Thorax. 2001;46:712–16. doi: 10.1136/thx.46.10.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcairn G, Reader S, Pavia D, et al. Deposition of corticosteroid aerosol in the human lung by Respimat® Soft Mist™ Inhaler compared to deposition by metered dose inhaler or by Turbuhaler® dry powder inhaler. J Aerosol Med. 2005;18:264–72. doi: 10.1089/jam.2005.18.264. [DOI] [PubMed] [Google Scholar]
- Schürmann W, Schmidtmann S, Moroni P, et al. Respimat® Soft Mist™ Inhaler versus hydrofluoroalkane metered dose inhaler. Patient preference and satisfaction. Treat Respir Med. 2005;4:53–61. doi: 10.2165/00151829-200504010-00006. [DOI] [PubMed] [Google Scholar]
- Spallek MW, Hochrainer D, Wachtel H. Optimizing nozzles for soft mist inhalers. Respiratory Drug Delivery VIII. 2002;2:375–8. [Google Scholar]
- Steed KP, Towse LJ, Freund B, et al. Lung and oropharyngeal deposition of fenoterol hydrobromide delivered from the prototype III hand-held multidose Respimat nebulizer. Eur J Pharm Sci. 1997;5:55–61. [Google Scholar]
- Van Noord JA, Smeets JJ, Creemers JPHM, et al. Delivery of fenoterol via Respimat®, a novel “soft mist” inhaler. Respiration. 2000;67:672–8. doi: 10.1159/000056298. [DOI] [PubMed] [Google Scholar]
- Vincken W, Bantje T, Middle MV, et al. Long-term efficacy and safety of ipratropium bromide plus fenoterol via Respimat® Soft Mist™ inhaler (SMI) versus a pressurised metered-dose inhaler in asthma. Clin Drug Invest. 2004;24:17–28. doi: 10.2165/00044011-200424010-00003. [DOI] [PubMed] [Google Scholar]
- Vincken W, Dewberry H, Moonen D. Fenoterol delivery by Respimat® soft mist inhaler versus CFC metered dose inhaler: cumulative dose-response study in asthma patients. J Asthma. 2003;40:721–30. doi: 10.1081/jas-120023495. [DOI] [PubMed] [Google Scholar]
- von Berg A, Jeena PM, Soemantri PA, et al. Efficacy and safety of ipratropium bromide plus fenoterol inhaled via Respimat® Soft Mist™ inhaler vs. a conventional metered dose inhaler plus spacer in children with asthma. Pediatr Pulmonol. 2004;37:264–72. doi: 10.1002/ppul.10428. [DOI] [PubMed] [Google Scholar]
- Wilkes W, Fink J, Dhand R. Selecting an accessory device with a metered-dose inhaler: variable influence of accessory devices on fine particle dose, throat deposition, and drug delivery with asynchronous actuation from a metered-dose inhaler. J Aerosol Med. 2001;13:351–60. doi: 10.1089/089426801316970312. [DOI] [PubMed] [Google Scholar]
- Wolff RK, Niven RW. Generation of aerosolized drugs. J Aerosol Med. 1994;7:89–104. doi: 10.1089/jam.1994.7.89. [DOI] [PubMed] [Google Scholar]
- Zierenberg G. Optimizing the in vitro performance of Respimat. 1999. J Aerosol Med. 12:S19–24. doi: 10.1089/jam.1999.12.suppl_1.s-19. [DOI] [PubMed] [Google Scholar]