Abstract
Background
Theophylline is a nonspecific inhibitor of phosphodiesterases that, despite exerting bronchodilator and anti-inflammatory effects, is a third-line therapy rarely used to treat chronic airflow limitation. We wished to evaluate the efficacy of oral theophylline as measured by improvements in trough (pre-dose) or peak (post-dose) FEV1 and FVC in patients with clinically stable COPD.
Design
Meta-analysis of randomized, placebo-controlled trials reported as of June 2005 in which theophylline was orally administered to stable COPD patients and the functional evaluations included pre- and post-theophylline values for FEV1 and FVC.
Results
A total of 18 trials were included in the meta-analysis. The weighted mean differences (WMD) with 95% confidence intervals (95% CI) for improvement over placebo in trough FEV1 and FVC were 0.108L (0.053–0.163) and 0.186L (0.036–0.336), respectively, while peak FEV1 and FVC improved by 0.096L (0.044–0.147) and 0.242L (0.11–0.374), respectively.
Conclusions
Treatment with oral theophylline improves both trough and peak FEV1 and FVC in clinically stable COPD patients. These results support previously reported benefits of theophylline in COPD.
Keywords: COPD, meta-analysis, theophylline
Introduction
Theophylline is a nonspecific inhibitor of phosphodiesterases. Since the original report of its efficacy as a bronchodilator, theophylline has been used extensively to treat COPD and asthma (Schultze-Werninghaus and Meier-Sydow 1982). More recently, the importance of its anti-inflammatory properties has been emphasized (Barnes 2003; Barnes et al 2005). Although its use in COPD is recommended in the GOLD Guidelines (Pauwels et al 2001), oral theophylline is rarely prescribed in the Western Hemisphere due to the belief that it is either poorly efficacious, unsafe, or cumbersome in the outpatient setting.
Most of the randomized, controlled trials of theophylline reported in stable COPD patients used a small sample size (Schmidt and Altschuler 1979; Alexander et al 1980; Anderson et al 1982; Marvin et al 1983; Mahler et al 1985; Dullinger et al 1986; Guyatt et al 1987; Chrystyn et al 1988; Berry et al 1991; Thomas et al 1992, 1995; Nishimura et al 1993; Fink et al 1994; Newman et al 1994; Shivaram et al 1997; Tsukino et al 1998; Rossi et al 2002; Broseghini et al 2005) and there is only one systematic review/meta-analysis of the published data (Ram et al 2005). Thus, controversy still exists about its potential risks and benefits. In addition, it is unclear whether any putative benefits of oral theophylline in COPD would be immediate (ie, improvement in peak pulmonary function after dosing), long-term (ie, improvement in trough pulmonary function over time), or both.
We wished to analyze the efficacy of oral theophylline in stable COPD patients. We conducted meta-analyses of the trough and peak FEV1 and FVC data reported and published as of June 2005 from randomized, placebo-controlled trials in which oral theophylline was administered to clinically stable COPD patients. To our knowledge, this approach of analyzing the effects of theophylline on peak and trough pulmonary function has not been reported previously.
Materials and methods
Types of trials and patients
All the trials included involved treatment with oral theophylline in a stable COPD population. Only those published trials involving patients with stable COPD who had pulmonary function tests (PFTs) performed before and after administration of theophylline were included. The articles were evaluated for comparisons of theophylline vs placebo. Specifically, trough and peak FEV1 and FVC results were of interest.
Search for trials
A search of the PubMed database was performed using the terms “theophylline + COPD” for English language articles published up to and including June 2005. The literature search was limited to randomized controlled trials in humans. The search yielded 66 articles. A review of these articles was conducted for trials involving patients with stable COPD who had PFTs measured as part of the trial. This yielded 17 articles. Of these, 3 did not provide either FEV1 or FVC data and were excluded from the analysis, and 4 additional papers were active-controlled only (ie, were not placebo controlled) and were also excluded. Additionally, we reviewed a recently published meta-analysis (Ram et al 2005). Since the selection criteria for inclusion of publications in this meta-analysis were similar to our criteria (albeit not identical), additional articles cited by Ram and colleagues that were not identified in our PubMed review were also included. This yielded 8 additional articles. Therefore, a total of 18 trials met the criteria for inclusion in our meta-analysis.
Extraction of data used for meta-analysis
Articles were individually reviewed for the following PFT results: trough FEV1, trough FVC (pre-dose values), peak FEV1, and peak FVC (highest values post-dose). Not all articles reported all four of these PFTs and in many cases they did not differentiate between trough and peak. If any of the four PFT results were reported, the article was accepted. In the cases where the authors did not differentiate between trough and peak, we determined this from the time the last dose of theophylline was taken and the time the PFT was done. The data were extracted from text, tables, and/or graphs.
The mean FEV1 (trough and/or peak) and FVC (trough and/or peak) in the theophylline group and the placebo group were recorded along with the standard deviation. Additionally, the number of patients participating in each group (n) was recorded (Table 1).
Table 1.
Characteristics of randomized controlled trials included in the meta-analysis
| Author and year | n | Mean age or rangea | Males/females | Theo dose (mg) | Treatment duration | Baseline FEV1b |
|---|---|---|---|---|---|---|
| Schmidt and Altschuler 1979 | 12 | 30–70 | NRc | 800/day | 3-month crossover | 1.0 L |
| Alexander et al 1980 | 40 | 59 | 40/0 | 400/day | 4-week crossover | <60% predicted |
| Anderson et al 1982 | 21 | 58 | 17/4 | 350–700/day | 8-day crossover | 48% predictedd |
| Marvin et al 1983 | 15 | 50–69 | 15/0 | 800/day | 10 days | 1.0 L |
| Mahler et al 1985 | 12 | 60 | 12/0 | Titrated to 10–20 mg/L | 4-week crossover | 1.36 L |
| Dullinger et al 1986 | 10 | 61 | NR | Titrated to 10–15 mg/L | 1-week crossover | < 1.0 L |
| Guyatt et al 1987 | 19 | 65 | 19/0 | Titrated to 65–100 mmol/L | 14 days | 1.0 L |
| Chrystyn et al 1988 | 33 | 53–73 | 30/3 | Titrated to 5–20 mg/L | 60 days | < 30% predicted |
| Berry et al 1991 | 12 | 63 | 12/0 | 750–1000 mg/day | 2 days | 1.4 L |
| Thomas et al 1992 | 12 | 63 | 6/6 | Titrated to 10–16.5 mg/L | 2-week crossover | 1.1 L |
| Nishimura et al 1993 | 12 | 64 | 11/1 | 400–600/day | 4-week crossover | 0.9 L |
| Newman et al 1994 | 12 | 62 | 11/1 | Titrated to 10–20 μg/ml | 4 weeks | 1.1 L |
| Fink et al 1994 | 22 | 68 | 17/5 | Titrated to >55 μmol/L | 4-week crossover | 1.0 L |
| Nishimura et al 1995 | 24 | 65 | 24/0 | Titrated to >10 μg/mL | 4-week crossover | 0.96 L |
| Shivaram et al 1997 | 17 | 66 | NR | 400/day | Single dose | 0.77 L |
| Tsukino et al 1998 | 21 | 65 | 21/0 | 600–800/day | 3-day crossover | 1.0 L |
| Rossi et al 2002 | 854 | 63 | 709/145 | Titrated to 8–20 mg/L | 12 months | 1.37 L |
| Broseghini et al 2005 | 13 | 67 | 10/3 | Titrated to 10–20 μg/mL | 2-week crossover | 1.3 L |
mean of whole study population
mean percent predicted in the study population
not reported
peak expiratory flow rates
Statistical analysis
Individual trial data were pooled using meta-analytical techniques. Meta-analyses involving continuous outcomes were based on comparisons of means for theophylline vs placebo. For continuous variables (such as trough FEV1, trough FVC, peak FEV1, and peak FVC), the results of individual studies were pooled using fixed-effect weighted mean difference (WMD) with the corresponding 95% confidence interval (CI). The WMD is a meta-analytical technique used to combine measures on continuous scales, where the mean, standard deviation and sample size for each group are known. The weight given to each study is determined by the precision (the inverse of the variance) of its estimate of effect. The WMD technique assumes that all of the trials measured the outcomes using the same scales. All statistical analyses were carried out using Comprehensive Meta-Analysis™ Version 2.
Results
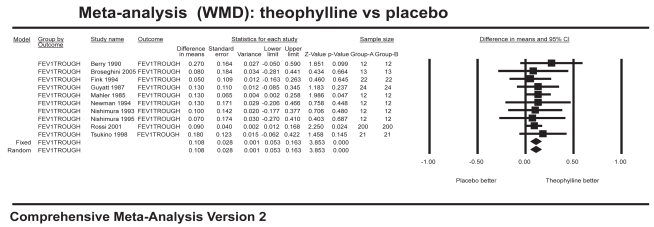
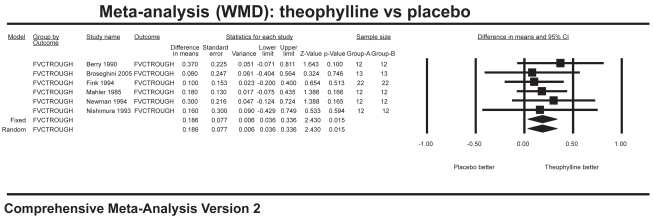
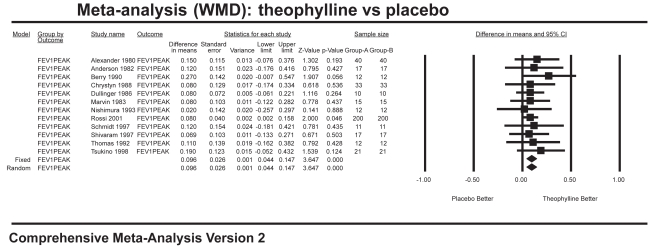
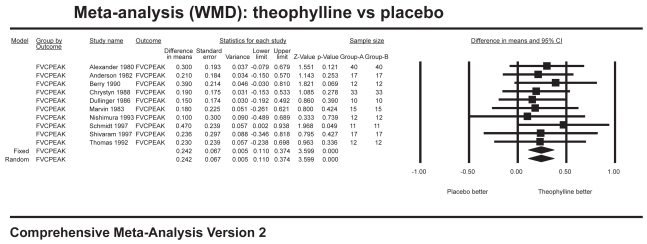
Ten studies with 704 patients combined contributed data for the analysis of trough FEV1 (Figure 1), which showed a significant improvement for theophylline of 108 mL over placebo (WMD 0.108 L; 95% CI=0.05–0.16). Six studies with 166 patients combined contributed data for the analysis of trough FVC (Figure 2), which showed a significant improvement of 186 mL over placebo (WMD 0.186 L; 95% CI=0.04–0.34). Twelve studies with 800 patients combined contributed data for the analysis of peak FEV1 (Figure 3), which showed a significant improvement of 96 mL over placebo (WMD 0.096 L; 95% CI=0.04–0.15). Ten studies with 358 patients combined contributed data for the analysis of peak FVC (Figure 4), which showed a significant improvement of 242 mL over placebo (WMD 0.242 L; 95% CI=0.11–0.37). For trough and peak FEV1, the largest contribution of data (400 patients) was derived from a single study (Rossi et al 2002) that showed a significant improvement over placebo of 90 mL and 80 mL, respectively.
Figure 1.
Meta-analysis on trough FEV1 showing results favoring theophylline.
Figure 2.
Meta-analysis on trough FVC showing results favoring theophylline.
Figure 3.
Meta-analysis on peak FEV1 showing results favoring theophylline.
Figure 4.
Meta-analysis on peak FVC showing results favoring theophylline.
Discussion
Our results show that oral theophylline confers a bronchodilator effect as measured by improvements of trough and peak FEV1 and FVC in clinically stable COPD patients. One limitation of our study that limits the interpretation of these results is that we did not analyze the incidence of adverse events reported by the patients who received theophylline. However, in a recent meta-analysis that reported similar improvements in pulmonary function to those we are reporting, preference for theophylline was higher than for placebo despite a higher incidence of adverse events (Ram et al 2005).
Our study dissects the improvements in trough and peak pulmonary function tests and shows that the benefits of theophylline are immediate (improvements in peak values) and also sustained (improvements in trough values).
There is debate around the meaning of improvements in trough vs peak pulmonary function, but the debate is more intense concerning the magnitude of such improvements that are clinically meaningful in COPD (Donohue 2004). It is generally agreed that for a particular improvement to be considered of value it needs to be accompanied by clinical improvement. The improvement of 108 mL seen in trough (pre-dose) FEV1 and of 186 mL seen in trough FVC, as well as the improvements of 96 mL and 242 mL in peak (post-dose) FEV1 and FVC, respectively, seem comparable to those achieved with other bronchodilators commonly used in COPD. For example, data presented at different US Food and Drug Administration (FDA) Advisory Committee meetings showed that salmeterol provoked an improvement of 90 mL in trough FEV1 and 200 mL in peak FEV1. Salmeterol 50 mg/fluticasone 250 mg combination therapy achieved 164 mL in trough FEV1 and 281 mL in peak FEV1 (http://www.fda.gov/ohrms/dockets/ac/02/transcripts/3830T1.htm). Tiotropium improved trough FEV1 by 110–130 mL (depending on the study) and peak FEV1 by 240–260mL (http://www.fda.gov/ohrms/dockets/ac/02/transcripts/3890t1.htm).
An additive benefit of comparable magnitude can be seen when oral theophylline is used with another bronchodilator (Table 2). This additive effect may be different from the putative synergistic mechanism proposed by Barnes et al (2003) for the effects of inhaled steroids in COPD (Lim et al 2000; Oliver et al 2001).
Table 2.
Studies that have demonstrated a benefit when theophylline is used with another bronchodilator in stable COPD patients
| Author (bronchodilator, bd) | Mean % increase FEV1 post-bd | Mean % increase FVC post-bd | Mean % increase FEV1 post-bd+theophylline | Mean % increase FVC post-bd+theophylline |
|---|---|---|---|---|
| Thomas et al 1992 (albuterol) | 16.2 | 16.3 | 31.3 | 24.9 |
| Tsukino et al1998 (ipratropium) | 17.4 | NR | 33.3 | NR |
| ZuWallack 2001 (salmeterol) | 8 | 13 | 17 | 26.6 |
| Bellia 2002 (oxitropium) | 5 | 4 | 16 | 13 |
| Dullinger et al 1986 (metaproterenol) | 18.5 | 11 | 18.5 | 14.4 |
In conclusion, it appears that in patients with clinically stable COPD, oral theophylline can improve FEV1 and FVC. Since blood levels need to be closely monitored and adjusted to avert serious, even potentially life-threatening side-effects, theophylline remains a third choice in the COPD therapeutic armamentarium. It remains to be shown whether safer sub-therapeutic doses of oral theophylline can exert any clinically meaningful benefit in COPD, as recently proposed (Barnes 2005).
References
- Alexander MR, Dull WL, Kasik JE. Treatment of chronic obstructive pulmonary disease with orally administered theophylline. A double-blind, controlled study. JAMA. 1980;244:2286–90. [PubMed] [Google Scholar]
- Anderson G, Peel ET, Pardoe T, et al. Sustained-release theophylline in chronic bronchitis. Br J Dis Chest. 1982;76:261–5. [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25:552–63. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med. 2003;167:813–18. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- Bellia V, Foresi A, Bianco S, et al. Efficacy and safety of oxitropium bromide, theophylline and their combination in COPD patients: a double-blind, randomized, multicentre study (BREATH Trial) Respir Med. 2002;96:881–9. doi: 10.1053/rmed.2002.1380. [DOI] [PubMed] [Google Scholar]
- Berry RB, Desa MM, Branum JP, et al. Effect of theophylline on sleep and sleep-disordered breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;143:245–50. doi: 10.1164/ajrccm/143.2.245. [DOI] [PubMed] [Google Scholar]
- Broseghini C, Testi R, Polese G, et al. A comparison between inhaled salmeterol and theophylline in the short-term treatment of stable chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2005;18:103–8. doi: 10.1016/j.pupt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Chrystyn H, Mulley BA, Peake MD. Dose response relation to oral theophylline in severe chronic obstructive airways disease. BMJ. 1988;297(6662):1506–10. doi: 10.1136/bmj.297.6662.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue JF. Therapeutic responses in asthma and COPD. Bronchodilators. Chest. 2004;126(Suppl):S125–37. doi: 10.1378/chest.126.2_suppl_1.125S. [DOI] [PubMed] [Google Scholar]
- Dullinger D, Kronenberg R, Niewoehner DE. Efficacy of inhaled metaproterenol and orally-administered theophylline in patients with chronic airflow obstruction. Chest. 1986;89:171–3. doi: 10.1378/chest.89.2.171. [DOI] [PubMed] [Google Scholar]
- Fink G, Kaye C, Sulkes J, et al. Effect of theophylline on exercise performance in patients with severe chronic obstructive pulmonary disease. Thorax. 1994;49:332–4. doi: 10.1136/thx.49.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Townsend M, Pugsley SO, et al. Bronchodilators in chronic air-flow limitation. Effects on airway function, exercise capacity, and quality of life. Am Rev Respir Dis. 1987;135:1069–74. doi: 10.1164/arrd.1987.135.5.1069. [DOI] [PubMed] [Google Scholar]
- Lim S, Jatakanon A, Gordon D, et al. Comparison of high dose inhaled steroids, low dose inhaled steroids plus low dose theophylline, and low dose inhaled steroids alone in chronic asthma in general practice. Thorax. 2000;55:837–41. doi: 10.1136/thorax.55.10.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler DA, Matthay RA, Snyder PE, et al. Sustained-release theophylline reduces dyspnea in nonreversible obstructive airway disease. Am Rev Respir Dis. 1985;131:22–5. doi: 10.1164/arrd.1985.131.1.22. [DOI] [PubMed] [Google Scholar]
- Marvin PM, Baker BJ, Dutt AK, et al. Physiologic effects of oral bronchodilators during rest and exercise in chronic obstructive pulmonary disease. Chest. 1983;84:684–9. doi: 10.1378/chest.84.6.684. [DOI] [PubMed] [Google Scholar]
- Newman D, Tamir J, Speedy L, et al. Physiological and neuropsychological effects of theophylline in chronic obstructive pulmonary disease. Isr J Med Sci. 1994;30:811–16. [PubMed] [Google Scholar]
- Nishimura K, Koyama H, Ikeda A, et al. Is oral theophylline effective in combination with both inhaled anticholinergic agent and inhaled beta 2-agonist in the treatment of stable COPD? Chest. 1993;104:179–84. doi: 10.1378/chest.104.1.179. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Koyama H, Ikeda A, et al. The additive effect of theophylline on a high-dose combination of inhaled salbutamol and ipratropium bromide in stable COPD. Chest. 1995;107:718–23. doi: 10.1378/chest.107.3.718. [DOI] [PubMed] [Google Scholar]
- Oliver B, Tomita K, Keller A, et al. Low-dose theophylline does not exert its anti-inflammatory effects in mild asthma through upregulation of interleukin-10 in alveolar macrophages. Allergy. 2001;56:1087–90. doi: 10.1034/j.1398-9995.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Ma P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798–825. [PubMed] [Google Scholar]
- Ram FS, Jardin JR, Atallah A, et al. Efficacy of theophylline in people with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Med. 2005;99:135–44. doi: 10.1016/j.rmed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Rossi A, Kristufek P, Levine BE, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058–69. doi: 10.1378/chest.121.4.1058. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Altschuler S. The usefulness of theophylline in nonasthmatic airway obstruction. Angiology. 1979;30:744–9. doi: 10.1177/000331977903001103. [DOI] [PubMed] [Google Scholar]
- Schultze-Werninghaus G, Meier-Sydow J. The clinical and pharmacological history of theophylline: first report on the bronchospasmolytic action in man by S. R. Hirsch in Frankfurt (Main) 1922. Clin Allergy. 1982;12:211–15. doi: 10.1111/j.1365-2222.1982.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Shivaram U, Cash ME, Mateo F, et al. Effects of high-dose ipratropium bromide and oral aminophylline on spirometry and exercise tolerance in COPD. Respir Med. 1997;91:327–34. doi: 10.1016/s0954-6111(97)90058-5. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pugsley JA, Stewart JH. Theophylline and salbutamol improve pulmonary function in patients with irreversible chronic obstructive pulmonary disease. Chest. 1992;101:160–5. doi: 10.1378/chest.101.1.160. [DOI] [PubMed] [Google Scholar]
- Tsukino M, Nishimura K, Ikeda A, et al. Effects of theophylline and ipratropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. Thorax. 1998;53:269–73. doi: 10.1136/thx.53.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZuWallack RL, Mahler DA, Reilly D, et al. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest. 2001;119:1661–70. doi: 10.1378/chest.119.6.1661. [DOI] [PubMed] [Google Scholar]