Abstract
Objective
The aim of the present study was to examine the relationship between serious arrhythmias in patients with psychotropic drug overdose and electrocardiography (ECG) findings that have been suggested previously to predict this complication.
Methods
Thirty-nine patients with serious arrhythmias (ventricular tachycardia, supraventricular tachycardia or cardiac arrest) after tricyclic antidepressant overdose or thioridazine overdose were compared with 117 controls with clinically significant overdose matched to each case for the drug ingested. These patients with psychotropic drug overdose had presented for treatment to the Department of Clinical Toxicology, Newcastle and to the Princess Alexandra Hospital, Brisbane. The heart rate, the QRS width, the QTc and QT intervals, the QT dispersion, and the R wave and R/S ratios in aVR on the initial ECGs were compared in cases and controls.
Results
The cases had taken dothiepin (16 patients), doxepin (six patients), thioridazine (five patients), amitriptyline (five patients), nortriptyline (three patients), imipramine (one patient) and a combination of dothiepin and thioridazine (three patients). In 20 of the 39 patients with arrhythmias, the arrhythmia had been a presumed ventricular tachycardia. Of the other 19 patients, 15 patients had a supraventricular tachycardia, two patients had cardiac arrests (one asystole, one without ECG monitoring) and two patients had insufficient data recorded to make classification of the arrhythmias possible. The QRS was ≥ 100 ms in 82% of cases but also in 76% of controls. QRS ≥ 160 ms had a sensitivity of only 13% and occurred in 2% of controls. QRS > 120 ms, QTc > 500 and the R/S ratio in aVR appeared to have a stronger association with the occurrence of arrhythmia: QRS > 120 ms (odds ratio [OR], 3.56; 95% confidence interval [CI], 1.46–8.68), QTc > 500 (OR, 3.07; 95% CI, 1.33–7.07), and R/S ratio in aVR > 0.7 (OR, 16; 95% CI, 3.47–74). Excluding thioridazine overdoses and performing the analysis for tricyclic antidepressant overdoses alone gave increased odds ratios for QRS > 120 ms (OR, 4.83; 95% CI, 1.73–13.5) and QTc > 500 (OR, 4.5; 95% CI, 1.56–13) but had little effect on that for the R/S ratio in aVR > 0.7 (OR, 14.5; 95% CI, 3.10–68).
Conclusion
ECG measurements were generally weakly related to the occurrence of arrhythmia and should not be used as the sole criteria for risk assessment in tricyclic antidepressant overdose. The frequently recommended practice of using either QRS ≥ 100 ms or QRS ≥ 160 ms to predict arrhythmias is not supported by our study. R/S ratio in aVR > 0.7 was most strongly related to arrhythmia but had estimated positive and negative predictive values of only 41% and 95%, respectively. The use of these specific predictors in other drug overdoses is not recommended without specific studies.
Keywords: arrhythmia, electrocardiography, overdose, sensitivity, specificity, thioridazine, tricyclic antidepressive agents
Introduction
Psychotropic drugs with cardiotoxic effects, taken frequently in overdose, are among the most common causes of death from overdose [1,2]. The majority of deaths occur out of hospital, however; the inhospital mortality is quite low (< 2%) [3-5]. As only a small proportion of these patients have life-threatening complications, efforts have been made to identify those at risk using findings on the electrocardiograph. The electrocardiography (ECG) findings examined have included the QRS duration, the QT interval duration, and the axis of the terminal 40 ms of the QRS complex represented by the height of the R wave and the R/S ratio in aVR [6-20]. As well as using ECG findings to predict risk of arrhythmias, some authors use ECG criteria to predict the risk of noncardiac complications such as coma or seizures. But the risk of seizures and other complications varies widely between antidepressants [4,21,22], and may be due to mechanisms unrelated to the cardiac effects of these drugs [23]. This fact is supported by the observation that the increase in seizure risk for dothiepin is independent of the ECG findings [4].
Another problem with ECG indicators of risk is that many studies have used nonstandardised observations. As there is substantial inter-rater variation in measuring even simple ECG findings in overdose [24], doubt must be cast on the validity of such observations. Second, the use of post hoc analysis means that the sensitivity and specificity of suggested 'cutpoints' usually have been overestimated. Finally, some studies have combined a number of trivial 'complications' as outcomes (e.g. ventricular ectopic activity) with more serious complications. These data cannot be used to predict only the more serious adverse events. The important clinical decision in the emergency setting is which patients should have continuous electrocardiographic monitoring for arrhythmias.
These ECG predictors have been based on studies of tricyclic antidepressant (TCA) poisoning. However, they are often loosely applied to patients who take drugs with apparently qualitatively similar cardiotoxicity, such as antipsychotic drugs, antihistamines and antiarrhythmic drugs. The most closely related drugs are antipsychotic drugs that have a similar tricyclic structure, such as phenothiazines. For example, thioridazine causes similar ECG changes to TCAs and commonly causes ventricular arrhythmias in overdose [25]. Experimentally, thioridazine also blocks ion channels in the heart and causes early after-depolarisations, in a similar manner to some TCAs [26].
The aim of the present study was to examine the usefulness of all the previously suggested ECG findings in predicting serious arrhythmias in patients with overdose of TCAs and of antipsychotic drugs. In addition, as QT dispersion has been used as a marker for arrhythmia in patients with ischaemic heart disease and cardiac failure [27-29], we examined the usefulness of QT dispersion as a predictor of arrhythmias in these overdoses.
Patients and methods
We identified patients with arrhythmias from two clinical databases. All cases recorded as having arrhythmias after TCA overdose or antipsychotic drug overdose were selected. No patients had a known cardiac history. While ingestion of other drugs was common, no patient had ingested other drugs that were believed to be a more probable cause of the arrhythmia in any of these cases. We have made the assumption that the purpose of the ECG is to identify patients at risk of any sustained arrhythmia, and therefore arrhythmias were defined as sustained tachyarrhythmias (supraventricular tachycardia [SVT] or ventricular tachycardia [VT]), complete heart block or asystole.
The cases recorded in the Department of Clinical Toxicology database, Newcastle include all patients who presented to hospital between 1987 and 1995. The Princess Alexandra Hospital, Brisbane only recorded data on patients who were admitted with overdose (not the substantial number who were discharged from the emergency department) between 1986 and 1995. Our institutions do not require Human Research Ethics Committee approval to collect, analyse or publish data involving audit of usual clinical practice.
For all patients who were recorded as having arrhythmias, the first ECG recorded was reviewed. If this ECG was reported as showing an arrhythmia, the first ECG taken as soon as possible after reversion of the arrhythmia was used (this affected 10 out of 39 cases).
Original medical records of all patients who had been recorded as having an arrhythmia were reviewed. Controls were selected from patients who had ingested the same drug, had a potentially severe TCA overdose or antipsychotic drug overdose (> 1500 mg ingested or significant toxicity – coma or seizures) and who did not experience arrhythmias. It was not possible to find three matched (for drug-ingested) controls for cases with nortriptyline overdoses or dothiepin and thioridazine in combination overdoses. Other TCA overdoses were used to match cases of nortriptyline. Dothiepin-only ingestions were used to match dothiepin and thioridazine combinations. The ECGs were reviewed by two cardiologists (SC and JL). Wide complex tachycardia were classified as VT except when the ECG morphologies in sinus rhythm and in tachycardia were not significantly different. These arrhythmias and narrow complex tachycardia were classified as SVT [30].
Every ECG was magnified (× 1.4) using a high-quality photocopier (Konica 3135, Konica, Tokyo, Japan). Intervals were measured manually by one observer blinded to the case or control status using the computer program SigmaScan (version 3.90; SigmaScan, SPSS, Chicago, IL, USA) and a Kurta IS-1 digitising pad (Altek Corporation, SIlver Spring, MD, USA).
The QT interval was measured from the earliest QRS deflection to the end of the T-wave, defined as the point of return of the T wave to the isoelectric baseline. If the end of the T wave could not be determined reliably, the lead was not included. The QTc was calculated using Bazett's formula [31]. The height of the R wave in aVR above the isoelectric line and the ratio of this to the depth of the S wave below the line were measured using the method described by Leibelt and colleagues [8]. The QT and JT dispersions were the difference between the minimum and maximum QT and JT intervals. The adjusted QT (and JT) dispersion is the QT (JT) dispersion divided by the square root of the number of leads used for QT (JT) measurements [32].
Statistical analysis
Subjects who had arrhythmias were compared with matched individuals who had ingested the same drug with respect to age, gender, and the amount ingested. To investigate the strength of the association between a variety of study factors and arrhythmia, the odds ratio (OR) of exposure to the study factor in those with and without the outcome was calculated using the Mantel–Haenszel method for matched sets. ORs were adjusted for age and gender, and the effect of other study factors using conditional logistic regression. ORs for QRS measurements were adjusted for the QTc duration, and other ECG findings were adjusted for the QRS duration to determine whether the associations were independent of other commonly used predictors.
Results
Thirty-nine patients developed significant arrhythmias (SVT, VT or cardiac arrest). All arrhythmias occurred within 24 hours of presentation and were attributed to the overdose. Three of these patients died: one with an asystolic arrest, one with VT followed by cardiac arrest, and one with refractory VT and hypotension. Arrhythmias occurred in 2% (13/630) of TCA overdoses and in 4% (6/147) of thioridazine overdoses in Newcastle. No arrhythmias occurred in patients ingesting other antipsychotic drugs (0/289). Arrhythmias were also uncommon in Brisbane; however, the total number of psychotropic drug overdoses in the area was not known.
On review, 20 patients had a presumed VT. In the other 19 patients, 15 patients had SVT, two patients had cardiac arrests (one asystole and one without ECG monitoring) and two patients had a tachyarrhythmia for which classification was not possible because the record of the arrhythmia was not in the chart. The drugs taken by cases with arrhythmia were dothiepin (16 patients), doxepin (six patients), thioridazine (five patients), amitriptyline (five patients), nortriptyline (three patients), imipramine (one patient), and a combination of dothiepin and thioridazine (three patients).
Descriptive data for patients with arrhythmias (cases) and controls are presented in Table 1. The cases and controls had a similar age, a similar gender and a similar dose ingested (although these were not matched a priori). The relationship between arrhythmia and various ECG findings is presented in Table 2 (analysed as continuous variables) and in Table 3 (using dichotomised variables based on previously described cutpoints or on the median). Adjustment for age and gender had no substantial effect on the ORs (data not shown). Adjustment for other ECG variables significantly lowered the ORs, however, indicating that they may be measuring different aspects of the same electrophysiological abnormality.
Table 1.
Comparison of study variables and electrocardiography measurements on initial electrocardiograms in those with and without arrhythmias
| Controls (n = 117) | Cases (n = 39) | Ventricular tachycardia (n = 20) | |
| Age (years) | 32 (14–71) | 31 (15–49) | 34 (18–49) |
| Male (%) | 46 | 41 | 30 |
| Amount (mg) | 2250 (450–10,000) | 2325 (500–6250) | 2375 (1000–6250) |
| Heart rate (beats/min) | 101 (62–151) | 111 (68–176) | 115 (68–163) |
| QRS (ms) | 100 (70–160) | 115 (80–205) | 120 (80–205) |
| QT (ms) | 390 (280–537) | 396 (255–550) | 400 (255–540) |
| QTc | 500 (404–735) | 538 (420–693) | 554 (420–693) |
| QT dispersion | 51 (10–190) | 67 (28–150) | 65 (36–150) |
| Corrected QT dispersion | 16 (3–55) | 20 (9–65) | 20 (11–43) |
| JT dispersion | 50 (20–154) | 63 (30–163) | 63 (30–160) |
| R wave in aVR (ms) | 1 (0–42) | 3.9 (0–61) | 5.5 (0–61) |
| R/S wave ratio in aVR | 0.07 (0–1.72) | 0.4 (0–1.97) | 0.52 (0–1.74) |
Values presented as median (range) except for gender.
Table 2.
Unadjusted odds ratios for each electrocardiography measurement from conditional logistic regression
| Odds ratio | 95% confidence interval | |
| Heart rate (beats/min) | 1.28 | 1.07–1.53 |
| QRS (ms) | 1.36 | 1.13–1.62 |
| QT (ms) | 1.04 | 0.98–1.11 |
| QTc | 1.08 | 1.02–1.14 |
| QT dispersion | 1.12 | 1.00–1.26 |
| Adjusted QT dispersion | 1.58 | 1.08–2.32 |
| JT dispersion | 1.12 | 1.00–1.25 |
| R wave in aVR (mm)a | 1.62 | 1.16–2.24 |
| R/S ratio in aVRb | 3 | 1.66–5.40 |
Odds ratios refer to a 10-unit change. a Odds ratio refers to a 5-unit change. b Odds ratio refers to a 0.5-unit change.
Table 3.
Relationship between dichotomised variables, based on measurements of initial electrocardiography and 'arrhythmia'
| Cases (%)(n = 39) | Controls (%)(n = 117) | Odds ratio (95% confidence interval) | Adjusted odds ratio (95% confidence interval) | |
| Heart rate > 100 beats/min | 64 | 51 | 1.68 (0.80–3.52) | 1.44 (0.65–3.18)a |
| QRS ≥ 100 ms | 82 | 76 | 1.47 (0.57–3.79) | 0.87 (0.31–2.47)b |
| QRS >120 ms | 28 | 9 | 3.56 (1.46–8.68) | 2.02 (0.66–6.17)b |
| QRS ≥ 160 ms | 13 | 2 | 14 (1.52–129) | 5.42 (0.50–59)b |
| QT > 400 ms | 38 | 35 | 1.17 (0.54–2.51) | 0.57 (0.23–1.43)a |
| QTc > 500 | 74 | 50 | 3.07 (1.33–7.07) | 1.48 (0.57–3.87)a |
| R wave in aVR > 3 mm | 56 | 29 | 33 (3.17–921)c | 13.0 (1.64–102)a |
| R/S ratio in aVR > 0.7 | 28 | 3 | 16 (3.47–74) | 8.88 (1.73–45)a |
| QT dispersion > 60 ms | 79 | 62 | 2.67 (1.03–6.91) | 1.50 (0.56–4.00)a |
a Adjusted for QRS. b Adjusted for QTc. c Exact confidence interval.
The QRS, the QTc and the R/S ratio in aVR appeared to be most strongly related to arrhythmia, although there was no clear separation of the groups. The raw data (Figs 1,2,3) indicate that the relationship may be stronger when TCAs were ingested as compared with thioridazine ingestion, but the number of thioridazine cases was too small to make firm conclusions. Excluding cases and controls with thioridazine overdose and repeating the analysis for TCA overdoses alone gave increased ORs for QRS > 120 ms (OR, 4.83; 95% confidence interval [CI], 1.73–13.5) and QTc > 500 (OR, 4.5; 95% CI, 1.56–13) but had little effect on that for the R/S ratio in aVR > 0.7 (OR, 14.5; 95% CI, 3.10–68). The ECG findings in patients with VT were not markedly different from those for all cases except for the relationship between QRS duration and arrhythmia, which may be stronger for TCA overdoses and wide complex tachycardia (Fig. 1).
Figure 1.
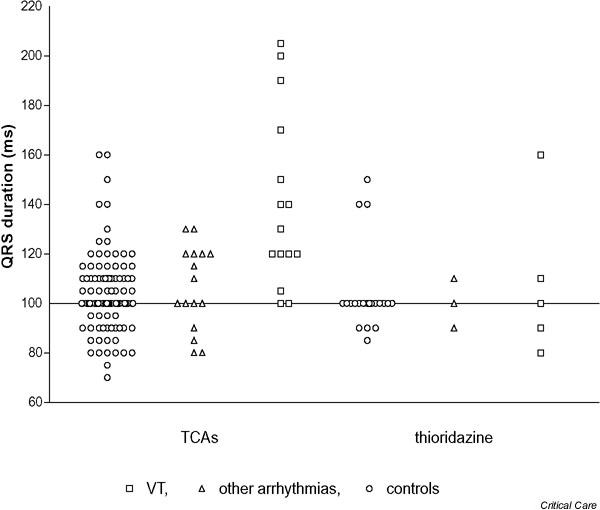
QRS duration on initial electrocardiography according to whether patients developed arrhythmias after tricyclic antidepressant (TCA) overdose or thioridazine overdose. VT, ventricular tachycardia.
Figure 2.
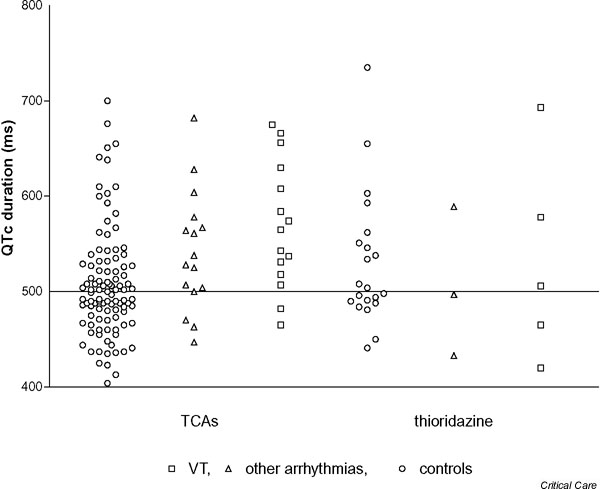
QTc duration on initial electrocardiography according to whether patients developed arrhythmias after tricyclic antidepressant (TCA) overdose or thioridazine overdose. VT, ventricular tachycardia.
Figure 3.
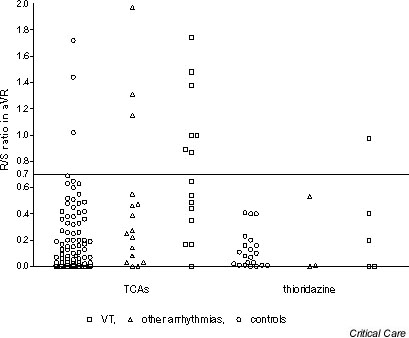
R/S ratio in aVR on initial electrocardiography according to whether patients developed arrhythmias after tricyclic antidepressant (TCA) overdose or thioridazine overdose. VT, ventricular tachycardia.
Discussion
In the present study we have shown that significant arrhythmias are an uncommon complication of TCA overdose or antipsychotic drug overdose. A large number of ECG findings are significantly related to this life-threatening complication. However, none of the previously suggested cutpoints were sensitive enough or specific enough to use as the sole method of risk determination. As part of an overall assessment of patients in the emergency setting, the QRS, the QTc and the terminal 40 ms right axis deviation (height of the R wave and R/S ratio in aVR) appear most strongly related to the risk of arrhythmias. They also appear to be more strongly related for TCA overdoses than for thioridazine overdoses. The calculated predictive values, presented in Table 4, illustrate the limited utility of even these ECG findings. The QT and JT dispersions, although related to the development of arrhythmias, were not significantly better than the other measures and are considerably more difficult to measure.
Table 4.
Calculated positive and negative predictive values for arrhythmia from electrocardiography cutpoints based on incidence of arrhythmias in significant tricyclic antidepressant poisonings (seizures, coma or ingested dose > 1.5 g) in Newcastle (7%)
| Positive predictive value (%) | Negative predictive value (%) | Likelihood ratio | |
| QRS ≥ 100 ms | 7.5 | 94.7 | 1.08 |
| QRS > 120 ms | 19 | 94.4 | 3.11 |
| QRS ≥ 160 ms | 32.8 | 93.7 | 6.5 |
| QTc (Bazett's) > 500 | 10.0 | 96.2 | 1.48 |
| R wave in aVR > 3 mm | 12.7 | 95.5 | 1.93 |
| R/S ratio in aVR > 0.7 | 41.2 | 94.7 | 9.33 |
It is difficult to compare our data directly with other data that have been published. Most authors have studied less serious outcomes and included less than 10 patients with life-threatening arrhythmias. Nevertheless, Boehnert and Lovejoy stated that "a QRS < 100 ms indicated a negligible risk of seizures or arrhythmias, a QRS ≥ 100 ms and < 160 ms indicated a moderate risk of seizures but a negligible risk of arrhythmias and a QRS ≥ 160 ms indicated a high risk of both complications" [6]. This has been widely quoted (and misquoted, as > 100 ms and 160 ms rather than ≥ 100 ms and 160 ms) [33,34], although not replicated by ourselves or other workers [8,11,15,17].
The present study provides further evidence that the R/S ratio in aVR may be a better predictor of complications [8,11,15,17]. Examination of the adjusted ORs indicates that many of the ECG findings may reflect the same underlying electrophysiological disturbance. The R/S ratio in aVR was the only ECG measurement that remained statistically significantly associated with arrhythmias after adjustment for another ECG measurement (QRS). It was suggested by the authors who described this association that the right bundle may be more susceptible to TCA-induced conduction block [13]. Thus, the increased R/S ratio in aVR may be providing additional information about the nature of electrophysiologic disturbance.
The strengths of our study include larger numbers of patients with significant arrhythmias, the use of blinded, standardised observations and our analysis of previously suggested thresholds. There are, however, some limitations of our study. We used a matched case–control design, in contrast to most previous studies that have examined cohorts. This was to allow us to perform a very detailed examination of each ECG, which would not have been practical with more than 1000 controls. This has allowed us to compare the performance of the different measurements and thresholds with much higher precision and control for drugs ingested. However, it means that the relatively poor sensitivity, specificity and predictive values presented in Table 4 are calculations where the controls are assumed to be typical of all patients ingesting these drugs without arrhythmias. This may be an overly conservative assumption as the rate of arrhythmias varies substantially between these drugs [4,25], and we did not include minor poisonings in our control group.
The use of these ECG predictors in patients who take closely related drugs such as thioridazine appears rational. These phenothiazines are also weak bases with a tricyclic structure; they block sodium and potassium channels in the heart and cause early after-depolarisations in animal studies [26,35], and they cause similar ECG changes and ventricular arrhythmias in overdose [25]. However, our study provides no support for this practice. The raw data illustrated in Fig 1,2,3 again illustrate that ECG abnormalities are common in thioridazine overdose, but these do not appear to show any significant correlation with the occurrence of arrhythmias. This surprising finding should ideally be confirmed in another larger study focusing solely on this drug group.
The accuracy of the ECG must be influenced by the time from ingestion. An ECG performed shortly after TCA ingestion is much more likely to be normal than an ECG 4 hours after ingestion. It is often recommended to perform ECGs more than once, but this clinical practice has not been assessed in any studies. Moreover, neither our study nor any previous studies have been large enough to evaluate the effect of elapsed time between the TCA ingestion and the ECG criteria.
Future studies will ideally evaluate a strategy that more closely resembles current expert clinical practice; for example, evaluating the best ECG predictors in combination at two different times. This should be tested in much larger, prospective cohort studies to see whether a strategy can be found that is sufficiently sensitive and yet is able to safely identify the majority of patients who are not at significant risk of arrhythmia. We would suggest that a good starting point would be to use the cutpoints with the highest likelihood ratios from our study (QRS > 120 ms, QTc > 500 and R/S ratio in aVR > 0.7) at defined times (e.g. on admission and 2 hours later). Only those without any abnormalities at any time would be considered low risk. Other patients would be monitored until their ECG abnormalities resolve, although the risk of arrhythmias occurring for the first time after 24 hours of monitoring is very low.
Using measures that are capable of being measured by an ECG machine in the emergency department would simplify matters greatly for such a study, which would need to be conducted in multiple centres to be completed within a feasible time frame. However, this would probably require purpose-built software for the R/S ratio in aVR, the measurement with the best predictive performance.
Implications for management
Our results show that there is no sufficiently good predictor(s) that can be applied to only the initial ECG to identify patients that should have continuous electrocardiographic monitoring for arrhythmias. The calculated negative predictive values (Table 4) mean that we would expect 4–6% of unmonitored patients to have arrhythmias, and this could not be considered acceptable. We would recommend a strategy similar to that we propose for a prospective trial. The trial should bear in mind that such a strategy, although often recommended, has never been validated. A more conservative strategy may be appropriate for other proarrhythmic drugs until more data are available on prediction for other drug classes. Based on the positive predictive values, specific treatment to prevent arrhythmias, such as systemic alkalinisation, would be justified for all patients with QRS > 120 ms or R/S ratio > 0.7 in aVR. As hypoxia and acidosis increase cardiac excitability directly, and seizures often precede cardiac arrests and arrhythmias [4,8], patients with hypoxia, acidosis, seizures or other significant noncardiac complications also should receive specific treatment.
Key messages
• Serious arrhythmias after TCA and thioridazine overdose occur in a small but significant minority of patients. The initial ECG is often used to predict the need for monitoring
• A QRS > 120 ms, QTc > 500 and an increased R/S ratio in aVR on the first ECG have the strongest association with the occurrence of arrhythmia in TCA overdose. However, predictive values have unacceptably high false positive and negative rates
• These measures had a much weaker association with arrhythmia in thioridazine than in TCA overdose (although numbers were small). Any predictive strategy needs validation in each drug class where it is applied
• We believe a more complex algorithm factoring in multiple measurements, multiple ECGs and the effect of time may provide a much more accurate approach to predicting the need for monitoring in TCA overdose
Competing interests
None declared.
Abbreviations
CI = confidence interval; ECG = electrocardiography; OR = odds ratio; SVT = supraventricular tachycardia; TCA = tricyclic antidepressant; VT = ventricular tachycardia.
References
- Crome P. The toxicity of drugs used for suicide. Acta Psychiatr Scand Suppl. 1993;371:33–37. doi: 10.1111/j.1600-0447.1993.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Buckley NA, Whyte IM, Dawson AH, McManus PR, Ferguson NW. Correlations between prescriptions and drugs taken in self-poisoning. Implications for prescribers and drug regulation. Med J Aust. 1995;162:194–197. [PubMed] [Google Scholar]
- Callaham M, Kassel D. Epidemiology of fatal tricyclic antidepressant ingestion: implications for management. Ann Emerg Med. 1985;14:1–9. doi: 10.1016/s0196-0644(85)80725-3. [DOI] [PubMed] [Google Scholar]
- Buckley NA, Dawson AH, Whyte IM, Henry DA. Greater toxicity in overdose of dothiepin than of other tricyclic antidepressants. Lancet. 1994;343:159–162. doi: 10.1016/s0140-6736(94)90940-7. [DOI] [PubMed] [Google Scholar]
- Strom J, Sloth MP, Nygaard NN, Bredgaard SM. Acute self-poisoning with tricyclic antidepressants in 295 consecutive patients treated in an ICU. Acta Anaesthesiol Scand. 1984;28:666–670. doi: 10.1111/j.1399-6576.1984.tb02142.x. [DOI] [PubMed] [Google Scholar]
- Boehnert MT, Lovejoy FH., Jr Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313:474–479. doi: 10.1056/NEJM198508223130804. [DOI] [PubMed] [Google Scholar]
- Thorstrand C. Clinical features in poisonings by tricyclic antidepressants with special reference to the ECG. Acta Med Scand. 1976;199:337–344. doi: 10.1111/j.0954-6820.1976.tb06745.x. [DOI] [PubMed] [Google Scholar]
- Liebelt EL, Francis PD, Woolf AD. ECG lead aVR versus QRS interval in predicting seizures and arrhythmias in acute tricyclic antidepressant toxicity. Ann Emerg Med. 1995;26:195–201. doi: 10.1016/s0196-0644(95)70151-6. [DOI] [PubMed] [Google Scholar]
- Hulten BA, Heath A. Clinical aspects of tricyclic antidepressant poisoning. Acta Med Scand. 1983;213:275–278. doi: 10.1111/j.0954-6820.1983.tb03733.x. [DOI] [PubMed] [Google Scholar]
- Foulke GE. Identifying toxicity risk early after antidepressant overdose. Am J Emerg Med. 1995;13:123–126. doi: 10.1016/0735-6757(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Foulke GE, Albertson TE. QRS interval in tricyclic antidepressant overdosage: inaccuracy as a toxicity indicator in emergency settings. Ann Emerg Med. 1987;16:160–163. doi: 10.1016/s0196-0644(87)80006-9. [DOI] [PubMed] [Google Scholar]
- Foulke GE, Albertson TE, Walby WF. Tricyclic antidepressant overdose: emergency department findings as predictors of clinical course. Am J Emerg Med. 1986;4:496–500. doi: 10.1016/S0735-6757(86)80002-X. [DOI] [PubMed] [Google Scholar]
- Niemann JT, Bessen HA, Rothstein RJ, Laks MM. Electrocardiographic criteria for tricyclic antidepressant cardiotoxicity. Am J Cardiol. 1986;57:1154–1159. doi: 10.1016/0002-9149(86)90691-0. [DOI] [PubMed] [Google Scholar]
- Bessen HA, Niemann JT. Improvement of cardiac conduction after hyperventilation in tricyclic antidepressant overdose. J Toxicol Clin Toxicol. 1985;23:537–546. doi: 10.3109/15563658508990655. [DOI] [PubMed] [Google Scholar]
- Wolfe TR, Caravati EM, Rollins DE. Terminal 40-ms frontal plane QRS axis as a marker for tricyclic antidepressant overdose. Ann Emerg Med. 1989;18:348–351. doi: 10.1016/s0196-0644(89)80566-9. [DOI] [PubMed] [Google Scholar]
- Caravati EM. The electrocardiogram as a diagnostic discriminator for acute tricyclic antidepressant poisoning. J Toxicol Clin Toxicol. 1999;37:113–115. doi: 10.1081/clt-100102510. [DOI] [PubMed] [Google Scholar]
- Caravati EM, Bossart PJ. Demographic and electrocardiographic factors associated with severe tricyclic antidepressant toxicity. J Toxicol Clin Toxicol. 1991;29:31–43. doi: 10.3109/15563659109038595. [DOI] [PubMed] [Google Scholar]
- Marshall JB, Forker AD. Cardiovascular effects of tricyclic antidepressant drugs: therapeutic usage, overdose, and management of complications. Am Heart J. 1982;103:401–414. doi: 10.1016/0002-8703(82)90281-2. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Capone RJ, Hunt JD. Cardiac complications following tricyclic antidepressant overdose. Issues for monitoring policy. JAMA. 1985;254:1772–1775. [PubMed] [Google Scholar]
- Berkovitch M, Matsui D, Fogelman R, Komar L, Hamilton R, Johnson D. Assessment of the terminal 40-millisecond QRS vector in children with a history of tricyclic antidepressant ingestion. Pediatr Emerg Care. 1995;11:75–77. doi: 10.1097/00006565-199504000-00004. [DOI] [PubMed] [Google Scholar]
- Wedin GP, Oderda GM, Klein-Schwartz W, Gorman RL. Relative toxicity of cyclic antidepressants. Ann Emerg Med. 1986;15:797–804. doi: 10.1016/s0196-0644(86)80375-4. [DOI] [PubMed] [Google Scholar]
- Crome P. Tricyclic antidepressant poisoning. Br Med J. 1979;1:1080–1081. doi: 10.1136/bmj.1.6170.1080-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires RF, Saederup E. Mono N-aryl ethylenediamine and piperazine derivatives are GABAA receptor blockers: implications for psychiatry. Neurochem Res. 1993;18:787–793. doi: 10.1007/BF00966774. [DOI] [PubMed] [Google Scholar]
- Buckley NA, O'Connell DL, Whyte IM, Dawson AH. Interrater agreement in the measurement of QRS interval in tricyclic antidepressant overdose: implications for monitoring and research. Ann Emerg Med. 1996;28:515–519. doi: 10.1016/s0196-0644(96)70115-4. [DOI] [PubMed] [Google Scholar]
- Buckley NA, Whyte IM, Dawson AH. Cardiotoxicity more common in thioridazine overdose than with other neuroleptics. J Toxicol Clin Toxicol. 1995;33:199–204. doi: 10.3109/15563659509017984. [DOI] [PubMed] [Google Scholar]
- Studenik C, Lemmens-Gruber R, Heistracher P. Proarrhythmic effects of antidepressants and neuroleptic drugs on isolated, spontaneously beating guinea-pig Purkinje fibers. Eur J Pharm Sci. 1999;7:113–118. doi: 10.1016/s0928-0987(98)00013-x. [DOI] [PubMed] [Google Scholar]
- van de Loo A, Arendts W, Hohnloser SH. Variability of QT dispersion measurements in the surface electrocardiogram in patients with acute myocardial infarction and in normal subjects. Am J Cardiol. 1994;74:1113–1118. doi: 10.1016/0002-9149(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Leitch J, Basta M, Dobson A. QT dispersion does not predict early ventricular fibrillation after acute myocardial infarction. Pacing Clin Electrophysiol. 1995;18:45–48. doi: 10.1111/j.1540-8159.1995.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- Griffith MJ, Garratt CJ, Mounsey P, Camm AJ. Ventricular tachycardia as default diagnosis in broad complex tachycardia. Lancet. 1994;343:386–388. doi: 10.1016/s0140-6736(94)91223-8. [DOI] [PubMed] [Google Scholar]
- Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- Day CP, McComb JM, Campbell RW. QT dispersion in sinus beats and ventricular extrasystoles in normal hearts. Br Heart J. 1992;67:39–41. doi: 10.1136/hrt.67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumack BH, Hess AJ, Gelmen CR. Poisindex (R) System. Englewood, CO: Micromedex Inc; 2001. [Google Scholar]
- Weisman RS, Howland MA, Hoffman RS, Cohen H. Goldfrank's Toxicologic Emergencies. 5. New York: McGraw-Hill; 1994. Cyclic antidepressants. [Google Scholar]
- Ogata N, Nishimura M, Narahashi T. Kinetics of chlorpromazine block of sodium channels in single guinea pig cardiac myocytes. J Pharmacol Exp Ther. 1989;248:605–613. [PubMed] [Google Scholar]


