Abstract
Acute Otitis Media occurs mostly after upper respiratory tract infection; the causative bacteria are those colonized in the nasopharynx. We studied 709 URI episodes and found that children with no bacteria in NP were at low risk while those with three pathogenic bacteria were at the greatest risk for AOM.
Keywords: Otitis Media, Nasopharyngeal Bacterial Colonization, Upper Respiratory Tract Infection
BACKGROUND
Acute Otitis Media (AOM) is one of the most common pediatric infectious diseases. Although the disease is primarily considered a bacterial infection, it is well known that viral upper respiratory tract infections (URI) predispose children to AOM and viruses alone can cause AOM [1]. In other words, AOM often occurs as a complication of URI. Respiratory virus infection disrupts the mucociliary system impairing the host’s primary mechanical defense from bacterial invasion. Viral infection also causes Eustachian tube dysfunction leading to reduced middle ear pressure, forcing mucus, nasopharyngeal secretions, and bacteria colonized in the nasopharynx (NP) into the middle ear [2].
Three most common AOM pathogenic bacteria are Streptococcus pneumoniae, Non-typeable (NT)-Haemophilus influenzae, and Moraxella catarrhalis. By 6 months of age healthy children are colonized with one or more of these three pathogens[3]. Children colonized with AOM pathogens at a young age (less than 3 months) are at two times greater risk of having AOM by 6 months of age [3]. Children are more likely to have AOM pathogenic bacteria cultured from their NP at the time of AOM diagnosis[4]. Nasopharyngeal bacterial colonization (NPC) during healthy state is different than NPC at the time of URI [5] and AOM [6]. Previous studies have compared NPC during health state and URI or AOM, but have not followed children for URI onset to the development of AOM complication. We studied the relationship between NPC during URI and subsequent AOM occurrence to determine if NPC data at URI onset can be used to predict the occurrence of AOM complicating URI.
METHODS
This study is a secondary analysis of data collected from January 2003 to March 2007 at the University of Texas Medical Branch, Galveston (UTMB) during a prospective, longitudinal study of virus-induced AOM (Chonmaitree-unpublished data). The primary study was designed to capture all URI episodes occurring during a one-year period in healthy children aged 6 to 35 months to study the rate and characteristics of AOM following URI. The study was approved by the UTMB Institutional Review Board. At enrollment, demographic and AOM risk factor information was collected. Parents were asked to notify the study office as soon as the child began to have a cold or URI symptoms (nasal congestion, rhinorrhea, cough, sore throat, or fever). Children were seen by a study physician as soon as possible after the onset of URI symptoms, followed a few days later (days 3–7 of the URI) and monitored closely for 3 weeks for AOM development.
AOM complicating URI was considered when the episode occurred within 21 days of the URI. AOM was defined by acute onset of symptoms (fever, irritability, or earache), signs of inflammation of the tympanic membrane and presence of fluid in the middle ear documented by pneumatic otoscopy and/or tympanometry. Children diagnosed with AOM were observed or given antibiotic therapy consistent with standard of care [7].
Included in this study were URI episodes for which the child was seen by the study physician and had NP swabs collected within 7 days of URI onset. Excluded from the analysis were: URI episodes with NP cultures taken within 7 days of antibiotic therapy and AOM episodes without preceding URI.
NP samples for bacterial cultures were collected at enrollment, during the first visit of each URI episode and at the time of AOM diagnosis using Mini-Tip Culturette® kits (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA). The specimens were submitted for routine bacterial cultures on blood and chocolate agar plates. Isolates of S. pneumoniae were identified by using the optochin disk susceptibility test (Taxo P, Becton Dickinson Microbiology Systems), S. pneumoniae isolates were not serotyped. M. catarrhalis by the API QuadFerm assay (bioMerieux, Inc., Hazelwood, MO, USA), and NT- H. influenzae by the Haemophilus ID Quad Plate with Growth Factors (Becton Dickinson Microbiology Systems).
Chi-square analysis was performed using STATA 9.0 © (Stata corporation, College Station, TX) statistical software. Logistic Regression modeling was performed using SAS 9.1 (SAS Institute Inc, Cary, NC) statistical software.
RESULTS
During the study period, there were a total of 1295 URI episodes documented in 294 patients enrolled and followed in our study. Of the total URI episodes, 867 were seen by the study group; 709 URI episodes from 198 patients met the inclusion criteria for this analysis. Of 198 patients, 49% were male, 58% Caucasian, 29% Black, 10% Bi-racial, 3% Asian. Forty-three percent were of Hispanic or Latino ethnicity. Median age at enrollment was 12 mos.
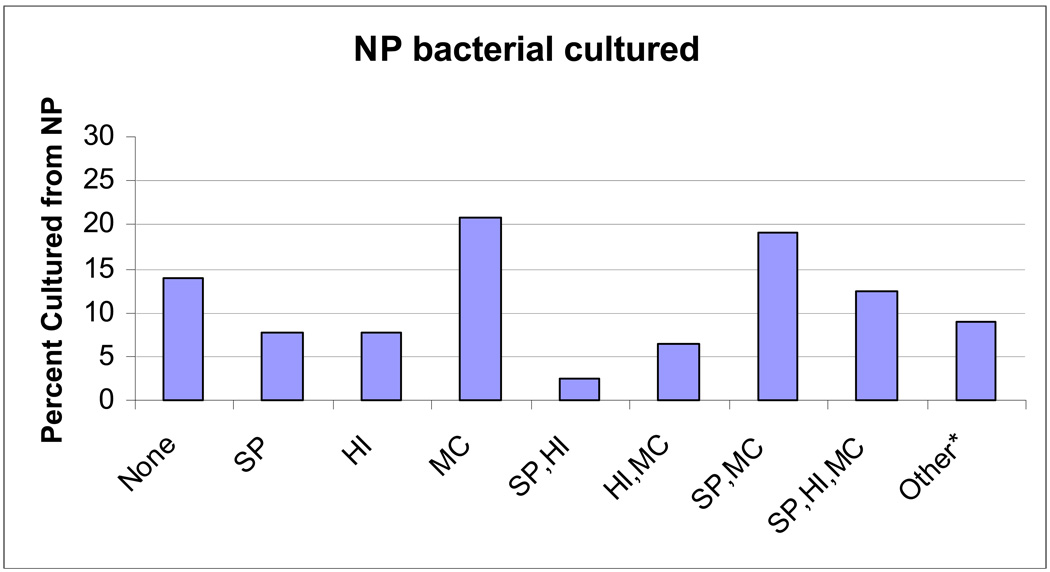
NP culture was positive for pathogenic bacteria in 607 (85.6%) of cases. Seventy-five percent of the cultures were performed on or before day 4 of illness. The proportion of AOM pathogenic bacteria isolated from the nasopharynx at the time of URI is shown in Figure 1. S. pneumoniae was isolated alone and in combination during 209 episodes (49%), NT- H. influenzae, 205 (34%) and M. catarrhalis, 417 (69%).
Figure 1.
AOM pathogenic bacteria isolated from the nasopharynx at the time of URI. *Other bacteria included Staphylococcus aureus, Streptococcus pyogenes, Streptococcus agalactiae, Diptheriods, and Bacillus species.
Abbreviations: Sp: Streptococcus pneumoniae, Hi: Haemophilus influenzae, Mc: Moraxella catarrhalis.
Two hundred forty-seven URI episodes (35%) were complicated by AOM (93 of them were bilateral). AOM diagnosis peaked on day 3 and 85% were within 7 days of URI onset. The odds ratios (OR) showed increased risk of AOM when NP were colonized with pathogenic bacteria (S. pneumoniae, NT- H. influenzae or M. catarrhalis), compared to no pathogen (Table 1a). AOM rates were adjusted for breastfeeding, day care attendance and cigarette smoke exposure. The three pathogenic bacteria either alone or in combination were more likely to be isolated from the NP in children with AOM than from those without AOM; S. pneumoniae: OR 1.8 (1.3–2.6, p<0.001), NT- H. influenzae: OR 2.2 (1.6–3.1, p<0.001)or M. catarrhalis: OR 1.9 (1.4–2.8, p<0.001). Of the AOM diagnosed, 168 (68%) were diagnosed on the day of NP swab collection. To determine the predictive value of NP bacterial culture on AOM occurrence prior to AOM diagnosis, we excluded the URI episodes for which AOM was diagnosed at the time of NP swab collection. In this subset of data (Table 1b), odd ratios and adjusted odds ratios were similar, however, reduced numbers changed the significant differences in a few categories. Overall, compared to a child with no bacteria in the NP, a child with two or more bacteria in the NP had 2.6 greater odds (95% CI 1.8 – 3.8, p< 0.001) of having AOM after controlling for breastfeeding, daycare attendance, cigarette smoke exposure and number of PCV7 doses (Data not shown).
Table 1.
| Table 1a. Risk of AOM Complicating URI by Pathogenic Bacteria Colonized in the Nasopharynx at the Time of URI. | |||||
|---|---|---|---|---|---|
| NP Bacterial colonization (n=646a) |
AOM Incidence (%) |
OR, (95% CI) |
p-value | Adjusted OR,b (95% CI) |
p-value |
| No Bacteria (102) | 10 | REF | REF | REF | REF |
| Sp only (55) | 29 | 3.7 (1.6–9.0) | <0.01 | 3.8 (1.6–9.1) | <0.01 |
| Hi only (54) | 43 | 6.8 (2.9–15.9) | <0.01 | 6.7 (2.8–15.9) | <0.01 |
| Mc only (148) | 32 | 4.4 (2.1–9.2) | <0.01 | 4.2 (2.0–8.8) | <0.01 |
| Sp, Hi (18) | 50 | 9.2 (3.0–28.5) | <0.01 | 8.0 (2.1–30.0) | <0.01 |
| Sp, Mc (136) | 41 | 6.4 (3.1–13.4) | <0.01 | 5.7 (2.7–12.2) | <0.01 |
| Hi, Mc (45) | 51 | 9.6 (4.0–23.0) | <0.01 | 8.4 (3.2–21.5) | <0.01 |
| Sp, Hi, Mc (88) | 51 | 9.6 (4.4–20.9) | <0.01 | 15.3 (6.0–39.6) | <0.01 |
| Table 1b. Risk of AOM Complicating URI by Pathogenic Bacteria Colonized in the Nasopharynx at the Time of URI (excluding NP swabs collected at the time of AOM diagnosis). | |||||
|---|---|---|---|---|---|
| NP Bacterial colonization (541a) |
AOM incidence % |
OR, (95% CI) |
p-value | Adjusted OR,b (95% CI) |
p-value |
| No Bacteria (97) | 5 | REF | REF | REF | REF |
| Sp only (46) | 15 | 3.3 (0.99 – 11.0) | 0.04 | 3.5 (1.0 – 12.3) | 0.05 |
| Hi only (39) | 21 | 4.7 (1.4–15.6) | <0.01 | 5.2 (1.5–17.9) | <0.01 |
| Mc only (119) | 16 | 3.5 (1.2–9.7) | <0.01 | 3.7 (1.3–10.3) | 0.01 |
| Sp, Hi (11) | 18 | 4.1 (0.7 – 24.2) | 0.09 | 10.5 (1.1 – 101.8) | 0.04 |
| Sp, Mc (96) | 17 | 3.7 (1.3–10.5) | 0.01 | 3.7 (1.2–10.9) | 0.02 |
| Hi, Mc (26) | 15 | 3.3 (0.8–13.4) | 0.07 | 3.6 (0.8–16.3) | 0.1 |
| Sp, Hi, Mc (53) | 19 | 4.3 (1.4–13.3) | <0.01 | 7.5 (1.9–30.4) | <0.01 |
63 cultures that grew other bacteria including Staphylococcus aureus, Group A beta helmolytic Streptococcus, Group B beta hemolytic Streptococcus, Diptheriods, and Bacillus species are not included. Multiple bacteria listed are from the same sample. In 168 (68%) cases AOM was diagnosed at the time of NP swab collection.
OR adjusted for breastfeeding, smoking and day care exposure
Abbreviations: OR: odd ratio, REF: reference rate for odds ratio, Sp: Streptococcus pneumoniae, Hi: Haemophilus influenzae, Mc: Moraxella catarrhalis.
57 cultures that grew other bacteria including Staphylococcus aureus, Group A beta helmolytic Streptococcus, Diptheriods, and Bacillus species are not included. Multiple bacteria listed are from the same sample.
OR adjusted for breastfeeding, smoking and day care exposure
Abbreviations: OR: odd ratio, REF: reference rate for odds ratio, Sp: Streptococcus pneumoniae, Hi: Haemophilus influenzae, Mc: Moraxella catarrhalis.
DISCUSSION
Of 709 URI episodes included in this study, approximately one-third resulted in AOM. We obtained NP cultures early in the course of URI and clearly showed what had long been speculated; presence of pathogenic bacteria in the NP during URI increases the risk for AOM complication. Children colonized with S. pneumoniae, NT- H. influenzae, and M. catarrhalis concurrently were at the highest risk for AOM, compared to children with no pathogenic bacteria in their NP. Our data suggest that NPC results at the early URI onset may be helpful in predicting the risk of AOM complicating URI.
NPC is dynamic and the rate of NP flora turnover may differ among children and among bacterial strains. [8] Up to 90% of children in day care carry AOM pathogens in their NP at some point. [9] OM non-prone children have the same rate of NPC as OM-prone children when healthy.[10] NPC changes as children progress from a healthy state through to URI or AOM. Harrison et at [5] found that during URI children have more bacterial types and higher bacterial colony counts in the NP. Syruanen et al [6] found that 87% of healthy S. pneumoniae carriers has S. pneumoniae at the time of AOM and only 26% of the non carriers has S. pneumoniae at the time of AOM. Interestingly, the majority of S. pneumoniae associated AOM were due to newly acquired S. pneumoniae strains, not the one found during the health state. This group found that S. pneumoniae AOM occurred 3.8 times more often if the child had newly acquired carriage than if they had an established one. These data demonstrate that in order to better understand the causal relationship between NPC and AOM, NP cultures should be obtained closer to the onset of AOM, ideally during URI.
Children in our study were seen and NP cultures obtained as early as possible after the onset of URI symptoms, usually within 2–5 days. Because the peak incidence of AOM complicating URI is also between 2–5 days of URI onset [11–13], AOM was already diagnosed at the initial visit in 68% of cases. Nevertheless, we were still able to compare NP cultures in 32% of children with URI prior to AOM development (cases diagnosed later in the first week through the third week of URI onset) to children with URI who never developed AOM. There was significant correlation between positive NPC with multiple pathogenic bacteria and AOM occurrence even after exclusion of cases diagnosed AOM at the time of NP culture collection.
There has been some evidence to suggest that children who have been immunized with PCV7 are more likely to have Staphylococcus aureus in the NP [14]. In our study 57 (8%) NP cultures grew S. aureus alone or in combination with other bacteria. There was no difference based PCV7 vaccination status.
It has now been accepted that viruses play a major role in the pathogenesis of AOM [1,15] and, AOM occurs mostly as a bacterial complication of viral URI. Viruses alone may also cause AOM [15]; this could be the case in AOM for which there was no bacteria colonized in NP in our study. In general, both the URI causative virus and the colonized bacteria together play major roles in AOM pathogenesis [1]. Therefore, effective prevention of AOM will need to include both prevention of viral URI and prevention and/or elimination of NPC with pathogenic bacteria. Further studies are required to better understand how these interventions effect bacterial and viral interactions in the pathogenesis of viral-induced AOM. Such understanding will lead to better ways for effective prevention of this highly prevalent pediatric disease.
Acknowledgements
We would like to thank M. Lizette Rangel, Kyralessa B. Ramirez, Liliana Najera, Rafael Serna, Michelle Tran and Syed Ahmad for their assistance with study subjects. This work was supported by the National Institutes of Health grants R01 DC005841, and DC 005841-02S1 (both to T.C.). The study was conducted at the General Clinical Research Center at the University of Texas Medical Branch at Galveston, funded by grant M01 RR 00073 from the National Center for Research Resources, NIH, USPHS.
Footnotes
Reprints not available
Presented in part at the 9th International Symposium of Recent Advances in Otitis Media, St. Pete Beach, FL June 3–7th 2007.
None of the Authors have any conflicts of interest to disclose
References
- 1.Chonmaitree T, Heikkinen T. Viruses and acute otitis media. Pediatr Infect Dis J. 2000;19:1005–1007. doi: 10.1097/00006454-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Bakaletz LO. Viral potentiation of bacterial superinfection of the respiratory tract. Trends Microbiol. 1995;3:110–114. doi: 10.1016/s0966-842x(00)88892-7. [DOI] [PubMed] [Google Scholar]
- 3.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 4.Faden H, Stanievich J, Brodsky L, Bernstein J, Ogra PL. Changes in nasopharyngeal flora during otitis media of childhood. Pediatr Infect Dis J. 1990;9:623–626. [PubMed] [Google Scholar]
- 5.Harrison LM, Morris JA, Telford DR, Brown SM, Jones K. The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position. FEMS Immunol Med Microbiol. 1999;25:19–28. doi: 10.1111/j.1574-695X.1999.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 6.Syrjanen RK, Auranen KJ, Leino TM, Kilpi TM, Makela PH. Pneumococcal acute otitis media in relation to pneumococcal nasopharyngeal carriage. Pediatr Infect Dis J. 2005;24:801–806. doi: 10.1097/01.inf.0000178072.83531.4f. [DOI] [PubMed] [Google Scholar]
- 7.Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 8.Trottier S, Stenberg K, Svanborg-Eden C. Turnover of nontypable Haemophilus influenzae in the nasopharynges of healthy children. J Clin Microbiol. 1989;27:2175–2179. doi: 10.1128/jcm.27.10.2175-2179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda K, Masuda R, Nishi J, Tokuda K, Yoshinaga M, Miyata K. Incidences of nasopharyngeal colonization of respiratory bacterial pathogens in Japanese children attending day-care centers. Pediatr Int. 2002;44:376–380. doi: 10.1046/j.1442-200x.2002.01587.x. [DOI] [PubMed] [Google Scholar]
- 10.Prellner K, Christensen P, Hovelius B, Rosen C. Nasopharyngeal carriage of bacteria in otitis-prone and non-otitis-prone children in day-care centres. Acta Otolaryngol. 1984;98:343–350. doi: 10.3109/00016488409107572. [DOI] [PubMed] [Google Scholar]
- 11.Heikkinen T. Temporal development of acute otitis media during upper respiratory tract infection. Pediatr Infect Dis J. 1994;13:659–661. doi: 10.1097/00006454-199407000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Koivunen P, Kontiokari T, Niemela M, Pokka T, Uhari M. Time to development of acute otitis media during an upper respiratory tract infection in children. Pediatr Infect Dis J. 1999;18:303–305. doi: 10.1097/00006454-199903000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Revai K, Dobbs LA, Nair S, Patel JA, Grady JJ, Chonmaitree T. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119:e1408–e1412. doi: 10.1542/peds.2006-2881. [DOI] [PubMed] [Google Scholar]
- 14.Bogaert D, van Belkum A, Sluijter M, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. The Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 15.Chonmaitree T. Viral and bacterial interaction in acute otitis media. Pediatr Infect Dis J. 2000;19:S24–S30. doi: 10.1097/00006454-200005001-00005. [DOI] [PubMed] [Google Scholar]