Abstract
Sulfated polysaccharides from the egg jelly of sea urchins act as species-specific inducers of the sperm acrosome reaction, which is a rare molecular mechanism of carbohydrate-induced signal-transduction event in animal cells. The sea urchin polysaccharides differ in monosaccharide composition (l-fucose or l-galactose), glycosylation, and sulfation sites, but they are always in the α-anomeric configuration. Herein, structural analysis of the polysaccharide from the sea urchin Glyptocidaris crenularis surprisingly revealed a unique sulfated β-d-galactan composed by (3-β-d-Galp-2(OSO3)-1→3-β-d-Galp-1)n repeating units. Subsequently, we used the G. crenularis galactan to compare different 2-sulfated polysaccharides as inducers of the acrosome reaction using homologous and heterologous sperm. We also tested the effect of chemically over-sulfated galactans. Intriguingly, the anomeric configuration of the glycosidic linkage rather than the monosaccharide composition (galactose or fucose) is the preferential structural requirement for the effect of these polysaccharides on sea urchin fertilization. Nuclear magnetic resonance and molecular dynamics indicate that sulfated α-galactan or α-fucan have less dynamic structural behavior, exhibiting fewer conformational populations, with an almost exclusive conformational state with glycosidic dihedral angles Φ/Ψ = −102°/131°. The preponderant conformer observed in the sulfated α-galactan or α-fucan is not observed among populations in the β-form despite its more flexible structure in solution. Possibly, a proper spatial arrangement is required for interaction of the sea urchin-sulfated polysaccharides with the specific sperm receptor.
The evolution of barriers to inter-specific hybridization is a crucial step in the fertilization of free-spawning marine invertebrates. In sea urchins the molecular recognition between sperm and egg ensures species recognition. The jelly coat surrounding sea urchin eggs is not a simple accessory structure; it is considerably complex on a molecular level and intimately involved in gamete recognition. It contains sulfated polysaccharides, sialoglycans, and peptides.
Structural changes in the sulfated polysaccharide from the egg jelly of sea urchins modulate cell-cell recognition and species specificity leading to exocytosis of the acrosomal vesicle, the acrosome reaction. This is a crucial event for the recognition between male and female gametes, leading to the fertilization success, and is also what prevents intercrosses. The sulfated polysaccharide from the egg jelly recognizes its specific receptor present in the sperm. Apart from the sialoglycans that act in synergy with the sulfated polysaccharides, other components of the egg jelly do not possess acrosome reaction-inducing activity (1). The sulfated polysaccharide-mediated mechanism of sperm-egg recognition co-exists with that of bindin and its receptor in the egg (2–4).
The sulfated polysaccharides from sea urchin show species-specific structures composed of repetitive units (mono-, tri-, and tetrasaccharides) that differ in the monosaccharide backbone (l-fucose or l-galactose), glycosidic linkage (3- or 4-linked), and sulfation (2- and/or 4-sulfation). However, they are always in the α-enantiomeric configuration (4, 5). Previous studies from our laboratory have demonstrated that sea urchin-sulfated polysaccharides induce the acrosome reaction in a species-specific way. In some cases the sperm from a certain species of sea urchin recognizes the sulfated polysaccharide containing a similar structure from a different species. For example, the egg jelly from Strongylocentrotus franciscanus contains a 2-sulfated, 3-linked α-fucan, but the sperm from this species recognizes a heterologous 2-sulfated, 3-linked α-galactan from Echinometra lucunter (6).
We now extended our studies to the sulfated polysaccharides of the sea urchin Glyptocidaris crenularis (7). Surprisingly, we observed that this species contains a unique sulfated β-d-galactan composed of repetitive disaccharide units alternating 2-sulfated and non-sulfated 3-linked units. This polymer is markedly distinct from all other sea urchin-sulfated polysaccharides described so far that are composed of units on α-l-configuration. Furthermore, this sea urchin does not contain sialoglycans, which are commonly found in the echinoderm egg jelly.
We used this new sulfated β-galactan to investigate the acrosome reaction in a further molecular detail using homologous and heterologous sperm. We tested three 2-sulfated polysaccharides that differ in their conformation (α or β) and monosaccharide composition (galactose or fucose) as inducers of the sperm acrosome reaction. We aimed to establish the structure versus biological activity of the echinoderm polysaccharides, including structural features at a conformational level.
EXPERIMENTAL PROCEDURES
Extraction of Sulfated Galactan from Egg Jelly of Sea Urchins
Mature females of G. crenularis were harvested by dredging in Noheji Bay, Japan. Eggs were spawned into sea water by intracelomic injection of 0.5 m KCl. The crude egg jelly was isolated by pouring eggs repeatedly through nylon mesh, prepared as a 30,000 × g supernatant, and stored at −20 °C, lyophilized, and dialyzed against distilled water (8). The acidic polysaccharides were extracted from the jelly coat by papain digestion and partially purified by ethanol precipitation, as described previously (9).
Purification of Sulfated Galactan
Approximately 20 mg of the crude polysaccharides from G. crenularis was applied to a Mono Q fast protein liquid chromatography (FPLC) column (HR5/5; Amersham Biosciences) equilibrated with 20 mm Tris-HCl (pH 8.0) and coupled to a FPLC system. The column was washed with 10 ml of the same solution and then eluted with a linear gradient of 0–3 m NaCl. Fractions of 0.5 ml were collected at a flow rate of 0.45 ml/min and checked for hexose (10), sialic acid (11), and metachromatic property (12). The NaCl concentration was estimated by conductivity. Fractions were pooled, dialyzed against distilled water, and lyophilized.
Chemical Analyses
After acid hydrolysis of the polysaccharide (5.0 m trifluoroacetic acid for 5 h at 100 °C), sulfate was measured by the BaCl2/gelatin method (13). The presence of hexoses and 6-deoxyhexoses in the acid hydrolysates was estimated by paper chromatography in 1-butanol/pyridine/water (3:2:1, v/v) for 48 h. In addition, alditol acetate derivatives were analyzed by gas-liquid chromatography/mass spectrometry (14).
Determination of d or l Configuration of Galactose
The enantiomeric form of the galactose was assigned based on the analysis of the acetylated (−)-2-butyl glycoside, as described (15). Galactose obtained after acid hydrolysis of the polysaccharide from G. crenularis (1 mg, see above) was mixed with 0.5 ml of (−)-2-butanol (Aldrich) containing 1 m HCl. After butanolysis for 18 h at 80 °C, the solution was neutralized with Ag2CO3, and the supernatant was concentrated and dissolved in 50 μl of distilled water. Thereafter, alditol acetate derivative was prepared (14) and analyzed on a DB-5 GLC column. The temperature of the column was programmed to increase in a linear gradient of 120 to 240 °C at 2 °C/min. The injector and detector temperatures were 220 and 260 °C, respectively. Appropriate controls of acetylated (−)-2-butyl-d- and l-galactosides were analyzed under the same conditions.
Agarose and Polyacrylamide Gel Electrophoresis
The sulfated galactan was analyzed by agarose gel electrophoresis, as described previously (16, 17). About 15 μg of the purified sulfated polysaccharide was applied to a 0.5% agarose gel and run for 1 h at 110 V in 0.05 m 1,3-diaminopropane acetate (pH 9.0). The sulfated polysaccharides in the gel were fixed with 0.1% N-cetyl-N,N,N-trymethylammonium bromide solution. After 12 h, the gel was dried and stained with 0.1% toluidine blue in acetic acid:ethanol:water (0.1:5:5, v/v).
The average molecular mass of the sulfated galactan was estimated by comparison with the electrophoretic mobility of standard compounds (18). The sulfated polysaccharides (∼10 μg of each) were applied to a 1-mm-thick 10% polyacrylamide slab gel in 0.02 m sodium barbital (pH 8.6). After electrophoresis (100 V for 30 min), the sulfated polysaccharides were stained with 0.1% toluidine blue in 1% acetic acid and washed for about 1 h in 1% acetic acid.
Desulfation and Oversulfation Procedures
Desulfation of the sulfated galactan was performed as described previously (16, 19). About 20 mg of the polysaccharide was dissolved in 5 ml of distilled water and mixed with 1 g (dry weight) of Dowex 50-W (H+, 200–400 mesh). After neutralization with pyridine, the solution was lyophilized. The resulting pyridinium salt was dissolved in 2.5 ml of dimethyl sulfoxide/methanol (9:1, v/v). The mixture was heated at 80 °C for 4 h, and the desulfated product was exhaustively dialyzed against distilled water and lyophilized. The extent of desulfation was estimated by the molar ratio of sulfate/total sugar. This method allowed us to detect desulfation up to a molar ratio of ≤0.1 sulfate/total sugar. About 5 mg of desulfated polysaccharide was obtained at the end of the reaction. Over-sulfated galactans were prepared through chemical sulfonation of the native polysaccharides (6). In a control reaction we followed sulfonation of chondroitin 4-sulfate. The reaction reached about 72.1% of the total sites available for sulfation on the entire chondroitin sulfate backbone, as indicated by NMR analysis. It was not possible to run NMR spectra of the over-sulfated galactans due to scarce material.
NMR Experiments
1H and 13C one- and two-dimensional spectra of the native sulfated galactan and of its desulfated derivative were recorded using a Bruker DRX 400 MHz apparatus with a triple resonance probe, as detailed previously (18). About 5 mg of each sample was dissolved in 0.5 ml of 99.9% deuterium oxide (Cambridge Isotope Laboratory, Cambridge, MA). All spectra were recorded at 50 °C with HOD suppression by presaturation. One-dimensional 1H NMR spectra were recorded with 16 scans. Two-dimensional 1H/1H COSY,2 TOCSY, NOESY, and 13C,1H HSQC spectra were recorded using states-TPPI (states-time proportion phase incrementation) for quadrature detection in the indirect dimension. TOCSY spectra were run with 4046 × 400 points with a spin-lock field of 10 kHz and a mixed time of 80 ms. NOESY spectra were recorded with a mixing time of 100 ms. 13C,1H HSQC spectra were run with 1024 × 256 points and GARP (globally optimized alternating phase rectangular pulses) for decoupling. Chemical shifts are relative to external trimethylsilylpropionic acid at 0 ppm for 1H and to methanol for 13C.
Molecular Dynamics
All calculations were performed using GROMACS simulation suite (20) and GROMOS96 force field (21). Briefly, structures of disaccharide units containing α-l-Galp-(1→3)-α-l-Galp, α-l-Galp-2(SO4)-(1→3)-α-l-Galp-2(SO4), β-d-Galp-(1→3)-β-d-Galp, β-d-Galp-2(SO4)-(1→3)-β-d-Galp, and β-d-Galp-(1→3)-β-d-Galp-2(SO4) were submitted to the PRODRG server (22), and the initial geometries and crude topologies were retrieved. These topologies were supplied with Löwdin HF/6–31G** atomic charges (23, 24) and submitted to conformational analysis by varying the ϕ and ψ dihedral angles from −180 to 180°, with a 30° step, in a total of 144 conformers for each linkage. Each conformation was further refined in a 20-ps molecular dynamics at 10 K, with an integration step of 0.5 fs (25). The relative stabilities of the conformations were used to construct relaxed energy contour plots. The minimum energy conformations described in these plots were submitted to 0.1 μs molecular dynamics (MD) simulations in aqueous solutions using the SPC water model (26) following a protocol previously described (23, 24, 27). A triclinic water box under periodic boundary conditions was employed using a 10-Å minimum distance from solute to the box faces. Counter ions (Na+) were added to neutralize the system. The Lincs method (28) was applied to constraint covalent bond lengths, allowing an integration step of 2 fs after an initial energy minimization using the Steepest Descents algorithm. All simulations applied the Particle-Mesh Ewald method (29). Temperature and pressure were kept constant by coupling carbohydrate, ions, and solvent to external temperature and pressure baths with coupling constants of t = 0.1 and 0.5 ps, respectively (26). The reference temperature was adjusted to 310 K. The relative orientation of a pair of contiguous carbohydrate residues is described by two torsional angles at the glycosidic linkage, denoted ϕ and ψ, as follows: ϕ = O-5-C-1-O-1-C-3′, and ψ = C-1-O-1-C-3′-C-2′.
Fertilization Block by Sperm Preincubation with Sulfated Galactans
G. crenularis sperm were collected as undiluted semen, stored on ice, and diluted in ice-chilled seawater shortly before use. Sulfated galactans (20 μl) were dissolved in sea water at the final concentration of 4 mg/ml and placed in a test tube. Thereafter, 100-fold diluted sperm suspension (5 μl) was added and incubated for 10 min at room temperature. Unfertilized G. crenularis eggs spawned by intercelomic injection with 0.5 m KCl were placed in a 48-well culture dish filled with 150 μl of seawater. After a 10-min incubation of sperm with sulfated galactan, such sperm (5 μl) were inseminated for 30 min at 20 °C. The percentage of fertilization (judged by the presence/absence of the fertilization envelope) was scored under the microscope by counting ∼200 eggs. Three independent experiments were performed.
Acrosome Reaction Assays
The method used to access the acrosome reaction was slightly modified from Su and Vacquier (30). Briefly, sperm were spawned by intracelomic injection of 0.5 m KCl (0.5 ml/animal), collected undiluted, and stored on ice before dilution. They were diluted 1:5 in 10 mm HEPES-buffered sea water (pH 7.9). Immediately after dilution, ∼25 μl of the sperm suspension were mixed with 50 μl of the polysaccharide solution. The sugar content of these solutions was previously quantified by the phenol-sulfuric acid assay (10). After 5 min on ice, sperm were fixed in 350 μl of 3.7% formaldehyde in seawater for 30 min, washed 2 times with 500 μl of phosphate-buffered saline and stained for at least 2 h with 1 unit of rhodamine phalloidin (Molecular Probes R415, Invitrogen) in 50 μl of 0.1 m glycine, 1 mg/ml bovine serum albumin, 0.02% sodium azide in phosphate-buffered saline (pH 7.4). The solution was then washed twice in 500 μl of phosphate-buffered saline and incubated with 30 μl of 4′6′-diamino-2-phenylindole (Sigma) for 6 min. The cells were washed twice in 500 μl of phosphate-buffered saline and then re-suspended in 30 μl of 70% glyceraldehyde in the same solution and mounted in a thin layer, and the coverslip was sealed. Sperm were scored blindly using a Zeiss Axioskop 2 plus fluorescent microscope. Photos were acquired with a Zeiss LSM 510 Meta confocal microscope (Jeha, Germany) in red (phalloidin), blue (4′6′-diamino-2-phenylindole), and transmitted light channels.
Measurements of Increases in Intracellular Ca2+
Intracellular calcium was measured by a methodology slightly modified from Hirohashi and Vacquier (8). Briefly, sperm from E. lucunter were collected on ice and in darkness and immediately used. Undiluted semen was suspended in 4 volumes of dye loading buffer (artificial sea water containing 10 mm HEPES, 1 mm CaCl2, and 0.1 mg/ml soybean trypsin inhibitor at pH 7.0), placed in dimethyl sulfoxide (final concentration 0.6%) containing fura-2/AM at a final concentration of 12 μm, and incubated for 4 h in darkness at 4 °C. The cells were washed twice with phosphate-buffered saline and grounded at 430 × g for 5 min. The final pellet was resuspended in fresh dye loading buffer without soybean trypsin inhibitor. Finally, 50 μl of fura-2-loaded sperm were placed in an 11-mm-diameter glass tube containing 1.5 ml of dye loading buffer without soybean trypsin inhibitor and mounted in a FP-6300 spectrofluorimeter from Jasco at 16 °C at continual agitation. The fluorescence intensity was measured at Ex/Em 340/500 nm to follow the effect of the sulfated polysaccharide in the sperm. The sugar content of the polysaccharide solutions was quantified by the phenol-sulfuric acid assay (10).
RESULTS AND DISCUSSION
The Egg Jelly of the Sea Urchin G. crenularis Contains a Sulfated d-Galactan
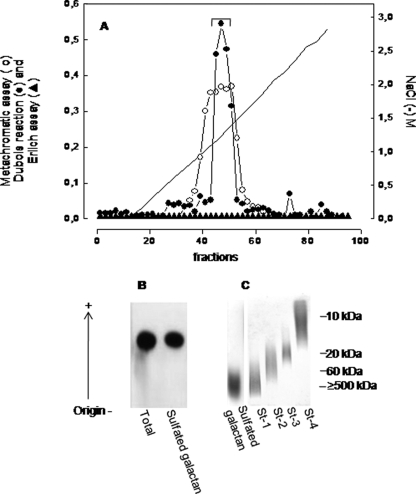
The polysaccharides extracted from the egg jelly of G. crenularis were purified by anion-exchange chromatography on a Mono Q column coupled to a fast protein liquid chromatography system, and fractions were monitored for hexose and metachromasia (Fig. 1A). We observed a single peak of sulfated polysaccharide, eluted from the column with ∼1.3 M NaCl, as indicated by the hexose assay (closed circles in Fig. 1A) and metachromasia (open circles). Different from other species of sea urchins (17, 31–33), no sialoglycan was detected in the egg jelly of this sea urchin, as indicated by the negative Erlich reaction (closed triangles in Fig. 1A).
FIGURE 1.
Purification and electrophoretic mobility of the sulfated galactan from the egg jelly of G. crenularis. A, total sulfated polysaccharide from the egg jelly (20 mg) was applied to a Mono Q fast protein liquid chromatography column (HR5/5) equilibrated with 20 mm Tris-HCl (pH 8.0). The column was developed by a linear gradient of 0–3.0 m NaCl in the same solution. Fractions were assayed by metachromasia using 1,9-dimethylmethylene blue (○), the Dubois reaction for hexose (●), and the Ehrlich assay for sialic acid (▲). The NaCl concentration was estimated by conductivity (–). Fractions containing the sulfated galactan (indicated by the horizontal bar) were pooled, dialyzed against distilled water, and lyophilized. B, total polysaccharides from the egg jelly and the purified sulfated galactan (15 μg of each) were applied to a 0.5% agarose gel, and electrophoresis was carried out for 1 h at 110 V in 0.05 m 1,3-diaminopropane:acetate (pH 9.0). Gels were fixed with 0.1% N-cetyl-N,N,N-trimethylammonium bromide solution. After 12 h, the gel was dried and stained with 0.1% toluidine blue in acetic acid/ethanol/water (0.1:1:5, v/v). C, the purified sulfated galactan (10 μg) were run on 6% polyacrylamide gels in 0.2 m sodium barbital (pH 8.6) and stained with 0.1% toluidine blue in 1% acetic acid. Molecular mass markers used were dextran sulfate (St-1, average molecular mass ≥500 kDa), chondroitin 6-sulfate (St-2, average molecular mass ∼60 kDa), chondroitin 4-sulfate (St-3, average molecular mass ∼40 kDa), and heparin (St-4, average molecular mass ∼10 kDa).
The purified sulfated polysaccharide from G. crenularis showed a single component on agarose gel electrophoresis (Fig. 1B), with a high molecular mass (Fig. 1C), as already observed for the sulfated polysaccharides from the egg jelly of other species of sea urchins (17, 31–33). Chemical analysis revealed the occurrence of galactose and sulfate, exclusively. After derivatization with butyl alcohol, the galactoside derivatives obtained showed the same retention times and peak areas as d-galactose standard, indicating that the galactose occurs in the G. crenularis exclusively in the d-enantiomeric form.
Thus, the egg jelly of the sea urchin G. crenularis contains a single fraction of sulfated d-galactan. It differs from the sulfated polysaccharides found in other species of sea urchins that contain fucose or galactose always in the l-configuration (17, 31–33).
The Sulfated Galactan from G. crenularis Has a Regular Disaccharide Repeating Unit Composed of Alternating 2-Sulfated and Non-sulfated 3-Linked β-Galactopyranosyl Units
For a detailed structural analysis of the sulfated galactan from G. crenularis, we employed one- and two-dimensional NMR spectroscopy of the native polysaccharide and of its desulfated derivative (Figs. 2–4).
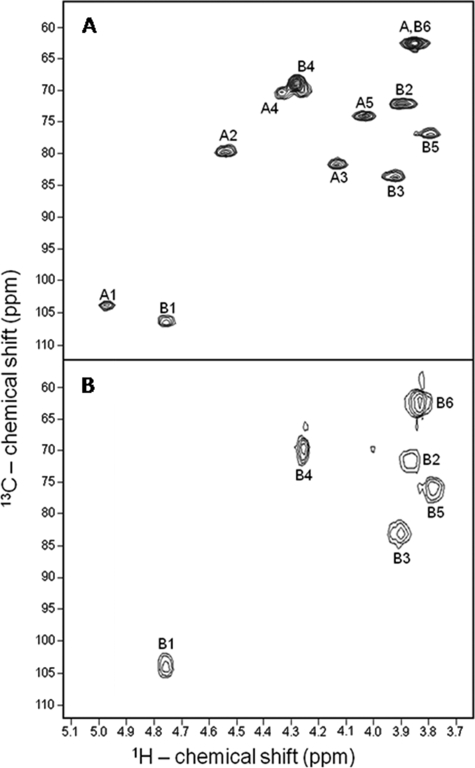
FIGURE 2.
One-dimensional 1H NMR spectra at 400 MHz of the native sulfated β-galactan from G. crenularis (A) and its desulfated derivative (B). About 5 mg of each polysaccharide were dissolved in 0.5 ml of D2O, and the one-dimensional NMR spectra were recorded at 50 °C. The residual water signal was suppressed by presaturation. 1H chemical shifts are relative to external trimethylsilylpropionic acid at 0 ppm. The signals designated with A and B correspond to the 2-sulfated and non-sulfated β-d-galactopyranosyl units, respectively, and numbers correspond to each hydrogen of the hexose ring. Numbers in panel A below the spectrum indicate integrals of A1 and B1 signals. The peak marked by the asterisk corresponds to a contaminant.
FIGURE 3.
13C,1H HSQC spectra of the sulfated β-galactan from G. crenularis (A) and its desulfated derivative (B). Chemical shifts are relative to external trimethylsilylpropionic acid at 0 ppm for 1H and methanol for 13C. The spin systems were denoted as A and B for 2-sulfated and non-sulfated β-galactopyranosyl units, respectively. Spectra were recorded at 50 °C.
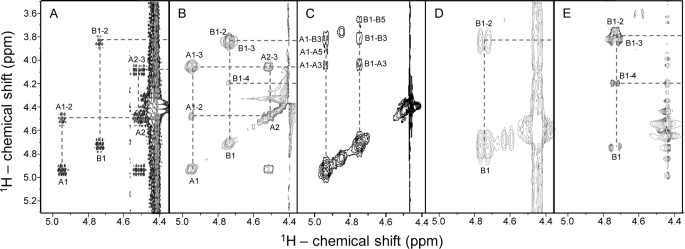
FIGURE 4.
Strips of the anomeric regions (expansions from 4.4 to 5.1 ppm) from the COSY (A and D), TOCSY (B and E), and NOESY (C) spectra of the sulfated β-galactan (A–C) from G. crenularis and its desulfated derivative (D and E). About 5 mg of each polysaccharide were dissolved in 0.5 ml of D2O, and the two-dimensional NMR spectra were recorded at 50 °C at 400 MHz. Chemical shifts are relative to external trimethylsilylpropionic acid at 0 ppm for 1H. The spin systems were denoted as A and B for 2-sulfated and non-sulfated β-galactopyranosyl units, respectively. The peak at 4.85/3.85 ppm in the NOESY spectrum (C) corresponds to a contaminant.
The 1H one-dimensional spectrum (Fig. 2A) and mainly the 13C,1H HSQC spectrum (Fig. 3A) of the native sulfated galactan showed equimolar proportions of two anomeric signals, denominated as A1 and B1. A1 exhibited a downfield shift of ∼0.2 ppm, possibly because of sulfation (34). This conclusion was reinforced by analysis of the 13C,1H HSQC spectrum of the native polysaccharide (Fig. 3A) and the disappearance of signal A1 after the desulfation reaction (Figs. 2B and 3B). These observations suggest that the sulfated galactan from G. crenularis contains equimolar proportions of non-sulfated and sulfated galactoses.
Correlation peaks in the two-dimensional COSY (Figs. 4, A and D) and TOCSY spectra (Figs. 4, B and E) allowed us to trace the entire spin systems of A and B signals in the native sulfated galactan and of B signal in the desulfated derivative. The COSY spectrum of the native sulfated galactan showed two correlation signals (A1–2 and B1–2) because of scalar-couplings between 1H-anomeric proton and H2 for both residues (Fig. 4A). A single B1–2 correlation signal is observed in the spectrum of the desulfated derivative (Fig. 4D). After identification of the cross-peaks in the COSY spectra, the TOCSY experiments (Figs. 4, B and E) allowed up to assign unequivocally the whole spin systems A and B and to obtain the 1H chemical shifts, as indicated in Table 1. Based on the 1H chemical shifts, we assigned the peaks of correlation in the 13C,1H HSQC spectra (Fig. 3) and obtained the values of 13C chemical shifts shown in Table 1. Analysis of these 13C chemical shifts revealed that the galactan contains 3-linked β-galactopyranosyl residues, as indicated by the typical low-field shift of carbon (∼10 ppm) in sites of glycosylation (Table 1). This 13C shift was also seen in reference compounds (Table 1).
TABLE 1.
1H and 13C chemical shifts (ppm) of the sulfated and desulfated β-d-galactan from G. crenularis and standard compounds
ND, not determined.
| Polysaccharide | Structure |
1H and 13C chemical shifta |
|||||
|---|---|---|---|---|---|---|---|
| H-1C-1 | H-2C-2 | H-3C-3 | H-4C-4 | H-5C-5 | H-6C-6 | ||
| ppm | |||||||
| Sulfated β-d-galactan from G. crenularis | 3-β-d-Galp-2(SO3−)-1 (unit A) | 4.94 | 4.52 | 4.11 | 4.37 | 4.02 | 3.82 |
| 104.1 | 80.2 | 81.8 | 70.5 | 74.3 | 62.5 | ||
| 3-β-d-Galp-1 (unit B) | 4.73 | 3.87 | 3.90 | 4.24 | 3.75 | 3.82 | |
| 107.2 | 72.0 | 83.5 | 69.1 | 77.0 | 62.5 | ||
| Desulfated β-d-galactan from G. crenularis | 3-β-d-Galp-1 (unit B) | 4.79 | 3.90 | 3.93 | 4.29 | 3.82 | 3.87 |
| 103.5 | 69.9 | 81.9 | 68.3 | 74.8 | 60.5 | ||
| Desulfated β-d-galactan from Codium isthmocladumb | 3-β-d-Galp-1 | 4.81 | 3.64 | 3.92 | 4.39 | 3.86 | 3.94-3.85 |
| 102.6 | 73.8 | 82.9 | 67.1 | 75.2 | 60.3 | ||
| 6-β-d-Galp-1 | 4.62 | 3.82 | 3.89 | 4.38 | 4.09 | 4.36-4.01 | |
| 103.1 | 70.1 | 70.2 | 67.0 | 73.9 | 69.9 | ||
| Sulfated β-d-galactan from Meretrix petechialisc | 3-β-d-Galp-2(SO3−)-1 | 4.83 | 4.45 | 3.97 | ND | ND | ND |
| Sulfated α-l-galactan from E. lucunterd | 3-α-l-Galp-2(SO3−)-1 | 5.47 | 4.66 | 4.23 | 4.33 | 4.35 | 3.82 |
| 97.2 | 76.2 | 75.9 | 72.5 | 69.5 | 63.8 | ||
| Desulfated α-l-galactan from E. lucunterd | 3-α-l-Galp-1 | 5.26 | 4.08 | 4.14 | 4.32 | 4.24 | 3.82 |
| 98.1 | 73.5 | 77.2 | 69.5 | 68.5 | 63.9 | ||
a Chemical shifts are relative to external trimethylsilylpropionic acid at 0 ppm for 1H and methanol for 13C. Values in boldface indicate sulfate position and in italics indicate glycosylated positions.
b Values are from Farias et al. (43).
c Data are from Amornrut et al. (38)).
d Values are from Alves et al. (17).
The spin systems A and B traced for the native sulfated galactan differ mainly because of typical 1H low-field shift (∼0.6 ppm) of H2 that indicates 2-sulfation (Table 1). The neighbors H1 and H3 protons showed characteristic ∼0.2-ppm low-field shifts. The spin systems B traced for native sulfated galactan and for its desulfated derivative show almost similar chemical shifts for 1H and 13C.
In summary, these results indicate that sulfated galactan from G. crenularis has equimolar proportions of 2-sulfated and non-sulfated β-galactopyranosyl units. It remains to clarify whether these units alternate along the polysaccharide chain or occur in a random distribution or even as clusters in the molecule. This aspect was investigated using two-dimensional NOESY spectrum of the native sulfated galactan (Fig. 4C). The spectrum revealed intraresidue NOEs between 1H-anomeric and H3 and H5, as typically found in β-galactopyranose residues in their regular chair conformation (35). Protons H1, H3, and H5 are on the same plane (equatorially or axially) and commonly show <5 Å space contacts, which allows detection of their NOE. But more significantly, the NOESY spectrum also showed inter-residue NOEs between H1 and H3 of the adjacent residue (A1-B3 and B1-A3). These observations indicated that the 2-sulfated and non-sulfated units intercalate along the polysaccharide chain as a repeating disaccharide unit. Overall, NMR analysis indicated that the sulfated galactan from the egg jelly of G. crenularis has the following disaccharide repeating units: (3-β-d-Galp-2(OSO3)-1→3-β-d-Galp-1)n (Fig. 5A).
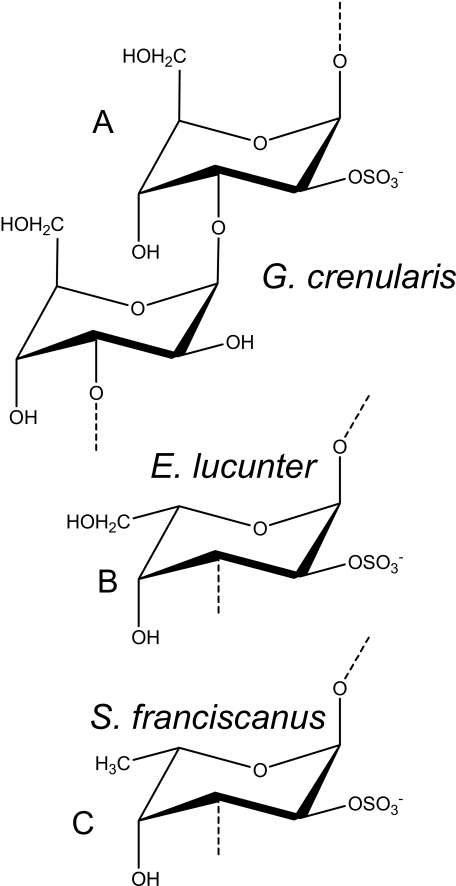
FIGURE 5.
Structures of 2-sulfated polysaccharides obtained from the egg jelly of different species of sea urchins. A, the sulfated β-d-galactan from G. crenularis is composed of the following disaccharide repeating unit: (3-β-d-Galp-2(SO4)-(1→3)-β-d-Galp-1)n. The polysaccharides from E. lucunter (B) and S. franciscanus (C) are composed of α-l-galactopyranosyl or α-l-fucopyranosyl residues, respectively, both 2-sulfated and 3-linked.
The Anomeric Configuration of the Glycosidic Linkage Is an Important Structural Feature for Recognition of Sulfated Polysaccharides by Sea Urchin Sperm
Previously, we tested a variety of sulfated polysaccharides with well defined structures as inducers of the acrosome reaction in sea urchin sperm. These studies indicated that sulfated polysaccharides show species specificity in inducing the sperm acrosome reaction, which is regulated by the structure of the saccharide chain and its sulfation pattern (3, 31–33). However, the glycosidic linkages in all these previously tested polysaccharides were in the α-anomeric configuration. The sulfated β-d-galactan from G. crenularis extends the possibility of determining the event at a further molecular detail.
Investigation of the acrosome reaction using sperm from G. crenularis was not feasible. There was no obvious difference in morphology and phalloidin staining between sperm treated with and without sulfated β-d-galactan or 10 μm ionomycin. Thus, the acrosome in this species is too small and difficult to visualize using microscopic methods. Attempts to determine the increase in intracellular Ca2+ using fura-2 loaded sperm were also unsuccessful. As an alternative, we used a fertilization inhibition assay. Preincubation of the G. crenularis sperm with homologous sulfated β-galactan blocks fertilization, possibly because of a premature acrosome reaction (see Table 2). In contrast, preincubation with the sulfated α-galactan from E. lucunter, composed of (3-α-l-Galp-2(OSO3)-1-)n (Fig. 5B) (17), did not affect the fertilization capacity of the G. crenularis sperm. Clearly, these results indicate that the effect of sulfated galactans on the sea urchin fertilization depends mostly on the anomeric configuration of the glycosidic linkage rather than charge density.
TABLE 2.
Fertilization block by pre-incubation of G. crenularis sperm with homologous and heterologous sulfated galactans
G. crenularis sperm were preincubated with sulfated galactan for 10 min. Thereafter, such sperm were inseminated for 30 min with homologous eggs. The percentages of fertilization (indicated by the presence of fertilization envelope) were then scored under microscopy by counting ∼200 eggs.
| Fertilization envelope formed |
||||
|---|---|---|---|---|
| Sulfated α-galactan from E. lucunter |
Sulfated β-galactan from G. crenularis |
|||
| 0 | 4 mg/ml | 0 | 4 mg/ml | |
| % of total eggs | ||||
| Experiment 1 | 98.7 | 93.0 | 98.5 | 3.3 |
| Experiment 2 | 99.1 | 96.7 | 98.0 | 0.5 |
| Experiment 3 | 98.2 | 98.1 | 98.3 | 0.5 |
| Mean ± S.D. | 98.7 ± 0.5 | 95.9 ± 2.6 | 98.3 ± 0.3 | 1.4 ± 0.9 |
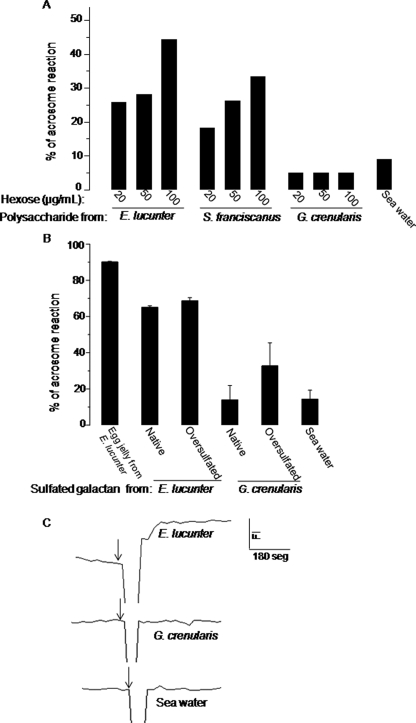
We further investigate the effect of sulfated galactans on sea urchin fertilization using sperm from E. lucunter, which express in their egg jelly a sulfated α-galactan (Fig. 5B) (17). Sperm from E. lucunter were equally sensitive to homologous 2-sulfated α-galactan and to heterologous 2-sulfated α-fucan from S. franciscanus (see structure in Fig. 5C) but not to the 2-sulfated β-galactan from G. crenularis, even when the polysaccharide was tested at high concentrations (Fig. 6A). This indicates that the receptor for egg jelly of E. lucunter sperm does not differentiate between the CH2OH of l-galactose and CH3 of l-fucose at position 6. These two polysaccharides present the same sulfation pattern and position of glycosylation but differ in the sugar moieties. In a similar way sperm from S. franciscanus were sensitive to the homologous sulfated α-fucan and to the heterologous E. lucunter-sulfated α-galactan (6, 36). Despite that, E. lucunter sperm markedly distinct between α- and β-galactans.
FIGURE 6.
Effects of native (A) or over-sulfated (B) 2-sulfated α-l-galactan, α-l-fucan, and β-d-galactan from the egg jellies of E. lucunter, S. franciscanus, and G. crenularis, respectively, as inducers of acrosome reaction (A and B) and on increases intracellular Ca2+ (C) of E. lucunter sperm. Sulfated polysaccharides or lyophilized egg jelly were dissolved in sea water and incubated with sperm from E. lucunter, and the acrosome reaction was detected using fluorescence phalloidin (see “Experimental Procedures”). Negative control was done with artificial sea water. Approximately 100–150 sperm were scored per data point. The concentrations of polysaccharides were normalized by hexose content. In panel B a fixed concentration of sulfated polysaccharides (100 μg/ml) was used in the assays. The results are representative from two experiments and are shown as the average and S.D. C, increases in intracellular Ca2+: each sulfated polysaccharide were added to a fura-2 loaded E. lucunter sperm suspension (arrows) at a final concentration of 100 μg/ml. Scale bars indicate the fluorescence intensity (FI, y axis) and 100 s (x axis).
Another plausible reason for the absence of effect of the sulfated β-galactan on E. lucunter sperm is its reduced charge density compared with the homologous polysaccharide (0.5 versus 1.0 sulfate/monosaccharide). We attempted to investigate this aspect using chemically over-sulfated galactan. In the 2-sulfated, 3-linked α-galactan from E. lucunter, the 4- and 6-positons are the only ones capable of additional sulfation. Oversulfation of this polysaccharide did not change its responsiveness to homologous sperm (Fig. 6B), indicating that increased sulfates do not increase or inhibit the biological activity. In contrast, over-sulfated β-galactan from G. crenularis induced acrosome reaction in E. lucunter sperm but at a significantly lower potency compared with the homologous α-galactan. Thus, the anomeric configuration of the glycosidic linkage is still a preferential structural requirement for the effect of these over-sulfated galactans on the acrosome reaction.
Another point to consider is that the sulfated β-galactan could increase intracellular Ca2+ of E. lucunter sperm but not up to the proper high concentration required to induce the complete acrosome reaction. In fact, a low molecular weight sulfated α-fucan increases intracellular Ca2+ and pH, which is enough to induce exocytosis of the acrosome vesicle, but only at a slower kinetics, which is not able to induce the complete acrosome reaction (8, 37). To test for a similar effect of the sulfated β-galactan in E. lucunter sperm, we measured the increase in intracellular Ca2+ using fura-2-loaded sperm after incubation with egg jelly polysaccharides (Fig. 6C). Increase of intracellular Ca2+ was detected when E. lucunter sperm were incubated with homologous polysaccharides but not with the sulfated β-galactan from G. crenularis (Fig. 6C).
In conclusion, studies with the sulfated β-galactan from G. crenularis extend the characterization of the induction of sea urchin acrosome reaction to a further molecular detail. It indicates that the echinoderm sperm are sensitive to polysaccharides with the appropriate anomeric configuration.
The Scalar Coupling Constants 3JH-H and 1JC-H Differ between Sulfated β-Galactans and Sulfated α-Galactan or α-Fucan
Conformational analysis is an important approach to extend the characterization of the biological effect of the sea urchin polysaccharides at molecular level. The differences in chemical structure may in fact determine spacing between sulfate groups required to match the interval between basic amino acid residues in the protein chain of the receptors.
This aspect was investigated by determining the scalar coupling constant of the 2-sulfated polysaccharides isolated from three species of sea urchins. The sulfated β-galactan from G. crenularis showed well defined doublets of 1H-1H couplings for the anomeric signals in the one-dimensional NMR spectra (Figs. 2, A and B) (Table 3). This pattern of 1H-1H coupling is evidenced by multiplets in all cross-peaks showed in the COSY spectrum (Fig. 4A) and by the doublets in the other homonuclear two-dimensional experiments (TOCSY and NOESY spectra, Fig. 4, B and C). Interesting, a similar pattern of coupled signals was also observed for another invertebrate sulfated β-galactans (38) but poorly noted in sulfated α-galactans and in a sulfated α-fucan (17, 39). These marked spin-spin couplings of the sulfated β-galactan from G. crenularis together with the presence of inter-residue NOEs exclusively between protons involved in the glycosidic bond, suggest a polysaccharide with dynamic behavior and the absence of a single preponderant conformation. This proposition is confirmed by the MD simulations, as discussed below.
TABLE 3.
3JH-H and 1JC-H (Hz) in the NMR spectra of sulfated α- or β-galactans and α-fucans
ND, not determined.
| Polysaccharide | Structure |
3JH-H |
||||
|---|---|---|---|---|---|---|
| H1–H2 | H2–H3 | H3–H4 | H4–H5 | H5–H6 | ||
| Hz | ||||||
| Sulfated β-d-galactan from G. crenularis | 3-β-d-Galp-2(SO3−)-1 (unit A) | 7.4 | 7.9 | 2.7 | ND | ND |
| 3-β-d-Galp-1 (unit B) | 7.01 | ND | ND | ND | ND | |
| Desulfated β-d-galactan from G. crenularis | 3-β-d-Galp-1 (unit B) | 7.29 | ND | 2.83 | ND | ND |
| Sulfated β-d-galactan from M. petechialisa | 3-β-d-Galp-2(SO3−)-1 | 7.6–7.8 | 7.8–8.2 | <1.5 | ND | ND |
| Sulfated α-l-galactan from E. lucunterb | 3-α-l-Galp-2(SO3−)-1 | 3.0 | 10.5 | ND | ND | 4.5 |
| Sulfated α-l-fucan from S. franciscanusb | 3-α-l-Fucp-2(SO3−)-1 | 3.1 | 9.5 | ND | ND | 4.9 |
| Sulfated α-l-fucan from Ludwigothurea griseac | 3-α-l-Fucp-2(SO3−)-1 | 3.5–4.0 | ≈10.0 | <3.0 | <3.0 | 6.7–7.0 |
|
1JC-H |
|||||||
|---|---|---|---|---|---|---|---|
| C1-H1 | C2-H2 | C3-H3 | C4-H4 | C5-H5 | C6-H6 | ||
| Hz | |||||||
| Sulfated β-d-galactan from G. crenularis | 3-β-d-Galp-2(SO3−)-1 (unit A) | 164.7 | 156.8 | 143.4 | 151.4 | 143.0 | 143.9 |
| 3-β-d-Galp-1 (unit B) | 163.5 | 141.2 | 143.1 | 150.5 | 139.9 | 143.9 | |
| Sulfated α-l-galactan from E. lucunterb | 3-α-l-Galp-2(SO3−)-1 | 177.5 | 151.3 | 146.2 | 146.5 | 146.8 | 143.3 |
| Sulfated α-l-fucan from S. franciscanusb | 3-α-l-Fucp-2(SO3−)-1 | 177.2 | 148.7 | 144.6 | 140.9 | 147.1 | 125.3 |
The scalar-coupling constants 3JH-H and 1JC-H observed for the sulfated β-galactan differ significantly compared with the values for sulfated α-galactan and α-fucan, especially 3JH-H (Table 3). Again, these different coupling-constant values reflect distinct conformations for these polysaccharides. The 2-sulfated and non-sulfated units found in the sulfated β-galactan from G. crenularis showed similar 3JH-H and 1JC-H values, with only discrete difference in the 1JC-H values (Table 3). It means that 2-sulfation does not significantly alter the glycosidic geometry of the β-galactopyranosyl residues.
MD of α- and β-Galactans
Differences in the scalar coupling constants between sulfated β-galactan and sulfated α-galactan or α-fucan suggest distinct conformations. To explore such behavior at the atomic level, we employed conformational calculations based on MD simulations, an important tool for the structural and conformational characterization of carbohydrates (40).
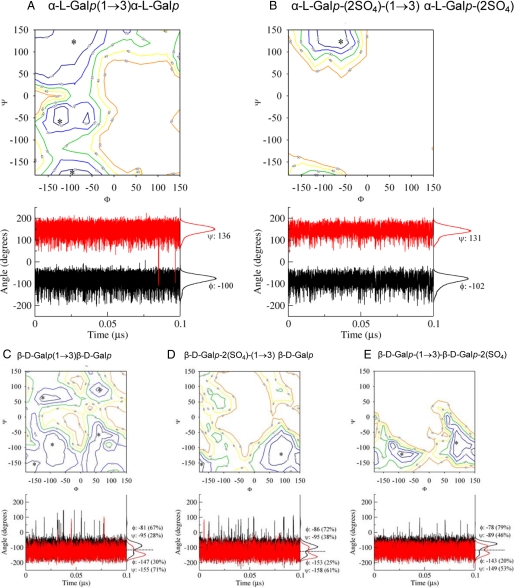
Initially, the conformational preference of the disaccharides α-l-Galp-(1→3)-α-l-Galp, α-l-Galp-2(SO4)-(1→3)-α-l-Galp-2(SO4), β-d-Galp-(1→3)-β-d-Galp, β-d-Galp-2(SO4)-(1→3)-β-d-Galp, and β-d-Galp-(1→3)-β-d-Galp-2(SO4) was analyzed employing relaxed contour plots (Fig. 7). These maps indicate that the non-sulfated galactans possess more flexible conformational behavior than their sulfated counterparts (Fig. 7, A versus B and C versus D and E), indicating that sulfate groups increase the rigidity of the polysaccharide chain, as already suggested (41). Additionally, the configuration of the glycosidic linkage appears to play an important role on the flexibility of sulfated galactans and fucans in aqueous solution, as the β-d-galactopyranosyl residues showed an increased flexibility when compared with α-l-galactopyranosyl units (Fig. 7, A and B versus C–E), which did not differ from α-l-fucopyranose (41).
FIGURE 7.
MD of galactans composed of the following disaccharide units: A, α-l-Galp-(1→3)-α-l-Galp; B, α-l-Galp-2(SO4)-(1→3)-α-l-Galp-2(SO4); C, β-d-Galp-(1→3)-β-d-Galp; D, β-d-Galp-2(SO4)-(1→3)-β-d-Galp; E, β-d-Galp-(1→3)-β-d-Galp-2(SO4). The contour plots are shown at every 10 kJ·mol−1 from 10 to 50 kJ·mol−1. Asterisks (*) indicate the input minimum energy conformations for MD refinement. The fluctuation and distribution of the ϕ (black) and (red) dihedral angles are also presented together with average and prevalence values (%) for each conformer population. The S.D. for all average values lies in ∼20°.
To refine the analysis obtained by relaxed contour plots data upon the addition of solvent molecules, each galactan minimum energy conformation was further submitted to a 0.1-μs MD simulation in aqueous solutions. The analysis of these simulations confirmed the observations that α-l-galactopyranosyl units have a more rigid structure than β-d-galactopyranoses. Thus, the α-l-galactopyranose disaccharides, as in the sulfated α-galactan from E. lucunter, present unique prevalent conformation in solution (glycosidic dihedral angles of Φ/Ψ = − 102°/131°, Fig. 7B). In contrast, β-d-galactopyranosyl disaccharides, as in the sulfated β-galactan from G. crenularis, present at least two main solution conformations for each dihedral angle (Figs. 7, C–E), indicating four possible conformations co-existing simultaneously in solution. As a consequence, the glycosidic linkage around the sulfated β-galactan has no prevalent conformation in aqueous solutions, and a series of conformational sub-states occur in equilibrium. Curiously, the conformers experimented by the β-configuration do not match the preponderant conformer observed for the α-forms.
MD simulations of the desulfated derivatives from the two enantiomeric forms of the galactans (Figs. 7, A versus C) indicated that α-galactopyranose still has less dynamic molecular behavior compared with β-galactopyranose. Although sulfate groups may promote steric/electrostatic hindrances, which restrict the glycosidic motions, the three-dimensional orders of these sulfated galactans are clearly dominated by the configuration of their glycosidic linkage.
Thus, the sulfated β-galactan from G. crenularis is a more dynamic and flexible polysaccharide than the sulfated α-galactan from E. lucunter or the sulfated α-fucan from S. franciscanus. But more significantly, the observation that only α-polysaccharides induce the acrosome reaction in sperm from E. lucunter and S. franciscanus can arise from the observation that the preponderant conformer population observed by the active sulfated α-polysaccharides is not observed among populations of the β-form despite its much more flexible structure in aqueous solution.
Major Conclusions
We extended our studies to the sea urchin G. crenularis, which inhabits high depth and low temperature seawater (7). The egg jelly of this sea urchin contains a sulfated β-galactan, which is constituted of the disaccharide repeating structure 3-β-d-Galp-2(OSO3)-1→3-β-d-Galp-1. This is the first report of a sulfated β-galactan with a regular and homogeneous disaccharide structure. The polymer is markedly distinct from all other sea urchin-sulfated polysaccharides described so far that are composed of units on α-l-configuration. Furthermore, this sea urchin does not contain sialoglycans, commonly found in the echinoderm egg jelly.
Sulfated β-galactans have been reported in marine green algae. In these organisms the polysaccharides have complex structures composed preponderantly of 4-sulfated, 3-linked β-d-galactopyranosyl units but with branching and mostly highly pyruvylated at the non-reducing terminal residues, forming cyclic ketals (42, 43). Red algae contain a linear sulfated galactan made of alternating 3-linked β-d-galactopyranosyl and 4-linked α-galactopyranosyl residues, but considerable structural variation in these sulfated galactans occurs among different species, including complex sulfation pattern, substitution by methyl groups or pyruvic acid, formation of anydro sugar, etc. (45).
We used this new sulfated β-galactan to investigate the acrosome reaction in a further molecular detail using homologous and heterologous sperm. We tested three 2-sulfated polysaccharides differing in their conformation (α or β) and monosaccharide composition (galactose or fucose) as inducers of the sperm acrosome reaction. We aimed to establish the structure versus biological activity of the echinoderm polysaccharides, including structural features at a conformational level. The sperm from G. crenularis react to the homologous sulfated β-galactan but not to the sulfated α-galactan from E. lucunter. The species specificity was confirmed as E. lucunter sperm react to the homologous sulfated α-galactan and also to a 2-sulfated α-fucan but not to the sulfated β-galactan from G. crenularis. In a similar way, sperm from S. franciscanus react equally to 2-sulfated α-fucan and α-galactan (6).
MD and NMR data of 3JH-H and 1JC-H strongly indicated that α- and β-isomers of sulfated galactan and fucan exhibit distinct conformational preferences. In particular, the preponderant conformer population experimented by the active sulfated α-galactan or α-fucan (Φ/Ψ = − 102°/131°) is not observed among populations of the β-form, despite its much more flexible structure in solution. Thus, the anomeric configuration of the glycosidic linkage rather than monosaccharide composition (galactose or fucose) is the main structural requirement to induce the acrosome reaction in G. crenularis, E. lucunter, and possibly in S. franciscanus sperm. Our hypothesis is that sulfated β-galactan from G. crenularis, besides being a more flexible structure compared with the α-polysaccharides, cannot assume the precise conformation necessary for recognition by the E. lucunter and S. franciscanus sperm. In an opposite way, the conformers found in sulfated α-galactan are not recognized by G. crenularis sperm. In conclusion, our results extend the observation about the structural stringency of the sea urchin polysaccharides as inducers of the sperm acrosome reaction to a conformational level.
Acknowledgments
We thank Dr. Ana-Paula Valente for help on the NMR experiments at the Bruker 400 MHz and Adriana A. Piquet for technical assistance. We also thank Dr. Keiichiro Kyozuka and Masahiko Washio (Research Center for Marine Biology, Asamushi, Tohoku University, Japan) for collection of sea urchin G. crenularis.
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
- COSY
- correlation spectroscopy
- NOESY
- nuclear overhauser effect (NOE) spectroscopy
- TOCSY
- total correlation spectroscopy
- HSQC
- heteronuclear single quantum coherence
- MD
- molecular dynamics.
REFERENCES
- 1.Hirohashi N., Vacquier V. D. (2002) J. Biol. Chem. 277, 8041–8047 [DOI] [PubMed] [Google Scholar]
- 2.Vacquier V. D., Moy G. W. (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 2456–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biermann C. H., Marks J. A., Vilela-Silva A. C., Castro M. O., Mourão P. A. S. (2004) Evol. Dev. 6, 353–361 [DOI] [PubMed] [Google Scholar]
- 4.Vilela-Silva A. C., Hirohashi N., Mourão P. A. (2008) Int. J. Dev. Biol. 52, 551–559 [DOI] [PubMed] [Google Scholar]
- 5.Mourão P. A. S. (2007) Braz. J. Med. Biol. Res. 40, 5–17 [DOI] [PubMed] [Google Scholar]
- 6.Hirohashi N., Vilela-Silva A. C., Mourão P. A., Vacquier V. D. (2002) Biochem. Biophys. Res. Commun. 298, 403–407 [DOI] [PubMed] [Google Scholar]
- 7.Hirai E. (1963) Sci. Rep. Tôhoku Univ. Ser. IV (Biol.). 29, 369–375 [Google Scholar]
- 8.Hirohashi N., Vacquier V. D. (2002) J. Biol. Chem. 277, 1182–1189 [DOI] [PubMed] [Google Scholar]
- 9.Albano R. M., Mourão P. A. S. (1986) J. Biol. Chem. 261, 758–765 [PubMed] [Google Scholar]
- 10.Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. (1956) Anal. Chem. 28, 350–356 [Google Scholar]
- 11.Kabat E. A., Mayer M. M. (1971) in Experimental Immunochemistry, pp. 505–513, Charles C. Thomas Publisher, Springfield, IL [Google Scholar]
- 12.Farndale R. W., Buttle D. J., Barrett A. J. (1986) Biochim. Biophys. Acta 883, 173–177 [DOI] [PubMed] [Google Scholar]
- 13.Saito H., Yamagata T., Suzuki S. (1968) J. Biol. Chem. 243, 1536–1542 [PubMed] [Google Scholar]
- 14.Kircher H. W. (1960) Anal. Chem. 32, 1103–1106 [Google Scholar]
- 15.Gerwig G. J., Kamerling J. P., Vliegenthart J. F. (1979) Carbohydr. Res. 77, 10–17 [DOI] [PubMed] [Google Scholar]
- 16.Vieira R. P., Mulloy B., Mourão P. A. (1991) J. Biol. Chem. 266, 13530–13536 [PubMed] [Google Scholar]
- 17.Alves A. P., Mulloy B., Diniz J. A., Mourão P. A. S. (1997) J. Biol. Chem. 272, 6965–6971 [DOI] [PubMed] [Google Scholar]
- 18.Pomin V. H., Pereira M. S., Valente A. P., Tollefsen D. M., Pavão M. S., Mourão P. A. S. (2005) Glycobiology 15, 369–381 [DOI] [PubMed] [Google Scholar]
- 19.Mourão P. A. S., Perlin A. S. (1987) Eur. J. Biochem. 166, 431–436 [DOI] [PubMed] [Google Scholar]
- 20.Van Der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A. E., Berendsen H. J. (2005) J. Comput. Chem. 26, 1701–1718 [DOI] [PubMed] [Google Scholar]
- 21.Van Gunsteren W. F., Billeter S. R., Eising A. A., Hunenberger P. H., Kruger P., Mark A. E., Scott W. R. P., Tironi I. G. (1996) Biomolecular Simulation: The GROMOS96 Manual and User Guide, University of Groningen, Groningen, The Netherlands and ETH, Zurich, Switzerland [Google Scholar]
- 22.Schüttelkopf A. W., van Aalten D. M. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 23.Verli H., Guimarães J. A. (2004) Carbohydr. Res. 339, 281–290 [DOI] [PubMed] [Google Scholar]
- 24.Becker C. F., Guimarães J. A., Verli H. (2005) Carbohydr. Res. 340, 1499–1507 [DOI] [PubMed] [Google Scholar]
- 25.Pol-Fachin L., Verli H. (2008) Carbohydr. Res. 343, 1435–1445 [DOI] [PubMed] [Google Scholar]
- 26.Berendsen H. J., Grigera J. R., Straatsma T. P. (1987) J. Phys. Chem. 91, 6269–6271 [Google Scholar]
- 27.Verli H., Guimarães J. A. (2005) J. Mol. Graph. Model. 24, 203–212 [DOI] [PubMed] [Google Scholar]
- 28.Hess B., Bekker H., Berendsen H. J., Fraaije J. G. (1997) J. Comput. Chem. 18, 1463–1472 [Google Scholar]
- 29.Darden T., York D., Pedersen L. (1993) J. Chem. Phys. 98, 10089–10092 [Google Scholar]
- 30.Su Y. H., Vacquier V. D. (2006) Mol. Biol. Cell 17, 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves A. P., Mulloy B., Moy G. W., Vacquier V. D., Mourão P. A. (1998) Glycobiology 8, 939–946 [DOI] [PubMed] [Google Scholar]
- 32.Vilela-Silva A. C., Alves A. P., Valente A. P., Vacquier V. D., Mourão P. A. (1999) Glicobiology 9, 927–933 [DOI] [PubMed] [Google Scholar]
- 33.Vilela-Silva A. C., Castro M. O., Valente A. P., Biermann C. H., Mourao P. A. (2002) J. Biol. Chem. 277, 379–387 [DOI] [PubMed] [Google Scholar]
- 34.Pomin V. H., Valente A. P., Pereira M. S., Mourão P. A. (2005) Glycobiology 15, 1376–1385 [DOI] [PubMed] [Google Scholar]
- 35.Ravenscroft N., Gamian A., Romanowska E. (1995) Eur. J. Biochem. 227, 889–896 [DOI] [PubMed] [Google Scholar]
- 36.Hirohashi N., Vacquier V. D. (2002) Biochem. Biophys. Res. Commun. 296, 833–839 [DOI] [PubMed] [Google Scholar]
- 37.Hirohashi N., Vacquier V. D. (2003) Biochem. Biophys. Res. Commun. 304, 285–292 [DOI] [PubMed] [Google Scholar]
- 38.Amornrut C., Toida T., Imanari T., Woo E. R., Park H., Linhardt R., Wu S. J., Kim Y. S. (1999) Carbohydr. Res. 321, 121–127 [DOI] [PubMed] [Google Scholar]
- 39.Pereira M. S., Vilela-Silva A. C., Valente A. P., Mourão P. A. (2002) Carbohydr. Res. 337, 2231–2238 [DOI] [PubMed] [Google Scholar]
- 40.Woods R. J. (1995) Curr. Opin. Struct. Biol. 5, 591–598 [DOI] [PubMed] [Google Scholar]
- 41.Becker C. F., Guimarães J. A., Mourão P. A., Verli H. (2007) J. Mol. Graph. Model. 26, 391–399 [DOI] [PubMed] [Google Scholar]
- 42.Bilan M. I., Vinogradova E. V., Shashkov A. S., Usov A. I. (2007) Carbohydr. Res. 342, 586–596 [DOI] [PubMed] [Google Scholar]
- 43.Farias E. H., Pomin V. H., Valente A. P., Nader H. B., Rocha H. A., Mourão P. A. (2008) Glycobiology 18, 250–259 [DOI] [PubMed] [Google Scholar]
- 44.Mulloy B., Ribeiro A. C., Alves A. P., Vieira R. P., Mourão P. A. (1994) J. Biol. Chem. 269, 22113–22123 [PubMed] [Google Scholar]
- 45.Usov A. I., Bilan M. I., Shashkov A. S. (1997) Carbohydr. Res. 303, 93–102 [DOI] [PubMed] [Google Scholar]