Abstract
ABCA12 (ATP binding cassette transporter, family 12) is a cellular membrane transporter that facilitates the delivery of glucosylceramides to epidermal lamellar bodies in keratinocytes, a process that is critical for permeability barrier formation. Following secretion of lamellar bodies into the stratum corneum, glucosylceramides are metabolized to ceramides, which comprise ∼50% of the lipid in stratum corneum. Gene mutations of ABCA12 underlie harlequin ichthyosis, a devastating skin disorder characterized by abnormal lamellar bodies and a severe barrier abnormality. Recently we reported that peroxisome proliferator-activated receptor (PPAR) and liver X receptor activators increase ABCA12 expression in human keratinocytes. Here we demonstrate that ceramide (C2-Cer and C6-Cer), but not C8-glucosylceramides, sphingosine, or ceramide 1-phosphate, increases ABCA12 mRNA expression in a dose- and time-dependent manner. Inhibitors of glucosylceramide synthase, sphingomyelin synthase, and ceramidase and small interfering RNA knockdown of human alkaline ceramidase, which all increase endogenous ceramide levels, also increased ABCA12 mRNA levels. Moreover, simultaneous treatment with C6-Cer and each of these same inhibitors additively increased ABCA12 expression, indicating that ceramide is an important inducer of ABCA12 expression and that the conversion of ceramide to other sphingolipids or metabolites is not required. Finally, both exogenous and endogenous ceramides preferentially stimulate PPARδ expression (but not other PPARs or liver X receptors), whereas PPARδ knockdown by siRNA transfection specifically diminished the ceramide-induced increase in ABCA12 mRNA levels, indicating that PPARδ is a mediator of the ceramide effect. Together, these results show that ceramide, an important lipid component of epidermis, up-regulates ABCA12 expression via the PPARδ-mediated signaling pathway, providing a substrate-driven, feed-forward mechanism for regulating this key lipid transporter.
The outermost layer of mammalian epidermis, the stratum corneum, is essential for permeability barrier function and critical for terrestrial life. The stratum corneum consists of terminally differentiated, anucleate keratinocytes, or corneocytes, surrounded by lipid-enriched lamellar membranes composed of three major lipids, ceramides, cholesterol, and free fatty acids (1). These lipids are delivered to the extracellular spaces of the stratum corneum through exocytosis of lamellar body contents from outermost stratum granulosum cells (2). Mature lamellar bodies contain primarily cholesterol, phospholipids, and glucosylceramides (3). Following lamellar body secretion, the secreted phospholipids and glucosylceramides are converted to free fatty acids and ceramides by phospholipases and β-glucocerebrosidase, respectively (1, 4). ABCA12 (ATP binding cassette transporter, family 12), a lipid transporter predominantly expressed in epidermis, has been shown to play a vital role in the formation of mature lamellar bodies (5, 6), although how this transporter is regulated remains unresolved.
ABCA12 is a member of the ABCA subfamily of transporters, which are involved in the transport of a variety of lipids (7). Mutations in ABCA1 cause Tangier disease, which is due to a defect in transporting cholesterol and phospholipids from intracellular lipid stores to apolipoproteins, particularly apolipoprotein A-I (8–11). Mutations in ABCA3 cause neonatal respiratory failure due to a defect in surfactant transport from alveolar type II cells into the alveolar space (12). Mutations in ABCA4 cause Stargardt's macular degeneration, with visual loss due to a defect in transporting phosphatidylethanolamine-retinylidene out of retinal pigment cells (13).
Recently, mutations in ABCA12 have been shown to cause harlequin ichthyosis and a subgroup of lamellar ichthyosis, two disorders of keratinization (5, 14, 15). ABCA12 mutations lead to an abnormality in lamellar body formation, a decrease in lamellar membranes in the extracellular spaces of the stratum corneum, an accumulation of glucosylceramide in the epidermis with a reduction in ceramide (16), and ultimately loss of permeability barrier function (17), which in harlequin ichthyosis can result in neonatal lethality (5, 15). Strikingly, genetic correction of ABCA12 deficiency in patients' keratinocytes by gene transfer normalized loading of glucosylceramides into lamellar bodies (5). These studies demonstrate a critical role for ABCA12 in epidermal physiology, specifically in the formation of mature lamellar bodies and subsequent permeability barrier homeostasis. Hence, it is crucial to understand how ABCA12 is regulated.
Our laboratory recently demonstrated that activation of peroxisome proliferator-activated receptor (PPARδ and PPARγ) or liver X receptor (LXR) stimulates ABCA12 expression in cultured human keratinocytes (18). Both PPARs and LXR are important lipid sensors that stimulate keratinocyte differentiation and enhance permeability barrier function (19). Additionally, PPARα and -δ as well as LXR activators stimulate ceramide synthesis in keratinocytes (20, 21). Likewise, ceramide synthesis increases in keratinocytes during differentiation, foreshadowing the formation of lamellar bodies (22, 23).
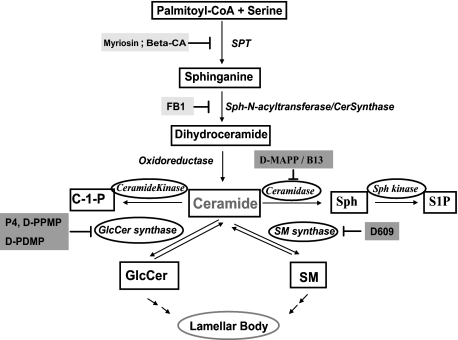
In addition to serving as structural membrane components, ceramides are also important signaling molecules that can induce growth arrest, differentiation, and apoptosis in various cells, including keratinocytes (24–26). Moreover, distal ceramide metabolites, sphingosine and sphingosine-1-phosphate (Fig. 1), are also important signaling molecules (27).
FIGURE 1.
The central role of ceramide in sphingolipid metabolism in keratinocytes. C1P, ceramide 1-phosphate; Sph, sphingosine; S1P, sphingosine-1-phosphate; GlcCer, glucosylceramide; SM, sphingomyelin.
It is well established that the expression of ABCA1 is regulated by cellular cholesterol levels in many cell types, including keratinocytes (28). Cholesterol, if metabolized to certain oxysterols, can activate LXR, which then stimulates ABCA1 expression and the transport of cholesterol out of cells (29). This example of feed-forward regulation leads us to hypothesize that either ceramide or a metabolite of ceramide might stimulate ABCA12 expression, thereby leading to an increase in the transport of glucosylceramides into maturing lamellar bodies. Here, we provide evidence that ceramide stimulates ABCA12 expression in keratinocytes via a mechanism involving PPARδ signaling.
EXPERIMENTAL PROCEDURES
Materials
D609 was purchased from BIOMOL International (Plymouth Meeting, PA). Fumonisin B1 and β-chloro-d-alanine hydrochloride (βCA) were purchased from Sigma. Myriocin was from Calbiochem. d-MAPP, l-MAPP, sphingosine, and sphinganine were from EMD Biosciences, Inc. (La Jolla, CA). Synthetic ceramides, N-hexanoyl-d-erythro-sphingosine (C6-Cer) and N-acetyl-d-erythro-sphingosine (C2-Cer), d-threo-P4 (P4), and d-threo-1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol·HCl (d-PPMP) were purchased from Matreya Inc. (Pleasant Gap, PA). C8-β-d-glucosyl ceramide was purchased from Avanti Polar lipids (Alabaster, AL). Molecular grade chemicals such as TRI reagent were obtained from either Sigma or Fisher. The iScriptTM cDNA synthesis kit for first strand cDNA synthesis was purchased from Bio-Rad. All reagents and supplies for real time PCR were purchased from Applied Biosystems (Foster City, CA). All other reagents for Western blot, including NuPAGE® Novex precast gradient gels (3–8% Tris-acetate), buffers, protein standards, and detection kits were purchased from Invitrogen.
Keratinocyte Culture
The second passage of human neonatal foreskin keratinocytes was seeded and maintained in 0.07 mm calcium chloride, serum-free 154CF with growth supplement (Cascade Biologics, Inc., Portland, OR). Once the cells were attached, the culture was switched to medium containing 0.03 mm calcium chloride, as described (18).
Trypan Blue Staining and TUNEL Assay
Trypan blue staining (Sigma) and TUNEL assay (TdT-FragELTM DNA fragmentation detection kit; Calbiochem) were carried out following the manufacturer's protocol.
Real Time PCR
Total RNA was isolated using TRI reagent, and first strand cDNA for PCR was synthesized using an iScriptTM cDNA synthesis kit, following the manufacturer's protocol. Relative mRNA levels of hABCA12 (full length), two major transcripts (hABCA12-L and -S), and an invariant transcript, cyclophilin, were determined as previously described (18). The primers used for human PPARα, -δ, and -γ, LXR-α, and LXR-β are listed in Table 1. Relative mRNA levels of ABCA1 were determined as described previously (28). The expression levels of each gene were normalized against cyclophilin using the comparative CT method and expressed as a percentage of control, with the control as 100%.
TABLE 1.
Oligonucleotide primer sequences for real time PCR
| Gene | Sequence of forward and reverse primers | GenBankTM accession number |
|---|---|---|
| PPARα | GGTGGACACGGAAAGCCCAC | NM_005036 |
| GGACCACAGGATAAGTCACC | ||
| PPARδ | CACACGGCGCCCTTTG | NM_006238 |
| CCTTCTCTGCCTGCCACAA | ||
| PPARγ | CTCATATCCGAGGGCCAA | U79012 |
| TGCCAAGTCGCTGTCATC | ||
| LXR-α | CGCACTACATCTGCCACAGT | NM_005693 |
| TCAGGCGGATCTGTTCTTCT | ||
| LXR-β | CGCTACAACCACGAGACAGA | NM_007121 |
| GAACTCGAAGATGGGGTTGA | ||
| Control siRNA | UAAGGUAUGAAGAGAUACUU | |
| GUAUCUCUUCAUAGCCUUAUU | ||
| CER1 siRNA | GGCCUGUUCUCCAUGUAUUUUU | |
| AAUACAUGGAGAACAGGCCUU |
siRNA Transfection
A control siRNA and human alkaline ceramidase 1-specific siRNA (sequences listed in Table 1) were used for transfecting cultured human keratinocytes. Human PPARδ siRNA (ON-TARGETplus SMARTpool) was purchased from Dharmacon, Inc. For human alkaline ceramidase 1 siRNA, cultured human keratinocytes were transfected at a concentration of 10 nm using Lipofectamine and PLUS reagents (Invitrogen) according to the manufacturer's instructions. For human PPARδ siRNA, cultured human keratinocytes were transfected at a concentration of 30 nm using SiLentFectTM Lipid (Bio-Rad), followed by 24-h treatment with C6-Cer. Cells were then harvested and subjected to mRNA measurement by reverse transcription-quantitative PCR.
Western Blot Analysis
Western blots were carried out according to the manufacturer's protocol. Briefly, for ABCA12, 80–100 μg of proteins prepared from keratinocytes were fractionated on precast gels (3–8%) and transferred to polyvinylidene difluoride membranes overnight at 4 °C. The proteins on the membrane were subsequently probed with monoclonal primary antibodies against human ABCA12 (30) and then visualized by secondary antibody using the ECL Western blotting detection system kit. Membranes were then exposed to CL-XPosure film. For PPARδ, similar procedures were used, except following treatment, nuclear proteins were prepared from keratinocytes, as described previously (31). An identical blot was probed with anti-β-actin antibody to verify the equal loading of protein.
Lipid Analysis
The endogenous ceramide levels in the total cellular lysates were measured as described previously (32). Lipid extracts were prepared from cellular homogenates by the method of Bligh and Dyer (33). Separation of individual lipid species was achieved by HPTLC, followed by quantification by scanning densitometry (32). Glucosylceramides and ceramides were separated first using chloroform/methanol/water (40:10:1, v/v/v) to 2.0 cm and to 5.0 cm and then chloroform/methanol/acetic acid (94:4:1, v/v/v) to 8.5 cm, followed by n-hexane-diethyl acetic acid (65:35:1, v/v/v) to the top of the plate.
Statistical Analysis
All data are expressed as mean ± S.E., except for ceramide quantification, which is expressed as mean ± S.D. Comparison between two groups is undertaken using two-tail and unpaired t test. Differences in values are considered significant if p is <0.05.
RESULTS
Exogenous Ceramides but Not Other Sphingolipids Stimulate ABCA12 mRNA
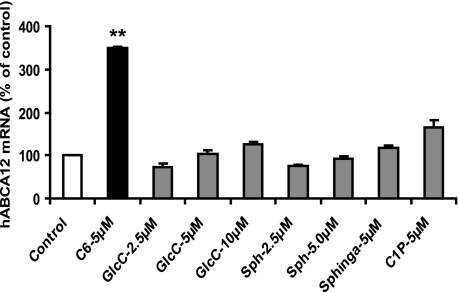
To determine whether sphingolipids regulate ABCA12 gene expression, we initially examined the effect of various exogenously added sphingolipids, including C6-Cer, C8-β-d-glucosyl ceramide, sphingosine, sphinganine, or ceramide 1-phosphate, on ABCA12 mRNA expression in cultured human keratinocytes (Fig. 2). C6-Cer, a synthetic ceramide that is cell-permeable, increased ABCA12 mRNA levels ∼3.5-fold. Similarly, another synthetic ceramide, C2-Cer, also increased ABCA12 mRNA levels (data not shown). In contrast, neither synthetic C8-β-d-glucosyl ceramide, sphingosine, sphinganine, nor ceramide 1-phosphate induces ABCA12 mRNA expression (Fig. 2). These results indicate that ceramides but not other major metabolites of ceramide elevate ABCA12 mRNA levels in human keratinocytes.
FIGURE 2.
Exogenous ceramide stimulates ABCA12 mRNA. Primary cultured human keratinocytes were incubated in the presence or absence of C6-Cer, glucosylceramide (GlcC), sphingosine (Sph), sphinganine (Sphinga), or ceramide 1-phosphate (C1P) for 16 h. ABCA12 and cyclophilin mRNA levels were then measured as described under “Experimental Procedures.” Data are expressed as a percentage of control (100%) and presented as mean ± S.E. (n = 3–4). Similar results were obtained when the experiment was repeated with a different batch of cells. **, p < 0.01; C: C6-Cer.
Ceramide Stimulates ABCA12 Expression in a Time- and Dose-dependent Manner
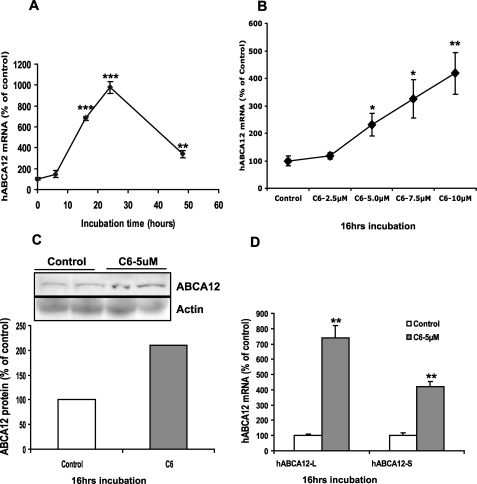
As shown in Fig. 3A, C6-Cer increased ABCA12 mRNA levels in a time-dependent fashion, with a large increase first seen at 16 h and a further increase by 24 h, which was diminished at 48 h. Further, C6-Cer also increased ABCA12 mRNA levels in a dose-dependent manner, with a half-maximal effect at 5 μm (Fig. 3B). Higher doses (≥12.5 μm) of C6-Cer were toxic to the cells (data not shown). Interestingly, the stimulatory effect of C6-Cer is specific for ABCA12, since C6-Cer did not induce the expression of another ABC transporter, ABCA1 (data not shown). Similarly, C2-Cer, another synthetic and cell-permeable ceramide, also increased ABCA12 mRNA in a similar dose- and time-dependent manner (data not shown). Furthermore, consistent with our mRNA data, an increase in ABCA12 protein mass (∼2-fold) was evident following C6-Cer treatment (Fig. 3C). Together, these results indicate that exogenous ceramide induces ABCA12 expression in cultured human keratinocytes.
FIGURE 3.
Ceramide stimulates ABCA12 mRNA expression in a time- and dose-dependent manner. Cultured human keratinocytes were incubated with C6-Cer (5 μm) for various periods of time (0, 9, 16, 24, and 48 h) (A). Alternatively, cells were incubated with various doses of C6-Cer (0–10 μm) for 16 h (B). Messenger RNA levels of full-length ABCA12 or cyclophilin were measured as described. Data are expressed as a percentage of vehicle control (100%) and presented as mean ± S.E. (n = 3–5). C, ABCA12 and β-actin protein levels were measured by Western blot following C6-Cer treatment for 16 h. Similar results were obtained when the experiment was repeated with a different batch of cells. D, messenger RNA levels of ABCA12 variants (ABCA12-L and -S) were measured, expressed as a percentage of vehicle control (100%), and presented as mean ± S.E. (n = 4). *, p < 0.05; **, p < 0.01; ***, p < 0.001; C: C6-Cer.
Finally, we reported previously that two major splicing transcripts of ABCA12, ABCA12-L and -S, are expressed in cultured human keratinocytes and that both are up-regulated by PPAR and LXR activation (18). We therefore examined whether C6-Cer also stimulates the expression of one or both of these transcripts. As we reported previously (18), the basal expression level of ABCA12-L mRNA is higher than ABCA12-S (CT, ∼24 versus ∼30). However, both transcripts are up-regulated by C6-Cer treatment (7- and 4-fold, respectively) (Fig. 3D). Thus, like PPAR and LXR activators, C6-Cer increases the expression of both ABCA12-L and -S transcripts in cultured human keratinocytes.
Glucosyltransferase Inhibitors Increase Endogenous Ceramide Levels and Induce ABCA12 Expression
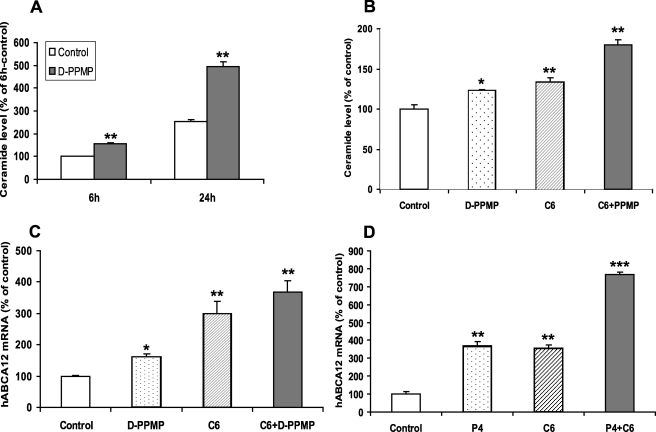
To further elucidate the mechanism responsible for ceramide-induced ABCA12 expression, we next examined ABCA12 mRNA levels after raising endogenous ceramide levels by inhibiting glucosylceramide synthase activity with three different inhibitors (Fig. 1). Treatment of cultured human keratinocytes with d-PPMP for a short (6 h) or long (24 h) period of time increased total intracellular ceramide levels (Fig. 4A) and decreased glucosylceramide levels by ∼58% (data not shown). Pertinently, treatment of cells with d-PPMP also increased ABCA12 mRNA levels (Fig. 4B), and co-treatment with d-PPMP and exogenous C6-Cer provoked a greater increase in ABCA12 mRNA level than was observed with either treatment alone, indicating an additive effect (Fig. 4B). Accordingly, although d-PPMP or C6-Cer alone increases intracellular ceramide levels, the combination of d-PPMP and C6-Cer results in a greater increase in ceramide levels (Fig. 4C), which could contribute to the greater increase in ABCA12 mRNA levels with combination treatment. Additionally, we also treated cells with P4, another inhibitor of glucosylceramide synthase, and observed a similar increase in ABCA12 mRNA levels and an additive effect with exogenous C6-Cer (Fig. 4D). Furthermore, dl-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol·HCl (DDMP), another inhibitor of glucosylceramide synthase, also increased ABCA12 mRNA levels (data not shown). These results indicate that the conversion of ceramide to glucosylceramide cannot be the basis for the C6-Cer-induced increase in ABCA12 expression, since one would have expected a decrease rather than an additive induction with simultaneous treatment with glucosylceramide synthase inhibitors. Together, these data indicate that glucosylceramide synthase inhibitors increase endogenous ceramide levels and increase ABCA12 mRNA levels and also that the conversion of ceramide to glucosylceramide is not required for the stimulation of ABCA12 expression.
FIGURE 4.
Glucosyltransferase inhibitors increase endogenous ceramide levels and induce ABCA12 mRNA. A, cultured human keratinocytes were incubated with vehicle control (open bars) or d-PPMP (10 μm; solid bars) for 6 or 24 h, and ceramide levels were determined as described under “Experimental Procedures.” Data are expressed as mean ± S.D. (n = 4). B, cells were incubated with vehicle, d-PPMP (5 μm; dotted bar), C6-Cer-5 μm (as positive control; hatched bar), or d-PPMP plus C6-Cer (solid bar) for 16 h. C, ceramide levels were determined after cells were incubated with vehicle, d-PPMP, C6-Cer, or d-PPMP plus C6-Cer for 6 h. Another set of cells were incubated with vehicle, C6-Cer (5 μm), P4 (10 μm), or P4 plus C6-Cer for 16 h (D). ABCA12 mRNA levels (B and D) were measured as described. Data are expressed as a percentage of control (100%) and presented as mean ± S.D. (n = 3–4). Experiments were repeated at least once with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001; C6: C6-Cer.
Sphingomyelin Synthase Inhibitors Increase Endogenous Ceramide Levels and Induce ABCA12 mRNA
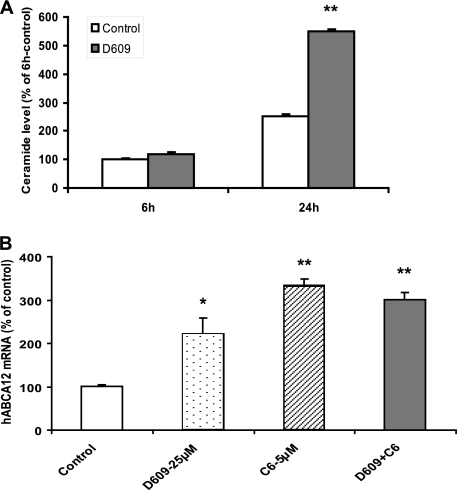
In addition to glucosylceramides, ceramides can also be readily converted into sphingomyelin, a step that can be blocked with D609, a sphingomyelin synthase inhibitor (Fig. 1). Therefore, we initially quantified intracellular ceramide levels following D609 treatment, and as predicted, after a 6- or 24-h incubation, D609 increased ceramide levels (Fig. 5A). Moreover, incubation with D609 increased ABCA12 mRNA levels. However, the combination of D609 with exogenous C6-Cer did not additively affect ABCA12 expression (Fig. 5B). Thus, increasing endogenous ceramide levels by inhibiting sphingomyelin synthase stimulates ABCA12 gene expression, similar to our observations with glucosylceramide synthase inhibition noted above. Additionally, these results demonstrate that the conversion of C6-Cer to sphingomyelin is not required for the ceramide-mediated increase in ABCA12 expression.
FIGURE 5.
Sphingomyelin synthase inhibitors increase endogenous ceramide levels and induce ABCA12 mRNA. Cultured human keratinocytes were incubated with vehicle (open bars) or d-609 (25 μm; solid bars) for 6 or 24 h, and ceramide levels were determined (n = 4) (A). Alternatively, cells were incubated with vehicle, D609 (25 μm; dotted bar), C6–5 μm (hatched bar), or D609 plus C6-Cer (solid bar) for 16 h. ABCA12 mRNA levels were measured as described (B). Data are expressed as a percentage of control (100%) and presented as mean ± S.E. (n = 3). Experiments were repeated at least once with similar results. *, p < 0.05; **, p < 0.01; C6: C6-Cer.
Ceramidase Inhibitors Increase Endogenous Ceramide Levels and Induce ABCA12 mRNA
In addition to serving as a substrate for the formation of complex sphingolipids (glucosylceramides and sphingomyelin), ceramides can be further hydrolyzed into sphingosine, catalyzed by a family of ceramidases (Fig. 1). Previously it has been reported that four of the five known ceramidase isoforms are widely expressed in cutaneous and extracutaneous tissues, including alkaline and acidic ceramidases (34), which can be blocked by d-MAPP and B13, respectively. Furthermore, as noted above, exogenous sphingosine failed to stimulate ABCA12 mRNA expression (Fig. 2).
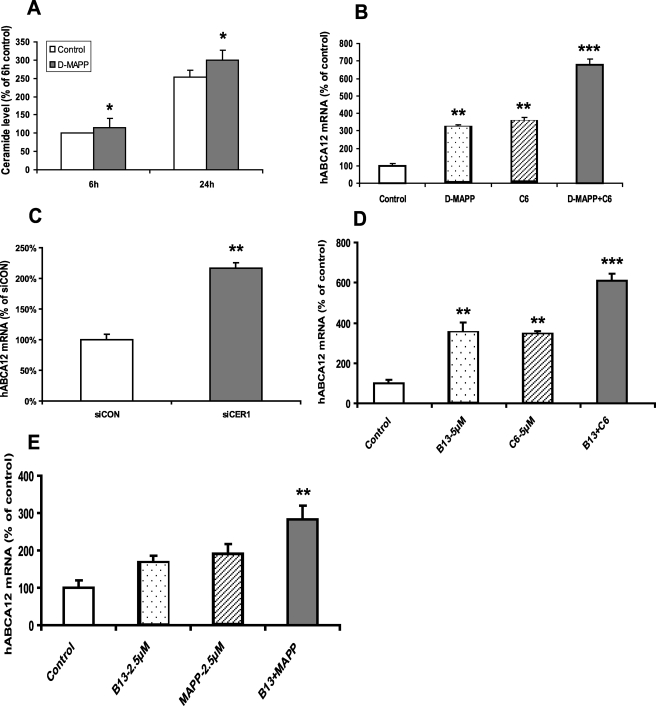
d-MAPP, an alkaline ceramidase inhibitor, has been shown to increase endogenous ceramide levels in a cancer cell line (35). As expected, d-MAPP treatment increased intracellular ceramide levels in cultured human keratinocytes (Fig. 6A). Moreover, d-MAPP increases ABCA12 mRNA levels, and the combination with C6-Cer was additive (Fig. 6B). In contrast, l-MAPP, a non-biological enantiomer of d-MAPP (35) that does not inhibit alkaline ceramidase activity, had no effect on ABCA12 expression (data not shown). Additionally, when alkaline ceramidase-1 mRNA was knocked down by siRNA transfection, a 2-fold increase in ABCA12 mRNA levels was observed in transfected cells compared with controls (Fig. 6C).
FIGURE 6.
Ceramidase inhibitors increase endogenous ceramide levels and induce ABCA12 mRNA. A, cultured human keratinocytes were incubated with vehicle (open bars) or d-MAPP (10 μm; solid bars) for 6 or 24 h, and ceramide levels were determined (n = 4). B, cells were incubated with vehicle, d-MAPP (10 μm; dotted bar), C6-Cer (5 μm; hatched bar), or d-MAPP plus C6-Cer for 16 h (n = 3). C, cells were transiently transfected with specific alkaline ceramidase-1 siRNA (solid bar) or control siRNA (open bar) (n = 4). D, cells were incubated with vehicle control, B13 (5 μm), C6-Cer (5 μm), or B13 plus C6-Cer for 16 h (n = 3). E, cells were incubated with vehicle, B13 (2.5 μm), d-MAPP (2.5 μm), or B13 plus d-MAPP for 16 h. ABCA12 mRNA levels were measured in cells (B–E), and data are expressed as a percentage of control (100%) and presented as mean ± S.E. Experiment was repeated at least once with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001; C6: C6-Cer.
B13, a compound that specifically inhibits cellular acidic ceramidase activity (36), resulting in the intracellular accumulation of ceramide (37), also induces ABCA12 mRNA levels and is additive to the effects of C6-Cer (Fig. 6D). Finally, whereas suboptimal concentrations of either d-MAPP or B13 only slightly increase ABCA12 mRNA levels, the combination of these two ceramidase inhibitors induces a significant increase in ABCA12 mRNA levels (Fig. 6E). Together, these results indicate that inhibition of ceramidase activity increases endogenous ceramide levels, leading to a stimulation of ABCA12 expression. Additionally, the metabolism of C6-Cer via the ceramidase pathway is not required for C6-Cer-induced ABCA12 gene expression.
Ceramides are well known to cause toxicity and apoptosis in some cell lines. Hence, during the course of our experiments, both trypan blue staining, a marker of cell toxicity, and a TUNEL assay, a marker of apoptosis, were performed. After 16 h of incubation, we observed that trypan blue staining was 82% in controls versus 84% in the C6-Cer (5 μm)-treated cells (n = 3; repeated once with similar results), indicating that C6-Cer did not cause cell toxicity. Similarly, following a 20-h incubation of cells with vehicle, C6-Cer (5 μm), d-PPMP (5 μm), d-MAPP (15 μm), or C6-Cer plus d-PPMP/d-MAPP, the apoptotic cell number was less than 3% in all groups (n = 3; repeated once with similar results), indicating that these treatments do not induce apoptosis in keratinocytes under these culture conditions. Thus, under our experimental condition, the cells are healthy and do not exhibit any increased toxicity or apoptosis.
Blocking de Novo Synthesis of Ceramide Does Not Affect ABCA12 mRNA Expression
Previous studies have shown that de novo ceramide synthesis is robust in epidermis/keratinocytes (32, 38). Since exogenously added ceramide induces ABCA12 expression, we next examined whether blocking ceramide biosynthesis would decrease ABCA12 expression. Myriosin and βCA are inhibitors of serine-palmitoyl transferase, the key enzyme for initiating de novo biosynthesis of ceramides (39, 40) (Fig. 1). However, treatment with either myriocin or βCA failed to alter ABCA12 expression (data not shown). Furthermore, fumonisin B1, a mycotoxin that inhibits the activity of another key enzyme required for ceramide synthesis (Fig. 1), ceramide synthase (sphingosine N-acyltransferase) (41, 42), also did not alter ABCA12 expression (data not shown). Previously, we reported that treatment with either βCA or fumonisin B1 decreases intracellular ceramide levels in cultured human keratinocytes (40). Thus, inhibiting de novo ceramide biosynthesis of ceramide did not affect ABCA12 expression.
Ceramide Induces ABCA12 Gene Expression via the PPARδ Signaling Pathway
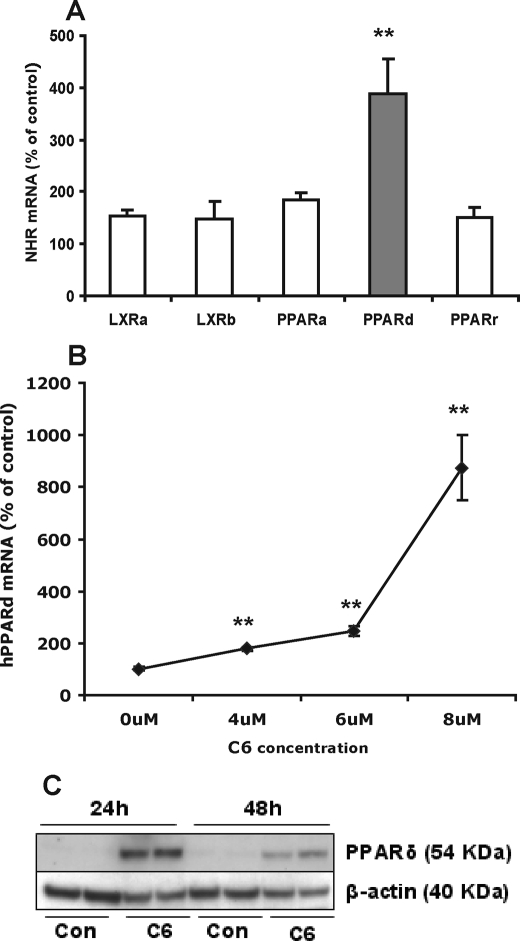
Previously, we reported that activation of PPARδ markedly stimulates ABCA12 expression in cultured human keratinocytes (18). Furthermore, activation of PPARδ also stimulates both ceramide and lamellar body formation (20). To investigate whether ceramide increases ABCA12 gene expression via the PPARδ pathway, we initially examined the effect of ceramide treatment on expression levels of PPAR (α, δ, and γ), or LXR (α and β). C6-Cer preferentially increased PPARδ mRNA levels (∼4 fold) (Fig. 7A). In contrast, C6-Cer had limited effects on PPARα, PPARγ, LXR-α, or LXR-β mRNA levels (Fig. 7A). Similar results were obtained when keratinocytes were incubated with C2-Cer (data not shown). C6-Cer also elevated PPARδ mRNA levels dose-dependently (Fig. 7B), and similar results were obtained for C2-Cer treatment (data not shown). Accordingly, C6-Cer markedly enhanced PPARδ protein levels (Fig. 7C).
FIGURE 7.
Exogenous C6-Cer preferentially stimulates PPARδ expression. Cultured human keratinocytes were incubated with C6-Cer (5 μm) or vehicle for 16 h, and mRNA levels of PPARα, PPARδ, PPARγ, LXR-α, and LXR-β were measured (A). Alternatively, cells were treated with vehicle or various doses (0, 4, 6, and 8 μm) of C6-Cer for 24 h (B), and PPARδ mRNA levels were measured, expressed as a percentage of control (100%) and presented as mean ± S.E. (n = 4). In an another set of cells, following C6-Cer (5 μm) or vehicle treatment for 24 or 48 h, PPARδ protein levels (C) were measured as described under “Experimental Procedures.” Experiments were repeated at least once with similar results. *, p < 0.05; **, p < 0.01. Con, control; C6: C6-Cer.
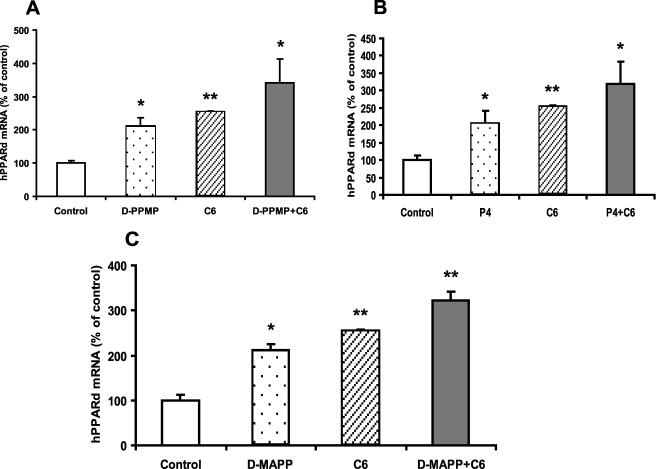
In parallel to what was seen with ABCA12, inhibitors of glucosyltransferase, such as d-PMPP and P4, increased PPARδ mRNA levels (Fig. 8, A and B). Furthermore, both d-PMPP (Fig. 8A) and P4 (Fig. 8B) were additive with C6-Cer in inducing PPARδ mRNA. Similarly, other inhibitors, such as d-MAPP, also increased PPARδ mRNA levels and were additive to C6-Cer (Fig. 8C). Thus, both exogenous and endogenous ceramides up-regulate PPARδ mRNA expression.
FIGURE 8.
Increasing endogenous ceramides by inhibiting glucosylceramide synthase or ceramidase stimulate PPARδ expression. Cultured human keratinocytes were incubated with vehicle, d-PPMP (5 μm), C6-Cer (5 μm), or d-PPMP plus C6-Cer for 16 h (A). Alternatively, cells were incubated with vehicle, P4 (10 μm), C6-Cer (5 μm), or P4 plus C6-Cer in the same medium for 16 h (B). In another set of experiments, cells were incubated with vehicle, d-MAPP (10 μm), C6-Cer (5 μm), or d-MAPP plus C6-Cer in the same medium for 16 h (C). PPARδ mRNA levels were measured, expressed as a percentage of control (100%), and presented as mean ± S.E. (n = 3). Experiments were repeated at least once with similar results. *, p < 0.05; **, p < 0.01; C: C6-Cer.
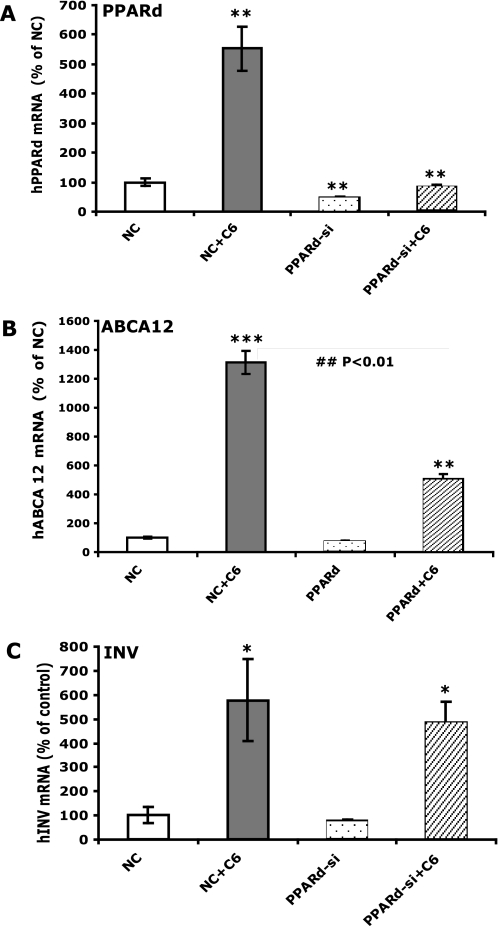
Finally, to determine if this increase in PPARδ expression could mediate the C6-Cer-induced increase in ABCA12 expression, we examined whether PPARδ knockdown by siRNA transfection would attenuate the increase in ABCA12 expression by exogenous ceramide. The siRNA knocked down PPARδ mRNA levels by ∼70% (Fig. 9A). In parallel, the PPARδ siRNA transfection greatly diminished the C6-Cer-induced increase in PPARδ mRNA levels (Fig. 9A). Accordingly, although C6-Cer significantly increased ABCA12 mRNA levels in keratinocytes transfected with negative control siRNA, this increase was markedly attenuated (∼70%) by PPARδ siRNA transfection (Fig. 9B). In contrast, the C6-Cer-induced increase in mRNA levels of involucrin, a differentiation marker of keratinocytes, was not significantly attenuated by PPARδ siRNA transfection (Fig. 9C). Together, these results indicate that the C6-Cer-induced increase in ABCA12 mRNA levels is mediated at least in part by PPARδ, since C6-Cer preferentially stimulated PPARδ expression, and knocking down PPARδ by siRNA transfection specifically attenuated the mRNA levels of ABCA12 but not other C6-Cer-inducible mRNA levels (involucrin).
FIGURE 9.
Decreasing PPARδ by siRNA specifically attenuates C6-Cer-induced ABCA12 gene expression. Cultured human keratinocytes were transiently transfected with a specific human PPARδ siRNA or negative control siRNA, followed by treatment with either vehicle or C6-Cer (5 μm) for 24 h. Messenger RNA levels of PPARδ (A), ABCA12 (B), and involucrin (C) were measured (n = 4) as described under “Experimental Procedures.” Data are expressed as a percentage of negative control (100%) and presented as mean ± S.E. Experiments were repeated twice with similar results. **, p < 0.01; ***, p < 0.001 (versus negative control). ##, p < 0.01 (versus vehicle). NC, negative control; PPARd-si, PPARδ siRNA; INV, involucrin; C6: C6-Cer.
DISCUSSION
ABCA12 plays an essential role in permeability barrier formation, since human mutations and knock-out mice have defective barriers, due to abnormal lamellar body formation (5, 14, 43). In this study, we demonstrate that ceramides, the precursor of glucosylceramides (Fig. 1), stimulate ABCA12 expression by a PPARδ-dependent mechanism.
First, we showed that exogenous, synthetic, cell-permeable ceramides, C6-Cer and C2-Cer, when incubated with human keratinocytes, increase the mRNA and protein levels of ABCA12. In contrast, sphingosine, sphinganine, glucosylceramide, or ceramide 1-phosphate did not alter ABCA12 gene expression. Second, increasing endogenous ceramide levels also stimulated ABCA12 expression. Blocking the conversion of ceramide to glucosylceramide by glucosylceramide synthase inhibitors, inhibiting sphingomyelin synthesis from ceramides by sphingomyelin synthase inhibitors, and blocking the hydrolysis of ceramides to sphingosine by ceramidase inhibitors all increased cellular ceramide levels and concomitantly increased ABCA12 mRNA levels. Thus, both short-chain, exogenous added synthetic ceramides and long-chain, endogenously produced ceramides stimulate ABCA12 gene expression. However, blocking de novo ceramide synthesis with a number of different inhibitors did not decrease ABCA12 expression. This may simply indicate that the basal expression of ABCA12 is regulated by other factors and is not dependent on ceramide levels. Alternatively, it is possible that inhibiting de novo ceramide synthesis diminishes a cellular pool that does not regulate ABCA12 expression.
Our studies further suggest that ceramides per se and not metabolites derived from ceramides (see Fig. 1) regulate ABCA12 gene expression. Blocking the conversion of ceramides into glucosylceramides or sphingomyelin by inhibitors did not decrease the ability of C6-Cer to increase ABCA12 mRNA levels. Similarly, blocking ceramidase activity, which is required for the formation of sphingosine and sphingosine 1-phosphate, also did not decrease the ability of C6-Cer to increase ABCA12 mRNA levels. Additionally, as noted above, sphingosine, sphinganine, ceramide 1-phosphate, and glucosylceramide did not alter ABCA12 gene expression. Together, these results suggest that ceramides directly regulate ABCA12 expression. However, since the specific molecular pathway by which ceramides increase ABCA12 expression has not been fully elucidated, one cannot rule out the formation of an alternative ceramide metabolite accounting for the effect.
The involvement of ceramides in gene regulation has been well documented. In cultured keratinocytes, synthetic C6-Cer and C2-Cer have been shown to inhibit cell proliferation and to promote cell differentiation (44). In addition, ceramide enhances glucosylceramide synthase activity via increasing gene transcription (45, 46) and induces acid sphingomyelinase expression (47, 48). Ceramides have also been shown to directly stimulate the transcription of cyclooxygenase-2 in human breast epithelial cells (49) and indirectly mediate the tumor necrosis factor α-induced increase in cyclooxygenase-2 expression in human alveolar epithelial cells (50). In this study, we demonstrated that ceramides stimulate the expression of ABCA12, an important transporter for glucosylceramides. One can view this as a feed-forward effect. As the keratinocyte begins to produce increasing quantities of ceramides that will lead to increased glucosylceramide synthesis, the ceramides signal the cell to increase ABCA12 levels, which will facilitate the transport of glucosylceramides into lamellar bodies. To further support this notion, Zuo et al. (16) recently revealed that loss of ABCA12 transport function by generating Abca12−/− mice causes a 90% reduction in epidermal linoleic esters of long-chain ω-hydroxy-ceramides, associated with an increase in their glucosyl ceramide precursors. Thus, the ceramides serve not only as a precursor of glucosylceramide formation but also as a signaling molecule that will allow the keratinocyte to transport the glucosylceramide to the appropriate location within the lamellar body.
To further elucidate the mechanism underlying the ceramide-induced stimulation of ABCA12 expression, we carried out time course studies and noted that the increase in ABCA12 mRNA occurs only after a ∼9-h incubation, suggesting a secondary rather than a direct stimulatory effect. Previous studies by our laboratory (18) have shown that activation of PPARδ stimulates ABCA12 expression. These observations prompted us to hypothesize that ceramide might induce ABCA12 expression via PPARδ. Our results demonstrate that PPARδ signaling does play an important role in the ceramide-induced stimulation of ABCA12 expression in keratinocytes. First, C6-Cer increases PPARδ expression at both mRNA and protein levels. Second, the stimulatory effect on PPARδ expression is not limited to exogenous ceramides but also was seen with increases in endogenously produced ceramides. In contrast, ceramides did not increase the expression of PPARα, PPARγ, LXR-α, or LXR-β. Third, PPARδ mRNA silencing by siRNA transfection results in a significant attenuation of the ceramide-induced increase in ABCA12 gene expression. This attenuation effect of ceramides on ABCA12 is specific, since the ability of ceramides to induce other genes, such as involucrin, was not altered by decreasing PPARδ.
Thus, the ceramide-induced stimulation of ABCA12 gene expression is an indirect effect and probably involves multiple steps. Studies have shown that the expression of PPARδ is increased by AP-1 activation (51). Moreover, numerous studies have shown that ceramides increase AP-1 activity in keratinocytes and other cells (51–55). We would therefore hypothesize that ceramides increase AP-1 activity, leading to the increased expression of PPARδ, which subsequently stimulates ABCA12 gene expression.
PPARδ is the most abundant PPAR isoform in keratinocytes and is found throughout all layers of the epidermis (56). In human keratinocytes, PPARδ plays an important role in regulating gene expression and regulates epidermal proliferation, differentiation, and wound healing (19, 57). For example, activation of PPARδ stimulates the expression of proteins, including involucrin, loricrin, filaggrin, and transglutaminase 1, required for the differentiation of keratinocytes into corneocytes (58, 59). PPARδ activation also improves epidermal permeability barrier homeostasis by stimulating epidermal lipid biosynthesis, including de novo ceramide synthesis (20). Finally, activation of PPARδ increases β-glucocerebrosidase activity in the stratum corneum (20), a key enzyme required for the conversion of glucosylceramides to ceramides and the formation of normal lamellar membranes. Whether the ability of ceramides to increase PPARδ expression will account for most of the observed effects of ceramides on keratinocytes remains to be determined. However, it should be noted that the increase in involucrin expression induced by ceramide is not mediated by PPARδ signaling. Nonetheless, our study demonstrated that PPARδ mediates the ceramide-induced ABCA12 expression in keratinocytes.
In summary, our results show that ceramide, an important lipid component of epidermis, up-regulates ABCA12 expression via the PPARδ signaling pathway, providing a substrate-driven, feed-forward mechanism for regulating this key lipid transporter required for lamellar body formation.
Acknowledgments
We thank Sally Pennypacker for the excellent technical support of cell culture.
This work was supported, in whole or in part, by National Institutes of Health Grants AR39448 and AR049932. This work was also supported by the Medical Research Service, Department of Veterans Affairs at San Francisco.
- PPAR
- peroxisome proliferator-activated receptor
- LXR
- liver X receptor
- βCA
- β-chloro-l-alanine hydrochloride
- C2-Cer
- N-acetyl-d-erythro-sphingosine
- C6-Cer
- N-hexanoyl-d-erythro-sphingosine
- d-MAPP
- d-erythro-2-tetradecanoylamino-1-phenyl-1-propanol
- l-MAPP
- l-erythro-2-tetradecanoylamino-1-phenyl-1-propanol
- P4
- d-threo-1-phenyl-2-palmitoyl-3-pyrrolidinopropanol
- d-PPMP
- d-threo-1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol·HCl
- TUNEL
- terminal dUTP nick-end labeling
- siRNA
- small interfering RNA.
REFERENCES
- 1.Feingold K. R. (2007) J. Lipid Res. 48, 2531–2546 [DOI] [PubMed] [Google Scholar]
- 2.Elias P. M., Feingold K. R. (ed) (2006) Epidermal Lamellar Body as A Multifunctional Secretory Organelle, pp. 261–272, Taylor & Francis, New York [Google Scholar]
- 3.Grayson S., Johnson-Winegar A. G., Wintroub B. U., Isseroff R. R., Epstein E. H., Jr., Elias P. M. (1985) J. Invest. Dermatol. 85, 289–294 [DOI] [PubMed] [Google Scholar]
- 4.Holleran W. M., Takagi Y., Uchida Y. (2006) FEBS Lett. 580, 5456–5466 [DOI] [PubMed] [Google Scholar]
- 5.Akiyama M., Sugiyama-Nakagiri Y., Sakai K., McMillan J. R., Goto M., Arita K., Tsuji-Abe Y., Tabata N., Matsuoka K., Sasaki R., Sawamura D., Shimizu H. (2005) J. Clin. Invest. 115, 1777–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hovnanian A. (2005) J. Clin. Invest. 115, 1708–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiyama M. (2006) Arch. Dermatol. 142, 914–918 [DOI] [PubMed] [Google Scholar]
- 8.Bodzioch M., Orsó E., Klucken J., Langmann T., Böttcher A., Diederich W., Drobnik W., Barlage S., Büchler C., Porsch-Ozcürümez M., Kaminski W. E., Hahmann H. W., Oette K., Rothe G., Aslanidis C., Lackner K. J., Schmitz G. (1999) Nat. Genet. 22, 347–351 [DOI] [PubMed] [Google Scholar]
- 9.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., Loubser O., Ouelette B. F., Fichter K., Ashbourne-Excoffon K. J., Sensen C. W., Scherer S., Mott S., Denis M., Martindale D., Frohlich J., Morgan K., Koop B., Pimstone S., Kastelein J. J., Genest J., Jr., Hayden M. R. (1999) Nat. Genet. 22, 336–345 [DOI] [PubMed] [Google Scholar]
- 10.Lawn R. M., Wade D. P., Garvin M. R., Wang X., Schwartz K., Porter J. G., Seilhamer J. J., Vaughan A. M., Oram J. F. (1999) J. Clin. Invest. 104, R25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denèfle P., Assmann G. (1999) Nat. Genet. 22, 352–355 [DOI] [PubMed] [Google Scholar]
- 12.Shulenin S., Nogee L. M., Annilo T., Wert S. E., Whitsett J. A., Dean M. (2004) N. Engl. J. Med. 350, 1296–1303 [DOI] [PubMed] [Google Scholar]
- 13.Allikmets R. (1997) Nat. Genet. 17, 122. [DOI] [PubMed] [Google Scholar]
- 14.Lefévre C., Audebert S., Jobard F., Bouadjar B., Lakhdar H., Boughdene-Stambouli O., Blanchet-Bardon C., Heilig R., Foglio M., Weissenbach J., Lathrop M., Prud'homme J. F., Fischer J. (2003) Hum. Mol. Genet. 12, 2369–2378 [DOI] [PubMed] [Google Scholar]
- 15.Kelsell D. P., Norgett E. E., Unsworth H., Teh M. T., Cullup T., Mein C. A., Dopping-Hepenstal P. J., Dale B. A., Tadini G., Fleckman P., Stephens K. G., Sybert V. P., Mallory S. B., North B. V., Witt D. R., Sprecher E., Taylor A. E., Ilchyshyn A., Kennedy C. T., Goodyear H., Moss C., Paige D., Harper J. I., Young B. D., Leigh I. M., Eady R. A., O'Toole E. A. (2005) Am. J. Hum. Genet. 76, 794–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo Y., Zhuang D. Z., Han R., Isaac G., Tobin J. J., McKee M., Welti R., Brissette J. L., Fitzgerald M. L., Freeman M. W. (2008) J. Biol. Chem. 283, 36624–36635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moskowitz D. G., Fowler A. J., Heyman M. B., Cohen S. P., Crumrine D., Elias P. M., Williams M. L. (2004) J. Pediatr. 145, 82–92 [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y. J., Lu B., Kim P., Paragh G., Schmitz G., Elias P. M., Feingold K. R. (2008) J. Invest. Dermatol. 128, 104–109 [DOI] [PubMed] [Google Scholar]
- 19.Schmuth M., Jiang Y. J., Dubrac S., Elias P. M., Feingold K. R. (2008) J. Lipid Res. 49, 499–509 [DOI] [PubMed] [Google Scholar]
- 20.Man M. Q., Choi E. H., Schmuth M., Crumrine D., Uchida Y., Elias P. M., Holleran W. M., Feingold K. R. (2006) J. Invest. Dermatol. 126, 386–392 [DOI] [PubMed] [Google Scholar]
- 21.Chang K. C., Shen Q., Oh I. G., Jelinsky S. A., Jenkins S., Wang W., Wang Y., Lacava M., Yudt M. R., Thompson C. C., Freedman L. P., Chung J. H., Nagpal S. (2008) Mol. Endocrinol. 22, 2407–2419 [DOI] [PubMed] [Google Scholar]
- 22.Lampe M. A., Williams M. L., Elias P. M. (1983) J. Lipid Res. 24, 131–140 [PubMed] [Google Scholar]
- 23.Vielhaber G., Pfeiffer S., Brade L., Lindner B., Goldmann T., Vollmer E., Hintze U., Wittern K. P., Wepf R. (2001) J. Invest. Dermatol. 117, 1126–1136 [DOI] [PubMed] [Google Scholar]
- 24.Geilen C. C., Bektas M., Wieder T., Kodelja V., Goerdt S., Orfanos C. E. (1997) J. Biol. Chem. 272, 8997–9001 [DOI] [PubMed] [Google Scholar]
- 25.Hannun Y. A., Obeid L. M. (2002) J. Biol. Chem. 277, 25847–25850 [DOI] [PubMed] [Google Scholar]
- 26.Uchida Y., Nardo A. D., Collins V., Elias P. M., Holleran W. M. (2003) J. Invest. Dermatol. 120, 662–669 [DOI] [PubMed] [Google Scholar]
- 27.Spiegel S., Milstien S. (2002) J. Biol. Chem. 277, 25851–25854 [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y. J., Lu B., Kim P., Elias P. M., Feingold K. R. (2006) J. Lipid Res. 47, 2248–2258 [DOI] [PubMed] [Google Scholar]
- 29.Venkateswaran A., Laffitte B. A., Joseph S. B., Mak P. A., Wilpitz D. C., Edwards P. A., Tontonoz P. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama M., Titeux M., Sakai K., McMillan J. R., Tonasso L., Calvas P., Jossic F., Hovnanian A., Shimizu H. (2007) J. Invest. Dermatol. 127, 568–573 [DOI] [PubMed] [Google Scholar]
- 31.Neish A. S., Khachigian L. M., Park A., Baichwal V. R., Collins T. (1995) J. Biol. Chem. 270, 28903–28909 [DOI] [PubMed] [Google Scholar]
- 32.Holleran W. M., Man M. Q., Gao W. N., Menon G. K., Elias P. M., Feingold K. R. (1991) J. Clin. Invest. 88, 1338–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 34.Houben E., Holleran W. M., Yaginuma T., Mao C., Obeid L. M., Rogiers V., Takagi Y., Elias P. M., Uchida Y. (2006) J. Lipid Res. 47, 1063–1070 [DOI] [PubMed] [Google Scholar]
- 35.Bielawska A., Greenberg M. S., Perry D., Jayadev S., Shayman J. A., McKay C., Hannun Y. A. (1996) J. Biol. Chem. 271, 12646–12654 [DOI] [PubMed] [Google Scholar]
- 36.Selzner M., Bielawska A., Morse M. A., Rudiger H. A., Sindram D., Hannun Y. A., Clavien P. A. (2001) Cancer Res. 61, 1233–1240 [PubMed] [Google Scholar]
- 37.Fishelevich R., Malanina A., Luzina I., Atamas S., Smyth M. J., Porcelli S. A., Gaspari A. A. (2006) J. Immunol. 176, 2590–2599 [DOI] [PubMed] [Google Scholar]
- 38.Holleran W. M., Williams M. L., Gao W. N., Elias P. M. (1990) J. Lipid Res. 31, 1655–1661 [PubMed] [Google Scholar]
- 39.Miyake Y., Kozutsumi Y., Nakamura S., Fujita T., Kawasaki T. (1995) Biochem. Biophys. Res. Commun. 211, 396–403 [DOI] [PubMed] [Google Scholar]
- 40.Uchida Y., Murata S., Schmuth M., Behne M. J., Lee J. D., Ichikawa S., Elias P. M., Hirabayashi Y., Holleran W. M. (2002) J. Lipid Res. 43, 1293–1302 [PubMed] [Google Scholar]
- 41.Merrill A. H., Jr., van Echten G., Wang E., Sandhoff K. (1993) J. Biol. Chem. 268, 27299–27306 [PubMed] [Google Scholar]
- 42.Merrill A. H., Jr., Sullards M. C., Wang E., Voss K. A., Riley R. T. (2001) Environ. Health Perspect. 109, Suppl. 2, 283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanagi T., Akiyama M., Nishihara H., Sakai K., Nishie W., Tanaka S., Shimizu H. (2008) Hum. Mol. Genet. 17, 3075–3083 [DOI] [PubMed] [Google Scholar]
- 44.Wakita H., Tokura Y., Yagi H., Nishimura K., Furukawa F., Takigawa M. (1994) Arch. Dermatol. Res. 286, 350–354 [DOI] [PubMed] [Google Scholar]
- 45.Liu Y. Y., Yu J. Y., Yin D., Patwardhan G. A., Gupta V., Hirabayashi Y., Holleran W. M., Giuliano A. E., Jazwinski S. M., Gouaze-Andersson V., Consoli D. P., Cabot M. C. (2008) FASEB J. 22, 2541–2551 [DOI] [PubMed] [Google Scholar]
- 46.Komori H., Ichikawa S., Hirabayashi Y., Ito M. (2000) FEBS Lett. 475, 247–250 [DOI] [PubMed] [Google Scholar]
- 47.Deigner H. P., Claus R., Bonaterra G. A., Gehrke C., Bibak N., Blaess M., Cantz M., Metz J., Kinscherf R. (2001) FASEB J. 15, 807–814 [DOI] [PubMed] [Google Scholar]
- 48.Murate T., Suzuki M., Hattori M., Takagi A., Kojima T., Tanizawa T., Asano H., Hotta T., Saito H., Yoshida S., Tamiya-Koizumi K. (2002) J. Biol. Chem. 277, 9936–9943 [DOI] [PubMed] [Google Scholar]
- 49.Subbaramaiah K., Chung W. J., Dannenberg A. J. (1998) J. Biol. Chem. 273, 32943–32949 [DOI] [PubMed] [Google Scholar]
- 50.Chen C. C., Sun Y. T., Chen J. J., Chang Y. J. (2001) Mol. Pharmacol. 59, 493–500 [DOI] [PubMed] [Google Scholar]
- 51.Tan N. S., Michalik L., Noy N., Yasmin R., Pacot C., Heim M., Flühmann B., Desvergne B., Wahli W. (2001) Genes Dev. 15, 3263–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westwick J. K., Bielawska A. E., Dbaibo G., Hannun Y. A., Brenner D. A. (1995) J. Biol. Chem. 270, 22689–22692 [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y., Nichols J. E., Valdez R., Mendelson C. R., Simpson E. R. (1996) Mol. Endocrinol. 10, 1350–1357 [DOI] [PubMed] [Google Scholar]
- 54.Quillet-Mary A., Jaffrézou J. P., Mansat V., Bordier C., Naval J., Laurent G. (1997) J. Biol. Chem. 272, 21388–21395 [DOI] [PubMed] [Google Scholar]
- 55.Reunanen N., Westermarck J., Häkkinen L., Holmström T. H., Elo I., Eriksson J. E., Kähäri V. M. (1998) J. Biol. Chem. 273, 5137–5145 [DOI] [PubMed] [Google Scholar]
- 56.Braissant O., Wahli W. (1998) Endocrinology 139, 2748–2754 [DOI] [PubMed] [Google Scholar]
- 57.Schmuth M., Jiang Y. J., Dubrac S., Elias P. M., Feingold K. R. (2008) J. Lipid Res. 49, 499–509 [DOI] [PubMed] [Google Scholar]
- 58.Westergaard M., Henningsen J., Svendsen M. L., Johansen C., Jensen U. B., Schrøder H. D., Kratchmarova I., Berge R. K., Iversen L., Bolund L., Kragballe K., Kristiansen K. (2001) J. Invest. Dermatol. 116, 702–712 [DOI] [PubMed] [Google Scholar]
- 59.Schmuth M., Haqq C. M., Cairns W. J., Holder J. C., Dorsam S., Chang S., Lau P., Fowler A. J., Chuang G., Moser A. H., Brown B. E., Mao-Qiang M., Uchida Y., Schoonjans K., Auwerx J., Chambon P., Willson T. M., Elias P. M., Feingold K. R. (2004) J. Invest. Dermatol. 122, 971–983 [DOI] [PubMed] [Google Scholar]