Abstract
Background
There is very little information on what is considered an adequate energy intake for mechanically ventilated, critically ill patients. The purpose of the present study was to determine this energy requirement by making use of patients' nutritional status.
Methods
The study was conducted in a multidisciplinary intensive care unit of Taichung Veterans General Hospital, Taiwan. Patients were hemodynamically stable and not comatose, and were requiring at least 7 days of mechanical ventilation. Fifty-four patients successfully completed this study. The resting energy expenditure was measured using indirect calorimetry. The total energy requirement was considered 120% of the measured energy expenditure. The daily nutrient intake was recorded. Nutritional status was assessed using single and multiple parameters, nitrogen balance, and medical records, and was performed within 24 hours of admission and after 7 days in the intensive care unit.
Results
Fifteen patients were being underfed (<90% of total energy requirement), 20 patients were in the appropriate feeding (AF) group (within ± 10% of total energy requirement), and 19 patients received overfeeding (>110% of total energy requirement). Patients in the underfeeding group received only 68.3% of their energy requirement, while the overfeeding group patients received up to 136.5% of their required calories. Only patients in the AF group had a positive nitrogen balance (0.04 ± 5.1) on day 7. AF group patients had a significantly higher Nutritional Risk Index value at day 7 than at day 1.
Conclusion
AF patients had more improvement in nutritional status than patients in the other feeding groups. To provide at least 120% of the resting energy expenditure seemed adequate to meet the caloric energy needs of hemodynamically stable, mechanically ventilated, critically ill patients.
Keywords: appropriate feeding, critically ill, energy requirement, nutritional status, overfeeding, underfeeding
Introduction
Sufficient nutrients are needed for critically ill patients to meet metabolic needs. Previous studies have shown that appropriate nutritional feeding during critical illness improves the success in weaning patients from mechanical ventilation and in reducing the length of hospitalization [1-5]. Underfeeding (UF) decreases the regeneration of respiratory epithelium and causes respiratory muscular weakness [6], and it may prolong mechanical ventilation by failing to restore respiratory muscle strength and endurance. Overfeeding (OF), in contrast, increases physiological stress and also prolongs mechanical ventilation by increasing carbon dioxide production, which increases the amount of ventilation necessary to maintain a steady state of arterial blood gases [7]. Unfortunately, severe protein-caloric malnutrition is still a major problem in many critically ill patients. Our previous study [8] even showed that 94% of intensive care unit (ICU) patients were malnourished after 14 days of admission. It thus becomes very important to determine adequate and appropriate energy needs for mechanically ventilated, critically ill patients.
Although appropriate feeding (AF) is required for mechanically ventilated, critically ill patients, there is very little information on the energy requirement for these patients. The purpose of the present study was to determine the energy requirement for mechanically ventilated, critically ill patients using the nutritional status.
Patients and methods
Patients
This study was conducted in the ICU of Taichung Veterans General Hospital, a 1359-bed teaching hospital in the central part of Taiwan. The study was approved by the Institutional Review Board of Chung Shan Medical University. Informed consent was obtained. Only patients that were hemodynami-cally stable and not comatose, and requiring at least 7 days of mechanical ventilation, were included.
Patients were excluded when they received a fraction of inspired oxygen > 60% at the time of indirect calorimetry measurement. Oliguric patients were also excluded since abnormal renal function would not give a 24-hour urine volume of sufficient steady state for confidence in values for urinary nitrogen excretion. One hundred and eighteen patients entered the present study. However, only fifty-four patients (40 men, 14 women) successfully completed the study. Medical records including diagnosis, severity of illness, length of ICU stay and length of hospital stay, and ventilatory dependency were obtained. The severity of illness of these patients was evaluated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score [9] on day 1 and day 7 of admission.
Energy requirements and nutrient intakes
The Deltatrac™ II MBM-200 Metabolic Monitor (Datex-Engstrom Division, Instrumentation Corp., Datex-Engstrom, Finland) was used to measure the actual energy expenditure (measured energy expenditure [MEE]) of patients on two occasions during the study (day 1 and day 6 or 7 of the study). The MEE was based on a single measure taken on day 1 and thereafter used as the goal. The Deltatrac™ II MBM-200 Metabolic Monitor can be used to perform indirect calorimetry with most commonly used ventilators.
Patients were without intermittent caloric intake for at least 4 hours prior to the indirect calorimetry measurement. However, patients receiving continuous enteral or parenteral infusions were not interrupted during the measurement. The patient was connected via an endotracheal tube to a spirometer filled with 100% O2 attached to a kymograph. As the patient breathed, the oxygen was consumed and CO2 was exhaled. The water and CO2 vapor were mechanically absorbed, so that volume changes in the spirometer were only due to the consumption of oxygen. The oxygen uptake by the lungs was determined from the amount of oxygen consumed from the spirometer. All measurements were performed by one trained technician. The flowmeter, and the CO2 and O2 analyzers are automatically calibrated before each measurement. Patients' oxygen consumption was measured for at least 40 min each time. Nitrogen excretion data was not available for nine patients. The Weir equation [10], which assumes that 12.3% of the total calories arise from protein metabolism, was then used to calculate nitrogen excretion. The total energy requirement was considered the MEE plus a 5% activity factor [11,12] and plus 15% for day-to-day variability [13].
Patients received nutrition support (enteral nutrition, total parenteral nutrition, or combined enteral nutrition plus total parenteral nutrition), which was based on the physicians' concerns for the clinical conditions of the patient. The composition of tube feeding formulas was prepared from various commercial products based on the patient's nutritional needs. The daily macronutrient (carbohydrate, protein, and fat) intake from nutritional support (enteral or parenteral nutrition) and the intravenous crystalloid infusions were recorded routinely by the ICU nurses and dietitians during the study. The amount of nutrient intake represented in the result was the mean amount of feed delivered in each group daily, up to and including the day of assessment. All of the nonfeed sources of macronutrients were also included in the calculations. Subjects were then divided into three groups based on the degree of feeding (UF, AF, and OF) as previously described [14]. UF was defined as a subject's actual average energy intake being less than 90% of total energy requirements. AF was defined as a subject's actual average energy intake being within ± 10% of total energy requirements. For OF, the actual average energy intake was larger than 110% of the total energy requirements.
Nutritional status assessment
Nutritional status was assessed within 24 hours of admission and after 7 days in the ICU by the trained technician. The patients' nutritional status was determined from anthropometric and biochemical measurements, and from medical records.
Anthropometric measurements included patients' height and weight on admission, triceps skinfold thickness (TSF), and mid-arm circumference (MAC). All the anthropometric measurements were performed by one trained technician. The body mass index, mid-arm muscle circumference, and arm muscle area were then calculated: body mass index = weight (kg)/height2(m), mid-arm muscle circumference (mm) = MAC (mm) - (TSF [mm] × 3.14), and arm muscle area (mm2) = (MAC [mm] - [TSF × 3.14])2 / 4π.
Biochemical measurements included serum albumin and pre-albumin, and total lymphocyte count (TLC). A 24-hour urine collection was obtained from patients for measurement of urine urea nitrogen (UUN) and of creatinine. The creatinine height index was calculated from 24-hour urinary creatinine excretion [15]. The nitrogen balance was derived from the daily volume of infusate minus the daily output of urinary nitrogen: nitrogen balance (g/day) = nitrogen intake - (UUN + 4 g obligatory loss). The following values were considered normal for our laboratory: serum albumin, 3.5–5.0 g/dl; prealbumin, 18–43 mg/dl; TLC > 1500/mm3; and creatinine height index > 90%.
Two multiparameter nutritional status indices, the Maastricht Index (MI) [16] and the Nutritional Risk Index (NRI) [17], were also used to assess the nutritional status of patients. The MI was calculated as follows: MI = 20.68 - (0.24 × albumin [g/l]) - (19.21 × prealbumin [g/l]) - (1.86 × lymphocytes [109/l]) - (0.04 × percentage ideal weight). Patients with MI > 0 were considered malnourished. The NRI was developed to assess malnutrition of surgical patients: NRI = (15.9 × plasma albumin [g/dl]) + 41.7 × (present weight / usual weight). A value of NRI > 100 indicates that the subject is not malnourished, 97.5–100 indicates the subject is mildly malnourished, 83.5 to < 97.5 indicates that the subject is moderately mal-nourished, and NRI < 83.5 indicates that the subject is severely malnourished.
Statistical analyses
Data were analyzed using the SigmaStat statistical software (version 2.03; Jandel Scientific, San Rafael, CA, USA). Differences in patients' anthropometric and biochemical values, nutritional status indices, and clinical outcome among groups were compared for significant differences using one-way analysis of variance. The Student t test was used to compare the difference between day 1 and day 7 within a group. Pearson correlation coefficients were determined to assess the relationship between energy requirement, nutrient intake, and clinical results. Statistical results were considered significant at P ≤ 0.05. Values are presented as means ± standard deviation.
Results
Characteristics of patients
Fifteen patients (14 men, one woman) were being underfed, 20 patients (17 men, three women) were in the AF group, and 19 patients (11 men, eight women) were overfed while they were in the ICU. Patients' ages ranged from 23 to 89 years, with a mean age of 61.9 ± 14.6 years, 68.0 ± 14.1 years and 72.2 ± 2.1 years for the UF group, the AF group and the OF group, respectively. There were no statistically significant differences among groups for age, height, and weight at admission. The diagnoses of patients at the time of admission in the ICU are presented in Table 1. The most common diagnoses were gastrointestinal disorders, malignant neoplasms, and pneumonia.
Table 1.
Diagnosis of patients at the time of admission in the intensive care unit
| Underfeeding (n = 15) | Appropriate feeding (n = 20) | Overfeeding (n = 19) | |
| Respiratory disease | 2 | 1 | 5 |
| Gastrointestinal disorder | 5 | 9 | 7 |
| Malignant neoplasms | 6 | 4 | 4 |
| Infection | 0 | 4 | 2 |
| Trauma | 1 | 2 | 1 |
| Burn | 1 | 0 | 0 |
Respiratory diseases include pneumonia; gastrointestinal disorders include peptic ulcers, enteritis, acute pancreatitis, acute cholecystitis, gastric ulcers, duodenal ulcers, peritonitis, and intestinal obstructions; malignant neoplasms include esophageal tumors, hypopharyngeal tumors, and colon cancer; infection includes deep neck infections, viral meningitis, and acute maxillary sinusitis; trauma includes pelvic open fractures, and diaphragm ruptures.
Energy requirements versus energy intakes
The type of feeding route, values of the respiratory quotient (RQ), the MEE, the total energy requirements and the nutrient intake are presented in Table 2. UF patients were more likely to be receiving combined nutrition (n = 8), while AF and OF patients were more likely to be receiving enteral (n = 7) and combined (n = 7) nutrition. OF patients had a mean RQ value greater than 1. UF patients had the highest MEE and total energy requirement.
Table 2.
The type of feeding route, energy requirement and actual macronutrient intakes of patients
| Underfeeding (n = 15) | Appropriate feeding (n = 20) | Overfeeding (n = 19) | |
| Feeding route | |||
| Enteral nutrition (number of subjects) | 2 | 7 | 7 |
| Parenteral nutrition (number of subjects) | 5 | 6 | 5 |
| Combined nutrition (number of subjects) | 8 | 7 | 7 |
| Energy expenditure | |||
| Respiratory quotient | 0.91 ± 0.19b | 0.97 ± 0.12a,b | 1.16 ± 0.32a |
| Measured energy expenditure* (kcal/day) | 1878.9 ± 359.5a | 1572.0 ± 256.0b | 1272.4 ± 194.9c |
| Total energy requirement† (kcal/day) | 2254.7 ± 431.3a | 1886.4 ± 307.2b | 1526.9 ± 233.8c |
| Total energy requirement† (kcal/kg/day) | 35.7 ± 7.3 | 30.6 ± 6.9 | 30.6 ± 7.0 |
| Nutrient intake | |||
| Energy (kcal) | 1541.1 ± 305.9b | 1869.6 ± 303.4a | 2084.9 ± 448.9a |
| Energy (kcal/kg per day) | 24.9 ± 6.0b | 30.3 ± 7.4b | 41.5 ± 11.5a |
| Carbohydrate (g) | 246.4 ± 61.8 | 273.9 ± 77.3 | 304.3 ± 84.4 |
| Carbohydrate (g/kg per day) | 4.0 ± 1.1b | 4.4 ± 1.3b | 6.0 ± 1.7a |
| % of total energy | 63.7 ± 10.4 | 58.5 ± 11.1 | 58.0 ± 6.2 |
| Protein (g) | 59.8 ± 16.8 | 68.1 ± 22.7 | 66.4 ± 16.3 |
| Protein (g/kg per day) | 1.0 ± 0.3 | 1.1 ± 0.5 | 1.3 ± 0.5 |
| % of total energy | 16.3 ± 6.6 | 14.7 ± 5.2 | 12.9 ± 2.4 |
| Fat (g) | 42.2 ± 20.7b | 57.0 ± 19.1a,b | 66.7 ± 11.8a |
| Fat (g/kg per day) | 0.7 ± 0.4b | 0.9 ± 0.3b | 1.4 ± 0.5a |
| % of total energy | 24.2 ± 10.9 | 27.7 ± 9.0 | 29.5 ± 5.7 |
Values presented as mean ± standard deviation. Values in a row with different superscript letters are significantly different, P ≤ 0.05. *Resting energy expenditure was measured using indirect calorimetry. †Total energy requirement = measured energy expenditure + physical activity (5%) + day-to-day variation (15%).
It is not surprising to find OF patients consuming significantly more calories than the other two groups. UF patients received, on average, only 68.3% of their energy requirements, while OF patients received up to 136.5% of their estimated caloric requirements. The energy intake of the OF patients was more than 40 kcal/kg per day on average. Looking at the individual data, three AF patients and nine OF patients had an energy intake of more than 40 kcal/kg per day. OF patients had a higher energy intake attributed to carbohydrate and fat, but not to protein. There was no significant difference, however, in the percentage of energy intakes from carbohydrate, protein, and fat.
OF patients received a significantly higher carbohydrate intake than the other two groups when intake is expressed per kilogram of body weight, although there was no significant difference in total carbohydrate intake among the three groups. It is worth noting that UF patients had a carbohydrate intake of more than 60% of the total calories. There was no significant difference in protein intake among the three groups. UF and AF patients had a mean protein intake of less than 1.2 g/kg per day. Five UF patients (33.3%), six AF patients (30%), and two OF patients (10.5 %) had an even lower protein intake, less than 0.8 g/kg per day. UF patients had a significantly lower fat intake than the OF patients. The percentage of calories from fat for all three groups was less than 30%.
Nutritional status assessment
Anthropometric measurements on day 1 and day 7 after admission to the ICU are presented in Table 3. The mean current weight did not significantly change during the study. On day 7 in the ICU, UF patients showed significant reductions in MAC, mid-arm muscle circumference, and arm muscle area when compared with that at day 1. AF and OF patients, however, showed slight increases in weight, body mass index and TSF at day 7.
Table 3.
Anthropometric and biochemical measurements of patients
| Underfeeding (n = 15) | Appropriate feeding (n = 20) | Overfeeding (n = 19) | ||||
| Measurement | Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 |
| Anthropometric measurements | ||||||
| Current weight (kg) | 64.5 ± 11.1 | 62.9 ± 9.0 | 63.1 ± 13.0 | 63.9 ± 12.9 | 52.7 ± 7.9 | 56.6 ± 9.4 |
| Body mass index (kg/m2) | 23.7 ± 3.6 | 23.1 ± 3.1 | 23.8 ± 4.2 | 24.1 ± 4.6 | 21.1 ± 4.0 | 23.1 ± 5.5 |
| Triceps skinfold (mm) | 11.6 ± 5.8 | 10.3 ± 5.6 | 11.5 ± 5.1 | 12.0 ± 5.9 | 10.8 ± 5.4 | 11.7 ± 7.7 |
| Mid-arm circumference (mm) | 282.3 ± 33.4a | 259.5 ± 37.2b | 271.5 ± 38.0 | 273.5 ± 42.0 | 245.9 ± 37.9 | 245.6 ± 34.8 |
| Mid-arm muscle circumference (mm) | 245.8 ± 26.1a | 227.2 ± 27.0b | 235.5 ± 30.7 | 235.7 ± 32.6 | 212.0 ± 29.5 | 208.8 ± 30.9 |
| Arm muscle area (mm2) | 4861.7 ± 998.9a | 4164.5 ± 968.0b | 4485.8 ± 1167.2 | 4503.2 ± 1250.1 | 3641.7 ± 988.2 | 3541.8 ± 989.9 |
| Biochemical measurements | ||||||
| Albumin (g/dl) | 2.5 ± 0.2 | 2.4 ± 0.4 | 2.3 ± 0.5 | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.4 ± 0.4 |
| Prealbumin (mg/dl) | 10.0 ± 2.8 | 10.5 ± 3.8 | 11.9 ± 6.5 | 11.5 ± 3.8 | 11.7 ± 6.9 | 14.1 ± 6.4 |
| Total lymphocyte count (mm3) | 727.3 ± 651.7 | 1094.4 ± 776.9 | 856.3 ± 994.4 | 1319.8 ± 1080.4 | 1064.3 ± 1305.0 | 915.0 ± 478.8 |
| Creatinine height index (%) | 76.2 ± 25.9 | 70.7 ± 36.6 | 68.9 ± 24.2 | 57.1 ± 29.0 | 67.2 ± 25.1 | 66.4 ± 23.3 |
| Nitrogen balance | -6.7 ± 6.0 | -7.6 ± 7.2 | -2.8 ± 4.5 | 0.04 ± 5.1 | -1.9 ± 5.1 | -1.7 ± 4.3 |
Values presented as mean ± standard deviation.
The results of biochemical measurements are also presented in Table 3. The albumin, prealbumin and TLC levels were, on average, below normal for patients in all three groups. Patients in all groups showed no significant changes in albumin, prealbumin and TLC by day 7. The mean percentage of the creatinine height index was less than 90% in the three groups, with no significant changes when comparing day 1 and day 7. Patients in the three groups had negative nitrogen balance at admission. Only AF patients showed a positive nitrogen balance on day 7.
The MI was calculated based on albumin, prealbumin, TLC, and percentage ideal body weight to classify patients as mal-nourished or nonmalnourished (Fig. 1). On day 1 and day 7 in the ICU, the MI values were 7.1 ± 1.5 versus 6.5 ± 2.4, 7.1 ± 2.3 versus 5.7 ± 2.5, and 8.2 ± 3.7 versus 8.8 ± 4.1 for patients in the UF group, the AF group, and the OF group, respectively. All patients had MI > 0 when admitted and on day 7, which indicates that patients were malnourished. There were no significant differences for the MI values on day 1 and day 7 among the three groups. Patients in all groups were classified as severely malnourishment based on the NRI on day 1 and day 7 (Fig. 2). Only AF patients had a significantly higher NRI value at day 7 than at day 1.
Figure 1.
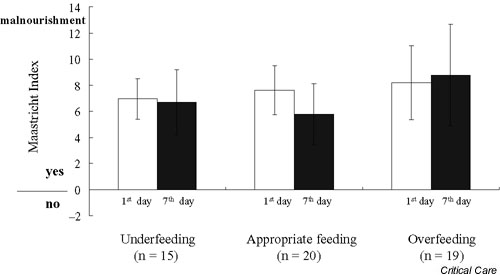
The Maastricht Index at day 1 (white bar) and at day 7 (black bar) after admission to the intensive care unit for patients in the underfeeding, appropriate feeding, and overfeeding groups.
Figure 2.
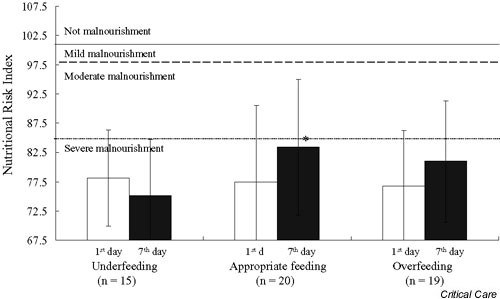
The Nutritional Risk Index at day 1 (white bar) and at day 7 (black bar) after admission to the intensive care unit for patients in the underfeeding, appropriate feeding, and overfeeding groups. * The value at day 7 is significantly different to that at day 1, P < 0.05.
Clinical outcome
The clinical outcome including the severity of the disease (APACHE II score), the length of hospital stay and the length of ICU stay, and the length of ventilator dependence are presented in Table 4. The severity of disease (APACHE II score) was not significantly different among the three groups at admission and 7 days after. In addition, there were no statistically significant differences among groups for length of ventilator dependency, ICU stay, and hospital stay. The length of ventilator dependency was significantly positively correlated with total energy intake (r = 0.494, P = 0.03) only in the AF group. The length of ICU stay was significantly positively correlated with carbohydrate intake (r = 0.525, P = 0.02) also only in the AF group.
Table 4.
Clinical outcome
| Underfeeding (n = 15) | Appropriate feeding (n = 20) | Overfeeding (n = 19) | |
| APACHE II score (day 1) | 19.9 ± 5.2 | 17.4 ± 4.0 | 17.7 ± 3.9 |
| APACHE II score (day 14) | 19.1 ± 7.0 | 17.3 ± 4.6 | 16.3 ± 5.0 |
| Length of hospital stay (days) | 79.2 ± 50.5 | 77.3 ± 69.1 | 74.1 ± 25.0 |
| Length of intensive care unit stay (days) | 45.2 ± 25.0 | 39.1 ± 19.9 | 31.0 ± 6.6 |
| Length of ventilatory dependence (day) | 65.2 ± 48.3 | 53.5 ± 28.1 | 58.0 ± 16.8 |
APACHE, Acute Physiology and Chronic Health Evaluation.
Discussion
The prevalence of malnutrition is a common problem in critically ill patients who are being ventilated mechanically [8,18-21]. Using single and multiple parameters to assess nutritional status, results showed our critically ill patients were in a severely malnourished state. Driver and LeBrun found that nutritional support was inadequate in almost all cases, reporting that only three out of 26 patients received sufficient energy intake [22]. Sufficient energy and nutrient consumption are needed for patients to improve their nutritional status and to increase their immunity. In the critically care setting, however, appropriate and adequate feeding is sometimes difficult. Previous studies [14,23] have shown that only 25–32% of patients received total calories within 10% of their required needs. The present study showed a higher percentage of patients (37%) were fed appropriately; however, there was still 28% and 35% of patients who were underfed and overfed, respectively.
A RQ value greater than 1 indicates that patients have been overfed. Indeed, the mean RQ value in the present AF and OF patients was close to or greater than 1, which might indicate a rise in CO2 production. Looking at the distribution of energy intake, the OF group received significantly higher amounts of carbohydrate than the other two groups. Excess carbohydrate intake leads to increasing CO2 production, which will delay success in weaning from mechanical ventilation and will prolong the length of hospitalization [7,24,25]. The present result did show that carbohydrate intake positively correlated with the length of the ICU stay. Thus, when there is a need for increasing energy intake, the energy source should be carefully chosen to avoid giving excess carbohydrate calories.
The optimal protein requirement for critically ill patients has been stated as 1.5–2.0 g/kg [26]. Smith and colleagues [27] indicated that 300 mg/kg nitrogen intake (~1.8 g/kg protein) was needed for gastroenterological patients to maintain nitrogen balance. Shaw and colleagues [28] showed, when providing 1.5 g/kg per day protein to severely septic patients, that maximal stimulation of protein synthesis could be achieved. Overall the present patients had a mean protein intake approximating 1.1 g/kg per day, which was obviously inadequate for this critically ill population to achieve a positive nitrogen balance. A negative nitrogen balance indicates protein catabolism and reflects inadequate protein intake. The goal of nutrition support is to achieve a nitrogen balance in the range of +2 to +4 g/day [29]. It was therefore not surprising to find that our patients had a negative nitrogen balance throughout the study.
The nitrogen balance reflects both protein and energy intake. We suggested that nitrogen balance studies should be routinely performed in a critical care setting in order to return patients back to an anabolic state. However, previous studies have indicated that the UUN is too insensitive for determining total urinary nitrogen, and would therefore interfere significantly with nitrogen balance predictions [30-32]. Because Kjeldahl nitrogen determination or pyrochemiluminescence are generally not routinely performed in the clinical laboratory in Taiwan, UUN was used as an estimate of total urinary nitrogen when we calculated the nitrogen output for the nitrogen balance. However, we used full 24-hour urine collection rather than spot samples to determine the UUN, and so a more accurate calculation of UUN could thus be made [33]. However, the severity, duration and influence of nitrogen balance on patients' clinical outcome still cannot be assessed from the data we collected. In addition, patients in the UF group showed significant decreases in MAC, mid-arm muscle circumference, and arm muscle area at day 7 in the ICU, indicating that patients were significantly losing their lean body mass and were in a catabolic state.
Previous authors have suggested adding approximately 20–25% to the resting energy expenditure for clinically stable patients in order to calculate total energy requirements [11-13]. In the present study, 20% was added to the resting energy expenditure. Shortcomings of the present study include the sample size and the length of observation (7 days), which may not be sufficient time to see any significant improvement in nutritional status in the critically ill. However, only patients receiving total energy intakes within ± 10% of 120% MEE achieved positive nitrogen balance by the end of the study. In addition, AF patients showed slight increases in body weight, body mass index, and TSF after 7 days' stay in the ICU, and a significantly improved NRI value on day 7. We know that critically ill patients with mechanical ventilation have greater fluid retention, not breathing spontaneously. They have more severe clinical conditions, are more sedated, and are in a relatively more motionless state than general hospitalized patients [34]. However, 120% MEE might be considered an appropriate and adequate total energy requirement for mechanically ventilated, critically ill patients.
In conclusion, critically ill patients were in a severely malnourished state. UF patients had more severe malnutrition than AF or OF patients. AF patients showed more improvement in nutritional status than patients in the other feeding groups. Providing at least 120% of the resting energy expenditure thus seemed adequate to meet the caloric energy needs of hemody-namically stable, mechanically ventilated, critically ill patients.
Competing interests
None declared.
Key messages
• Only patients in the appropriate feeding group improved their nitrogen balance positively by day 7 of admission, and had a significantly higher Nutritional Risk Index at day 7 than on day 1
• Patients in the appropriate feeding group had a greater improvement in nutritional status than patients in the other feeding groups
• To provide at least 120% of the resting energy expenditure seemed adequate to meet the caloric energy needs of hemodynamically stable, mechanically ventilated, critically ill patients
Abbreviations
AF = appropriate feeding; APACHE = Acute Physiology and Chronic Health Evaluation; ICU = intensive care unit; MAC = mid-arm circumference; MEE = measured energy expenditure; MI = Maastricht Index; NRI = Nutritional Risk Index; OF = overfeeding; RQ = respiratory quotient; TLC = total lymphocyte count; TSF = triceps skinfold thickness; UF = underfeeding; UUN = urine urea nitrogen.
References
- Bassili HR, Deitel M. Effect of nutritional support on weaning patients off mechanical ventilators. J Parenter Enteral Nutr. 1981;5:161–163. doi: 10.1177/0148607181005002161. [DOI] [PubMed] [Google Scholar]
- Larca L, Greenbaum DM. Effectiveness of intensive nutritional regimes in patients who fail to wean from mechanical ventilation. Crit Care Med. 1982;10:297–300. doi: 10.1097/00003246-198205000-00001. [DOI] [PubMed] [Google Scholar]
- Askanazi J, Starker PM, Olsson C, Hensle TW, Lockhart SH, Kinney JM, La Sala PA. Effect of immediate postoperative nutritional support on length of hospitalization. Ann Surg. 1986;2:236–239. doi: 10.1097/00000658-198603000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benotti PN, Bistran B. Metabolic and nutritional aspects of weaning from mechanical ventilation. Crit Care Med. 1989;17:181–185. doi: 10.1097/00003246-198902000-00017. [DOI] [PubMed] [Google Scholar]
- Garrel DR, Davignon I, Lopez D. Length of care in patients with severe burns with or without early enteral nutritional support. J Burn Care Rehabil. 1991;12:85–90. doi: 10.1097/00004630-199101000-00021. [DOI] [PubMed] [Google Scholar]
- Askanazi J, Weissman C, Rosenbaum SH, Hyman AI, Milic-Emili J. Nutrition and the respiratory system. Crit Care Med. 1982;10:163–172. doi: 10.1097/00003246-198203000-00005. [DOI] [PubMed] [Google Scholar]
- Dark DS, Pingleton SK, Kerby GR. Hypercapnia during weaning: a comparison of nutritional support. Chest. 1985;88:141–143. doi: 10.1378/chest.88.1.141. [DOI] [PubMed] [Google Scholar]
- Huang YC, Yen CL, Cheng CH, Jih KS, Kan MN. Nutritional status of mechanically ventilated critically ill patients: comparison of different type of nutritional support. Clin Nutr. 2000;19:101–107. doi: 10.1054/clnu.1999.0077. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- Weir JB de V. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1941;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinamer DL, Phang PT, Jones RL, Grace M, King EG. Twenty-four hour energy expenditure in critically ill patients. Crit Care Med. 1987;15:637–643. doi: 10.1097/00003246-198707000-00002. [DOI] [PubMed] [Google Scholar]
- Weissman C, Kemper M, Elwyn DH, Askanazi J, Hyman AI, Kinney JM. The energy expenditure of the mechanically ventilated critically ill patient: an analysis. Chest. 1986;89:254–259. doi: 10.1378/chest.89.2.254. [DOI] [PubMed] [Google Scholar]
- Weissman C, Kemper M, Hyman AI. Variation in the resting metabolic rate of mechanically ventilated critically ill patients. Anesthesia Analgesia. 1989;68:457–461. [PubMed] [Google Scholar]
- McClave SA, Lowen CC, Kleber MJ, Nicholson JF, Jimmerson SC, McConnell JW, Jung L. Are patients fed appropriately according to their caloric requirements? J Parenter Enteral Nutr. 1998;22:375–381. doi: 10.1177/0148607198022006375. [DOI] [PubMed] [Google Scholar]
- Bistrian BR, Blackburn GL, Sherman M, Scrimsaw NS. Therapeautic index of nutritional depletion in hospitalized patients. Surg Gynecol Obstet. 1975;141:512–516. [PubMed] [Google Scholar]
- de Jong PCM, Wesdorp RIC, Volovics A, Roufflart M, Greep JM, Soeters PB. The value of objective measurements to select patients who are malnourished. Clin Nutr. 1985;4:61–66. doi: 10.1016/0261-5614(85)90043-3. [DOI] [PubMed] [Google Scholar]
- Hall JC. The use of internal validity in the construct of an index of undernutrition. J Parenter Enteral Nutr. 1990;14:582–587. doi: 10.1177/0148607190014006582. [DOI] [PubMed] [Google Scholar]
- Driver AG, Le Brun M. Iatrogenic malnutrition in patients receiving ventilatory support. JAMA. 1980;244:2195–2196. [PubMed] [Google Scholar]
- Bassili HR, Deitel M. Effect of nutritional support on weaning patients off mechanical ventilators. J Parenter Enteral Nutr. 1981;5:161–163. doi: 10.1177/0148607181005002161. [DOI] [PubMed] [Google Scholar]
- Larca L, Greenbaum DM. Effectiveness of intensive nutritional regimes in patients who fail to wean from mechanical ventilation. Crit Care Med. 1982;10:297–300. doi: 10.1097/00003246-198205000-00001. [DOI] [PubMed] [Google Scholar]
- Christman JW, McCain RW. A sensible approach to the nutritional support of mechanically ventilated critically ill patients. Intensive Care Med. 1993;19:129–136. doi: 10.1007/BF01720527. [DOI] [PubMed] [Google Scholar]
- Driver AG, LeBrun M. Iatrogenic malnutrition in patients receiving ventilatory support. JAMA. 1980;244:2195–2196. [PubMed] [Google Scholar]
- Makk LJ, McClave SA, Creech PW, Johnson DR, Short AF, Whitlow NL, Priddy FS, Sexton LK, Simpson P. Clinical application of the metabolic cart to the delivery of total parenteral nutrition. Crit Care Med. 1990;18:1320–1327. doi: 10.1097/00003246-199012000-00003. [DOI] [PubMed] [Google Scholar]
- Covelli HD, Black JW, Olsen MS, Beekman JF. Respiratory failure precipitated by high carbohydrate loads. Ann Intern Med. 1981;95:579–581. doi: 10.7326/0003-4819-95-5-579. [DOI] [PubMed] [Google Scholar]
- Askanazi J, Rosenbaum SH, Hyman AI, Silverberg PA, Milic-Emili J, Kinney JM. Respiratory changes induced by the large glucose loads of total parenteral nutrition. JAMA. 1980;243:1444–1447. [PubMed] [Google Scholar]
- ASPEN Board of Directions. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. J Parenter Enteral Nutr. 1993;17(Suppl):1–52. [PubMed] [Google Scholar]
- Smith RC, Burkinshaw L, Hill GL. Optimal energy and nitrogen intake for gastroenterological patients requiring intravenous nutrition. Gastroenterology. 1982;82:445–452. [PubMed] [Google Scholar]
- Shaw JHF, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients: the response to glucose infusion and total parenteral nutrition. Ann Surg. 1986;205:288–294. doi: 10.1097/00000658-198703000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case KO, Cuddy PG, McGurk EPD. Nutrition support in the critically ill patient. Crit Care Nurs Q. 2000;22:75–89. doi: 10.1097/00002727-200002000-00008. [DOI] [PubMed] [Google Scholar]
- Konstantinides FN, Boehm KA, Radmer WJ, Storm MC, Adderly JT, Weisdorf SA, Cerra FB. Pyrochemiluminescence: real-time, cost-effective method for determining total urinary nitrogen in clinical nitrogen-balance studies. Clin Chem. 1988;34:2518–2520. [PubMed] [Google Scholar]
- Loder PB, Kee AJ, Horsburgh R, Jones M, Smith RC. Validity of urinary urea nitrogen as a measure of total urinary nitrogen in adult patients requiring parenteral nutrition. Crit Care Med. 1989;17:309–312. doi: 10.1097/00003246-198904000-00002. [DOI] [PubMed] [Google Scholar]
- Konstantinides FN, Konstantinides NN, Li JC, Myaya ME, Cerra FB. Urinary urea nitrogen: too insensitive for calculating nitrogen balance studies in surgical clinical nutrition. J Parenter Enteral Nutr. 1991;15:189–193. doi: 10.1177/0148607191015002189. [DOI] [PubMed] [Google Scholar]
- Ford EG, Jennings LM, Andrassy RJ. Hourly urine nitrogen values do not reflect 24-hour totals in injured children. Nutr Clin Pract. 1987;2:195–198. [Google Scholar]
- Damask MC, Schwarz RY, Weissman C. Energy measurements and requirements of critically ill patients. Crit Care Clin. 1987;3:71–96. [PubMed] [Google Scholar]


