Abstract
Src family kinases (SFKs) signal in response to E-cadherin to support cadherin adhesion and the integrity of cell-cell contacts (McLachlan, R. W., Kraemer, A., Helwani, F. M., Kovacs, E. M., and Yap, A. S. (2007) Mol. Biol. Cell 18, 3214–3223). We now identify the actin-regulatory protein, cortactin, as a target of E-cadherin-activated SFK signaling. Tyr-phosphorylated cortactin was found at cell-cell contacts in established epithelial monolayers, and cortactin became acutely tyrosine-phosphorylated when E-cadherin adhesion was engaged. In all circumstances, cortactin tyrosine phosphorylation was blocked by inhibiting SFK signaling. Importantly, Tyr-phosphorylated cortactin was necessary to preserve the integrity of cadherin contacts and the perijunctional actin cytoskeleton. Moreover, expression of a phosphomimetic cortactin mutant could prevent SFK blockade from disrupting cadherin organization, thereby placing cortactin functionally downstream of SFK signaling at cadherin adhesions. We conclude that SFK and cortactin constitute an important signaling pathway that functionally links E-cadherin adhesion and the actin cytoskeleton.
Functional cooperation between cadherin adhesion receptors and the actin cytoskeleton is commonly believed to play a key role in the morphogenesis of cell-cell interactions (1, 2). This functional interplay, and the biochemical mechanisms that underpin it, are much more complex than previously realized. Increasingly it is apparent that a range of distinct actin regulators can be recruited to cadherin adhesions depending on the biological context of cell-cell interactions (2). It is likely that the choice of actin regulator(s) recruited determines the dynamics and organization of the actin cytoskeleton at those contacts, with morphogenetic implications for the formation, modeling, and turnover of cell-cell interactions. Identifying the actin regulators that influence cell-cell interactions and how they cooperate with adhesion receptors are important open issues.
Adhesion-activated cell signaling provides a useful paradigm to analyze how classical cadherins regulate the actin cytoskeleton (2, 3). Over the past several years, a range of signal transduction pathways have been identified that are stimulated upon productive engagement of cadherins, such as E-, C-, and N-cadherin (reviewed in Ref. 3). Among these signals are Rho family GTPases, lipid kinases, and protein-tyrosine kinases. Of the latter, we recently identified Src family kinase (SFK)5 activity as a component of E-cadherin signaling (4). SFK was stimulated in an E-cadherin-dependent fashion when cells assembled contacts with one another. Indeed, binding to recombinant cadherin ligands was sufficient to activate SFK, implying that the cadherin itself can serve as a receptor to transduce an adhesive signal to SFK. Furthermore, inhibiting SFK signaling perturbed cadherin adhesion and the integrity of cell-cell contacts. This suggested a model where adhesive ligation of E-cadherin stimulated an SFK signaling cascade to ultimately support cell-cell interactions. An important challenge now is to identify targets of cadherin-activated SFK signaling that contribute to cadherin biology.
In this work, we tested whether the actin-binding protein, cortactin, might be just such a target. A multidomain scaffolding protein, cortactin regulates the actin cytoskeleton by interacting with a range of other actin-regulatory proteins (5, 6). It is best understood to participate in actin filament assembly (6) by promoting Arp2/3-mediated actin nucleation and also by stabilizing nascent Arp2/3-generated actin filament branches (7). Cortactin exerts many of these effects through direct interactions with actin filaments and Arp2/3 (8) as well as indirectly by associating with proteins such as N-WASP and WIP, which can themselves activate Arp2/3. Consistent with this, cortactin is often found at sites in the cortex where Arp2/3 drives membrane protrusion, such as lamellipodia and invadopodia (9).
Cortactin is also found at cadherin-based cell-cell contacts where a biochemical complex with E-cadherin or N-cadherin has been detected by co-immunoprecipitation analysis (10–12). Moreover, ligation of the cadherin with recombinant ligands could induce formation of a complex with cortactin and also recruited cortactin to the cortex at the sites of adhesion (10, 12). Cortactin is found with Arp2/3 at newly forming E-cadherin adhesive contacts, and disruption of cortactin by RNAi or dominant-negative mutants perturbs efficient assembly of cadherin-based contacts (10). At N-cadherin adhesions, cortactin promotes adhesive strengthening and its surface expression (12). Overall, these reports identified a role for cortactin in modulating cadherin biology, likely through regulation of the cadherin-based actin cytoskeleton.
Of note, cortactin was first identified as a substrate for v-Src (13) and as a target of fibroblast growth factor-stimulated SFK signaling (14). Tyrosine phosphorylation of cortactin is implicated in cellular events that are accompanied by extensive remodeling of the actin cytoskeleton, such as cell migration and invasion (6, 15, 16). Building on our earlier experience in epithelial cells (4, 10), we now report that E-cadherin ligation induces the tyrosine phosphorylation of cortactin through an SFK-dependent signaling pathway. Furthermore, we demonstrate that phosphorylation at the key Tyr-421, Tyr-466, and Tyr-482 residues is necessary to maintain the integrity of established cell-cell contacts and their perijunctional actin cytoskeleton.
EXPERIMENTAL PROCEDURES
Plasmids and siRNA
Plasmids for FLAG-tagged wild-type mouse cortactin and the Y421F/Y466F/Y482F (3YF) cortactin mutant have been described (8, 10, 17). Mutations of Y421D, Y466D, and Y482D were introduced using a two-step PCR-based mutagenesis method adopted from Ref. 18. In brief, the first PCR round amplified two DNA fragments with overlapping sequences that incorporated mutations. The second PCR round used these two fragments as template to generate a full-length mutagenized DNA fragment. A dominant-negative Src construct (K295M, Y527F) tagged with enhanced GFP was a kind gift of Dr. Margaret Frame (Beatson Institute, Glasgow) (4). Cortactin RNAi oligonucleotide duplexes (CTTN-HSS103233) and scrambled control RNA (106203312) were from Invitrogen. E-cadherin siRNA is as follows: sense strand, 5′-GCAUGGACUCAGAAGACAGtt-3′; and antisense strand, 5′-CUGUCUUCUGAGUCCAUGCtg-3′. SMARTpool siRNA directed against human E-cadherin were purchased from Dharmacon and used following the manufacturer's instructions.
Cell Culture, Transfections, and hE/Fc
MCF7 cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, non-essential amino acids, 2 mm l-glutamine, 100 mg/ml streptomycin, and 100 units/ml penicillin. hE/Fc cell culture, purification, and preparation of hE/Fc-coated substrata were as described previously (19, 20). Transient transfections were performed with Lipofectamine (Invitrogen) according to the manufacturer's instructions. To achieve effective knockdown of E-cadherin or cortactin, cells were retransfected with siRNA 48 h after the initial transfections. Clones were screened for expression by immunofluorescence and Western analysis.
Antibodies and Fluorescent Stains
The following primary antibodies were used in this study: mouse monoclonal HECD1 against human E-cadherin (a kind gift from Dr. M. Wheelock, University of Nebraska, with the permission of Dr. M. Takeichi, RIKEN Center for Developmental Biology), rabbit polyclonal E-cadherin antibody (10), mouse monoclonal 4F11 against human cortactin (8), rabbit polyclonal against Tyr(P)-421-cortactin (Invitrogen), rabbit polyclonal against Tyr(P)-466-cortactin (17), mouse monoclonal against β-tubulin (Sigma), mouse monoclonal against the FLAG epitope (Sigma), mouse monoclonal against GFP (Invitrogen), Alexa Fluor 594-phalloidin (Invitrogen), and species-specific secondary antibodies conjugated with Alexa Fluor 488, Alexa Fluor 594, and horseradish peroxidase (Invitrogen). Alexa Fluor 488- and 594-conjugated phalloidin (Invitrogen) was used to stain F-actin.
Immunofluorescence Microscopy and Analysis
Samples were prepared for immunofluorescence analysis as described previously (20). Fixed material was mounted in 1% N-propyl gallate in 50% glycerol:phosphate-buffered saline for epi-illumination and spinning disc confocal microscopy; fixed material was examined either by epi-illumination using Olympus AX70 or IX81 microscopes or with a PerkinElmer spinning disc confocal microscope, and images were captured using Hamamatsu Orca 1 ER cameras driven by Metamorph software. All images were acquired within the linear range of the cameras. Figures were arranged for presentation in Adobe Photoshop CS2.
ImageJ was used to measure the average E-cadherin contact width and intensity using a “line-scanning method.” In brief, a fixed length line (65 pixels) was placed orthogonal to individual E-cadherin contacts. The ImageJ “Plot Profile” function was applied and plots of intensity (0–255) along the line distance (0–65) were obtained. Contact width was estimated by calculating +2 S.D. that covers 95% of contact pixel intensity.
ImageJ was also used to quantify the ratio of the intensity of phosphocortactin to the intensity of E-cadherin at each pixel along a defined contact. All images for a single antibody were taken at the same exposure and an identical spinning disc microscope setting. To decrease nonspecific staining, the average background fluorescence for the image was determined and subtracted from intensities for all pixels. An ImageJ built-in calculator was then used to calculate the ratio of phosphocortactin to the intensity of E-cadherin.
Immunoprecipitations
Cells were lysed in 1 ml of lysis buffer (1% Nonidet P-40, 150 mm NaCl, 50 mm Tris-HCl, pH 7.4, 1 mm EDTA, 50 mm sodium fluoride, 2 mm sodium vanadate, 0.1% bovine serum albumin, and Complete protease inhibitors (Roche Applied Science)). Protein complexes were immunoprecipitated with either HECD1 E-cadherin monoclonal antibody or an antibody against the FLAG epitope bound to protein A-agarose beads. Twenty percent of each immunoprecipitation reaction was then separated by SDS-PAGE. Immune complexes were blotted for E-cadherin, cortactin, and phosphocortactin.
Calcium Chelation Assay
Cells were grown to 100% confluence and then incubated in 2 mm EGTA solution for 15–30 min or until cell-cell contacts were disrupted. Following washing, cells were incubated with unsupplemented culture medium containing 2 mm CaCl2 for a time course from 0 to 90 min before paraformaldehyde fixation or lysis in Laemmli sample buffer.
Drug Inhibition of Src Family Kinase Activity
To inhibit Src family kinase activities, 10 μm PP2 (Merck) or SU6656 (Merck) was added to MCF7 cell monolayers. The nonfunctional PP2 analog PP3 and solvent DMSO were also introduced as controls.
RESULTS
Junctional Tyrosine-phosphorylated Cortactin in Established Epithelial Monolayers
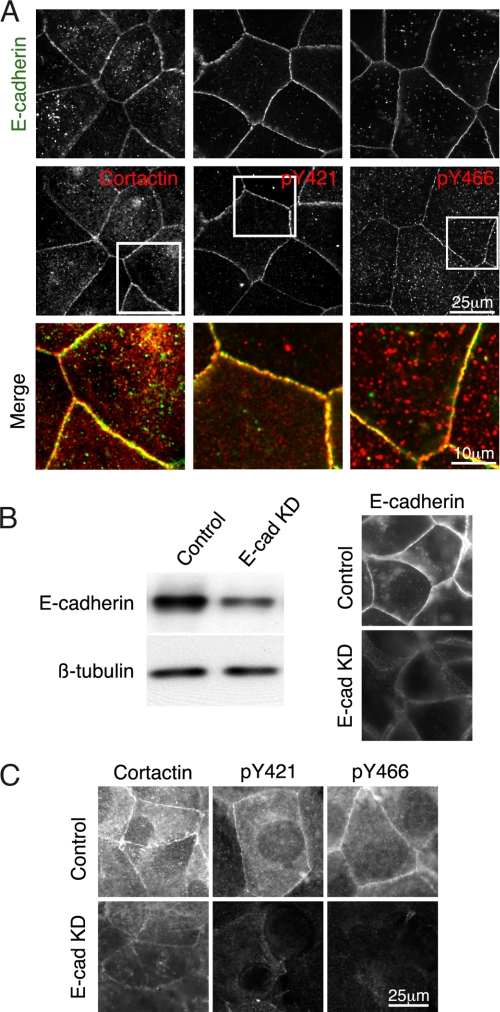
Cortactin is phosphorylated by SFK at Tyr-421, Tyr-466, and Tyr-482 (21, 22). Accordingly, we first used antibodies specific for either the phosphorylated Tyr-421 (Tyr(P)-421)- or phosphorylated Tyr-466 (Tyr(P)-466) residue to characterize the subcellular localization of Tyr(P)-cortactin in MCF7 epithelial monolayers (Fig. 1). Confluent MCF7 monolayers formed cohesive, linear cell-cell contacts enriched in E-cadherin that co-stained for cortactin (Fig. 1A) (10). Both Tyr(P)-421- and Tyr(P)-466-cortactin clearly co-accumulated with E-cadherin, indicating that these established cadherin-based contacts were enriched in Tyr(P)-cortactin (Fig. 1A). Punctate Tyr(P)-cortactin staining was also identified in the cytoplasm, possibly decorating vesicles. Similar junctional staining for cortactin and Tyr(P)-421- and Tyr(P)-466-cortactin was also detected in confluent Madin-Darby canine kidney cells (data not shown). We found that probing for Tyr(P)-421- and Tyr(P)-466-cortactin yielded similar results throughout this study.
FIGURE 1.
E-cadherin is required for junctional accumulation of tyrosine-phosphorylated cortactin. A, confluent MCF7 cell monolayers were fixed and stained for E-cadherin, cortactin, as well as phosphorylated cortactin using antibodies against its phosphorylated tyrosines 421 and 466 (pY421 and pY466), respectively. Cortactin and Tyr(P)-cortactin staining (red) were identified in most cell-cell contacts that also co-stained with E-cadherin (green). High magnification overlay (merge) images of the regions outlined by squares show extensive co-localization between E-cadherin and Tyr(P)-cortactin at cell-cell contacts. B, MCF7 cell monolayers were transfected with siRNA duplexes to deplete E-cadherin (E-cad KD) as well as with a scrambled siRNA as a control. After 72 h, cells were lysed and immunoblotted for E-cadherin. Depletion of E-cadherin was also confirmed by immunofluorescence staining of endogenous E-cadherin. C, control and E-cadherin KD cells were immunostained for cortactin and phosphotyrosines 421 and 466. Staining for cortactin and Tyr(P)-cortactin was reduced in E-cadherin KD cells.
E-cadherin ligation activates a number of intracellular signaling pathways, including Src family tyrosine kinases (4). As a first test of whether E-cadherin might be necessary for junctional Tyr(P)-cortactin, we depleted E-cadherin in MCF7 cells using either single siRNA duplexes (Fig. 1B) or an siRNA SMARTpool (data not shown). Although E-cadherin depletion was moderate, junctional staining for Tyr(P)-421- and Tyr(P)-466-cortactin was substantially reduced (Fig. 1C). This suggested that E-cadherin might be necessary for tyrosine phosphorylation of cortactin at cell-cell contacts. Junctional cortactin staining was also decreased (Fig. 1C), however, making it difficult to determine whether cadherin adhesion triggered a pathway leading to tyrosine phosphorylation of cortactin or simply served to localize cortactin itself at cell-cell contacts.
E-cadherin Adhesion Induces Tyrosine Phosphorylation of Cortactin
To pursue this issue, we then asked whether the assembly of cell-cell contacts affects the tyrosine phosphorylation status of cortactin. We acutely disrupted MCF7 interactions by chelating extracellular calcium and then monitored contacts as they reassembled after restoration of calcium (Fig. 2, A–C). Cells began to re-establish E-cadherin-based contacts within 15 min of adding Ca2+. Tyr(P)-cortactin was not detectable in very early contacts, but began to accumulate with E-cadherin at ∼30 min and persisted as contacts became more extensive and linear (Fig. 2A). In Western blot analysis of total cell lysates (Fig. 2B), we found that Tyr(P)-cortactin levels were relatively low after chelation of extracellular calcium and then began to rise significantly ∼30 min after replenishment of Ca2+, consistent with the appearance of junctional Tyr(P)-cortactin detected by immunofluorescence. Characteristically, the rise in total Tyr(P)-cortactin levels appeared biphasic, with an initial rise at ∼30 min, followed by a second peak at ∼90 min, although the precise timing of these peaks showed some inter-experiment variation, perhaps due to variations in culture density.
FIGURE 2.
E-cadherin ligation induces tyrosine phosphorylation of cortactin. A–C, tyrosine phosphorylation of cortactin during assembly of cell-cell contacts. MCF7 cell monolayers were incubated with 2 mm EGTA until their cell-cell contacts were disrupted. EGTA was then replaced with fresh medium containing 2 mm calcium (+Ca) to allow E-cadherin-based contacts to reassemble. Cells were examined after incubation with 2 mm EGTA (−Ca) or 0–90 min after restitution of calcium (2 mm, +Ca). A, fixed samples were immunostained for E-cadherin and phosphorylated cortactin (pY466). Arrows mark Tyr(P)-cortactin in newly forming cadherin contacts. B, total cell lysates were immunoblotted for Tyr(P)-466-cortactin and cortactin. C, E-cadherin (E-cad) or control IgG immunoprecipitates (IP) were probed for Tyr(P)-466-cortactin, cortactin, and E-cadherin. D, ligation of E-cadherin is sufficient to induce cortactin tyrosine phosphorylation. MCF7 cells were allowed to adhere to hE/Fc- or poly-l-lysine (PLL)-coated substrata. Cells were lysed and immunoblotted for phosphorylated cortactin (pY466) or cortactin immediately after isolation (0′) or at various times after adhesion to substrata. Representative immunoblots and quantitative analysis are shown. Tyr(P)-466-cortactin band intensity was normalized to the total cortactin band intensity for each time point. Data are mean ± S.E. (n = 3).
We then used co-immunoprecipitation analysis to test whether tyrosine phosphorylation of the cadherin-associated cortactin pool changed as cells assembled contacts with one another. As shown in Fig. 2C, a low level of Tyr(P)-cortactin was detectable in E-cadherin immune complexes after chelation of calcium, but this rose significantly ∼45 min after restoration of extracellular Ca2+. Interestingly, total levels of cortactin protein in the immune complexes did not change greatly during the course of these experiments. Overall, these findings implied that the assembly of cell-cell contacts triggered a signaling pathway leading to tyrosine phosphorylation of E-cadherin-associated cortactin.
Cell-cell contact can induce signaling through cadherin receptors themselves, but also by a variety of other juxtacrine signaling pathways (3). To test directly whether ligation of E-cadherin was able to induce the tyrosine phosphorylation of cortactin, we allowed MCF7 cells to adhere to substrata coated with the recombinant ligand hE/Fc, which consists of the complete ectodomain of human E-cadherin expressed as an Fc fusion protein. In previous studies, we have shown that adhesion to hE/Fc stimulates cell signaling through Rac (19), Cdc42 (23), and SFK (4) themselves. Tyr(P)-cortactin was largely undetectable in freshly isolated non-adherent MCF7 cells, but increased as cells adhered to hE/Fc (Fig. 2D). Interestingly, the induction of tyrosine phosphorylation was biphasic, with an early peak at ∼30 min and a later, further rise in Tyr(P)-cortactin levels. Adhesion to the nonspecific ligand poly-l-lysine produced no comparable rise in Tyr(P)-cortactin levels, corrected for protein loading. Similarly, when we presented ligands immobilized on 10-mm latex beads, we found that Tyr(P)-cortactin accumulated at the sites of adhesion to hE/Fc-coated beads to a much greater extent than at sites of binding to concanavalin A-coated beads.6 Together, these findings indicate that ligation of E-cadherin alone is sufficient to activate a signaling pathway that induces tyrosine phosphorylation of cortactin.
Tyrosine-phosphorylated Cortactin Is Necessary for the Integrity of E-cadherin Contacts and the Perijunctional Actin Cytoskeleton
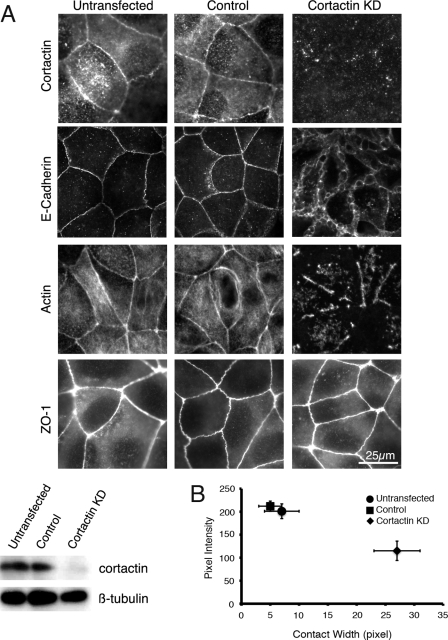
We then used cortactin knockdown and reconstitution to test the functional significance of Tyr(P)-cortactin. Transfection of siRNA reduced overall cortactin levels in confluent MCF7 cells by ∼90% (Fig. 3A), and cortactin was largely undetectable at the cell-cell contacts (Fig. 3A). Although cortactin KD cells made contacts with one another, E-cadherin staining was clearly perturbed. Instead of the crisp, linear staining seen in controls, cortactin-depleted cells showed broadened zones, where junctional E-cadherin staining was replaced by fragmented streaks and loose networks distributed extensively throughout the lateral cell-cell interfaces (Fig. 3A). To quantify the alterations in cadherin staining at contacts, we measured the width and the average pixel intensity of E-cadherin staining at cell-cell contacts. As shown in Fig. 3B, cortactin KD cells showed consistently broader cadherin staining at cell-cell contacts associated with a reduction in average pixel intensity. The perijunctional actin cytoskeleton was also greatly perturbed by cortactin KD (Fig. 3A). Whereas control cells showed an intense band of F-actin staining at the apical interface between cells, cortactin KD cells showed intermittent perijunctional staining, often with extensive discontinuities at the contacts.
FIGURE 3.
Cortactin is necessary for the integrity of E-cadherin cell-cell contacts in MCF7 monolayers. A, MCF7 cell monolayers were transfected with siRNA duplexes to deplete cortactin (cortactin KD) as well as with scrambled RNA duplexes as controls. After 72 h, cells were fixed and immunostained for cortactin, E-cadherin, F-actin (phalloidin), or ZO-1. Depletion of cortactin was also confirmed by immunoblotting for cortactin in cell lysates. Compared with untransfected or scrambled RNA-transfected control cells, E-cadherin and perijunctional F-actin staining was substantially perturbed in the cortactin KD cells. B, morphological changes in E-cadherin staining were quantitated by measuring E-cadherin contact pixel intensity and contact width as described under “Experimental Procedures.” Compared with untransfected or scrambled RNA-transfected control cells, depletion of cortactin exhibited a consistent pattern of weaker intensity and wider contacts.
Exogenous expression of mouse cortactin, which was resistant to the human-specific siRNA (Fig. 4A), restored both linear E-cadherin staining and the integrity of the perijunctional actin cytoskeleton (Fig. 4A), confirming that these alterations were due to cortactin depletion. Quantitation demonstrated that exogenous mouse cortactin restored both the width of cadherin staining and the fluorescence intensity (Fig. 4B).
FIGURE 4.
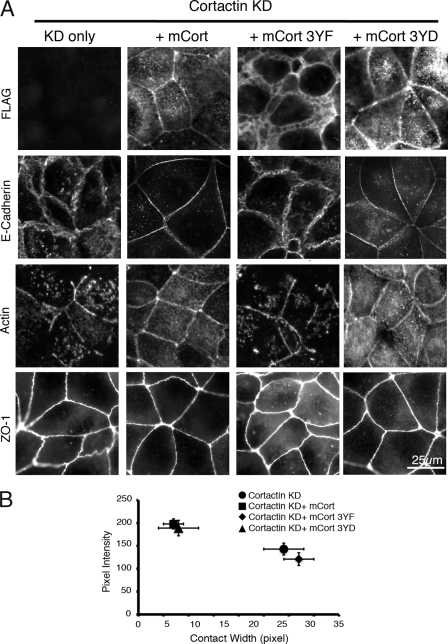
Tyrosine-phosphorylated cortactin is necessary for integrity of E-cadherin-based cell-cell contacts. A, MCF7 cell monolayers were transfected with siRNA against cortactin (cortactin KD). After 72 h, cells were then transfected with plasmids expressing FLAG epitope-tagged wild-type mouse cortactin (mCort) and mouse cortactin with tyrosines 421, 466, and 482 mutated to either phenylalanine (mCort 3YF) or aspartic acid (mCort 3YD). Cells were then fixed and immunostained for cortactin, E-cadherin, F-actin (phalloidin), or ZO-1. Reconstitution with wild-type mouse cortactin restored linear E-cadherin contact integrity as well as perijunctional F-actin staining. Expression of the mouse cortactin 3YF mutant failed to rescue defects in cortactin KD cells, although it remained localized at the cell-cell contacts. Expressing mouse cortactin bearing phosphomimetic mutations (mCort 3YD) restored both E-cadherin integrity and linear junctional F-actin staining. B, E-cadherin-based cell-cell contacts were quantitatively analyzed by measuring E-cadherin contact pixel intensity and contact width as described under “Experimental Procedures.” The intensity and width of E-cadherin staining were restored by reconstitution with wild-type mouse cortactin and cortactin 3YD but not by mouse cortactin 3YF. Data are mean ± S.E. (n = 30).
To test the significance of tyrosine-phosphorylated cortactin, we reconstituted cortactin expression in cortactin KD cells either with a phosphorylation-incompetent cortactin mutant (3YF) in which Tyr-421, Tyr-466, and Tyr-482 were mutated to phenylalanines or with a phosphomimetic mutant (3YD) in which these tyrosine residues were replaced by aspartic acid (Fig. 4). All transgenes expressed polypeptides of the expected molecular mass (data not shown). In contrast to wild-type cortactin, reconstitution with cortactin 3YF did not restore the cortactin KD phenotype. E-cadherin staining at contacts remained dispersed (Fig. 4A) and was quantitatively indistinguishable from KD cells (Fig. 4B). Similarly, perijunctional F-actin staining remained fragmented and discontinuous (Fig. 4A). The linear integrity of both E-cadherin and perijunctional F-actin staining was restored by the 3YD mutant (Fig. 4, A and B). Overall, these findings suggest strongly that cortactin is necessary for the integrity of E-cadherin and actin cytoskeletal organization at cell-cell contacts in established epithelial monolayers. They indicate further that tyrosine phosphorylation of cortactin was necessary for these effects to occur.
Interestingly, immunofluorescence staining for the tight junction marker ZO-1 was much less disrupted by cortactin depletion than that for E-cadherin. Subtle irregularity in ZO-1 staining was evident in both cortactin KD cells (Figs. 3A and 4A) and KD cells reconstituted with the 3YF mutant (Fig. 4A) compared with the more linear staining seen in control cells (Fig. 3A) and in KD cells expressing the 3YD mutant (Fig. 4A). However, the overall integrity of ZO-1 staining was not compromised, in contrast to the dramatic changes seen in E-cadherin organization. This suggested that cortactin has a greater impact on E-cadherin adhesions than on tight junctions.
Cortactin Is an Effector of E-cadherin-activated Src Signaling
Together, these observations suggested that tyrosine-phosphorylated cortactin was a functionally important downstream effector of cadherin signaling. To further characterize the relevant signaling pathways, we sought to identify protein-tyrosine kinases critical for E-cadherin to signal to cortactin. We focused on SFK activity, given its roles in both E-cadherin signaling (4) and cortactin regulation (24, 25).
First we tested whether SFK activity was required for homophilic cadherin ligation to increase tyrosine-phosphorylated cortactin (Fig. 5A). Freshly isolated MCF7 cells were allowed to adhere to hE/Fc-coated substrata for 30 or 90 min, when cadherin-induced cortactin tyrosine phosphorylation was clearly detectable (Fig. 2A); Western blots of cell lysates were then probed for Tyr(P)-cortactin. Tyrosine phosphorylation of cortactin at both time points was significantly reduced when adhesion assays were performed in the presence of SFK inhibitors PP2 (Fig. 5A) and SU6656 (data not shown). In contrast, PP3 did not affect the tyrosine phosphorylation of cortactin when cells adhered to hE/Fc (Fig. 5A). These findings strongly suggest that SFK activity is, directly or indirectly, necessary for E-cadherin homophilic ligation to tyrosine phosphorylate cortactin.
FIGURE 5.
Src kinase signaling is required for E-cadherin to induce tyrosine phosphorylation of cortactin. A, MCF7 cells were allowed to adhere to hE/Fc-coated substrata for 30 or 90 min in the presence of the Src inhibitor PP2, the non-inhibitory analog PP3, or DMSO. Cells were then lysed and immunoblotted for phosphorylated cortactin (pY466) and cortactin. Representative immunoblots for Tyr(P)-cortactin and total cortactin are shown. The Tyr(P)-cortactin response was quantitated by measuring band intensities that were then normalized by expression as a percentage of the average band intensity in DMSO controls at 30 min. Data are mean ± S.E. (n = 3). B, confluent MCF7 cells were treated with Src kinase inhibitors PP2 and SU6656 or controls (PP3, DMSO). Cells were then fixed and immunostained for E-cadherin and phosphorylated cortactin (pY421 and pY466). In contrast to cells treated with DMSO or PP3, PP2 and SU6656 substantially reduced Tyr(P)-421 and Tyr(P)-466 staining at E-cadherin contacts. Loss of junctional Tyr(P)-466 staining was further quantitatively analyzed as described under “Experimental Procedures.” Data are mean ± S.E. (n = 20). Cells were also lysed and immunoblotted with Tyr(P)-421, Tyr(P)-466, and cortactin to further confirm the inhibition of cortactin phosphorylation by PP2 and SU6656. C, MCF7 cells were transfected with plasmids expressing GFP or GFP-tagged dominant-negative c-Src (DN Src). Cells were fixed at confluence and immunoblotted for Tyr(P)-421. Asterisks mark transfected cells. Compared with the GFP controls, Tyr(P)-421 staining at junctional contacts was largely undetectable in cells expressing dominant-negative c-Src. Tyr(P)-421-cortactin staining in cell-cell contacts was also quantitated by counting the percentage of contacts that stained for Tyr(P)-421-cortactin. Data are mean ± S.E. (n = 20).
We then examined whether inhibiting SFK signaling affected Tyr(P)-cortactin at cell-cell contacts in confluent MCF7 monolayers. Western analysis of cell extracts showed that Tyr(P)-421- and Tyr(P)-466-cortactin levels were substantially reduced in monolayers treated with either PP2 or SU6656 (Fig. 5B). Furthermore, the prominent junctional staining of Tyr(P)-421-cortactin and Tyr(P)-466-cortactin was greatly decreased in cells treated with either drug (Fig. 5B). Tyr(P)-cortactin staining was lost before E-cadherin integrity was disrupted in these acutely treated cultures; E-cadherin staining was perturbed with more prolonged treatment (data not shown) as reported previously (4).
To confirm a role for Src signaling, we transiently transfected MCF7 cells with a dominant-negative c-Src mutant (c-Src-mf, bearing point mutations K295M and Y527F, which open up the Src structure but ablate kinase activity (26)), which we recently showed perturbs E-cadherin junctions (4). E-cadherin contacts appeared fragmented in cells expressing this c-Src mutant compared with controls stably expressing GFP alone (data not shown), and Tyr(P)-cortactin staining at the cell-cell contacts was significantly reduced (Fig. 5C). Overall, these results identify a necessary role for SFK activity in mediating tyrosine phosphorylation of cortactin by E-cadherin adhesion.
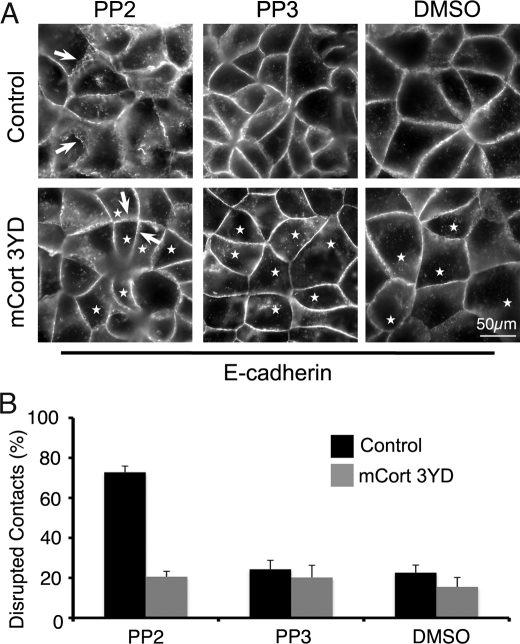
We then used the cortactin 3YD mutant to test whether cortactin was a functionally important effector of E-cadherin-activated SFK signaling (Fig. 6). We reasoned that if this were the case, then expression of a phosphomimetic cortactin transgene should rescue the effects of blocking SFK signaling. As previously observed (4), treatment of cells with either PP2 or SU6656 (data not shown) perturbed junctional integrity (Fig. 6A), causing E-cadherin staining at contacts to become fragmented and discontinuous compared with the linear staining seen in controls. In contrast, expression of cortactin 3YD restored the linear integrity of contacts (Fig. 6A). Quantitation revealed that 3YD reduced the number of PP2-treated cells with discontinuous contacts to levels comparable with control cells not treated with PP2 (Fig. 6B).
FIGURE 6.
Phosphomimetic cortactin mutant rescues the junctional effect of SFK inhibition. Control MCF7 cells or cells transiently expressing the phosphomimetic cortactin mutant (mCort 3YD) were incubated overnight with PP2, PP3, or DMSO. Cells were then fixed and immunostained for E-cadherin. A, PP2-treated control cells showed disrupted cell-cell contacts bearing many gaps and discontinuities (arrows), whereas contact integrity was restored in cells expressing mouse cortactin 3YD (arrows). Asterisks mark transfected cells. B, contact integrity was quantitated by assessing the number of contacts that showed disruption, expressed as a proportion (%) of the total number of contacts. Data are mean ± S.E. (n = 200).
Tyrosine Phosphorylation and Cortactin-Cadherin Association
Finally, we used co-immunoprecipitation analysis to test whether tyrosine phosphorylation affected the biochemical interaction between E-cadherin and cortactin (Fig. 7). Cortactin co-immunoprecipitated with E-cadherin in established, confluent MCF7 monolayers (Fig. 7A), consistent with previous observations in other cellular contexts (10, 12), and Tyr(P)-cortactin was also identified in these immune complexes (Fig. 7A). Incubation of cells with PP2 substantially reduced the amount of Tyr(P)-cortactin associated with E-cadherin, but did not affect the total amount of cortactin in the immune complexes (Fig. 7A). Furthermore, when reconstituted into cortactin KD cells, the cortactin 3YF mutant co-immunoprecipitated E-cadherin as efficiently as did wild-type mouse cortactin (Fig. 7B), consistent with the observation that this mutant still localized to disrupted E-cadherin adhesions (Fig. 4A). Overall, this suggests that phosphorylation of these canonical tyrosine residues is not necessary for cortactin to interact with the E-cadherin molecular complex.
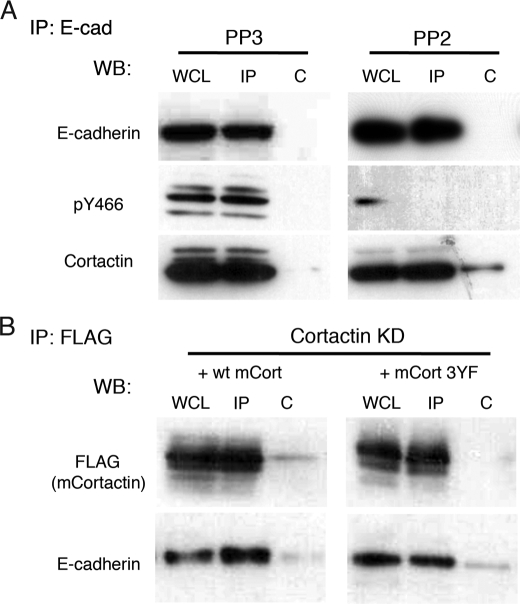
FIGURE 7.
Src kinase activity is necessary for tyrosine phosphorylation of cortactin, but not for its association with E-cadherin. A, E-cadherin was immunoprecipitated from confluent MCF7 cell monolayers treated with either PP2 or PP3 (IP: E-cad). Whole cell lysates (WCL) and immunoprecipitates (IP) were Western-blotted (WB) for E-cadherin, cortactin, and tyrosine-phosphorylated cortactin (pY466). The negative control for immunoprecipitation was naïve rabbit antisera. E-cadherin was able to immunoprecipitate both cortactin and tyrosine-phosphorylated cortactin. Treatment of PP2 substantially reduced levels of Tyr(P)-466-cortactin, but did not affect the amount of cortactin detected in E-cadherin immunoprecipitates. B, MCF7 cells depleted of cortactin by siRNA were transfected with plasmids expressing either FLAG-tagged mouse wild-type cortactin (wt mCort) or the mouse cortactin 3YF mutant. Cortactin-binding protein complexes were immunoprecipitated using FLAG antibodies (IP: FLAG). Whole cell lysates and immunoprecipitates were Western-blotted for E-cadherin and FLAG (cortactin). Negative control for immunoprecipitation was carried out using anti-hemagglutinin monoclonal antibody. Both wild-type and 3YF cortactin were able to immunoprecipitate E-cadherin, suggesting that tyrosine phosphorylation of cortactin is not necessary for its interaction with E-cadherin.
DISCUSSION
Src family kinases signal at E-cadherin-based cell-cell contacts (4, 27). Our current findings now identify cortactin as an important target for SFK signaling at these sites and demonstrate that its tyrosine phosphorylation is necessary for the integrity of both cadherin adhesive junctions and the perijunctional actin cytoskeleton. Overall, these observations lead to the notion that SFK and cortactin form key elements of a membrane-local signaling pathway that is activated by E-cadherin ligation to drive cooperation between adhesion receptors and the perijunctional actin cytoskeleton.
Several observations collectively establish cortactin as a target for E-cadherin-activated SFK signaling. First, in established monolayers, tyrosine-phosphorylated cortactin was consistently found at E-cadherin-based cell-cell contacts and was lost when E-cadherin was depleted by RNAi. Furthermore, co-immunoprecipitation analysis showed that Tyr(P)-cortactin interacts directly or indirectly with E-cadherin in confluent monolayers. Second, adhesive engagement of E-cadherin induced cortactin to become tyrosine-phosphorylated. This was suggested when cells were allowed to reassemble contacts with one another and confirmed when cellular cadherin was ligated with recombinant ligands.
In all these circumstances, cortactin phosphorylation was inhibited by blocking SFK signaling, either pharmacologically or by expression of a dominant-negative c-Src mutant. Thus SFK signaling was necessary for E-cadherin to induce tyrosine phosphorylation of cortactin. We do not yet know which SFK member is responsible for mediating E-cadherin signaling to cortactin. Both c-Src and Yes are expressed in the MCF7 cells used in our experiments,7 and c-Src RNAi reduces Tyr(P)-cortactin staining at cell-cell contacts.8 It remains to be determined, however, whether these SFKs have distinct or redundant roles in signaling from E-cadherin to cortactin.
Importantly, cortactin was necessary for the integrity of both cell-cell contacts and the perijunctional actin cytoskeleton. This observation extends earlier evidence that cortactin participates in the assembly of cadherin-based cell-cell contacts (10, 12), to suggest that it plays an ongoing role to maintain the integrity of cell-cell junctions. Moreover, phosphorylation of cortactin at the best characterized Src target sites was necessary for cortactin to support contact integrity. Consistent with this, blocking SFK signaling also causes E-cadherin to be reduced at cell-cell contacts (4) and decreases perijunctional actin staining.9 Although cortactin tyrosine phosphorylation can alter the expression of N-cadherin in fibroblasts (11), the surface levels of E-cadherin were not affected in our experiments.8 Interestingly, tight junction integrity was not affected by cortactin manipulation to the same extent as was E-cadherin organization. Overall, this implies that Tyr(P)-cortactin is necessary for the integrity of E-cadherin-based cell-cell interactions and further suggests that cortactin exerts a degree of selectivity for cadherin interactions rather than indiscriminately affecting all cell-cell junctions.
The notion that cortactin is a downstream effector of SFK in cadherin signaling is further supported by the fact that expression of the phosphomimetic cortactin 3YD mutant could rescue the integrity of cell-cell contacts when SFK signaling was blocked. The effect of SFK inhibition on cadherin contacts was somewhat less dramatic than that of cortactin KD. There may be several reasons for this difference. Unphosphorylated cortactin may also be functional, although tyrosine phosphorylation is clearly needed for its full impact on cadherin junctions. Additionally, cortactin is unlikely to be the sole target for SFK signaling at cadherin contacts: phosphatidylinositol 3-kinase and other effectors are found at cell-cell contacts and respond to SFK (4, 28). Furthermore, other signals, including Ser/Thr kinases (29) and Rho family GTPases (8), can influence cortactin. SFK and cortactin are then likely to constitute one key module, but not the sole element, in the complex network of signals and effectors that ultimately control junctional integrity and morphogenesis.
Although Tyr(P)-cortactin was clearly required to maintain the integrity of cell-cell contacts, the tyrosine phosphorylation events that we studied did not appear to be necessary for cortactin to interact with E-cadherin. This carries two implications. First, it suggests that the impact of Tyr(P)-cortactin on cadherin biology is not simply due to regulation of the cortactin-cadherin interaction. Instead, it is likely that tyrosine phosphorylation regulates the ability of cortactin to interact with other proteins once it is incorporated into the cadherin complex. We do not yet know what these interacting proteins may be. One interesting possibility is p120-catenin, which associates directly with the cadherin cytoplasmic tail. p120-catenin can also bind directly to cortactin (30), although whether this interaction occurs when p120-catenin is associated with E-cadherin is not yet reported. Another possibility is the Arp2/3 actin nucleator. Src-phosphorylated cortactin potentiates actin nucleation in vitro by promoting the association of cortactin with Nck and N-WASP to form a trimeric complex that activates Arp2/3 (24). As Arp2/3 can interact with the E-cadherin molecular complex (20, 31), it is possible that cadherin-bound Tyr(P)-cortactin modulates Arp2/3-mediated actin assembly at cell-cell contacts.
Second, this observation implies that cortactin might be tyrosine-phosphorylated after it recruits to E-cadherin. Indeed, this is suggested by our observation that although cell contact assembly greatly increased tyrosine phosphorylation of cadherin-associated cortactin, the total amount of cortactin found in cadherin complexes did not change significantly. In this regard, it is interesting to note that Src (32) can also immunoprecipitate with E-cadherin. This suggests the possibility that the cadherin might serve to bring these two proteins together, thereby facilitating tyrosine phosphorylation of cortactin. This would be consistent with increasing evidence that classical cadherins can scaffold signaling complexes at the plasma membrane.
However, it is important to emphasize that although our data indicate a key role for SFK in E-cadherin-to-cortactin signaling, they do not establish whether SFK are directly responsible for phosphorylating cadherin-bound cortactin. The Tyr-421 and Tyr-466 residues monitored in our experiments are established sites for SFK, but can also be phosphorylated by Abelson family kinases (Abl, Arg) and FER (6), which are also found in MCF7 cells.10 FER has been reported to mediate cortactin tyrosine phosphorylation in response to N-cadherin engagement (11). Moreover, both SFK and Abelson kinases are involved in tyrosine phosphorylation of cortactin in platelet-derived growth factor-stimulated fibroblasts; indeed, SFK may lie upstream of Abelson in this pathway (33). Nonetheless, although we cannot exclude roles for Abelson kinases or FER in E-cadherin signaling, it is clear that these kinases do not significantly compensate when Src signaling is disrupted.
SFK exert profound, but complex, effects on cadherin biology (reviewed in Ref. 27). It is well documented that expression of oncogenic, constitutively active Src mutants perturbs cadherin adhesion and disrupts the integrity of cadherin-based cell-cell contacts (34, 35). Conversely, loss-of-function studies have demonstrated a positive contribution of SFK to cadherin function. The integrity of cell-cell contacts and the ability of cadherins to support contact formation are compromised when SFK signaling is perturbed both in mammalian cells (4, 36) and in Drosophila embryos (37, 38). Overall, these observations suggest that physiological levels of SFK signaling serve to support cell-cell contacts, whereas pathological overstimulation may disrupt their integrity. Our current results imply that cortactin is a key component of physiological E-cadherin-activated SFK signaling. This extends earlier evidence that cortactin supports cadherin-based cell-cell interactions (10, 12) and complements the demonstration that cortactin is a target for Src signaling in osteoclasts that are necessary for podosome formation (15, 16). Additionally, overexpression of cortactin can promote cell migration and is often found in malignant tumors (6). Whether dysregulation of cortactin by SFK signaling may contribute to cadherin dysfunction in human disease is thus an interesting issue for future investigation.
Acknowledgments
We thank all the members of our laboratories for the kind encouragement and support during the course of this work. Confocal microscopy was performed at the Australian Cancer Research Foundation (ACRF)/Institute for Molecular Bioscience Dynamic Imaging Facility for Cancer Biology, established with the support of the Australian Cancer Research Foundation.
This work was supported in part by the National Health and Medical Research Council of Australia.
F. Helwani, unpublished data.
S. Wu and R. McLachlan, unpublished data.
G. Ren, unpublished data.
R. McLachlan, unpublished data.
S. Verma, unpublished data.
- SFK
- Src family kinase
- RNAi
- RNA interference
- DMSO
- dimethyl sulfoxide
- siRNA
- small interfering RNA
- GFP
- green fluorescent protein
- KD
- knockdown.
REFERENCES
- 1.Yap A. S., Brieher W. M., Gumbiner B. M. (1997) Annu. Rev. Cell Dev. Biol. 13, 119–146 [DOI] [PubMed] [Google Scholar]
- 2.Mège R. M., Gavard J., Lambert M. (2006) Curr. Opin. Cell Biol. 18, 541–548 [DOI] [PubMed] [Google Scholar]
- 3.Yap A. S., Kovacs E. M. (2003) J. Cell Biol. 160, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLachlan R. W., Kraemer A., Helwani F. M., Kovacs E. M., Yap A. S. (2007) Mol. Biol. Cell 18, 3214–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly R. J. (2004) Biochem. J. 382, 13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ammer A. G., Weed S. A. (2008) Cell Motil. Cytoskeleton 65, 687–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver A. M., Karginov A. V., Kinley A. W., Weed S. A., Li Y., Parsons J. T., Cooper J. A. (2001) Curr. Biol. 11, 370–374 [DOI] [PubMed] [Google Scholar]
- 8.Weed S. A., Karginov A. V., Schafer D. A., Weaver A. M., Kinley A. W., Cooper J. A., Parsons J. T. (2000) J. Cell Biol. 151, 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weed S. A., Parsons J. T. (2001) Oncogene 20, 6418–6434 [DOI] [PubMed] [Google Scholar]
- 10.Helwani F. M., Kovacs E. M., Paterson A. D., Verma S., Ali R. G., Fanning A. S., Weed S. A., Yap A. S. (2004) J. Cell Biol. 164, 899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Sayegh T. Y., Arora P. D., Fan L., Laschinger C. A., Greer P. A., McCulloch C. A., Kapus A. (2005) Mol. Biol. Cell 16, 5514–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Sayegh T. Y., Arora P. D., Laschinger C. A., Lee W., Morrison C., Overall C. M., Kapus A., McCulloch C. A. (2004) J. Cell Sci. 117, 5117–5131 [DOI] [PubMed] [Google Scholar]
- 13.Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3328–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhan X., Hu X., Hampton B., Burgess W. H., Friesel R., Maciag T. (1993) J. Biol. Chem. 268, 24427–24431 [PubMed] [Google Scholar]
- 15.Tehrani S., Faccio R., Chandrasekar I., Ross F. P., Cooper J. A. (2006) Mol. Biol. Cell 17, 2882–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luxenburg C., Parsons J. T., Addadi L., Geiger B. (2006) J. Cell Sci. 119, 4878–4888 [DOI] [PubMed] [Google Scholar]
- 17.Head J. A., Jiang D., Li M., Zorn L. J., Schaefer E. M., Parsons J. T., Weed S. A. (2003) Mol. Biol. Cell 14, 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barettino D., Feigenbutz M., Valcárcel R., Stunnenberg H. G. (1994) Nucleic Acids Res. 22, 541–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs E. M., Ali R. G., McCormack A. J., Yap A. S. (2002) J. Biol. Chem. 277, 6708–6718 [DOI] [PubMed] [Google Scholar]
- 20.Kovacs E. M., Goodwin M., Ali R. G., Paterson A. D., Yap A. S. (2002) Curr. Biol. 12, 379–382 [DOI] [PubMed] [Google Scholar]
- 21.Huang C., Liu J., Haudenschild C. C., Zhan X. (1998) J. Biol. Chem. 273, 25770–25776 [DOI] [PubMed] [Google Scholar]
- 22.Huang C., Ni Y., Wang T., Gao Y., Haudenschild C. C., Zhan X. (1997) J. Biol. Chem. 272, 13911–13915 [DOI] [PubMed] [Google Scholar]
- 23.Kraemer A., Goodwin M., Verma S., Yap A. S., Ali R. G. (2007) Am. J. Physiol. Cell Physiol. 292, C1061–C1069 [DOI] [PubMed] [Google Scholar]
- 24.Tehrani S., Tomasevic N., Weed S., Sakowicz R., Cooper J. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11933–11938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H., Parsons J. T. (1993) J. Cell Biol. 120, 1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timpson P., Jones G. E., Frame M. C., Brunton V. G. (2001) Curr. Biol. 11, 1836–1846 [DOI] [PubMed] [Google Scholar]
- 27.McLachlan R. W., Yap A. S. (2007) J. Mol. Med. 85, 545–554 [DOI] [PubMed] [Google Scholar]
- 28.Pang J. H., Kraemer A., Stehbens S. J., Frame M. C., Yap A. S. (2005) J. Biol. Chem. 280, 3043–3050 [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Quiles N., Ho H. Y., Kirschner M. W., Ramesh N., Geha R. S. (2004) Mol. Cell. Biol. 24, 5269–5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boguslavsky S., Grosheva I., Landau E., Shtutman M., Cohen M., Arnold K., Feinstein E., Geiger B., Bershadsky A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10882–10887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma S., Shewan A. M., Scott J. A., Helwani F. M., den Elzen N. R., Miki H., Takenawa T., Yap A. S. (2004) J. Biol. Chem. 279, 34062–34070 [DOI] [PubMed] [Google Scholar]
- 32.Bennett H. L., Brummer T., Jeanes A., Yap A. S., Daly R. J. (2008) Oncogene 27, 2693–2704 [DOI] [PubMed] [Google Scholar]
- 33.Boyle S. N., Michaud G. A., Schweitzer B., Predki P. F., Koleske A. J. (2007) Curr. Biol. 17, 445–451 [DOI] [PubMed] [Google Scholar]
- 34.Behrens J., Vakaet L., Friis R., Winterhager E., Van Roy F., Mareel M. M., Birchmeier W. (1993) J. Cell Biol. 120, 757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuyoshi N., Hamaguchi M., Taniguchi S., Nagafuchi A., Tsukita S., Takeichi M. (1992) J. Cell Biol. 118, 703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calautti E., Cabodi S., Stein P. L., Hatzfeld M., Kedersha N., Paolo Dotto G. (1998) J. Cell Biol. 141, 1449–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi F., Endo S., Kojima T., Saigo K. (1996) Genes Dev. 10, 1645–1656 [DOI] [PubMed] [Google Scholar]
- 38.Takahashi M., Takahashi F., Ui-Tei K., Kojima T., Saigo K. (2005) Development 132, 2547–2559 [DOI] [PubMed] [Google Scholar]