Abstract
Stem/progenitor cells reside throughout the adult CNS and are actively dividing in the subventricular zone (SVZ) and the dentate gyrus (DG) of the hippocampus. This neurogenic capacity of the SVZ and DG is enhanced following traumatic brain injury (TBI) suggesting that the adult brain has the inherent potential to restore populations lost to injury. This raises the possibility of developing strategies aimed at harnessing the neurogenic capacity of these regions to repair the damaged brain. One strategy is to enhance neurogenesis with mitogenic factors. As basic fibroblast growth factor (bFGF) is a potent stem cell mitogen, we set out to determine if an intraventricular administration of bFGF following TBI could affect the levels of injury-induced neurogenesis in the SVZ and DG, and the degree to which this is associated with cognitive recovery. Specifically, adult rats received a bFGF intraventricular infusion for 7 days immediately following TBI. BrdU was administered to animals daily at 2–7 days post-injury to label cell proliferation. At 1 or 4 weeks post-injury, brain sections were immunostained for BrdU and neuronal or astrocytic markers. We found that injured animals infused with bFGF exhibited significantly enhanced cell proliferation in the SVZ and the DG at 1 week post-TBI as compared to vehicle-infused animals. Moreover, following bFGF infusion, a greater number of the newly generated cells survived to 4 weeks post-injury, with the majority being neurons. Additionally, animals infused with bFGF showed significant cognitive improvement. Collectively, the current findings suggest that bFGF-enhanced neurogenesis contributes to cognitive recovery following TBI.
Keywords: Neurogenesis, bFGF, Traumatic brain injury, Hippocampus, SVZ, Morris water maze
Introduction
Over the last two decades it has become established that the mature mammalian brain continually generates new neurons in the regions of subventricular zone (SVZ) and the dentate gyrus (DG) of the hippocampus throughout life (Altman and Das, 1965; Lois and Alvarez-Buylla, 1993). More recently, it has become evident that heightened levels of neurogenesis in the SVZ and DG, over that seen in the normal uninjured brain, have been observed in response to several forms of brain insult including traumatic brain injury (TBI). Specifically, studies from our laboratory and others have shown that TBI stimulates cell proliferation within the DG at all ages (Chiruma-milla et al., 2002; Sun et al., 2005; Dash et al., 2001), and that these newly generated cells mature into dentate granular neurons which are capable of integrating into the existing neuronal circuitry (Sun et al., 2007). Furthermore, this injury-enhanced cell proliferation has been linked to the cognitive recovery processes (Sun et al., 2007) observed post-TBI. Collectively, these results suggest that the mature brain retains a degree of innate repair and regenerative potential to restore damaged neuronal populations through endogenous neuro-genesis. Consequently, strategies aimed at harnessing further these endogenous repair processes may have significant therapeutic potential for treating the injured brain.
As a result of both in vivo and in vitro studies, it has been established that several factors regulate CNS cell proliferation and neuronal generation (Gould and Cameron, 1996; Palmer et al., 1999). Among these factors, growth factors have been widely accepted as important mediators for neurogenesis. More specifically, basic fibroblast growth factor (bFGF) has been shown to be a potent mitogenic factor for neural stem and progenitor cells both in vitro and in vivo. For example, in vitro studies have demonstrated that cultured hippocampal neural progenitor cells divide only in response to bFGF (Ray et al., 1993; Vicario-Abejon, 2004). Additionally, in vivo studies have shown that while bFGF expression levels are elevated during brain development, they diminish with aging (Shetty et al., 2005; Caday et al., 1990). This reduction in bFGF levels with CNS maturity, however, is reversed in response to various forms of brain insult (Kumon et al., 1993; Logan et al., 1992). Furthermore, both intraven-tricular and subcutaneous administration of bFGF to normal adult animals enhance the proliferation of endogenous neural progenitors in the DG and SVZ (Kuhn et al., 1997; Wagner et al., 1999). It has also been reported that bFGF null mice fail to exhibit an injury-induced progenitor proliferative response, which can be restored by the administration of exogenous bFGF (Yoshimura et al., 2001; Yoshimura et al., 2003). Taken together, these studies demonstrate the important role of bFGF in regulating neurogenesis and mediating brain repair processes.
To explore the therapeutic potential of bFGF for brain repair, the present study was undertaken to examine the effect of an exogenous administration of bFGF on insult/trauma induced cell proliferation in the SVZ and the DG. Furthermore, the degree to which bFGF administration affected the maturational fate and survival of newly generated cells following insult was assessed. Additionally, the extent to which an infusion of bFGF can ameliorate cognitive deficits associated with TBI was ascertained by comparing cognitive recovery in bFGF infused animals versus those receiving vehicle. Collectively, these studies establish a crucial link between the therapeutic manipulation of neurogenesis and improved cognitive function following brain injury.
Materials and methods
Animals
A total of 42 three month old male Sprague–Dawley rats (Harlan Inc., Indiana) weighing approximately 300 g at the beginning of this study were used. Animals were housed in the animal facility, with a 12-hour light/dark cycle, water and food provided ad libitum. All procedures were approved by our Institutional Animal Care and Use Committee.
Surgical procedure and BrdU injection
Animals were subjected to a moderate lateral fluid percussion injury (FPI) following a previously described protocol (Sun et al., 2005). Briefly, adult rats were anesthetized in a Plexiglass chamber with 3% isofluorane, intubated and ventilated with 2% isofluorane in a gas mixture (30% oxygen, 70% N2), and secured in a stereotaxic frame. After a midline incision and exposure of the skull, a 4.9 mm craniotomy was made on the left parietal bone half way between the lambda and bregma sutures. ALuer lockfitting was then cemented to the skull and a 2.2±0.02 ATM fluid pulse was administered, using a pre-calibrated fluid percussion injury device. Sham control rats were subjected to the same surgical procedure without receiving the fluid pulse. After injury, the Luer lock fitting was removed, and anesthesia was switched off. Animals were examined for the recovery of mobility and consciousness by recording paw and tail reflex time as well as righting time (the time lapse that an animal to return to a spontaneous upright position from being placed on its back). Fifteen minutes after the injury, animals were re-anesthetized with 2% isofluorane and an Alzet brain infusion cannula (Brain Infusion Kit II, DURECT, Cupertino, CA) was stereotactically implanted into the posterior lateral ventricle ipsilateral to the injury site (coordinates: AP −0.8 mm, lateral 1.4 mm, 3.5 mm beneath the pial surface). The cannula was attached to an Alzet mini-osmotic pump (Model 1007D) which was placed subcutaneously on the back of the neck. Recombinant human bFGF (Roche, CA) was reconstituted in sterile artificial CSF (148 mM NaCl, 3 mM KCl, 1.4 mM CaCl2, 0.8 mM MgCl2, 1.5 mM Na2HPO4, 0.2 mM NaH2PO4, pH 7.4) containing 100 µg/ml bovine serum albumin and 10 µg/ml heparin for a final concentration of 33 µg/ml. This bFGF solution was infused for 7 consecutive days at a flow rate of 0.5 µl/h (approximately 400 ng/day). A total of 14 rats received bFGF infusion whereas 14 injured animals and 14 sham uninjured animals received a vehicle solution infusion. In order to determine the extent of the proliferative response to TBI, animals receive daily single i.p. injections of BrdU (50 mg/kg) for five consecutive days beginning at 48 h after injury. This group of animals was sacrificed at 7 days post-growth factor infusion. To determine the maturational fate and survival of cells generated following bFGF infusion, a second group of animals was allowedtosurvive for 4weeks before sacrificing. These animals were also assessed for cognitive recovery using the Morris Water Maze test.
Tissue processing
At 1 or 4 weeks post-TBI, animals were euthanized with sodium pentobarbital, transcardically perfused with phosphate-buffer saline (PBS) followed by 4% paraformaldehyde in PBS and the brains dissected and post-fixed in 4% paraformaldehyde for 48 h at 4 °C. Brains were cut coronally at 60 µm with a vibratome throughout the rostro-caudal extent of the brain. Sections were collected in 24-well plates filled with PBS plus 0.01% sodium azide and stored at 4 °C.
BrdU immunostaining and immunofluorescent double-labeling
To assess the number of BrdU-labeled cells, every 4th section was processed for BrdU immunohistochemistry. BrdU staining was performed following our previously published protocol (Sun et al., 2007). Briefly, the sections were washed with PBS, DNAwas denatured with 50% formamide for 60 min at 60 °C followed by a rinse in 2× SSC and then incubated with 2 N HCl for 30 min at 37 °C. After denaturing, the sections were washed with PBS and endogenous peroxidase was blocked using 3% H2O2. After overnight serum blocking with 5% normal horse serum, sections were incubated with mouse anti-BrdU antibody (1:200, Dako) in PBST (PBS with 0.4% Triton) plus 5% normal horse serum at 4 °C for 48 h. After rinsing with PBST, sections were incubated with HRP-conjugated anti-mouse-IgG (1:200, Santa Cruz, CA) overnight at 4 °C and visualized with 5.5 diaminobenzidine (DAB). Sections were mounted on glass slides, lightly counterstained with Cresyl violet and coverslipped.
To determine the maturational fate of the newly generated cells, parallel sections were processed for immunofluorescent double labeling using antibodies against BrdU and markers for mature neuron (NeuN) and astrocytes (GFAP). The staining procedure was similar to BrdU staining procedure described above. The primary antibodies used were mouse anti-NeuN (1:500, Chemicon), rabbit anti-GFAP (1:1000, Dako) and mouse anti-BrdU (1:200, Dako) or rat anti-BrdU (1:200, Immunologicals Direct, Oxford, UK). Secondary antibodies used were Alexa Fluor 488 anti-rat IgG, Alex Fluor 488 or 568 anti-mouse IgG or Alex Fluor 568 anti-rabbit IgG (1:200, Molecular Probes). Briefly, after DNA denaturing, endogenous perox-idase and serum blocking, sections were incubated with primary antibodies for 72 h at 4 °C with constant agitation. After washing, sections were then incubated with secondary antibodies overnight at 4 °C. Finally, sections were mounted on glass slides and coverslipped with Vectorshield (Vector Lab).
Stereological quantification of BrdU-labeled proliferating cells
To quantify the number of BrdU-positive cells in the dentate gyrus and SVZ, sections were examined with an Olympus Image System CAST program (Olympus, Demark). Ten 60 µm thick sections spaced 240 µm apart at the level of DG spanning from −2.56 mm to −5 mm of bregma and 10 sections through rostro-caudal extension of SVZ at the level from +1.7 mm to −0.8 mm of bregma (coordinates as identified in the rat atlas) were examined by a “blinded” observer using unbiased stereological methods. Using a 60× oil immersion lens, BrdU-positive cells were counted within the counting frame, ignoring cells in the upper and lower most focal planes and focusing through the thickness of the section (optical dissector principle) (Coggeshall and Lekan, 1996) to avoid over-sampling errors. Cells in the region of the subgranule zone and granular region were counted together as the granule cell layer.
Quantification for double-labeled cells
To quantify the percentage of BrdU-positive cells that have differentiated to the varying cell types, sections were examined by confocal microscopy (Leica TCS SP2). In the hippocampus, the entire granule cell layer was assessed and every BrdU-labeled cell was examined to assess the co-labeling of BrdU with cell type specific markers. A minimum of 100 BrdU+ cells from at least three sections per brain were examined for each marker. Each BrdU+ cell was manually examined in its full z dimension, and only those cells for which the BrdU+ nucleus was unambiguously associated with a given cell type-specific marker was considered double-labeled. The percentage of double-labeled cells was calculated as the number of BrdU+/ GFAP+ or BrdU+/NeuN+ cells against the total number of BrdU+ cells.
To examine the survival of the newly generated cells, the total number of BrdU+ cells in the entire granule cell layer from animals sacrificed at 4 weeks post-injury was quantified using stereological method described above and compared to the total number of BrdU+ cells in the same region at 7 days post-injury. The ratio indicated the survival rate of newly-generated cells.
Ki67 immunostaining
To assess whether TBI as well as bFGF treatment affect cell proliferation in the hippocampus at a later time point post-injury, brain sections were taken from animals which underwent the MWM test (and were sacrificed at 4 weeks post-injury) and were stained with the proliferation marker, Ki67. The immunostaining procedure for Ki67 was similar to BrdU immunostaining described above except the denaturing procedure with 50% formamide and 2 N HCl was omitted. Antibodies used were the rabbit anti-Ki67 (1:500, Abcam) primary antibody and the biotinylated goat anti-rabbit IgG (1:200, Jackson Lab) secondary antibody, followed by the ABC kit and DAB substrate. To quantify the number of Ki67+ cells, 4 animals in each experimental group (three sections per brain) were examined and every single Ki67+ cells in the ipsilateral SGZ and GCL were counted using a 60× oil immersion objective. The number of Ki67 in each group was averaged and presented as the number of cells per section.
Quantification of neuronal cell numbers in the hippocampus
To examine whether a bFGF infusion affects hippocampal neuronal survival after injury, we counted the number of dentate granule neurons in the ipsilateral hippocampus as well as the number of pyramidal neurons in the ipsilateral CA3 and hilus regions where neurons are most susceptible to LFPI injury using the stereology optical fractionator method. Ten 60 µm thick sections at 240 µm apart through the rostro-caudal extent of the hippocampus at the level from −2.56 mm to −5 mm of bregma from animals which were perfused at 4 weeks following injury were mounted on microscope slides and processed for Giemsa histochemical staining. The number of neurons in the DG, CA3 and hilus regions was counted by a “blinded” observer. Only neurons which had clear nucleoli within a defined nuclear membrane were counted.
Stereological assessment
The optical fractionator method was used to estimate the total number of BrdU+ cells in the SVZ and DG, as well the total number of neurons in the hippocampal CA3, hilus and DG. This design-based stereological method, which is commonly used in neuroscience studies of this nature (Tran et al., 2006; Grady et al., 2003; Sun et al., 2007; Keuker et al., 2001), provides an estimate of cell numbers that is not influenced by the size, shape, spatial orientation, and spatial distribution of the cells under study (Keuker et al., 2001). Briefly, the region of interest was outlined using a 4× objective. A 60× oil immersion objective was used for cell counting. In the examining region, an optical dissector counting frame was used to count BrdU+ cells or Giemsa stained neuronal nuclei at predetermined regular x, y intervals. The area (a) of the counting frame was known relative to the stage-stepping intervals over the section, the sampling fraction (asf)=a (frame)/a (x,y step). The dissector height (h) was known relative to the section thickness (t). With these parameters, the number of total cell counts (N) was estimated as N=(ΣQ)(t/h)(1/asf)(1/ssf), where ssf was the section-sampling fraction (=0.25 in this study), and ΣQ was the number of cells counted.
Morris Water Maze (MWM)
To test whether bFGF infusion can affect the cognitive recovery of rats following TBI, animals were tested on hippocampal-dependent tasks using the MWM at days 21–25 post-injury. MWM testing was performed following our previously published protocol (Sun et al., 2007). In the MWM performance, goal latency and path length are equally sensitive measures (Hamm, 2001). We used goal latency as the primary dependent variable. Path length (to reach the goal in the MWM) and swim speed were also analyzed. Prior to MWM testing, a visual platform test was performed to confirm that the visual system of the animal was not impaired. Briefly, the animals were placed in a large circular tank containing opaque water and allowed to swim freely to find the hidden goal platform (1 cm below the water surface) in order to escape from the water. Each animal was tested four times each day. For each trial, the animal was randomized to one of four starting positions (N, E, S, W). MWM performance was recorded using a computerized video tracking system (Columbus Instruments) and latency to find the platform, total distance swum to reach the goal platform, and swim speed were calculated for each trial. Upon finding the platform, animals were left there for 30 s before being removed from the maze and placed in a warming cage to dry. Animals that did not find the platform after 120 s were placed on the platform for 30 s and then removed from the maze. Animals unable to swim were removed immediately from the water.
Statistical analysis
The data generated was analyzed using SPSS software. For cell quantification a one-way ANOVA with post-hoc Fisher LSD test or the Student t-test with an applied Bonferroni correction for multiple groups was utilized, with p value less than 0.05 taken as statistically significant. For MWM data analysis, the data was analyzed using a split-plot ANOVA [Treatment×Day] comparing effect of group on goal latency. A Fisher LSD test was performed to allow for pairwise group contrasts. Swim speed was also analyzed using a one-way ANOVA. Data are presented as mean±SEM in all figures.
Results
The current study aimed to assess the extent to which an intraventricular infusion of bFGF modifies the endogenous neurogenic response of the CNS following traumatic brain injury and whether aspects of this response are associated with cognitive recovery.
Righting response
To assess whether all injured animals received a similar level of injury, we first compared the post-injury righting response in animals which received bFGF infusion with animals received vehicle. The return of response, which correlates with neuromotor deficits, is generally regarded as an indicator for determining injury severity (Morehead et al., 1994; Hamm, 2001). The mean (SEM) duration of suppression of the righting response after injury was 6.95±0.52 min for the vehicle-infused group and 7.27±0.34 min for the bFGF-infused group. A t-test on this data indicated that the groups did not significantly differ on the duration of the righting response (t14 =−0.51, p=0.62), supporting the conclusion that both groups received a similar severity of TBI.
Intraventricular infusion of bFGF enhances TBI-induced cell proliferation in the SVZ and dentate gyrus
In previous studies, we have reported that TBI significantly enhances cell proliferation in the neurogenic regions of the brain, i.e., the SVZ and the dentate gyrus (Chirumamilla et al., 2002; Sun et al., 2005). To explore whether the injury-induced proliferative response in these neurogenic regions can be further augmented with exogenous manipulations, animals were subjected to a moderate LFPI followed by a 7-day infusion of recombinant bFGF into the lateral ventricle ipsilateral to the injury site. BrdU was injected daily to label the proliferating cells in these two regions. Animals were then sacrificed at 7 days post-injury and processed for BrdU immunohistochemistry.
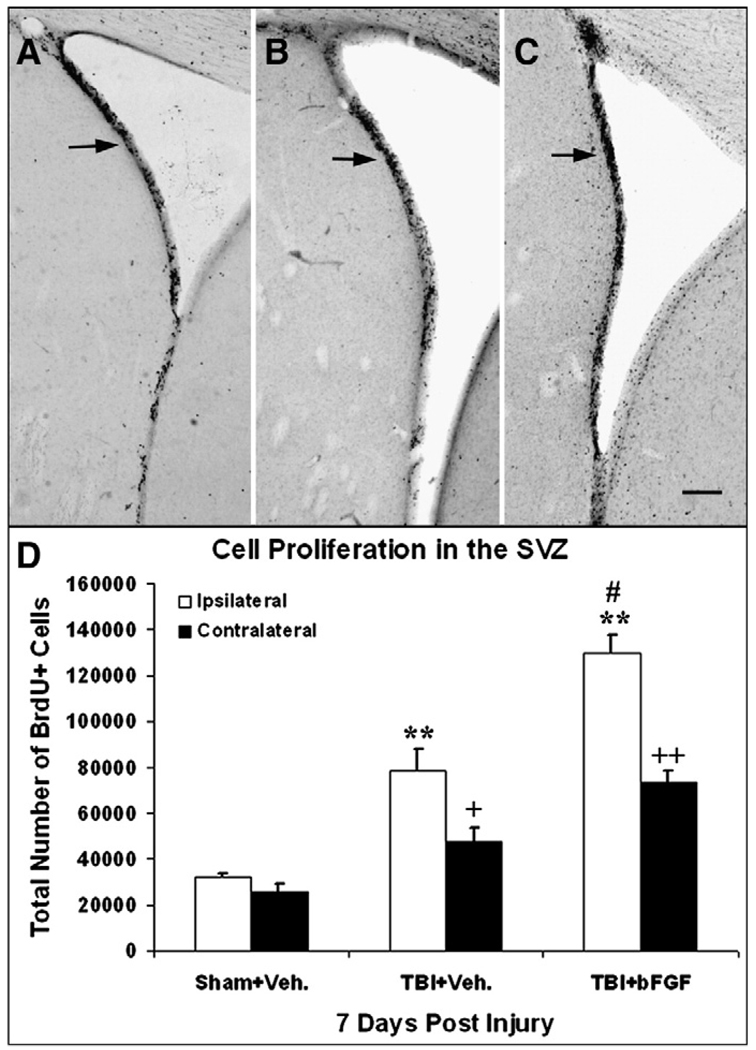
In the SVZ, BrdU immunohistochemistry revealed enhanced levels of BrdU+ proliferating cells in animals subjected to LFPI (receiving a vehicle or bFGF infusion) as compared to sham animals (Figs. 1A–C arrows). Unbiased stereological quantitative analysis for the total number of BrdU+ cells showed that injured animals with vehicle or bFGF infusion have a significantly higher number of proliferating cells in both ipsilateral and contralateral SVZ in comparison to sham animals (p<0.01, Fig. 1D). Moreover, injured animals receiving bFGF infusion had a significantly higher number of BrdU+ cells in both sides of the SVZ, particularly in the ipsilateral side, when compared to injured vehicle treated animals (p<0.01, Fig. 1D).
Fig. 1.
Growth factor infusion enhances cell proliferation in the SVZ. Coronal sections of the ipsilateral SVZ taken from the following animals at 7 days post-injury: (A) a sham animal that received an intraventricular infusion of vehicle only; (B) an injured animal that received an intraventricular infusion of vehicle; and (C) an injured animal that received an intraventricular infusion of bFGF. Increased BrdU-labeling (black cell nuclei: arrow) was observed in the injured animal and further enhanced in the injured animal that received bFGF as compared to the sham animal. Bar=500 µm. (D) Quantification analysis of the degree of cell proliferation in the SVZ. Compared to sham animals, injured animals with vehicle or bFGF infusion had significantly enhanced cell proliferation in the SVZ both ipsilateral (**p<0.01) and contralateral side to the injury site (+p<0.05; ++p<0.01). Injured animals with bFGF infusion had significantly more BrdU+ cells in the ipsilateral SVZ compared to injured animals with vehicle infusion (#p<0.01).
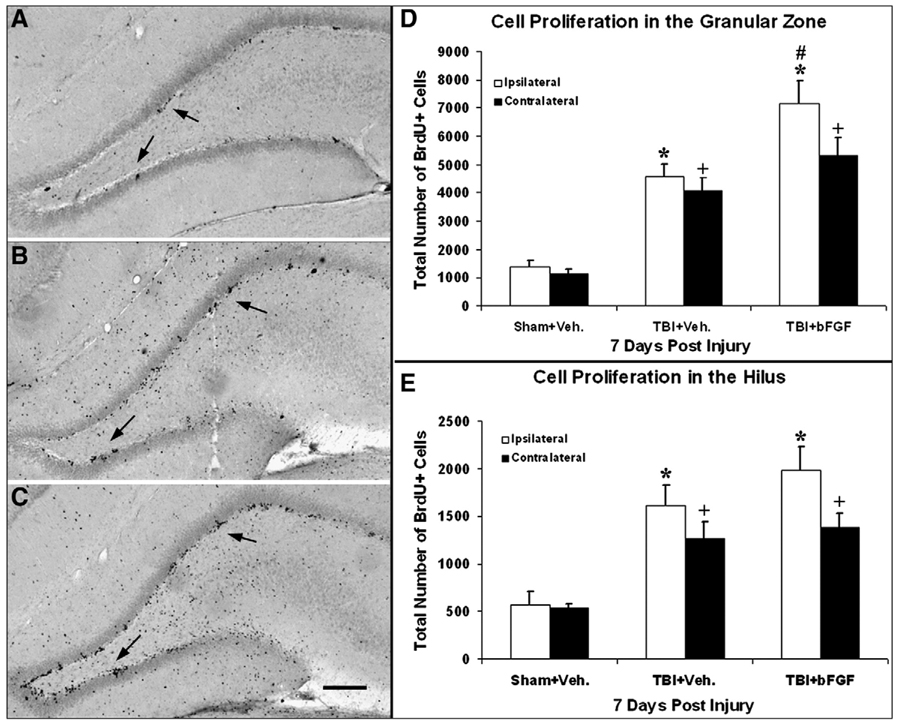
In the DG, BrdU staining displayed clustered BrdU+ cells predominately located in the SGZ, with enhanced levels of cell proliferation in injured animals (infused with vehicle or bFGF) as compared to sham animals (Figs. 2A–C arrows). Stereological quantification analysis of subregions within the DG showed that the total number of BrdU+ cells was significantly increased in the hilus and granular zone (GZ, including subgranular zone and granular cell layer) of both hemispheres in injured animals infused with vehicle or bFGF as compared to sham animals (p<0.05, Figs. 2D and E). Furthermore, injured animals which received bFGF infusion had a significantly greater number of BrdU+ cells in the ipsilateral GZ as compared to injured vehicle-treated animals (p<0.05, Fig. 2D).
Fig. 2.
Growth factor infusion enhances cell proliferation in the DG. Coronal sections of the ipsilateral DG taken from the following animals at 7 days post-injury: (A) sham with vehicle infusion; (B) injured with vehicle infusion; and (C) injured with bFGF infusion. Increased numbers of BrdU+ cells were observed in the injured animals with either vehicle or bFGF infusions compared to the sham (black dots indicated by arrow). BrdU+ cells in the DG were clustered and mainly located in the SGZ. Bar=200 µm. (D) Quantification analysis of the degree of cell proliferation in the DG. Compared to shams, injured animals with vehicle or bFGF infusion had significantly more proliferating cells in the ipsilateral granular zone (*p<0.05) and the contralateral side (+p<0.05). Injured animals which received bFGF had significantly higher number of BrdU+ cells in the ipsilateral granular zone compared to injured animals with vehicle (#p<0.05). (E) Quantification analysis of the degree of cell proliferation in the hilus region. Compared to sham animals, proliferation cells in both the ipsi- and contralateral hilus were significantly higher in the injured animals with either vehicle or bFGF (*/+p<0.05).
Collectively, these data indicate that post-TBI administration of bFGF further enhances the injury-induced cell proliferative response in the SVZ and DG of adult rats following TBI.
Cells generated by an infusion of bFGF survive for an extended period
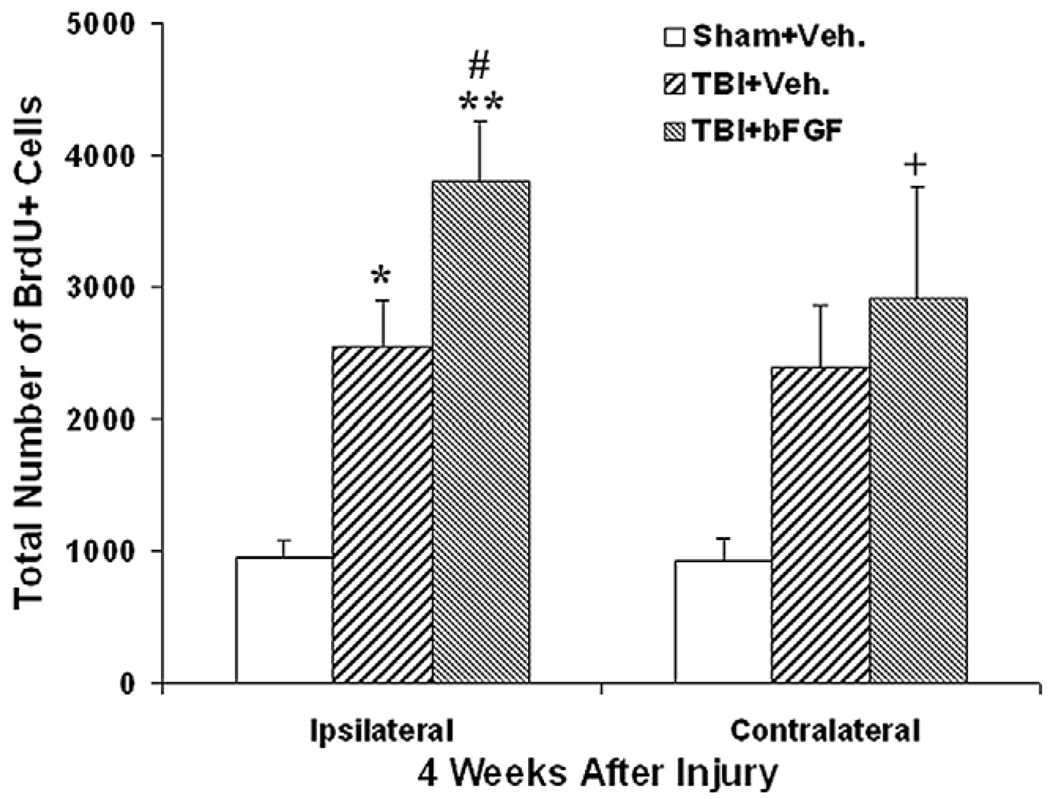
To examine whether cells generated following injury and bFGF infusion can survive for an extended period, we quantified the total number of BrdU+ cells at 4 weeks post-injury for animals infused with either vehicle or bFGF and shams. Because new cells generated from the SVZ constantly migrate out to the olfactory bulb, the number of BrdU+ cells in the SVZ at 4 weeks does not accurately represent the number of survival cells, therefore, for this study, only BrdU+ cells in the dentate gyrus were quantified. Brains were taken from animals which were tested for cognitive function using Morris Water Maze tests (see below). Stereological cell quantification was done in a similar manner as performed on the 7 days post-injury groups described above. In contrast to the number of BrdU+ cells present at 7 days post-injury, a substantial proportion of the newly generated cells were lost by 4 weeks post-injury and this decline was more significant in the injured animals as compared to shams. Specifically, in the sham group 70% and 82% of BrdU+ cells in the ipsilateral and contralateral DG, respectively, survived for 4 weeks post-injury. Whereas, in injured vehicle-treated animals, 56% and 59% of newly generated cells in the ipsilateral and contralateral DG, respectively, survived at 4 weeks. This is comparable to injured animals treated with bFGF, which displayed 53% survival in the ipsilateral DG and 55% in the contralateral DG. Because bFGF was infused for only 7 days in this study and consequently was absent during the peak time of cell death, which was approximately 2 weeks after generation, bFGF did not have an effect in encouraging the extended survival of newly generated cells following TBI. However, because of the larger pool of newly generated cells in the injured groups, by 4 weeks post-TBI, the total number of surviving BrdU+ cells in the granular zone in injured animals receiving either vehicle or FGF were significant higher when compared to the sham animals in both ipsilateral side (p<0.01, Fig. 3) and contralateral side (p<0.05, Fig. 3). Moreover, injured animals treated with bFGF had a significantly higher number of BrdU+ cells in the ispilateral GZ than vehicle treated animals (p<0.05, Fig. 3).
Fig. 3.
Infusion of bFGF enhances the survival of newly generated cell in the DG following injury. Graph showing the number of BrdU+ cells in the granular zone at 4 weeks post-injury in sham animals, injured animals infused with vehicle or injured animals infused with bFGF. Notice that at this time point post-injury, a significantly higher number of BrdU+ cells were observed in injured animals treated with vehicle or those treated with bFGF in both ipsi- and contralateral sides (*/+p<0.05; **/++p<0.01). Moreover, injured animals which received bFGF infusion had a higher number of BrdU+ cells than vehicle treated animals, and this was significant in the ipsilateral side (#p=0.05).
A significant number of neurons are generated in the DG following an infusion of bFGF
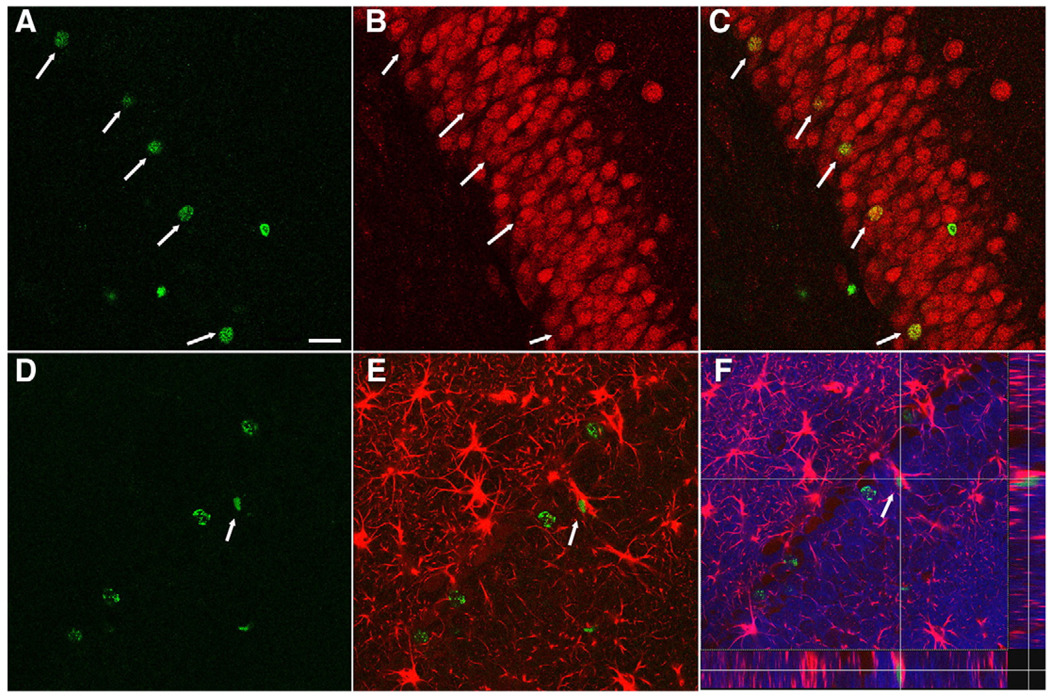
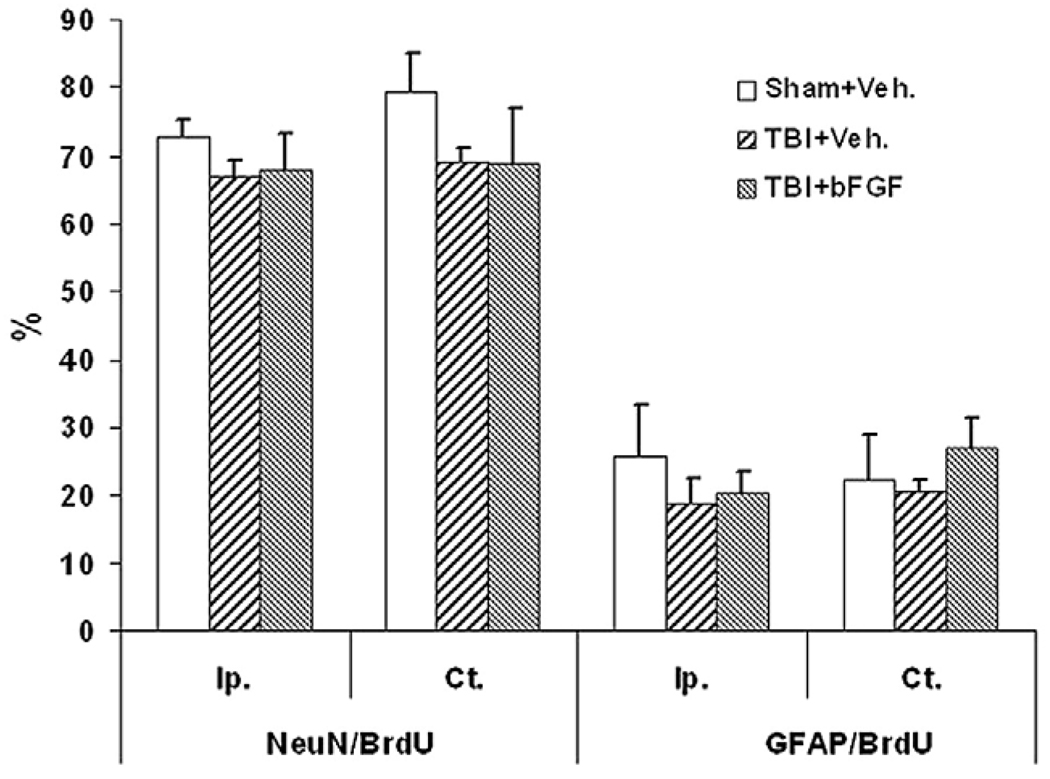
Previously, we reported that the majority of the cells that were generated in the DG following injury, which had survived for an extendedperiod, becamemature granularneurons (Sun et al., 2007).To examine whether a post-injury intraventricular infusion of bFGF affects the maturational fate of the newly generated cells, coronal hippocampal sections taken from animals which received bFGF or vehicle infusion and survived for 4 weeks post-TBI were processed for immunofluorescent double-labeling of BrdU with either NeuN or GFAP (Fig. 4). In the granular zone, many newly generated BrdU+ cells were co-labeled with the mature neuronal marker, NeuN (Figs. 4A–C) or the astrocytic marker, GFAP (Figs. 4D–F). The percentage of newly generated cells in the ipsilateral granular zone (SGZ+GCL) which had differentiated into mature neurons as demonstrated by double-labeling of BrdU and NeuN was 72.7±2.7% for shams, 66.7±2.6% for injury/vehicle infused and 67.8±5.4% for injury/bFGF-infused animals. The percentage of cells that had differentiated into astrocytes as shown as BrdU/GFAP double-labeling was 25.8±7.7% for shams, 19±3.8% for injury/vehicle infused, and 20.4±3.2% for injury/bFGF-infused animals. In the contralateral side of granular zone, similar percentages of BrdU/ NeuN and BrdU/GFAP double-labeled cells were found as in the ipsilateral side (Fig. 5). While there are no significant differences in the percentage of cells that take a neuronal or glial phenotype between the groups, when these percentages are extrapolated to the total number of proliferating cells found in the DG following bFGF infusion at 4 weeks (Fig. 3), it is estimated that 2574 new neurons are added to the granule cell population in injured animals treated with bFGF as compared to 1706 and 699 new neurons in the injured treated with vehicle and sham animals respectively.
Fig. 4.
Newly generated cells in the DG following injury differentiate into both neurons and astrocytes. Confocal microscopic images of the DG at 4 weeks following injury showing double-labeling of BrdU+ cells with the neuronal marker NeuN and the astrocytic marker GFAP. (A–C) Arrows indicate BrdU-positive cells (green, A) in the DG were co-stained with the mature neuronal marker NeuN (red, B) and merged as yellow (C). (D–F) Arrow indicates co-localization of a BrdU-labeled cell (green, D) with GFAP (red, E) in the granular cells layer throughout z-axis (F, DAPI-blue). Scale bar=50 µm.
Fig. 5.
The maturational fate of the newly generated cells in the DG at 4 weeks post-injury is not affected by bFGF infusion. Quantitative analysis at 4 weeks following TBI showing that the percentage of BrdU+ cells double-labeled with NeuN was similar in sham animals, and injured animals with vehicle infusion or injured animals with bFGF infusion, in both ipsilateral and contralateral DG. Moreover, the percentage of BrdU/ GFAP labeled cells in the ipsilateral GZ was similar in sham groups as compared with injured vehicle and bFGF groups. No statistic significance was found between groups.
In the hilus region, we found many BrdU/GFAP double-labeled cells but only a few BrdU/NeuN double-labeled cells (data not shown). Specifically, in sham, injury vehicle and injured bFGF groups 38.6±9.9%, 35.9±1.81% and 36.6±6% BrdU+ cells were co-labeled with GFAP, respectively, in the ipsilateral hilus region. The contralateral hilus region had a similar rate of BrdU/GAFP co-labeling for each group. In contrast, only 7.8±1.7% BrdU+ cells were found to be co-labeled with NeuN in the sham group and less than 2% BrdU/NeuN double-labeled cells were found in both injured groups.
These data suggested that bFGF had no influence on the endogenous cell fate choice made by these cells in the DG. Nevertheless, since a bFGF infusion generated many more proliferating cells than found in the other animal groups, the total number of neurons added into the existing pool of cells in the granular zone of the DG was significantly higher.
Intraventricular infusion of bFGF has no effect on cell proliferation at 4 weeks post-injury
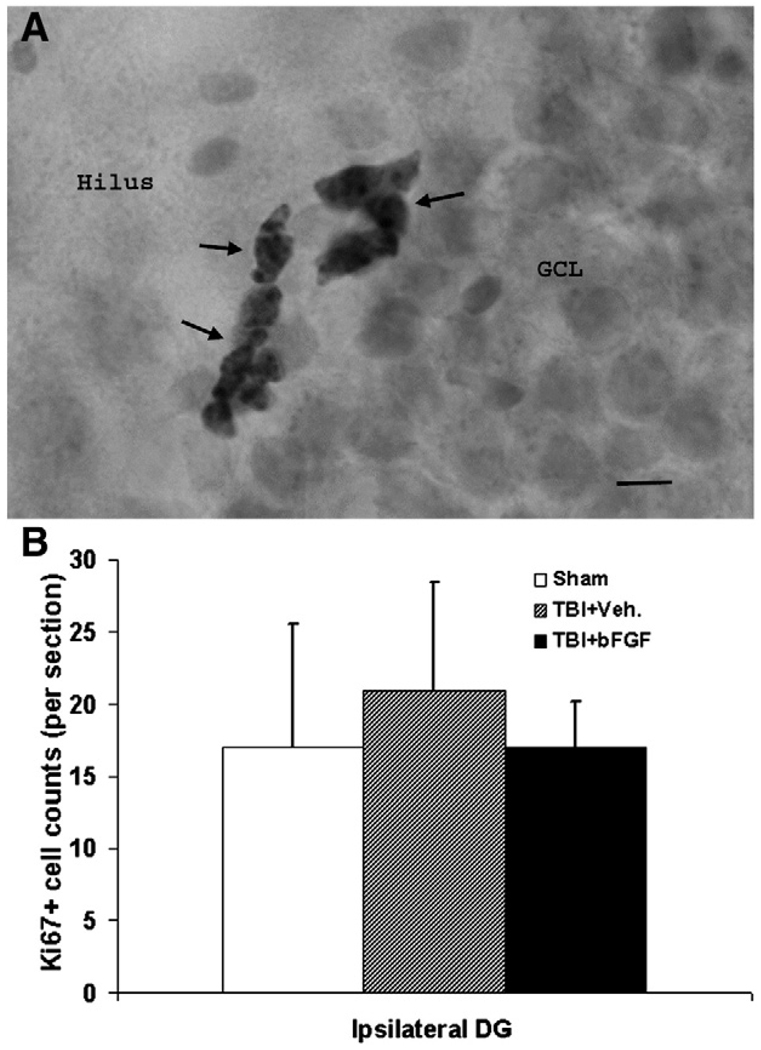
Previously we had reported that the level of TBI-induced cell proliferation peaked at 2 days post-injury and decreased with time so as to return to near sham levels by 2 weeks post-injury (Sun et al., 2005). Since bFGF further enhanced cell proliferation, we sought to determine the length of time this FGF treatment could affect cell proliferation in vivo. Specifically, using the proliferation marker Ki67, we surveyed the degree of cell proliferation in the hippocampus in animals sacrificed at 4 weeks post-TBI. Ki67 antigen is a cell cycle related nuclear protein expressed by proliferating cells in all phases of the active cell cycle. By examining the location of Ki67+ cells in the hippocampus of these animals, we found that at the time of perfusion they were mostly located in the SGZ (Fig. 6A). Moreover, at 4 weeks post-injury, the level of cell proliferation as established by the number of Ki67+ cell was similar in sham and injured animals that were infused either vehicle or bFGF (Fig. 6B). This data suggested that neither TBI nor bFGF treatment has any prolonged effect on cell proliferation in the hippocampus.
Fig. 6.
Intraventricular infusion of bFGF for 7 days does not have prolonged effect on cell proliferation in the DG. (A) Microscopic image of the DG which stained with proliferation marker Ki67 showing clustered Ki67+ cells were located in the SGZ between the GCL and hilus. Scale bar=20 µm. (B) Graph showing the number of Ki67+ proliferation cells in the ipsilateral DG in sham, injured animals received either bFGF or vehicle infusion at 4 weeks following TBI. Notice that there was no significant difference in all three groups.
Intraventricular infusion of bFGF improves recovery of TBI-induced cognitive deficits
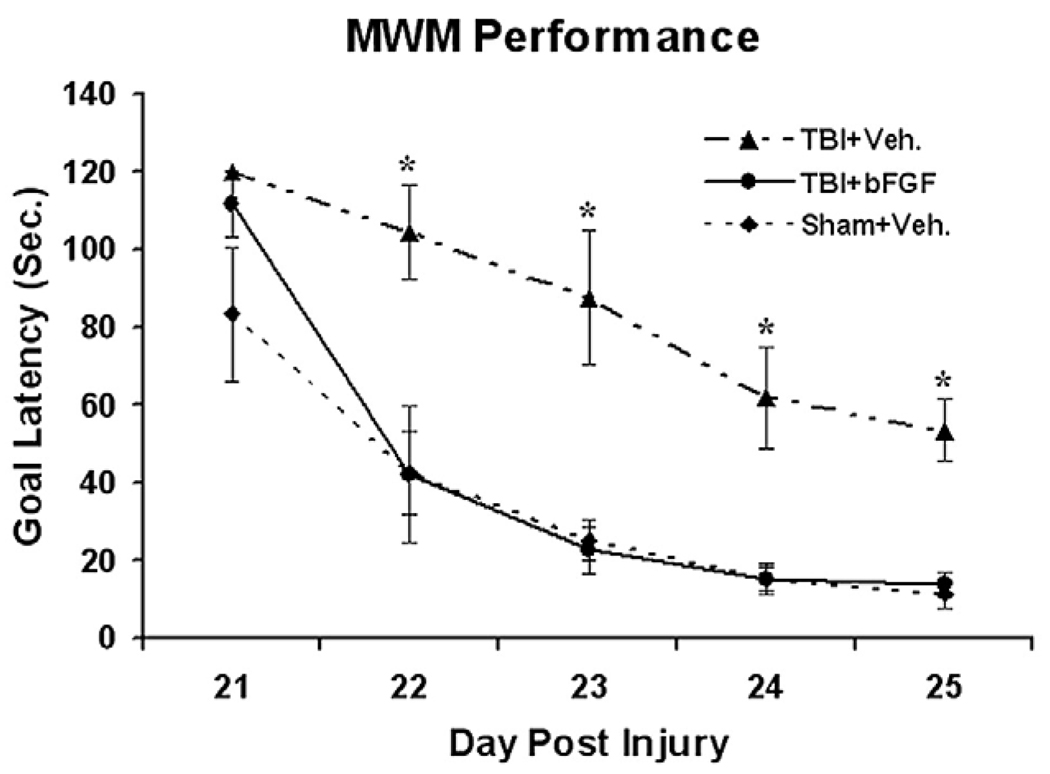
Moderate lateral fluid percussive injury induces cognitive deficits which recover with time. We previously found that the time course of the spontaneous cognitive recovery is associated with the TBI-induced neurogenesis and the integration of newly generated granule neurons into the hippocampus (Sun et al., 2007). Since a post-TBI infusion of bFGF was found to enhance neurogenesis in the DG and SVZ, the cognitive recovery of these injured animals was assessed using the Morris Water Maze (MWM) test. Specially, MWM was assessed in sham animals as well as TBI animals which received intraventricular infusions of bFGF or vehicle for 7 days post-injury. These tests were performed daily from 21–25 days post-TBI. The mean latency (s) to reach the goal platform for each group is presented in Fig. 7. We found that injured animals which received a bFGF infusion had a significantly shorter latency to reach the platform than vehicle treated animals (p<0.01, Fig. 7) suggesting that bFGF treated animals displayed better performance or quicker learning in the MWM. The data were analyzed by a split-plot analysis of variance (ANOVA, Group×Day). The results of ANOVA revealed a significance Group effect (F2,21 = 39.19, p<0.001), Day effect (F4,84 = 73.18, p<0.001), and Day×Group interaction (F8,84=3.23, p<0.003). To examine specific group differences, Fisher LSD tests were performed. This analysis found that the TBI+Vehicle group had significantly longer goal latencies than TBI+bFGF and Sham+Vehicle groups (p<0.001). In addition the TBI+bFGF group did not differ in goal latency from the Sham+Vehicle group (p=0.37). No differences were observed in swim speed between groups indicating that motor impairments did not contribute to the different latencies. Taken together, these data demonstrate that injured animals display a significant improvement in cognitive function when treated with an intraventricular infusion of bFGF.
Fig. 7.
Growth factor infusion improves cognitive recovery following TBI. Graph compared MWM performance of injured rats infused with either bFGF or vehicle, to sham animals infused with vehicle alone. Injured rats infused with bFGF showed a significant improvement of cognitive recovery as compared to injured rats with vehicle (*p<0.01, n=10 in each group). This cognitive recovery, as characterized by shorter goal latency in the water maze performance reached similar levels to that observed in sham animals through days 22–25 following injury.
Post-TBI infusion of bFGF increases the total number of dentate granule neurons but does not affect neurons in the CA3 and hilus
Since bFGF displays both mitogenic and neurotrophic functions, we attempted to verify which of these properties were likely to participate in the cognitive recovery observed following bFGF infusion in injured animals. Since bFGF infusion significantly enhances cell proliferation in the DG, we wanted to attest whether bFGF treatment would affect the total number of DG granule cells. As hippocampal neurons in the CA3 and hilus regions are particularly vulnerable to secondary insults following TBI and their loss contributes to cognitive deficits as assessed by MWM, we also sought to determine whether a post-injury administration of bFGF protects these neuronal populations from injury-induced damage. Using unbiased stereological method, the total number of neurons in the ipsilateral dentate granule layer, CA3 and hilus regions was quantified at 4 weeks post-injury in animals which went through MWM tests.
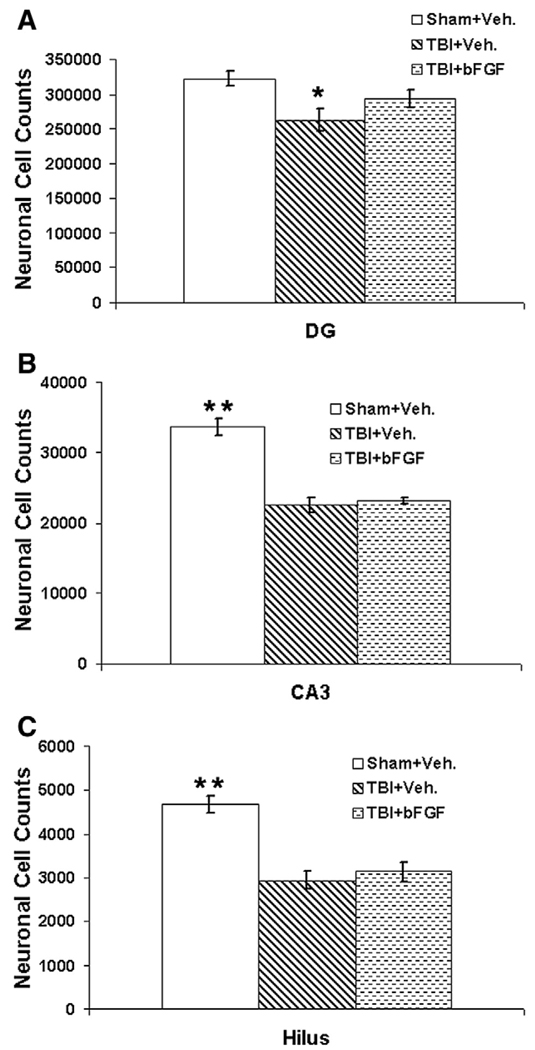
In the DG, injured animals which received vehicle infusion had significant neuronal cell loss as compared to sham animals (p=0.02, Fig. 8A), whereas injured animals which received bFGF infusion had slightly but not significantly low neuronal cell counts as compared to shams (p=0.125, Fig. 8A). Moreover, injured animals receiving bFGF had a higher number of dentate granule neurons as compared to injured animals infused with vehicle, however, the difference was not significant (p=0.187). In both the CA3 and hilus regions, injured animals treated with either bFGF or vehicle had significant neuronal cell loss compared to sham animals (p<0.01, Figs. 8B, C). Injured animals treated with vehicle had slightly but not significantly more cell loss in both regions as compared to bFGF treated animals (p>0.05, Figs. 8B, C). This data suggests that an intraventricular infusion of bFGF increases the total number of dentate granule neurons, which is likely due to the addition of large numbers of newly generated granule neurons induced by bFGF treatment. The data also suggests that the short term bFGF treatment following TBI does not have a significant neural protective effect on the CA3 and hilus neurons in the injured hippocampus.
Fig. 8.
Growth factor bFGF preserves the total number of dentate granule cells but does not have a neuroprotective effect on the survival of neurons in the CA3 and hilus region following injury. Four weeks after FPI, injured animals infused with either vehicle had significant neuronal cell lose in the ipsilateral dentate gyrus (A), CA3 region (B) and hilus region (C) as compared to sham animals (*p<0.05, **p<0.01, n=4 in each group). Compared to sham animals, injured animals which received bFGF infusion also had significantly neuronal cell lose in the CA3 and hilus regions (B and C, **p<0.05) but not in the DG (A). No significant difference was observed in the number of total neuronal counts between injured animals infused with bFGF and vehicle treated animals in all three regions (A–C).
Discussion
The current study demonstrates that traumatic brain injury induces cell proliferation in the subventricular zone and the dentate gyrus of the hippocampus which can be further augmented with an exogenous application of bFGF. Specifically, an intraventricular infusion of bFGF immediately following TBI significantly increased the number of newly generated cells in the subventricular zone and the dentate gyrus. Moreover, by determining the cell fate of these newly generated cells it was ascertained that this growth factor treatment generated a larger pool of neurons than that found in injury alone or with a vehicle infusion. Furthermore, bFGF administration improved cognitive functional recovery in injured animals as assessed by Morris Water Maze tests. As a post-injury bFGF infusion did not prevent injury-induced neuronal cell loss of hippocampal neurons in the CA3 and hilus regions but rather increased the total number of dentate granule neurons, it is likely that the mechanism of bFGF-enhanced cognitive recovery is independent of its neuroprotective effect.
The injury-induced proliferation response observed in this study is in agreement with our previous investigations into endogenous repair mechanisms of the brain following TBI as well as reports from other labs (Dash et al., 2001; Chirumamilla et al., 2002; Sun et al., 2007). The current study extends these observations by assessing cell proliferation in the DG and SVZ in both the ipsilateral and contralateral hemispheres. Importantly, we demonstrate that this injury-induced cell proliferation within the SVZ and DG can be further enhanced by intraventricular administration of bFGF. This observation is somewhat at odds with the findings of Kuhn et al. (1997) that bFGF infusion solely enhances cell proliferation in the SVZ without affecting cell proliferation or differentiation in the DG. However, the current observation as well of those of others has shown that bFGF treatment does indeed enhance neurogenesis in the dentate gyrus (Wagner et al., 1999; Tureyen et al., 2005). This disparity could be due to differences in sampling or quantification methods, dose of bFGF, timing or length as well as route of growth factor administration.
In the hippocampus in normal adult rodents, newly born cells are likely to degenerate within approximately 1–2 weeks of their formation (Cameron and McKay, 2001; Dayer et al., 2003). Moreover, the percentage of surviving newly born cells at 1 month after generation in the normal animals varies in different studies and in difference species. For example, Dayer et al. (2003) have reported a 50% survival rate by the time of 1 month in normal rats, whereas Kempermann et al. reported a varying degree of survival in different aged mice from near 25% in 2 month old animals (Kempermann et al., 2003) to 43% in 6 month old and 61% in 18 months old (Kempermann et al., 1998). In the current study, we found that following injury, bFGF or vehicle injured animals had slightly lower percentage of survival rate than the sham (56–59% versus 70–80%). This data was compatible to what we reported previously (Sun et al., 2007). In the current study, the lower survival rate observed in injured animals may due to the shortage of neurotrophic support for far too many newly generated cells and/or the less conducive injured environment for cell survival. Compared to other published data, the higher percentage of survival in the sham animals in this study is likely due to the differences in animal species and age as well as the tissue sampling and quantification methods. While the current study does not provide definitive proof that the cognitive recovery observed following bFGF infusion is the direct result of this enhanced neurogenesis, the large numbers of newly generated cells that mature into neurons within the DG, survive for an extended period and increase the total number of dentate granule neurons provide compelling evidence that these events are related. Independent of the precise mechanism, this study is the first of its kind to demonstrate that a post-injury intraven-tricular infusion of bFGF can improve cognitive recovery following traumatic brain injury.
Although novel and of significant clinical relevance, the current observations are hardly unexpected. Basic FGF is a well-known mitogen for both neuronal and non-neuronal cells, showing both multifunctional and pleiotropic activities in vitro and in vivo. For example, during development, bFGF provides important extracellular signals for regulating the proliferation and fate determination of stem and progenitor cells in the CNS (Calof, 1995; Maric et al., 2007; Raballo et al., 2000). In vitro, bFGF provides mitogenic and differentiating signals for neuroblasts and glial cells, as well as supporting cultured neural stem cell proliferation and survival (Gritti et al.,1996; Palmer et al., 1999; Qian et al., 1997). In addition to its effects on neuronal cells, bFGF induces astrocyte and oligodendrocyte proliferation as well (Bogler et al., 1990; Fressinaud et al., 1993; Hagood et al., 2006). In vivo, bFGF has been shown to increase cell proliferation in the SVZ in normal animals when administered intraventricularly (Kuhn et al., 1997) or subcutaneously (Wagner et al., 1999). Basic FGF can also restore neurogenesis in the hippocampus and subventricular zone in aged animals (Jin et al., 2003; Rai et al., 2007). Collectively, these observations demonstrate that the proliferation, differentiation and survival of cells within the CNS are crucially dependent on signals provided by bFGF.
In the mature brain, bFGF is expressed in both glia and neurons. Relevant to the current study, however, is the fact that bFGF and its receptor FGFR1 are expressed at high levels in subependymal cells (Gonzalez et al., 1995). Moreover, bFGF immunoreactive cells are present in the DG subgranular zone (Weickert et al., 2005). The anatomical distribution of bFGF and its receptor FGFR1 in these neurogenic regions suggests a role for bFGF in endogenous neurogen-esis in the adult brain. Indeed, using bFGF gene knock out mice, Yoshimura et al. (2001; 2003) have shown that bFGF is necessary to stimulate cell proliferation and differentiation of neuroprogenitor cells in the adult hippocampus. Specifically, bFGF knock out mice exhibited diminished hippocampal DG neurogenesis in response to seizure or trauma, which can be reversed by administration of bFGF. In a related study, (Tao et al., 1997) showed that neurogenesis in the hippocampus and cerebellum of newborn rats can be inhibited with a neutralizing monoclonal antibody against bFGF. Taken together, these studies demonstrate the importance of bFGF in the regulation of neurogenesis within the neurogenic regions of the brain.
In addition to its mitogenic function, bFGF is also a well-known neurotrophic factor for cells of the CNS. In vitro, bFGF protects neurons from glutamate toxicity (Freese et al., 1992; Casper and Blum, 1995) and prevents hippocampal cell death caused by hypoxia (Akaneya et al., 1993) and hypoglycemia (Cheng and Mattson, 1991). In vivo, bFGF protects hippocampal CA1 neuronal cell loss following forebrain ischemia (Nakata et al., 1993). However, these neuroprotective effects of bFGF treatment in animals are not consistently observed following TBI. For example, (Dietrich et al., 1996) found that intravenous administration of bFGF significantly decreased cortical neuronal cell death and contusion volume followinga mildto moderate parasagittal fluid percussion injury. On the contrary, others have reported that bFGF treatment attenuated post-TBI memory dysfunction but had no effect on hippocampal neuronal cell loss or cortical contusion volume in a lateral fluid percussion model (McDermott et al., 1997) or a cortical contusion injury model (Yan et al., 2000). Likewise, in the current study, we found that an intraventricular infusion of bFGF attenuated cognitive deficits of injured animals in the absence of significant protective effects on hippocampal CA3 and hilus neurons. Taken together, these studies suggest that the neuroprotective effect of bFGF on neuronal preservation following TBI is rather limited, and the beneficial effect of bFGF treatment on cognitive function is not due to its protection on cyto-architecture but rather through other mechanisms. Other possible mechanisms by which bFGF may be aiding in cognitive recovery when given systemically include synaptic plasticity such as long term potentiation and memory and learning (Schuman, 1999), modulatory effects on axonal branching and arborization (Ramirez et al., 1999), by affecting ion channel function and synaptic efficacy (Fitzsimonds and Poo, 1998). In vitro evidence have demonstrated that bFGF can enhance synaptogenesis (Li et al., 2002) and promote branching and growth of axons in cultured rat hippocampal neurons including dentate gyrus neurons (Aoyagi et al., 1994; Patel and McNamara, 1995) though these effects are in a dose dependent manner and require continuous presence of bFGF (Patel and McNamara, 1995; Aoyagi et al., 1994). Furthermore, the beneficial effect of bFGF on functional recovery may due to the increased connectivity. Monfils et al. (2008) have shown that bFGF induces functional recovery in motor cortex injured animals through stimulating reconnection of cortical spinal projections. Although we cannot discount these possibilities, in our study bFGF is infused focally into the ventricular system which mostly affects areas around the lateral ventricle. Therefore, it is reasonable to assume that its beneficial effects are related to its actions on cells within proximity of the ventricles, i.e., those within the SVZ and DG.
The results of current study demonstrated that an intraventricular infusion of bFGF immediately following brain injury significantly enhance endogenous neurogenesis and has profound beneficial effects on the cognitive recovery of injured animals. Independent of its mode of action, the therapeutic potential of bFGF should be explored further as a viable option for treating patients suffering from traumatic brain injury.
Acknowledgments
This study was funded by the National Institutes of Health Grant RO1. NS055086 (D. Sun). Microscopy work was performed at the VCU — Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS center core grant 5P30NS047463.
References
- Akaneya Y, Enokido Y, Takahashi M, Hatanaka H. In vitro model of hypoxia: basic fibroblast growth factor can rescue cultured CNS neurons from oxygen-deprived cell death. J Cereb. Blood Flow Metab. 1993;13:1029–1032. doi: 10.1038/jcbfm.1993.130. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Aoyagi A, Nishikawa K, Saito H, Abe K. Characterization of basic fibroblast growth factor-mediated acceleration of axonal branching in cultured rat hippo-campal neurons. Brain Res. 1994;661:117–126. doi: 10.1016/0006-8993(94)91188-6. [DOI] [PubMed] [Google Scholar]
- Bogler O, Wren D, Barnett SC, Land H, Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caday CG, Klagsbrun M, Fanning PJ, Mirzabegian A, Finklestein SP. Fibroblast growth factor (FGF) levels in the developing rat brain. Brain Res. Dev. Brain Res. 1990;52:241–246. doi: 10.1016/0165-3806(90)90240-y. [DOI] [PubMed] [Google Scholar]
- Calof AL. Intrinsic and extrinsic factors regulating vertebrate neurogenesis. Curr. Opin. Neurobiol. 1995;5:19–27. doi: 10.1016/0959-4388(95)80082-4. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Casper D, Blum M. Epidermal growth factor and basic fibroblast growth factor protect dopaminergic neurons from glutamate toxicity in culture. J. Neurochem. 1995;65:1016–1026. doi: 10.1046/j.1471-4159.1995.65031016.x. [DOI] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. NGF and bFGF protect rat hippocampal and human cortical neurons against hypoglycemic damage by stabilizing calcium homeostasis. Neuron. 1991;7:1031–1041. doi: 10.1016/0896-6273(91)90347-3. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J. Comp. Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Finklestein SP. Posttreatment with intravenous basic fibroblast growth factor reduces histopathological damage following fluid-percussion brain injury in rats. J. Neurotrauma. 1996;13:309–316. doi: 10.1089/neu.1996.13.309. [DOI] [PubMed] [Google Scholar]
- Fitzsimonds RM, Poo MM. Retrograde signaling in the development and modification of synapses. Physiol. Rev. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- Freese A, Finklestein SP, DiFiglia M. Basic fibroblast growth factor protects striatal neurons in vitro from NMDA-receptor mediated excitotoxicity. Brain Res. 1992;575:351–355. doi: 10.1016/0006-8993(92)90104-h. [DOI] [PubMed] [Google Scholar]
- Fressinaud C, Laeng P, Labourdette G, Durand J, Vallat JM. The proliferation of mature oligodendrocytes in vitro is stimulated by basic fibroblast growth factor and inhibited by oligodendrocyte-type 2 astrocyte precursors. Dev. Biol. 1993;158:317–329. doi: 10.1006/dbio.1993.1191. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA. Regulation of neuronal birth, migration and death in the rat dentate gyrus. Dev. Neurosci. 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. J. Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Gritti A, Parati EA, Cova L, Frolichsthal P, Galli R, Wanke E, Faravelli L, Morassutti DJ, Roisen F, Nickel DD, Vescovi AL. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 1996;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagood SK, McGinn MJ, Sun D, Colello RJ. Characterizing the mitogenic effect of basic fibroblast growth factor in the adult rat striatum. J. Neurotrauma. 2006;23:205–215. doi: 10.1089/neu.2006.23.205. [DOI] [PubMed] [Google Scholar]
- Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Keuker JI, Vollmann-Honsdorf GK, Fuchs E. How to use the optical fractionator: an example based on the estimation of neurons in the hippocampal CA1 and CA3 regions of tree shrews. Brain Res. Brain Res. Protoc. 2001;7:211–221. doi: 10.1016/s1385-299x(01)00064-2. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumon Y, Sakaki S, Kadota O, Matsuda S, Fujita H, Yoshimura H, Sakanaka M. Transient increase in endogenous basic fibroblast growth factor in neurons of ischemic rat brains. Brain Res. 1993;605:169–174. doi: 10.1016/0006-8993(93)91369-4. [DOI] [PubMed] [Google Scholar]
- Li AJ, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur. J. Neurosci. 2002;16:1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- Logan A, Frautschy SA, Gonzalez AM, Baird A. A time course for the focal elevation of synthesis of basic fibroblast growth factor and one of its high-affinity receptors (flg) following a localized cortical brain injury. J. Neurosci. 1992;12:3828–3837. doi: 10.1523/JNEUROSCI.12-10-03828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D, Fiorio PA, Chang YH, Barker JL. Self-renewing and differentiating properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J. Neurosci. 2007;27:1836–1852. doi: 10.1523/JNEUROSCI.5141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KL, Raghupathi R, Fernandez SC, Saatman KE, Protter AA, Finklestein SP, Sinson G, Smith DH, McIntosh TK. Delayed administration of basic fibroblast growth factor (bFGF) attenuates cognitive dysfunction following parasagittal fluid percussion brain injury in the rat. J. Neurotrauma. 1997;14:191–200. doi: 10.1089/neu.1997.14.191. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Driscoll I, Vavrek R, Kolb B, Fouad K. FGF-2-induced functional improvement from neonatal motor cortex injury via corticospinal projections. Exp. Brain Res. 2008;185:453–460. doi: 10.1007/s00221-007-1172-0. [DOI] [PubMed] [Google Scholar]
- Morehead M, Bartus RT, Dean RL, Miotke JA, Murphy S, Sall J, Goldman H. Histopathologic consequences of moderate concussion in an animal model: correlations with duration of unconsciousness. J. Neurotrauma. 1994;11:657–667. doi: 10.1089/neu.1994.11.657. [DOI] [PubMed] [Google Scholar]
- Nakata N, Kato H, Kogure K. Protective effects of basic fibroblast growth factor against hippocampal neuronal damage following cerebral ischemia in the gerbil. Brain Res. 1993;605:354–356. doi: 10.1016/0006-8993(93)91766-l. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MN, McNamara JO. Selective enhancement of axonal branching of cultured dentate gyrus neurons by neurotrophic factors. Neuroscience. 1995;69:763–770. doi: 10.1016/0306-4522(95)00281-m. [DOI] [PubMed] [Google Scholar]
- Qian X, Davis AA, Goderie SK, Temple S. FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron. 1997;18:81–93. doi: 10.1016/s0896-6273(01)80048-9. [DOI] [PubMed] [Google Scholar]
- Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J. Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- Ramirez JJ, Finklestein SP, Keller J, Abrams W, George MN, Parakh T. Basic fibroblast growth factor enhances axonal sprouting after cortical injury in rats. Neuroreport. 1999;10:1201–1204. doi: 10.1097/00001756-199904260-00008. [DOI] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M, Gage FH. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM. Neurotrophin regulation of synaptic transmission. Curr. Opin. Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J. Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Tao Y, Black IB, Cicco-Bloom E. In vivo neurogenesis is inhibited by neutralizing antibodies to basic fibroblast growth factor. J. Neurobiol. 1997;33:289–296. [PubMed] [Google Scholar]
- Tran LD, Lifshitz J, Witgen BM, Schwarzbach E, Cohen AS, Grady MS. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J. Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Bowen KK, Sailor KA, Dempsey RJ. EGF and FGF-2 infusion increases post-ischemic neural progenitor cell proliferation in the adult rat brain. Neurosurgery. 2005;57:1254–1263. doi: 10.1227/01.neu.0000186040.96929.8a. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C. Long-term culture of hippocampal neurons. Curr. Protoc. Neurosci. 2004 doi: 10.1002/0471142301.ns0302s26. Chapter 3:Unit. [DOI] [PubMed] [Google Scholar]
- Wagner JP, Black IB, Cicco-Bloom E. Stimulation of neonatal and adult brain neurogenesisbysubcutaneous injection ofbasic fibroblast growth factor. J. Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Kittell DA, Saunders RC, Herman MM, Horlick RA, Kleinman JE, Hyde TM. Basic fibroblast growth factor and fibroblast growth factor receptor-1 in the human hippocampal formation. Neuroscience. 2005;131:219–233. doi: 10.1016/j.neuroscience.2004.09.070. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Yu J, Kline AE, Letart P, Jenkins LW, Marion DW, Dixon CE. Evaluation of combined fibroblast growth factor-2 and moderate hypothermia therapy in traumatically brain injured rats. Brain Res. 2000;887:134–143. doi: 10.1016/s0006-8993(00)03002-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Teramoto T, Whalen MJ, Irizarry MC, Takagi Y, Qiu J, Harada J, Waeber C, Breakefield XO, Moskowitz MA. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J. Clin. Invest. 2003;112:1202–1210. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]