Abstract
Background
NXL104 is a novel-structure β-lactamase inhibitor with potent activity against both class A and class C enzymes. Among the class A carbapenemases, KPC-type enzymes are now spreading rapidly and KPC-related carbapenemase resistance is an emerging phenomenon of great clinical importance. The activity of NXL104 against KPC β-lactamases was examined.
Methods
Enzymatic activity of purified recombinant KPC-2 was measured with nitrocefin as reporter substrate and inhibition by NXL104 was measured by determination of IC50 values. Antimicrobial susceptibility testing of various β-lactams combined with a fixed concentration of NXL104 at 4 mg/L against strains producing KPC enzymes was performed by the broth microdilution method.
Results
NXL104 was a potent inhibitor of KPC-2 with an IC50 of 38 nM. NXL104 restored the antimicrobial activity of ceftazidime, ceftriaxone, imipenem and piperacillin against Enterobacteriaceae strains producing KPC-2 or KPC-3. MIC values of ceftazidime against KPC producers were reduced by up to 1000-fold by combination with NXL104.
Conclusions
NXL104 inhibitory activity is unique in terms of spectrum, encompassing class A extended-spectrum β-lactamases, class C enzymes and class A carbapenemases. Given the limited therapeutic options available for infections caused by multiresistant Enterobacteriaceae isolates, NXL104 β-lactamase inhibitor is a promising agent to be used in combination with a β-lactam to protect its antibacterial activity.
Keywords: β-lactamase, carbapenem resistance, KPC inhibition
Introduction
Owing to their broad spectrum antibacterial activity and to their stability against most common β-lactamases, carbapenems play a major role in the treatment of severe and difficult-to-treat infections caused by Gram-negative bacteria.1 The carbapenem group of antibiotics is often considered as the last resort treatment for nosocomial infections caused by multiresistant Gram-negative organisms, when the other β-lactams are no longer useful. Hence, the recent reports of resistance to carbapenems are of great concern.2
Within the heterogeneous group of carbapenemases, the Ambler class A KPC family represents a relatively recent and emerging concern. KPC-type enzymes in carbapenem-resistant Klebsiella pneumoniae strains were first reported in 2001 in North Carolina, USA3 and until 2005 were limited to sporadic occurrences in the eastern USA; they are now endemic in some facilities in New York4 and show an expanding geographic range. Bacterial strains producing KPC enzymes have been reported from a few patients in Europe (France and UK),5,6 Central and South America (Colombia, Brazil and Puerto Rico)7–9 and China,10 while hospital outbreaks have been described in Israel11 and in Greece.12 Essentially limited to isolates of K. pneumoniae in initial surveillance reports, KPC β-lactamases have since been identified in a variety of Enterobacteriaceae species, as well as in Pseudomonas aeruginosa.13 The analysis of genetic structures surrounding blaKPC genes have evidenced the presence of a novel Tn3-based transposon, Tn4401, which is likely to be the origin of blaKPC mobilization and further insertion into various plasmids of non-clonally related organisms.14 Seven KPC variants have so far been described (KPC-2 to -8, KPC-1 being synonymous with KPC-2).15 This small group of enzymes displays a broad substrate spectrum with high hydrolytic efficiency against most clinically relevant β-lactam antibacterial agents, including the carbapenems.3 KPC enzymes are only poorly inhibited by marketed β-lactamase inhibitors, therefore the addition of clavulanate, tazobactam or sulbactam generally does not rescue the β-lactam antibacterial activity. KPC enzymes are thus characterized by a broad-spectrum hydrolytic activity, and high interspecies and intercontinental dissemination potential.
NXL104 is a new non-β-lactam inhibitor of β-lactamases currently in clinical development (Figure 1). It displays a broad-spectrum inhibition profile against both class A and class C enzymes,16 and a variable level of activity against class D enzymes. Both types of enzyme are inactivated very efficiently with low IC50 (concentration resulting in 50% inhibition) values, low turnover numbers and form highly stable complexes. NXL104 has virtually no intrinsic antibacterial activity, but efficiently protects β-lactams from hydrolysis in a variety of strains producing class A and class C enzymes, including extended-spectrum β-lactamases.17
Figure 1.
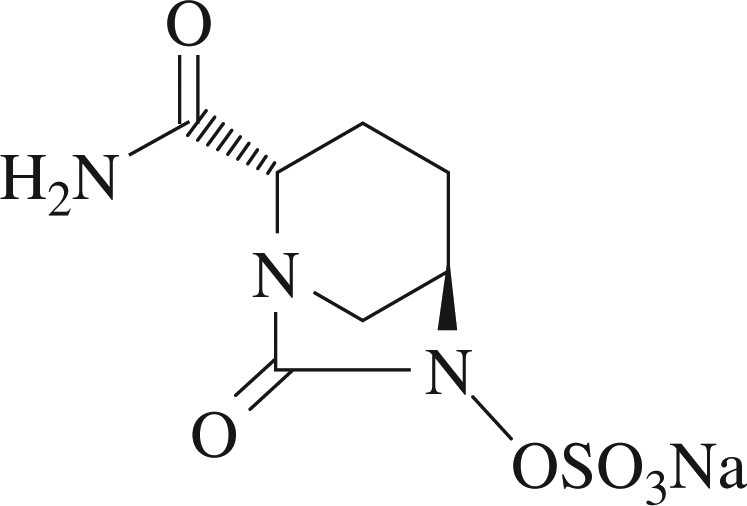
Chemical structure of NXL104.
The objective of this study was to evaluate the inhibition of the KPC-2 enzyme by NXL104 and to determine the in vitro antibacterial activity of various β-lactam/NXL104 combinations against highly resistant strains producing KPC carbapenemases.
Materials and methods
Bacterial strains and MIC determination
Four bacterial strains producing KPC enzymes were isolated in French hospitals in the Paris area between 2002 and 2005: Escherichia coli 2138 and Enterobacter cloacae 750618 (Pitié-Salpétrière hospital); E. cloacae MAC19 (Tenon hospital); and K. pneumoniae YC5 (Kremlin-Bicêtre hospital). Two other KPC producers tested were US isolates (VAKP and VA8).
MIC values were determined using the CLSI microdilution broth methods for antimicrobial susceptibility testing with cation-adjusted Mueller–Hinton broth.20 Bacterial inocula were adjusted to contain 5 × 105 cfu/mL. MIC values were interpreted according to breakpoints established by the CLSI guideline.21
The following commercially available antimicrobials were used: ceftazidime pentahydrate; piperacillin; ceftriaxone; amoxicillin; and imipenem. The commercially available β-lactam inhibitors lithium clavulanate and tazobactam were assessed. NXL104 was from Novexel SA. Inhibitors were added to β-lactam antibiotics at a fixed concentration of 4 mg/L.
β-Lactamase assay
Purified KPC-2 enzyme was kindly provided by S. Petrella and W. Sougakoff;18 purity as assessed by SDS–PAGE was >95%. The steady-state kinetic parameters (Km and kcat) were determined by measuring the initial hydrolysis rates of nitrocefin at 485 nm on a SOFTmax® Pro spectrophotometer (Molecular Devices), in 50 mM phosphate buffer (pH 7.0) containing 0.1 mg/mL BSA to prevent enzyme denaturation. Reactions were performed in a total volume of 200 µL at 37°C. Inhibition of enzyme activity was determined in the same conditions with 100 µM nitrocefin as reporter substrate and after a 5 min or a 30 min pre-incubation of 3 nM KPC-2 with various concentrations of inhibitors. IC50s corresponded to the concentration of inhibitor needed to reduce the initial rate of hydrolysis of nitrocefin by 50%. The kinetic parameters (Km and kcat) and IC50s were determined by non-linear fitting to the Michaelis–Menten equation using GraFit (version 5.0) software from Erithacus (Horley, Surrey, UK), and final values were determined by averaging results from at least three independent experiments.
Results and discussion
Inhibition of enzyme activity
The kcat and Km values of KPC-2 for nitrocefin were 59 ± 5 s−1 and 52 ± 2 µM, respectively, in close agreement with published values.3 NXL104 demonstrated potent inhibition of KPC-2 activity when tested after a 5 min and a 30 min pre-incubation step, with IC50 values of 170 and 35 nM, respectively. Reference inhibitors were substantially less active, with IC50 values in the micromolar range: ≥100 and 6.5 µM for clavulanate after 5 and 30 min of incubation, respectively; and 50 and 9.2 µM, respectively, for tazobactam. Complete inhibition of KPC-2 could not be achieved with clavulanate, even at the highest concentrations tested; ∼25%–50% KPC-2 activity remained with clavulanate in the concentration range 5–100 µM. In contrast, KPC-2 activity was abolished by NXL104 at 1 µM. Inhibitory activity measured for clavulanate was consistent with published data.3
Antimicrobial activity of various β-lactam/NXL104 combinations against strains producing KPC enzymes
Six different clinical isolates of Enterobacteriaceae species (three K. pneumoniae, one E. coli and two E. cloacae strains) known to produce a KPC enzyme were tested for their susceptibility to different β-lactam antibiotics (Table 1). They were all found to be highly resistant to the penicillins and cephalosporins tested, since MIC values were ≥2048 mg/L with amoxicillin and piperacillin, and in the range 64 to >2048 mg/L for ceftazidime and ceftriaxone. Imipenem MICs were in the CLSI guidelines resistant range of ≥16 mg/L, except for K. pneumoniae VA8, which was susceptible to this carbapenem (MIC of 1 mg/L).
Table 1.
In vitro antibacterial activity of different β-lactam/β-lactamase inhibitor combinations against strains of Enterobacteriaceae species
| MIC in mg/L (fold decrease with NXL104) |
||||||
|---|---|---|---|---|---|---|
| Antimicrobial/β-lactamase inhibitora | K. pneumoniae VA8 (KPC-3)b | K. pneumoniae VAKP (KPC-2) | K. pneumoniae YC (KPC-2) | E. coli 2138(KPC-2, TEM-1) | E. cloacae 7506(KPC-2, TEM-1, KLUC-2) | E. cloacae MAC (KPC-3, TEM-1, OXA-9) |
| NXL104 | >128 | >128 | >128 | >128 | >128 | 128 |
| AMX | >2048 | >2048 | >2048 | >2048 | >2048 | >2048 |
| AMX + CLA | 32 | >2048 | >2048 | >2048 | >2048 | >2048 |
| AMX + NXL104 | 64 (64) | 128 (≥32) | 256 (≥16) | 128 (≥32) | 512 (≥8) | 256 (≥16) |
| PIP | 2048 | 2048 | >2048 | >2048 | >2048 | >2048 |
| PIP + TZB | 256 | 1024 | >2048 | >2048 | >2048 | >2048 |
| PIP + NXL104 | 4 (512) | 8 (256) | 8 (≥512) | 4 (≥1024) | 32 (≥128) | 16 (≥256) |
| CAZ | 512 | 512 | 1024 | 64 | 512 | 1024 |
| CAZ + TZB | 2 | 64 | 256 | 32 | 512 | 512 |
| CAZ + CLA | 0.25 | 16 | 128 | 32 | 512 | 512 |
| CAZ + NXL104 | 0.5 (1024) | 0.5 (1024) | 1 (1024) | 0.25 (256) | 8 (64) | 8 (128) |
| CRO | 128 | 256 | >2048 | >2048 | >2048 | >2048 |
| CRO + NXL104 | 0.06 (2048) | 0.06 (4096) | 0.25 (≥16 384) | 0.06 (≥65 536) | 1 (≥4096) | 0.5 (≥8192) |
| IPM | 1 | 16 | 32 | 16 | 128 | 64 |
| IPM + NXL104 | 0.5 (2) | 0.5 (32) | 0.25 (64) | 0.5 (32) | 0.5 (256) | 2 (32) |
CAZ, ceftazidime; PIP, piperacillin; CRO, ceftriaxone; AMX, amoxicillin; IPM, imipenem; CLA, clavulanate; TZB, tazobactam.
aInhibitors were added at fixed 4 mg/L concentration.
bEnzymes possessed by each strain are shown in parentheses.
NXL104, clavulanate or tazobactam was added to β-lactam antibiotics at a fixed concentration of 4 mg/L. Clavulanate and tazobactam were able to afford the β-lactam antibiotics some protection from hydrolysis; however, it was only for the K. pneumoniae VA8 isolate treated with ceftazidime that these inhibitors reversed MICs to the susceptible range. For the five other strains producing KPC enzymes, clavulanate (when added to amoxicillin or ceftazidime) and tazobactam (when added to piperacillin or ceftazidime) could not lower the MIC values below the susceptibility breakpoint. In contrast, NXL104 efficiently protected piperacillin, ceftazidime, ceftriaxone and imipenem from β-lactamase disruption, the addition of NXL104 restoring the susceptibility of KPC producers to all of these antibiotics. In the presence of NXL104, the MIC of the antibiotic fell by up to 1000-fold for ceftazidime and up to 65 000-fold for ceftriaxone. When combined with NXL104, amoxicillin activity was improved but was not restored to susceptible levels. This may reflect a high efficiency of KPCs against amoxicillin, as KPC enzymes have been shown to exhibit high catalytic efficiencies for penicillin substrates.3
In summary, the data provided in this study show that NXL104 is a potent inhibitor of KPC enzymes. NXL104 rescued the antibiotic activity of various β-lactam classes, exemplified by piperacillin, ceftazidime, ceftriaxone and imipenem against E. coli, K. pneumoniae and E. cloacae strains producing KPC enzymes. The addition of NXL104 resulted in a reduction of MIC values to below susceptibility breakpoints in all six isolates tested, including those exhibiting extremely high resistance levels. Although clavulanate and tazobactam showed some in vitro efficacy against the K. pneumoniae isolate producing KPC-3 that had retained imipenem susceptibility, both inhibitors were inactive against the imipenem-resistant isolates.
Clinically, KPC-positive organisms tend to be highly resistant to multiple classes of antibiotics; high-level resistance to quinolones, aminoglycosides and polymyxins is frequently observed among KPC-positive K. pneumoniae.4,22 In addition, infections due to organisms producing KPC carbapenemases have been associated with high mortality in affected patients.4,23 In the context of the limited therapeutic options to treat infections caused by multiresistant Gram-negatives, NXL104 is a promising agent that could be used to protect β-lactam antibiotics from hydrolysis. The combination ceftazidime/NXL104 is currently under clinical development and has demonstrated a remarkable tolerance profile in healthy volunteers.24
Funding
The work was fully funded by Novexel SA.
Transparency declarations
All the authors are employees of Novexel and hold shares in the company.
Acknowledgements
The authors are grateful to G. Arlet, R. Bonomo, P. Nordmann, S. Petrella, L. Poirel and W. Sougakoff for kindly providing bacterial strains producing KPC enzymes.
References
- 1.Dhaloff A, Janjic N, Echols R. Redefining penems. Biochem Pharmacol. 2006;71:1085–95. doi: 10.1016/j.bcp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JM, Bonomo RA. The threat of antibiotic resistance in Gram-negative pathogenic bacteria: β-lactams in peril! Curr Opin Microbiol. 2005;8:515–24. doi: 10.1016/j.mib.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151–61. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New-York city. Arch Intern Med. 2005;165:1430–5. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 5.Naas T, Nordmann P, Vedel G, et al. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother. 2005;49:4423–4. doi: 10.1128/AAC.49.10.4423-4424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodford N, Zhang J, Warner M, et al. Arrival of Klebsiella pneumoniae producing KPC carbapenemase in the United Kingdom. J Antimicrob Chemother. 2008;62:1261–4. doi: 10.1093/jac/dkn396. [DOI] [PubMed] [Google Scholar]
- 7.Villegas MV, Lolans K, Correa A, et al. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother. 2006;50:2880–2. doi: 10.1128/AAC.00186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peirano G, Seki LM, Val Passos VL, et al. Carbapenem-hydrolyzing β-lactamase KPC-2 in Klebsiella pneumoniae isolated in Rio de Janeiro, Brazil. J Antimicrob Chemother. 2009;63:265–8. doi: 10.1093/jac/dkn484. [DOI] [PubMed] [Google Scholar]
- 9.Wolter DJ, Kurpiel PM, Woodford N, et al. Surveillance of carbapenem resistant Pseudomonas aeruginosa from Puerto Rico Medical Center Hospitals: dissemination of KPC and IMP-18 β-lactamases. Antimicrob Agents Chemother. 2009;53:557–62. doi: 10.1128/AAC.01172-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei ZQ, Du XX, Yu YS, et al. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother. 2007;51:763–5. doi: 10.1128/AAC.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leavitt A, Navon-Venezia S, Chmelnitsky I, et al. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob Agents Chemother. 2007;51:3026–9. doi: 10.1128/AAC.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maltezou HC, Giakkoupi P, Maragos A, et al. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece) J Infection. 2009;58:213–9. doi: 10.1016/j.jinf.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Villegas MV, Lolans K, Correa A, et al. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing β-lactamase. Antimicrob Agents Chemother. 2007;51:1553–5. doi: 10.1128/AAC.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naas T, Cuzon G, Villegas MV, et al. Genetic structures at the origin of acquisition of the β-lactamase blaKPC gene. Antimicrob Agents Chemother. 2008;52:1257–63. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2008;52:809. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnefoy A, Dupuis-Hamelin C, Steier V, et al. In vitro activity of AVE1330A, an innovative broad-spectrum non-β-lactam β-lactamase inhibitor. J Antimicrob Chemother. 2004;54:410–7. doi: 10.1093/jac/dkh358. [DOI] [PubMed] [Google Scholar]
- 17.Livermore DM, Mushtaq S, Warner M, et al. NXL104 combinations versus Enterobacteriaceae with CTX-M extended-spectrum β-lactamases and carbapenemases. J Antimicrob Chemother. 2008;62:1053–6. doi: 10.1093/jac/dkn320. [DOI] [PubMed] [Google Scholar]
- 18.Petrella S, Ziental-Gelus N, Mayer C, et al. Genetic and structural insights into the dissemination potential of the extremely broad-spectrum class A beta-lactamase KPC-2 identified in an Escherichia coli strain and an Enterobacter cloacae strain isolated from the same patient in France. Antimicrob Agents Chemother. 2008;52:3725–36. doi: 10.1128/AAC.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dortet L, Radu I, Gautier V, et al. Intercontinental travels of patients and dissemination of plasmid-mediated carbapenemase KPC-3 associated with OXA-9 and TEM-1. J Antimicrob Chemother. 2008;61:455–7. doi: 10.1093/jac/dkm455. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Seventh Edition: Approved Standard M7-A7. Wayne, PA, USA: CLSI; 2006. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement M100-S18. Wayne, PA, USA: CLSI; 2008. [Google Scholar]
- 22.Chiang T, Mariano N, Urban C, et al. Identification of carbapenem-resistant Klebsiella pneumoniae harboring KPC enzymes in New Jersey. Microb Drug Resist. 2007;13:235–9. doi: 10.1089/mdr.2007.767. [DOI] [PubMed] [Google Scholar]
- 23.Marchaim D, Navon-Venezia S, Schwaber MJ, et al. Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob Agents Chemother. 2008;52:1413–8. doi: 10.1128/AAC.01103-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merdjan H, Tarral A, Girard AM, et al. Safety, single dose pharmacokinetics, and pharmacodynamics of β-lactamase inhibitor NXL104 in healthy young male adults. Abstracts of the Forty-seventh Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. Washington, DC, USA: American Society for Microbiology; 2007. p. 26. Abstract A-809. [Google Scholar]


