Abstract
α-Dystroglycan (α-DG) represents a highly glycosylated cell surface molecule that is expressed in the epithelial cell-basement membrane (BM) interface and plays an essential role in epithelium development and tissue organization. The α-DG–mediated epithelial cell-BM interaction is often impaired in invasive carcinomas, yet roles and underlying mechanisms of such an impaired interaction in tumor progression remain unclear. We report here a suppressor function of laminin-binding glycans on α-DG in tumor progression. In aggressive prostate and breast carcinoma cell lines, laminin-binding glycans are dramatically decreased, although the amount of α-DG and β-dystroglycan is maintained. The decrease of laminin-binding glycans and consequent increased cell migration were associated with the decreased expression of β3-N-acetylglucosaminyltransferase-1 (β3GnT1). Forced expression of β3GnT1 in aggressive cancer cells restored the laminin-binding glycans and decreased tumor formation. β3GnT1 was found to be required for laminin-binding glycan synthesis through formation of a complex with LARGE, thus regulating the function of LARGE. Interaction of the laminin-binding glycans with laminin and other adhesive molecules in BM attenuates tumor cell migratory potential by antagonizing ERK/AKT phosphorylation induced by the components in the ECM. These results identify a previously undescribed role of carbohydrate-dependent cell-BM interaction in tumor suppression and its control by β3GnT1 and LARGE.
Keywords: glycosylation, cell adhesion, basement membrane, carcinoma
Interaction of epithelial cells with basement membrane (BM) is mediated by cell adhesion molecules, which operate at the interface of epithelial cell-ECM and regulate cell growth, motility, and differentiation by integrating signals from ECM or soluble factors (1–3). One of the most important epithelial cell-BM interactions is mediated by α-dystroglycan (α-DG) on epithelial cells (4).
α-DG is a cell surface receptor for several major BM proteins, including laminin, perlecan, and agrin. A laminin G-like domain in all these glycoproteins binds to a unique glycan structure attached to α-DG, and this interaction has been shown to be critical in assembling BM (5, 6). This unique glycan structure is referred to as laminin-binding glycans hereafter. α-DG is not attached directly to the plasma membrane but is bound to it through attachment to the transmembrane protein β-dystroglycan (β-DG), which binds to the cytoplasmic protein dystrophin, which, in turn, binds to the actin cytoskeleton and many adaptor molecules involved in cellular signaling (4, 5).
α-DG is highly glycosylated and contains both N-linked glycans and mucin type O-glycans. The mucin type O-glycans are clustered in a mucin-like domain at the N-terminal of mature α-DG, which includes unique O-mannosyl glycans and sialic acid α2→3Galβ1→4GlcNAcβ1→2Manα1→Ser/Thr (7). Defects in glycosylation of the O-mannosyl glycans have been shown to cause muscular dystrophy (8). So far, 7 glycosyltransferases or glycosyltransferase-like genes, including POMT1, POMT2, POMGnT1, Fukutin, Fukutin-related protein, LARGE, and LARGE2, have been found to be involved in α-DG functional glycosylation (4). Among these molecules, LARGE is of particular interest because it was shown to be functionally able to bypass the O-mannose glycosylation defects in cells derived from patients with severe muscular dystrophy (9, 10). LARGE was discovered as a gene defective in meningioma (11) and was shown to be a causative gene for muscular dystrophy (LARGEmyd) in mice (12) and in humans (13). LARGE displays 2 distinct structural domains homologous to UDP-glucose protein glucosyltransferase (14) and β3-N-acetylglucosaminyltransferase 1 (β3GnT1) (11). β3GnT1 was originally identified by expression cloning as an enzyme that synthesizes i-antigen, a linear poly-N-acetyllactosamine, on human fetal red blood cell (15). β3GnT1 is widely distributed in various tissues and is structurally distinct from the other members of β3GnT gene family, and it was proposed that β3GnT1 might functionally differ from those enzymes (16). The significance of β3GnT1 in biosynthesis of laminin-binding glycans attached to α-DG has not been investigated.
Despite the critical function of α-DG glycosylation in the muscular system, not much is known about cancer development. Several reports have shown that defects in α-DG are associated with breast, colon, oral, and prostate carcinomas (17–20). However, the molecular cause for the α-DG defects found in various carcinomas and the mechanistic link between α-DG defects and tumor progression are not known.
In the present study, we found that β3GnT1 plays a critical role in the synthesis of the laminin-binding glycans on α-DG by collaboration with LARGE or LARGE2. We also showed that reduced expression of β3GnT1 leads to diminished synthesis of laminin-binding glycans, higher migration of cancer cells, and increased tumor formation. Restoration of laminin-binding glycans by forced expression of β3GnT1 results in reduced cell migration, and thus a reduction in tumor formation. These findings identified the key role of α-DG glycans and β3GnT1 in tumor suppression.
Results and Discussion
Loss of α-DG Laminin-Binding Glycans Correlates with Malignant Phenotype of Human Prostate and Breast Carcinoma Cells.
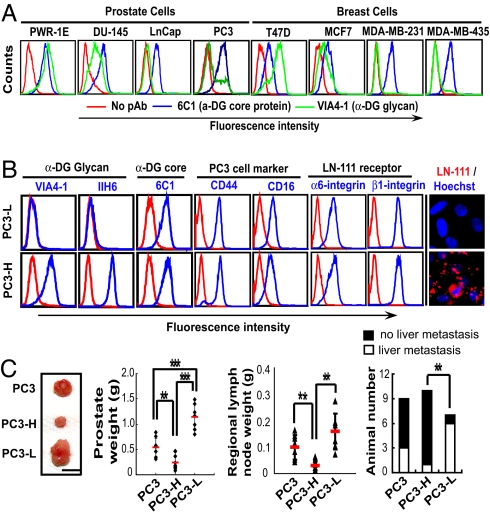
To determine whether the loss of laminin-binding glycans or loss of α-DG core protein is associated with tumor progression, we examined the expression of α-DG core protein and its glycosylation in normal and carcinoma cell lines of human prostate and breast. Diminished expression of laminin-binding glycans was detected by VIA4–1 antibody for migratory and invasive carcinoma cells, whereas only a small variation of α-DG core protein expressed was detected by 6C1 antibody (Fig. 1A), which recognizes α-DG core protein but has not been used previously for cancer cell staining. Consistently, a great decrease of laminin-binding glycans was detected by IIH6 antibody in human prostate carcinoma specimens, whereas the expression of α-DG and β-DG was unchanged and laminin expression was distributed in the ECM of prostate tumor [supporting information (SI) Fig. S1]. Two antibodies, IIH6 and VIA4–1, used in this study bind laminin-binding glycans present in α-DG (21). These results suggest that loss of laminin-binding glycans is associated with tumor progression. This finding clarifies that the decrease of α-DG in various carcinomas shown in previous reports using only IIH6 and VIA4–1 antibody (17–20) is mainly attributable to a specific reduction of laminin-binding glycan formation on α-DG.
Fig. 1.
Reverse association of laminin-binding glycan expression with malignancy of human prostate and breast carcinoma cells. (A) FACS analysis showed that invasive human prostate (DU-145, LNCaP, and PC-3) and breast (MCF7, MDA-MB-231, and MDA-MB-435) carcinoma cells showed reduced expression of laminin-binding glycans on α-DG compared with noninvasive human prostate cells (PWR-1E) and breast carcinoma (T47D) cells. α-DG protein and laminin-binding glycans were detected by 6C1 and VIA4–1 antibodies, respectively. (B) FACS analysis of expression of laminin-binding glycans (VIA4–1 and IIH6), α-DG core protein (6C1), CD44 and CD16, and α6- and β1-integrins of 2 subpopulations of PC3 cells. Laminin-111 binding to the cells was examined by immunofluorescence staining. (C) Tumor formation by subpopulations of PC3 cells in the orthotopic prostate. The weight of the prostate and regional lymph nodes and the tumor colony in the liver were examined 7 weeks after the inoculation of PC3 cells into the prostate of SCID mice. Representative primary tumors are shown on the left. (Scale bar: 1 cm.)
To evaluate the role of glycans attached to α-DG in tumor progression, we used human prostate carcinoma PC3 cells, which express varying amounts of laminin-binding glycans attached to α-DG (Fig. 1A). After 2 consecutive cell sortings, PC3 cells were separated into those expressing substantial (PC3-H) and minimal (PC3-L) amounts of laminin-binding glycans (Fig. 1B). Both PC3-H and PC3-L cells expressed equivalent amounts of α-DG core protein as assessed by 6C1 and PC3 cell markers CD44 and CD16 (Fig. 1B). Laminin-111 bound to PC3-H but not to PC3-L, although both cell types express comparable amounts of α6- and β1-integrin subunits, which when associated form a major receptor for laminin-111 (Fig. 1B). α-DG from PC3-H cells displayed a broad high molecular weight band that binds to IIH6 and laminin, whereas α-DG from PC3-L had a narrow molecular weight distribution and failed to bind to either IIH6 or laminin (Fig. S2A). PC3-H and PC3-L express, on the other hand, almost identical carbohydrate backbone structures, because no difference was observed on the staining with various lectins and antibody against heparan sulfate (Fig. S2B). These results indicate that PC3-H and PC3-L cells distinctly differ in the expression of laminin-binding glycans attached to α-DG.
Strikingly, PC3-H cells produced much smaller tumors after being inoculated in the orthotopic prostate of SCID mice and less metastasis to the draining lymph nodes and liver compared with PC3-L and the parental PC3 cells (Fig. 1C). Combined, these results indicate that the amount of laminin-binding glycans is inversely correlated to the progression of prostate carcinoma.
Expression of β3GnT1 Directs the Synthesis of Laminin-Binding Glycans and Suppresses Tumor Formation.
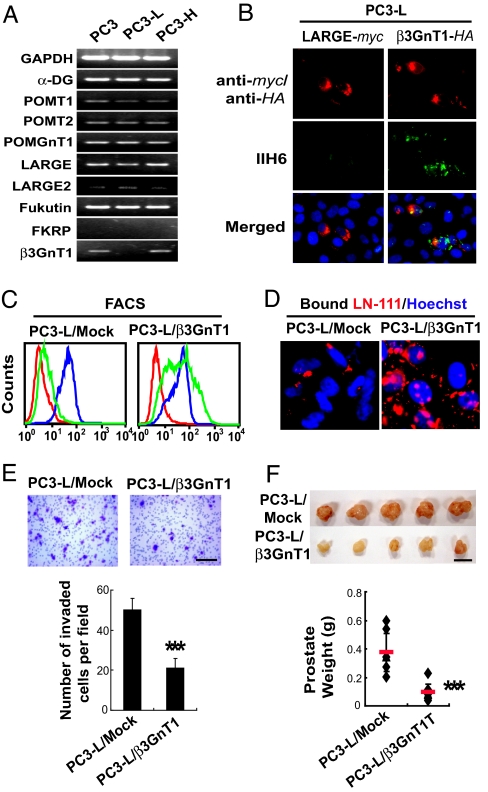
The previously discussed results suggested that glycosyltransferases(s) responsible for the glycan formation may be deficient in PC3-L cells. Unexpectedly, the transcripts for β3GnT1, a glycosyltransferase having partial homology to LARGE, were not detected in PC3-L cells but were expressed in parental PC3 and PC3-H cells, whereas no difference was detected for α-DG and all the other enzymes putatively involved in laminin-binding glycan formation (Fig. 2A). This finding prompted us to study the role of β3GnT1 in the biosynthesis of laminin-binding glycans. Surprisingly, transfection with β3GnT1 but not with LARGE cDNA restored expression of IIH6- and VIA4–1–positive laminin-binding glycans at the cell surface of transfected PC3-L cells and the capacity for laminin binding (Fig. 2 B–D and Fig. S2C). The results indicated that expression of LARGE alone is insufficient to form the laminin-binding glycans in the absence of β3GnT1. Transfection with β3GnT1 cDNA significantly reduced the invasion potential of PC3-L cells (Fig. 2E) and suppressed the tumor formation in prostates (Fig. 2F). Combined, these results indicated that β3GnT1 is required for the expression of laminin-binding glycans, thus suppressing tumor formation.
Fig. 2.
Expression of β3GnT1 directs the formation of the key laminin-binding glycans and suppresses tumor formation. (A) RT-PCR analysis of various transcripts encoding proteins implicated in the synthesis of α-DG and its functional glycosylation. (B) β3GnT1 but not LARGE expression restores laminin-binding glycans in PC3-L cells, as detected by IIH6. (C) Analysis of the expression of laminin-binding glycans (VIA4-1 in green) and α-DG (6C1 in blue) in PC3-L cells stably expressing β3GnT1 (PC3-L/β3GnT1) and mock-transfected PC3-L cells. Red is control. (D) Immunofluorescent detection of soluble laminin-111 binding to the stable transfectants. (E) Invasion assay of PC3-L/β3GnT1 and PC3-L/Mock using a transwell. The invaded cells were stained with crystal violet and counted. Small dots are pores on the membrane. (Scale bar: 200 μm.) (F) Primary tumors formed by PC3-L/Mock and PC3-L/β3GnT1 in orthotopic SCID mouse prostates 4 weeks after inoculation of the cells. (Scale bar: 1 cm.)
β3GnT1 Associates with LARGE Directing α-DG Functional Glycosylation.
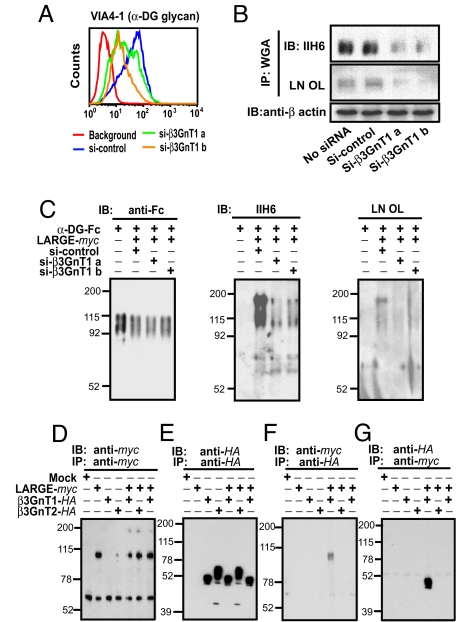
Despite the fact that β3GnT1 is known to have homology with the second catalytic domain of LARGE, no information was available before this study about the involvement of β3GnT1 in the glycosylation of α-DG. In support of the previously discussed findings, siRNA-mediated knockdown of β3GnT1 in PC3-H cells resulted in substantially decreased laminin-binding glycans on α-DG (Fig. 3 A and B). This effect was not attributable to nonspecific knockdown of LARGE or LARGE2 as judged from quantitative RT-PCR (Fig. S3A). Knockdown of LARGE, POMT1, or α-DG by siRNA decreased the laminin-binding glycans on α-DG (Fig. S3B). Importantly, knockdown of β3GnT1 by siRNA also reduced the laminin-binding glycan formation induced by overexpression of LARGE (Fig. 3C) or LARGE2 (Fig. S3C) in HEK293 cells, indicating that overexpressed LARGE or LARGE2 requires endogenous β3GnT1 to function. Expression of β3GnT1 alone in CHO cells barely increased laminin-binding glycans; however, expression of LARGE alone increased laminin-binding glycans, presumably through collaborating with endogenous β3GnT1 in CHO cells (Fig. S3D). Moreover, increased expression of β3GnT1 substantially augmented the LARGE-mediated formation of α-DG laminin-binding glycans (Fig. S3E). By contrast, increased expression of β3GnT2, which is another β3-N-acetylglucosaminyltransferase not related to β3GnT1 (22, 23), had no such effect (Fig. S3E), despite the fact that β3GnT1, LARGE, and β3GnT2 can all facilitate the formation of i-antigen (Fig. S3F). When β3GnT1 and LARGE or LARGE2 were coexpressed, β3GnT1 and LARGE or LARGE2 were coimmunoprecipitated (Fig. 3 D, E, F and G and Fig. S3G). This association can take place only when β3GnT1 and LARGE or LARGE2 are expressed in the same cells, because mixing both proteins did not form any complexes [Fig. 3 F and G, Right (far lanes) and Fig. S3G]. The association was not detected with β3GnT2 (Fig. 3 F and G). Together, these results indicate that β3GnT1 is a regulator for LARGE or LARGE2 by forming a complex and is required for formation of laminin-binding glycans on α-DG.
Fig. 3.
β3GnT1 facilitates functional glycosylation of α-DG by cooperating with LARGE. PC3-H cells were treated with siRNA to knock down β3GnT1, and the expression of laminin-binding glycans on α-DG was examined by FACS (A) and immunoblotting (B). (C) Effects of knockdown of endogenous β3GnT1 by siRNA on the laminin-binding glycan formation induced by LARGE overexpression in α-DG–Fc secreted from HEK293 cells. (D-G) β3GnT1-HA, LARGE-myc, and β3GnT2-HA were separately expressed or coexpressed in CHO cells and immunoprecipitated. Dissociated immunoprecipitates were separated, blotted, and incubated with specific anti-tag antibodies. LARGE-myc and β3GnT1-HA in far right lanes were prepared by mixing the lysates derived from cells separately expressing LARGE-myc or β3GnT1-HA.
Immunofluorescent examination showed colocalization of intact β3GnT1, LARGE and GMP130 in the Golgi apparatus (Fig. S4B and Fig. S4C). Mutation of the DXD motif (24) (Fig. S4A) abolished the activities of both enzymes in the formation of laminin-binding glycans (Fig. S4D) and i-antigen (Fig. S3F) and resulted in mislocalization of the mutated enzyme (Fig. S4E). Apparently, β3GnT1 does not function as a chaperone, because β3GnT1 and LARGE independently migrate to the Golgi apparatus, in contrast to Cosmc, which functions as a chaperone for core 1-forming enzyme (25).
Laminin-binding glycans, formed by β3GnT1 and LARGE together, are unique, structurally differ from i-antigen and linear poly-N-acetyllactosamine, and they mostly reside in locations other than N-glycans and sulfated glycans, because treatment with endo-β-galactosidase endo-F and sodium chlorate yielded no decrease in binding of laminin to α-DG (Fig. S4F). The majority, if not all, of laminin-binding glycans are located on α-mannosyl oligosaccharides, because knockdown of POMT1 significantly reduced the expression levels of laminin-binding glycans (Fig. S3B). Consistent with this observation, Lec15 cells that express little dolichol mannose-phosphate, which is required for POMT1 function, displayed a decreased amount of the glycans (14), which can be restored by transfection of DPM2 cDNA (Fig. S4G), and POMT1-deficient mice had no laminin-binding glycans and died before birth (26). Further studies are necessary to elucidate the structure of the laminin-binding glycans.
Glycosylated α-DG Suppresses ECM-Induced Carcinoma Cell Locomotion Through Attenuating Integrin-Dependent Signaling Pathways.
Tumors derived from PC3-H and PC3-L cells showed similar expression patterns for Ki-67, a proliferation marker; for p63, a prostate basal cell marker that is absent in carcinoma cells (27); and for laminin enriched in the BM of normal glands (Fig. S5A). Tumors derived from PC3-H cells contain greater amounts of IIH6-positive glycans than those derived from PC3-L cells, although both α-DG and β-DG expression levels were similar in the 2 tumors (Fig. S5B). Similarly, PC3-H and PC3-L cells exhibited almost identical growth in anchorage-dependent and anchorage-independent conditions (Fig. S5 C and D). These results showed that PC3-H and PC3-L proliferate at a similar rate.
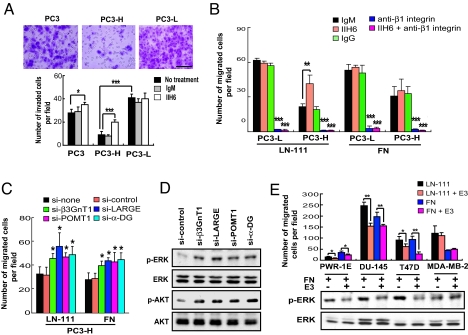
Using a Boyden chamber assay, PC3-H cells were much less invasive and the decreased invasion was significantly reversed by pretreatment with IIH6 antibody, which blocks laminin binding to α-DG glycans (21), whereas the invasion by PC3-L cells was not attenuated by this treatment (Fig. 4A). In addition, PC3-H cells migrated much slower than PC3-L cells in a wound-healing assay (Fig. S5E). These results strengthen our finding that decreased expression of α-DG laminin-binding glycans correlates with higher invasive and migratory potential of carcinoma cells.
Fig. 4.
Laminin-binding glycans on α-DG attenuate cell migration and integrin-dependent signaling pathways. (A) Cell invasion assay with and without antibody treatment. The invaded cells were stained with crystal violet and counted. (Scale bar: 500 μm.) (B) Cell migration assay of PC3-L and PC3-H cells on laminin-111 (LN-111) and fibronectin (FN). Effects of pretreatment with IIH6, anti-β1 integrin, and anti-β1 integrin plus IIH6 on the cell migration are also shown. (C) Effects of siRNA-mediated knockdown of α-DG, POMT1, β3GnT1, or LARGE on the cell migration on LN-111 and FN. (D) ERK and AKT kinase activation levels in PC3-H cells treated with siRNA as indicated. Antibodies specific to phosphorylated ERK (p-ERK), phosphorylated AKT (p-AKT), total ERK (ERK), and total AKT (AKT) were used. (E) Effects of LN-111 E3 fragment on cell migration and ERK phosphorylation. (E, Upper) Cell migration was measured using a transwell. E3 alone did not induce cell migration. (E, Lower) Phosphorylation of ERK of various prostate and breast carcinoma cells treated for 20 min with FN alone or with FN plus E3 and analyzed by immunoblotting (see Fig. S6E).
A cell adhesion assay revealed that PC3-L and PC3-H exhibit comparable adhesion toward laminin-111 or type IV collagen-coated dishes (Fig. S6A). Both cell types also express almost equal amounts of transcripts for matrix metalloproteinase (MMP)-2 and MMP-9, 2 MMPs implicated in tumor invasion (Fig. S6B) (28). We thus reasoned that their distinct invasion capacity is likely related to their migratory activity. Indeed PC3-H cells migrated much slower than PC3-L cells on both laminin-111 and fibronectin, the major ECM motility factors in BM and stroma, respectively (Fig. 4B). Pretreatment with IIH6 antibody, which blocks laminin-111 binding to α-DG, increased PC3-H cell migration on laminin-111 but not on fibronectin, whereas such treatment had no effect for the migration of PC3-L. Moreover, migration of both cell types was completely inhibited by anti-β1 integrin antibody, and this inhibition was not reversed by IIH6 antibody (Fig. 4B). Conversely, down-regulation of laminin-binding glycans by siRNA-mediated knockdown of α-DG, POMT1, β3GnT1, or LARGE increased the migration of PC3-H cells on laminin-111 and fibronectin (Fig. 4C). These results demonstrated antimigratory activity of laminin-binding glycans attached to α-DG. While we were preparing our article, a report appeared showing that down-regulation of LARGE resulted in higher migration of carcinoma cells (29).
Integrins are known to mediate ECM-induced cell motility through activating Ras/MAPK and FAK-AKT signaling pathways (30), and β-DG binds to multiple adaptor molecules involved in β1 integrin-mediated signaling (31, 32). We thus examined phosphorylation of ERK and AKT in PC3-H cells treated with or without siRNA against α-DG, POMT1, β3GnT1, and LARGE. The amount of LARGE2 is minimal compared with the amount of LARGE in PC3H cells (Fig. S3A); thus, the role of LARGE2 was not evaluated. Individual down-regulation of α-DG, POMT1, β3GnT1, or LARGE increased the phosphorylated AKT and ERK in PC3-H cells (Fig. 4D and Fig. S6D). Moreover, the phosphorylation of ERK and AKT was much lower in tumors derived from PC3-H and PC3-L/β3GnT1 cells than in those derived from PC3-L and PC3-L/Mock cells (Fig. S6C). These results indicate that functionally glycosylated α-DG attenuates the downstream activation of integrin-mediated signals, most likely through sequestering MEK from active ERK at focal adhesions as previously suggested (32).
Binding of Glycosylated α-DG to the ECM Ligand Is Required for Antimigratory Activity.
To analyze whether β3GnT1-regulated laminin-binding glycans have a more general role in cell migration, we examined the expression of transcripts for 8 glycosyltransferases in various prostate and breast carcinoma cells. In contrast to higher levels of β3GnT1 expression in low-migratory PWR-1E, T47D, and MCF7 cells, markedly reduced expression of β3GnT1 was detected for highly migratory LNCap, PC3, MDA-MB-231, and MDA-MB-435 cells (Fig. S7A). The expression level of β3GnT1 correlates with the amount of laminin-binding glycans in all the cells tested, as seen in Fig. 1A. siRNA-mediated down-regulation of α-DG or β3GnT1 significantly increased the migratory potential on both laminin-111 and fibronectin for all the laminin-binding glycan-positive cells tested (Fig. S7B). Transfection of breast carcinoma MDA-MB-435 cells with β3GnT1 restored the laminin-binding glycan presentation, resulting in decreased migration on both laminin-111 and fibronectin (Fig. S7 C and D). Together, these results support our conclusion on a previously undescribed suppressor function of β3GnT1 and laminin-binding glycans in carcinoma cell locomotion.
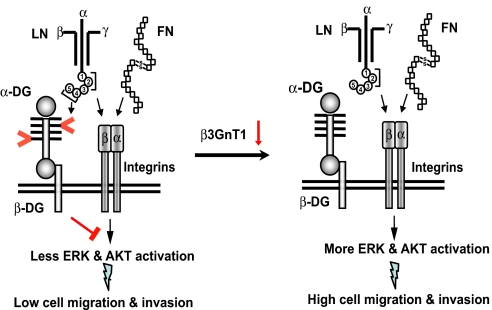
It has been shown that an E3 fragment (globular domains 4 and 5) and globular domains 1–3 of laminin α-chain bind to the laminin-binding glycans on α-DG and integrin, respectively (6, 33). The addition of a laminin-111 E3 fragment attenuated the migration of the laminin-binding glycan-positive cell lines PWR-IE, DU-145, and T47D on laminin-111 and fibronectin (Fig. 4E). This attenuation was associated with the decrease in ERK phosphorylation in these cells (Fig. 4E, Bottom and Fig. S6E). By contrast, the laminin-111 E3 fragment had no effect on migration on both laminin-111 and fibronectin or on phosphorylation of ERK for MDA-MB-231 cells (Fig. 4E), which lack laminin-binding glycans. Combined, these results indicate that binding of α-DG glycans to laminin, and most likely to perlecan and agrin, counteracts the signals initiated by integrin binding to extracellular molecules (Fig. 5).
Fig. 5.
Binding of glycosylated α-DG to ligands has antimigratory activity. A proposed model for the function of a laminin-binding glycan on α-DG in cell locomotion is presented. Interaction of intact ECM molecules such as laminin (LN) and fibronectin (FN) with integrin initiates kinase activation and promotes cancer cell migration and proliferation. (Left) By contrast, binding of laminin-binding glycans on α-DG (shown by > and <) to receptors such as E3 fragment induces signals that counteract the downstream signaling cascades initiated by integrin-ECM interaction. Such balanced signaling is proposed for maintaining the nonmalignant phenotype of a cell. (Right) Once laminin-binding glycans are decreased by down-regulation of β3GnT1, α-DG no longer plays a role in counteracting integrin-mediated signaling, thus resulting in a migratory aggressive cell phenotype. Down-regulation of other glycosyltransferase(s) also plays a role in this regulation. Different globular domains (1–5) of laminin α-chain are shown by circled numbers.
In conclusion, our study demonstrates a critical role of β3GnT1 in attenuating cancer cell locomotion by regulating the synthesis of laminin-binding glycans on α-DG and possibly on other glycoproteins. The previously undescribed tumor suppressor function of a carbohydrate significantly extends our understanding of the roles of glycosylation in pathogenesis over previous findings (34). Further studies will be important to determine if overexpression of β3GnT1 in vivo leads to control of tumor growth in animal models and patients.
Materials and Methods
Details on reagents, cell culture, and tissue specimens; cloning, mutation, and RT-PCR analyses; cell sorting, immunofluorescence, and immunohistochemistry; siRNA-mediated knockdown; cell proliferation assay; colony formation assay; cell adhesion assay; wound healing assay; laminin-binding assay; invasion and migration assays; orthotopic tumor formation assay; immunoprecipitation and immunoblotting; expression of laminin-binding glycans in CHO mutant Lec15 cells; and statistical analysis can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Drs. Erkki Ruoslahti, Ze'ev Ronai, Sara Courtneidge, Kay-Hooi Khoo, and Seung Ho Lee for useful discussion and critical reading of the manuscript; Drs. Sharon Krag, Taroh Kinoshita, Tamao Endo, and Misa Suzuki for useful reagents; Elise Lamar for editing the manuscript; and Aleli Morse for organizing the manuscript. The work was supported by Grant CA48737 (to M.F.) and, in part, by Grant CA71932 (to M.F. and M.N.F.) from the National Cancer Institute; Grant-in-Aid 18390113 (to J.N.) from the Japan Society for the Promotion of Science; and Grant 20790278 (to M.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904515106/DCSupplemental.
References
- 1.Bhowmick NA, Neilson EG, Morse HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taddei I, et al. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White DE, et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cells. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Barresi R, Campbell KP. Dystroglycan: From biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 5.Grady RM, et al. Role for α-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat Cell Biol. 1999;1:215–220. doi: 10.1038/12034. [DOI] [PubMed] [Google Scholar]
- 6.Larsen M, Artym VV, Gree JA, Yamada KM. The matrix reorganized: Extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Chiba A, et al. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of α-dystroglycan with laminin. J Biol Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- 8.Muntoni F, Torell S, Brockington M. Muscular dystrophies due to glycosylation defects. Neurotherapeutics. 2008;5:627–632. doi: 10.1016/j.nurt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barresi R, et al. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 2004;10:696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- 10.Kanagawa M, et al. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 2004;117:953–964. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Payrard M, et al. The human LARGE gene from 22qf2.3q13.1 is a new, distinct member of the glycosyltransferase gene family. Proc Natl Acad Sci USA. 1999;96:598–603. doi: 10.1073/pnas.96.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE. Mutant glycosyltransferase and altered glycosylation of α-dystroglycan in the myodystrophy mouse. Nat Genet. 2001;28:151–154. doi: 10.1038/88865. [DOI] [PubMed] [Google Scholar]
- 13.Longman C, et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of α-dystroglycan. Hum Mol Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- 14.Patnaik SK, Stanley P. Mouse LARGE can modify complex N- and mucin O-glycans on α-dystroglycan to induce laminin binding. J Biol Chem. 2005;280:20851–20859. doi: 10.1074/jbc.M500069200. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki K, et al. Expression cloning of cDNA encoding a human β-1,3-N-acetylglucosaminyltransferase that is essential for poly-N-acetyllactosamine synthesis. Proc Natl Acad Sci USA. 1997;94:14294–14299. doi: 10.1073/pnas.94.26.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda M. β1,3-N-acetylglucosaminyltransferase (iGnT) In: Taniguchi N, Honde K, Fukuda M, editors. Handbook of Glycosyltransferases and Their Related Genes. Berlin: Springer; 2002. pp. 114–124. [Google Scholar]
- 17.Muschler J, et al. A role for dystroglycan in epithelial polarization: Loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- 18.Jing J, et al. Aberrant expression, processing and degradation of dystroglycan in squamous cell carcinomas. Eur J Cancer. 2004;40:2143–2151. doi: 10.1016/j.ejca.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Sgambato A, et al. Dystroglycan expression is reduced during prostate tumorigenesis and is regulated by androgens in prostate cancer cells. J Cell Physiol. 2007;213:528–539. doi: 10.1002/jcp.21130. [DOI] [PubMed] [Google Scholar]
- 20.Martin PT. Congenital muscular dystrophies involving the O-mannose pathway. Curr Mol Med. 2007;7:417–425. doi: 10.2174/156652407780831601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiraishi N, et al. Identification and characterization of three novel β1,3-N-acetylglucosaminyltransferases structurally related to the β1,3-galactosyltransferase family. J Biol Chem. 2001;276:3498–3507. doi: 10.1074/jbc.M004800200. [DOI] [PubMed] [Google Scholar]
- 23.Yeh JC, et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 24.Wiggins CA, Munro S. Activity of the yeast MNN1α1,3-masnnosyltransferases. Proc Natl Acad Sci USA. 1998;95:7945–7950. doi: 10.1073/pnas.95.14.7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willer T, et al. Targeted disruption of the Walker-Warburg syndrome gene Pomt1 in mouse results in embryonic lethality. Proc Natl Acad Sci USA. 2004;101:14126–14131. doi: 10.1073/pnas.0405899101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Signoretti S, et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergers G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Bernabe DB, et al. Loss of α-dystroglycan laminin binding in epithelium-derived cancers is caused by silencing of LARGE. J Biol Chem. 2009;284:11279–11284. doi: 10.1074/jbc.C900007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 31.Ferletta M, et al. Opposing roles of integrin α6Aβ1 and dystroglycan in laminin-mediated extracellular signal-regulated kinase activation. Mol Biol Cell. 2003;14:2088–2103. doi: 10.1091/mbc.E03-01-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spence HJ, Dhillon AS, James M, Winder SJ. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 2004;5:484–489. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ido H, et al. Molecular dissection of the α-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J Biol Chem. 2004;279:10946–10954. doi: 10.1074/jbc.M313626200. [DOI] [PubMed] [Google Scholar]
- 34.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.