Abstract
Coastal ecosystems and the services they provide are adversely affected by a wide variety of human activities. In particular, seagrass meadows are negatively affected by impacts accruing from the billion or more people who live within 50 km of them. Seagrass meadows provide important ecosystem services, including an estimated $1.9 trillion per year in the form of nutrient cycling; an order of magnitude enhancement of coral reef fish productivity; a habitat for thousands of fish, bird, and invertebrate species; and a major food source for endangered dugong, manatee, and green turtle. Although individual impacts from coastal development, degraded water quality, and climate change have been documented, there has been no quantitative global assessment of seagrass loss until now. Our comprehensive global assessment of 215 studies found that seagrasses have been disappearing at a rate of 110 km2 yr−1 since 1980 and that 29% of the known areal extent has disappeared since seagrass areas were initially recorded in 1879. Furthermore, rates of decline have accelerated from a median of 0.9% yr−1 before 1940 to 7% yr−1 since 1990. Seagrass loss rates are comparable to those reported for mangroves, coral reefs, and tropical rainforests and place seagrass meadows among the most threatened ecosystems on earth.
Keywords: ecosystem decline, global trajectories, habitat loss, marine habitat
Coastal ecosystems such as salt marshes, coral reefs, mangroves, and seagrasses have declined, leading to growing concern because they have recognized ecological and economic values (1–5). Seagrasses, marine flowering plants that include the widely distributed genera Zostera, Thalassia, and Posidonia, form some of the most productive ecosystems on earth, rivaling even crops of corn and sugar cane (6). Further, seagrass meadows provide high-value ecosystem services such as supporting commercial fisheries worth as much as $3500 ha−1 yr−1 (7), subsistence fisheries that support entire communities (8), nutrient cycling (9, 10), sediment stabilization (11), and globally significant sequestration of carbon (12). Seagrasses and the services they provide are threatened by the immediate impacts of coastal development and growing human populations as well as by the impacts of climate change and ecological degradation (11, 13). Seagrass losses also disrupt important linkages between seagrass meadows and other habitats (14), and their ongoing decline is likely producing much broader and long-lasting impacts than the loss of the meadows themselves.
Previous efforts to assess general trends in seagrass abundance have been based on a few case studies with limited quantitative data for the time periods studied (15–19) or on extrapolations from a few reported regional rates (11, 15, 19). These assessments vary in their conclusions, ranging from those asserting widespread and abrupt declines, as reported in several recent studies (16–18), to those reporting less dramatic declines on the order of 2–5% yr−1 (19) and occasional increases at local scales (20, 21). To expand on these efforts, we synthesized quantitative data from 215 sites with a total of 1,128 observations around the world covering the time period 1879–2006, creating the most comprehensive data set compiled to date (Table S1). Our results extend previous findings in showing that seagrass areal cover is declining across the globe and that the rate of loss is accelerating.
Results
Our analysis of the change in areal extent of seagrass populations demonstrates that, since the earliest records in 1879, seagrass meadows have declined in all areas of the globe where quantitative data are available, including both high and low latitudes. Comparing all sites across their total study length, there were significantly more declines in seagrass meadows than predicted by chance: 58% of sites declined, 25% increased, and 17% exhibited no detectable change (Table 1; χ2 = 5.9, P < 0.002, df = 2). Over the entire time period of our analysis, there was a mean decline in seagrass area of 1.5% yr−1 (median = 0.9% yr−1). Not only are the rates of loss high, but the total seagrass area lost is large. Overall, the measured area of seagrass loss was 3,370 km2 between 1879 and 2006 (i.e., 27 km2 yr−1), representing 29% of the maximum area measured (11,592 km2). In addition, the difference in area lost among sites that declined was more than 10 times greater than that among sites that increased (Table 1). Bootstrap analysis supported the robustness of these results; subsampling recovered similar overall rates of change independent of subsample size (Fig. S1). Extrapolation to the global scale must be qualified by limited seagrass mapping efforts in turbid water systems and in some geographic regions that have received less attention from the scientific community. Thus, global estimates of total seagrass area remain poorly resolved; however, based on actual mapped areas and inferring additional unmapped area (19), the current estimate of the total area of seagrasses is ≈177,000 km2. Extrapolating our conservative net loss (29%) to this global scale suggests that more than 51,000 km2 of seagrass meadows have been lost during the past 127 years.
Table 1.
Percentage rate of change for seagrass meadows globally
| Trajectory* | Median % rate of change, μ (N) | Proportion in category, % | Mean % rate of change, μ (±SE, N) | Net maximum measured area, km2 | Net change in study areas, km2 (% of maximum) | Mean study length, yr |
|---|---|---|---|---|---|---|
| Declining | −3.7 (126) | 58 | −6.9 (±0.9, 116) | 9,147 | −3,662 (40) | 25 |
| Increasing | 5.4 (53) | 25 | 11.8 (±3.6, 43) | 879 | 314 (36) | 20 |
| No detectable change | −0.06 (36) | 17 | −0.2 (±0.2, 36) | 1,565 | −19 (1) | 14 |
| Overall | −0.9 (215) | 100 | −1.5 (±1.1, 196) | 11,592 | −3,367 (29) | 22 |
Rate of change expressed as μ, % yr−1.
*Meadows were categorized as declining (<90% of initial area), increasing (final area >110% of initial area), or having no detectable change (final area within ±10% of initial area).
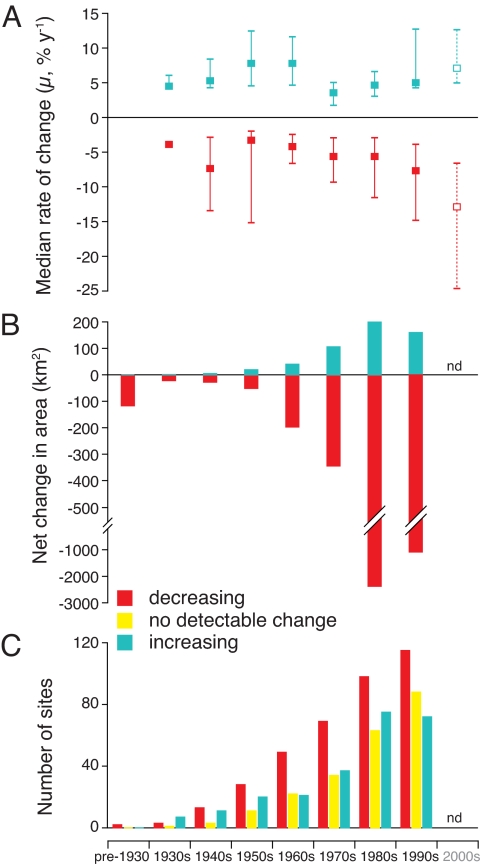
In addition, decadal time-course analysis reveals that the rate of decline in seagrass meadows has accelerated over the past 8 decades (Fig. 1). The median rate of decline was <1% yr−1 before 1940 but was 5% yr−1 after 1980 (Fig. 1A). The largest losses occurred after 1980 (Fig. 1B): in total, a loss of 35% of seagrass area. The acceleration in detected rates of decline cannot be attributed to increased sampling effort; there was a net change of −37 km2 site−1 decade−1 after 1980, twice the rate of loss before 1980 (−18 km2 site−1 decade−1). Comparing decadal trends, there was again a significantly greater number of sites experiencing decreases compared with increases (Wilcoxon signed pair ranked test, P = 0.002) (Fig. S2; χ2 = 23.7, P < 0.0001, df = 2). The median rate of change from 1879–2006 for sites with increased seagrass area was 5.4% yr−1 (mean: 11.8 ± 3.6% yr−1), which includes reports of the formation of 23 seagrass meadows where previously absent. As with loss rates, the rate of increase also accelerated from 4.3% between 1970 and 1980 to 8.4% in the period from 1990 to the 2000s. To date, however, the observed increase in seagrass area has been small compared with the total area lost globally (Table 1); details are available in Table S2.
Fig. 1.
Decadal trends in seagrass areal extent. Sites were categorized as declining in area, as increasing in area, or as having no detectable change (i.e., ±10% of initial area). Values for the 2000s (dotted line) include 2000–2006 data only. nd, not determined because of incomplete data. (A) Median % rate of change (μ) by decade across sites. Error bars represent 25% and 75% quartiles. (B) Measured net change in seagrass area, calculated as the net change across each decade. (C) Number of sites in each category (decreasing, increasing, or no change) by decade.
Evidence of causes of decline was available for 77 of 128 declining sites. Among these, 2 major causes of seagrass loss were indicated: (i) direct impacts from coastal development and dredging activities (21 sites) and (ii) indirect impacts from declining water quality (35 sites). Only 6 sites with decreases were classified as being caused by natural processes such as storm damage or biological disturbance. Of the 51 sites with increases, 29 had attributed causes, including 11 increases attributable to improved water quality and habitat remediation. Among the remaining increasing sites, recoveries from historical declines attributable to storm damage or episodes of wasting disease were the most common explanations.
Discussion
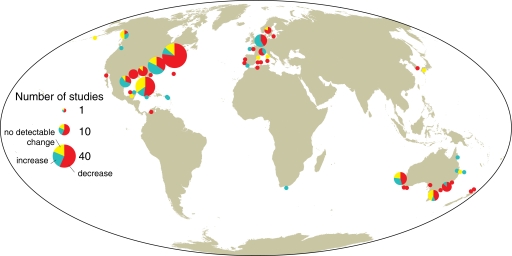
Our analysis included data from all 6 global seagrass bioregions (22), although sites were not distributed evenly. Europe, North America, and Australia were well represented (Fig. 2), reflecting monitoring efforts in these relatively affluent regions and their strong focus on coastal issues. Major gaps in information exist for West Africa, northeast South America, and the northwest Pacific area of the United States, where seagrasses are typically restricted in distribution. However, the largest data gap exists in the tropical Indo-Pacific region (from East Africa to Hawaii), where seagrasses are widespread and abundant. Seagrasses in this region perform vital ecosystem services for local human populations, support numerous elements of local economies (8), and are food for endangered species such as dugong and green turtle (22). Furthermore, this region has the highest number of seagrass species, including several endemic species (22). Given the rapid population growth and development pressures in the Indo-Pacific, there is a pressing need to acquire more data on seagrass extent in this important region to aid in evaluating the status of seagrasses.
Fig. 2.
Global map indicating changes in seagrass area plotted by coastline regions. Changes in seagrass areal extent at each site are defined as declining (red) or increasing (green) when areal extent changed by >10%, or no detectable change (yellow) when final area was within ±10% of the initial area. There were 131 sites in North America, 34 sites in Europe, and 40 sites in Australia.
Seagrass losses have been attributed to a broad spectrum of anthropogenic and natural causes (11). Because seagrass meadows are often dominated by a single seagrass species, they are susceptible to pandemic disease outbreaks like the “wasting disease” of the 1930s that killed as much as 90% of all eelgrass (Zostera marina) in the North Atlantic Ocean (23) or stand diebacks that killed more than 4,000 ha of turtlegrass (Thalassia testudinum) in Florida Bay (24). Destructive fishing practices, boat propellers, coastal engineering, cyclones, and tsunamis also cause direct and immediate seagrass loss (3, 4, 11). More indirect and potentially more damaging are the impacts of water quality degradation resulting from increased nutrient additions and sediment runoff in human-altered watersheds. In addition, the indirect effects of aquaculture and invasive species have been observed to affect seagrasses (25, 26). Other indirect effects from overfishing have caused the loss of predators, which can cascade down the food web and lead to the loss of the herbivores that clean seagrasses of fouling algae, resulting in seagrass loss (16, 27, 28). Lastly, global climate change is predicted to have deleterious effects on seagrasses (29) and is emerging as a pressing challenge for coastal management.
Worldwide, seagrasses are experiencing all 5 of the most serious threats to marine biodiversity (30); overexploitation, physical modification, nutrient and sediment pollution, introduction of nonnative species, and global climate change. Seagrass declines have been attributed to all these threats, often in combination. Managing seagrass meadows requires an integrated approach (31), including efforts to avoid excessive nutrient and organic inputs from agricultural, aquaculture, and urban sources and to prevent sediment loading, which causes a deterioration in the submarine light climate so critical for seagrass growth. Best practices should also seek to avoid mechanical damage through anchors, propellers, and fishing gear. Responsible stewardship that promotes favorable growing conditions will confer seagrass meadows with resistance and resilience against pressures that cannot be managed locally, such as those associated with climate change.
Evidence of outcomes from improved management practices are emerging. For example, a concerted effort to reduce point sources of nutrients in Tampa Bay, Florida, over the past 2 decades has resulted in a 50% reduction in total nitrogen loads and an ≈50% increase in water clarity, leading to the recovery of 27 km2 of seagrasses since 1982 (32). Likewise, mitigation measures adopted in Mondego Bay, a highly eutrophic estuary in Portugal, reduced nitrogen loads and increased seagrass area from 0.02 km2 (1997) to 1.6 km2 (2002) by altering estuarine hydraulics and controlling seagrass habitat destruction by fishing practices (33). These system-wide management strategies are improvements on the attempts over past decades to restore seagrass through transplantation. Numerous transplant projects have been attempted worldwide as mitigation measures for seagrass losses (34). However, transplant projects have involved only a few seagrass species and at spatial scales that have failed to alter the trajectories of seagrass loss significantly (34). Science-based protection and management approaches supporting a combination of statutory authorities and consensus planning must be designed to diminish the cumulative effect of stressors and accommodate the broad range of impacts on seagrass meadows to protect them from further losses (35, 36).
Our report of mounting seagrass losses reveals a major global environmental crisis in coastal ecosystems, for which seagrasses are sentinels of change (11). Seagrasses are sensitive integrators of changes in water quality, sediment loading, and other inputs that accumulate as a result of human modification of watersheds and receiving coastal water bodies (37). Seagrass meadows signal the early stages of eutrophication because they give way to faster growing plant competitors like macroalgae and microalgae as water quality decreases (38). More importantly, in contrast to coral reefs, which also herald environmental change but occupy a relatively small portion of the world's oceans, seagrasses are global in extent except for the highest polar regions.
The extent and rate of seagrass losses reported here have had significant ecological consequences. Losses of seagrass meadows will continue to reduce the energy subsidies they provide to other ecosystems such as adjacent coral reefs or distant areas such as deep-sea bottoms, diminishing the net secondary productivity of these habitats (14). Seagrass losses also threaten the future of endangered species such as Chinook salmon (39) and the habitat for many other organisms. Seagrass losses decrease primary production, carbon sequestration, and nutrient cycling in the coastal zone (5). If the current rate of seagrass loss is sustained or continues to accelerate, the ecological losses will also increase, causing even greater ill-afforded economic losses.
Severe impacts to seagrass meadows have received limited public attention compared with changes to other coastal (11, 40) and terrestrial ecosystems, despite the fact that the overall mean rate of seagrass loss calculated here is similar to that of mangrove forests (1.8% yr−1) (41) and even faster than that of tropical forests (0.5% yr−1) (42). Reported changes in Indo-West Pacific (43) coral cover are lower, declining at 0.72% yr−1 among reefs repeatedly monitored over the period 1997–2004. Mean decline rates reported in most coral reef studies (1 to 9% yr−1) (44, 45) are based on changes in percent coral cover, as opposed to the actual areal extent of the coral reef ecosystem; however, rates of seagrass meadow and coral reef declines can be considered roughly equivalent, given that seagrass meadows are expected to have a concomitant decline in percent cover as total area declines (46). The cumulative effect of the reported losses in seagrass, mangrove, coral reef, and coastal wetland habitats signals a serious deterioration of coastal environments around the world.
Materials and Methods
We compiled a database that incorporated existing quantitative data on seagrass areal extent from published studies, reports, web sites, online databases, and unpublished but audited sources (see SI Data Sources). Sources were identified by conducting a Web of Science search in February 2006 and then again in October 2006 using the following search term: (seagrass* or SAV or submerged aquatic vegetation) and (loss* or change* or recovery or stability or dynamic* or impact* or map* or decline* or increase* or gain*). This search returned 2,346 references (from which we excluded reports referring to “freshwater species”). We also requested relevant data on the Seagrass Forum listserv in October 2006.
To ensure that reported changes in areal extent were not simply attributable to seasonal variation, we included only studies with at least 2 estimates of areal extent that covered more than 2 years. If the date of a study was not specific within a year, it was assigned to the midyear point (i.e., 1980.5). A known location for each study is referred to as the “site,” and each measurement of seagrass area at a site is referred to as an “event.” The trajectory of each study was determined as the overall percent rate of change, either positive (i.e., more seagrass area measured) or negative (i.e., less seagrass area measured), across the entire time period of each study and across each decade of the study. At each site, we classified seagrass meadows as declining or increasing if the areal extent changed by >10% or as no detectable change if the areal extent changed by ≤10% [which is typically within the error of assessment techniques (47)]. Departure from even partitioning of meadow trajectories was calculated using a χ2 test. The final database comprises 215 sites with 1,128 events from 70 sources (Table S1). Several data verification steps were conducted, including independent checks of 63% of all site entries (136 sites).
We conducted 2 types of analyses: (i) trajectories were analyzed using the initial and final observations of seagrass area at each site to represent overall trends at sites irrespective of the time period, and (ii) trajectories were analyzed decade by decade to account for trends across decades (“decadal analysis”) (see SI Decadal Analysis; Fig. S3). Percentage rates of change (the trajectory, μ, % yr−1) for sites were calculated over time interval, t, from the initial to final reported areas (Ao and At, respectively) as μ = [ln(At/Ao)/t] × 100. In addition to the specific rate of change, the net change in area (final area minus initial area) was calculated for each site and for each site in each decade that the study traversed. Trajectories and net change in reported area were calculated across the total time span of each data set and for each decade of the data set (see SI Decadal Analysis; Fig. S3). A test of the relative proportion of sites experiencing decreases as opposed to increases in each decade was conducted by comparing the departure from a 1:1 relationship between these increases and decreases using a χ2 test and a Wilcoxon signed pair ranked test.
All records of seagrass area before 1930 were grouped for the decade analysis because of limited sample size. The 215 sites assessing change in area of seagrass meadows were not distributed randomly because some regions of the world (the eastern coast of North America, Europe, and southern Australia) have been sampled more intensely than others, irrespective of regional seagrass abundance. In addition to this geographic bias in available data (i.e., developed regions of the world were unavoidably overrepresented), there was a historical bias. More data were available after 1980 (>80% of records), reflecting recent increased research and monitoring effort (see SI Observational Effort). To address the influence of sample size effects, bootstrap resampling was used, and we observed the overall trend in μ, as the median rate of change, to be independent of sample size. Bootstrap analysis of μ was conducted for the overall data by random subsampling of 10–80 records in steps of 10, for 100 replicate random samples of each subsample size. For each replicate of a given subsample size, a median value and mean value of μ were calculated and plotted with the 25th and 75th percentiles and maximum and minimum or plus and minus SEs to assess the central tendency for random subsets of samples taken from the total data set (Fig. S1). Because of the lag in reporting changes in measured seagrass areal extent (estimated at >5 years from final date included in the data), the data available for the current decade should be considered incomplete.
Two global estimates of seagrass area that can be substantiated at present are (i) the measured global seagrass area, which is the area for which mapping polygons have been established (124,000 km2; these authors also extrapolate an estimate of expected total area, including unmapped seagrass as 177,000 km2) (19), and (ii) the potential global seagrass area determined by light regimen, bathymetry, and seagrass light requirements (4,300,000 km2) (48). Although these 2 estimates of global seagrass area result in an extremely wide range (35-fold), further refinement of these global estimates is not possible without more data. Estimates of global seagrass loss calculated in this study were based on the estimated total area value of 177,000 km2 (19) that we view as a minimum value for total global seagrass area. We note that seagrass meadows grow in turbid, deep, or remote waters in many parts of the world, making mapping their extent problematic.
Supplementary Material
Acknowledgments.
Ashley Simmons, Karen McGlathery, Richard Pearson, and Simon Robson provided comments on the manuscript. This work was conducted as a part of the Global Seagrass Trajectories Working Group supported by the National Center for Ecological Analysis and Synthesis, a center funded by National Science Foundation (Grant DEB-00–72909), the University of California at Santa Barbara, and the State of California.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905620106/DCSupplemental.
References
- 1.Costanza R, et al. The value of the world's ecosystem services and natural capital. Nature. 1997;387:253–260. [Google Scholar]
- 2.Halpern BS. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 3.Howarth R, et al. Nutrient pollution of coastal rivers, bays and seas. Issues in Ecology. 2000;7:1–14. [Google Scholar]
- 4.Valiela I. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnol Oceanogr. 1997;42:1105–1118. [Google Scholar]
- 5.Worm B. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 6.McRoy CP, McMillan CA. In: Seagrass Ecosystems. McRoy CP, Helfferich C, editors. New York: Marcel Dekker; 1977. pp. 53–87. [Google Scholar]
- 7.Watson RA, Coles RG, Lee Long WJ. Simulation estimates of annual yield and landed value for commercial penaeid prawns from a tropical seagrass habitat. Australian Journal of Marine and Freshwater Research. 1993;44:211–219. [Google Scholar]
- 8.de la Torre-Castro M, Ronnback P. Links between humans and seagrasses—an example from tropical East Africa. Ocean & Coastal Management. 2004;47:361–387. [Google Scholar]
- 9.McGlathery KJ, Sundbäck K, Anderson IC. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Mar Ecol Prog Ser. 2007;348:1–18. [Google Scholar]
- 10.Romero J, Lee K-S, Pérez M, Mateo MA, Alcoverro T. In: Seagrasses: Biology, Ecology and Conservation. Larkum WD, Orth RJ, Duarte CM, editors. Dordrecht, The Netherlands: Springer; 2006. pp. 227–254. [Google Scholar]
- 11.Orth RJ, et al. A global crisis for seagrass ecosystems. Bioscience. 2006;56:987–996. [Google Scholar]
- 12.Duarte CM, Middelburg J, Caraco N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences. 2005;2:1–8. [Google Scholar]
- 13.Duffy JE. Biodiversity and the functioning of seagrass ecosystems. Mar Ecol Prog Ser. 2006;311:233–250. [Google Scholar]
- 14.Heck KLJ, et al. Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems. 2008;11:1198–1210. [Google Scholar]
- 15.Duarte CM. The future of seagrass meadows. Environ Conserv. 2002;29:192–206. [Google Scholar]
- 16.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 17.Lotze HK, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfi JM, et al. Global trajectories of the long-term decline of coral ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 19.Spalding M, Taylor M, Ravilious C, Short FT, Green E. In: World Atlas of Seagrasses. Green EP, Short FT, editors. Berkley, CA: Univ of California Press; 2003. pp. 5–26. [Google Scholar]
- 20.Kendrick GA, Hegge BJ, Wyllie A, Davidson A, Lord DA. Changes in seagrass cover on Success and Parmelia Banks, Western Australia between 1965 and 1995. Estuarine, Coastal and Shelf Science. 2000;50:341–353. [Google Scholar]
- 21.Preen AR, Lee Long WJL, Coles RG. Flood and cyclone related loss, and partial recovery of more than 1000 km2 of seagrass in Hervey Bay, Queensland, Australia. Aquatic Botany. 1995;52:3–17. [Google Scholar]
- 22.Short FT, Carruthers TJB, Dennison WC, Waycott M. Global seagrass distribution and diversity: A bioregional model. J Exp Mar Biol Ecol. 2007;350:3–20. [Google Scholar]
- 23.Tutin TG. Zostera L. The Journal of Ecology. 1942;30:217–226. [Google Scholar]
- 24.Robblee MB, et al. Mass mortality of the tropical seagrass Thalassia testudinum in Florida Bay (USA) Mar Ecol Prog Ser. 1991;71:297–299. [Google Scholar]
- 25.Ruiz JM, Perez M, Romero J. Effects of fish farm loadings on seagrass (Posidonia oceanica) distribution, growth and photosynthesis. Mar Pollut Bull. 2001;42:749–760. doi: 10.1016/s0025-326x(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 26.Williams SL. Introduced species in seagrass ecosystems: Status and concerns. J Exp Mar Biol Ecol. 2007;350:89–110. [Google Scholar]
- 27.Duffy JE. Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecol Lett. 2005;8:301–309. [Google Scholar]
- 28.Myers RA. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science. 2007;315:1846–1850. doi: 10.1126/science.1138657. [DOI] [PubMed] [Google Scholar]
- 29.Brouns JJ. In: Impacts of Climate Change on Ecosystems and Species: Marine and Coastal Ecosystems. Pernetta JC, Leemans R, Elder D, Humphrey S, editors. Vol 2. Gland, Switzerland: International Union for Conservation of Nature; 1994. pp. 59–72. [Google Scholar]
- 30.Norse EA. Global Marine Biological Diversity: A Strategy for Building Conservation into Decision Making. Washington, DC: Island Press; 1993. [Google Scholar]
- 31.Borum J, Duarte CM, Krause-Jensen D, Greve TM. European Seagrasses: An Introduction to Monitoring and Management. Copenhagen: The M&MS Project; 2004. p. 95. [Google Scholar]
- 32.Greening HS, Janicki A. Toward reversal of eutrophic conditions in a subtropical estuary: Water quality and seagrass response to nitrogen loading reductions in Tampa Bay, Florida, USA. Environ Manage. 2006;38:163–178. doi: 10.1007/s00267-005-0079-4. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso PG, Brandão A, Pardal MA, Raffaelli D, Marques JC. Resilience of Hydrobia ulvae populations to anthropogenic and natural disturbances. Mar Ecol Prog Ser. 2005;289:191–199. [Google Scholar]
- 34.Paling EI, Fonseca M, van Katwijk MM, van Keulen M. Seagrass restoration. In: Perillo GME, Wolanski E, Cahoon DR, Brinson M, editors. Coastal Wetlands: An Integrated Ecosystems Approach. Amsterdam: Elsevier; 2009. pp. 687–713. [Google Scholar]
- 35.Coles R, Fortes M. In: Global Seagrass Research Methods. Short FT, Coles RG, editors. Amsterdam: Elsevier; 2001. pp. 445–463. [Google Scholar]
- 36.Kenworthy WJ, Wyllie-Echeverria S, Coles RG, Pergent G, Pergent-Martini C. In: Seagrasses: Biology, Ecology and Conservation. Larkum WD, Orth RJ, Duarte CM, editors. Dordrecht, The Netherlands: Springer; 2006. pp. 595–623. [Google Scholar]
- 37.Dennison WC, et al. Assessing water quality with submerged aquatic vegetation. Bioscience. 1993;43:86–94. [Google Scholar]
- 38.Duarte CM. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia. 1995;41:87–112. [Google Scholar]
- 39.Hughes AR, Williams SL, Duarte CM, Heck KL, Waycott M. Associations of concern: Declining seagrasses and threatened dependent species. Frontiers in Ecology and the Environment. 2009;7:242–246. [Google Scholar]
- 40.Duarte CM, Dennison WC, Orth RJ, Carruthers TJB. The charisma of coastal ecosystems. Estuaries and Coasts. 2008;31:233–238. [Google Scholar]
- 41.Valiela I, Bowen JL, York JK. Mangrove forests: One of the world's threatened major tropical environments. Bioscience. 2001;51:807–815. [Google Scholar]
- 42.Achard F, et al. Determination of deforestation rates of the world's humid tropical forests. Science. 2002;297:999–1002. doi: 10.1126/science.1070656. [DOI] [PubMed] [Google Scholar]
- 43.Bruno JF, et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 2007;5:1220–1227. doi: 10.1371/journal.pbio.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 45.Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 46.Duarte CM, Fourqurean JW, Krause-Jensen D, Olesen B. In: Seagrasses: Biology, Ecology and Conservation. Larkum WD, Orth RJ, Duarte CM, editors. Dordrecht, The Netherlands: Springer; 2006. pp. 271–294. [Google Scholar]
- 47.Kendrick GA, Eckersley J, Walker DI. Landscape-scale changes in seagrass distribution over time: A case study from Success Bank, Western Australia. Aquatic Botany. 1999;65:293–309. [Google Scholar]
- 48.Gattuso JP, et al. Light availability in the coastal ocean: Impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences. 2006;3:489–513. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.