Abstract
Background and Aims
Many species of lichen-forming fungi contain yellow or orange extracellular pigments belonging to the dibenzofurans (usnic acid), anthraquinones (e.g. parietin) or pulvinic acid group. These pigments are all equally efficient light screens, leading us to question the potential ecological and evolutionary significance of diversity in yellow and orange lichen substances. Here the hypothesis is tested that the different pigments differ in metal-binding characteristics, which suggest that they may contribute to adaptation to sites differing in pH and metal availability.
Methods
UV spectroscopy was used to study the dissociation and the pH dependence of the metal-binding behaviour of seven isolated lichen substances in methanol. Metals applied were selected macro- and micro-nutrients (Cu2+, Fe2+, Fe3+, Mg2+, Mn2+ and Zn2+).
Key Results
All the pigments studied are strong to moderate acids with pKa1 values between 2·8 and 4·5. Metal complexation is common in the lichen substances studied. Complexation takes place under acidic conditions with usnic acid, but under alkaline conditions with parietin and most compounds of the pulvinic acid group. The pulvinic acid derivative rhizocarpic acid forms metal complexes both in the acidic and the alkaline range.
Conclusions
Metal complexation by lichen substances could be a prerequisite for lichen substance-mediated control of metal uptake. Assuming such an effect at pH values where the affinity of the metal for the lichen substance is intermediate would explain the strong preference of lichens with usnic or rhizocarpic acids to acidic substrata. Moreover, it would explain the preference of lichens with parietin and some lichens with compounds of the pulvinic acid group either for nutrient-rich substrata at low pH or for calcareous substrata.
Key words: Anthraquinones, dibenzofurans, pulvinic acid derivatives, usnic acid, parietin, dissociation constant (pKa1), metal complexation, lichenized Ascomycetes, lichen ecology, nutrients, UV spectroscopy
INTRODUCTION
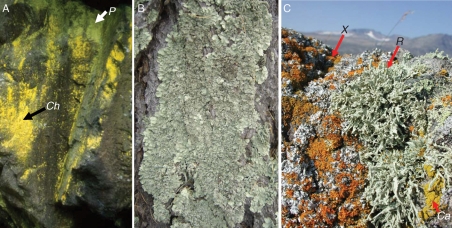
Many lichens produce lichen substances that absorb ultraviolet radiation, visible light usable for photosynthesis (PAR), or both ultraviolet radiation and PAR (Rikkinen, 1995; Huneck and Yoshimura, 1996). Photoprotection by lichen substances, melanins, calcium oxalate crystals or layers of dead fungal cells is essential for lichens occurring in environments exposed to high solar irradiation (Bjerke and Dahl, 2002; Nybakken et al., 2004; Hauck et al., 2007a). Most PAR-absorbing lichen substances are yellow or orange crystals (Fig. 1) that are produced by the fungus, but are located on the surfaces of the fungal or algal cell walls.
Fig. 1.
Examples of lichen symbioses with yellow lichen substances: (A) Chrysothrix chlorina (Ch) with the pulvinic acid derivatives calycin, leprapinic acid and vulpinic acid as well as Psilolechia lucida (P) with the pulvinic acid derivative rhizocarpic acid growing on siliceous rock; (B) Flavopunctelia soredica with the dibenzofuran usnic acid growing on acidic conifer bark; (C) Candelariella coralliza (Ca) with pulvinic acid and its derivatives calycin and pulvinic acid dilactone, Ramalina polymorpha (R) with usnic acid and Xanthoria candelaria (X) with the anthraquinone parietin co-occurring on nutrient-rich siliceous rock. Photographs by M. Hauck (A, B) and E. Timdal (C).
Chemically, these pigments belong to three different groups of substances, viz. dibenzofurans, anthraquinones and pulvinic acid derivatives (Huneck and Yoshimura, 1996). The faintly yellow xanthones form an additional group occurring, for example, in the lichen genera Buellia or Lecidella that are not treated in the present study. The yellow usnic acid, which is one of the most common lichen substances occurring in many different lichens, is a dibenzofuran (Cocchietto et al., 2002; Ingólfsdóttir, 2002). The anthraquinones include several orange substances that are synthesized by lichens, such as chrysophanol, emodin, fallacinol, parietin or parietinic acid (Huneck and Yoshimura, 1996). Parietin is the most common anthraquinone in lichens, which occurs in species of the families Teloschistaceae, Brigantiaceae, Letroutiaceae and Psoraceae (Solhaug and Gauslaa, 1996; Hafellner, 1997; Johansson et al., 2005) and furthermore in the non-lichenized Plectomycetes (Engstrom et al., 1980), which are thought to have lichenized ancestors (Lutzoni et al., 2001). Pulvinic acid and its derivatives, such as calycin, epanorin, pulvinic acid lactone as well as leprapinic, pinastric, rhizocarpic and vulpinic acids form a group of mostly yellow pigments (Huneck and Yoshimura, 1996). While anthraquinones and dibenzofurans are synthesized through the acetyl-polymalonyl pathway, pulvinic acid is formed on the shikimic acid pathway (Huneck, 1999).
Alhough it is well known that many lichens contain yellow or orange pigments (Fig. 1), it has not yet been studied why different substances occur in different lichen symbioses. To achieve protection against energy-rich solar irradiation, the evolution of just one pigment with absorption maxima in the blue and ultraviolet range of the electromagnetic spectrum would theoretically have been sufficient. The existence of more than one substance with such properties in lichens could be the result of the repeated development of the lichen symbiosis in the course of evolution (Gargas et al., 1995) or suggest that the yellow pigments have other functions in addition to light absorption that may have contributed to the evolution of different substances.
In the present paper, the hypothesis is tested that the evolution of different yellow and orange lichen substances is a response to habitats differing in pH and in the availability of metal ions. This hypothesis was tested because the acidity tolerance of lichens was recently shown to be influenced by the content of lichen substances (Hauck, 2008a; Hauck and Jürgens, 2008). Lichens with the dibenzofuran usnic acid were shown to prefer ambient pH values close to the first dissociation constant (pKa1) of this compound (Hauck and Jürgens, 2008). Furthermore, lichen substances were found to be involved in metal homoeostasis of lichens (Hauck and Huneck, 2007a, b; Hauck et al., 2007b; Hauck, 2008b). Since both the dissociation and the affinity of lichen substances to metal ions depend on pH (Sharma and Jannke, 1966; Takani et al., 2002), it is reasonable to assume that the evolution of different yellow lichen substances may be an adaptation to different pH conditions. Therefore, pKa1 values of yellow lichen substances were determined and the pH dependence of the formation of complexes between these metabolites and metal ions was studied using UV spectroscopy. The results of these experiments were compared with published preferences for ambient pH values of lichens producing yellow or orange lichen substances (Wirth, 1995).
MATERIALS AND METHODS
Determination of pKa1 values
Dissociation constants of lichen substances were determined in methanol using an Uvikon 922 UV spectrophotometer (Bio-Tek Kontron, Neufahrn, Germany). Methanol was used as a solvent, because most lichen substances are highly soluble in it and because important physico-chemical properties of methanol influencing the dissociation of dissolved acids resemble those of water. Water, which would be the most realistic solvent in terms of the ecological relevance of the dissociation data, could not be used for the determination of the dissociation constants because of the low solubility of most lichen substances in water. Methanol was preferred over Brij 35, recently applied by Hidalgo et al. (2002, 2005) for the determination of dissociation constants of lichen substances, because many lichen substances are more soluble in methanol than in Brij 35. Furthermore, spectroscopic results obtained in preliminary tests with different solvents were more highly reproducible with methanol than with Brij 35, which might be due to the micellar nature of the Brij 35 solutions. Acetonitrile (Wang et al., 2001) was not used, because its aprotic character suppresses the dissociation of acids and leads, therefore, to very high dissociation constants (Jover et al., 2008), which probably do not realistically reflect their dissociation behaviour in the aqueous environment found in the lichen thallus (Hauck and Jürgens, 2008). Compared with water, methanol is similar with regard to two important characters controlling the dissociation of acids dissolved in these liquids, namely the proticity and the Lewis basicity (donor number 19·0 kcal mol−1 for methanol versus 18·0 kcal mol−1 for water; Stangret, 2002) so that the use of methanol was obvious.
Isolated lichen substances were dissolved in methanol in a concentration of 10 µm. The pH was adjusted with buffer solutions to pH values ranging from 1 to 13 (Fixanal, Sigma-Aldrich, Steinheim, Germany). The methanol solution and the buffer were mixed in a ratio of 1 to 3, so that the final concentration of the lichen substances amounted to 3·3 µm. Lichen substances included commercially available dibenzofuran (+)-usnic acid (Sigma-Aldrich) and anthraquinone parietin (Carl Roth, Karlsruhe, Germany). Five substances of the pulvinic acid group were obtained from the collections of isolated lichen substances of S. Huneck (leprapinic, pinastric, pulvinic and rhizocarpic acids) or G. B. Feige (vulpinic acid) now deposited at the Botanical Museum, Berlin-Dahlem, Germany. Solutions were stirred and left at room temperature for 1 d before measurements were taken.
Absorbance between 220 nm and 500 nm was recorded every 1 nm at pH 1 and pH 13 to detect the absolute and relative absorption maxima of the individual lichen substances. Absorbance versus pH was then measured at the absorption maxima at 20 °C. Wavelengths selected for these measurements were as follows: (+)-usnic acid, λ=325 nm; parietin, 438 nm; leprapinic acid, 371 nm; pinastric acid, 347 nm; pulvinic acid, 347 nm; rhizocarpic acid, 325 nm; vulpinic acid, 346 nm. All measurements were replicated six times. Since the buffer solutions could not completely compensate for changes in the pH after the addition of the lichen substance–methanol solutions, the pH was measured subsequent to the spectroscopic analysis with an MP 120 pH meter with an InLab 413 electrode (Mettler-Toledo, Greifensee, Switzerland).
The dissociation constants can be deduced from the plots of the absorbance taken up against the pH. The graphs were fitted with Spline approximations using Xact 7·12 software (SciLab GmbH, Hamburg, Germany). The turning points of these graphs (i.e. the points where the second derivation is zero) equal the dissociation constants (Sharma and Jannke, 1966; Jankulovska et al., 2006). Mean curves calculated from the replicate measurements of the individual lichen substances were used for the determination of the dissociation constants. As the lichen substances dissociate incompletely, only the pKa1 values are thought to be physiologically relevant (Hauck and Jürgens, 2008). Therefore, the determination of dissociation constants was limited to the pKa1 values.
pH dependence of metal complexation
Complex formation of metal ions with each lichen substance was investigated for selected macro- and micro-nutrients (Cu2+, Fe2+, Fe3+, Mg2+, Mn2+ and Zn2+). These ions were supplied as acetylacetonate salts, because the corresponding acid, acetylacetone, is a weak acid [pKa (in water)=9·0] (Pearson and Dillon, 1953) and is therefore less likely to disturb the formation of metal complexes than would be the case if, for example, chloride, nitrate or sulfate salts had been applied. The pH was adjusted with Fixanal buffer solutions varying between pH 2 and pH 12. Isolated lichen substances were dissolved in methanol. Final concentrations both of the lichen substances and the metal ions amounted to 3·3 µm. Absorbance maxima were determined by measuring the absorbance of all pairs of metals and lichen substances between λ=220 and 900 nm at pH 12, as maximum complex formation was assumed at high pH (Takani et al., 2002). Wavelengths selected for recording absorbance versus pH are compiled in Table 1. Solutions with lichen substances and metal ions were measured against a blank differing only in the lack of the lichen substance. Five replicate measurements were carried out and mean curves were calculated from these replicate measurements. The pH of all solutions was determined after the spectroscopic measurements.
Table 1.
Wavelengths (in nm) applied in the UV spectroscopic study of complex formation of metal ions with lichen substances
| Cu2+ | Fe2+ | Fe3+ | Mg2+ | Mn2+ | Zn2+ | |
|---|---|---|---|---|---|---|
| Usnic acid | 282 | 281 | 282 | 286 | 281 | 281 |
| Parietin | 297 | 256 | 295 | 256 | 295 | 294 |
| Leprapinic acid | 293 | 291 | 293 | 293 | 292 | 294 |
| Pinastric acid | 293 | 293 | 293 | 293 | 293 | 294 |
| Pulvinic acid | 293 | 294 | 294 | 294 | 294 | 293 |
| Rhizocarpic acid | 293 | 292 | 289 | 293 | 292 | 292 |
| Vulpinic acid | 294 | 294 | 293 | 294 | 294 | 295 |
RESULTS
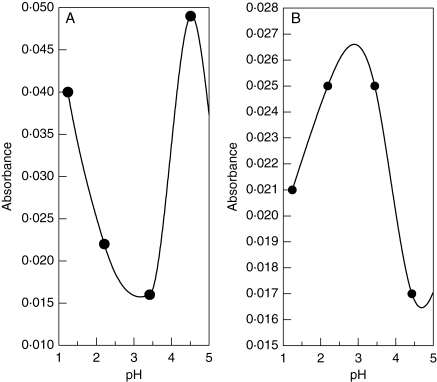
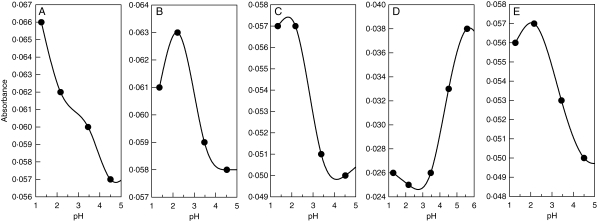
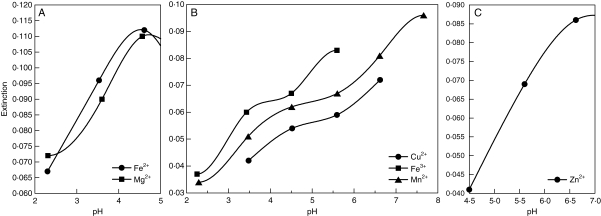
All lichen substances studied are strong to moderate acids with pKa1 values varying between 2·8 and 4·5 (Table 2 and Figs 2 and 3). The UV spectroscopic analyses of metal–lichen substance solutions suggest that complex formation took place in all pairwise combinations of metal ions (Cu2+, Fe2+, Fe3+, Mg2+, Mn2+ and Zn2+) and lichen substances (Figs 4–7).
Table 2.
pKa1 values of yellow or orange lichen substances in methanol
| Lichen substance | pKa1 |
|---|---|
| Usnic acid | 4·0 |
| Parietin | 3·9 |
| Leprarinic acid | 3·2 |
| Pinastric acid | 2·8 |
| Pulvinic acid | 2·8 |
| Rhizocarpic acid | 4·5 |
| Vulpinic acid | 3·4 |
Fig. 2.
UV spectroscopic results showing the dissociation of (A) the dibenzofuran usnic acid and the (B) anthraquinone parietin between pH 1 and pH 5. Turning points indicate pKa1 values.
Fig. 3.
UV spectroscopic results showing the dissociation of pulvinic acid (A) and its derivatives leprapinic acid (B), pinastric acid (C), rhizocarpic acid (D), and vulpinic acid (E) between pH 1 and pH 5 (A–C, E) or pH 1 and pH 6 (D). Turning points indicate pKa1 values.
Fig. 4.
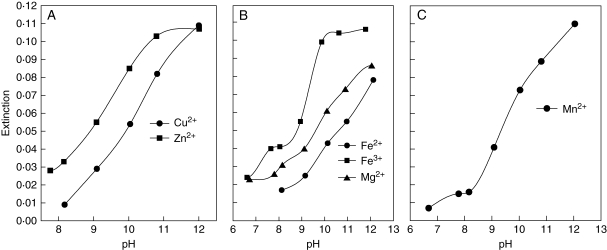
pH dependence of metal-binding by usnic acid: (A) Fe2+, Mg2+; (B) Cu2+, Fe3+, Mn2+; (c) Zn2+.
Fig. 5.
pH dependence of metal-binding by parietin: (A) Cu2+, Zn2+; (B) Fe2+, Fe3+, Mg2+; (C) Mn2+.
Fig. 6.
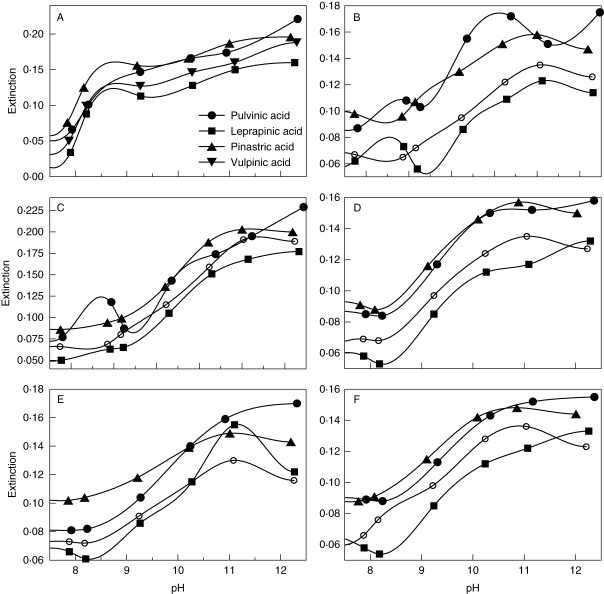
pH dependence of metal-binding by pulvinic acid and its derivatives leprapinic, pinastric and vulpinic acids: (A) Cu2+, (B) Fe2+, (C) Fe3+, (D) Mg2+, (E) Mn2+, (F) Zn2+.
Fig. 7.
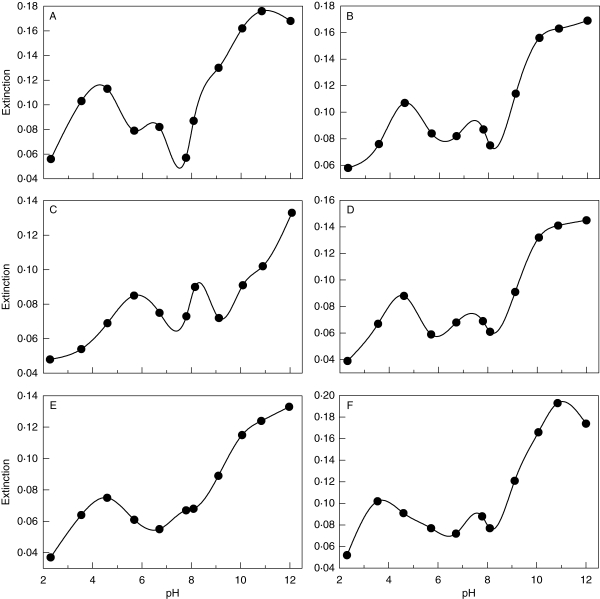
pH dependence of metal-binding by the pulvinic acid derivative rhizocarpic acid: (A) Cu2+, (B) Fe2+, (C) Fe3+, (D) Mg2+, (E) Mn2+, (F) Zn2+.
Metal complexation by usnic acid was primarily observed under acidic conditions (Fig. 4). Absorbance of solutions containing Fe2+, Fe3+, Mg2+ or Mn2+ and usnic acid started to increase between pH 2·0 and pH 2·5 (Fig. 4A and B). Maximum absorbance indicating complete metal binding was achieved at pH 4·5 for Fe2+ and Mg2+ (Fig. 4A), pH 5·5 for Fe3+ and pH 7·5 for Mn2+ (Fig. 4B). Absorbance of the solutions with Cu2+ and usnic acid increased between pH 3·5 and pH 6·5 (Fig. 4B), whereas the absorbance of solutions with Zn2+ and usnic acid increased between pH 4·5 and pH 6·5 (Fig. 4C).
In contrast to the usnic acid, complex formation between parietin and metal ions was found under alkaline conditions (Fig. 5). The absorbance of parietin solutions mixed with Fe2+, Mg2+ or Mn2+ increased at a pH >6·5 (Fig. 5B, C). If parietin was mixed with Cu2+, Fe3+ or Mn2+, absorbance started to increase around pH 8 (Fig. 5A, B). In all cases, maximum absorbance was reached at pH 12 (Fig. 5).
The pigments of the pulvinic acid group formed complexes with metal ions either in the alkaline range (leprapinic, pinastric, pulvinic and vulpinic acids; Fig. 6) or both at low and high pH values (rhizocarpic acid; Fig. 7). While all the compounds of the pulvinic acid group investigated showed increased absorbance values at pH >8 with Mn2+ and Zn2+, the metal-binding behaviour differed between the individual lichen substances for Cu2+, Fe2+, Fe2+ and Mg2+. Leprapinic, pinastric, pulvinic, and vulpinic acids exhibited increasing absorbance values with Cu2+ at pH >7 and with Mg2+ at pH >8 (Fig. 6). With Fe2+, the absorbance of the leprapinic, pulvinic and vulpinic acids solutions increased at pH >8, whereas that of the pinastric acid solution increased at pH >7. With Fe3+, absorbance increased at pH >8 with pulvinic acid, at pH >6 with leprapinic and pinastric acids, and at pH >5 with vulpinic acid (Fig. 6). Rhizocarpic acid exhibited two different pH ranges with increasing absorbance values (Fig. 7). With Cu2+, Fe2+, Mg2+, Mn2+ and Zn2+, absorbance increased from pH 2 to pH 4 or pH 2 to pH 5 and from pH 8 to pH 12. With Fe3+, increasing absorbance was found between pH 2 and pH 5·5 and between pH 9 and pH 12 (Fig. 7).
DISCUSSION
pKa1 values of lichen substances
Dissociation constants have rarely been determined for lichen substances. Published values are available for the dibenzofuran usnic acid (Sharma and Jannke, 1966), the depsidone lobaric acid (Hidalgo et al., 2005), the anthraquinones parietin, emodin and chrysophanol (Wang et al., 2001) and the pulvinic acid derivatives calycin and rhizocarpic acid (Hidalgo et al., 2002). However, the dissociation constants of these compounds were determined in different solvents, limiting comparisons of the results (Bordwell, 1988; Jover et al., 2008). Therefore, pKa1 values of usnic and rhizocarpic acids as well as parietin were re-determined in the present study despite the results of Sharma and Jannke (1966), Wang et al. (2001) and Hildalgo et al. (2002).
The present method for the determination of dissociation constants is most similar to that of Sharma and Jannke (1966) for usnic acid. The difference in the dissociation constants determined in the present study (pKa1=4·0) and by Sharma and Jannke (1966) (pKa1=4·4) can be attributed to the different solvents, as Sharma and Jannke (1966) used a mixture of chloroform and methanol, whilst pure methanol was the solvent in the present study. The similarity of these results for usnic acid also supports the validity of the present method.
The dissociation constants for rhizocarpic acid differ more between the present study (pKa1=4·5) and Hidalgo et al. (2002) (pKa1=5·1). However, the experimental conditions also differed more strongly than in the comparison made for usnic acid. Hidalgo et al. (2002) used Brij 35 as a solvent, which is chemically quite different from methanol. This has also to be kept in mind when the dissociation constants determined in the present study for compounds of the pulvinic acid group are compared with the pKa of 4·9 published by Hidalgo et al. (2002) for calycin, a pulvinic acid derivative not included in the present study. The present result for parietin (pKa1=3·9) is very different from that of Wang et al. (2001), who published a pKa1 of 8·5 in acetonitrile. This difference for parietin is likely to be a solvent effect, as pKa values in acetonitrile are generally considerably higher than in methanol, because the former is an aprotic solvent, whereas the protic nature of the latter promotes the dissociation of dissolved molecules (Jover et al., 2008). Furthermore, the higher Lewis basicity of methanol (donor number 19·0 kcal mol−1) than of acetonitrile (14·1 kcal mol−1) supports dissociation (Arnaud-Neu et al., 2003).
Relations between pKa1 values of lichen substances and ambient pH values of lichens producing these lichen substances
Hauck and Jürgens (2008) suggested that the acidity tolerance of usnic acid-containing lichens is limited by the ability of protonated usnic acid to shuttle protons into the cytoplasm. The ability of usnic acid to pass phospholipid membranes and thereby to shuttle protons had already been experimentally proven by Abo-Khatwa et al. (1996) and Bačkor et al. (1997). Abo-Khatwa et al. (1996) also found protonophoric properties in the pulvinic acid derivative vulpinic acid, which was also treated in the present study, and in the depside atranorin. The protonophoric properties of lichen substances apparently limit the tolerance of lichens to the acidity of precipitation or leachates from the substratum, as increasing acidity in the environment leads (in the small water-soluble fraction of the lichen substance) to a shift of the equilibrium between the protonated lichen substance and its conjugated base towards the former. If a large proportion of the water-soluble lichen substance is charged with protons in the apoplasts, substances capable of crossing the membrane are assumed to cause an acidification of the cytoplasm and thereby the death of the cells (Hauck and Jürgens, 2008). Support for this assumption was found in the case of usnic acid, as Hauck and Jürgens (2008) established a relationship between the pKa1 value of usnic acid and the pH preferences of lichens producing usnic acid. Moreover, lichens with their natural content of usnic acid were found to be less tolerant of acidic solution with SO2 than lichens where the usnic acid was extracted at the beginning of the experiment (Hauck and Jürgens, 2008). These results explain why usnic acid-containing lichens decline at an environmental pH <3·5 (Hauck and Jürgens, 2008), where the protonated form of water-soluble usnic acid prevails over the usneate ion.
Given the finding of Abo-Khatwa et al. (1996) that protonophoric properties are not limited to usnic acid, but are perhaps a common character of lichen substances, the pKa1 value of lichen substances becomes ecologically significant, as it determines the lower limit of the environmental pH range a lichen is able to tolerate. Studies of usnic acid suggest that lichens can tolerate ambient pH values slightly below the pKa1 values of their lichen substances. This would mean that all lichens with usnic acid, parietin or the studied compounds of the pulvinic acid group are generally able to grow in acidic environments corresponding to pKa1 values between 2·8 and 4·5 (Table 2). Inferred from the pKa1 values, the tolerance of lichens producing yellow or orange lichen substances to acidic solutions may increase in the order rhizocarpic acid<usnic acid<parietin<vulpinic acid<leprapinic acid<pinastric and pulvinic acids.
Metal complexation by lichen substances
The results of the UV spectroscopy suggest that complex formation of lichen substances with metals is common. This contrasts with a relatively small number of previously published reports on the formation of metal–lichen substance complexes. Crystallographic data that give direct evidence of the formation and molecular structure of such complexes are only available for a few substances (Alagna et al., 1990; Ribár et al., 1993; Takani et al., 2002). One of them is a complex of Cu2+ and an usnic acid derivative showing that each Cu2+ ion is co-ordinated with two molecules of usnic acid (Takani et al., 2002). Purvis et al. (1987, 1990) provided indirect evidence of the formation of complexes of Cu2+ with the depsidones norstictic and psoromic acids. Spectroscopic data suggesting the formation of metal–lichen substance complexes have been published repeatedly (e.g. Syers 1969; Iskandar and Syers 1972). One of these studies dealt with a complex of Fe3+ and parietin (Engstrom et al., 1980). Arakawa et al. (2001) demonstrated the formation of complexes of the anthraquinone chrysophanol with Al3+, Ni2+ and Zn2+.
Prior to this study, the pH dependence of complex formation between metals and lichen substances has only been investigated in a single UV spectroscopic experiment. This was conducted with Cu2+ and usnic acid and showed the same sigmoid increase of absorbance at pH >3·5 as in the present study despite the application of different solvents (methanol versus chloroform) (Takani et al., 2002). The pH dependence is attributable to the release of 1 mol protons per 1 mol Cu2+ during the formation of the complex (Takani et al., 2002).
Significance of metal binding by lichen substances for metal homeostasis
Based on their spectroscopic detection of the Fe3+–parietin complex, Engstrom et al. (1980) were the first to suggest a potential role of lichen substances in the metal homeostasis of lichens; however, they did not conduct any experiments to test this hypothesis. Such a function of lichen substances was later substantiated in a case study with Hypogymnia physodes (Hauck, 2008b). The lichen substances produced by H. physodes selectively reduced the intracellular uptake of Cu2+ and Mn2+ (Hauck, 2008b), which, at high concentrations, are known to limit the abundance of this lichen in the field (Hauck, 2003; Hauck and Paul, 2005). The uptake of Fe2+ and Zn2+, which usually do not occur in high limiting concentrations at the sites of H. physodes, was not influenced by the content of lichen substances (Hauck, 2008b). Experiments with isolated lichen substances suggested that depsidone physodalic acid is the most effective metabolite at modifying metal uptake among the seven lichen substances known from H. physodes (Hauck and Huneck, 2007a).
The mechanisms by which lichen substances affect metal uptake in lichens are not yet known. Engstrom et al. (1980) hypothesized that lipophilic Fe3+–parietin complexes could cross phospholipid membranes and could, thus, support the uptake of Fe3+. Purvis et al. (1987, 1990) found that complexes of the depsidones norstictic and psoromic acids with Cu2+ remained in the apoplast and apparently served in the immobilization of Cu2+ in lichen thalli from cupriferous substrata.
The pH dependence of metal binding by lichen substances found in the present study and by Takani et al. (2002) suggests that lichen substances may promote metal uptake by temporary intracellular binding at a pH range with modest affinity of the metal to the lichen substance. This hypothesis is supported by the fact that the ambient optimum pH of 4·0–4·5 for usnic acid-containing lichens (Hauck and Jürgens, 2008) is in a range where important nutrients, including Cu2+, Fe2+, Fe3+, Mg2+ and Mn2+, have a medium–high affinity to usnic acid (Fig. 4). The spectroscopic data suggest that complete binding of metal ions to lichen substances at high pH values could hamper intracellular cation uptake. This is surely an advantage at sites with high metal concentrations (Purvis et al., 1987, 1990), but could lead to deficiencies at sites with low nutrient availability. Rapid binding of nutrients is an important issue for lichens that acquire most of their mineral nutrients during phases with precipitation or when the substratum is wet. Carboxylic and hydroxylic groups in the cell wall or in exopolysaccharide matrices are known to function as cation exchange sites in the apoplast of lichens (Brown and Brown, 1991; Paul et al., 2003), from which delayed uptake into the cytoplasm occurs (Hauck et al., 2006). Lichen substances could be an additional means of enabling temporary extracellular metal binding for subsequent intracellular uptake.
Assuming that pH ranges with complete binding of metal ions to lichen substances are unsuited for lichens producing these lichen substances at sites with normal metal concentrations, the spectroscopic data suggest that usnic acid hampers the uptake of Fe2+/3+ and Mg2+ at pH >5, the uptake of Cu2+ and Zn2+ at pH >7 and the uptake of Mn2+ at pH >8 (Fig. 4). In lichens producing parietin (Fig. 5) or with most compounds of the pulvinic acid group (Fig. 6), a reduction of intracellular metal uptake is only to be expected in the alkaline range at pH >11, which is not ecologically relevant.
The pulvinic acid derivative rhizocarpic acid differs from all other lichen substances studied in having two pH ranges with increasing absorbance values, suggesting the formation of two different types of complexes (Fig. 7). This could imply that rhizocarpic acid facilitates the uptake of these metals at these two different pH ranges. Alternatively, strong binding of metal ions at the acidic binding site could rule out the occurrence of species with rhizocarpic acid at medium or high pH. The preference of rhizocarpic acid-producing lichens for nutrient-poor, acidic sites (Purvis et al., 1992; Wirth, 1995) suggests that the latter alternative is the case.
Potential role of yellow lichen substances at determining site preferences of lichens
The dissociation and metal-binding behaviour of yellow and orange lichen substances can be used for a hypothetical model to explain the ability of lichens with such pigments to colonize substrata of different pH or metal availability. Lichens with usnic acid are excluded from sites with pH <3·5, as the protonophoric activity of usnic acid is increased, if the pH falls significantly below the pKa1 value of 4·0 (measured in methanol; Hauck and Jürgens, 2008). At pH >3·5, lichens could benefit from the production of usnic acid, as the uptake of Mg2+ and transition metals could be promoted by temporary binding. The high affinity to metal ions at pH >5 would explain the upper pH limit of usnic acid-containing lichens (Hauck and Jürgens, 2008). Only a few species of lichenized ascomycetes with usnic acid occur on alkaline substrata, such as the saxicolous Lecanora muralis as well as the terricolous Cladonia convoluta or Vulpicida tilesii and V. tubulosus (Purvis et al., 1992; Mattsson, 1993; Wirth, 1995). However, the occurrence of these species at high ambient pH values does not contradict the assumption that metal-binding by usnic acid determines the upper pH limit of lichens, because (a) the mentioned terricolous lichens are only loosely attached to the soil and can, therefore, be assumed to take up most of their nutrients from the atmosphere, and (b) these lichens contain other lichen substances with intermediate affinity to metal ions under slightly alkaline conditions, including pinastric and vulpinic acids in V. tilesii and V. tubulosus (Fig. 6) and fumarprotocetraric acid in C. convoluta (M. Hauck et al., unpublished UV spectroscopic data). Lecanora muralis primarily contains usnic acid in the apothecia, which have no physical contact to the substratum, and produces less usnic acid on highly alkaline mortar than on more acidic sedimentary rock (Huneck et al., 2004). Furthermore, usnic acid is only one of several lichen substances in L. muralis (Huneck et al., 1979, 2004), which may influence metal uptake into this species.
The pKa1 of 3·9 of parietin together with the metal-binding at high pH values (Fig. 5) may explain the wide pH range of substrata that are inhabited by parietin-containing lichens (Wirth, 1995). The largest genera of lichenized fungi producing parietin include the Teloschistaceae Caloplaca, Teloschistes and Xanthoria s.l. In Caloplaca, species of both acidic and alkaline substrata are found (Purvis et al. 1992; Wirth, 1995; Brodo et al., 2001). Furthermore, Xanthoria elegans colonizes acidic rock and limestone (Purvis et al., 1992). The spectroscopic results show that parietin is not able to support the uptake of metals at low pH (Fig. 5). This suggests that parietin-containing lichens of acidic substrata may be restricted to nutrient-rich sites because a support of metal uptake is not necessary in these conditions, whereas lichens with usnic acid prefer nutrient-poor sites. On calcareous soils and rock, the capability of parietin to complex metal ions at high pH can be expected to facilitate the uptake of micronutrients and Mg2+ under conditions of poor availability of these metals, a problem that generally excludes calcifuge plants from calcareous sites (Tyler and Ström, 1995).
Similar to parietin, substances of the pulvinic acid group putatively enable lichens to colonize a wide range of substrata. Among the compounds of the pulvinic acid group studied, the physico-chemical properties of leprapinic, pinastric, pulvinic and vulpinic acids differ significantly from those of rhizocarpic acid. The low pKa1 values of leprapinic, pinastric, pulvinic and vulpinic acids between 2·8 and 3·4 allow lichens containing these pigments to grow on very acidic substrata, such as conifer bark as well as nutrient-poor, acid siliceous rock and sandy soils. Metal adsorption of these acids is limited to the alkaline range. This suggests that lichens with leprapinic, pinastric, pulvinic or vulpinic acids are generally able to colonize sites of a wide pH range. Indeed, lichens with these pigments include marked acidophytes (Calicium, Chaenotheca, Chrysothrix, Letharia and Vulpicida) as well as species of calcareous rock (Candelariella aurella and C. medians) (Purvis et al., 1992; Wirth, 1995; Brodo et al., 2001). In the genus Candelariella, both species from acidic and calcareous substrates are known. As with the parietin-containing lichens, the acidophytes among the species of Candelariella (e.g. Candelariella coralliza, C. kuusamoensis, C. vitellina) are confined to nutrient-rich sites (Wirth, 1995). Vulpicida produces usnic acid in addition to pulvinic acid derivatives, the former being capable of binding metals under the acidic conditions preferred by most species of the genus (Mattsson, 1993). The species of Calicium, Chaenotheca and Chrysothrix with pulvinic acid lack usnic acid. However, these species apparently have extremely low nutrient requirements, as they are very sensitive to eutrophication (Wirth, 1995).
CONCLUSIONS
The pKa1 value of lichen substances has already been shown to determine the lower limit of the ambient pH range preferred by species of lichen-forming fungi in a case study with usnic acid (Hauck and Jürgens, 2008). The present results suggest that the dissociation behaviour of yellow or orange pigments generally allows all lichens producing these metabolites to grow on acidic substrata, at least as long as they are not acidified by atmospheric deposits. The different metal-binding characteristics of the various pigments can be used to explain different site preferences of the species of lichen-forming fungi producing these pigments. The high affinity of metal ions to usnic acid under alkaline conditions could explain why most usnic acid-containing lichens are calcifuge. At moderately acidic sites, lichens may benefit from temporary binding of nutrients to usnic acid. Such benefit is not possible for parietin and most compounds of the pulvinic acid group studied, as metal binding fails to occur at low pH. On the one hand, this could explain the preference of many lichens with these pigments for nutrient-rich sites at low pH, whereas lichens with usnic acid generally prefer nutrient-poor sites. On the other hand, metal-binding at low pH would probably cause nutrient deficiency for lichens at high pH. This may explain the occurrence of lichens with parietin, pulvinic acid and some related substances, but not of lichens with usnic acid or rhizocarpic acid on neutral or alkaline substrata, including limestone.
While a limitation of the acidity tolerance by usnic acid and the control of metal homoeostasis by lichen substances have been experimentally proven (Hauck, 2008b; Hauck and Jürgens, 2008), other aspects related to the present conclusions deserve further study to detect the mechanisms behind the correlations demonstrated in here. The hypothesis that temporary binding of metals to extracellular lichen substances facilitates metal uptake, but permanent binding of metals to lichen substances at high pH impairs intracellular metal uptake has still to be substantiated by experimental work involving lichen thalli in addition to experiments based on isolated lichen substances.
ACKNOWLEDGEMENTS
The study has been supported by a grant of the Deutsche Forschungsgemeinschaft to M. Hauck (Ha 3152/8-1). We are thankful to Dr H. J. M. Sipman (Botanical Museum, Berlin-Dahlem) for his help with supplying us with samples of isolated lichen substances from the collections of S. Huneck and G. B. Feige. Dr E. Timdal (Botanical Museum, Oslo) is thanked for providing the photograph in Fig. 1C.
LITERATURE CITED
- Abo-Khatwa AN, Al-Robai AA, Al-Jawhari DA. Lichen acids as uncouplers of oxidative phosporylation of mouse-liver mitochondria. Natural Toxins. 1996;4:96–102. doi: 10.1002/19960402nt7. [DOI] [PubMed] [Google Scholar]
- Alagna L, Prosperi T, Tomlinson AAG, Kjøsen H, Mo F. An EXAFS, and preliminary X-ray crystallographic, investigation of an iron-containing product from the lichen Cladonia deformis. Biochimica et Biophysica Acta. 1990;1036:71–77. doi: 10.1016/0304-4165(90)90215-i. [DOI] [PubMed] [Google Scholar]
- Arakawa R, Sasao A, Abura T, Suzuki T, Fujitake N. Studies of complex formation between anthraquinones and metal ions by electrospray ionization mass spectrometry. European Journal of Mass Spectrometry. 2001;7:467–471. [Google Scholar]
- Arnauld-Neu F, Delgado R, Chaves AS. Critical evaluation of stability constants and thermodynamic functions of metal complexes of crown ethers. Pure and Applied Chemistry. 2003;75:71–102. [Google Scholar]
- Bačkor M, Gaburjáková J, Hudák J, Ziegler W. The biological role of secondary metabolites from lichens. 1. The influence of usnic acid on bimolecular lipid membranes. Acta Facultatis Rerum Naturalium Universitatis Comenianae. 1997;29:67–71. [Google Scholar]
- Bjerke JW, Dahl T. Distribution patterns of usnic acid-producing lichens along local radiation gradients in West Greenland. Nova Hedwigia. 2002;75:487–506. [Google Scholar]
- Bordwell FG. Equilibrium acidities in dimethyl sulfoxide solution. Accounts of Chemical Research. 1988;21:456–463. [Google Scholar]
- Brodo IM, Sharnoff SD, Sharnoff S. Lichens of North America. New Haven, CT: Yale University Press; 2001. [Google Scholar]
- Brown DH, Brown RM. Mineral cycling and lichens: the physiological basis. Lichenologist. 1991;23:293–307. [Google Scholar]
- Cocchietto M, Skert N, Nimis PL, Sava G. A review on usnic acid, an interesting natural compound. Naturwissenschaften. 2002;89:137–146. doi: 10.1007/s00114-002-0305-3. [DOI] [PubMed] [Google Scholar]
- Engstrom GW, McDorman DJ, Maroney MJ. Iron chelating capability of physcion, a yellow pigment from Aspergillus ruber. Journal of Agricultural and Food Chemistry. 1980;28:1139–1141. [Google Scholar]
- Gargas A, DePriest PT, Grube M, Tehler A. Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny. Science. 1995;268:1492–1495. doi: 10.1126/science.7770775. [DOI] [PubMed] [Google Scholar]
- Hafellner J. A world monograph of Brigantiaea (lichenized Ascomycotina, Lecanorales) Symbolae Botanicae Upsalienses. 1997;32(1):35–74. [Google Scholar]
- Hauck M. Epiphytic lichen diversity and forest dieback: the role of chemical site factors. Bryologist. 2003;106:257–269. [Google Scholar]
- Hauck M. Susceptibility to acidic precipitation contributes to the decline of the terricolous lichens Cetraria aculeata and Cetraria islandica in central Europe. Environmental Pollution. 2008;152:731–735. doi: 10.1016/j.envpol.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Hauck M. Metal homeostasis in Hypogymnia physodes is controlled by lichen substances. Environmental Pollution. 2008;b 153:304–308. doi: 10.1016/j.envpol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Hauck M, Huneck S. Lichen substances affect metal adsorption in Hypogymnia physodes. Journal of Chemical Ecology. 2007;a 33:219–223. doi: 10.1007/s10886-006-9225-6. [DOI] [PubMed] [Google Scholar]
- Hauck M, Huneck S. The putative role of fumarprotocetraric acid in the manganese tolerance of the lichen Lecanora conizaeoides. Lichenologist. 2007;b 39:301–304. [Google Scholar]
- Hauck M, Jürgens S-R. Usnic acid controls the acidity tolerance of lichens. Environmental Pollution. 2008;156:115–122. doi: 10.1016/j.envpol.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Hauck M, Paul A. Manganese as a site factor for epiphytic lichens. Lichenologist. 2005;37:409–423. [Google Scholar]
- Hauck M, Paul A, Spribille T. Uptake and toxicity of manganese in epiphytic cyanolichens. Environmental and Experimental Botany. 2006;56:216–224. [Google Scholar]
- Hauck M, Dulamsuren Ch, Mühlenberg M. Lichen diversity on steppe slopes in the northern Mongolian mountain taiga and its dependence on microclimate. Flora. 2007;a 202:530–546. [Google Scholar]
- Hauck M, Huneck S, Elix JA, Paul A. Does secondary chemistry enable lichens to grow on iron-rich substrates? Flora. 2007;b 202:471–478. [Google Scholar]
- Hidalgo ME, Fernández E, Ponce M, Rubio C, Quilhot W. Photophysical, photochemical, and thermodynamic properties of shikimic acid derivatives: calycin and rhizocarpic acid (lichens) Journal of Photochemistry and Photobiology B: Biology. 2002;66:213–217. doi: 10.1016/s1011-1344(02)00264-6. [DOI] [PubMed] [Google Scholar]
- Hidalgo ME, Bascunan L, Quilhot W, Fernández E, Rubio C. Spectroscopic and photochemical properties of the lichen compound lobaric acid. Photochemistry and Photobiology. 2005;81:1447–1449. doi: 10.1562/2005-05-17-RA-530. [DOI] [PubMed] [Google Scholar]
- Huneck S. The significance of lichens and their metabolites. Naturwissenschaften. 1999;86:559–570. doi: 10.1007/s001140050676. [DOI] [PubMed] [Google Scholar]
- Huneck S, Yoshimura I. Identification of lichen substances. Berlin: Springer; 1996. [Google Scholar]
- Huneck S, Schreiber K, Höfle G, Snatzke G. Neodihydromurol- und Murolsäure, zwei neue γ-Lactoncarbonsäuren aus Lecanora muralis. Journal of the Hattori Botanical Laboratory. 1979;45:1–23. [Google Scholar]
- Huneck S, Lumbsch HT, Porzel A, Schmidt J. Die Verteilung von Flechteninhaltsstoffen in Lecanora muralis und Lecidea inops und die Abhängigkeit der Usninsäure-Konzentration vom Substrat und von den Jahreszeiten bei Lecanora muralis. Bibliotheca Lichenologica. 2004;88:211–221. [Google Scholar]
- Ingólfsdóttir K. Usnic acid. Phytochemistry. 2002;61:729–736. doi: 10.1016/s0031-9422(02)00383-7. [DOI] [PubMed] [Google Scholar]
- Iskandar IK, Syers JK. Metal-complex formation by lichen compounds. Journal of Soil Science. 1972;23:255–265. [Google Scholar]
- Jankulovska M, Spirevska I, Šoprajanova L. Determination of the dissociation constants of some newly synthesized derivatives of 1,2,4-triazoline-3-thione in sodium hydroxide media. Bulletin of the Chemists and Technologists of Macedonia. 2006;25:99–106. [Google Scholar]
- Johansson S, Søchting U, Elix JA, Wardlaw JH. Chemical variation in the lichen genus Letrouitia (Ascomycota, Letrouitiaceae) Mycological Progress. 2005;4:139–148. [Google Scholar]
- Jover J, Bosque R, Sales J. QSPR prediction of pKa for benzoic acids in different solvents. QSAR and Combinatorial Sciences. 2008;27:563–581. [Google Scholar]
- Lutzoni F, Pagel M, Reeb V. Major fungal lineages are derived from lichen symbiotic ancestors. Nature. 2001;411:937–940. doi: 10.1038/35082053. [DOI] [PubMed] [Google Scholar]
- Mattsson J-E. A monograph of the genus Vulpicida (Parmeliaceae, Ascomycetes) Opera Botanica. 1993;119:1–61. [Google Scholar]
- Nybakken L, Solhaug KA, Bilger W, Gauslaa Y. The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats. Oecologia. 2004;140:211–216. doi: 10.1007/s00442-004-1583-6. [DOI] [PubMed] [Google Scholar]
- Paul A, Hauck M, Fritz E. Effects of manganese on element distribution and structure in thalli of the epiphytic lichens Hypogymnia physodes and Lecanora conizaeoides. Environmental and Experimental Botany. 2003;50:113–124. [Google Scholar]
- Pearson RG, Dillon RL. Rates of ionization of pseudo acids. IV. Relation between rates and equilibria. Journal of the American Chemical Society. 1953;75:2439–2443. [Google Scholar]
- Purvis OW, Elix JA, Broomhead JA, Jones GC. The occurrence of copper-norstictic acid in lichens from cupriferous substrata. Lichenologist. 1987;19:193–203. [Google Scholar]
- Purvis OW, Elix JA, Gaul KL. The occurrence of copper-psoromic acid in lichens from cupriferous substrata. Lichenologist. 1990;22:345–354. [Google Scholar]
- Purvis OW, Coppins BJ, Hawksworth DL, James PW, Moore DM, editors. The lichen flora of Great Britain and Ireland. London: Natural History Museum; 1992. [Google Scholar]
- Ribár B, Kapor A, Argay G, Engel P, Djarmati Z, Jankov RM. Crystal structure of usnic acid sodium salt 2 ½ hydrate. Journal of Crystallographic and Spectroscopic Research. 1993;23:107–111. [Google Scholar]
- Rikkinen J. What's behind the pretty colours? A study on the photobiology of lichens. Bryobrothera. 1995;4:1–239. [Google Scholar]
- Sharma RK, Jannke PJ. Acidity of usnic acid. Indian Journal of Chemistry. 1966;4:16–18. [Google Scholar]
- Solhaug KA, Gauslaa Y. Parietin, a photoprotective secondary product of the lichen Xanthoria parietina. Oecologia. 1996;108:412–418. doi: 10.1007/BF00333715. [DOI] [PubMed] [Google Scholar]
- Stangret J. Donor properties of water in organic solvents derived from infrared spectra of HDO. Journal of Molecular Structure. 2002;643:29–35. [Google Scholar]
- Syers JK. Chelating ability of fumarprotocetraric acid and Parmelia conspersa. Plant and Soil. 1969;31:205–208. [Google Scholar]
- Takani M, Yajima T, Masuda H, Yamauchi O. Spectroscopic and structural characterization of copper(II) and palladium(II) complexes of a lichen substance usnic acid and its derivatives: possible forms of environmental metals retained in lichens. Journal of Inorganic Biochemistry. 2002;91:139–150. doi: 10.1016/s0162-0134(02)00439-7. [DOI] [PubMed] [Google Scholar]
- Tyler G, Ström L. Differing organic acid exudation pattern explains calcifuge and acidifuge behaviour of plants. Annals of Botany. 1995;75:75–78. doi: 10.1016/S0305-7364(05)80011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Yang G, Song X. Determination of pKa values of anthraquinone compounds by capillary electrophoresis. Electrophoresis. 2001;22:464–469. doi: 10.1002/1522-2683(200102)22:3<464::AID-ELPS464>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Wirth V. Die Flechten Baden-Württembergs. 2nd edn. Stuttgart: Ulmer; 1995. [Google Scholar]