Abstract
Background and Aims
Exogenous ethylene has recently gained commercial interest as a sprouting inhibitor of onion bulbs. The role of ethylene in dormancy and sprouting of onions, however, is not known.
Methods
A cultivar (Allium cepa ‘Copra’) with a true period of dormancy was used. Dormant and sprouting states of onion bulbs were treated with supposedly saturating doses of ethylene or with the ethylene-action inhibitor 1-methylcyclopropene (1-MCP). Initial sprouting was determined during storage at 18 °C by monitoring leaf blade elongation in a specific size class of leaf sheaths. Changes in ATP content and sucrose synthase activity in the sprout leaves, indicators of the sprouting state, were determined. CO2 and ethylene production of onion bulbs during storage were recorded.
Key results
Exogenous ethylene suppressed sprout growth of both dormant and already sprouting onion bulbs by inhibiting leaf blade elongation. In contrast to this growth-inhibiting effect, ethylene stimulated CO2 production by the bulbs about 2-fold. The duration of dormancy was not significantly affected by exogenous ethylene. However, treatment of dormant bulbs with 1-MCP caused premature sprouting.
Conclusions
Exogenous ethylene proved to be a powerful inhibitor of sprout growth in onion bulbs. The dormancy breaking effect of 1-MCP indicates a regulatory role of endogenous ethylene in onion bulb dormancy.
Key words: Bulb dormancy, Allium cepa, onion, sprout growth, ethylene, CO2 production, respiration, 1-methylcyclopropene
INTRODUCTION
Sprouting limits the storability of onion (Allium cepa) bulbs. At harvest, onion bulbs are usually dormant. Depending on genotype and storage conditions sprout growth is initiated after a certain period of storage (Komochi, 1990). Hormonal control involving a gradual increase of the ratio of sprouting promoters to inhibitors may underlie the loss of dormancy with time (Gubb and MacTavish, 2002). However, the specific roles of different hormones, and especially of ethylene, in the regulation of dormancy and sprouting of onion bulbs is not known. Application of the ethylene-releasing agent ethephon to dry onions generally enhanced sprouting (Abdel-Rahman and Isenberg, 1974; Miedema and Kamminga, 1994; Benkeblia and Selselett-Attou, 1999). In contrast, application of ethephon during bulb development in the field apparently reduced sprouting during storage (Thomas and Rankin, 1982). On the other hand, treatment of dry bulbs with the ethylene-action inhibitor 1-methylcyclopropene (1-MCP) reduced sprout growth in bulbs stored at 4 °C or 12 °C, but not when stored at 20 °C (Chope et al., 2007). Notwithstanding these conflicting scientific reports it has been shown in commercial onion stores that continuous application of ethylene retards sprout growth during cold storage (Johnson, 2006).
Progress in onion dormancy research has been hampered, at least in part, by inadequate experimental methodology and using inappropriate genotypes to study dormancy and sprout growth. Since dormancy of onion genotypes after harvest can vary between none and several weeks it is imperative to select a genotype with a true period of dormancy (Yasin and Bufler, 2007). Moreover, dormancy release in a population of onion bulbs, even from a single harvest, is not uniform and can stretch over several weeks (Yasin and Bufler, 2007). During bulb growth and development sprout leaf initials are formed at the apex of the compressed stem, differentiating into small sprout leaves which encircle the growing point and enclose younger leaves within (Brewster, 1994). These sprout leaves consist of a proximal leaf sheath and a distal leaf blade, separated by a pore (Fig. 1A) through which the next youngermost sprout leaf will ultimately emerge. When sprouting is initiated the leaf blade elongates but not the leaf sheath (De Mason, 1990); elongation of the leaf sheath may be delayed by several weeks or even months, depending on storage conditions (G. Bufler, unpubl. res.). Thus, by monitoring the length of the leaf blade of a specific size class of leaf sheaths initial sprout growth can be detected and tracked during the course of an experiment (Yasin and Bufler, 2007). Lang et al. (1987) proposed that dormancy was defined by the non-growth of leaf blades. Using this approach, it has been shown that extension of leaf blades in bulbs of Allium cepa ‘Copra’ was initiated about 8 weeks after harvest and was simultaneous with an increase in respiratory and sink activity in sprout leaves (Yasin and Bufler, 2007).
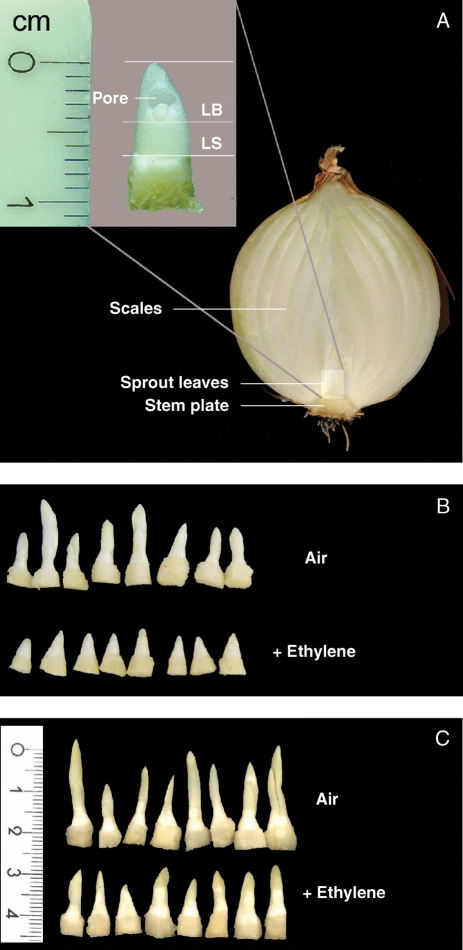
Fig. 1.
Photographs and digital scans of sprout leaves isolated from onion bulbs after different treatments. (A) Longitudinal cut through a dormant onion bulb showing the stem plate, with the approximate location of sprout leaves. Insert: dormant bud with sprout leaf visible in the size class (leaf sheath length 2·0–3·5 mm) used in this study. LS, Leaf sheath; LB, leaf blade. (B) Sprout leaves (leaf sheaths 2·0–3·5 mm) isolated from onion bulbs stored at 18 °C and continuously treated with air or 7·2 ± 1·4 µL L−1 ethylene until 14 weeks after harvest. Ethylene treatment was started during bulb dormancy 2 weeks after harvest. (C) Sprout leaves (leaf sheaths 2·0–3·5 mm) isolated from sprouting onion bulbs after 4 weeks continuous treatment at 18 °C with air or 7·2 ± 1·4 µL L−1 ethylene. Ethylene treatment was started 12 weeks after harvest.
The present study was undertaken to clarify the role of ethylene in onion bulb dormancy and sprout elongation using the precise methods indicated above for tracking release from dormancy and initial sprout elongation. The effects of both exogenous ethylene and 1-MCP were investigated.
MATERIALS AND METHODS
Plant material
Bulbs of Allium cepa L. ‘Copra’ were grown from seed or transplants at the Experimental Station of Horticulture, University of Hohenheim. Common agricultural practices of fertilization and plant disease control were adopted. Bulbs were harvested when 70–80 % of the plants had collapsed foliage. They were subsequently dried for 2 weeks in shallow trays in a ventilated and temperature-controlled room at 25 ± 2 °C. After drying the foliage was cut off and the bulbs stored at 18 ± 2 °C.
Treatment with ethylene during dormancy
This experiment was carried out in autumn 2006 (Fig. 2) and repeated with minor modifications in autumn 2007 (Fig. 3), producing similar results. Two weeks after harvest onion bulbs were divided between eight 60-L plastic barrels connected to a gas flow-through system. Flow rates (between 10 and 20 L h−1) were adjusted manually during the course of the experiment using needle valves to keep the CO2 concentration in each of the containers below 0·5 %. The barrels were in a temperature-controlled room (18 ± 2 °C). Four barrels were ventilated with air and the other four containers were ventilated with 7·2 ± 1·4 µL L−1 ethylene in 2006 and 10·6 ± 1·4 µL L−1 ethylene in 2007. Most known ethylene effects are saturated between 5 and 10 µL L−1 (Abeles et al., 1992). The slight fluctuation in the ethylene concentration in each season was due to the ethylene source during the treatment period; however, the actual ethylene concentration was identical in each of the four barrels. Ethylene was supplied from a compressed nitrogen/ethylene gas mixture subsequently mixed with air. The ethylene concentration in the gas lines entering and leaving the storage barrels was analysed in 1-mL gas samples at weekly intervals by gas chromatography using a Shimadzu GC-6A (Duisburg, Germany) equipped with a flame ionization detector, activated Al2O3 in a 1·4-m stainless steel column, and nitrogen as the carrier gas. Ethylene concentration in the air supply was below detection (ethylene detection limit approx. 0·005 µL L−1). Similarly, the CO2 production of the onion bulbs was determined at weekly intervals by analysing 1-mL gas samples using gas chromatography using a Shimadzu GC-3BT equipped with a thermal conductivity detector, activated charcoal in a 0·7-m stainless-steel column, and helium as the carrier gas. Relative humidity inside the storage room and storage barrels was kept below approx. 80 % and 90 %, respectively.
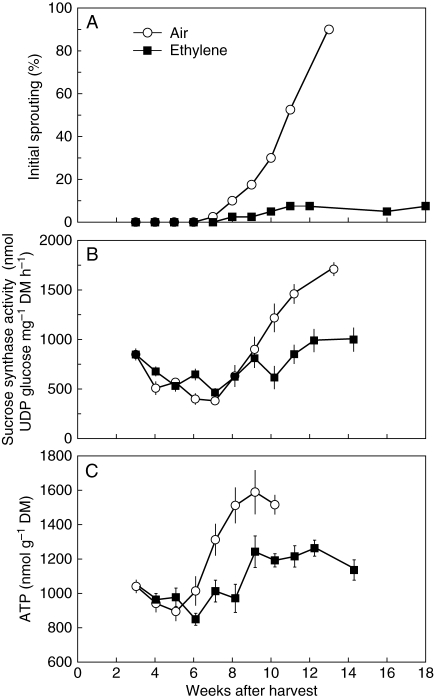
Fig. 2.
Changes in (A) percentage initial sprouting, (B) sucrose synthase activity and (C) ATP content in sprout leaves of onion bulbs during storage at 18 °C in air or 7·2 ± 1·4 µL L−1 ethylene. Each data point represents the mean of 40 bulbs (A; n = 40) or ten sprout leaves (B, C; n =10). Vertical bars indicate ± s.e.
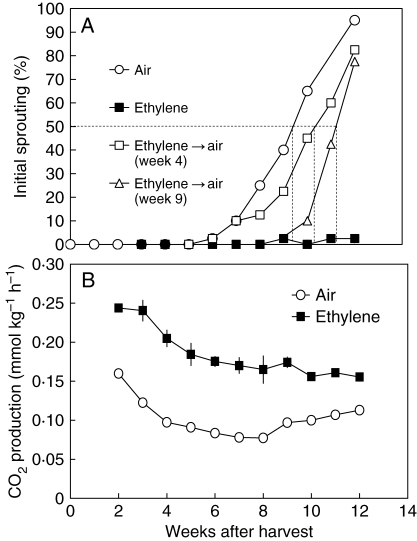
Fig. 3.
Changes in (A) percentage initial sprouting and (B) CO2 production of onion bulbs during storage at 18 °C in air or 10·6 ± 1·4 µL L−1 ethylene. Batches of bulbs were transferred at 4 weeks or 9 weeks after harvest from ‘ethylene’ into ‘air’. Dashed lines indicate time of 50 % initial sprouting. Each data point in (A) represents the mean of 40 bulbs (n = 40); each data point in (B) represents the mean of four storage barrels (n = 4). Vertical bars indicate ± s.e. In the case of the air treatment error bars are too small to be visible.
Depending on the experiment, at certain time points, batches of bulbs were transferred from air to ethylene treatment and vice versa.
Treatment with ethylene during sprouting
This experiment was carried out only in autumn 2006. Sprouting bulbs, previously stored for 12 weeks at 18 ± 2 °C were placed at the same temperature in 60-L plastic barrels and connected to the flow-through system as described above. Measurement of sprout leaf elongation and CO2 and ethylene concentration was carried out at weekly intervals as described above.
Treatment with 1-MCP
This experiment was carried out in autumn 2004 following a preliminary experiment in 2003 which had similar results. There were two dates of treatment in 2004, 2 and 4 weeks after harvest. Since there was no significant difference between the two dates of treatment only data from the second date of treatment (2004) are presented. Four weeks after harvest, onion bulbs were placed at 20 °C in four sealed 50-L plastic containers. The weight of bound 1-MCP (SmartFresh® powder; a.i. 0·14 %) required to obtain 0·25 µL L−1 in a 50-L container was placed in a 10-mL glass syringe and sealed with a septum. After injecting 0·8 mL of warm water and dissolving thoroughly, the entire contents of a syringe (gas and liquid) were injected into each of the four 50-L plastic containers. Control bulbs were enclosed in another four 50-L plastic containers, but without receiving any further treatments. After 5 h incubation onion bulbs from each treatment were removed from the containers and stored in plastic bins at 18 ± 2 °C.
Bulb sampling
At weekly intervals, 40 bulbs were sampled from each treatment (ten bulbs from each of four 60-L plastic containers). After dissecting the centre of the bulbs, excising the appropriate size of sprout leaves and measuring leaf blade length (see below), sprout leaves still attached to a small part of the stem plate (smaller than can be seen in Fig. 1A) were individually frozen in liquid nitrogen, lyophilized and stored at –30 °C until use.
Determination of initial sprouting
Initial sprout growth was determined as previously described (Yasin and Bufler, 2007). When isolating appropriate sprout leaves for freezing in liquid nitrogen outer sprout leaves with leaf sheaths >3·5 mm in length were excised and discarded; only sprout leaves with leaf sheaths between 2·0 mm and 3·5 mm in length (Fig. 1A) were used to monitor leaf blade length using calipers. At harvest (70–80 % foliage collapsed) bulbs were assumed to be dormant. A one-tailed confidence interval (n = 40; P = 0·05) of the mean leaf blade length from 40 randomly chosen bulbs at harvest or 1 week after harvest was used to define the exclusion limit for dormant bulbs; i.e. bulbs at subsequent sampling dates containing sprout leaves (size class of leaf sheaths 2·0–3·5 mm) with leaf blade lengths exceeding the calculated exclusion limit were denoted as ‘initially sprouting’ and hence used to calculate percentage initial sprouting. Exclusion limits in 2004, 2006 and 2007 were determined as 4·3 mm, 5·3 mm and 4·8 mm, respectively.
Determination of sucrose synthase activity
Sucrose synthase activity (E.C.2·4·13) was extracted and determined as described previously (Yasin and Bufler, 2007). Lyophilized sprout leaves of individual bulbs were powdered in liquid nitrogen and extracted on ice (100 µL mg−1 tissue; 50 mm Hepes–KOH, 5 mm MgCl2·6H2O, 1 mm EDTA, 2·5 mm DTT, 0·05 % TritonX-100, pH 7·5). After centrifugation, an aliquot of the supernatant was dialysed in a micro-dialyser capsule equipped with a Zellutrans dialysis membrane (MWCO 12 000–14 000; Carl Roth GmbH, Karlsruhe, Germany) against buffer (50 mm Hepes–KOH, 5 mm MgCl2·6H2O, 2·5 mm DTT, pH 7·5). Sucrose synthase activity in the extract was determined in the cleaving direction as described by Pak et al. (1995). Assay conditions have been optimized to yield maximum enzyme activities. Sucrose synthase activity was expressed on a dry-matter basis as nmol UDP-glucose mg dry matter−1 h−1. Sucrose synthase activities represent mean values of sprout leaves isolated from ten individual bulbs (n = 10).
Extraction and determination of ATP and ADP
Adenine nucleotides were extracted on ice from powdered lyophilized sprout leaves (10–20 mg) using 5 % (w/v) trichloroacetic acid containing 2 mm EDTA (Yasin and Bufler, 2007). ATP contents were analysed by a luminometric method using an ATP monitoring kit (BioThema AB, Handen, Sweden) based on the firefly luciferase reaction. ATP contents represent mean values of sprout leaves isolated from ten individual bulbs (n = 10).
Statistical analysis
Standard error of means (s.e.) are based on measurements of ten individual bulbs (n = 10) in the case of sucrose synthase activity and ATP content, and on four storage barrels (n = 4) in the case of CO2 production rate. Percentage initial sprouting is derived from the calculation of confidence limits (n = 40, P = 0·05) of the blade length of dormant bulbs as described above. When appropriate, treatment means of blade lengths (n = 40) were subjected to Student–Newman–Keuls test following ANOVA.
RESULTS
Ethylene treatment
Initial sprouting of previously dormant onion bulbs stored in air started about 7 weeks after harvest and reached almost 100 % 13 weeks after harvest (Fig. 2A). In contrast, 92·5 % of dormant onion bulbs continuously treated with 7·2 µL L−1 ethylene did not sprout up to 18 weeks after harvest when the experiment was discontinued (Figs 1B and 2A). Sucrose synthase activity in sprout leaves of air-stored bulbs increased simultaneously to the initiation of sprout growth (Fig. 2B), indicating increased sink activity in this bulb part (Pak et al., 1995; Yasin and Bufler, 2007). Increase of ATP content, another indicator of initiated sprout growth (Yasin and Bufler, 2007), also occurred in the sprout leaves of air-stored bulbs when sprouting was initiated (Fig. 2C). Continuous ethylene treatment, however, largely reduced these increases in sucrose synthase activity and ATP content in sprout leaves (Fig. 2B, C).
The sprouting-inhibiting effect of ethylene disappeared when ethylene was removed, regardless of the time of removal (Fig. 3A). If ethylene treatment was short (2 weeks) and supplied during dormancy (between 2 and 4 weeks after harvest), sprout growth was initiated at about the same time as for air-treated bulbs, though the time to reach 50 % sprouting bulbs was increased by about 1 week (Fig. 3A). If ethylene treatment was extended to 9 weeks after harvest and then stopped, initial sprout growth was detectable 10 weeks after harvest (Fig. 3A). In contrast to its inhibiting effect on sprouting, ethylene significantly stimulated CO2 production; 10·6 µL L−1 ethylene approximately doubled the CO2 production rate of dormant onion bulbs, although this effect diminished during later stages of the treatment (Fig. 3B). If ethylene treatment of onion bulbs was started 12 weeks after harvest when sprouting was fully initiated it also inhibited sprout growth significantly (Table 1 and Fig. 1C) and stimulated CO2 production, both in a reversible manner (data not shown). Ethylene-enhanced CO2 production is a common phenomenon in vegetative tissue (Abeles et al., 1992).
Table 1.
Blade lengths of sprout leaves isolated from sprouting bulbs after various times of treatment, starting 12 weeks after harvest, with ethylene (7·2 ± 1·4 µL L−1) or air at 18 °C (leaf sheath length 2·0–3·5 mm)
| Blade length (mm) |
||
|---|---|---|
| Days of treatment | Air | Ethylene |
| 0 | 7·8a | – |
| 14 | 10·3b | 8·2a |
| 28 | (10·8 b)* | 8·1a |
Each value represents the mean of 40 bulbs (n = 40).
Numbers followed by the same letter are not significantly different (P = 0·05; Student–Newman–Keuls test).
*Exact monitoring of leaf blade length at a defined leaf sheath length becomes invalid at this stage of sprout development in air because leaf sheath elongation has started (see Materials and Methods). This is indicated by an apparently stagnating blade length at a fixed sheath length even though sprout leaf growth is in progress.
1-MCP treatment
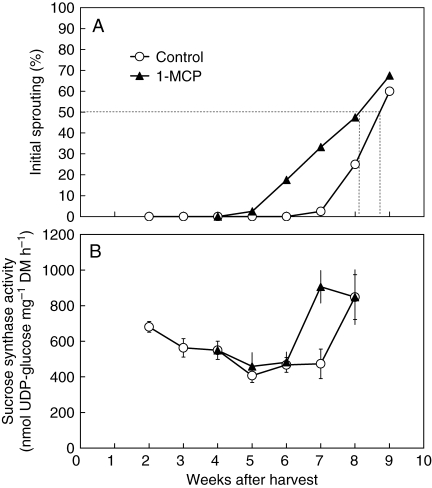
Onion bulbs produce very low amounts of ethylene (air-stored bulbs in these experiments: between 0·002 and 0·005 µL kg−1 h−1). To check a possible role of endogenous ethylene in onion bulb dormancy, onion bulbs were treated with the ethylene action inhibitor 1-MCP (Sisler and Serek, 1997). Treatment of dormant bulbs 4 weeks after harvest with 1-MCP caused premature sprouting; initial sprouting of untreated control bulbs started 7 weeks after harvest, whereas in bulbs treated with 1-MCP sprout growth was inititated 5 weeks after harvest (Fig. 4A). This hastening effect of 1-MCP on sprout growth initiation corresponded to a premature increase of sucrose synthase activity 6 weeks after harvest (Fig. 4B). However, the proportion of bulbs sprouting increased more slowly in the 1-MCP treatment than in the control; bulbs treated with 1-MCP reached 50 % initial sprouting 3·2 weeks after the start of sprouting (week 5) in contrast to 1·7 weeks for control bulbs (week 7; Fig. 4A).
Fig. 4.
Changes in (A) percentage initial sprouting of onion bulbs and (B) sucrose synthase activity in sprout leaves after treatment with 1-MCP or untreated (control). Treatment with 1-MCP was 4 weeks after harvest. Dashed lines indicate 50 % initial sprouting. Each data point represents the mean of 40 bulbs (A; n = 40) or ten sprout leaves (B; n =10). Vertical bars indicate ± s.e.
DISCUSSION
These results indicate that ethylene has at least two effects on onion bulbs after harvest: (1) it inhibits sprout elongation and (2) it interferes with dormancy. While the two effects are experimentally entangled it is important to note that each is related to an entirely different physiological process.
Exogenous ethylene inhibited sprout growth of onion bulbs when supplied during sprouting (Table 1) and when supplied during dormancy (Figs 2A and 3A). It is suggested that in both cases ethylene action was primarily on sprout leaf elongation and not on dormancy as could be supposed in the latter case. This view is supported by the observation that after stopping ethylene ventilation during dormancy (week 4) the increase in percentage of sprouting bulbs occurred at the same time as in air-stored bulbs, i.e. at the end of natural dormancy (Fig. 3A). Thus, it seems that the presence of ethylene prevented sprout growth after the bulbs were released naturally from dormancy. Translated into the terminology of Lang et al. (1987) this means that in the course of continuous ethylene treatment endogenous bulb dormancy was superseded by ethylene-induced eco-dormancy. As soon as the restraint imposed by exogenous ethylene was removed sprout growth could ensue.
Ethylene is known to inhibit growth of stems, leaves and roots (Abeles et al., 1992). For example, inhibition of potato sprouting by continuous application of ethylene has long been known (Rylski et al., 1974) and has found commercial application (Prange et al., 1998). The nature of growth inhibition of onion sprout leaves by ethylene is not known, except that ethylene inhibits leaf blade elongation (Fig. 1B, C). Recently, it has been demonstrated that growth of dark-grown arabidopsis seedlings is inhibited by ethylene concentrations as low as 0·2 nL L−1 (Binder et al., 2004) and its implications for ethylene signalling were discussed (Chen et al., 2005). Ethylene production of onion bulbs during dormancy and initial sprout growth was very low (between 0·003 and 0·005 µL kg−1 h−1) but is reported to increase during storage (Abdel-Rahman and Isenberg, 1974). Whether endogenous ethylene is involved in the control of sprout growth during later stages of sprout development, e.g. when sprout leaves press through the tightly closed neck of the bulb, would be an interesting aspect to investigate.
1-MCP has been a powerful tool to demonstrate ethylene effects in various stages of plant development (Huber, 2008). Treatment of dormant onion bulbs with 1-MCP caused breaking of dormancy 2 weeks before natural dormancy release was detectable (Fig. 4A). However, the dormancy-breaking effect of 1-MCP was not very strong, as indicated by the relatively slow increase in percentage of sprouting bulbs compared with untreated bulbs. Possibly only some of the bulbs responded to 1-MCP owing to the non-uniform sprouting behaviour of onion bulbs. On the other hand, a 2-week exposure of dormant onion bulbs to 10·6 µL L−1 exogenous ethylene did not affect the duration of dormancy compared with air-treated bulbs (Fig. 3A). It seems, therefore, that relatively low concentrations of endogenous ethylene may be somehow involved in the regulation of dormancy but relatively high concentrations of exogenous ethylene are not. If, however, the ethylene treatment was extended to 9 weeks after harvest, the time to reach 50 % initial sprouting was shorter (2 weeks versus 3 weeks) than for the air treatment (Fig. 3A). In this case all or most of the bulbs may have exited endogenous dormancy 9 weeks after harvest, only prevented from sprout leaf elongation by the presence of ethylene. Like onion bulbs, potato tubers are low producers of ethylene. In developing potato microtubers continuous treatment with inhibitors of ethylene action also resulted in premature sprouting, suggesting a critical role of endogenous ethylene in tuber dormancy (Suttle, 1998). A 3-d exposure of potato tubers to 2 µL L−1 ethylene, however, reduced the length of dormancy significantly (Rylski et al., 1974). From these and other data it was concluded that, depending on the concentration and duration of exposure, exogenous ethylene can either hasten or delay tuber sprouting (Rylski et al., 1974; Suttle, 2004). At present a similar conclusion cannot be drawn for onion bulb dormancy.
Although there may be some similarities in the dormancy physiology of onion bulbs and potato tubers, onion bulb dormancy is much less investigated. Nonetheless, the results in this study clearly indicate that exogenous ethylene suppresses sprout growth of onion bulbs by inhibiting leaf blade elongation. The role of ethylene in dormancy control, however, is far from clear. Endogenous ethylene may be involved in maintenance of bulb dormancy as suggested by the dormancy breaking effect of 1-MCP. It seems likely, however, that ethylene may be one factor in a complex interaction of growth substances still to be identified.
ACKNOWLEDGEMENTS
I am greatly indebted to Dr Jim Brewster for improving the English version of the manuscript. I thank Mrs Christiane Beierle for excellent technical assistance and Dr Josef Streif, KOB Bavendorf, for technical advice. This work was supported by the Fachverband Deutsche Speisezwiebel e.V.
LITERATURE CITED
- Abdel-Rahman M, Isenberg FMR. The role of exogenous plant regulators in the dormancy of onion bulbs. The Journal of Agricultural Science. 1974;82:113–116. [Google Scholar]
- Abeles FB, Morgan PW, Saltveit ME., Jr . Ethylene in plant biology. 2nd edn. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Benkeblia N, Selselett-Attou G. Role of ethylene on sprouting of onion bulbs (Allium cepa L) Acta Agriculturae Scandinavica, Section B, Soil and Plant Science. 1999;49:122–124. [Google Scholar]
- Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB. Short-term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiology. 2004;136:2921–2927. doi: 10.1104/pp.104.050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL. Onions and other vegetable alliums. Wallingford: CAB International; 1994. [Google Scholar]
- Chen Y-F, Etheridge N, Schaller GE. Ethylene signal transduction. Annals of Botany. 2005;95:901–915. doi: 10.1093/aob/mci100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chope GA, Terry LA, White PJ. The effect of 1-methylcyclopropene (1-MCP) on the physical and biochemical characteristics of onion cv. SS1 bulbs during storage. Postharvest Biology and Technology. 2007;44:131–140. [Google Scholar]
- De Mason DA. Morphology and anatomy of Allium. In: Rabinowitch HD, Brewster JL, editors. Onions and allied crops. Vol. 1. Boca Raton, FL: CRC Press; 1990. pp. 27–51. [Google Scholar]
- Gubb IR, MacTavish HS. Onion pre- and postharvest considerations. In: Rabinowitch HD, Currah L, editors. Allium crop science: recent advances. Wallingford: CABI Publishing; 2002. pp. 233–265. [Google Scholar]
- Huber DJ. Suppression of ethylene responses through application of 1-methylcyclopropene: a powerful tool for elucidating ripening and senescence mechanisms in climacteric and nonclimacteric fruits and vegetables. HortScience. 2008;43:106–111. [Google Scholar]
- Johnson J. Onion storage revolution? The Vegetable Farmer. 2006;2:25–26. [Google Scholar]
- Komochi S. Bulb dormancy and storage physiology. In: Rabinowitch HD, Brewster JL, editors. Onions and allied crops. Vol. 1. Boca Raton, FL: CRC Press; 1990. pp. 89–111. [Google Scholar]
- Lang GA, Early JD, Martin GC, Darnell RL. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience. 1987;22:371–377. [Google Scholar]
- Miedema P, Kamminga GC. Bulb dormancy in onion. II. The role of cytokinins in high-temperature imposed sprout inhibition. Journal of Horticultural Science. 1994;69:41–45. [Google Scholar]
- Pak C, van der Plas L, Douwe de Boer A. Importance of dormancy and sink strength in sprouting of onions (Allium cepa) during storage. Physiologia Plantarum. 1995;94:277–283. [Google Scholar]
- Prange RK, Kalt W, Daniels-Lake BJ, Liew CL, Page RT, Walsh JR, et al. Using ethylene as a sprout control agent in stored ‘Russet Burbank’ potatoes. Journal of the American Society for Horticultural Science. 1998;123:463–469. [Google Scholar]
- Rylski I, Rappaport L, Pratt HK. Dual effects of ethylene on potato dormancy and sprout growth. Plant Physiology. 1974;53:658–662. doi: 10.1104/pp.53.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiologia Plantarum. 1997;100:577–582. [Google Scholar]
- Suttle JC. Involvement of ethylene in potato microtuber dormancy. Plant Physiology. 1998;118:843–848. doi: 10.1104/pp.118.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle JC. Physiological regulation of potato tuber dormancy. American Journal of Potato Research. 2004;81:253–262. [Google Scholar]
- Thomas TH, Rankin WEF. Effect of ethephon on bulbing, bull-necking, yield and sprouting during storage of two onion cultivars (Allium cepa L.) Journal of Horticultural Science. 1982;57:465–467. [Google Scholar]
- Yasin HJ, Bufler G. Dormancy and sprouting in onion (Allium cepa L.) bulbs. I. Changes in carbohydrate metabolism. Journal of Horticultural Science & Biotechnology. 2007;82:89–96. [Google Scholar]