Abstract
Background and Aims
The grass Alloteropsis semialata is the only plant species with both C3 and C4 subspecies. It therefore offers excellent potential as a model system for investigating the genetics, physiology and ecological significance of the C4 photosynthetic pathway. Here, a molecular phylogeny of the genus Alloteropsis is constructed to: (a) confirm the close relationship between the C3 and C4 subspecies of A. semialata; and (b) infer evolutionary relationships between species within the Alloteropsis genus.
Methods
The chloroplast gene ndhF was sequenced from 12 individuals, representing both subspecies of A. semialata and all four of the other species in the genus. ndhF sequences were added to those previously sequenced from the Panicoideae, and used to construct a phylogenetic tree.
Key Results
The phylogeny confirms that the two subspecies of A. semialata are among the most recently diverging lineages of C3 and C4 taxa currently recognized within the Panicoideae. Furthermore, the position of the C3 subspecies of A. semialata within the Alloteropsis genus is consistent with the hypothesis that its physiology represents a reversion from C4 photosynthesis. The data point to a similar evolutionary event in the Panicum stenodes–P. caricoides–P. mertensii clade. The Alloteropsis genus is monophyletic and occurs in a clade with remarkable diversity of photosynthetic biochemistry and leaf anatomy.
Conclusions
These results confirm the utility of A. semialata as a model system for investigating C3 and C4 physiology, and provide molecular data that are consistent with reversions from C4 to C3 photosynthesis in two separate clades. It is suggested that further phylogenetic and functional investigations of the Alloteropsis genus and closely related taxa are likely to shed new light on the mechanisms and intermediate stages underlying photosynthetic pathway evolution.
Key words: Alloteropsis semialata, Panicoideae; Poaceae, ndhF, C4 photosynthesis, C3 photosynthesis, photosynthetic pathway evolution, molecular phylogeny
INTRODUCTION
Despite the multitude of changes to leaf anatomy and biochemistry required for the transition from C3 to C4 photosynthesis, this evolutionary event is estimated to have occurred independently in >48 plant lineages (Sage, 2004). Such a remarkable example of convergent evolution suggests either the action of very strong selective pressures, or that genetic mechanisms underlying the C3 to C4 transition are less complex than currently thought. Investigation of these selective pressures and genetic mechanisms requires comparison of closely related C3 and C4 plants in order to minimize confounding effects of different genetic backgrounds. C3 and C4 eudicot species have been used for this purpose (e.g. Brown et al., 2005), but studies are also required in the grasses, half of whose species use the C4 pathway (Sage et al., 1999). Grasses have great ecological and commercial importance, covering a fifth of the vegetated land surface (Matthews, 1983) and providing more than half of digestible energy in human diets, as well as pasture for livestock (Evans, 1993). However, closely related C3 and C4 grasses are rare because the ancient origins of C4 photosynthesis within the Poaceae mean that substantial divergence has occurred between C3 and C4 lineages (Giussani et al., 2001; Grass Phylogeny Working Group, 2001; Christin et al., 2008). Alloteropsis semialata is unique in having both C3 and C4 subspecies, and therefore could provide a novel model system for comparative studies of C3 and C4 grasses.
Gibbs-Russell (1983) designated the C3 and C4 variants of A. semialata as subspecies rather than separate species because herbarium specimens included apparent intermediates between the C3 and C4 forms and therefore suggested there was gene flow between them. However, recent molecular phylogenies have shown some grass genera to be polyphyletic (e.g. Panicum; Duvall et al., 2001; Aliscioni et al., 2003), indicating that species assignment based on classical taxonomy can be misleading. The taxonomic relationship between the C3 and C4 subspecies of A. semialata therefore requires corroboration from molecular studies. In total, the Alloteropsis genus consists of five species and belongs to the grass subfamily Panicoideae (Clayton and Renvoize, 1986). This subfamily has considerable commercial importance, since its species include key food crops, such as maize (Zea mays) and sugarcane (Saccharum sp.), as well as biofuel crops such as switchgrass (Panicum virgatum) and Miscanthus (Clayton and Renvoize, 1986). Furthermore, Panicoideae is an intriguing group for studying photosynthetic pathway evolution since it has C3 and all three sub-types of C4 photosynthesis represented among its species (Hattersley and Watson, 1992). A recent phylogeny indicates that the genus Alloteropsis occurs within a clade consisting of both C3 and C4 species (Christin et al., 2008). Therefore, establishing relationships among this group and the five Alloteropsis species could illuminate the evolutionary routes leading to particular photosynthetic pathways.
Here, the aim was to: (a) confirm the close relationship between the C3 and C4 subspecies of A. semialata; and (b) infer evolutionary relationships between species within the Alloteropsis genus. A molecular phylogeny was constructed using the chloroplast locus ndhF, chosen because it has previously been sequenced for >150 Panicoideae species, and shows sufficiently high rates of molecular evolution to allow resolution between grass taxa at the species level (Clark et al., 1995; Giussani et al., 2001; Aliscioni et al., 2003).
MATERIALS AND METHODS
DNA preparation
The specimens used for sequencing are detailed in the Supplementary Information (Table S1, available online). For DNA extraction from living specimens or from silica-dried leaf material, leaves were ground in liquid nitrogen, and genomic DNA was purified using the Plant DNAzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Whole genomic DNA from herbarium specimens was obtained from the DNA-Bank at the Royal Botanic Gardens, Kew, UK.
The ndhF gene was amplified via PCR using a Taq-mediated protocol (BioTaq, Bioline, London). Primer sequences were specified by Olmstead and Sweere (1994) and Aliscioni et al. (2003), or optimized for the Alloteropsis genus specifically for this project (Supplementary Information Table S2, available online). For DNA extracted from live specimens, ndhF was amplified in two overlapping fragments, 1F/1318R and 972F/2110R or 1143F/2110R. DNA from the herbarium specimens was degraded, so ndhF in these cases was amplified in smaller, overlapping fragments, 1F/536R, 216F/757R, 536F/972R, 757F/1318R, 1143F/1660R and 1606F/2110R. PCR was performed in 30 or 50 µL reactions in a DNA engine tetrad thermocycler (MJ Research, Cambridge, MA). PCR products were separated from the reaction mixture by band cutting after electrophoresis on a 1·5 % agarose gel and purification using Wizard SV gel and a PCR clean-up system (Promega, Madison, WI). PCR products were then quantified by comparison with DNA ladders of known size and concentration (GeneRuler 1 kb or 100 bp DNA ladder, Fermentas, Vilnius, Lithuania).
PCR products were sequenced in both directions using fluorescent dye terminators (ABI PRISM BigDye Terminator v1.1 Cycle Sequencing Kit, Applied Biosystems, Foster City, CA) in 10 µL reactions following conditions recommended by the manufacturer. For the short (<600 bp) PCR products derived from herbarium specimens, sequencing primers used were the same as the PCR primers. For longer fragments, internal sequencing primers were also included. Extension products were precipitated using the manufacturer's ethanol/EDTA/sodium acetate-mediated protocol, suspended in 10 µL of Hi-Di formamide (Applied Biosystems) and electrophoresed on an ABI 3730 capillary sequencer (Applied Biosystems). Forward and reverse sequences were manually edited and checked using Bioedit v.7.0.5.2. (Hall, 1999), and overlapping fragments were assembled into the full ndhF sequence.
Phylogenetic analysis
For preliminary analysis, the 12 sequences obtained from Alloteropsis specimens were aligned manually with 137 of the 156 ndhF sequences used by Aliscioni et al. (2003). Taxa used by Aliscioni et al. (2003) were excluded if information on their photosynthetic pathway was unavailable, or if the available ndhF sequences were incomplete (Panicum piausense, P. pedersenii and Tatianyx arnacites). A full list of all taxa used, together with the accession numbers of ndhF sequences and information on the photosynthetic pathway, is provided (Supplementary Information Table S3, available online). Gaps were treated as missing data in all analyses. Alternative evolutionary models for the resulting 149 taxa matrix were compared using ModelTest 3·7 (Posada and Crandall, 1998), which showed that the transversional nucleotide substitution model with gamma distribution of variation among sites and fixed proportion of invariant sites (TVM I + G) was optimal. Distances were calculated under this model with the neighbour-joining (NJ) method (Saitou and Nei, 1987), as implemented in PAUP v.4.0 b10 (Swofford, 2002), and NJ bootstrapping was then conducted using 1000 replicates. Thysanolaena maxima, Danthoniopsis dinteri, Zeugites pittieri and Chasmanthium laxum were chosen as outgroup taxa, following Aliscioni et al. (2003).
The preliminary NJ tree confirmed the position of Alloteropsis within the well-supported x = 9 clade of the Panicoideae and also that multiple accessions of an individual Alloteropsis species or subspecies were monophyletic. Therefore, Bayesian inference (BI) and maximum parsimony (MP) trees were constructed using only taxa from the x = 9 clade. To avoid taxonomic bias in further analysis of the x = 9 clade, a single specimen of each Alloteropsis species or subspecies was chosen. For A. cimicina and A. papillosa, one sequence of each was chosen at random: A. cimicina specimen A and A. papillosa specimen B (Supplementary Information Table S1). For A. semialata subspecies, sequences were chosen to represent the accessions used for other experiments (Ripley et al., 2007, 2008; Ibrahim et al., 2008; Osborne et al., 2008), and therefore A. semialata subsp. semialata specimen A and A. semialata subsp. eckloniana specimen B (Supplementary Information Table S1) were used, although selection of alternative sequences did not change tree topology (data not shown). Based on the NJ analysis, four species were chosen as outgroup taxa for analysis of the x = 9 clade: Zea mays, Sorghum bicolour, Panicum pilosum and Paspalum vaginatum.
For the BI tree, evolutionary models were selected using MrModelTest 2·2 (Nylander, 2004), which showed that the best model was the general time-reversible nucleotide substitution model with gamma distribution of variation among sites and fixed proportion of invariant sites (GTR I + G). Outgroup taxa were chosen as above. This model was implemented in MrBayes v.3.1 (Huelsenbeck and Ronquist, 2001) using 1 million generations, four chains, two runs, and trees sampled every 100 generations. The temperature parameter for chain heating was set to 0·05 to improve the acceptance rate for swapping between chains, and other parameters used default settings. The first 2500 trees from each run were discarded and the remainder combined to form a consensus tree.
The MP tree used the same data set as the BI tree. A parsimony ratchet (Nixon, 1999) as implemented in PAUPrat (Sikes and Lewis, 2001) with 15 replicates of 200 iterations, and 15 % of characters re-weighted at each iteration, was used to find the optimal tree in PAUP* v.4.0 b10. A total of 2905 of the 3000 resulting trees were shortest and of equal length, and were combined to form a strict consensus tree. Bootstrapping was then performed using 1000 replicates with 1000 random addition sequence replicates per bootstrap replicate and tree bisection–reconnection (TBR) branch swapping. The number of rearrangements per addition sequence replicate in bootstrapping was limited to 106 to save computing time.
RESULTS
Alloteropsis sequences
In total, ndhF sequences ranging in length from 2070 to 2084 bp were obtained from 12 Alloteropsis specimens (Supplementary Information Table S1). The sequence for A. paniculata excluded 21 bp between nucleotide positions 520 and 541 where sequencing failed. Sequences were identical in the three accessions of A. cimicina, and did not differ at more than two nucleotide positions between accessions within A. papillosa, A. semialata subsp. semialata or A. semialata subsp. eckloniana. In addition, the ndhF sequence for A. semialata subsp. semialata specimen A was completely identical to that reported for A. semialata by Christin et al. (2008).
Preliminary NJ tree
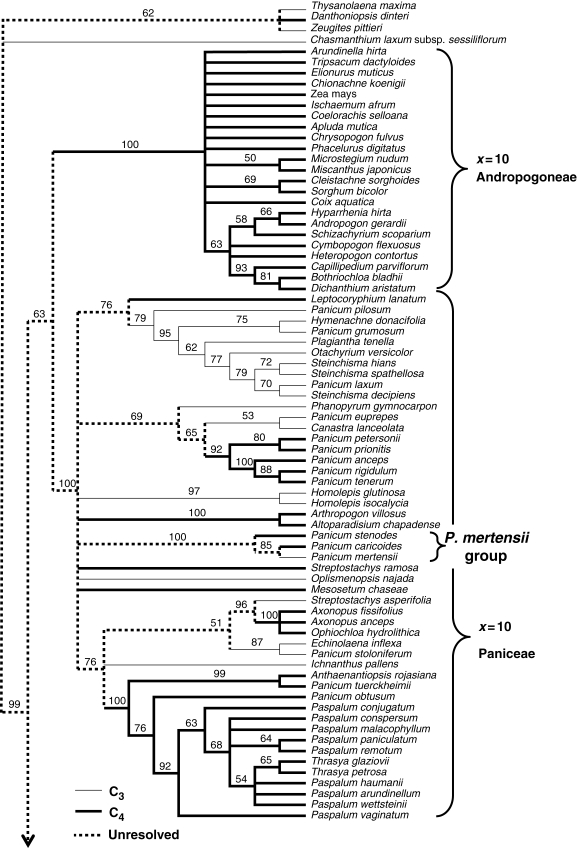
For preliminary analysis, an NJ tree was constructed using ndhF sequences from all Alloteropsis specimens and 137 other Panicoideae species (Fig. 1). The data matrix contained 149 taxa and 2073 characters. The topology of the NJ tree is congruent with the ndhF tree resolved by Aliscioni et al. (2003), and the combined ndhF and rbcL tree resolved by Christin et al. (2008; Fig. 1). The three main clades, Andropogoneae, x = 10 Paniceae and x = 9 Paniceae, are therefore well supported (Fig. 1). Alloteropsis has a basal chromosome number of nine (Liebenberg and Fossey, 2001) and, as expected, is resolved within the x = 9 Paniceae clade (Fig. 1). Additionally, replicate specimens for each Alloteropsis taxon emerged as sister taxa on the NJ tree (Fig. 1). Further analysis was therefore conducted using one specimen of each Alloteropsis taxon together with other species resolved in the x = 9 clade.
Fig. 1.
Neighbour–joining cladogram for ndhF sequences in the Panicoideae. Percentage bootstrap values (1000 replicates) are shown above branches when they are >50 %. Major groupings of species are indicated. C3 taxa are shown with narrow lines, C4 taxa with bold lines, and the unresolved photosynthetic type of ancestral lineages with dotted lines. The figure continues on the next page.
Multiple transitions between C3 and C4 photosynthesis are apparent across the NJ tree and the small, well-supported clade of C3 and C4 Panicum species (P. mertensii, P. caricoides and P. stenodes), the P. mertensii group, is of particular interest (Fig. 1). This clade was also identified in the MP ndhF tree of Aliscioni et al. (2003); however, these authors did not describe the photosynthetic pathway of these species, which represent the most recently diverging examples of C3 and C4 Panicum species within the phylogeny (Fig. 1). Furthermore, placement of the C4 species P. stenodes basal to the clade suggests that P. mertensii may represent a reversion from C4 to C3 photosynthesis (Fig. 1).
The x = 9 Paniceae clade
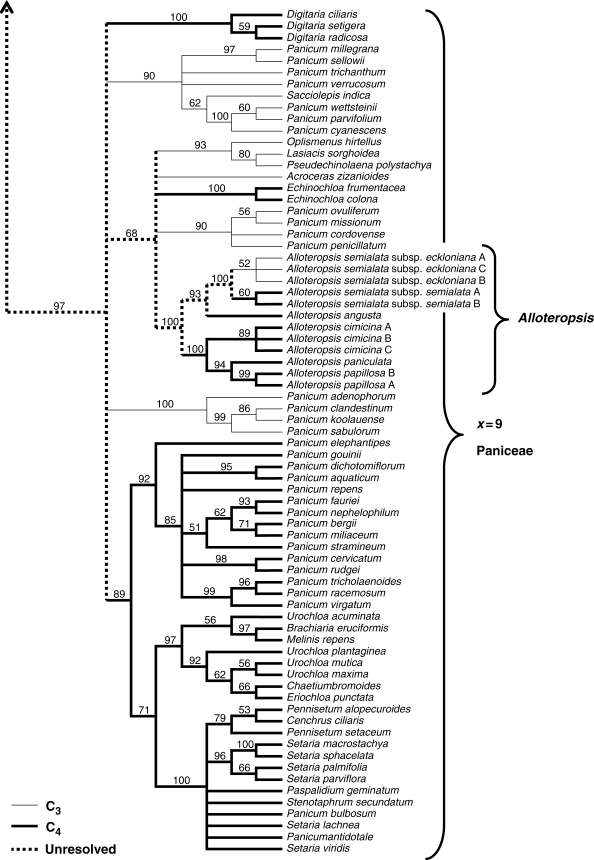
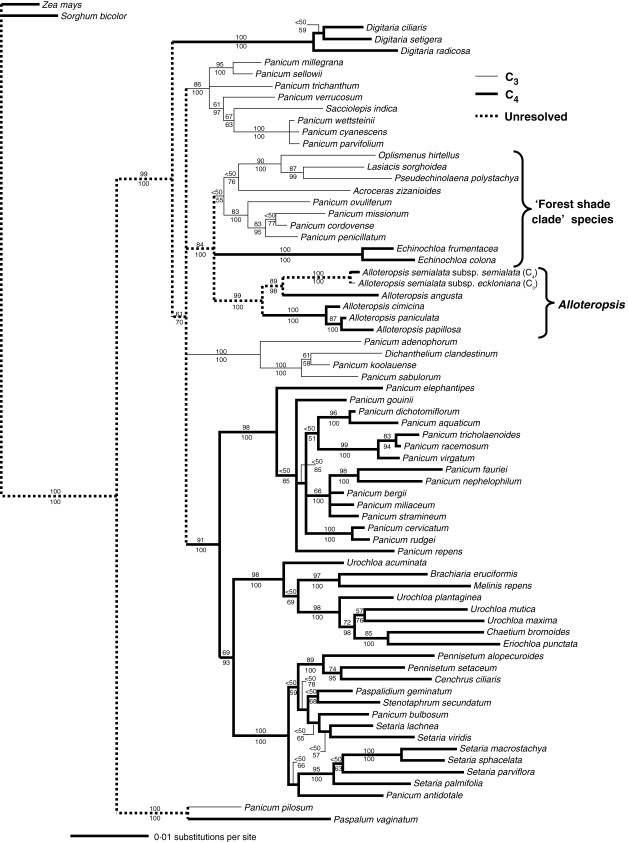
The BI and MP trees for the x = 9 Paniceae clade used 71 taxa and 2073 characters. The data set contained 1512 invariant characters and 306 parsimony-informative characters. The MP ratchet recovered 2905 trees all with a length of 803 steps, consistency index (CI) of 0·501 and retention index (RI) of 0·749 when uninformative characters were excluded. The topologies of BI and MP trees were identical, and the consensus BI tree shown in Fig. 2 therefore includes both posterior probabilities for clades and bootstrap values from the MP analysis.
Fig. 2.
Consensus Bayesian inference tree for the x = 9 clade of the Paniceae tribe. Values above and below branches indicate percentage bootstrap values from MP analysis and Bayesian posterior probabilities, respectively. C3 taxa are shown with narrow lines, C4 taxa with bold lines, and the unresolved photosynthetic type of ancestral lineages with dotted lines.
Tree topology in the x = 9 Paniceae is similar to the preliminary NJ tree, and shows that Alloteropsis forms a monophyletic group (Fig. 2). This clade forms a polytomy with two sister clades that were together designated the ‘forest shade clade’ by Giussani et al. (2001) (Fig. 2). The Alloteropsis–forest shade group itself is well supported, although relationships within the group and among major clades in the x = 9 Paniceae are poorly resolved (Fig. 2). Within the genus Alloteropsis, species are split among two sister groups, with A. semialata more closely related to A. angusta than the other three Alloteropsis species (Fig. 2). In addition, A. semialata is a monophyletic species with the C3 and C4 subspecies as sister taxa (Fig. 2). Furthermore, the branch length between the two subspecies of A. semialata is short, indicating that they have undergone recent divergence.
Photosynthetic pathway evolution in Alloteropsis
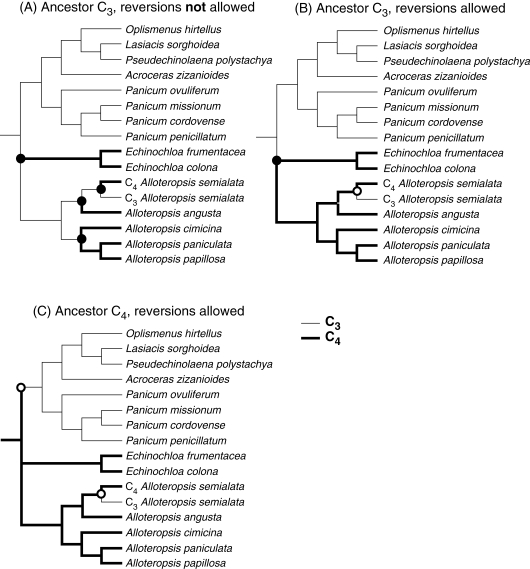
Poor resolution between clades in the x = 9 Paniceae means that the photosynthetic pathway in the common ancestor of the Alloteropsis–forest shade group is undetermined (Fig. 2). However, regardless of whether the common ancestor is C3 or C4, the most parsimonious explanation of photosynthetic pathway evolution in the Alloteropsis–forest shade group is that the C3 subspecies of A. semialata represents a reversion from C4 photosynthesis (Fig. 3). If the ancestor to the group was C3, and reversions from C4 photosynthesis have not occurred, then there must have been a minimum of four origins of C4 photosynthesis within the clade (Fig. 3A). In contrast, if reversions have taken place, then a single origin and one subsequent reversion from C4 photosynthesis could have occurred (Fig. 3B). If the common ancestor to the group was C4, then reversions of photosynthetic type must have happened, and at least two incidences of this would have occurred within the group (Fig. 3C). The hypothesis of reversions from C4 to C3 photosynthesis therefore requires two fewer transitions in the photosynthetic pathway to explain the ndhF data than the alternative hypothesis of multiple C4 origins. The combined ndhF and rbcL phylogeny of Christin et al. (2008) indicates that Echinochloa occurs as sister to Panicum ovuliferum, and therefore represents a unique origin of C4 photosynthesis. However, this relationship is only weakly supported (Christin et al., 2008) and, regardless of the position of Echinochloa, the most parsimonious explanation for photosynthetic pathway evolution within the Alloteropsis genus requires a C4 to C3 transition to account for C3 physiology in A. semialata subsp. eckloniana (Fig. 3).
Fig. 3.
Possible routes of photosynthetic pathway evolution in the Alloteropsis–forest shade group. The relationship between taxa is taken from Fig. 2. C3 taxa are shown with narrow lines, and C4 taxa with bold lines. Circles at nodes represent transitions in the photosynthetic pathway, where grey is a transition to C4 and white is a transition to C3.
DISCUSSION
Reversion from C4 to C3 photosynthesis
The ndhF phylogeny confirms that the two subspecies of A. semialata are among the most recently diverging lineages of C3 and C4 taxa currently recognized within the Panicoideae, and therefore that they can serve as a model system in which to compare gene expression, physiology and ecology of C3 and C4 grasses (Ripley et al., 2007, 2008; Ibrahim et al., 2008; Osborne et al., 2008). Our data are also consistent with the hypothesis that the C3 subspecies of A. semialata represents an evolutionary reversion from C4 photosynthesis, and highlight the possibility of an additional reversal in the P. stenodes–P. caricoides–P. mertensii clade. These interpretations of the molecular data are consistent with observations of atypical leaf anatomy in the C3 subspecies of A. semialata, where the inner bundle sheath cells contain a large number of chloroplasts, and mesophyll cells show a radiate arrangement around the vascular bundles (Ueno and Sentoku, 2006), traits that are usually associated with the C4 pathway. Radiate mesophyll cells also occur in P. mertensii (Renvoize, 1987). However, the relatively common occurrence of this anatomical arrangement among C3 Paniceae species (Renvoize, 1987) suggests that caution should be exercised in interpreting it as evidence for photosynthetic pathway evolution.
Species of the P. mertensii clade do not share clear distinctive morphological characters; however, their leaf structure is intriguing because the C3 species P. mertensii has lanceolate leaf laminae while its two C4 counterparts have more linear laminae with a tendency to roll inwards (Aliscioni et al., 2003). A similar difference in leaf morphology exists between the C3 and C4 subspecies of A. semialata (Gibbs-Russell, 1983), highlighting potential links between photosynthetic pathway evolution and changes to leaf anatomy and morphology.
Although the possibility of evolutionary reversions from C4 to C3 photosynthesis has been suggested for some time (Brown, 1977; Ellis, 1984; Hattersley and Watson, 1992; Pyankov et al., 2001), the phenomenon has been controversial when interpreting molecular phylogenies (e.g. Duvall et al., 2001; Giussani et al., 2001). The data suggest that reversal of the photosynthetic pathway may occur, highlighting extraordinary lability for such a complex character. This interpretation assumes that the origin and loss of C4 photosynthesis are equally likely. However, alternative scenarios are also possible: strong selection pressure towards gaining the pathway would reduce the probability of reversals; conversely, a complex character could be more likely to be lost than gained (e.g. Dollo parsimony). Such reversal of complex characters to ancestral states is receiving renewed interest in the wider field of evolutionary biology (Porter and Crandall, 2003; Whiting et al., 2003), and reversions from the C4 to C3 photosynthetic pathway within the Panicoideae could serve as a further important example of this phenomenon.
Evolution of the C4 photosynthetic pathway
The most striking feature of the Alloteropsis–forest shade group is its remarkable degree of diversity in photosynthetic metabolism. As well as the C3 and C4 taxa indicated in Fig. 2, multiple subtypes of C4 photosynthesis are present. Echinochloa frumentacea and E. colona are both of the NADP-malic enzyme (NADP-ME) subtype (Gutierrez et al., 1974), while A. semialata subsp. semialata is of the phosphoenolpyruvate carboxylase (PCK) type (Prendergast et al., 1987; Ueno and Sentoku, 2006). Intriguingly, even more diversity is suggested by intermediate biochemical features in E. frumentacea, which contains a significant amount of PCK enzyme despite being an NADP-ME-type C4 species (Voznesenskaya et al., 2006). Furthermore, A. semialata subsp. semialata exhibits higher NADP-ME activity than a ‘classic’ PCK species, Urochloa maxima (= Panicum maximum, Ueno and Sentoku, 2006). Photosynthetic biochemistry for other C4 Alloteropsis species has not been analysed but, based on leaf anatomy, A. papillosa, A. paniculata and A. cimicina are predicted to be NAD-ME type (Hattersley and Watson, 1992), while A. angusta is expected to be NADP-ME type (Ellis, 1977). However, the inference of biochemistry in A. angusta should be treated with caution since it shares similar leaf anatomy to A. semialata, which was also initially classified as NADP-ME on the basis of anatomical observations alone (Ellis, 1977).
The split of Alloteropsis into two sister groups in the ndhF phylogeny is consistent with their leaf anatomy, since the Kranz sheaths in both A. semialata and A. angusta are derived from the mestome sheath (XyMS– type anatomy; Hattersley and Watson, 1976), while in A. papillosa, A. paniculata and A. cimicina they are derived from the parenchyma sheath (XyMS+ type anatomy; Hattersley and Watson, 1976; Brown, 1977; Ellis, 1977, 1981). These differences in leaf anatomy correspond to the segregation of Alloteropsis into two species complexes, an ‘Alloteropsis’ group consisting of A. semialata and A. angusta, and a ‘Coridochloa’ group of A. cimicina, A. paniculata and A. papillosa (Hattersley and Watson, 1992), and the ndhF phylogeny therefore supports such a grouping.
XyMS– leaf anatomy is typically associated with a single bundle sheath and the NADP-ME subtype of C4 biochemistry (Hattersley and Watson, 1992). However, A. semialata subsp. semialata is atypical because it has a complete layer of parenchyma cells surrounding the photosynthetically active mestome sheath (Ellis, 1974), and shows PCK-type biochemistry (Ueno and Sentoku, 2006). Leaf anatomy in A. angusta is similar to that in A. semialata subsp. semialata, but the parenchyma sheath cells are much reduced (Ellis, 1977). Placement of A. semialata and A angusta as sister species therefore suggests that their leaf anatomies represent intermediate stages during transition from typical XyMS+ to typical XyMS– anatomy through a pathway involving transfer of photosynthetic activity from parenchyma sheath cells to the mestome sheath, and subsequent loss of the parenchyma. In this scheme, A. angusta is further advanced towards conventional XyMS– physiology than the C4 subspecies of A. semialata. However, this hypothetical evolutionary scenario depends critically on the type of photosynthetic biochemistry operating in A. angusta.
Future directions
The present data suggest that coupling further phylogenetic and functional investigations of the Alloteropsis–forest shade group could provide dramatic and revealing insights into the genetic basis of photosynthetic pathway evolution. However, the current data should be treated with caution because phylogenies based on chloroplast genes reveal only maternal evolutionary history, and can differ from the underlying species phylogeny due to hybridization and introgression (Small et al., 2004). These processes seem particularly likely in Alloteropsis, since previous surveys of chromosome number have shown that the C3 subspecies of A. semialata is always diploid (2n = 18), while the C4 subspecies is always hexaploid or higher (2n = 54, 72, 108), raising the possibility of a historical hybridization event accompanied by genome duplication (Liebenberg and Fossey, 2001). The possibility that C4 photosynthesis in A. semialata subsp. semialata arose via hybridization with a C4 sister species therefore cannot be excluded. However, even if this hybridization event occurred, the most parsimonious explanation for the C3 photosynthetic pathway in A. semialata subsp. eckloniana remains unchanged; that it evolved via reversal from a C4 progenitor. This scenario for the evolutionary loss of a complex trait, followed by its re-acquisition, is unlikely, but not without precedent in evolutionary biology (Whiting et al., 2003).
Clarification is also required of phylogenetic relationships among anatomical variants of A. semialata subsp. semialata (Renvoize, 1987), and the C3–C4 intermediates that intergrade morphologically with the A. semialata subspecies (Hattersley and Watson, 1992). These occupy an approximately intermediate geographical distribution (Hattersley and Watson, 1992), and may represent intermediates in an actively evolving transition between photosynthetic types, or hybridization between the subspecies (Hattersley and Watson, 1992).
Further molecular markers from different cellular compartments will therefore be critical to improve robustness of the phylogeny, and unravel any hybridization events. In addition, the species of Paniceae sequenced thus far are biased towards New World taxa, and the addition of more African species would also be likely to improve resolution within the Alloteropsis–forest shade group. Finally, the atypical leaf anatomy and PCK-type C4 biochemistry in A. semialata mean that assigning C4 subtypes to the rest of the genus based on leaf anatomy is ill advised. It is suggested that the biochemical characterization of other Alloteropsis species, particularly A. angusta, is likely to provide novel insights into the C4 evolutionary pathway.
SUPPLEMENTARY DATA
Supplementary data is available at Annals of Botany Online and consists of the following: Table S1, details of Alloteropsis specimens used for sequencing; Table S2, primer sequences for amplification and sequencing of ndhF; and Table S3, details of taxa used in constructing the ndhF phylogeny.
ACKNOWLEDGEMENTS
We thank the DNA Bank at Kew Gardens, Richmond, Surrey; Mary Namaganda (Makerere Herbarium, Kampala, Uganda); and Gabriel Crowley and Joe Holtum (James Cook University, Cairns and Townsville, Australia) for providing DNA, leaf and plant samples, respectively. We also thank Andy Krupa (molecular work), Jake Gratten (phylogenetic analysis) and Tony Dold (herbarium work) for their valuable help, Elizabeth Kellogg and Steve Renvoize for their comments on the work, and Maxim Kapralov for critical comments on the manuscript. This work was funded by a UK Natural Environment Research Council (NERC) postgraduate award (D.G.I.); a Royal Society University Research Fellowship (C.P.O.); and grants from the South African National Research Foundation (NRF) and Rhodes University Joint Research Committee (JRC) (B.S.R.).
LITERATURE CITED
- Aliscioni SS, Giussani LM, Zuloaga FO, Kellogg EA. A molecular phylogeny of Panicum (Poaceae: Paniceae): tests of monophyly and phylogenetic placement within the Panicoideae. American Journal of Botany. 2003;90:796–821. doi: 10.3732/ajb.90.5.796. [DOI] [PubMed] [Google Scholar]
- Brown NJ, Parsley K, Hibberd JM. The future of C4 research – maize, Flaveria or Cleome? Trends in Plant Science. 2005;10:215–221. doi: 10.1016/j.tplants.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Brown WV. The Kranz syndrome and its subtypes in grass systematics. Memoirs of the Torrey Botanical Club. 1977;23:1–97. [Google Scholar]
- Brown WV, Smith BN. The genus Dichanthelium (Gramineae) Bulletin of the Torrey Botanical Club. 1975;102:10–13. [Google Scholar]
- Christin PA, Besnard G, Samaritani E, Duvall MR, Hodkinson TR, Savolainen V, Salamin N. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Current Biology. 2008;18:37–43. doi: 10.1016/j.cub.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Clark LG, Zhang WP, Wendel JF. A phylogeny of the grass family (Poaceae) based on ndhF sequence data. Systematic Botany. 1995;20:436–460. [Google Scholar]
- Clayton WD, Renvoize SA. Genera Graminum. Grasses of the world. Kew Bulletin, Additional Series. 1986;13:1–389. [Google Scholar]
- Duvall MR, Noll JD, Minn AH. Phylogenetics of Paniceae (Poaceae) American Journal of Botany. 2001;88:1988–1992. [PubMed] [Google Scholar]
- Ellis RP. The significance of the occurrence of both Kranz and non-Kranz leaf anatomy in the grass species Alloteropsis semialata. South African Journal of Science. 1974;70:169–173. [Google Scholar]
- Ellis RP. Distribution of the Kranz syndrome in the southern African Eragrostoideae and Panicoideae according to bundle sheath anatomy and cytology. Agroplantae. 1977;9:73–110. [Google Scholar]
- Ellis RP. Relevance of comparative leaf anatomy in taxonomic and functional research on the South African Poaceae. South Africa: University of Pretoria; 1981. DSc Thesis. [Google Scholar]
- Ellis RP. Eragrostis walterii, a first record of non-Kranz leaf anatomy in the subfamily Chloridoideae (Poaceae) South African Journal of Botany. 1984;3:380–386. [Google Scholar]
- Evans LT. Crop evolution, adaptation and yield. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Gibbs-Russell GE. The taxonomic position of C3 and C4 Alloteropsis semialata (Poaceae) in southern Africa. South African Journal of Botany. 1983;2:250–250. [Google Scholar]
- Giussani LM, Cota-Sanchez JH, Zuloaga FO, Kellogg EA. A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. American Journal of Botany. 2001;88:1993–2012. [PubMed] [Google Scholar]
- Grass Phylogeny Working Group. Phylogeny and subfamilial classification of the grasses (Poaceae) Annals of the Missouri Botanical Garden. 2001;88:373–457. [Google Scholar]
- Gutierrez M, Gracen VE, Edwards GE. Biochemical and cytological relationships in C4 plants. Planta. 1974;119:279–300. doi: 10.1007/BF00388331. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hattersley PW, Watson L. C4 grasses: an anatomical criterion for distinguishing between NADP-malic enzyme species and PCK or NAD-malic enzyme species. Australian Journal of Botany. 1976;24:297–308. [Google Scholar]
- Hattersley PW, Watson L. Diversification of photosynthesis. In: Chapman GP, editor. Grass evolution and domestication. Cambridge: Cambridge University Press; 1992. pp. 38–116. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Ibrahim DG, Gilbert ME, Ripley BS, Osborne CP. Seasonal differences in photosynthesis between the C3 and C4 subspecies of Alloteropsis semialata are offset by frost and drought. Plant, Cell and Environment. 2008;31:1038–1050. doi: 10.1111/j.1365-3040.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- Liebenberg EJL, Fossey A. Comparative cytogenetic investigation of the two subspecies of the grass Alloteropsis semialata (Poaceae) Botanical Journal of the Linnean Society. 2001;137:243–248. [Google Scholar]
- Matthews E. Global vegetation and land use: new high-resolution data bases for climate studies. Journal of Climate and Applied Meteorology. 1983;22:474–487. [Google Scholar]
- Nixon KC. The Parsimony Ratchet, a new method for rapid parsimony analysis. Cladistics. 1999;15:407–414. doi: 10.1111/j.1096-0031.1999.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Nylander JAA. MrModeltest v2.2. Uppsala University, Uppsala, Sweden: Evolutionary Biology Centre; 2004. [Google Scholar]
- Olmstead RG, Sweere JA. Combining data in phylogenetic systematics – an empirical approach using 3 molecular data sets in the Solanaceae. Systematic Biology. 1994;43:467–481. [Google Scholar]
- Osborne CP, Wythe EJ, Ibrahim DG, Gilbert ME, Ripley BS. Low temperature effects on leaf physiology and survivorship in the C3 and C4 subspecies of Alloteropsis semialata. Journal of Experimental Botany. 2008;59:1743–1754. doi: 10.1093/jxb/ern062. [DOI] [PubMed] [Google Scholar]
- Porter ML, Crandall KA. Lost along the way: the significance of evolution in reverse. Trends in Ecology and Evolution. 2003;18:541–547. [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Prendergast HDV, Hattersley PW, Stone NE. New structural biochemical associations in leaf blades of C4 grasses (Poaceae) Australian Journal of Plant Physiology. 1987;14:403–420. [Google Scholar]
- Pyankov VI, Artyusheva EG, Edwards GE, Black CC, Soltis DE. Phylogenetic analysis of the tribe Salsoleae (Chenopodiaceae) based on ribosomal ITS sequences: implications for the evolution of photosynthesis types. American Journal of Botany. 2001;88:1189–1198. [PubMed] [Google Scholar]
- Renvoize SA. A survey of leaf-blade anatomy in grasses XI. Paniceae. Kew Bulletin. 1987;42:739–768. [Google Scholar]
- Ripley BS, Gilbert ME, Ibrahim DG, Osborne CP. Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. Journal of Experimental Botany. 2007;58:1351–1363. doi: 10.1093/jxb/erl302. [DOI] [PubMed] [Google Scholar]
- Ripley BS, Abraham TI, Osborne CP. Consequences of C4 photosynthesis for the partitioning of growth: a test using C3 and C4 subspecies of Alloteropsis semialata under nitrogen limitation. Journal of Experimental Botany. 2008;59:1705–1714. doi: 10.1093/jxb/erm210. [DOI] [PubMed] [Google Scholar]
- Sage RF. The evolution of C4 photosynthesis. New Phytologist. 2004;161:341–370. doi: 10.1111/j.1469-8137.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- Sage RF, Li M, Monson RK. The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego: Academic Press; 1999. pp. 551–584. [Google Scholar]
- Saitou N, Nei M. The neighbor joining method – a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sikes DS, Lewis PO. PAUPRat: PAUP* implementation of the parsimony ratchet. 2001 Beta software, version 1. Distributed by the authors. Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT, USA http://users.iab.uaf.edu/~derek_sikes/software2.htm. (accessed 19 May 2008) [Google Scholar]
- Small RL, Cronn RC, Wendel JF. Use of nuclear genes for phylogeny reconstruction in plants. Australian Systematic Botany. 2004;17:145–170. [Google Scholar]
- Spangler R, Zaitchik B, Russo E, Kellogg E. Andropogoneae evolution and generic limits in Sorghum (Poaceae) using ndhF sequences. Systematic Botany. 1999;24:267–281. [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Ueno O, Sentoku N. Comparison of leaf structure and photosynthetic characteristics of C3 and C4 Alloteropsis semialata subspecies. Plant, Cell and Environment. 2006;29:257–268. doi: 10.1111/j.1365-3040.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Chuong SDX, Edwards GE. Functional characterization of phosphoenolpyruvate carboxykinase type C4 leaf anatomy: immuno-, cytochemical and ultrastructural analyses. Annals of Botany. 2006;98:77–91. doi: 10.1093/aob/mcl096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting MF, Bradler S, Maxwell T. Loss and recovery of wings in stick insects. Nature. 2003;421:264–267. doi: 10.1038/nature01313. [DOI] [PubMed] [Google Scholar]