Abstract
Background
Since the identification of the genes controlling the root acquisition of iron (Fe), the control of inter- and intracellular distribution has become an important challenge in understanding metal homeostasis. The identification of the yellow stripe-like (YSL) transporter family has paved the way to decipher the mechanisms of long-distance transport of Fe.
Scope
Once in the plant, Fe will systematically react with organic ligands whose identity is poorly known so far. Among potential ligands, nicotianamine has been identified as an important molecule for the circulation and delivery of metals since it participates in the loading of copper (Cu) and nickel in xylem and prevents Fe precipitation in leaves. Nicotianamine is a precursor of phytosiderophores, which are high-affinity Fe ligands exclusively synthesized by Poaceae species and excreted by roots for the chelation and acquisition of Fe. Maize YS1 is the founding member of a family of membrane transporters called YS1-like (YSL), which functions in root Fe–phytosiderophore uptake from the soil. Next to this well-known Fe acquisition role, most of the other YSL family members are likely to function in plant-wide distribution of metals since (a) they are produced in vascular tissues throughout the plant and (b) they are found in non-Poaceae species that do not synthesize phytosiderophores. The hypothesized activity as Fe–nicotianamine transporters of several YSL members has been demonstrated experimentally by heterologous expression in yeast or by electrophysiology in Xenopus oocytes but, despite numerous attempts, proof of the arabidopsis YSL substrate specificity is still lacking. Reverse genetics, however, has revealed a role for AtYSL members in the remobilization of Cu and zinc from senescing leaves, in the formation of pollen and in the Fe, zinc and Cu loading of seeds.
Conclusions
Preliminary data on the YSL family of transporters clearly argues in favour of its role in the long-distance transport of metals through and between vascular tissues to eventually support gametogenesis and embryo development.
Key words: Metals, iron, nicotianamine, yellow stripe-like, YS1-like, circulation, transport, phytosiderophore, xylem, phloem
INTRODUCTION
Cell life is reliant on iron (Fe) for its unique property of being able to catalyse oxidation/reduction reactions. Fe serves as a prosthetic group in proteins to which it is associated either directly or through a haem or an iron-sulfur cluster. Since in solution it exists under two redox states, reduced ferrous and oxidized ferric, Fe can lose or gain an electron within metalloproteins. A wide range of metabolic pathways rely on Fe redox enzymes, including the electron transfer chains of respiration and photosynthesis (cytochromes), the biosynthesis of DNA (ribonucleotide reductase), lipids (lipoxygenase) and hormones [1-aminocyclopropane 1-carboxylic acid (ACC) oxidase], the detoxification of reactive oxygen species (ROS) (peroxidase, catalase) and the nitrogen assimilation (nitrite and nitrate reductase). These cellular processes take place in distinct intracellular compartments, which therefore need to be provided with an adequate amount of Fe.
Fe is relatively insoluble in its inorganic state, and therefore is scarcely available to plant roots. As a result, plants have developed specialized systems to optimize Fe uptake in case of Fe shortage (Curie and Briat, 2003). To increase soil Fe solubility, Poaceae, that include the major crop plants, rely on chelation of Fe by small metabolites generically termed phytosiderophores (PS) that they synthesize and secrete. Dicotyledonous and non-grass monocotyledonous plants, however, improve Fe solubility by lowering rhizospheric pH through the extrusion of organic acids and protons. While the first class of plants acquires Fe as Fe(III)–PS complexes, the latter reduces solubilized Fe(III) through activation of a ferric-chelate reductase and take Fe up in its reduced state by the ferrous Fe transporter IRT1 (Eide et al., 1996; Vert et al., 2002; Vacchina et al., 2003). The dogma of the classification of plants in two distinct uptake strategies has been recently disturbed by the finding that rice, a Poaceae, can induce both Fe(II) uptake by OsIRT1 and Fe(III)–PS uptake in response to Fe starvation (Ishimaru et al., 2006). These sophisticated physiological mechanisms, as well as root morphological changes necessary to increase foraging, enable plants to adapt to an ever-changing environment for Fe nutrition. Severe Fe deficiency limits plant growth and provokes the development of a characteristic interveinal chlorosis. Conversely, an excess of Fe in the organism can be detrimental. Indeed, the same chemical properties that make Fe a central player in many essential enzymatic reactions, can lead to harmful effects. Iron, specially Fe(II), catalyses the production of reactive oxygen species, including the nasty hydroxyl radical OH•, through the Fenton reaction. Because free radicals can react with many cell components and generate some so-called oxidative stress, cells prevent noxious effects of excessive Fe in the cell by storing it either associated with organic acids in the vacuole or engulfed in ferritin, a ubiquitous Fe storage protein, which in plants is located in plastids (Briat et al., 2006).
Iron chelation too is a central process in Fe homeostasis because it can be viewed both as a way to enhance its availability by helping its solubilization, or as a way to scavenge Fe in a non-Fenton active form. Indeed, Fe reactivity is such that Fe exists mainly in cells as highly stable complexes with organic ligands or inorganic phosphate. Yet very little is known about the nature of the Fe species in the different cell compartments. Furthermore, circulation of Fe throughout the plant and distribution to tissues and organelles relies on transmembrane transporters whose nature depends on the type of Fe substrate. Contrary to the transporters responsible for the entry of Fe from the soil solution into the root, candidate transporters that mediate long-distance transport within the plant have only been discovered in recent years. This review focuses on one such candidate family of proteins, the yellow stripe-like (YSL) transporters, and present data supporting the view that YSLs mediate long-distance transport of specific Fe species, along with other metals, within the plant. Because internal transport of Fe is tightly integrated with Fe speciation, recent advances are also highlighted and old data on the nature and physiological role of a plant-specific Fe ligand, nicotianamine (NA), revisited.
THE NA MOLECULE: BIOCHEMICAL PROPERTIES
The metabolite NA, ubiquitous in the plant kingdom, is indispensable for a plant to complete its life cycle. NA (molecular weight 303) results from the enzymatic condensation of three amino-carboxylpropyl groups of three S-adenosyl-methionine molecules, by NA synthase. During the enzymatic reaction, three covalent bonds are broken to release three amino-carboxypropyl groups from S-adenosyl-methionine and three new covalent bonds are formed, including an internal cyclization, leading to the formation of an azetidin ring. The presence of three amino and three carboxy groups in the molecule allows the formation of an hexadentate co-ordination that drives the formation of very stable octahedral chelates with a central metal ion (Fig. 1A). In vitro, NA is able to form stable complexes with manganese (Mn), Fe(II), cobalt (Co), zinc (Zn), nickel (Ni) and copper (Cu), in increasing order of affinity (Benes et al., 1983; Anderegg and Ripperger, 1989). It has been shown only recently that NA can complex Fe(III) in vitro with a high affinity (Fig. 1B; von Wiren et al., 1999). In that study, based on potentiometric and spectrophotometric measurements of the chelation capabilities of NA, computer analyses were performed to simulate the pH-dependent stability of different metal–NA complexes. Several important conclusions were drawn: (a) for all the metals considered, the stability of the complexes was maximal at pH values above 6·5, indicating that NA would be more likely a ‘symplastic’ chelator of metals; (b) among essential metals Cu is the exception as the Cu–NA complex is very stable in mild acidic conditions, which is a strong argument in favour of the possible occurrence of a Cu–NA complex in an ‘apoplastic’ environment such as the xylem sap; (c) in vitro, NA can chelate Fe(III) with a high affinity, although the kinetic stability of this complex is much lower than Fe(II)–NA; (d) for pH values around 5·5 organic acids like citrate would be the main chelators of metal ions whereas in conditions of neutrality NA would be almost the exclusive chelator; (e) when mixing NA and deoxymugineic acid (DMA; a PS derived from NA) with Fe(III), DMA would complex Fe in the pH range 3·5–5·5 whereas above this value NA would prevail, which illustrates that ligand exchanges can occur when switching from an apoplastic to a symplastic environment (von Wiren et al., 1999). These observations are based on computer simulations and it is only very recently that the metal–NA complexes have been studied in vitro with mass spectrometry techniques, namely electrospray ionization time-of-flight mass spectrometry (Rellan-Alvarez et al., 2008). In that work, the formation of different metal–NA complexes has been analysed by measuring free NA and metal–NA complexes at different pH conditions. The authors have confirmed biochemically, without ambiguity, the computer simulations proposed above concerning the pH-dependent stability of the several metal–NA complexes, the high stability of Fe(II)–NA and finally the possibility of ligand exchange of Fe from NA to citrate at pH 5·5. In summary, NA has ideal structural features for a stable metal chelator, through the formation of hexadentate octahedral complexes. The different complexes studied either through computational prediction (modellization) and/or in vitro are more stable in neutral conditions, which implies that NA is likely to be a symplastic metal chelator. The capacity of NA to chelate Fe(III), somehow controversial, has been actually reported and demonstrated in different ways (von Wiren, 1999; Rellan-Alvarez et al., 2008). However, the stability of this complex is low, in comparison with the surprisingly high stability of Fe(II)–NA. On the basis of these data, two questions can be raised. (1) When metal–NA complexes are formed, how can they dissociate in a given cellular fluid? Clearly the switch of pH can be a way to dissociate the complexes, with a concomitant ligand exchange. (2) In the case of Fe (and possibly Cu), what is the importance of the redox status? Depending of the situation it can be potentially important to keep a stable Fe(II)–NA complex or to switch to the less stable Fe(III)–NA complex by oxidation.
Fig. 1.
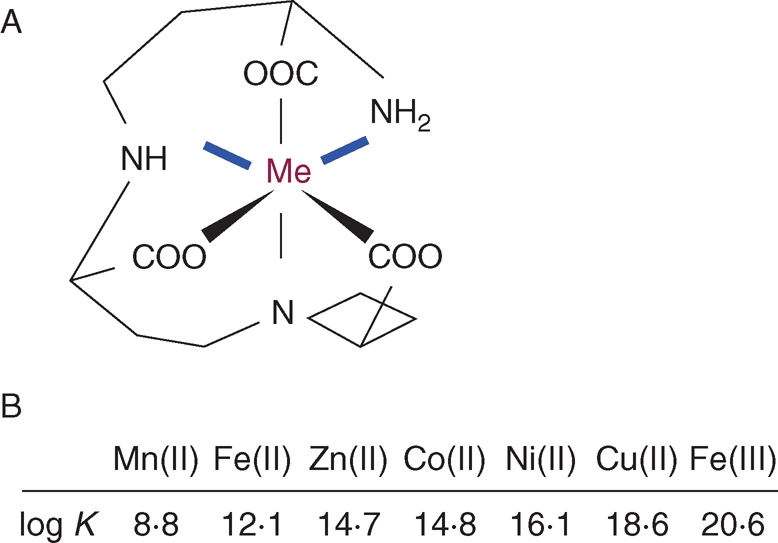
Biochemical properties of nicotianamine: (A) proposed chemical structure of the nicotianamine–metal complex; (B) in vitro affinity constants of complexes of nicotianamine with various metals. Me, Metal.
ROLE OF NA IN THE LONG-DISTANCE TRANSPORT OF METALS
Translocation of Cu revealed by chloronerva
In the last two decades, most of the information on the role of NA has been obtained with the NA-free tomato mutant chloronerva, which bears a single base change in the apparently single NAS gene (Ling et al., 1999). This mutant displays a strong interveinal chlorosis in young leaves, reminiscent of an Fe deficiency, reduced growth and a complete sterility. These phenotypes can be reverted by exogenous supply of NA or by grafting. To what extent is this mutant affected in the long-distance circulation of metals? The measurement of the main essential metal ions, Fe, Zn, Mn and Cu, has revealed that, surprisingly, the different organs of this mutant contained significantly more Fe than the control plants, irrespective of the Fe nutritional status (Pich et al., 1994). This high Fe content, even in young leaves, could be attributed to the constitutive activity of the root Fe acquisition systems, the proton extrusion and the ferric reductase (Stephan and Grun, 1989). This result indicates actually that the long-distance transport of Fe is not impaired in this mutant. The main disorder in metal distribution in chloronerva concerns Cu. During growth Cu accumulates in the roots whereas leaves suffer from severe Cu deficiency, which increases for each new leaf produced (Pich et al., 1994; Pich and Scholz, 1996). Accordingly, the Cu-containing enzymes superoxide dismutases (cytosolic and chloroplastic) and plastocyanin were nearly absent in leaves of chloronerva (Herbik et al., 1996). Finally, the measurement of Cu concentration and translocation rates in the xylem sap were three to five times lower than in the wild-type genotype and the addition of NA could restore part of the normal Cu concentration (Pich and Scholz, 1996). Taken together, examples of experimental evidence are largely in favour of a direct role of NA in the root-to-shoot translocation of Cu, via the xylem sap. This assumption is strengthened by the demonstration that the Cu–NA complex is completely stable at the pH of the xylem sap that ranges between 5 and 6. However, the final proof of such a role of NA would be the identification of the Cu–NA complex in the xylem sap. The main challenge in this case is the ability to identify, isolate and purify metal complexes from a bulk of organic molecules with different sizes and origins, in order to develop the MS analyses further. The development of hyphenated analytical techniques, based on the coupling of chromatographic separations to a parallel element-specific detection (ICP MS) or a molecule-specific detection (ESI MS/MS) should help to solve these technical difficulties (reviewed in Szpunar et al., 2003).
The use of metal hyperaccumulators as models for high-capacity xylem transport
Plant hyperaccumulators have developed the ability to translocate massive amounts of heavy metals from the roots to the shoots where they are stored in non-toxic forms. Thlaspi caerulescens, a Cd/Zn/Ni hyperaccumulator, has been used as a model to study the speciation of Ni in the xylem sap by mass spectrometry approaches. Root exudates from Ni-exposed plants were analysed as follows: first, the samples were chromatographed by size-exclusion coupled to ICP MS, to detect the Ni-containing fractions. In a second chromatography of the same sample, the corresponding Ni-enriched fractions were collected and concentrated. Thirdly, electrospray ionization mass spectrometry was used to identify Ni complexes, based on the typical pattern of an Ni-containing molecule: two peaks separated by two mass units in the proportion 70 : 30 that corresponds to the natural abundance of the two major Ni isotopes. Only one molecule fitted to these criteria with an m/z of 360 and was proven to be the Ni–NA complex (Vacchina et al., 2003; Ouerdane et al., 2006). Thus, in vivo, NA was able to form a stable complex with Ni, that could represent up to 25 % of the total Ni (Mari et al., 2006; Ouerdane et al., 2006). These results clearly showed a role of NA in the chelation and long-distance transport of a metal in plants and established a new role of NA in the transport of Ni in a hyperaccumulator plant.
NA and metal movement in the phloem sap
The chelation properties of NA are the highest at neutral and mild basic pH, in terms of affinity and, more importantly, stability against other ligands like organic acids, making this molecule ‘made-to-measure’ for phloem transport (Stephan et al., 1996). Obtaining reasonable amounts of phloem sap is, however, extremely problematic, except in some specific cases like Ricinus communis seedlings, cucurbits and oilseed rape by wounding of fruits and exudation (for a review, see Kehr and Rep, 2007). As for the xylem sap, NA has been detected in the phloem sap of Ricinus hypocotyls (Stephan and Scholz, 1993; Schmidke et al., 1999) and Brassica napus (Mendoza-Cozatl et al., 2008). The concentration in the phloem sap of Ricinus was estimated to be 200 µm (which is 10 times higher than the usual concentration in the xylem) and, intriguingly, this concentration coincided with the aggregate concentration of Fe, Mn, Zn and Cu (Stephan and Scholz, 1993). The fact that the stoechiometry of NA and metals approximates one is not a proof that the metals only occur as complexes with NA. Actually, in this phloem sap Fe appears to be mostly bound to the protein fraction, in particular to a specific protein called ITP (iron transport protein) belonging to the late embryogenesis abundant family (Krueger et al., 2002). A putative role of NA, that remains to be proven, would be to serve as a shuttle to load and/or unload Fe from ITP. There are, anyway, some indirect physiological data that indicate a role of NA in phloem loading, or more generally in entering a symplastic route.
ROLE OF NA IN THE INTRACELLULAR TRANSPORT OF METALS
NA and Fe: the elusive couple
The chlorosis of chloronerva is only visible in young leaves and disappears with age. In parallel, the concentration of NA is always highest in meristematic tissues (apical zones of shoots and roots). Since the differentiation of xylem is slower than that of phloem, young leaves and apical meristems are mostly fed by phloem or provascular tissues. The over-expression of a nicotianamine amino transferase (NAAT) in tobacco mimics the chloronerva phentotype due to the total enzymatic consumption of NA (Takahashi et al., 2003). In this mutant, the distribution of 59Fe was monitored in detached leaves fed with different Fe chelates. When provided alone, Fe was restricted to the main veins whereas a co-incubation of Fe and NA induced a widespread distribution of Fe in the mesophyll cells. Although this does not demonstrate the role of NA in phloem loading, it highlights the importance of this molecule in the distribution of Fe at the cellular level or in its entry in the ‘symplastic compartment’. The latter statement would imply that NA is involved in the uptake of metals, Fe for instance, on the plasma membrane. This appealing hypothesis was challenged by Pich and Schloz (1991) who compared the Fe acquisition of protoplasts prepared from chloronerva and its wild-type parent Bonner Beste. The authors showed that the plasma membrane ferric reductase activity was identical in both genotypes and, moreover, that the Fe uptake was higher in chloronerva protoplasts. The addition of NA to the uptake medium had no effect on the absorption of Fe, showing that NA is not essential for the transport of Fe at the plasma membrane level. Finally, the comparison of Fe content between protoplasts and whole leaves, which is an approximation of the apoplastic Fe concentration, has shown that the lack of NA in cells provokes a high accumulation of Fe in symplastic and apoplastic pools. In conclusion, if NA is not directly involved in the uptake of Fe at the cell level, it is essential for the regulation of its distribution between apoplastic and symplastic compartments.
Distribution of NA in organelles, role in deficiency and excess of Fe
To move one step further, the ‘symplastic compartment’ embraces the cytoplasm and several organelles that do contain Fe atoms, mainly bound to proteins. Is NA involved in the regulation/delivery/distribution of Fe between these internal compartments? The distribution of metals between organelles of a cell is currently an important challenge in the field of metal homeostasis. New discoveries in this field require the development of high quality cell fractionation protocols and metal imaging. Concerning NA, the measurement in fractions enriched in any organelles has never been reported to the best of our knowledge. However, anti-NA antibodies have been obtained and used in immuno-histochemical approaches (Pich et al., 1997). In root tips of the tomato cultivar Bonner Beste, the vacuole lumen and, to a lesser extent, the cytosol were stained whereas the other compartments of the cells were devoid of labelling. It was further shown that the Fe nutritional status had a marked effect on the distribution of the NA labelling. In leaves and roots of Bonner Beste grown in control conditions (10 µm Fe) most of the labelling was cytosolic, whereas in Fe-loaded plants (100 µm) a strong labelling of the vacuoles was detected in electron-dense protein-rich structures (Pich et al., 2001). These results indicate that at the cellular level NA could be involved in the detoxification of high Fe concentrations by sequestration in the vacuole. This hypothesis was further strengthened using two pea mutants, bronze (brz) and degenerated leaves (dgl) that over-accumulate Fe in the leaves. In leaves and roots of both mutants the labelling of the vacuole was much stronger than in the corresponding wild-type genotypes. The NA content in the leaves and roots, measured by HPLC, confirmed that the mutants contained 10–20 times more NA than the control. These observations, together with the finding that the Fe(II)–NA complex is a poor Fenton reagent (von Wiren et al., 1999), are good indications that NA may play an important role in the detoxification of excess Fe, by chelation and sequestration in the vacuole. It is not clear, however, if NA is involved in Fe detoxification, if the mutant chloronerva suffers from Fe toxicity. Nevertheless, the distribution of Fe in chloronerva, at the cellular level, is strongly altered. As revealed by energy dispersive X-ray micro analysis (EDXMA), in the leaves, Fe accumulates in electron-dense deposits in the stroma of chloroplasts and in the phloem (Becker et al., 1995). These deposits are not attributed to Fe–ferritins since ferritin proteins do not accumulate, but rather to Fe-phosphate depositions. In root cells, Fe accumulates in the cytosol and vacuole whereas, in Bonner Beste, Fe was mainly detected in the cell walls. It thus appears that in standard Fe conditions, the main role of NA would be to keep Fe in a soluble form, enabling its correct distribution within the different compartments of the cell; the lack of NA would induce Fe precipitations in chloroplast and in the phloem sap, lowering its availability and mobility. In conditions of Fe toxicity, NA would participate in the detoxification mechanisms by chelation and further sequestration in the vacuole.
THE YSL TRANSPORTERS
The YSL family of transporters represents a serious candidate for the transport of NA–metal chelates across plant cell membranes. Although final proof is still missing, experimental evidence points to a role of the YSL proteins in the long-distance and intracellular transport of metals, specially Fe, complexed to NA.
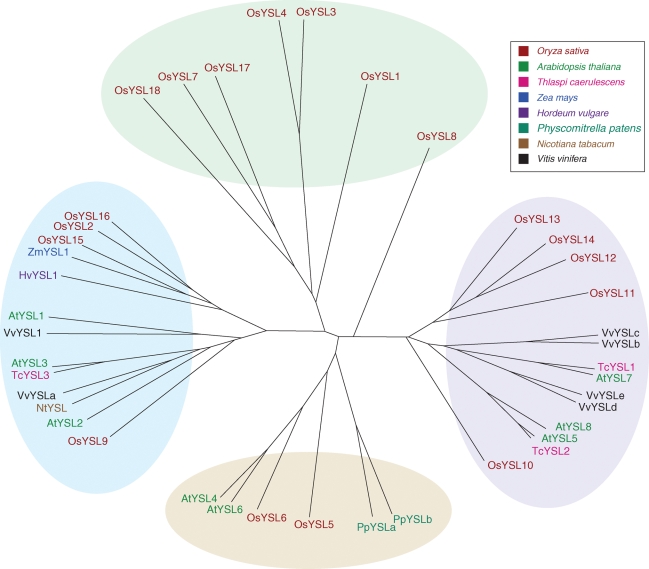
The YSL transporters belong to the oligopeptide transporters (OPT), a poorly characterized family of proteins involved in the transport of tri-, tetra-, penta- and hexapeptides as well as amino acids derivatives, with representatives only in plants, fungi, bacteria and archaebacteria (Yen et al., 2001). Within the OPT family, the YSL form a separate cluster that is <20 % similar at the protein level to the plant OPT cluster. Figure 2 indicates that YSL proteins are distributed in four sub-families, which contains exclusively Graminae members, suggesting for these proteins a function specific to Graminae.
Fig. 2.
Phylogenetic tree of the yellow stripe 1-like family of proteins. Calculation and drawing of the tree were carried out with the Dendroscope program (http://www-ab.informatik.uni-tuebingen.de/software/dendroscope/welcome.html; Huson et al., 2007).
Role of YS1, the YSL-founding member, in Fe acquisition by Poaceae roots
Consistent with it belonging to the OPT family, maize yellow stripe 1 (YS1), the founding member of the YSL cluster, transports Fe–PS complexes. PS of the mugineic acid family are low-molecular-weight amino acid derivatives, that derive from NA after a deamination step by the NAAT, followed by a reduction step by the deoxymugineic acid synthase (DMAS; Mori and Nishizawa, 1987; Bashir et al., 2006). Only graminaceous plants possess NAAT and DMAS genes. Thus while all plants produce NA, only Graminae can transform NA into PS. In response to Fe deficiency, PS are synthesized and secreted in the soil where they solubilize ferric hydroxides through efficient chelation. The YS1 transporter then takes up the resulting Fe(III)–PS complex into the cytosol of the root epidermis (Curie et al., 2001; Schaaf et al., 2004; Murata et al., 2006). Maize plants that have a loss-of-function mutation in the YS1 gene harbour leaf interveinal chlorosis, hence the name ‘yellow stripe’, and poor growth, due to the lack of Fe–PS entry in the plant (von Wiren et al., 1994; Curie et al., 2001). ZmYS1, and its orthologue in barley HvYS1, are up-regulated in Fe-deficient roots (Curie et al., 2001; Roberts et al., 2004; Murata et al., 2006). Immuno-histological staining with an anti-HvYS1 antibody and transient expression of hvYS1::GFP fusion demonstrated that the HvYS1 transporter is located on the plasma membrane of epidermal cells (Murata et al., 2006). Transport activity determined by two-electrode voltage clamp analysis in Xenopus leavis oocytes showed that ZmYS1 transports Fe(III)–PS with a Km of 5–10 µm, and that this transport depends on a proton co-transport and on the membrane potential (Schaaf et al., 2004). The observation that ZmYS1 transport capacity is increased at acidic pH does not fit with ZmYS1 being required in Fe-limiting conditions, which in nature occurs notably in highly basic calcareous soils. However, Fe–PS transport may still operate at alkaline pH as long as a negative membrane potential is achieved and plasma membrane proton ATPase activity is enhanced to avoid local proton limitation. Among the 18 members of the family of YSL transporters in rice, OsYSL15 is the best suited to fill the function of Fe acquisition from the soil. Indeed, OsYSL15 is expressed in root epidermis and up-regulated under Fe limitation. OsYSL15 is the family member that is most closely related to ZmYS1 (Fig. 2).
ZmYS1 and hvYS1 have a distinct substrate specificity regarding both metal and ligand. Analyses by electrophysiology in Xenopus oocytes showed that hvYS1 transports only Fe(III) chelated to PS whereas ZmYS1 transports various ions including Fe(III), Fe(II), Ni(II), Zn(II), Cu(II), Mn(II) and Cd(II) (Schaaf et al., 2004; Murata et al., 2006). Experiments of domain swapping between these two YS1 transporters indicate that the determinants of the substrate specificity lie in the outer membrane loop located between putative transmembrane domains 6 and 7 of the proteins (Harada et al., 2007). The physiological relevance of the broad spectrum of ZmYS1 specificity is not clear, except for Zn: Zn deficiency too was shown to stimulate PS secretion in barley and rice (Suzuki et al., 2006, 2008). Consistently the maize ys1 mutant was reported to absorb less 62Zn(II)–DMA than wild-type plants (Wiren et al., 1996).
Fe uptake and Fe circulation: two different functions for proteins of the same family
Several pieces of information, however, led to the idea that the role of the YSL cluster of OPT transporters may not be restricted to the uptake of Fe–PS in roots. (a) The identification of eight YS1-like sequences in the genome of A. thaliana (Curie et al., 2001), which as a dicotyledonous plant does not synthesize mugineic acids. (b) The finding that ZmYS1 can also transport Fe(II)–NA and Ni(II)–NA complexes (Schaaf et al., 2004), which is consistent with the fact that NA and PS are chemically related, share a similar high affinity for various metal cations and thus represent close analogue transport substrates. Because NA is ubiquitously synthesized by plants, together (a) and (b) have led to the hypothesis that YSL proteins could transport metal–NA chelates. Based on the recognized physiological function of NA in Fe/metal circulation as mentioned above, it is likely that the function of the YSLs in dicots is to contribute to Fe/metal long-distance circulation/distribution within the plant.
The situation in grasses is not as simple due to the coexistence of PS and NA in the plant, suggesting that YSL transporters in grasses could be involved either in root Fe acquisition, internal Fe circulation, or both. Rice for instance clearly possesses members dedicated to the two functions: OsYSL15 for root Fe–PS uptake whose gene is strictly expressed in Fe-deficient root epidermis; and OsYSL2, 13, 14 that may be involved in the movement of metals within the plant based on their gene expression pattern restricted to the aerial parts (Koike et al., 2004; Murata et al., 2006; Nishizawa et al., 2006). In maize, the Fe–PS uptake-encoding gene ZmYS1 is not only expressed in root epidermis, but is also strongly expressed in shoots of Fe-deficient plants (Curie et al., 2001). Since Fe–NA complexes are also substrates of ZmYS1, this suggests that ZmYS1 could transport Fe–NA complexes within the plant. Alternately, ZmYS1 could transport Fe–PS complexes in shoots since PS production is also detectable in shoots of Poaceae, although their role is not clear. The observation of PS in xylem and phloem saps of barley suggests that PS could play a similar role as NA as a metal ligand in the saps (Mori et al., 1991). Moreover, a recent report indicates that endogenous deoxymugineic acid increases in shoots of Zn-deficient rice where it stimulates root-to-shoot Zn translocation (Suzuki et al., 2008). Finally, it is also conceivable that PS are secreted in the apoplastic space of the shoots to chelate stored Fe(III) and that the Fe(III)–PS complex formed is taken up in the cytosol by a YSL transporter, in a way comparable to the uptake of Fe(III)–PS by YS1 in roots.
Transport activity and substrate specificity of the YSL proteins
Transport activity of the YSL proteins has been tested by heterologous expression in yeast metal transport defective mutants as well as in Xenopus oocytes. The yeast fet3fet4 mutant is altered in the low and high affinity Fe uptake activities and thus does not grow on a low Fe supply. Typically, ZmYS1 restores growth of fet3fet4 when Fe in the medium is provided as a complex of Fe(III)–DMA, Fe(III)–NA or Fe(II)–NA, but not Fe(III)–citrate (Curie et al., 2001; Schaaf et al., 2004). Likewise, the two other putative transporters of Fe(III)–PS from the soil, HvYS1 and OsYSL15, complement the Fe uptake function of the fetfet4 mutant albeit with a substrate specificity narrowed to Fe(III)–DMA (Murata et al., 2006; Nishizawa, et al., 2006). Among the 17 other rice members, the eight arabidopsis and the three reported Thlaspi caerulescens members, however, only TcYSL3 was shown unambiguously to complement fet3fet4 (Gendre et al., 2007). TcYSL3 expression in the fet3fet4 strain was shown to mediate Fe(II)–NA and Ni(II)–NA influx. In arabidopsis, it was reported that AtYSL1 fails to restore fet3fet4 growth on any Fe source (Le Jean et al., 2005) and we have experienced the same failure for several other AtYSLs (A. Schikora, J. Misson, D. Couch, S. Mari and C. Curie, unpubl. res.). Contradictory results have been obtained for AtYSL2. DiDonato et al. (2004) reported that expressing AtYSL2 in fet3fet4 conferred a growth advantage to the mutant when Fe is provided as Fe(II)–NA. These results were contested in a carefully controlled study from Schaff et al. (2005) in which AYSL2, unlike ZmYS1, failed to complement Fe transport in yeast, even when expressed from various expression vectors. Thus except for TcYSL3, showing functional complementation in yeast has been challenging for the putative Fe–NA transporters whereas all the known uptake transporters of Fe–PS from the soil to the root do show transport activity in yeast. As a consequence, Fe–PS and Fe–NA transport activities are likely to have different characteristics such as membrane potential requirements. The fact that TcYSL3 manages to mediate Fe–NA uptake may be related to Thlaspi caerulescens being a metal hyperaccumulator. Indeed, it has been established that hyperaccumulation is associated with higher expression of metal homeostasis genes expressed in the related non-extremophile species. The enhanced expression of the HMA4 gene, encoding a heavy metal ATPase, in the Zn and Cd hyperaccumulator Arabidopsis halleri is attributable to a combination of a larger copy number and the presence of naturally evolved stronger promoter cis-regulatory sequences, in comparison to Arabidopsis thaliana (Hanikenne et al., 2008). The three TcYSL genes are also expressed several-fold more in T. caerulescens than their homologues in A. thaliana (Gendre et al., 2007). In addition to higher expression, it is possible that a stronger transport activity may account for the hyperaccumulation/hypertolerance processes in these plants, therefore enabling measurable transport activity in yeast. Another possibility is that the YSLs that do not complement yeast are located on an intracellular membrane. However, it is not the case at least for AtYSL2 since the AtYSL2::GFP fusion protein was shown to be addressed at the plasma membrane of xylem parenchyma cells in the root (DiDonato et al., 2004). Whether YSL proteins could perform metal efflux was tested with AtYSL2. ZmYS1 and AtYSL2 were co-expressed in the fet3fet4 yeast mutant, but when cells were grown in the presence of Ni–NA, a substrate efficiently transported by ZmYS1, no efflux of Ni was detected (Schaaf et al., 2005). Moreover, since all functionally characterized members of the OPT and YSL families function with inwardly directed polarity, all members of the YSL family may function in the influx of metal–NA or metal–PS complexes. Given the affinity of NA for several metal ions, especially Cu and Zn, the possibility that YSL proteins could transport metal–NA complexes other than Fe–NA was tested by expression in yeast strains mutated for zinc (zrt1zrt2) or copper (ctr1) transport activities. However, except for a weak complementation of ctr1 by AtYSL2 (DiDonato et al., 2004), which suffers the same bias as the fet3fet4 complementation experiment, no growth restoration was observed with several AtYSL proteins (Schaaf et al., 2005; J. Misson, A. Schikora, M. Le Jean, C. Curie, unpubl. res.). Finally, proper controls of accumulation in yeast such as expression of tagged YSL versions are still to be provided in order to rule out the trivial problem of protein production in yeast, which is often observed with transporters.
Similar to heterologous expression in yeast, expression in Xenopus oocytes successfully showed transport activity of the YSL members involved in uptake from the soil, i.e. ZmYS1, HvYS1 and OsYSL15 (Schaaf et al., 2004; Murata et al., 2006; Nishizawa et al., 2006). However, among the other members, only OsYSL2 mediates transport activity in oocytes, with a substrate specificity restricted to Fe(II)–NA and Mn(II)–NA (Koike et al., 2004). AtYSL2, on the other hand, did not mediate Fe(II)–NA-, Fe(III)–NA- or Ni(II)–NA-inducible currents when assayed in oocytes (Schaaf et al., 2005). A question therefore arises whether the YSL proteins could transport only the amino acid derivative moiety of the complex. The observation that ZmYS1 expression in oocytes mediates currents induced by metal–DMA complexes but not by DMA alone argues against this idea (Schaaf et al., 2004); however, the final proof awaits direct measurement of radiolabelled PS uptake in oocytes.
Role of the YSLs in metal homeostasis: hints from their gene expression
Since the metal substrate specificity of the YSL transporters, when known, is quite broad, gene expression modulation has been analysed with regard to the content of various metals, which may give an idea of the physiological role of these proteins. As expected considering their function of Fe(III)–PS uptake in grasses under Fe-deficient conditions, ZmYS1, HvYS1 and OsYSL15 are rather strongly up-regulated in response to low Fe supply (Curie et al., 2001; Koike et al., 2004; Murata et al., 2006). On the contrary, expression of AtYSL1–8, TcYSL3, 5, 7 and most of the OsYSL genes except OsYSL2, are little or not at all modulated by the plant metal status. The subclass AtYSL1–3 is repressed about 2-fold by Fe deficiency and induced by Fe excess (DiDonato et al., 2004; Le Jean et al., 2005; Schaaf et al., 2005; Waters et al., 2006). Among the rice YSL genes, OsYSL2 is strongly expressed in leaves in response to Fe deficiency (Koike et al., 2004). Regulation of the YSL genes expression by metals other than Fe has also been investigated. So far, only AtYSL2 has given interesting results since it was shown to be repressed strongly by Zn deficiency (Schaaf et al., 2005) and mildly by Cu excess (DiDonato et al., 2004). In the latter treatment, however, because Cu provokes Fe deficiency though competition, Cu-induced expression is likely to be indirect.
Tissue localization of the YSL genes
Genes encoding Fe–PS transporters are, as expected, expressed in root epidermis. The other YSL genes, which comprise all the genes of dicots, have three main territories of expression: vascular tissues throughout the plant, pollen grains and seeds. Microarray databases also indicate a marked increase of expression in senescent leaves for most AtYSL genes, a pattern that was confirmed experimentally for AtYSL1 and AtYSL3 (Waters et al., 2006). These expression patterns imply roles for YSL transporters in long-distance transport through the saps, microsporogenesis/fertility and seed development, as well as metal remobilization during senescence.
Membrane localization of the YSL proteins: putative role in metal movement to or from the vacuole
Comparison of Fe and NA localization between tomato chloronerva or pea bronze/dgl mutants and their respective wild-type parent highlights a role for NA in sub-cellular allocation of Fe. If YSL transporters participate in the import/export of Fe–NA into/from the organelles, one might expect to find YSL proteins on intracellular membranes. However, membrane targeting of the large majority of the YSL family members is predicted to be in the plasma membrane. Experimental evidence supports this view for HvYS1, TcYSL3, 5 and AtYSL2 (DiDonato et al., 2004; Murata et al., 2006; Gendre et al., 2007). As expected, given its role in Fe(III)–mugineic acid uptake from the soil, HvYS1 was shown to be located in the plasma membrane of root epidermal cells of Fe-deficient barley plants (Murata et al., 2006). AtYSL2 was shown to localize to the lateral membranes of pericycle cells, a localization that is compatible with a role in loading or unloading of root vessels (DiDonato et al., 2004). Interestingly, AtYSL4 and AtYSL6, and only they among the AtYSL family, came out of an arabidopsis tonoplastic proteomic study (Jaquinod et al., 2007). Confirmation of these proteomic data is crucial to determine whether or not YSL transporters are involved in intracellular compartmentalization of metals. As potential H+/metal–NA co-transporters, AtYSL4 and AtYSL6 are likely to function in the influx from the acidic vacuole to the cytoplasm. Alternately, since neutral vacuoles are also present in cells, AtYSL4 and AtYSL6 could cause metal–NA complexes to flow out of the cytoplasm to the neutral vacuolar compartments. Pich et al. (1997) were able to detect accumulation of NA in vacuoles of leaves and roots of plants treated with an excess of Fe. It would therefore be interesting to test whether AtYSL4 and AtYSL6 contribute to this NA movement. AtYSL4 and AtYSL6, together with the two rice members OsYSL5 and OsYSL6, form a separate cluster in the YSL family (Fig. 2) whose function in metal homeostasis has not yet been documented by reverse genetics approaches.
Physiological role of the YSL transporters investigated by reverse genetics
Given how difficult it is to show transport activity of the YSL proteins by heterologous expression, YSL function in planta has been investigated by reverse genetics. However, knocking out a single YSL gene did not produce plants with visible phenotypes (DiDonato et al., 2004; Le Jean et al., 2005; Waters et al., 2006; A. Schikora, J. Misson, D. Couch, S. Mari, C. Curie, unpubl. res.). Moreover, to our knowledge, very few double knockout combinations do show macroscopic phenotypes, and only when the combination involves mutations in genes of the same AtYSL sub-class (Fig. 3, G. Cassin, A. Schikora, D. Couch, S. Mari, C. Curie, P. Czernic, unpubl. res.). Remarkably, crossing loss-of-function mutants of genes harbouring the same territories of expression, such as AtYSL1 and AtYSL7 in pollen, did not produce abnormal phenotypes. Conversely, plants inactivated in two genes that belong to the same sub-class but have non-overlapping expression territories, such as AtYSL5 and AtYSL7, develop a strong phenotype (G. Cassin, A. Schikora, D. Couch, S. Mari, C. Curie, P. Czernic, unpubl. res.). These observations imply a distinct role for each sub-class of YSL. Either they function in different pathways of metal transport, or they transport in different directions such as loading versus unloading of the vessels. Alternately YSL transporters could have different substrate specificities in terms of the metal or the ligand involved in the transported complex.
Fig. 3.
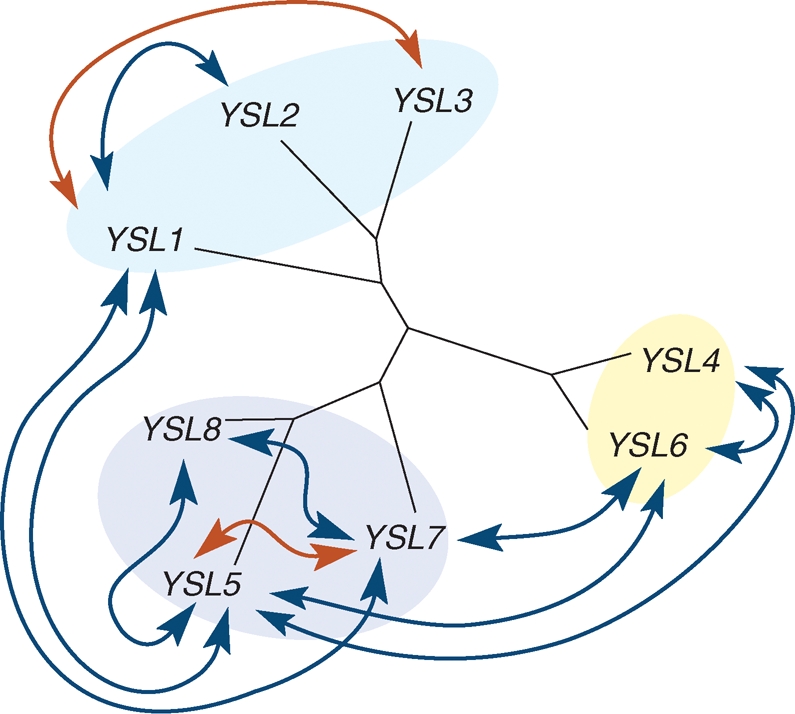
Crosses between AtYSL gene knockouts lead to macroscopic phenotypes only when affecting proteins of the same AtYSL subclass. Arrows indicate the combinations of genes knocked out in double mutants: blue, combinations with no apparent phenotype; red, combinations affecting plant growth and development.
Role of the YSLs in the development of reproductive organs
Current reports on the characterization of Arabidopsis ysl knockouts point to an involvement of the YSL transporters in the development of pollen grains and seeds. This is consistent with the strong expression of the YSL genes observed in these organs and with the fact that pollen and seeds represent the main sink for Fe in arabidopsis (H. Roschzttardtz Choucroun, unpubl. res.). Moreover this phenotype is reminiscent of the one observed in tobacco NAAT overexpressor plants that lack NA (Takahashi et al., 2003).
The double ysl1ysl3 loss-of-function mutant illustrates the role of the YSL proteins in pollen development (Waters et al., 2006). Both AtYSL1 and AtYSL3 genes are expressed in pollen grains, albeit at different stages since AtYSL1 expression is only detected in pollen of flower buds and later on localizes to the vascular tissue of the anther filament (Le Jean et al., 2005), whereas AtYSL3 is strongly expressed in pollen of open flowers (Waters et al., 2006). Consistently, anthers of ysl1ysl3 plants produce very few pollen grains, most of which are not viable. Fe supplementation greatly increases pollen production of ysl1ysl3 plants, indicating that Fe deficiency is causing the defect, although confirmation by the measurement of metal content in pollen grains was not provided in that study. Our unpublished research as well as publicly available transcriptomic studies (https://www.genevestigator.ethz.ch) indicate that in addition to that of AtYSL1 and AtYSL3, expression of AtYSL2, 5, 6 and 7 is also enhanced in stamen. Takahashi et al. (2003) have observed that anthers of tobacco 35S-naat plants have a reduced concentration of Fe and produce very few pollen grains. The requirement of NA for proper Fe content in pollen is also illustrated by the tobacco NAS overexpressor that produces pollen grains with 2·5 times more Fe than wild-type tobacco. The finding that the double ko ysl1ysl3 mimics part of the phenotypic defects of a NA-less mutant is compatible with a role for AtYSL1, 3 in the delivery of Fe to pollen as an Fe–NA complex. Likewise, the arabidopsis ysl5ysl7 double mutant produces few viable pollen grains although, as for 35S-naat plants, development of the female gametophyte is also affected (G. Cassin, unpubl. res.).
Role of YSL transporters in seed formation
Seeds of the ysl1 loss-of-function mutant contain less Fe and NA as wild-type seeds, regardless of the Fe concentration provided in the culture medium (Le Jean et al., 2005). AtYSL1 is expressed in the chalazal endosperm of the seed, a tissue that is thought to contribute to the nutrition of the developing embryo. Moreover, AtYSL1 is expressed in the silique vascular tissue and increases strongly in the peduncle of the silique between fertilized pistil and mature silique stage. Direct proof of the Fe–NA transport activity of AtYSL1 is lacking, but the phenotype of the ysl1 mutant is consistent with it being involved in the delivery of Fe to the seeds as an Fe–NA complex. OsYSL2, a rice member that mediates Fe(II)–NA and Mn(II)–NA transport activity in Xenopus oocytes, also shows a strong promoter activity in developing seeds. OsYSL2 is phylogenetically most related to the arabidopsis AtYSL1-3 sub-class, to which it is between 60 % and 65 % similar at the protein level and represents a good candidate transporter in rice to provide NA–metal chelates to the seed. Confirmation of this hypothesis still awaits the characterization of null OsYSL2 alleles or OsYSL2 RNAi transgenic rice. Imaging 55Fe circulation in such rice genotypes using a positron-emitting tracer imaging system (PETIS), as shown for 62Zn translocation in rice (Suzuki et al., 2008), would nicely document the role of OSYSL2 in Fe delivery to the seed. The double ysl1ysl3 is almost sterile but, surprisingly, the few seeds produced contain the same amount of Fe as the single ysl1 ko (Waters et al., 2006), suggesting that YSL3 does not contribute to the seed Fe content. Cu, however, is drastically reduced in seeds of ysl1ysl3 plants contrary to ysl1 seeds. Whether this phenotype is already observed in a ysl3 single mutant remains to be determined.
Role of the YSLs in metal remobilization from senescent leaves
Remobilization of metal reserves from leaves and hulls (siliques envelope) to supply seeds has been estimated in a recent study to represent at most 40 % of the seed content in arabidopsis (Waters and Grusak, 2008). This implies that continuous uptake during seed filling also constitutes the major source of metal supply to the seed. Based on public microarray data, it is remarkable that five out of eight arabidopsis YSL genes, as well as the AtNAS3 gene, are most strongly expressed in senescent leaves. The activity of AtYSL1 and AtYSL3 promoters is enhanced in leaves during senescence (Waters et al., 2006). The fact that two non-allelic Atysl1 loss-of-function mutants produce seeds containing half of the wild-type Fe concentration (Le Jean et al., 2005) is compatible with a loss of Fe remobilization capacity from senescing leaves. Alternately, the strong expression of AtYSL1 in vascular tissues of the silique peduncle supports a role for AtYSL1 of providing to the developing seed a pool of Fe that could originate from either remobilized seeds, or continuous uptake by the plant, or both. Analysis of the ysl1ysl3 double mutant strengthens this view, at least for Cu. While leaves of wild-type arabidopsis lose almost 60 % of Cu content between the 4th and the 5th week of growth, leaves of ysl1ysl3 lose only 10 % in the same time frame (Waters et al., 2006). Since the Cu concentration of ysl1ysl3 seeds is 5-fold lower than that of wild-type seeds, it suggests that AtYSL1 and AtYSL3 function in Cu remobilization from leaves to seeds. However, this is only estimated by the measurement of the net content change as opposed to showing metal movement between organs, which will require the use of radioactive metal isotopes. Another interesting avenue to address the role of YSL transporters in metal remobilization from leaves to flowers and seeds is the technique of grafting by which a ysl mutant floral stem could be grafted on a wild-type rosette or vice versa.
CONCLUSIONS
In conclusion, the biochemical properties of NA and the physiological data, mainly on NA-free mutants, indicate that NA is very probably a Cu chelator in the xylem sap for its translocation from the roots to the shoots. Furthermore, NA is very likely an important molecule for the phloem loading and or unloading processes, for Cu, Fe and Zn, although the technical difficulty of obtaining reasonable amounts of phloem sap, compatible with biochemical approaches, has not allowed further developments in this direction so far. The different techniques developed on chloronerva, based on Fe imaging and immuno-histochemical localization of NA have pointed to a role in Fe homeostasis, most likely by keeping Fe in a soluble and available form and maybe also in detoxification mechanisms.
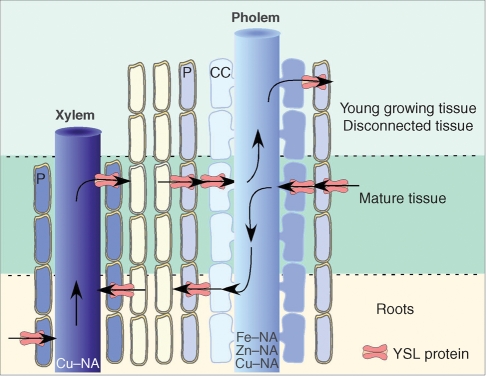
Studies on ZmYS1 and HvYS1, transporters of Fe(III)–PS from the soil to root epidermis in Poaceae, have provided the basic knowledge of the transport activity of this family of proteins. Assuming that most members of the YSL family, as exemplified by TcYSL3 and OsYSL2, are plasma membrane-located proteins involved in NA–metal transport in the same direction, several roles can be proposed for YSL proteins in metal homeostasis. A common feature of the YSL genes is that expression is limited to the vascular system. The precise localization of the expression, to one type of vessel has not been systematically reported, except for AtYSL1 (xylem parenchyma) and OsYSL2 (phloem companion cells). In other cases the expression seems to be spread around or between the phloem and xylem, like TcYSL3, AtYSL3 (S. Mari, unpubl. res.) or AtYSL5 (J. Misson, unpubl. res.). If YSL proteins are competent in NA–metal uptake, they could participate (a) in the unloading of metals from the xylem sap through the uptake by xylem parenchyma cells and (b) in the loading of phloem sieve tubes via uptake by phloem-associated cells (Fig. 4). We also propose, for those genes expressed around both types of vessels, a function in xylem–phloem exchange. This function can be important in all supporting tissues like petioles and stems for the fine balance of NA–metal nutrition and also in young organs or tissues where xylem is either not differentiated yet or interrupted as for seed coat and embryo.
Fig. 4.
Scheme of the hypothetical transport activity of YSL proteins in and around the vessels. Xylem-to-phloem exchange is proposed in young growing tissues whereas phloem-to-xylem exchange is thought to occur in roots. Assuming that all YSL transporters follow the proton gradient and function as importers, the proteins have been drawn as to perform inward polarity transport in parenchyma cells surrounding the vessels or in phloem companion cells. Only Cu–NA is likely to be stable at the pH of the xylem sap, whereas Cu–NA, Fe–NA and Zn–NA are stable at the symplastic pH and thus may circulate in phloem. P, Parenchyma; CC, companion cell.
In roots, Ni and most likely Cu are transported in the xylem sap chelated to NA. Among YSL proteins, AtYSL2, AtYSL3 and TcYSL3 are expressed in the root central cylinder. It is probable that these proteins are involved in the loading of NA–metal complexes in xylem, by mediating the uptake of the complexes in cells associated with the direct loading of the xylem vessels (Fig. 4). It cannot be excluded that these YSL proteins can function in the nutrition of root cells via the phloem since it is not clear whether these genes are exclusively expressed around one type of vessel or the other, or both.
In leaves, the expression of AtYSL1 and AtYSL3 is increased during senescence and the double ysl1ysl3 mutant is strongly affected in the remobilization of Cu and Zn. These genes may thus participate directly in the loading of Cu–NA and Zn–NA complexes in the phloem or, alternately, function in the re-direction of these metals from the xylem stream towards phloem. Since AtYSL1 appears to be expressed preferentially in xylem parenchyma cells, such a xylem-to-phloem exchange would reconcile a xylem-associated expression with the observed phloem-associated phenotype (lack of remobilization from senescent tissue or defect of seed filling).
AtYSL1 is highly expressed in the anther filament. As proposed for the leaves, its function could be in the long-distance transport of NA–metal that precedes delivery to pollen. After anther dehiscence, AtYSL1 promoter activity increases in senescing stamens (Le Jean et al., 2005), suggesting that a remobilization of metals involving AtYSL1 could take place. The strong expression of several YSL genes in pollen grains indicates a role for these proteins in pollen metal homeostasis, possibly in the uptake of NA–metal complexes directly from the tapetum. In seeds, AtYSL1 and AtYSL3 are required to maintain the amount of Fe, Zn and Cu as well as NA (Le Jean et al., 2005; Waters et al., 2006), which supports the view that these metals are delivered to the seeds or absorbed by the developing embryo as NA–metal complexes. As the filling of seeds supposes that, at a given point, the xylem path is interrupted in favour of a symplastic movement, we propose that these genes play an important role in circulating metals from the xylem to the phloem, i.e. from an apoplastic to a symplastic path. In future developments in this field, MS techniques could be used to demonstrate to which metal NA is bound in phloem and xylem exudates. In addition, imaging of the repartition of metal pools between organs and saps and comparison of these pools between ysl mutants represent a promising avenue to gain further insight into the role of the YSL transporters in the circulation of essential and toxic metals in the plant.
ACKNOWLEDGEMENTS
We thank Cécile Fizames for performing phylogenetic analyses of the YSL protein family. This work was supported by the Centre National pour la Recherche Scientifique (CNRS) and the Institut National pour la Recherche Agronomique (INRA). It was also funded by a ToxNuc-E contract from the Réseau Inter-Organismes (RIO) and the ‘CIDS’ contract from the Agence Nationale pour la Recherche (ANR).
LITERATURE CITED
- Anderegg G, Ripperger H. Correlation between metal complex formation and biological activity of nicotianamine analogues. Journal of the Chemical Society – Chemical Communications. 1989;10:647–650. [Google Scholar]
- Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, et al. Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. Journal of Biological Chemistry. 2006;281:32395–32402. doi: 10.1074/jbc.M604133200. [DOI] [PubMed] [Google Scholar]
- Becker R, Fritz E, Manteuffel R. Subcellular localization and characterization of excessive iron in the nicotianamine-less tomato mutant chloronerva. Plant Physiology. 1995;108:269–275. doi: 10.1104/pp.108.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes I, Schreiber K, Ripperger H, Kirsceiss A. Metal complex formation of nicotianamine, a possible phytosiderophore. Experientia. 1983;39:261–262. [Google Scholar]
- Briat JF, Cellier F, Gaymard F. Ferritins and iron accumulation in plant tissues. In: Barton L, Abadia J, editors. Iron nutrition in plants and rhizospheric microorganisms. Amsterdam: Kluwer Academic Publishers; 2006. [Google Scholar]
- Curie C, Briat JF. Iron transport and signaling in plants. Annual Review of Plant Biology. 2003;54:183–206. doi: 10.1146/annurev.arplant.54.031902.135018. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- DiDonato RJ, Jr, Roberts LA, Sanderson T, Eisley RB, Walker EL. Arabidopsis Yellow Stripe-Like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. The Plant Journal. 2004;39:403–414. doi: 10.1111/j.1365-313X.2004.02128.x. [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proceedings of the National Academy of Sciences of the USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre D, Czernic P, Conejero G, Pianelli K, Briat JF, Lebrun M, et al. TcYSL3, a member of the YSL gene family from the hyper-accumulator Thlaspi caerulescens, encodes a nicotianamine-Ni/Fe transporter. The Plant Journal. 2007;49:1–15. doi: 10.1111/j.1365-313X.2006.02937.x. [DOI] [PubMed] [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, et al. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- Harada E, Sugase K, Namba K, Iwashita T, Murata Y. Structural element responsible for the Fe(III)-phytosiderophore specific transport by HvYS1 transporter in barley. FEBS Letters. 2007;581:4298–4302. doi: 10.1016/j.febslet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Herbik A, Giritch A, Horstmann C, Becker R, Balzer HJ, Baumlein H, et al. Iron and copper nutrition-dependent changes in protein expression in a tomato wild type and the nicotianamine-free mutant chloronerva. Plant Physiology. 1996;111:533–540. doi: 10.1104/pp.111.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R. Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics. 2007;8:460. doi: 10.1186/1471-2105-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ The Plant Journal. 2006;45:335–346. doi: 10.1111/j.1365-313X.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- Jaquinod M, Villiers F, Kieffer-Jaquinod S, Hugouvieux V, Bruley C, Garin J, et al. A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Molecular & Cellular Proteomics. 2007;6:394–412. doi: 10.1074/mcp.M600250-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Rep M. Protein extraction from xylem and phloem sap. Methods in Molecular Biology. 2007;355:27–35. doi: 10.1385/1-59745-227-0:27. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Suzuki M, Inoue H, Itai RN, Takahashi M, Nakanishi H, et al. Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. Journal of Experimental Botany. 2005;56:1305–1316. doi: 10.1093/jxb/eri131. [DOI] [PubMed] [Google Scholar]
- Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, et al. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. The Plant Journal. 2004;39:415–424. doi: 10.1111/j.1365-313X.2004.02146.x. [DOI] [PubMed] [Google Scholar]
- Krueger C, Berkowitz O, Stephan UW, Hell R. A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. Journal of Biological Chemistry. 2002;277:25062–25069. doi: 10.1074/jbc.M201896200. [DOI] [PubMed] [Google Scholar]
- Le Jean M, Schikora A, Mari S, Briat JF, Curie C. A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. The Plant Journal. 2005;44:769–782. doi: 10.1111/j.1365-313X.2005.02569.x. [DOI] [PubMed] [Google Scholar]
- Ling HQ, Koch G, Baumlein H, Ganal MW. Map-based cloning of chloronerva, a gene involved in iron uptake of higher plants encoding nicotianamine synthase. Proceedings of the National Academy of Sciences of the USA. 1999;96:7098–7103. doi: 10.1073/pnas.96.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari S, Gendre D, Pianelli K, Ouerdane L, Lobinski R, Briat JF, et al. Root-to-shoot long-distance circulation of nicotianamine and nicotianamine-nickel chelates in the metal hyperaccumulator Thlaspi caerulescens. Journal of Experimental Botany. 2006;57:4111–4122. doi: 10.1093/jxb/erl184. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cozatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, et al. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus: a role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. The Plant Journal. 2008;54:249–259. doi: 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nishizawa NK. Methionine as a dominant precursor of phytosiderophores in graminae plants. Plant Cell Physiology. 1987;28:1081–1092. [Google Scholar]
- Mori S, Nishizawa NK, Hayashi H, Chino M, Yoshimura E, Ishihara J. Why are young rice plants highly susceptible to iron deficiency? Plant and Soil. 1991;130:143–156. [Google Scholar]
- Murata Y, Ma JF, Yamaji N, Ueno D, Nomoto K, Iwashita T. A specific transporter for iron(III)-phytosiderophore in barley roots. The Plant Journal. 2006;46:563–572. doi: 10.1111/j.1365-313X.2006.02714.x. [DOI] [PubMed] [Google Scholar]
- Nishizawa N. Montpellier, France: 2006. Abstract 13th International Symposium on Iron Nutrition in Plants. 3–7 July 2006. [Google Scholar]
- Ouerdane L, Mari S, Czernic P, Lebrun M, Lobinski R. Speciation of non-covalent nickel species in plant tissue extracts by electrospray Q-TOF MS/MS after their isolation by 2D Size-Exclusion–Hydrophilic Interaction LC (SEC–HILIC) monitored by ICP MS. Journal of Analytical Atomic Spectrometry. 2006;21:676–683. [Google Scholar]
- Pich A, Scholz G. Nicotianamine and the distribution of iron into apoplast and symplast of tomato (Lycopersicon esculentum Mill.). II. Uptake of iron by protoplasts from the variety Bonner Beste and its nicotianamine-less mutant chloronerva and the compartmentation of iron in leaves. Journal of Experimental Botany. 1991;42:1517–1523. [Google Scholar]
- Pich A, Scholz G. Translocation of copper and other micronutrients in tomato plants (Lycopersicon esculentum Mill): nicotianamine-stimulated copper transport in the xylem. Journal of Experimental Botany. 1996;47:41–47. [Google Scholar]
- Pich A, Scholz G, Stephan UW. Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of two tomato genotypes: nicotianamine as possible copper translocator. Plant and Soil. 1994;165:189–196. [Google Scholar]
- Pich A, Hillmer S, Manteuffel R, Scholz G. First immunohistochemical localization of the endogenous Fe2+-chelator nicotianamine. Journal of Experimental Botany. 1997;48:759–767. [Google Scholar]
- Pich A, Manteuffel R, Hillmer S, Scholz G, Schmidt W. Fe homeostasis in plant cells: does nicotianamine play multiple roles in the regulation of cytoplasmic Fe concentration? Planta. 2001;213:967–976. doi: 10.1007/s004250100573. [DOI] [PubMed] [Google Scholar]
- Rellan-Alvarez R, Abadia J, Alvarez-Fernandez A. Formation of metal-nicotianamine complexes as affected by pH, ligand exchange with citrate and metal exchange: a study by electrospray ionization time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2008;22:1553–1562. doi: 10.1002/rcm.3523. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Pierson AJ, Panaviene Z, Walker EL. Yellow stripe1: expanded roles for the maize iron-phytosiderophore transporter. Plant Physiology. 2004;135:112–120. doi: 10.1104/pp.103.037572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wiren N. ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. Journal of Biological Chemistry. 2004;279:9091–9096. doi: 10.1074/jbc.M311799200. [DOI] [PubMed] [Google Scholar]
- Schaaf G, Schikora A, Häberle J, Vert GA, Ludewig U, Briat JF, et al. A putative function of the Arabidopsis Fe-Phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant and Cell Physiology. 2005;46:762–774. doi: 10.1093/pcp/pci081. [DOI] [PubMed] [Google Scholar]
- Schmidke I, Kruger C, Frommichen R, Scholz G, Stephan U. Phloem loading and transport characteristics of iron in interaction with plant- endogenous ligands in castor bean seedlings. Physiologia Plantarum. 1999;106:82–89. [Google Scholar]
- Stephan UW, Grun M. Physiological disorders of the nicotianamine- auxotroph tomato mutant chloronerva at different levels of iron nutrition. II. Iron deficiency response and heavy metal metabolism. Biochemie und Physiologie der Pflanzen. 1989;185:3–4. [Google Scholar]
- Stephan UW, Scholz G. Nicotianamine: mediator of transport of iron and heavy metals in the phloem? Physiologia Plantarum. 1993;88:522–529. [Google Scholar]
- Stephan UW, Schmidke I, Stephan VW, Scholz G. The nicotianamine molecule is made-to-measure for complexation of metal micronutrients in plants. Biometals. 1996;9:84–90. [Google Scholar]
- Suzuki M, Takahashi M, Tsukamoto T, Watanabe S, Matsuhashi S, Yazaki J, et al. Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. The Plant Journal. 2006;48:85–97. doi: 10.1111/j.1365-313X.2006.02853.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Tsukamoto T, Inoue H, Watanabe S, Matsuhashi S, Takahashi M, et al. Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Molecular Biology. 2008;66:609–617. doi: 10.1007/s11103-008-9292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar J, Lobinski R, Prange A. Hyphenated techniques for elemental speciation in biological systems. Applied Spectroscopy. 2003;57:102A–112A. doi: 10.1366/000370203321558128. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, et al. Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. The Plant Cell. 2003;15:1263–1280. doi: 10.1105/tpc.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchina V, Mari S, Czernic P, Marques L, Pianelli K, Schaumloffel D, et al. Speciation of nickel in a hyperaccumulating plant by high-performance liquid chromatography-inductively coupled plasma mass spectrometry and electrospray MS/MS assisted by cloning using yeast complementation. Analytical Chemistry. 2003;75:2740–2745. doi: 10.1021/ac020704m. [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, et al. IRT1, an arabidopsis transporter essential for iron uptake from the soil and for plant growth. The Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters BM, Grusak MA. Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the mutant line ysl1ysl3. New Phytology. 2008;177:389–405. doi: 10.1111/j.1469-8137.2007.02288.x. [DOI] [PubMed] [Google Scholar]
- Waters BM, Chu HH, Didonato RJ, Roberts LA, Eisley RB, Lahner B, et al. Mutations in Arabidopsis yellow stripe-like1 and yellow stripe-like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiology. 2006;141:1446–1458. doi: 10.1104/pp.106.082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wiren N, Mori S, Marschner H, Romheld V. Iron inefficiency in maize mutant ys1 (Zea mays L. cv Yellow-Stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiology. 1994;106:71–77. doi: 10.1104/pp.106.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wiren N, Marschner H, Romheld V. Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiology. 1996;111:1119–1125. doi: 10.1104/pp.111.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wiren N, Klair S, Bansal S, Briat JF, Khodr H, Shioiri T, et al. Nicotianamine chelates both FeIII and FeII: implications for metal transport in plants. Plant Physiology. 1999;119:1107–1114. doi: 10.1104/pp.119.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen MR, Tseng YH, Saier MH., Jr Maize Yellow Stripe1, an iron-phytosiderophore uptake transporter, is a member of the oligopeptide transporter (OPT) family. Microbiology. 2001;147:2881–2883. doi: 10.1099/00221287-147-11-2881. [DOI] [PubMed] [Google Scholar]