Abstract
Background and Aims
While invasive species may escape from natural enemies in the new range, the establishment of novel biotic interactions with species native to the invaded range can determine their success. Biological control of plant populations can be achieved by manipulation of a species' enemies in the invaded range. Interactions were therefore investigated between a native parasitic plant and an invasive legume in Mediterranean-type woodlands of South Australia.
Methods
The effects of the native stem parasite, Cassytha pubescens, on the introduced host, Cytisus scoparius, and a co-occurring native host, Leptospermum myrsinoides, were compared. The hypothesis that the parasitic plant would have a greater impact on the introduced host than the native host was tested. In a field study, photosynthesis, growth and survival of hosts and parasite were examined.
Key Results
As predicted, Cassytha had greater impacts on the introduced host than the native host. Dead Cytisus were associated with dense Cassytha infections but mortality of Leptospermum was not correlated with parasite infection. Cassytha infection reduced the photosynthetic rates of both hosts. Infected Cytisus showed slower recovery of photosystem II efficiency, lower transpiration rates and reduced photosynthetic biomass in comparison with uninfected plants. Parasite photosynthetic rates and growth rates were higher when growing on the introduced host Cytisus, than on Leptospermum.
Conclusions
Infection by a native parasitic plant had strong negative effects on the physiology and above-ground biomass allocation of an introduced species and was correlated with increased plant mortality. The greater impact of the parasite on the introduced host may be due to either the greater resources that this host provides or increased resistance to infection by the native host. This disparity of effects between introduced host and native host indicates the potential for Cassytha to be exploited as a control tool.
Key words: Biological control, Cassytha pubescens, Cytisus scoparius, Leptospermum myrsinoides, parasitic plant, plant interactions, plant invasion, Scotch broom
INTRODUCTION
Invasive species become a management problem in natural ecosystems when an increase in their distribution and abundance results in disruption to ecological processes and the emergent properties of these systems. The ability of an invader to increase into a new range depends upon the opportunities the invaded community provides for the invader and the characteristics of the invading species (Shea and Chesson, 2002). The role of biotic interactions in the invasion process has long been considered important (see, for example, Darwin, 1859; Elton, 1958) but has less frequently been addressed in empirical research.
Two hypotheses have been proposed to explain how biotic interactions influence the invasive process. The enemy release hypothesis (ERH) proposes that plants invading a new range are able to spread and increase in abundance as they escape from population regulation by their co-evolved natural enemies that occur in their native range (Maron and Vila, 2001; Keane and Crawley, 2002; Parker and Hay, 2005). In contrast, the biotic resistance hypothesis (BRH) suggests that strong biotic interactions with native competitors, pathogens and/or herbivores in the invaded community can prevent invading species from establishing (Elton, 1958; Maron and Vila, 2001; Parker and Hay, 2005). The ERH predicts that generalist enemies in the new range will prefer native over introduced plants, and there will be no specialist enemies of the introduced plant present, thereby conferring a competitive advantage to the invading species. However, there is little empirical support for this (Maron and Vila, 2001; Agrawal and Kotanen, 2003; Colautti et al., 2004; Parker and Hay, 2005; Parker et al., 2006; Parker and Gilbert, 2007), as generalist enemies can have similar or greater effects on introduced species than on native species (but see Nunez et al., 2008). In the case of invertebrate herbivores of plants, however, there is evidence supporting both the ERH and BRH hypotheses. Specialist herbivores can regulate plant populations in their native range, whilst in the invaded range specialist herbivores tend to be absent (ERH); however, generalist herbivores provide resistance to invasion (BRH) (Strong et al., 1984; Liu and Stiling, 2006). Similar patterns have been observed for plant pathogens, with invasive and native species being equally or less susceptible to generalist pathogens (Agrawal et al., 2005; Parker and Gilbert, 2007), and some evidence of escape by introduced species from specialist pathogens of their native range (Mitchell and Power, 2003).
Classical biological control of invasive species exploits the ERH by introducing specialist enemies into the invaded range. Effective biological control agents have high host specificity and cause direct damage to the target species through continuous and prolonged attack (Myers and Bazely, 2003); however, the ecological drawbacks to this control method include the introduction of further exotic species into new ranges. If introduced species are more susceptible to native enemies than native species, then the introduction of native enemies into new populations of invading species may provide a viable control strategy (Colautti et al., 2004). This strategy would have an advantage if native plants have more resistance to the native enemy than the invading species, as predicted by the BRH.
Cytisus scoparius (Scotch broom; hereafter Cytisus) is a leguminous shrub native to Europe that is invasive in Australia, New Zealand and North America (Syrett et al., 1999). Population densities of Cytisus are higher in Australia than in the native range, although population longevity and plant size do not differ between the introduced and native ranges (Paynter et al., 2003). Phytophagous insects significantly inhibit the growth of Cytisus in its native range (Waloff and Richards, 1977). Similar herbivores, however, have little impact on introduced populations in the United States (Bossard and Rejmánek, 1994), New Zealand or Australia, despite the fact that generalist phytophagous insects are common on Cytisus in Australia and New Zealand (Memmott et al., 2000). The introduction of specialist insects from the species native range has also met with only limited success to date in invasive populations (Bossard and Rejmánek, 1994; Syrett et al., 1999). The species may encounter little biotic resistance in the communities it invades, at least in Australia and New Zealand, where it is a superior resource competitor with at least some of the native shrubs with which it co-occurs (Bellingham, 1998; Fogarty and Facelli, 1999).
A new potential enemy of Cytisus in the temperate woodlands of southern Australia has recently been observed. The native parasitic vine, Cassytha pubescens (Dodder laurel; hereafter Cassytha), occurs at high densities on Cytisus and observations suggest that infection by the parasite results in damage to this host. Parasitic plants can affect host productivity by extracting water, nutrients and organic compounds from the host's vascular system and also by impacting on host physiology and thus impairing the host's ability to acquire resources (Press et al., 1999). The extent to which this impacts on host performance depends upon the degree of autotrophy of the parasite, the relative ability of host and parasite sinks to attract resources (Graves, 1995; Press et al., 1999) and the tolerance or resistance to infection of the host species (Koskela et al., 2001, 2002; Rispail et al., 2007).
Cassytha is a stem hemi-parasite with a broad host range, although it is mostly confined to woody shrubs (McLuckie, 1924). Host–parasite associations can range from highly host-specific, to generalist, where a parasite may infect many species across taxonomic and functional groups. Most parasites display some host preference, where performance is poorer on non-preferred species and parasite density is disproportional to host availability (Kelly et al., 1988; Horning, 1995; Musselman and Press, 1995). Variation in the observed effects of a parasite on its host may be due to the combined influence of the virulence of the parasite and the susceptibility of the host (Keith et al., 2004). Typically, increasing negative effects of a parasite on its host are observed on host plants upon which parasites can achieve the highest biomass (Atsatt and Strong, 1970; Kelly et al., 1988; Graves, 1995; Marvier, 1998; Cameron et al., 2008). Hosts with a high nutrient content such as legumes are preferred hosts for several parasitic species (Matthies, 1996; Pate and Bell, 2000; Pennings and Callaway, 2002; Suetsugu et al., 2008; but see Marvier, 1998). Parasite growth is often greater on nitrogen-fixing hosts (Press et al., 1993; Seel and Press, 1993) but the impacts on the host are also greater (Jeschke et al., 1994; Matthies, 1996; Jeschke and Hilpert, 1997).
In this study, the impacts of infection by the native parasitic plant, Cassytha pubescens, on a co-occurring native species, Leptospermum myrsinoides (hereafter Leptospermum), and a novel host, the introduced species, Cytisus scoparius, are examined. The hypothesis is tested that the parasite, as a native generalist, will have a greater impact on the novel host Cytisus than the native host Leptospermum, with which it has co-existed, and possibly co-evolved. It is predicted that the parasite will be able to achieve higher growth rates on the introduced species and will consequently have greater impacts on the physiology and growth of this species than its native host.
MATERIALS AND METHODS
Study site
The study was undertaken in the Mount Lofty Ranges of South Australia (138·69°N, 35·03°E). The study area is secondary Eucalyptus obliqua woodland with an overstorey of scattered E. obliqua trees and an understorey dominated by sclerophyllous shrubs. Cytisus forms dense stands in places, particularly under remnant E. obliqua and occurs scattered throughout regenerating native shrubs. The perennial ground layer comprises sedges, grasses and low sclerophyllous shrubs.
The climate at the study site is Mediterranean-type, with cool, wet winters and hot, dry summers. Soils are sandy loams to sandy clays with low nitrogen and phosphorous content and a pH of 5–6 (H. T. Tsang, University of Adelaide, unpubl res.).
Study species
Cassytha pubescens R.Br. (Lauraceae) is a stem-twining, parasitic vine. The plant is free-living following emergence from the seed but after approx. 6 weeks is completely dependent on a host plant for growth, survival and reproduction (McLuckie, 1924). The leaves are reduced to small scales and the photosynthetic tissue of the plant occurs in the stem. The stems branch extensively and may twine around each other or over one to several host plants. The habit and morphology of Cassytha are similar to the widespread parasitic genus Cuscuta (Convolvulaceae).
Cytisus scoparius (L.) Link (Leguminosae) is a tall, woody, short-lived shrub native to the Mediterranean region. From a supposed initial introduction to Australia in about 1800 (Waterhouse, 1988), by the mid-1800s the plant had been introduced to most of the south-east of Australia as either an ornamental or hedging plant (Hosking et al., 1996). The plant is invasive in the Mount Lofty Ranges where it occurs in disturbed woodlands in the higher rainfall areas. In these woodland systems, Cytisus has high rates of growth and photosynthesis in the spring months but during summer the plants lose their leaves, although the stems remain photosynthetic (Fogarty and Facelli, 1999).
Leptospermum myrsinoides Schltdl. (Myrtaceae) is a medium to tall woody, sclerophyllous shrub that occurs naturally throughout south-eastern Australia. It occurs sparsely throughout the Mount Lofty Ranges but can become dominant in more open areas of heath vegetation, particularly on sandy soils (Specht and Rundel, 1990). Leptospermum was selected as a native host for this study as Cassytha grows at high density on this species, this species occurs at similar densities as Cytisus at the study site (see Results), and the species has a similar growth habit to Cytisus. Although it would have been preferable to compare Cassytha impacts on a native legume with the introduced legume Cytisus, the parasite did not grow vigorously or at high densities on native legumes at the study site.
Field survey
An area of natural vegetation was surveyed to compare the vigour of Cassytha on the two target host species and to qualify the condition of infected host plants. In June 2007, 24 2 × 2 m quadrats were sampled located randomly throughout the study site in the disturbed woodland. Within these areas, data were collected from all shrubs taller than 10 cm (only the data for Cytisus and Leptospermum are presented here). The height of each shrub and the width of the shrub at the widest point and perpendicular to this were measured to calculate the approximate volume of each shrub. The vigour of each shrub was scored qualitatively as dead, mostly dead, partly alive, or all alive. Mostly dead shrubs had >50 % of their stems or leaves dead or discoloured. Partly alive shrubs had some dead or discoloured biomass but this was not >50 % of the biomass. Cassytha was scored as present on the shrub only when haustorial attachments were observed on the target shrub. Cassytha cover was scored visually as low, moderate or high density. Low-density infections covered <10 % of the host shrub and Cassytha was usually present as a few stems. Moderate density infections covered up to 30 % of the host shrub and high density infections covered >30 % of the shrub with the Cassytha growing in dense coiling mats. The vigour of Cassytha on each shrub was scored as dead, vigorous (actively growing, green stems), or low vigour (stems yellowish and no active growth visible).
Cassytha pubescens growth
At the same study site, the growth of Cassytha was measured, as stem elongation, on 20 individuals of the two host species selected as described below. The parasite stems were marked by a small paint mark, 30 mm from the stem tip. At least ten Cassytha shoots were marked on each host where actively growing stem tips could be located. The stems were marked in winter (late June 2007) and measured in early spring (September 2007). New actively growing Cassytha stems on each host were marked in the same way and growth of newly marked stems remeasured in mid-spring (October 2007) and again in summer (December 2007). It was noted whether stems were coiled around the host or self-coiled (coiled around each other).
Above-ground biomass
The above-ground biomass of 20 uninfected and 20 infected host plants of each species and Cassytha on infected plants was harvested. It was ensured that the Cassytha on the infected plant was not attached to other hosts and occurred at moderate to high densities. This restricted plant choice and prevented all plants being selected of approximately equal size. Uninfected plants were selected to match the range of sizes of infected plants. The plants were harvested in December 2007 and January 2008. The biomass was oven-dried at 70 °C and separated into stem, leaf and parasite stem components before weighing. Cytisus stem biomass was further subdivided into woody stems and photosynthetic stems.
Photosynthesis
Eight Cytisus infected by Cassytha and eight non-infected plants and six each of Leptospermum were selected for physiological measurements. All host plants were mature with moderate to high intensity infections of Cassytha and situated in open areas that were unshaded for at least half of the day. Measurements of net assimilation rates and transpiration were made on plants during October 2007 (spring) using a CIRAS-2 infra-red gas analyser (PP Systems, Amesbury, MA, USA). For Cytisus, the first fully expanded leaf was measured from the top of the plant on the northern side of the shrub. Leptospermum leaves grow in small, erect clusters so measurements were made on the first fully expanded cluster of leaves on the uppermost, northern side of the shrub. For Cassytha measurements, two green portions of stem were selected that were located below the young, soft growing tips. Leaf area was determined by tracing leaves on graph paper before insertion into the leaf chamber. A standardized stem area was used for Cassytha, as stems are of similar diameter. Measurements were made on well-watered plants (natural rainfall) under saturating irradiance, with photon flux density greater than 1400 µmol m−2 s−1. Measurements were made between 0830 and 1030 h, on plants that had been in unshaded conditions for at least 30 min.
To determine whether plants infected with Cassytha became stressed, photosystem II (PSII) efficiency was measured during the afternoon and recovery assessed after a short period in the dark. PSII efficiency was measured on the same set of plants as above, after plants had been exposed to full sun for at least the previous 4 h. Measurements were made with a Mini-PAM Portable Chlorophyll Fluorometer fitted with a 2030-B leaf-clip holder (Heinz Walz GmbH, Effeltrich, Germany). A single measurement of yield was made between 1400 and 1500 h under ambient light conditions. The leaves or stems were then covered in aluminium foil. After 30 min the foil was removed and yield was remeasured. The measurements were repeated pre-dawn to determine the maximum quantum yield after an extended recovery period overnight. Plants were sampled at three separate times in October and November 2007.
Statistical analyses
The categorical survey data were analysed using nominal logistic models. For host biomass data, ANCOVA was used to compare photosynthetic tissue allocation between infected and uninfected plants, using woody stem biomass as a covariate. For Cytisus, allocation to leaves between infected and uninfected plants was also compared with photosynthetic stem biomass as the covariate. ANCOVA was also used to compare Cassytha biomass on the two host species, with total host above-ground biomass as the covariate. Analysis of variance was used to compare transpiration rates and maximum photosynthetic rates between infected and uninfected plants, testing each species separately. Repeated measures ANOVA (MANOVA) was used to compare yield measures for uninfected control plants, host plants infected by Cassytha and the parasite. Matched pair t-tests were used to assess short-term recovery in each treatment category, testing a one-tailed probability that yield increased after the recovery period. The statistical package JMP Ver. 4·0·3 (SAS Institute Inc., 2000) was used for analyses.
RESULTS
Survey
Cytisus scoparius occurred more frequently within the surveyed plots than Leptospermum, due mainly to an abundance of Cytisus seedlings. However, the volume of both target host plants per unit area was similar (Cytisus volume mean ± s.e., 0·61 ± 0·14 m3 m−2, n = 603; Leptospermum, 0·49 ± 0·10 m3 m−2, n = 133). Cassytha occurred in 65 % of sampled plots. On a biomass basis, the availability of the two species as hosts for Cassytha was similar; however, on an individual plant basis, Cytisus was a more available host than Leptospermum. Cassytha utilized Leptospermum as a host at higher proportions than its availability in each plot. Leptospermum comprised 13 % of the individuals sampled and was host to 22 % of the Cassytha sampled. Conversely, Cytisus comprised 61 % of all shrubs sampled and was host to 65 % of the parasite sampled. Cassytha preferentially infected taller plants of both species [likelihood ratio (LR) χ2 = 91·73, d.f. = 1, P < 0·0001] with a height (mean ± s.e.) of infected plants of 0·97 ± 0·03 m and uninfected plants 0·61 ± 0·02 m.
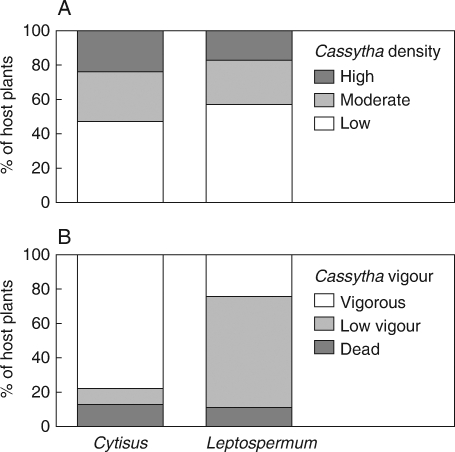
There was no significant difference in the density of Cassytha on the two host species (LR χ2 = 1·81, d.f. = 4, P = 0·40). Approximately half of the infected plants had infections of Cassytha classified as moderate to high intensity (Fig. 1A). Cassytha vigour varied with host species (LR χ2 = 66·36, d.f. = 4, P < 0·0001). A high proportion of the Cassytha growing on Cytisus hosts was vigorous whereas Leptospermum had higher proportions of non-vigorous Cassytha (Fig. 1B).
Fig. 1.
Frequency histograms of the proportions of Cassytha pubescens in different density classes on the two host species Cytisus scoparius and Leptospermum myrsinoides (A), and the vigour of Cassytha on the two host species (B).
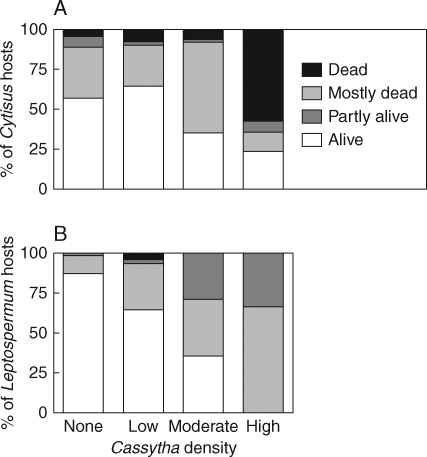
Host condition varied with the density of Cassytha (Cytisus LR χ2 = 100·45, d.f. = 9, P < 0·0001; Leptospermum LR χ2 = 49·73, d.f. = 9, P < 0·0001). The highest proportion of dead Cytisus occurred where Cassytha density was high (Fig. 2A). All Leptospermum plants with high density infections of Cassytha had at least some dead biomass but mortality was not associated with the presence of the parasite (Fig. 2B).
Fig. 2.
Frequency histograms of the proportions of Cytisus scoparius (A), and Leptospermum myrsinoides (B), in different condition classes infected by Cassytha pubescens of increasing density.
Cassytha growth
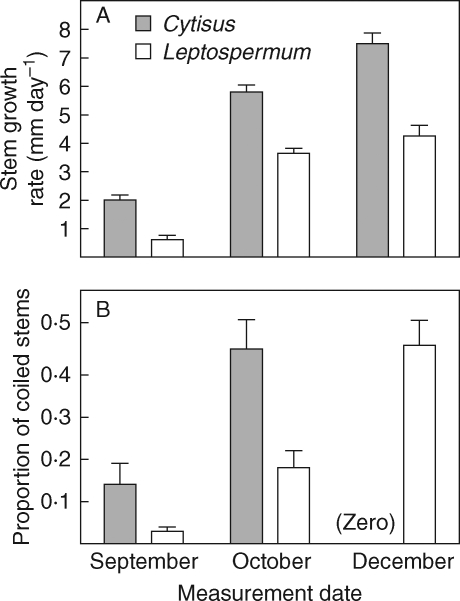
Cassytha stem growth rates were significantly higher on Cytisus hosts than on Leptospermum hosts over the three periods when measurements were made (Fig. 3A). Although Cassytha growth rates continued to increase on Cytisus hosts across the three measurement periods, on Leptospermum hosts growth rate did not increase from October to December (MANOVA time × species interaction, numerator d.f. = 2, denominator d.f. = 19, exact F = 27·96, P < 0·0001). New growth of Cassytha on Cytisus hosts coiled around host stems early in the growing season, whilst new growth of Cassytha stems coiled around Leptospermum stems later in the season (Fig. 3B). Most of the new growth on Cytisus later in the season was self-coiling, with the stems growing upright and self-coiling.
Fig. 3.
Cassytha pubescens stem growth rate (A) and proportion of stems coiled onto host (B) on Cytisus scoparius and Leptospermum myrsinoides hosts over three growth periods (means + s.e., n = 20 for each host species).
Host and parasite biomass
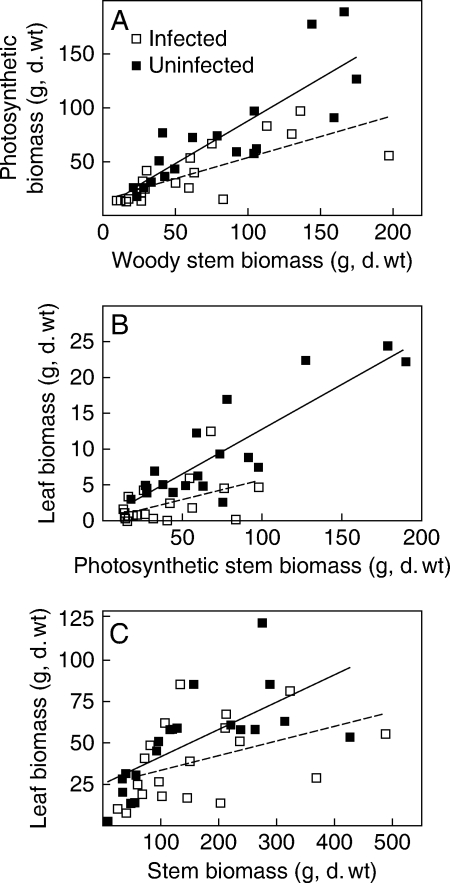
Leaves comprise a very small proportion of the total biomass of Cytisus with most of the photosynthetic biomass occurring in the stems. Infected plants had significantly less photosynthetic biomass than uninfected plants (ANCOVA, F = 2·27, d.f. = 1, P < 0·03; Fig. 4A). For infected plants, leaves contributed significantly less to the photosynthetic biomass than in uninfected plants (ANCOVA, F = 19·97, d.f. = 1, P < 0·0013; Fig. 4B). Infected Leptospermum had lower leaf biomass than uninfected plants but this difference was not significant (ANCOVA, F = 3·68, P = 0·063; Fig. 4C).
Fig. 4.
Biomass of plants infected by Cassytha pubescens and uninfected plants showing the relationship between (A) woody biomass and photosynthetic biomass of Cytisus scoparius (n = 19), (B) photosynthetic biomass and leaf biomass of Cytisus, and (C) stem biomass and leaf biomass of Leptospermum myrsinoides (n = 20). Regression lines are shown for illustrative purposes only.
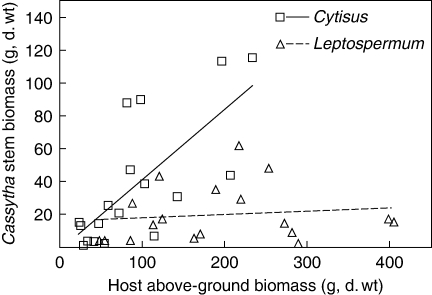
There was significantly less Cassytha biomass on Leptospermum than Cytisus (ANCOVA, d.f. = 1, F = 9·537, P = 0·004). The biomass of Cassytha increased linearly with Cytisus individual biomass but there was no significant linear relationship between Cassytha and Leptospermum biomass (Fig. 5). Cassytha comprised 35 ± 7 % (mean ± s.e.) of the total above-ground biomass of the Cytisus host–parasite association. For many infected Cytisus plants, the biomass of the parasite was greater than the photosynthetic biomass (stems and leaves) of the host plant (mean ± s.e., 104 ± 31 %). The proportion of Cassytha biomass in relation to Leptospermum hosts was lower than that for Cytisus, comprising 11 ± 2 % (mean ± s.e.) of the total above-ground biomass and 73 ± 23 % of the leaf biomass.
Fig. 5.
Relationship between Cassytha pubescens biomass and the total above-ground biomass of host plants, Cytisus scoparius and Leptospermum myrsinoides. Linear regression lines: Cytisus, n = 18, F = 17·48, P < 0·001; Leptospermum, n = 18, F = 0·22, P = 0·65.
Photosynthesis
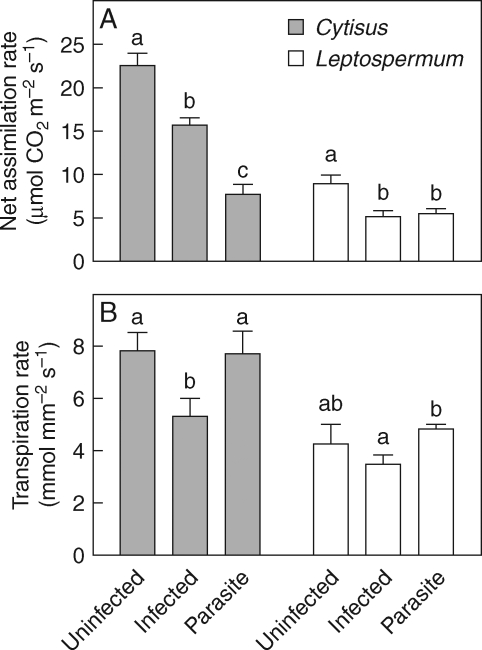
For both host species, plants infected by the parasite had significantly lower photosynthetic rates than uninfected plants (Table 1 and Fig. 6A). Infected Cytisus had significantly lower transpiration rates per unit leaf area than uninfected plants, although this difference was not observed between infected and uninfected Leptospermum (Fig. 6B).
Table 1.
Results of ANOVA testing for differences in physiological parameters between the parasite Cassytha pubescens, host infected by the parasite and uninfected plants
| Host species | Parameter | d.f. | F-statistic | P value |
|---|---|---|---|---|
| Cytisus scoparius | Net assimilation | 2 | 42·20 | <0·0001 |
| Transpiration | 2 | 3·38 | 0·053 | |
| Leptospermum myrsinoides | Net assimilation | 2 | 7·16 | 0·007 |
| Transpiration | 2 | 8·17 | 0·031 |
Fig. 6.
(A) Net assimilation rates and (B) transpiration rates for Cytisus scoparius and Leptospermum myrsinoides infected by Cassytha pubescens, and uninfected hosts (means + s.e., Cytisus n = 8; Leptospermum n = 6). Different letters indicate significant differences between plant treatments (separate Tukey–Kramer pairwise comparisons for each species).
Cassytha growing on Cytisus had significantly lower photosynthetic rates than either infected or uninfected Cytisus (Fig. 6A). In contrast, photosynthetic rates of parasites growing on Leptospermum were similar to those of infected plants, but significantly lower than those of uninfected Leptospermum. On both host species, the transpiration rate of Cassytha was significantly higher than the infected host (Fig. 6B).
Photosynthetic efficiency
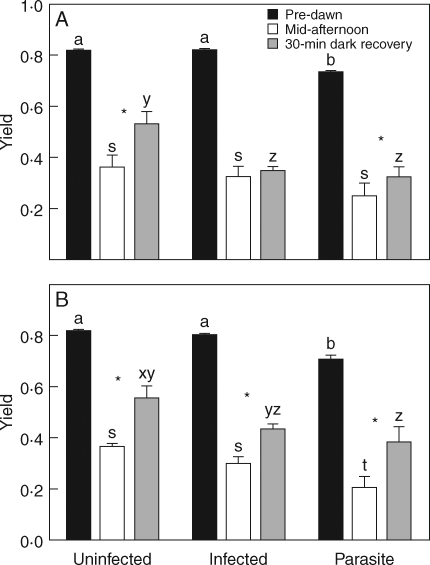
Cassytha had significantly lower pre-dawn yield than either of the host species on which it was growing (Fig. 7A, B). Mid-afternoon yield of Cassytha was lower than Leptospermum hosts but not Cytisus hosts (Fig. 7A, B). Short-term recovery of Cassytha PSII efficiency occurred on both host species with measured yield significantly greater after a short recovery period.
Fig. 7.
Pre-dawn yield, mid-afternoon yield and yield after a 30-min dark recovery period in the mid-afternoon for (A) Cytisus scoparius and (B) Leptospermum myrsinoides plants, infected by Cassytha pubescens, uninfected, and the parasite (means + 1 s.e., Cytisus n = 8; Leptospermum n = 6). Asterisks indicate significant differences between measurement times (not including pre-dawn measurements; separate Tukey–Kramer pairwise comparisons).
There was no difference in the mid-afternoon or pre-dawn yield of Cytisus plants that were infected by Cassytha and uninfected plants. However, the short-term recovery of Cytisus was faster in uninfected plants than in infected plants as infected plants showed no significant increase in yield after a short recovery period (Table 2).
Table 2.
Results of matched pair t-tests of the null hypothesis that yield is not higher after a period of 30 min dark recovery for the parasite Cassytha pubescens, two host species infected by the parasite and uninfected controls
| Host species | Treatment | d.f. | P > t |
|---|---|---|---|
| Cytisus scoparius | Uninfected | 7 | 0·027 |
| Infected | 7 | 0·283 | |
| Parasite | 7 | 0·031 | |
| Leptospermum myrsinoides | Uninfected | 5 | 0·004 |
| Infected | 5 | 0·03 | |
| Parasite | 5 | 0·008 |
There was no difference in the mid-afternoon or pre-dawn yield of Leptospermum plants that were infected by Cassytha and uninfected plants. Both infected and uninfected Leptospermum showed some short-term recovery in photosynthetic efficiency with significantly higher yields after a short recovery period (Table 2).
DISCUSSION
The present results show that the native generalist parasite Cassytha has a greater effect on the physiology and vigour of a novel host, Cytisus, than on a co-occurring, native host, Leptospermum. Although the parasite shows a slight preference for the native host it grows to high densities on both host species. However, host susceptibility to infection and the virulence of the parasite was greater on the introduced host than the native host. Although tolerance and resistance to infection may explain the reduced impacts of Cassytha on the native host, the higher impacts on the introduced host could also be the result of the higher resources that this host provides. When these resources support improved parasite growth, this can lead to a decline in host performance.
It was found that the assimilation rates of Cassytha were similar to its native host Leptospermum although lower than the introduced host, Cytisus. Parasite assimilation rates recorded during this study were much higher than those previously published for the South African species Cassytha ciliolata and C. filiformis (De La Harpe et al., 1981) and the morphologically similar genus Cuscuta (Stewart and Press, 1990). These authors considered that the autonomous carbon gain of Cassytha and Cuscuta was barely sufficient to replace respiratory losses. Despite the capacity for Cassytha to assimilate carbon, the parasite may still obtain a proportion of carbon from its host, other studies showing that from 28 % to 99 % of hemi-parasite carbon may be host derived (Graves et al., 1989, 1990; Marshall and Ehleringer, 1990).
Cassytha performance, in terms of net assimilation rates, growth rates and accumulated biomass, were poorer on the native host than on the introduced host. The parasite may have greater access to the resources that Cytisus provides due to either the higher nutrient status of this species or the reduced resistance to transfer of resources across the haustorium. Experimental manipulations of host nutrient status have demonstrated that holo-parasite performance increases with increases in host nitrogen (Marshall et al., 1994; Jeschke and Hilpert, 1997; Pennings and Simpson, 2008). However, where a parasite and its host have co-evolved, such as is potentially the case of the Cassytha–Leptospermum association, the development of host resistance mechanisms, particularly those that result in the degeneration or obstruction of haustorial tissue may have greater effects on nutrient transfer and hence parasite performance (Jiang et al., 2008). Chemical defences produced by the host may also have detrimental effects on parasite metabolism, particularly when they interfere with photosynthetic performance (Cameron et al., 2008).
Although both host species were affected by Cassytha infection, Leptospermum appeared to be less susceptible than Cytisus. Transpiration, allocation to leaf biomass and survival were not significantly reduced in infected Leptospermum relative to uninfected plants. In addition, infected plants rapidly recovered PSII efficiency. Cytisus was more negatively impacted by Cassytha. Photosynthesis, transpiration, allocation to photosynthetic biomass and survival were significantly reduced in infected plants.
Significant decreases in the photosynthesis of infected Cytisus and Leptospermum were measured. Parasites can reduce host carbon fixation by lowering host stomatal conductance, impacting host photosynthetic metabolism or changing host biomass, biomass allocation or architecture (Graves, 1995; Press et al., 1999; Watling and Press, 2001). The present results suggest there could be some stomatal limitation to photosynthesis as infected plants had lower transpiration rates than uninfected plants. However, other direct impacts of the parasite on photosynthetic metabolism through reductions in carboxylation efficiency and thylakoid capacity (e.g. Watling and Press, 2000; Cameron et al., 2008) require further examination. The slower rates of recovery of PSII efficiency in infected Cytisus indicate irradiance stress. The reduced photosynthetic rate in infected plants can increase the likelihood of photoinhibition (Watling and Press, 2001) but there were no long-term photoinhibitory effects as PSII efficiency recovered overnight. In addition, changes in the proportion of photosynthetic biomass, particularly in Cytisus, could have large impacts at the whole plant level. Heavy infestations can also create a shading effect, reducing the light available for photosynthesis. This ‘strangling’ effect has been observed in the parasite Cuscuta (Jeschke and Hilpert, 1997), which has a similar growth habit to Cassytha.
Suppression of photosynthesis can result in lower growth rates and biomass accumulation by the host but resource extraction by the parasite may also have the same effects. As parasite biomass increases in relation to host biomass, resource extraction may become more important (Graves et al., 1989). For Cytisus, parasite biomass frequently exceeded host photosynthetic biomass, therefore resource extraction in heavily infected plants could significantly increase mortality rates. Although Cassytha reached high densities on both host species, the present results suggest these infections develop at different rates. Cassytha stems coiled around Cytisus early in the growing season and around Leptospermum late in the growing season. The extraction of resources from Cytisus therefore commences earlier enabling the parasite to grow beyond the host plant, resulting in high parasite loads relative to host biomass. Conversely parasitic biomass on Leptospermum was typically confined to the host plant stems. Slow growth rates of Cassytha on Leptospermum may enable the host species to persist, with the high intensity infections developing over several years. High intensity infections of Cassytha were associated with mortality of Cytisus but not Leptospermum. In this dynamic process, Cassytha growth on Cytisus is rapid and the survey results show that these high parasite densities are correlated with host mortality.
Conclusions
Significant differences were found in the impacts of a parasitic plant on the growth and physiology of two host species under field conditions. There is evidence that declines in host condition may be the result of suppression of photosynthesis by the parasite but resource extraction by the parasite could also have significant impacts. In addition, the less affected native host, Leptospermum, may have evolved some resistance mechanisms to Cassytha infection. These mechanisms can operate at different stages of the infection process and result in degeneration or obstruction of the haustoria, chemical and physical barriers to haustorial penetration and/or abortion of parasitic tissues (Bringmann et al., 1999; Bouwmeester et al., 2003; Cameron et al., 2006; Rispail et al., 2007; Rümer et al., 2007).
The ERH predicts that invasive species will thrive in invaded communities when they are released from the controlling effects of their native enemies. Cytisus has encountered a new parasite, Cassytha, in its invaded range which has stronger impacts on this species than another co-occurring native species. However, Cytisus continues to spread in native vegetation which suggests that other factors favour its performance. The acquired native enemy, Cassytha may not be abundant enough to resist initial invasion by Cytisus. In this system biotic resistance is unlikely to prevent invasions, but this process may be an effective regulator of populations of invading species (Levine et al., 2004). It may be feasible for introduced species to be maintained at population levels where they have reduced detrimental effects on the invaded community. A native enemy, such as the parasitic plant Cassytha could be an effective natural biocontrol agent for Cytisus, with the advantage of having no lethal impact on native species. Further research is required to examine the interaction between the parasite and native and introduced hosts to evaluate the potential for introduction of the parasite into native vegetation invaded by Cytisus.
ACKNOWLEDGEMENTS
We thank R. Cirocco for processing samples and L. Pound and R. Faast for their comments on the manuscript. This work was supported by Australian Research Council Linkage Project (grant number LP 0667863) with the Adelaide and Mount Lofty Ranges and South Australian Murray Darling Basin Natural Resources Management Boards, SA Water, Forestry SA, and SA Department of Water, Land and Biodiversity Conservation as industry partners.
LITERATURE CITED
- Agrawal AA, Kotanen PM. Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecology Letters. 2003;6:712–715. [Google Scholar]
- Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos J. Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology. 2005;86:2979–2989. [Google Scholar]
- Atsatt PR, Strong DR. The population biology of annual grassland hemiparasites. I. The host environment. Evolution. 1970;24:278–291. doi: 10.1111/j.1558-5646.1970.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Bellingham PJ. Shrub succession and invasibility in a New Zealand montane grassland. Australian Journal of Ecology. 1998;23:562–573. [Google Scholar]
- Bossard CC, Rejmánek M. Herbivory, growth, seed production, and resprouting of an exotic invasive shrub, Cytisus scoparius. Biological Conservation. 1994;67:193–200. [Google Scholar]
- Bouwmeester HJ, Matusova R, Sun ZK, Beale MH. Secondary metabolite signalling in host–parasitic plant interactions. Current Opinion in Plant Biology. 2003;6:358–364. doi: 10.1016/s1369-5266(03)00065-7. [DOI] [PubMed] [Google Scholar]
- Bringmann G, Schlauer J, Ruckert M, et al. Host-derived acetogenins involved in the incompatible parasitic relationship between Cuscuta reflexa (Convolvulaceae) and Ancistrocladus heyneanus (Ancistrocladaceae) Plant Biology. 1999;1:581–584. [Google Scholar]
- Cameron DD, Coats AM, Seel WE. Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Annals of Botany. 2006;98:1289–1299. doi: 10.1093/aob/mcl218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DD, Geniez J-M, Seel WE, Irving LJ. Suppression of host photosynthesis by the parasitic plant Rhinanthus minor. Annals of Botany. 2008;101:573–578. doi: 10.1093/aob/mcm324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. Is invasion success explained by the enemy release hypothesis? Ecology Letters. 2004;7:721–733. [Google Scholar]
- Darwin CR. The origin of species. London: John Murray; 1859. [Google Scholar]
- De La Harpe AC, Visser JH, Grobbelaar N. Photosynthetic characteristics of some South African parasitic flowering plants. Zeitschrift fuer Pflanzenphysiologie. 1981;103:265–276. [Google Scholar]
- Elton CS. The ecology of invasions by animals and plants. London: Methuen; 1958. [Google Scholar]
- Fogarty G, Facelli JM. Growth and competition of Cytisus scoparius, an invasive shrub, and Australian native shrubs. Plant Ecology. 1999;144:27–35. [Google Scholar]
- Graves JD. Host–plant responses to parasitism. In: Press MC, Graves JD, editors. Parasitic plants. London: Chapman and Hall; 1995. pp. 206–225. [Google Scholar]
- Graves JD, Press MC, Stewart GR. A carbon balance model of the Sorghum-Striga-Hermonthica host–parasite association. Plant, Cell & Environment. 1989;12:101–107. [Google Scholar]
- Graves JD, Wylde A, Press MC, Stewart GR. Growth and carbon allocation in Pennisetum typhoides infected with the parasitic angiosperm Striga hermonthica. Plant, Cell & Environment. 1990;13:367–373. [Google Scholar]
- Horning KM. Host preference in Cuscuta attenuata: an assessment of host use relative to host availability. Bulletin of the Ecological Society of America. 1995;76:120–121. [Google Scholar]
- Hosking JR, Smith JMB, Sheppard AW. The biology of Australian weeds 28. Cytisus scoparius (L.) Link subsp. scoparius. Plant Protection Quarterly. 1996;11:102–108. [Google Scholar]
- Jeschke WD, Hilpert A. Sink-stimulated photosynthesis and sink-dependent increase in nitrate uptake: nitrogen and carbon relations of the parasitic association Cuscuta reflexa–Ricinus communis. Plant, Cell & Environment. 1997;20:47–56. [Google Scholar]
- Jeschke WD, Baumel P, Rath N, Czygan FC, Proksch P. Modeling of the flows and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb and its host Lupinus albus L. 2. Flows between host and parasite and within the parasitized host. Journal of Experimental Botany. 1994;45:801–812. [Google Scholar]
- Jiang F, Jeschke WD, Hartung W, Cameron DD. Does legume nitrogen fixation underpin host quality for the hemiparasitic plant Rhinanthus minor? Journal of Experimental Botany. 2008;59:917–925. doi: 10.1093/jxb/ern015. [DOI] [PubMed] [Google Scholar]
- Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution. 2002;17:164–170. [Google Scholar]
- Keith AM, Cameron DD, Seel WE. Spatial interactions between the hemiparasitic angiosperm Rhinanthus minor and its host are species-specific. Functional Ecology. 2004;18:435–442. [Google Scholar]
- Kelly CK, Venable DL, Zimmerer K. Host specialization in Cuscuta costaricensis – an assessment of host use relative to host availability. Oikos. 1988;53:315–320. [Google Scholar]
- Koskela T, Salonen V, Mutikainen P. Interaction of a host plant and its holoparasite: effects of previous selection by the parasite. Journal of Evolutionary Biology. 2001;14:910–917. [Google Scholar]
- Koskela T, Puustinen S, Salonen V, Mutikainen P. Resistance and tolerance in a host plant–holoparasitic plant interaction: genetic variation and costs. Evolution. 2002;56:899–908. doi: 10.1111/j.0014-3820.2002.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Levine JM, Adler PB, Yelenik SG. A meta-analysis of biotic resistance to exotic plant invasions. Ecology Letters. 2004;7:975–989. [Google Scholar]
- Liu H, Stiling P. Testing the enemy release hypothesis: a review and meta-analysis. Biological Invasions. 2006;8:1535–1545. [Google Scholar]
- McLuckie J. Studies in parasitism. I. Proceedings of the Linnean Society of New South Wales. 1924;49:55–78. A contribution to the physiology of the genus Cassytha, Part 1. [Google Scholar]
- Maron JL, Vila M. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos. 2001;95:361–373. [Google Scholar]
- Marshall JD, Ehleringer JR. Are xylem-tapping mistletoes partially heterotrophic? Oecologia. 1990;84:244–248. doi: 10.1007/BF00318279. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Dawson TE, Ehleringer JR. Integrated nitrogen, carbon, and water relations of a xylem-tapping mistletoe following nitrogen fertilization of the host. Oecologia. 1994;100:430–438. doi: 10.1007/BF00317865. [DOI] [PubMed] [Google Scholar]
- Marvier MA. Parasite impacts on host communities: plant parasitism in a California coastal prairie. Ecology. 1998;79:2616–2623. [Google Scholar]
- Matthies D. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: heterotrophic benefit and parasite-mediated competition. Oikos. 1996;75:118–124. [Google Scholar]
- Memmott J, Fowler SV, Paynter Q, Sheppard AW, Syrett P. The invertebrate fauna on broom, Cytisus scoparius, in two native and two exotic habitats. Acta Oecologica–International Journal of Ecology. 2000;21:213–222. [Google Scholar]
- Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- Musselman LJ, Press MC. Introduction to parasitic plants. In: Press MC, Graves JD, editors. Parasitic plants. London: Chapman and Hall; 1995. pp. 1–13. [Google Scholar]
- Myers JH, Bazely DR. Ecology and control of introduced plants. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Nunez MA, Relva MA, Simberloff D. Enemy release or invasional meltdown? Deer preference for exotic and native trees on Isla Victoria, Argentina. Austral Ecology. 2008;33:317–323. [Google Scholar]
- Parker IM, Gilbert GS. When there is no escape: the effects of natural enemies on native, invasive, and noninvasive plants. Ecology. 2007;88:1210–1224. doi: 10.1890/06-1377. [DOI] [PubMed] [Google Scholar]
- Parker JD, Hay ME. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecology Letters. 2005;8:959–967. doi: 10.1111/j.1461-0248.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- Parker JD, Burkepile DE, Hay ME. Opposing effects of native and exotic herbivores on plant invasions. Science. 2006;311:1459–1461. doi: 10.1126/science.1121407. [DOI] [PubMed] [Google Scholar]
- Pate JS, Bell TL. Host associations of the introduced annual root hemiparasite Parentucellia viscosa in agricultural and bushland settings in Western Australia. Annals of Botany. 2000;85:203–213. [Google Scholar]
- Paynter Q, Downey PO, Sheppard AW. Age structure and growth of the woody legume weed Cytisus scoparius in native and exotic habitats: implications for control. Journal of Applied Ecology. 2003;40:470–480. [Google Scholar]
- Pennings S, Simpson J. Like herbivores, parasitic plants are limited by host nitrogen content. Plant Ecology. 2008;196:245–250. [Google Scholar]
- Pennings SC, Callaway RM. Parasitic plants: parallels and contrasts with herbivores. Oecologia. 2002;131:479–489. doi: 10.1007/s00442-002-0923-7. [DOI] [PubMed] [Google Scholar]
- Press MC, Parsons AN, Mackay AW, Vincent CA, Cochrane V, Seel WE. Gas-exchange characteristics and nitrogen relations of two Mediterranean root hemiparasites – Bartsia trixago and Parentucellia viscosa. Oecologia. 1993;95:145–151. doi: 10.1007/BF00649518. [DOI] [PubMed] [Google Scholar]
- Press MC, Scholes JD, Watling JR. Parasitic plants: physiological and ecological interactions with their hosts. In: Press MC, Scholes JD, Barker MG, editors. Physiological plant ecology: the 39th symposium of the British Ecological Society held at the University of York, UK, 7–9 September 1998. Oxford: Blackwell Science; 1999. pp. 175–197. [Google Scholar]
- Rispail N, Dita MA, Gonzalez-Verdejo C, et al. Plant resistance to parasitic plants: molecular approaches to an old foe. New Phytologist. 2007;173:703–711. doi: 10.1111/j.1469-8137.2007.01980.x. [DOI] [PubMed] [Google Scholar]
- Rümer S, Cameron DD, Wacker R, Hartung W, Jiang F. An anatomical study of the haustoria of Rhinanthus minor attached to roots of different hosts. Flora – Morphology, Distribution, Functional Ecology of Plants. 2007;202:194–200. [Google Scholar]
- Seel WE, Press MC. Influence of the host on 3 sub-Arctic annual facultative root hemiparasites. 1. Growth, mineral accumulation and aboveground dry-matter partitioning. New Phytologist. 1993;125:131–138. doi: 10.1111/j.1469-8137.1993.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends in Ecology & Evolution. 2002;17:170–176. [Google Scholar]
- Specht R, Rundel P. Sclerophylly and foliar nutrient status of Mediterranean-climate plant communities in Southern Australia. Australian Journal of Botany. 1990;38:459–474. [Google Scholar]
- Stewart GR, Press MC. The physiology and biochemistry of parasitic angiosperms. Annual Review of Plant Physiology and Plant Molecular Biology. 1990;41:127–151. [Google Scholar]
- Strong DR, Lawton JH, Southwood R. Insects on plants: community patterns and mechanisms. London: Blackwell Scientific; 1984. [Google Scholar]
- Suetsugu K, Kawakita A, Kato M. Host range and selectivity of the hemiparasitic plant Thesium chinense (Santalaceae) Annals of Botany. 2008;102:49–55. doi: 10.1093/aob/mcn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrett P, Fowler SV, Coombs EM, et al. The potential for biological control of Scotch broom (Cytisus scoparius) (Fabaceae) and related weedy species. Biocontrol News and Information. 1999;20:17–34. [Google Scholar]
- Waloff N, Richards OW. The effect of insect fauna on growth mortality and natality of broom, Sarothamnus scoparius. Journal of Applied Ecology. 1977;14:787–798. [Google Scholar]
- Waterhouse BM. Broom (Cytisus scoparius) at Barrington Tops, New South Wales. Australian Geographical Studies. 1988;26:239–248. [Google Scholar]
- Watling JR, Press MC. Infection with the parasitic angiosperm Striga hermonthica influences the response of the C3 cereal Oryza sativa to elevated CO2. Global Change Biology. 2000;6:919–930. [Google Scholar]
- Watling JR, Press MC. Impacts of infection by parasitic angiosperms on host photosynthesis. Plant Biology. 2001;3:244–250. [Google Scholar]