Abstract
Background and Aims
Fodder provision in species-rich grasslands, i.e. herbage growth, proportion of leaf, and leaf and stem digestibility, is difficult to predict for short periods of time, such as between two defoliations or less. The value of two methods based on plant traits for evaluating these agronomic properties was examined.
Methods
One method is based on plant trait measurements on the plant community (leaf dry matter content, plant height, flowering date); the other is on vegetation composition expressed as plant functional types (acquisitive versus conservative PFTs) established by measuring leaf dry matter content on pure grass stands. The experiment consisted of 18 fields with three different defoliation regimes (combinations of cutting and grazing) and two levels of fertilization. To establish a growth curve over the first growth cycle, herbage was sampled about 10 times in spring.
Key Results
Coefficients of correlation between agronomic properties of the vegetation and its functional composition were higher when the latter was assessed through PFT and an indicator of the plant nutrient status (Ni) instead of measured plant traits. The date at which the ceiling yield occurred for the standing herbage mass or only the leaf component, which varied by up to 500 degree-days between treatments, and the leaf proportion, depended entirely on the PFT, and largely so for the leaf digestibility. The standing herbage mass at the time of ceiling yield depended only on Ni, or mainly so in the case of the daily herbage growth rate. Similar plant digestibility between plant communities was found at flowering time, although there were big differences in PFT composition. The shape of the growth curve was flatter when there was great functional diversity in the plant community.
Conclusions
The PFT composition and the Ni were more reliable than the plant functional traits measured in the field for evaluating herbage growth pattern and digestibility in spring.
Key words: Grass, fertilization, digestibility, ceiling yield, growth, botanical composition, functional diversity
INTRODUCTION
Due to the large number of species growing together in species-rich grasslands, fodder provision is poorly assessed using the concepts and methods of ecophysiology alone (Lavorel and Garnier, 2002). In such conditions, functional traits have been used successfully for describing the effect of land management on ecosystem processes, productivity and nutrient cycling on coarse space and time scales (e.g. Diaz et al., 2007). The approach has been found useful for estimating fodder provision (Hodgson et al., 2005a, b; Quetier et al., 2007a, b), but it has usually been used on an annual scale, whereas for managing grasslands used for feeding domestic herbivores data are also needed on a seasonal or even weekly scale. Moreover, the method should consider factors other than herbage productivity, e.g. dates at which ceiling yield occurs, leaf proportion and leaf and stem digestibility (Parsons, 1988). Thus, the main purpose of this paper was to overcome these limitations, especially on managed grasslands for which fertilizer applications and defoliation regimes interact together to determine the structure and composition of the vegetation (Grime, 1973; Sanderson et al., 2004).
To assess the effect of vegetation characteristics on ecosystem productivity, a current approach is to use measurements of plant functional traits (Grime et al., 1988) directly linked to the functions of plant growth and development or strongly correlated to other variables which are indirectly related to these functions (Weiher et al., 1999). Functional composition of the vegetation can be characterized using several methods based on plant traits. Two of them, one taxon-explicit and the other not were compared (Lavorel et al., 2008).
One consists of measurements of a trait for each species in a plant community for calculating a weighted value on the basis of the mass ratio hypothesis (Grime, 1998). In this paper, four plant effect traits were selected for which justifying hypotheses can be found in the literature: leaf dry matter content (LDMC); specific leaf area (SLA); plant height; and flowering time. LDMC is an estimator for plant tissue density, usually well correlated to SLA (Wilson et al., 1999). Fast-growing species have low tissue density, low LDMC, high SLA and a short organ lifespan (Ryser, 1996). LDMC was found to be a good indicator of lamina digestibility, with which it is negatively correlated (Al Haj Khaled et al., 2006). Plant height (H) is an indicator of species competitiveness (Hodgson et al., 2005a) and is correlated with growth rate (Diekmann and Falkengren-Grerup, 2002). Flowering time is a key plant feature for understanding the evolution of accumulation of herbage mass (Robson et al., 1988) and its digestibility (Demarquilly, 1989). It determines the date on which the ceiling yield (peak biomass) occurs and the changes in the leaf proportion which have a big influence on the digestibility of the standing herbage (Calvière and Duru, 1999). Since these three plant traits are sensitive to nutrient availability (Al Haj Khaled et al., 2005; Mokany and Ash, 2008) resulting from soil fertility and fertilizer use, values measured in the field can be expected to show if such differences between plant communities exist. In the same way, flowering time can be expectedto reflect the pattern of grass growth over time. In brief, it was hypothesized that the four plant traits are complementary for assessing the different agricultural characteristics of grassland communities.
The second method consists of using a pre-existing functional classification of species into plant functional types (PFT) on the basis of LDMC measured in standardized conditions (i.e. pure stands with the same high N supply, without any competition with other species). It has previously been found that this trait is significantly correlated with flowering times and leaf lifespan, two plant characteristics which have a fundamental effect on plant growth pattern, and are relatively insensitive to nutrient availability (Al Haj Khaled, 2005; Al Haj Khaled et al., 2006).
The first objective was to compare the ability of both methods based on measured plant traits or plant indicators to predict the different components of fodder provision (herbage growth rate and pattern: dates at which canopy closure and ceiling yield occur, herbage digestibility). This comparison was made assuming average plant traits and their distribution within a grassland community (Lavorel et al., 2008). For the latter, it was hypothesized that the greater the functional diversity, the flatter should be the shape of growth curve. The second objective was to assess whether it is better for scientific reasons, such as the difference in plant traits between functional groups, or for the sake of simplification, to consider only the dominant functional group (grass species) for making measurements.
Firstly, the relationship is examined between agronomic characteristics of the vegetation with the weighted plant traits, then with the plant functional type composition of the community, together with an indicator of nutrient availability. Then the relationship is analysed between the functional diversity within a plant community and the shape of the growth curve over the spring growth period. The results are discussed to ascertain which method performed best for predicting the different components of fodder provision.
MATERIALS AND METHODS
Experimental design
An experiment consisting of a set of 18 grassland communities sampled on four livestock farms to cover a wide range of management practices in the central Pyrenees was set up in 2004. It is located close to the village of Ercé in the French Pyrenees (0°E, 44°N, 600–1000 m a.s.l.). The mean air temperature is 12 °C and the mean annual rainfall 1200 mm at 650 m a.s.l. During the study period, it was found that there were no significant differences in temperatures between 650 and 950 m a.s.l., probably because the grasslands were spread from the bottom to the top of a south-facing slope. The soil is a brunisol developed on alluvium. Grasslands were chosen to represent the field diversity in terms of defoliation management (grazing and/or cutting) and fertilization practices (spreading farmyard manure or not).
There were three defoliation regimes [meadows cut twice per year and grazed in autumn by cows (M); meadows grazed at low sward height (<5 cm) in spring then cut and grazed (GM); pastures which were only grazed two or three times per year by cows before and after summer pasturing (P)], and two fertility levels (denoted + and –) defined by the nutrient index for estimating the nutrient availability (see below) determined the year before. There were three replications. The main grasses were for the three defoliation regimes, respectively: (1) Lolium perenne and Poa trivialis; (2) Dactylis glomerata and Holcus lanatus; (3) Agrostis capillaris and Festuca rubra. In each grassland field, plots of around 35 m2 were fenced off before taking measurements to preserve plant material from grazing and cutting. In this way, it was possible to focus on the after-effects of these management practices to study the pattern of spring herbage growth and composition.
Functional composition of the vegetation
Floristic composition was measured by harvesting 12 samples (100 cm2 each) around the time of peak biomass. All the samples of the same community were pooled and the different species were separated and identified. The floristic composition of each community was obtained through the list of species and their relative abundance, based on their oven-dry mass divided by the total sampled dry biomass. This allowed the proportion of grasses to be calculated together with the composition of the grasses in PFTs (Table 1). Two main PFTs were recorded following Ansquer et al. (2004): (1) Holcus lanatus, Lolium perenne, Anthoxanthum odoratum, Arrhenatherum elatius, Dactylis glomerata, Festuca arundinacea, Poa trivialis; (2) Agrostis capillaris Bromus erectus, Festuca rubra, Phleum pratense, Trisetum flavescens, Briza media, Cynosurus cristatus. Based on the classification of Grime et al. (1988), five of the seven species of PFT 1 have a competitive (C) or ruderal (R) plant strategy (or between C or R and C-S-R), while six of the seven species of PFT 2 have a stress-tolerant plant strategy (S) and/or are intermediate between S and C-S-R. Whatever the method considered, the meaning of each trait has to be evaluated for the different plant life forms (grasses and dicotyledons) growing within a given plant community. Flowering time and plant height are usually similar for the two forms (Ansquer, 2006) but this is not the case for LDMC (Al Haj Khaled et al., 2005). Thus it is necessary to consider whether it is acceptable to measure plant traits on the grass component alone for assessing the agronomic properties of the entire sward.
Table 1.
Characterization of the 18 grasslands studied: management practices, functional descriptors of vegetation and weighted plant traits
| Functional descriptors of vegetation |
Weighted plant traits |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant nutrient index | Proportion of grasses (%) | Proportion of PFT 2 (%) | Proportion of PFT 2 (classes)* | Whole plant species |
Grass species |
||||||||
| Grassland names | Organic fertilization | Defoliation | No. of species | LDMC (g kg−1) | Height (cm) | Flowering date (°Cd) | LDMC (g kg−1) | Height (cm) | Flowering date (°Cd) | ||||
| Angladure | Yes | M | 15 | 0·79 | 28 | 0 | 1 | 202 | 16 | 732 | 237 | 19 | 955 |
| Moulaque | Yes | M | 19 | 0·75 | 87 | 5 | 1 | 232 | 22 | 1104 | 243 | 23 | 1131 |
| Ajas 1† | Yes | M | 17 | 0·67 | 79 | 0 | 1 | 246 | 21 | 1053 | 256 | 22 | 1140 |
| Carré | Yes | M | 21 | 0·61 | 63 | 0 | 1 | 239 | 16 | 928 | 265 | 17 | 1023 |
| Campagn | Yes | M | 19 | 0·70 | 64 | 1 | 1 | 223 | 18 | 1041 | 261 | 21 | 1138 |
| Ajas 2 | Yes | M | 23 | 0·53 | 56 | 35 | 2 | 273 | 15 | 988 | 270 | 18 | 1194 |
| Campl 1 | Yes | GM | 15 | 0·86 | 73 | 1 | 1 | 210 | 21 | 1080 | 227 | 22 | 1202 |
| Rives | Yes | GM | 19 | 0·68 | 67 | 4 | 1 | 254 | 17 | 1143 | 272 | 18 | 1110 |
| Coste 1 | No | GM | 23 | 0·72 | 66 | 7 | 1 | 232 | 17 | 1048 | 251 | 19 | 1185 |
| Campl 2 | Yes | GM | 21 | 0·59 | 68 | 17 | 2 | 211 | 13 | 1102 | 236 | 15 | 1202 |
| Routies | Yes | GM | 34 | 0·55 | 43 | 9 | 1 | 197 | 10 | 970 | 267 | 13 | 985 |
| Coste 2 | No | GM | 41 | 0·68 | 30 | 29 | 2 | 229 | 14 | 952 | 289 | 16 | 1120 |
| Giron 1 | No | P | 20 | 0·80 | 80 | 67 | 3 | 256 | 11 | 1491 | 268 | 12 | 1507 |
| Peyche 1 | No | P | 13 | 0·83 | 88 | 11 | 2 | 244 | 17 | 1246 | 250 | 18 | 1256 |
| Lassus 1 | No | P | 25 | 0·83 | 93 | 25 | 2 | 225 | 17 | 1331 | 227 | 18 | 1329 |
| Giron 2 | No | P | 38 | 0·41 | 56 | 87 | 3 | 257 | 6 | 1327 | 312 | 7 | 1441 |
| Peyche 2 | No | P | 35 | 0·56 | 44 | 64 | 3 | 242 | 8 | 1232 | 293 | 10 | 1407 |
| Lassus 2 | No | P | 39 | 0·63 | 63 | 61 | 3 | 280 | 13 | 1184 | 300 | 13 | 1363 |
°Cd, degree-days; M, meadow; GM, meadow grazed in spring; P, pastures.
* 1, PFT 2 <10 %; 2, 10– < 60 %; 3, >60%.
† 1 and 2 indicates two facies within the same grassland field.
Plant functional traits (LDMC, SLA, plant height) were measured in situ in spring 2004 following a standardized protocol (Cornelissen et al., 2003). Flowering time was noted when 50 % of the reproductive stems reached this stage. It was expressed in degree-days (°Cd) from the 1 February (Al Haj Khaled, 2005). A weighted plant functional trait was calculated by weighting the average value of each species by its abundance (Vile et al., 2006). The functional diversity index (FD) was based on the variation of the species within the range of the plant traits (Mason et al., 2003).
 |
xi is the trait value for a species i, wi its relative abundance and N′ the number of species for which measurements were made.
Herbage mass and digestibility
Herbage yields were obtained by clipping three randomized subplots of 0·25 m2 at 1 cm above ground level. Measurements were made on about ten sampling dates from 24 February to 20 July. On a sub-sample of herbage, grasses were separated from dicotyledons, and, for the former, green and senescent laminae were separated from sheath, stem and inflorescence. Biomass was oven dried for 72 h at 70 °C and weighed. The samples were cut close to peak biomass and submitted to near infrared reflectance spectroscopy (NIRS) analysis to estimate the plant digestibility (NIRS system monochromator 5000). The calibration used was developed from that of the Aufrère (1982) reference laboratory (Biston and Dardenne, 1985).
Plant nutrient index for assessing nutrient availability
Plant nutrient status was assessed through plant nutrient indices. These indices were for nitrogen (NNi) and phosphorus (NPi). NNi was calculated as the ratio between the actual %N (%Na) and the critical %N (%Nc) which corresponded to: %Nc = 4·8 (DM)−0·32 reported by Lemaire and Gastal (1997). It is an estimate of the fraction of actual/potential growth (the latter being limited only by the season's weather) as restricted by the ability of the soil and fertilizer to provide a particular nutrient, in this case N. NPi was computed as proposed by Duru and Ducrocq (1997). A synthetic nutrient index (Ni) was calculated from the values of these two indices according to Duru and Ducrocq (1997): Ni = NNi × (0·3 NPi + 0·7). A value of Ni of 1 means that herbage growth was not limited by nutrients.
Growth curve and statistical analyses
Yield measurements were used to establish growth curves for the above-ground herbage mass and several fractions: senescent, grass, stem or leaf components. A third order polynomial equation (y = ax3 + bx2 + c) was fitted to the data, making sure that the coefficients were significant, and expressing × in °Cd (Duru et al., 2000) to account for differences in temperature over the growing season (S+ software). This equation was used to calculate the following four variables: (1) the date on which the LAI reached 4, indicating canopy closure (Simon and Lemaire, 1987) using SLA and leaf mass of each species; (2) the date on which peak herbage mass occurred, as the value of x for which the derivative (y′) = 0; (3) the peak biomass: ymax = axmax3 + bxmax2 + c; (4) the herbage growth rate, y′ being the derivative of y. The model failed for one to three grassland fields, depending on the plant component considered. In this case, regression analysis between plant traits and variables computed from growth curves was done, excluding data from these fields.
To assess whether the herbage growth pattern depended on plant functional diversity, the functional diversity indices for LDMC (database) and a parameter describing the shape of growth curves around the peak of herbage mass for the grass component were related. Using polynomial fitting, the biomass was calculated at the peak and 200 °Cd before and after the peak and the variation of herbage mass around the peak as a percentage. To evaluate the ceiling yield of the grass leaves, a specific method was used because there were only slight variations in mass over the growth period. Firstly, only data were used for which the coefficient of variation computed between the different sampling dates was >20 % (i.e. 16 plant communities). Then, moving averages were computed on three consecutive dates for smoothing the growth curves (Hunt, 1982), and the date at which the peak occurred was determined.
To show whether the method used for characterizing the plant community was taxon-specific, it is necessary to evaluate the appropriateness of only considering the grass component for assessing the agronomical properties of the entire vegetation. This was done by considering the aggregated plant traits for the whole observed species or only grass species.
Stepwise regressions were computed to determine which of the two vegetation functional descriptors [the plant nutrient index (Ni) and the plant functional type (% PFT 2)] and which of the four plant traits (LDMC, SLA, height, flowering time) had a significant effect upon the set of agronomic characteristics. As the regressions of agronomic characteristics on SLA were never significant, results are given only for the three other plant traits. ANOVA was performed to compare agronomic characteristics by classes of PFT. Logarithmic transformations were undertaken on data expressed as percentages to achieve normality of residuals as required by the method.
RESULTS
Overview of results
There were differences between the grassland communities for all agronomic characteristics (Table 2). The herbage accumulation rates varied by up to 3-fold and the herbage mass at the peak by up to 2-fold. The differences in temperature sums between plant communities to reach peak herbage mass were around 600 °Cd for the whole plant community, the grass component and the grass leaves. The date on which the LAI = 4 varied by >500 °Cd. On the other hand, differences were observed at the peak for the leaf proportion and the percentage of senescent material (not shown). For the grass component, the differences in plant digestibility were about 100 and 60 g kg−1 for the stems and leaves or for the stems and the whole grass component, respectively. The stem digestibility was always lower than that of the leaves, and positively correlated with it (P < 0·001; r = 0·72; n = 18). The digestibility of grasses was positively correlated to the leaf proportion (P < 0·007; r = 0·63; n = 17).
Table 2.
Relationship between herbage agronomic characteristics, plant traits and functional descriptors of vegetation
| No. of fields | Range of variations of agronomic characteristics |
Regression analysis between agronomic characteristics and weighted plant traits† |
Regression analysis‡ between agronomic characteristics and functional descriptors of vegetation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of agronomic characteristics | Mean | Min | Max | Species | LDMC | Height | Flowering date | R2 | Ni | Proportion of PFT 2 (%) | R2 | |||
| Rate of growth and standing biomass | Rate of growth over the linear phase (g m−2 Cd−1) | 17 | 0·658 | 0·243 | 0·900 | G | n.s.§ | *** (+) | n.s. | 0·66 | *** (+) | * (–) | 0·81 | |
| W | n.s.§ | *** (+) | n.s. | 0·57 | ||||||||||
| Herbage mass at the peak (g m−2) | 17 | 544 | 264 | 716 | G | n.s. | ** (+) | n.s. | 0·53 | *** (+) | n.s. | 0·73 | ||
| W | n.s. | ** (+) | n.s. | 0·51 | ||||||||||
| Pattern (°Cd) | Date when LAI = 4 | 18 | 450 | 292 | 838 | G | n.s.§ | *** (–) | n.s. | 0·60 | * (+) | *** (+) | 0·58 | |
| W | n.s.§ | *** (–) | n.s. | 0·61 | ||||||||||
| Date of peak | 17 | 1282 | 1070 | 1686 | G | n.s. | *** (–) | ** (+)§ | 0·82 | n.s. | *** (+) | 0·75 | ||
| W | n.s. | *** (–) | ** (+)§ | 0·85 | ||||||||||
| Date of peak: grass leaf | 16 | 822 | 450 | 1738 | G | ** (+) | n.s. | n.s. | 0·40 | n.s. | *** (+) | 0·79 | ||
| Composition of the grass component at the peak | % leaves | 18 | 34 | 10 | 94 | G | n.s.§ | *** (–) | n.s. | 0·58 | n.s. | *** (+) | 0·66 | |
| Digestibility (g kg−1) | ||||||||||||||
| Whole plant | 17 | 510 | 431 | 619 | G | n.s. | * (–) | n.s. | 0·28 | n.s. | n.s. | / | ||
| Leaf | 18 | 667 | 559 | 756 | G | n.s.§ | *** (+) | n.s. | 0·61 | * (+) | *** (–) | 0·79 | ||
| Stem | 18 | 437 | 378 | 474 | G | ** (–) | n.s.‡ | n.s. | 0·44 | * (+) | n.s. | 0·42 | ||
W, Whole plant species; G, grass species; °Cd, degree-days.
*, P < 0·05; **, P < 0·01; ***, P < 0·001; n.s., not significant; /, not calculated. Signs + and − indicate that the agronomic characteristics respond positively or negatively to an increase in Ni or PFT 2 (%).
† See Table 1 for plant trait values.
‡ Significance for Ni and PFT 2 (%), and coefficient of determination (R2) calculated considering one or both variables.
§ There was significant correlation (P < 0·05) with the trait considered alone.
Although the proportion of the grass component varied greatly from one field to another (Table 1), the standing herbage mass, the date at which the peak occurred and the growth rate calculated on the grass fraction were positively correlated with those of the whole plant community (P < 0·001; r = 0·96 for the date, r = 0·77 for the standing herbage mass at the peak and r = 0·82 for the herbage growth rate). The peak of herbage mass of grasses (green fraction) occurred on average almost twice as late as that of leaves (ratio = 0·56 ± 0·2).
Considering only the grass species instead of the whole set of species increased the values of the three plant traits by 9 % (flowering) or 11 % (LDMC) (Table 1). However, the coefficients of correlation between grass and all species were higher for height and flowering (r = 0·98 and 0·90, respectively; P < 0·001) than for LDMC (r = 0·61; P < 0·072). This indicates that similar statistical results can be expected for the grass component and the whole plant community for height and flowering time, but not LDMC. As some plant traits were significantly correlated (LDMC and flowering time: P < 0·0022; LDMC and height: P < 0·007), one plant trait would usually be expected to be significant in regression analysis.
Relationships between agronomic characteristics of the vegetation and weighted plant functional traits
There were only weak correlations between two plant traits (LDMC and H) and standing herbage mass at the peak and herbage growth rate (Table 2). The date at which the peak occurred was negatively correlated with the average plant height and positively correlated with flowering times. However, in both sentences, only H was significant in the regression analysis.
The leaf and stem digestibility of grass was negatively correlated with LDMC and positively with H (Table 2): the thicker the tissue, the lower was its digestibility. However, only H or LDMC were significant in regression analysis, respectively, for leaf and stem. The percentage of grass leaf was negatively correlated with the plant height and positively with the LDMC. The plant digestibility calculated from the digestibility of the plant components and the percentage of leaf were weakly negatively correlated to the plant height.
Relationships between agronomic characteristics of the vegetation and its PFT composition
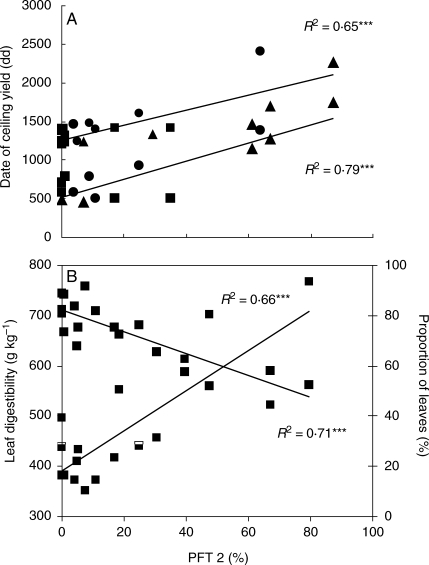
Stepwise regression distinguished three types of agronomic characteristic according to whether they depended on plant nutrient index, plant functional type, or both (Table 2). The standing herbage mass at the peak, as well as the stem digestibility of grasses, were significantly correlated only with the plant nutrient index. On the other hand, the date on which the peak occurred for the whole sward or the leaf for the grass component (Fig. 1A) as well the leaf proportion (Fig. 1B) for grasses, significantly depended on the proportion of PFT 2. For the former, the difference was >800 °Cd when the vegetation composition expressed as PFT 2 content increased from 0 to 80 %. On the other hand, the herbage growth rate, and the date when the canopy closed (LAI = 4) were mainly or secondarily correlated with Ni (Table 2), respectively. The leaf digestibility (grass species) depended mainly on the PFT plant community composition, and it decreased when the proportion of PFT2 increased (Fig. 1B).
Fig. 1.
Vegetation characteristics according to the proportion of PFT 2 (%): (A) dates (degree-days) at which the ceiling yield occurred for grass leaf (closed symbols) and whole grass herbage (open symbols) for meadow (squares), grazed meadow (triangles) and pasture (circles); (B) leaf digestibility (closed squares) and leaf proportion (open squares) for grasses at the peak of biomass
Finally, plant nutrient index performed well alone for herbage growth rate and standing herbage mass at the peak. For the other characteristics, in particular those related to the composition of the biomass and the herbage growth pattern, such as the canopy closure date and the date of the peak, the PFT composition was the most suitable variable, explaining 58–81 % of the variance of these characteristics. For those agronomic characteristics that depended only on functional composition, the plant community was compared ranked in three classes according to the PFT 2 proportion (Table 1). There were significant differences (P ≤ 0·01) between classes 1 and 3 (Table 3). Differences reached at least 300 °Cd for key stages that drive the herbage growth pattern (date of canopy closure, dates at which the whole plant or leaf ceiling yield occurred). On the other hand, the leaf proportion varied from one to three, and the difference in leaf digestibility was 140 g kg−1.
Table 3.
Herbage growth pattern and composition according to functional composition of the vegetation (proportion of PFT 2)
| Type of agronomic characteristics | Agronomic characteristics | No. of grasslands | Proportion of PFT 2 (%) expressed in three classes† |
|||
|---|---|---|---|---|---|---|
| P | 1: <10 % | 2: 10–60 % | 3: >60% | |||
| Pattern of herbage mass (date of peak in °Cd) | Green herbage mass | 17 | *** | 1198a | 1262ab | 1523b |
| Green herbage mass (grasses) | 16 | ** | 1213a | 1271a | 1520b | |
| Green leaf mass (grasses) | 16 | ** | 596a | 674a | 1001b | |
| Pattern (closure of the canopy in °Cd) | Date at which LAI = 4 | 18 | ** | 359a | 450ab | 607b |
| Quality of grasses at the peak | Leaf mass (%) | 18 | ** | 0·20a | 0·28ab | 0·70b |
| Leaf digestibility (g kg−1) | 18 | *** | 710a | 670a | 570b | |
**, P ≤ 0·01; ***, P ≤ 0·001; for each PFT treatment, data having a different letter in the same line were significantly different.
† See Table 1.
Relationships between agronomic characteristics of the vegetation and the functional diversity within the plant community
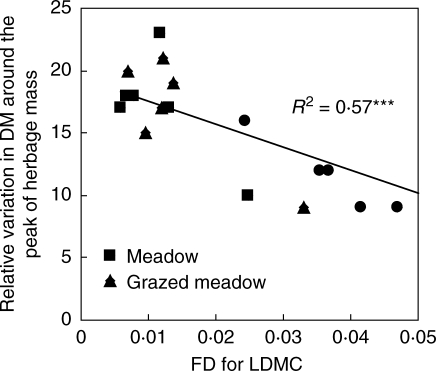
The variation in herbage mass around the peak decreased significantly when the functional diversity indices increased, whatever the plant trait considered (LDMC, H, flowering times) or the method for LDMC (measured versus calculated using the plant trait database). Correlations between the variation in herbage mass around the peak and the functional indices were greater for LDMC (r = 0·75, P < 0·001 whatever the method used), than for H (r = 0·66, P < 0·004) and flowering time (r = 0·49, P < 0·046). Pastures have the lowest variation in yield around the peak of herbage mass (Fig. 2). Such a correlation was also found between LDMC and the shape of leaf grass growth pattern, but it was weaker (r = 0·49 instead of 0·76).
Fig. 2.
Variation in herbage mass around the peak (percentage) according to functional diversity index (FD) for LDMC in meadows and grazed meadows.
DISCUSSION
Comparison of measured plant traits for assessing herbage growth pattern and digestibility
Each plant trait considered alone was correlated to several agronomic properties, but the weighted plant height was the one which was correlated with all of them except the date of the peak for grass leaf. When considering the three plant traits together, the weighted plant height was always significant considered alone except for predicting the date of the peak and the stem digestibility. The relevance of weighted plant height for assessing net above-ground productivity was shown previously (Garnier et al., 2007). It is a major trait characterizing plants' ability to compete for light (Westoby, 1998). The fact that plant functional traits involved in the capture–conservation trade-off are also correlated with agronomic characteristics such as productivity along a fertility gradient had already been observed (e.g. Wilson et al., 1999; Lavorel et al., 2005). However, the correlation coefficients calculated between weighted plant height at the vegetative stage or LDMC and the herbage growth rate or standing herbage mass at the peak were weaker than expected (Hooper et al., 2002; Garnier et al., 2004), probably because the range of management practices or abiotic conditions encountered in other studies was wider than in the present one.
The present results, showing that LDMC is a good predictor of plant digestibility for a set of plant communities submitted to different defoliation regimes, are in agreement with those of Louault et al. (2005). The digestibility of the plant components at peak biomass has a greater effect than the leaf percentage on the whole plant digestibility. Indeed, the correlations between LDMC and plant digestibility resulted from the strong correlation among each of these plant characteristics and the fibre content (van Arendonk and Poorter, 1994; Al Haj Khaled et al., 2006). The fact that flowering times were well correlated to dates at which the above-ground biomass peaks were reached is consistent with the ecophysiology of grasses (Robson et al., 1988).
The correlations among a set of agronomic characteristics and the plant traits measured on the whole plant species or only on the grass species were similar (Table 2) in spite of large differences in the proportion of grasses. Concerning agronomic properties, the present results confirmed those previously found for leaf proportion (Calvière and Duru, 1999) and herbage growth rate (Negi et al., 1992). On the other hand, the similarity observed in plant traits between species having contrasting life forms was also observed for plant phenology and leaf traits in other growing conditions (Campanella and Bertiller, 2008). Indeed, defoliation regime and nutrient availability act as influential factors that impose very similar plant behaviour, at least at the plant group level, determining specific local community structure and composition (Holdaway and Sparrow, 2006). These results justify characterizing the plant community only through its grass PFT composition to assess its agronomic characteristics.
Value of an approach based on functional composition and plant nutrient status
The main value of the present results is to evaluate the value of the plant trait approach for assessing fodder provision on a short time scale. It has mostly been used on a yearly time scale using plant trait measurement in standardized conditions (Pontes et al., 2007b) or at the field level (Hodgson et al., 2005b; Quetier et al., 2007a). In the present study, two time scales were considered – one the whole growing period, and the other the period on either side of the peak of herbage mass (around ± 200 °Cd, i.e. 10–20 d).
The first new finding of the present study for the whole growing period is that herbage growth rate depends mainly on plant nutrient availability assessed through plant nutrient content, and secondarily on PFT established from the LDMC in standardized growing conditions, whereas the dates at which the ceiling yields occurred for the standing herbage mass or for the leaf component depend mainly on the latter, and are consistent with the literature. Indeed, there was a slight difference in the radiation use efficiency (around 15 %) between species having an acquisitive (PFT 1) and conservative (PFT 2) resource strategy for grasses growing in pure stands with a high N supply (Al Haj Khaled, 2005); PFTs 1 and 2 corresponding roughly to competitive and stress-tolerant plant strategies, respectively, according to the classification of Grime et al. (1988) (see Materials and methods). Species from nutrient-rich habitats often show a high relative growth rate (Poorter et al., 1990; van der Werf et al., 1993) and produce more biomass, even with equal nutrient availability (Schippers and Olff, 2000). On the other hand, PFT composition of the plant community performed better than LDMC measurement for assessing certain agronomic characteristics that are only slightly dependent on plant nutrient status. There are several reasons for this. First, the plant characteristics (phenology, leaf lifespan, tissue composition at vegetative stage) that drive the agronomic properties depend little on nutrient availability in standardized growing conditions and are correlated to LDMC (Al Haj Khaled et al., 2005, 2006; Pontes et al., 2007b); hence assessing weighted LDMC using a plant trait database identified these properties at plant community level, whatever the nutrient availability. Secondly, LDMC measurements in the field are sensitive to growing conditions, especially nutrient availability (van der Werf et al., 1993), so that they record a mixture of growth and plant development characteristics. Hence LMDC is less suitable than PFT composition of the plant community for assessing those agronomic characteristics that are mainly plant development-dependent. This result partly refutes the conclusions of Mokany and Ash (2008) who recommended choosing a fertility treatment as similar as possible to those of a natural community when the values of the plant traits used are obtained from pot experiments. The grass PFTs established on an LDMC basis appeared suitable to account for the variation in agronomic characteristics related to variation of production and leaf digestibility for the whole growing period. The plant features measured on the sward (dates of canopy closure, dates at which the peak occurred for leaf and whole herbage mass) were all strongly correlated with the functional composition of the vegetation. Indeed, the grass PFTs differ significantly in their average leaf lifespan (500 and 900 °Cd for PFT 1 and 2, respectively; Ansquer et al., 2004), which is consistent with differences observed for the dates at which the peak of grass leaf mass occurred (around 600 and 1000 °Cd according to the PFT 2 proportion; Table 3). On the other hand, previous work has shown that the date of flowering varied according to the plant functional type (by around 400 °Cd between PFT 1 and 2), but not nutrient availability (Al Haj Khaled, 2005). The present results confirmed this difference that was around 300 °Cd between M+ or GM+ and P– treatments. Furthermore, the date at which the peak of standing herbage mass occurred was later than the flowering dates by 380 °Cd on average. However, this difference was larger when the plant nutrient status was high (P < 0·001), varying from 150 °Cd up to 900 °Cd. The plant communities composed of species with a resource conservation strategy have more leaves than those composed of species with a resource capture strategy. Comparisons of plant communities having different functional composition showed a significant effect of PFT on leaf digestibility. These results are consistent with those obtained previously in the field (Bruinenberg et al., 2002). They are also consistent with lignin and (hemi)cellulose concentrations analysed at the juvenile stage on grasses belonging to different PFTs (van Arendonk and Poorter, 1994).
The second new finding shows the effect of functional diversity on fodder provision on a short time scale. In most studies it has been found that functional diversity may increase the ecosystem's productivity (Hector et al., 1999) and enhance its resistance against invasive species (Naeem et al., 2000; Pokorny et al., 2005). As far as is known, this is the first time that this approach has shown the role of functional diversity on the shape of the growth curve given flexibility in management defoliation, i.e. extending the cutting/grazing dates without too much effect on the amount of herbage yield (Duru et al., 2008).
Value of the approach for deciding management practices
On a broad spatial scale, abiotic factors are usually the main drivers of growth (Diaz et al., 2007). The present results show that management, through the fertilization and defoliation regime, allows big changes in the fodder provision characteristics without any great variation in other factors such as elevation and pH, resulting from the sampling choice. The effects of management on herbage productivity and quality are well known (Frame, 1989) but those on the timing of growth, e.g. the time of regrowth at which the ceiling yield occurs, much less so. Thus there is scope for creating functional diversity between and within grassland fields (Andrieu et al., 2008).
The difference in the timing of reproductive development between species contributes to a range of earliness, e.g. a difference of 30 d between Lolium perenne and Agrostis (McCall and Bishop-Hurley, 2003) that can be an asset to a farm (White et al., 2004). This range of variation is consistent with the present findings for corresponding species belonging to PFT 1 and 2. Thus, for the set of grassland communities which differed most in terms of PFT composition (see Table 1), differences in the dates at which canopy closure and ceiling yield occurred varied by around 250 and 600 °Cd, respectively (Table 2). Even for the subset of grasslands having similar plant nutrient indices (M + , GM+ and P + , P > 0·5), there were significant differences (300 °Cd; P < 0·05) in the dates at which the ceiling yield occurred. These differences were mainly due to an indirect effect of the defoliation regime. Indeed, cutting favoured PFT 1, composed of taller species able to compete for light during the reproductive phase (Duru et al., 2005).
CONCLUSIONS
There are consistent results between plant features assessed at species level, mediated through PFTs, and grassland characteristics at plant community level. Hence, ranking of grassland communities for the date at which the ceiling yield of plant components occurs, the shape of the growth curve around the peak biomass, as well as for tissue composition, can be predicted well by the PFT composition of the vegetation, independently of nutrient availability for plant growth. Conversely, the plant nutrient status and, to a lesser extent, the plant functional composition are the most reliable functional descriptors of the vegetation for predicting the herbage accumulation rate.
ACKNOWLEDGEMENTS
The presented results were funded by the EU project VISTA (Vulnerability of Ecosystem Services to Land Use Change in Traditional Agricultural Landscapes) (Contract no. EVK2-2001-15 000356).
LITERATURE CITED
- Al Haj Khaled R. L'évaluation des caractéristiques agronomiques d'espèces par leurs traits de vie comme étape préalable au diagnostic des communautés à flore complexe. France: INPL, Nancy; 2005. PhD Thesis. [Google Scholar]
- Al Haj Khaled R, Duru M, Theau J P, Plantureux S, Cruz P. Variation of leaf traits through seasons and N-availability levels and its consequences for ranking grassland species. Journal of Vegetation Science. 2005;16:391–398. [Google Scholar]
- Al Haj Khaled R, Duru M, Decruyenaere V, Jouany C, Cruz P. Using leaf traits to rank native grasses according to their nutritive value. Rangeland Ecology & Management. 2006;59:648–654. [Google Scholar]
- Andrieu N, Coléno FC, Duru M. L'organisation du système fourrager source de flexibilité face aux variations climatiques. In: Dedieu B, Chia E, Leclerc B, Moulin CH, Tichit M, editors. L'élevage en mouvement – flexibilité et adaptation des exploitations d'herbivores. Versailles: Editions Quae; 2008. pp. 95–110. [Google Scholar]
- Ansquer P. Caractérisation agro-écologique des végétations prairiales naturelles en réponses au pratiques agricoles: apports pour la construction d'outils de diagnostic. Thèse, INP Toulouse; 2006. [Google Scholar]
- Ansquer P, Theau J P, Cruz P, Viegas J, Al Haj Khaled R, Duru M. Caractérisation de la diversité fonctionnelle des prairies à flore complexe: vers la construction d'outils de gestion. Fourrages. 2004;179:353–368. [Google Scholar]
- van Arendonk JJCM, Poorter H. The chemical composition and anatomical structure of leaves of grass species differing in relative growth rate. Plant, Cell & Environment. 1994;17:963–970. [Google Scholar]
- Aufrère J. Etude de la prévision de la digestibilité des fourrages par une méthode enzymatique. Annales de Zootechnie. 1982;31:111–130. [Google Scholar]
- Biston R, Dardenne P. Application de la spectrométrie de réflexion dans le proche infra-rouge: prévision de la qualité des fourrages en vue de leur exploitation rationnelle. Bulletin Recherches Agronomiques de Gembloux. 1985;20:23–41. [Google Scholar]
- Bruinenberg MH, Valk H, Korevaar H, Struik PC. Factors affecting digestibility of temperate forages from seminatural grasslands: a review. Grass and Forage Science. 2002;57:292–301. [Google Scholar]
- Calvière I, Duru M. The effect of N and P fertilizer application and botanical composition on the leaf/stem ratio patterns in spring in Pyrenean meadows. Grass and Forage Science. 1999;54:255–266. [Google Scholar]
- Campanella MV, Bertiller MB. Plant phenology, leaf traits and leaf litterfall of contrasting life forms in the arid Patagonian Monte, Argentina. Journal of Vegetation Science. 2008;19:75–85. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. Handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Demarquilly C. In: Deroches R, editor. The feeding value of forages; Proceedings of the. XVI International Grassland Congress. INRA et AFPF; 1989. pp. 1817–1823. [Google Scholar]
- Diaz S, Lavorel S, de Bello F, Quetier F, Grigulis K, Robson TM. Land change science special feature: incorporating plant functional diversity effects in ecosystem service assessments. Proceedings of the National Academy of Sciences of the USA. 2007;104:20684–20689. doi: 10.1073/pnas.0704716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann M, Falkengren-Grerup U. Prediction of species response to atmospheric nitrogen deposition by means of ecological measures and life history traits. Journal of Ecology. 2002;90:108–120. [Google Scholar]
- Duru M, Ducrocq H. A nitrogen and phosphorus herbage nutrient index as a tool for assessing the effect of N and P supply on the dry matter yield of permanent pastures. Nutrient Cycling in Agroecosystems. 1997;47:59–69. [Google Scholar]
- Duru M, Delprat V, Fabre C, Feuillerac E. Effect of nitrogen fertiliser supply and winter cutting on morphological composition and herbage digestibility of a Dactylis glomerata L. sward in spring. Journal of the Science Food and Agriculture. 2000;80:33–42. [Google Scholar]
- Duru M, Tallowin J, Cruz P. Functional diversity in low-input grassland farming systems: characterisation, effect and management. In: Lillak R, Viiralt R, Linke A, Geherman V, editors. Integrating efficient grassland farming and biodiversity. Tartu, Estonia: 2005. pp. 199–210. EGF Symposium 10. [Google Scholar]
- Duru M, Cruz P, Magda D. La conduite des couverts prairiaux, source de flexibilité. In: Dedieu B, Chia E, Leclerc B, Moulin CH, Tichit M, editors. L'élevage en mouvement – flexibilité et adaptation des exploitations d'herbivores. Versailles: Editions Quae; 2008. pp. 57–72. [Google Scholar]
- Frame J. Herbage productivity of a range of grass species under a silage cutting regime with high fertilizer nitrogen application. Grass Forage Science. 1989;44:267–276. [Google Scholar]
- Garnier E, Cortez J, Billès G, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Garnier E, Lavorel S, Ansquer P, et al. Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: a standardized methodology and lessons from an application to 11 European sites. Annals of Botany. 2007;99:967–985. doi: 10.1093/aob/mcl215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP. Control of species diversity in herbaceous vegetation. Journal of Environmental Management. 1973;1:151–167. [Google Scholar]
- Grime JP. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. Journal of Ecology. 1998;86:902–910. [Google Scholar]
- Grime JP, Hodgson JG, Hunt R. Comparative plant ecology. In: Unwin H, editor. Functional approach to common British species. London: Unwin Hyman; 1988. pp. ix–742. [Google Scholar]
- Hector A, Schmid B, Beierkuhnlein C. Plant diversity and productivity experiments in European grasslands. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Montserrat-Marti G, Cerabolini B, et al. A functional method for classifying European grasslands for use in joint ecological and economic studies. Basic and Applied Ecology. 2005;a 6:119–131. [Google Scholar]
- Hodgson JG, Montserrat-Marti G, Tallowin J, et al. How much will it cost to save grassland diversity? Biological Conservation. 2005;b 122:263–273. [Google Scholar]
- Holdaway RJ, Sparrow AD. Assembly rules operating along a primary riverbed-grassland successional sequence. Journal of Ecology. 2006;94:1092–1102. [Google Scholar]
- Hooper DU, Solan M, Symstad A, et al. Species diversity, functional diversity, and ecosystem functioning. In: Loreau M, Naeem S, Inchausti P, editors. Biodiversity and ecosystem functioning – synthesis and perspectives. Oxford: Oxford University Press; 2002. pp. 195–208. [Google Scholar]
- Hunt R. Plant growth curves: the functional approach to plant growth analysis. London: Edward Arnold; 1982. [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. [Google Scholar]
- Lavorel S, Diaz S, Cornelissen JH, et al. Plant functional types: are we getting any closer to the Holy Grail? In: Canadell J, Pitelka LF, Pataki D, editors. Terrestrial ecosystems in a changing world. Berlin: Springer-Verlag; 2005. [Google Scholar]
- Lavorel S, Grigulis K, McIntyre S, et al. Assessing functional diversity in the field – methodology matters! Functional Ecology. 2008;22:134–147. [Google Scholar]
- Lemaire G, Gastal F. N uptake and distribution in plant canopies. In: Lemaire G, editor. Diagnosis of the nitrogen status in the crops. Berlin: Springer Verlag; 1997. pp. 3–44. [Google Scholar]
- Louault F, Pillar VD, Garnier E, Soussana JF. How much will it cost to save grassland diversity? Journal of Vegetation Science. 2005;16:151–160. [Google Scholar]
- Mason WH, MacGillivray K, Steel JB, Wilson JB. An index of functional diversity. Journal of Vegetation Science. 2003;14:571–578. [Google Scholar]
- McCall DG, Bishop-Hurley GJ. A pasture growth model for use in a whole-farm dairy production model. Agricultural Systems. 2003;76:1183–1205. [Google Scholar]
- Mokany K, Ash J. Are traits measured on pot grown plants representative of those in natural communities? Journal of Vegetation Science. 2008;19:119–126. [Google Scholar]
- Naeem S, Knops JMH, Tilman D, Howe KM, Kennedy T, Gale S. Plant diversity increases resistance to invasion in the absence of covarying extinsic factors. Oikos. 2000;91:97–108. [Google Scholar]
- Negi GCS, Rikhari HC, Singh SP. Phenological features in relation to growth forms and biomass accumulation in an alpine meadow of the Central Himalaya. Vegetatio. 1992;101:161–170. [Google Scholar]
- Parsons AJ. The effect of season and managment on the grass growth of grass sward. In: Jones MB, Lazenby A, editors. The grass crop. London: Chapman & Hall; 1988. pp. 129–178. [Google Scholar]
- Pokorny ML, Sheley RL, Zabinski CA, Engel RE, Svejcar TJ, Borkowski JJ. Plant functional group diversity as a mechanism for invasion resistance. Restoration Ecology. 2005;13:448–459. [Google Scholar]
- Pontes L, Carrere P, Andueza D, Louault F, Soussana JF. Seasonal productivity and nutritive value of temperate grasses found in semi-natural pastures in Europe: responses to cutting frequency and N supply. Grass and Forage Science. 2007;a 62:485–496. [Google Scholar]
- Pontes L, Soussana JF, Louault F, Andueza D, Carrere P. Leaf traits affect the above-ground productivity and quality of pasture grasses. Functional Ecology. 2007;b 21:844–853. [Google Scholar]
- Poorter H, Remkes C, Lambers H. Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiology. 1990;94:621–627. doi: 10.1104/pp.94.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quétier F, Thébault A, Lavorel S. Plant traits in a state and transition framework as markers of ecosystem response to land-use change. Ecological Monographs. 2007;a 77:33–52. [Google Scholar]
- Quétier F, Lavorel S, Thuiller W, Davies I. Plant-trait-based modeling assessment of ecosystem-service sensitivity to land-use change. Ecological Applications. 2007;b 17:2377–2386. doi: 10.1890/06-0750.1. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Ryle GJA, Woledge J. The grass plant – its form and function. In: Jones MB, Lazenby A, editors. The grass crop. London Chapman & Hall; 1988. pp. 25–84. [Google Scholar]
- Ryser P. The importance of tissue density for growth and life span of leaves and roots: a comparison of five ecologically contrasting grasses. Functional Ecology. 1996;10:717–723. [Google Scholar]
- Sanderson MA, Skinner RH, Barker DJ, Edwards GR, Tracy BF, Wedin DA. Plant species diversity and management of temperate forage and grazing land ecosystems. Crop Science. 2004;44:1132–1144. [Google Scholar]
- Schippers P, Olff H. Biomass partitioning, architecture and turnover of six herbaceous species from habitats with different nutrient supply. Plant Ecology. 2000;149:219–231. [Google Scholar]
- Simon JC, Lemaire G. Tillering and leaf area index in grass in the vegetative phase. Grass and Forage Science. 1987;42:373–380. [Google Scholar]
- Vile D, Shipley B, Garnier E. Ecosystem productivity can be predicted from potential relative growth rate and species abundance. Ecological Letters. 2006;9:1061–1067. doi: 10.1111/j.1461-0248.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O. Challenging Theophrastus: a common core list of plant traits for functional ecology. Journal of Vegetation Science. 1999;10:609–620. [Google Scholar]
- van der Werf A, van Nuenen M, Visser AJ, Lambers H. Effects of N-supply on the rates of photosynthesis and shoot and root respiration of inherently fast- and slow-growing monocotyledonous species. Physiologia Plantarum. 1993;89:563–569. [Google Scholar]
- Westoby M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant and Soil. 1998;199:213–227. [Google Scholar]
- White TA, Barker DJ, Moore KJ. Vegetation diversity, growth, quality and decomposition in managed grasslands. Agricultural Ecosystems and Environment. 2004;101:73–84. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist. 1999;143:155–162. [Google Scholar]