Abstract
Background and aims
In recent years, Cyperus rotundus has become a problem weed in lowland rice (Oryza sativa) grown in rotation with vegetables in the Philippines. As the growth of C. rotundus is commonly suppressed by prolonged flooding, the ability of the weed to grow vigorously in flooded as well as upland conditions suggests that adapted ecotypes occur in these rotations. Studies were conducted to elucidate the mechanisms that permit C. rotundus to tolerate flooded soil conditions.
Methods
Upland and lowland ecotypes of C. rotundus were compared in terms of growth habit, carbohydrate reserves and metabolism, and activities of enzymes involved in alcoholic fermentation – alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC).
Key Results
The lowland ecotype has much larger tubers than the upland ecotype. Prior to germination, the amylase activity and total non-structural carbohydrate content in the form of soluble sugars were greater in the tubers of lowland plants than in those of upland C. rotundus. At 24 h after germination in hypoxic conditions, PDC and ADH activities in the lowland plants increased, before decreasing at 48 h following germination. In contrast, ADH and PDC activities in the upland plants increased from 24 to 48 h after germination.
Conclusions
Tolerance of lowland C. rotundus of flooding may be attributed to large carbohydrate content and amylase activity, and the ability to maintain high levels of soluble sugars in the tubers during germination and early growth. This is coupled with the modulation of ADH and PDC activities during germination, possibly to control the use of carbohydrate reserves and sustain substrate supply in order to avoid starvation and death of seedlings with prolonged flooding.
Key words: Anoxia, ethanol fermentation, flooding tolerance, nutsedge, Cyperus rotundus, Pasteur effect, weed ecology
INTRODUCTION
Grain yield losses due to weeds in lowland rice fields range from 20 % to 60 % in transplanted crops and from 30 % to 80 % in direct-seeded rice (Janiya, 2002). In lowland rice systems, grasses such as barnyardgrass (Echinochloa crus-galli), broadleaf weeds such as Monochoria vaginalis, and annual sedges such as Cyperus difformis are major weeds in the Philippines. Cyperus rotundus is a perennial sedge that thrives under upland conditions, and is suggested to be the ‘world's worst weed’, but grows slowly when soils are flooded or waterlogged (Holm et al., 1977). Recent studies, however, report that C. rotundus has become a major problem in rain-fed lowland rice grown in rotation with dry-season vegetables in central Luzon, Philippines (Baltazar et al., 1999). In the 1970s, C. rotundus was reported only as a minor weed in rice (Pablico and Moody, 1985), whereas in recent years it has become an increasingly serious problem in rice grown in rotation with vegetables. Densities of C. rotundus have increased from 15 plants m−2 in 1998 to more than 50 plants m−2 in 2005 (Baltazar et al., 1999, 2006), indicating ecotypes of C. rotundus that are well adapted to lowland flooded conditions now exist in these areas. The species has recently been reported as a weed of rice in 21 countries (Rao et al., 2007).
Ecotypic variation in C. rotundus in Asia and elsewhere in the world has been reported (Holm, et al., 1977; Chavez and Moody, 1986; Wills, 1998). Morphological variation among C. rotundus populations has been observed, with lowland ecotypes being taller and having bigger tubers than upland ecotypes (Komai et al., 1983). In further studies, Baltazar et al. (1997) found that, compared with upland C. rotundus plants, lowland plants were taller and had greater biomass, more offshoots, and longer and wider leaves, and the tubers were two- to threefold larger and heavier. Genetic variation between lowland and upland C. rotundus was observed among populations from North America, India and Brazil (Okoli et al., 1997), and among populations from rain-fed areas in central Luzon, Philippines, where rice–vegetable rotations are practiced (Casimero et al., 1999).
Increasing populations of C. rotundus in rice fields add to existing weed control problems, with a consequent increase in costs. Furthermore, carry-over of tubers into the vegetable crop is likely to result in the build-up of this species in vegetables. Populations of C. rotundus in vegetable crops in farmers' fields are reported to be about 200 plants m−2, and farmers can spend about US$400 ha−1, or 20 % of their production costs, to control this weed during a season (Islam et al., 2005).
The proliferation of C. rotundus in flooded rice fields in the Philippines suggests that ecotypes may have acquired some adaptive mechanisms for growth in an oxygen-deficient environment. Higher plants have an absolute requirement for oxygen for growth and, as gases diffuse 10 000 times slower in water than in air (Mohanty et al, 2000), flooding results in decreased oxygen availability, ranging from deficiency (hypoxia) to absence (anoxia) in highly reduced soils. When exposed to anaerobic conditions, plants may shift metabolism from aerobic respiration to an alcoholic fermentation pathway as an adaptive mechanism for survival under anaerobic conditions. Key enzymes for ethanol fermentation are alcohol dehydrogenase (ADH; alcohol : NAD+ oxidoreductase, E.C. 1·1·1) and pyruvate decarboxylase (PDC; E.C. 4·1·1·1). An increase in the activities of these enzymes under anaerobic conditions has been reported in rice (Ellis and Setter, 1999) and maize (Laszlo and St. Lawrence, 1983). Pyruvate decarboxylase catalyses the decarboxylation of pyruvate to yield carbon dioxide and acetaldehyde. The acetaldehyde produced is then reduced to ethanol by the second enzyme, ADH, with NADH being oxidized in the process. PDC is considered the key regulator of ethanol production because it is present in lower amounts than ADH, and its activity is almost the same as the rate of ethanol fermentation in vivo (Tadege et al., 1998). ADH is the most studied enzyme in relation to anaerobiosis and has been reported to increase in most plants in response to low oxygen stress (Kennedy et al., 1992).
As the fermentation pathway uses glucose as a substrate, an adequate supply of fermentable sugars, or the ability of plants to mobilize starch or stored carbohydrates into simple sugars through induction of amylases or other degradation enzymes when oxygen is deficient, is important for survival in flooded environments. In rice, a flood-tolerant plant, the use of carbohydrates during anoxia involves sugar degradation, the induction of amylase and starch degradation into simple sugars (Guglielminetti et al., 1995).
The present studies were conducted to decipher the morphological and physiological basis of adaptation of C. rotundus to flooding during its establishment by comparing lowland and upland ecotypes with respect to: (1) levels of non-structural carbohydrate concentrations (starch and sugars) in the tubers before and during flooding; (2) differential abilities to mobilize starch into soluble sugars during flooding as depicted by differences in total amylase activities and soluble sugar concentrations; and (3) the ability to up-regulate the alcohol fermentation pathway to generate energy required for growth and maintenance metabolism during hypoxia, as demonstrated by changes in activities of PDC and ADH.
MATERIALS AND METHODS
Collection and study sites
Cyperus rotundus tubers were collected from lowland fields, used for growing rice under almost continuously flooded conditions, and upland fields used for growing vegetables in Pampanga Province in central Luzon, Philippines, in June 2005 and in January and July 2006. Additional collections were made from lowland and upland fields in each of six provinces: Nueva Ecija, Laguna, Tarlac, Pangasinan, Pampanga and Iloilo. Tubers were collected randomly within a particular field. All subsequent studies were conducted at the International Rice Research Institute (IRRI), Los Baños, Philippines, from June 2005 to September 2006.
Morphological characterization of tubers
Tubers were collected from lowland and upland fields. From each field, 150 ungerminated tubers (50 tubers in each of three replicates) were picked at random, washed thoroughly and their fresh weights recorded. The morphology of the lowland and upland ecotypes was also compared visually for general appearance, and tuber fresh weights were determined.
Seedling growth and hypoxic treatments
The tubers were germinated and then grown in plastic trays (45 × 30 × 15 cm). Lowland tubers were germinated in saturated soil, whereas upland tubers were germinated in moist and well-drained soil. At 7 d after sprouting, seedlings with emerging roots and shoots but with unfolded leaves were subjected to hypoxia as described by Ellis and Setter (1999), with slight modifications. Seven-day-old seedlings of both upland and lowland C. rotundus were placed in airtight Erlenmeyer flasks containing water through which nitrogen gas was flushed until the concentration of oxygen fell below 0·05 mol m−3 (hypoxia). After hypoxia treatment for 24 to 48 h, the roots were excised from the seedling, ground in cold extraction buffer (1 g root tissue per 6 mL extraction buffer) in a mortar and pestle, and assayed for ADH and PDC activities. The extraction buffer consisted of 100 mm N-tris (hydroxymethyl) methyl-2-aminoethanesulfonic acid (TES), pH 7·7, 2 mm magnesium chloride hexahydrate, 1 mm disodium EDTA dihydrate, 1·25 % Triton X-100 and 4 mm dithiothreitol (DTT).
Ungerminated tubers collected from the sampling site and tubers from plants raised in the greenhouse from which roots were excised (0, 24 and 48 h hypoxia treatments) were used in the determination of total carbohydrate, starch and soluble sugar concentrations, amylase activity and total protein concentration.
Total non-structural carbohydrates, soluble sugars and starch concentrations
Tubers were peeled, cut into 1-mm pieces, oven-dried at 70 °C for 24 h, ground into fine powder and analysed for total soluble sugar concentration based on the anthrone reaction following the procedure of Fales (1951). Soluble sugars were extracted with 80 % ethanol twice at 80 °C for 10 min. Portions of the extracts were added to anthrone reagent and the absorbance was read on a Beckman Coulter DU 800 spectrophotometer at 620 nm. The amount of the total soluble sugars was obtained from a standard curve using known concentrations of glucose. The residue obtained after extraction of soluble sugars was used for starch analysis following the method of McCready et al. (1950). Diluted perchloric acid was used to bring about hydrolysis of starch and the released sugar was assayed based on the anthrone reaction described above. The amount of total non-structural carbohydrates in the tubers of upland and lowland C. rotundus under various hypoxic treatments was determined by summing up the concentrations of soluble sugar and starch.
Amylase activity and protein concentrations
Extraction buffer, consisting of 0·02 m sodium phosphate buffer, pH 6·9, with 0·006 m NaCl, 1 mm CaCl2 and 10 mm DTT, was added to 200 mg of ground tuber tissues (6 mL g−1 sample) and the mixture was ground in a mortar and pestle on ice. The extracts were centrifuged at 13 000 g for 3 min at 4 °C using a Beckman Coulter Allegra 21R centrifuge. The supernatant was transferred to Eppendorf tubes for determination of amylase activity and protein concentration. The method of Bernfeld (1955) was adapted for the determination of total amylase activity. The samples were mixed with 1 % starch as substrate and incubated at 25 °C for 3–4 min to achieve temperature equilibration. At timed intervals, 0·5 mL of starch solution was added to each sample at 25 °C and allowed to stand for 3 min for a reaction to take place. One mL of dinitrosalicylic colour reagent was added to each sample, incubated for 5 min in a water bath at 100 °C, cooled to room temperature, and then mixed with 10 mL of distilled water. The solution was mixed well and the amount of maltose released from the reaction was determined by reading the absorbance at 540 nm against a standard curve. The amount of maltose (μmoles) released in each sample was calculated. One unit of enzyme activity (U) is defined as one mmole of maltose released from starch by 1 mg of protein min−1 at 25 °C and pH 6·9. The protein content of all enzyme preparations was measured using the Bradford (1976) method with 3 % bovine serum albumin (BSA) as the standard.
ADH extraction and assay
ADH was extracted from the excised root tissues using the extraction buffer described above and as described by Valdez (1995). From the crude extract, 200 µL was placed in Eppendorf tubes containing BSA. The remaining extract was centrifuged and analysed for protein concentration using the Bradford (1976) method with BSA as the standard. The aliquot for ADH assay was centrifuged at 13 000 g for 3 min at 4 °C, and the supernatant transferred to an Eppendorf tube and kept on ice until the assay.
Alcohol dehydrogenase was assayed in the acetaldehyde-to-ethanol direction using the method optimized by Valdez (1995). The assay mixture consisted of 830 µL of 62·4 mm TES at pH 7·0, 20 µL crude extract, 50 µL of 3·4 mm nicotinamide adenine dinucleotide, reduced form (NADH), and 100 µL of 200·32 mm acetaldehyde. The enzyme activity was measured by following the disappearance of NADH at 340 nm at 30 °C for 7 min. One unit of the enzyme was defined as the amount of enzyme catalysing the oxidation of 1 mmole of NADH per minute at 30 °C. ADH activity was expressed as specific activity.
PDC extraction and assay
The ground root extract was placed in 1·5-mL Eppendorf tubes with sufficient BSA to give a final concentration of 1 %. The pH was adjusted to 6·0 with 2-[(N-morpholino] ethanesulfonic acid (MES; 1 mL of the extract requires 0·2 mL of 250 mm MES containing 2·5 mm Mg+2 as a co-factor to adjust its pH to 6·0), and thiamine pyrophosphate (TPP) was added to a final concentration of 0·5 mm (1·2 mL of extract at pH 6·0 needs 0·06 mL of 10 mm TPP to give a final concentration of 0·5 mm TPP). The extracts were centrifuged at 13 000 g for 3 min at 4 °C using a Beckman Coulter Allegra 21R centrifuge. The supernatant was transferred to Eppendorf tubes for enzyme assay and detrmination of protein concentration.
PDC activity was assayed as described by Valdez (1995) with modifications. The enzyme extract (supernatant) was incubated at 25 °C for 1 h prior to the assay. The assay mixture was prepared in a 1 mL-capacity cuvette in the following order: 500 µL of 125 mm MES buffer (containing 2·5 mm MgCl2), 100 µL of 5 mm TPP, 100 µL of 500 mm oxamate, 100 µL enzyme extract, 50 µL ADH (10 IU), 50 µL of 3·4 mm reduced β-nicotinamide adenine dinucleotide (NADH), and 100 µL of 100 mm pyruvate. After the addition of NADH, the mixture was stirred (Vortex-2 Genie, Scientific Industries) and allowed to stand for 2 min for non-specific oxidation to take place, after which pyruvate was added. The absorbance was read using a Beckman Coulter DU 800 spectrophotometer at 340 nm at 30 °C continuously for 10 min. One unit of enzyme activity (U) is defined as 1 mmole of NADH oxidized by 1 mg of protein per minute.
Statistical analysis
Analysis of variance (ANOVA) was conducted for the parameters of tuber biomass, carbohydrate and sugar concentrations, and activities of ADH, PDC and amylase using a split-split-plot design, with the ecosystem (upland and lowland) as the main plot, the locations within each ecosystem as the subplot, and the hypoxia treatment as the sub-subplots. Three replicates were used and each replication was an average of three repeat measurements.
RESULTS
Morphology of lowland and upland tubers
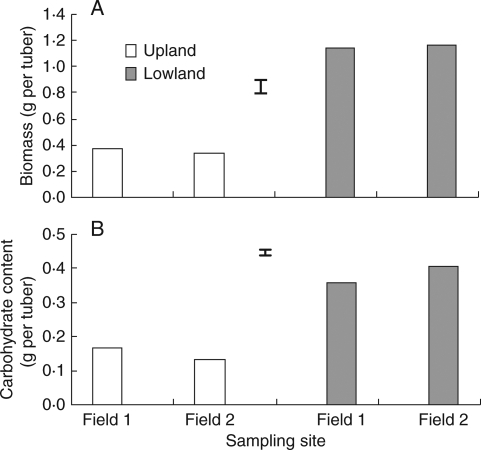
The biomass of lowland tubers was approximately three times higher than that of upland tubers, about 1·15 g per tuber vs 0·36 g per tuber (Figs. 1 and 2A). These findings agreed with those of previous studies that report that lowland C. rotundus tubers have greater biomass than those of upland plants (Baltazar et al., 1997). The tubers of C. rotundus serve not only as the vegetative propagules but also as storage organs for non-structural carbohydrates, which are the substrates for aerobic and anaerobic respiration.
Fig. 1.
Tubers of (A) upland and (B) lowland C. rotundus collected from respective field sites.
Fig. 2.
(A) Tuber biomass and (B) total non-structural carbohydrate content per tuber of upland and lowland C. rotundus. Data are means of three replicates and vertical bars indicate the l.s.d. at P < 0·05.
Concentrations of soluble sugars and starch
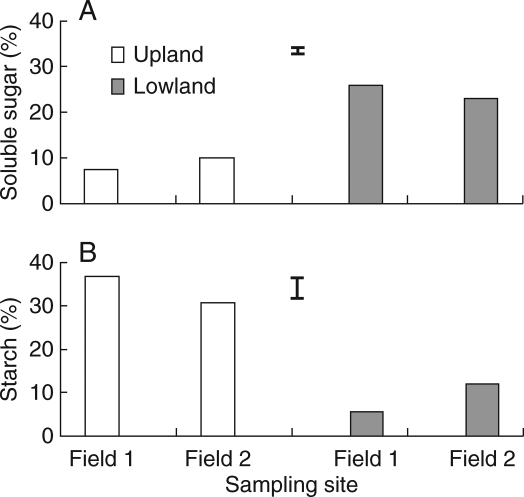
The total non-structural carbohydrate (soluble sugars and starch) content in lowland tubers was about twice that of upland tubers (Fig. 2B). Carbohydrate reserves in lowland tubers were largely in the form of soluble sugars, which were also about three times the amount found in upland tubers (Fig. 3A). Upland tubers, however, stored carbohydrates largely in the form of starch (Fig. 3B).
Fig. 3.
Concentrations of (A) soluble sugars and (B) starch in upland and lowland C. rotundus tubers. Data are means of three replicates and vertical bars indicate the l.s.d. at P < 0·05.
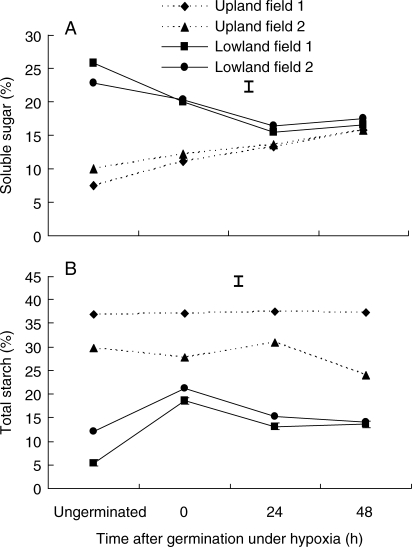
When incubated under hypoxic conditions following sprouting, the soluble sugar concentration of lowland tubers decreased during germination for up to 24 h and then levelled off (Fig. 4A). In contrast, soluble sugar concentration in upland tubers increased for up to 48 h during germination under hypoxia, but was consistently lower than that of lowland tubers. This increase in soluble sugars may suggest inability of upland ecotypes to use them for growth under anoxia. Unlike soluble sugars, starch concentration ( %) in lowland tubers was consistently lower and decreased even further in the first 48 h of germination under hypoxia (Fig 4B); however, no significant change in the amount of starch was observed in upland tubers during hypoxia for up to 48 h (Fig. 4B). These findings suggest that lowland ecotypes predominantly store their energy reserves in the form of soluble sugars and are relatively more efficient in using both soluble sugars and stored starch for growth under hypoxia.
Fig. 4.
Changes in (A) soluble sugar and (B) starch concentrations in ungerminated tubers and after 0, 24 and 48 h of germination under hypoxia in upland and lowland C. rotundus tubers. Data are means of three replicates and vertical bars indicate the l.s.d. at P < 0·05.
Amylase activity
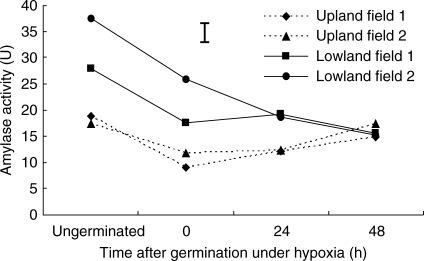
Total amylase activity was greater in lowland tubers than in upland tubers before and during germination. After imbibition, total amylase activity progressively decreased with time in lowland tubers and then remained more-or-less unchanged for up to 48 h following germination (Fig. 5).
Fig. 5.
Changes in total amylase activity in ungerminated tubers of upland and lowland C. rotundus and after 0, 24 and 48 h of germination under hypoxia. Data are means of three replicates with three measurements within each replicate, and vertical bars indicate the l.s.d at P < 0·05.
ADH and PDC activities in tubers following 24 h of hypoxia
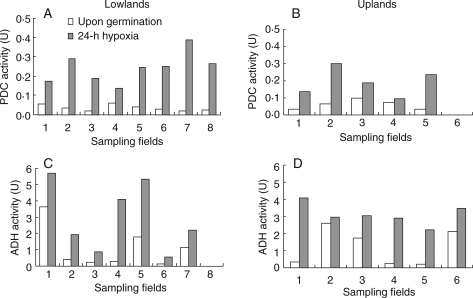
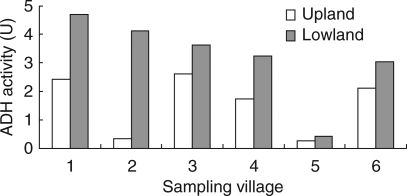
ADH and PDC activities in lowland and upland C. rotundus tubers collected from several locations increased significantly when both ecotypes were subjected to hypoxia for 24 h following germination (Fig. 6); however, this increase in activity was substantially greater in lowland tubers (Fig. 6A, C) than in upland tubers (Fig 6B, D). Activity of ADH was again compared in another set of tubers collected from either upland or lowland fields within each of six villages, then germinated and incubated for 24 h under hypoxia. Lowland tubers consistently had higher ADH activity than upland plants (Fig. 7).
Fig. 6.
PDC activities in C. rotundus tubers collected from different field sites at 0 h and 24 h following hypoxia: (A) lowland tubers from eight fields (means across fields: 0 h = 0·036 U, 24 h = 0·243 U; l.s.d.0·05 = 0·07) and (B) five upland fields (means: 0 h = 0·052 U, 24 h = 0·164 U; l.s.d.0·05 = 0·069); and ADH activities in (C) seven lowland fields (means 0 h = 1·087 U, 24 h = 2·943 U; l.s.d.0·05 = 1·174), and (D) six upland fields (means 0 h = 1·222 U, 24 h = 3·123 U; l.s.d.0·05 = 1·131).
Fig. 7.
ADH activities in sprouted C. rotundus tubers collected from upland and lowland sites within each of six villages, germinated and then incubated for 24 h under hypoxia. Mean values: upland = 1·59, lowland = 3·20; l.s.d.0·05 = 1·64.
ADH and PDC activities in roots following 24 and 48 h of hypoxia
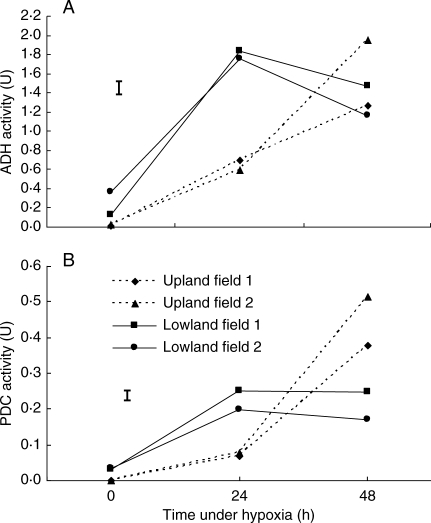
Upon germination, ADH activity in the roots of 7-d-old seedlings was detected in both ecotypes. At 24 h following germination under hypoxia, a sharp increase in ADH activity was manifested by both ecotypes, although the ADH activity in the lowland ecotypes was more than twice that of the upland ecotypes (Fig. 8A). At 48 h following germination, ADH activity in the upland ecotypes continued to increase, whereas ADH activity in the lowland ecotypes started to decrease.
Fig. 8.
Changes in (A) alcohol dehydrogenase and (B) pyruvate decarboxylase activities in roots of 7-d-old seedlings of upland and lowland C. rotundus after 0, 24 and 48 h of incubation under hypoxia using pre-germinated tubers. Data are means of three replications and vertical bars indicate the l.s.d. at P < 0·05.
Similarly, PDC activity in the roots of 7-d-old seedlings of both upland and lowland ecotypes increased after 24 h under hypoxia following germination (Fig. 8B). PDC activity after 24 h of hypoxia was significantly higher in lowland ecotypes than in upland plants. However, after 48 h of germination under hypoxia, activity of PDC levelled off in lowland seedlings but continued to increase in upland seedlings in a similar way to ADH (Fig. 8B). After 48 h under hypoxia, PDC activity in upland seedlings was significantly higher than in lowland seedlings.
DISCUSSION
As tubers provide storage organs for carbohydrates, the bigger tubers of lowland plants could be an important morphological adaptation of C. rotundus to flooding, in which more energy resources are needed as metabolism shifts toward the less efficient anaerobic respiration. Flood-tolerant plants have been reported to respond to anoxia by storing larger quantities of carbohydrates in their underground parts to provide energy during extended periods of flooding and, as a consequence, these plants have bigger rhizomes or tubers than flood-intolerant plants (Bucher and Kuhlemeier, 1993).
Our study showed that lowland tubers contain more total non-structural carbohydrates than upland tubers, and this is stored mostly in the form of soluble sugar reserves that would be readily available as substrates for anaerobic respiration under flooded conditions. In contrast, upland tubers stored carbohydrates predominantly in the form of starch. To avoid sugar starvation and, as a consequence, rapid cell death, degradation of starch to simple sugars is probably the most important metabolic process for survival under hypoxia or anoxia (Loreti et al., 2003). Rice, a flood-tolerant plant, contains a complete set of enzymes for starch breakdown in the endosperm, including amylases; these enzymes are absent in flood-intolerant plants such as barley, wheat, oat, rye and corn (Perata et al., 1997). Even some rice cultivars such as ‘IR22’, however, were observed to be intolerant of anoxia due to slow starch hydrolysis in the endosperm (Huang et al., 2003).
Higher levels of amylase activity in lowland tubers than in upland tubers before and during germination probably help in maintaining a high concentration of soluble sugars in lowland tubers, which will ensure a constant supply of soluble sugars to fuel the ethanol fermentation pathway. Perata et al. (1993) emphasized the importance of substrate availability during germination under anoxia or hypoxia. Amylase activity in upland tubers did not increase before and during germination and remained constant up to 48 h following germination. When plants are experiencing low-oxygen stress, however, fermentable substrates are not replenished, and the high rate of fermentation in upland seedlings would lead to rapid depletion of available substrates, as ATP production through alcohol fermentation is 18-fold less efficient than in aerobic respiration (Greenway and Setter, 1996). This would ultimately result in plant death if hypoxia is prolonged.
The decrease in the amounts of soluble sugars in lowland tubers upon germination and during hypoxia suggests that catabolism of soluble sugar is active. This indicates a pronounced Pasteur effect in lowland tubers when oxygen is limited. A slow decrease in soluble sugars after 48 h of hypoxia suggests that the use of soluble sugars by lowland ecotypes is highly regulated during hypoxia, such that a high steady-state sugar level in lowland tubers is sustained. Modulation in the consumption of carbohydrate reserves by lowland ecotypes will prevent the depletion of substrates during prolonged periods of hypoxia. The contrasting observation that soluble sugar concentration in upland tubers increased before and during germination, and up to 48 h following germination under hypoxia, could not be attributed to starch breakdown but is presumed to be due to reduced use of the metabolite. Similar observations have been made in other species, including rice and maize (Atwell et al., 1985).
Our data showed that both C. rotundus ecotypes can induce ADH and PDC activities when experiencing hypoxic stress. Activities of both enzymes in lowland seedlings were substantially higher in germinated tubers after 24 h under hypoxia, but subsequently levelled out. However, activities in upland seedlings were lower earlier but increased progressively with time, and were either similar to or greater than those of lowland ecotypes after 48 h of hypoxia (Fig. 8). This increase in enzyme activities, presumably coupled with a parallel increase in anaerobic respiration, could result in early exhaustion of energy resources, particularly soluble sugars, in upland ecotypes. Increased rates of ADH and PDC activities and ethanol fermentation to compensate for low energy production does not improve plant survival under hypoxic stress but leads to a depletion of available substrates (Tadege et al., 1998). Plants can germinate under hypoxia, however, if sufficient substrates are available (Perata et al., 1993) and therefore plant survival is enhanced over extended periods of oxygen deficiency (Tadege et al., 1998).
The responses in ADH and PDC activities indicate that lowland C. rotundus down-regulates the rate of ethanol fermentation following increased enzyme activities after 24 h of hypoxia. This could be in order to optimize the use of available carbohydrates as an adaptive mechanism to germinate and thrive under flooded environments. The levelling-off of ADH and PDC activities in roots, coupled with high amounts of soluble sugar and high amylase activity in tubers, ensures enough supply of soluble sugars for a longer duration to allow seedlings to survive and grow to the surface of flooded rice fields. This probably enables lowland C. rotundus to avoid depletion of substrates and energy sources during long periods of exposure to oxygen deficiency. Similar observations on other plants have been reported by Gibbs and Greenway (2003).
The results of our studies show that tolerance of lowland C. rotundus of hypoxia is due to a combination of several factors: (1) larger tubers with higher soluble sugar content than upland C. rotundus before germination; (2) the ability to mobilize more starch to soluble sugars and probably regulate its utilization during germination; and (3) the ability to activate but modulate the fermentation pathway through regulation of ADH and PDC activities during germination. This ensures the sustainability of substrate supply for a longer duration and avoids substrate depletion. Further, after comparing flood tolerant and intolerant species of Iris, Hanhijärvi and Fagerstedt (1995) concluded that the greater carbohydrate reserves in the rhizomes of the tolerant species allow maintenance of glycolysis and ethanolic fermentation during flooding.
In contrast, flood-intolerant upland C. rotundus has (1) smaller tubers that are less than half the size of those of lowland C. rotundus and with lower carbohydrate content, which is mainly stored in the form of starch in ungerminated tubers; (2) low levels of amylase activity under hypoxia to convert starch into soluble sugars, and an inability to use starch and sugars during germination; and (3) increased rates of fermentation under prolonged flooding (48 h after germination), through a progressive increase in ADH and PDC activities (Fig. 8) but with no apparent reduction in soluble sugars (Fig 4).
Knowledge of the adaptive mechanism of C. rotundus to flooding will help us understand the species' response to a wide range of environmental conditions. Data from this research can be used to provide basic information that may help enhance flood tolerance in crops. For management strategies, our data can be used as a basis in developing ecological weed-control approaches such as flooding and other kinds of habitat-manipulation management strategies. The studies suggest, however, that because C. rotundus may have acquired flood-tolerance mechanisms, water management or flooding may no longer be a viable management option against this weed. Because of their increased population density and occurrence within rice crops, tubers carried over from the rice crop to the vegetable crop and vice versa will result in a population build-up of C. rotundus. Hence, to prevent further population build-up, there is a need to develop alternative management strategies to prevent C. rotundus from infesting rainfed rice–vegetable areas and other rainfed environments with similar crop rotations.
ACKNOWLEDGMENTS
These studies are part of the M.S. theses of J. T. Peña-Fronteras and M. C. Villalobos and the research was partly funded through the Irrigated Rice Research Consortium of the International Rice Research Institute (IRRI), the SEAMEO Regional Center for Graduate Study and Research in Agriculture (SEARCA), and the United Board on Christian Higher Education in Asia (UBCHEA) through Central Philippine University. We thank Evangelina S. Ella, Ofelia Namuco, Teodoro Migo, Joel Janiya, James Egdane, Flor Bariuan and Gina Vergara for technical assistance, and Bill Hardy, Michael Thomson and two anonymous reviewers for their useful comments on the manuscript.
LITERATURE CITED
- Atwell B, Greenway H, Ward G, Waters I. A study of impaired growth of roots of Zea mays seedlings at low oxygen concentrations. Plant, Cell and Environment. 1985;8:179–188. [Google Scholar]
- Baltazar AM, Martin EC, Casimero MC, Bariuan FV, Obien SR, De Datta SK. Characterization of purple nutsedge in rainfed rice-onion systems. Proceedings of 16th Asian-Pacific Weed Science Society Conference; September 8–12, 1997; Kuala Lumpur, Malaysia. Kuala Lumpur, Malaysia: Asian-Pacific Weed Science Society; 1997. pp. 326–329. [Google Scholar]
- Baltazar AM, Martin EC, Casimero MC, Bariuan FV, Obien SV, De Datta SK. Major weeds and their dominance patterns in rainfed rice–onion cropping system. Philippine Agricultural Scientist. 1999;82:166–177. [Google Scholar]
- Baltazar AM, Johnson DE, Ismail A, Janiya JD, Bariuan FV, Peña JT, Villalobos MC, Merca FE. Control of sedges in rice: wetland purple nutsedge. In: Kim KU, Labrada R, editors. Management of sedge weeds in rice; Proceedings of the Workshop on Management of Sedge Weeds in Rice; November 6, 2005; Ho Chi Minh City, Vietnam. Daegu: Kyungpook National University, Korea; 2006. pp. 43–52. [Google Scholar]
- Bernfeld P. Amylases, α and β. In: Colowick S, Kaplan N, editors. Methods in enzymology. Vol 1. New York: Academic Press; 1955. pp. 149–150. [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bucher M, Kuhlemeier C. Long-term anoxia tolerance. Multi-level regulation of gene expression in the amphibious plant Acorus calamus L. Plant Physiology. 1993;103:441–448. doi: 10.1104/pp.103.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimero MC, Baltazar AM, Manuel JS, Obien SR, De Datta SK. Morphologic and genetic variations in upland and lowland ecotypes of purple nutsedge. Proceedings of the 17th Asian- Pacific Weed Science Society Conference; November 22–27, 1999; Bangkok, Thailand. Bangkok, Thailand: Asian-Pacific Weed Science Society: 1999. pp. 134–139. [Google Scholar]
- Chavez RC, Moody K. Ecotypic variation in Cyperus rotundus. In: Pancho JV, Sastoutomo SS, Tjitrosemito S, editors. Proceedings of the Symposium in Weed Science; Bogor, Indonesia: Regional Center for Tropical Biology; 1986. pp. 123–126. BIOTROP Special Publication 24. [Google Scholar]
- Ellis MH, Setter TL. Hypoxia induces anoxia tolerance in completely submerged rice seedlings. Journal of Plant Physiology. 1999;154:219–230. [Google Scholar]
- Fales F. The assimilation and degradation of carbohydrates by yeast cells. Journal of Biological Chemistry. 1951;193:113–124. [PubMed] [Google Scholar]
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology. 2003;30:1–47. doi: 10.1071/PP98095. [DOI] [PubMed] [Google Scholar]
- Greenway H, Setter TL. Is there anaerobic metabolism in submerged rice plants? A view point. In: Singh VP, Singh RK, Singh BB, Zeigler RS, editors. Physiology of stress tolerance in rice. Manila, Philippines: Narendra Deva University of Agriculture and Technology and International Rice Research Institute; 1996. pp. 11–30. [Google Scholar]
- Guglielminetti L, Perata P, Alpi A. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiology. 1995;108:735–741. doi: 10.1104/pp.108.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanhijärvi AM, Fagerstedt KV. Comparison of carbohydrate utilization and energy charge in the yellow flag iris (Iris pseudocorus) and garden iris (Iris germanica) under anoxia. Physiologia Plantarum. 1995;93:493–497. [Google Scholar]
- Holm L, Plucknett DL, Pancho JV, Herberger J. The world's worst weeds: distribution and biology. Honolulu, Hawaii: The University Press; 1977. [Google Scholar]
- Huang S, Greenway H, Colmer T. Anoxia tolerance in rice seedlings: exogenous glucose improves growth of an anoxia-'intolerant', but not of a ‘tolerant’ genotype. Journal of Experimental Botany. 2003;54:2362–2373. doi: 10.1093/jxb/erg252. [DOI] [PubMed] [Google Scholar]
- Islam Md N, Baltazar AM, Manuel JS, Martin EC, Ramos JM, De Datta SK, Rezaul Karim AMN. Tuber production, population dynamics, and management of purple nutsedge in a rice-onion rotation system. Proceedings of 20th Asian-Pacific Weed Science Society Conference; November 7–11, 2005; Ho Chi Minh City, Vietnam. Ho Chi Minh City, Vietnam: Agricultural Publishing House; 2005. pp. 541–549. [Google Scholar]
- Janiya JD. Weed management in major crops in the Philippines. Los Baños, Laguna, Philippines: Weed Science Society of the Philippines; 2002. Yield losses, major weed species, and suggested management systems in selected major crops: rice; pp. 17–37. [Google Scholar]
- Kennedy RA, Rumpho ME, Fox TC. Anaerobic metabolism in plants. Plant Physiology. 1992;100:1–6. doi: 10.1104/pp.100.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai K, Iwamura JC, Kiatsoonthorn V, Ueki K. Geographical variation of Cyperus rotundus L. and ecological properties. Proceedings of 9th Asian-Pacific Weed Science Society Conference; November 28–December 2, 1983; Manila. Manila, Philippines: Asian-Pacific Weed Science Society; 1983. pp. 66–70. [Google Scholar]
- Laszlo A, St., Lawrence P. Parallel induction and synthesis of PDC and ADH in anoxic maize roots. Molecular and General Genetics. 1983;192:110–117. [Google Scholar]
- Loreti EJ, Yamaguchi J, Alpi A, Perata P. Sugar modulation of alpha-amylase genes under anoxia. Annals of Botany. 2003;91:143–148. doi: 10.1093/aob/mcf117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready R, Guggolz J, Silviera V, Owens H. Determination of starch and amylase in vegetables. Analytical Chemistry. 1950;22:1156–1158. [Google Scholar]
- Mohanty H, Mallik S, Grover A. Prospects of improving flooding tolerance in lowland rice varieties by conventional breeding and genetic engineering. Current Science. 2000;78:131–140. [Google Scholar]
- Okoli CAN, Shilling DG, Smith RS, Bewick TA. Genetic diversity in purple nutsedge (Cyperus rotundus L.) and yellow nutsedge (Cyperus esculentus L.) Biological Control. 1997;8:111–118. [Google Scholar]
- Pablico PP, Moody K. A wet season lowland rice (Oryza sativa) weed survey in central and southern Luzon. Philippine Journal of Weed Science. 1985;12:39–49. [Google Scholar]
- Perata P, Geshi N, Yamaguchi J, Akazawa T. Effect of anoxia on the induction of alpha-amylase in cereal seeds. Planta. 1993;191:402–408. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. Mobilization of endosperm reserves in cereal seeds under anoxia. Annals of Botany. 1997;79:49–56. [Google Scholar]
- Rao AN, Johnson DE, Sivaprasad B, Ladha JK, Mortimer AM. Weed management in direct-seeded rice. Advances in Agronomy. 2007;93:153–255. [Google Scholar]
- Tadege M, Brandle R, Kuhlemeier C. Anoxia tolerance in tobacco roots: effect of overexpression of pyruvate decarboxylase. The Plant Journal. 1998;14:327–335. [Google Scholar]
- Valdez AP. The importance of ethanol fermentation to anoxia tolerance during submergence in rice (Oryza sativa L.) Philippines: University of the Philippines Los Baños; 1995. PhD Thesis. [Google Scholar]
- Wills GD. Comparison of purple nutsedge from around the world. Weed Technology. 1998;12:491–503. [Google Scholar]