Abstract
Background
Under hypoxic conditions, plant mitochondria preserve the capacity to oxidize external NADH, NADPH and tricarboxylic acid cycle substrates. Nitrite serves as an alternative electron acceptor at the level of cytochrome oxidase, with possibly complex III and the alternative oxidase also being involved. Nitric oxide is a significant product of the reaction, which has a high affinity for cytochrome c oxidase, inhibiting it. The excess NO is scavenged by hypoxically induced class 1 haemoglobin in the reaction involving ascorbate.
Scope
By using nitrite, mitochondria retain a limited capacity for ATP synthesis. NADH, produced from glycolysis during anaerobiosis and oxidized in the mitochondrial electron transport chain, should shift the composition of metabolites formed during anaerobiosis with increased conversion of pyruvate to alanine and greater involvement of other transamination reactions, such as those involving γ-aminobutyric acid formation.
Conclusions
Anaerobic mitochondrial metabolism may have a more significant role than previously thought in alleviating the effects of anoxia on plant cells. There is a need to re-examine mitochondrial carbon and nitrogen metabolism under anoxia to establish the extent of this involvement.
Key words: Electron transport, haemoglobin, hypoxia, mitochondria, nitric oxide, nitrite reduction
INTRODUCTION
Plants experience hypoxia even during their normal growth and development. There are examples of this in seeds and closely packed tissues (Porterfield et al., 1999; Rolletschek et al., 2002). Hypoxic stress affects mitochondrial function both via oxygen limitation and increased production of nitric oxide (NO) (Dordas et al., 2003), which inhibits cytochrome c oxidase (COX) at the oxygen-binding site (Cooper, 2002). Mitochondrial oxygenic respiration declines below oxygen levels required to saturate terminal oxidases. The oxygen Km for the alternative oxidase (AOX) is in the order of 10 µm (Millar et al., 1994; Affourtit et al., 2001b), limiting AOX function under low oxygen conditions. AOX can, however, play a role in NO tolerance under normoxic and moderately hypoxic conditions since NO does not inhibit its activity and up-regulates AOX synthesis (Huang et al., 2002). Millar et al. (1994) report the Km of COX for oxygen as 0·14 µm, but others suggest that it varies within the range of 0·08–0·16 µm (Hoshi et al., 1993). NO inhibition raises the Km to 1 µm or even higher (Cooper, 2002). As a working definition for this review, we define anoxia (or anaerobiosis) as a condition whereby the oxygen concentrations in the cytoplasm are such that COX cannot effectively donate electrons to oxygen, while hypoxia is a condition whereby COX has at least a limited capacity to use oxygen but several other oxidases such as AOX are inhibited. The actual oxygen concentrations corresponding to these conditions can change depending on diffusion parameters, NO levels and other factors.
There is abundant evidence that plant mitochondria can function even under strict anoxic conditions (Fox and Kennedy, 1991). Exposure to anoxia results in some changes in enzyme composition in mitochondria (Couee et al., 1992), but mitochondria preserve their ultrastructure and functionality, particularly when anaerobic plants are exposed to nitrate (Vartapetian et al., 2003). The latter observation was interpreted as an indication of the role of nitrate as a terminal electron acceptor under anoxia but never proved. Another interpretation is that nitrate is part of a more extensive cycle where nitrite serves as an intermediate electron acceptor by supporting NADH oxidation (Igamberdiev and Hill, 2004). This view assumes that mitochondrial nitrite reduction to NO may be linked to ATP synthesis contributing to the functionality of these organelles in anoxic conditions (Igamberdiev et al., 2005; Stoimenova et al., 2007).
The discovery of a hypoxically induced plant haemoglobin (Hb) (Taylor et al., 1994) has led to a number of studies showing that expression of this protein improved the energy and redox status of the hypoxic cell, leading to increased cell and plant survival. Scavenging NO formed in the hypoxic response was believed to be a major function of this Hb, but how this related to the improved energy status of the cell was unclear. The finding that anoxic plant mitochondria can drive ATP synthesis using nitrite to form NO provided a link between the energy metabolism of the hypoxic cell and the presence of Hb. This led us to examine whether some of the metabolic changes occurring during anaerobiosis could be explained on the basis of altered mitochondrial metabolism resulting from nitrite-driven ATP synthesis. The functioning of mitochondria under anaerobic conditions and the related reorganization of nitrogen and carbon metabolism accompanying this operation will be the topic of this review with the intention of stimulating further research and debate on the contribution of mitochondrial metabolism to the anaerobic response.
NITRATE AND NITRITE REDUCTION UNDER ANAEROBIC CONDITIONS
The presence of nitrate during hypoxia reduces the amount of fermentative end products produced, helps maintain a higher free nucleoside triphosphate concentration and increases the rate of overall recovery from hypoxia (Fan et al., 1988). Nitrate reductase is up-regulated under hypoxia at both the transcriptional and enzymatic levels, with nitrite reduction being suppressed at the nitrite reductase step (Botrel et al., 1996), although some anaerobic induction of nitrite reductase has been demonstrated in hypoxia-tolerant rice (Mattana et al., 1994). Nitrate, via up-regulation of nitrate reductase, is actively metabolized to nitrite. The limitation at the common nitrite reductase step turns nitrite to other reducing systems, where the mitochondrial system seems to be the most active (Planchet and Kaiser, 2006). A part of nitrite formed under hypoxia may still be reduced to NH4+, which is likely to be important for the increased amino acid production observed under hypoxia.
Mitochondria from tobacco, pea, barley and arabidopsis roots produce NO at a rate of 1–10 nmol mg−1 protein h−1 (Gupta et al., 2005). This represents about 1 % of the mitochondrial electron transport under anoxia, when measured as NADH oxidation (Stoimenova et al., 2007). One possible explanation is that a significant part of NO may not be detected due to immediate, efficient scavenging (Vanin et al., 2004). Secondly, NO may not be the only product of nitrite reduction. In this regard, nitrous oxide (N2O) is a likely by-product as it is formed in significant amounts in bacterial and fungal systems involving cytochrome-dependent nitrite reduction (Dalber et al., 2005). Plants can emit N2O (Smart and Bloom, 2001) and participation of mitochondrial electron transport in this process is possible. While the COX-catalysed reaction is slow (Cooper, 2002), other cytochrome-containing systems, including complex III, need to be tested for this activity. Ascorbate itself can slowly catalyse this reaction in the presence of reduced quinones forming dehydroascorbate (Alegria et al., 2004).
Estimates of the end-products of nitrate reduction suggest that only 1·3–4 % of NADH recycled during hypoxia is connected with the reduction of nitrate to NH4+ (Gibbs and Greenway, 2003). There are, however, experimental estimates that up to one-third of NAD recycling may result from nitrate reduction (Fan et al., 1997). There are a number of possible explanations for the differences in the two estimates, among them being cation balance within the cell. Another possible explanation could be linked to reduction of nitrate to NO or even dinitrogen gas (Morard et al., 2004). When nitrite accumulates, it can be used by cytosolic nitrate reductase as a substrate to produce NO, with the reaction competitively inhibited by nitrate (Rockel et al., 2002). The rate of NO production, however, is only 1–2 % of the maximal rate of the actual nitrate reduction reaction (Yamasaki et al., 2001; Rockel et al., 2002). A plasma membrane-bound nitrite:NO reductase (Ni-NOR), has been reported (Stöhr et al., 2001), but there has been no further work on this enzyme to properly evaluate its properties. In acidic and reducing environments, NO can be formed by non-enzymatic reduction of nitrous acid, when the latter reacts with ascorbate, producing dehydroascorbate and NO. Conditions appear to be favourable in aleurone layers for this conversion (Bethke et al., 2004). The NO-generating activity of xanthine oxidoreductase with nitrite is also detectable and increases at low oxygen tensions (Godber et al., 2000).
All these reactions are clearly established in plant systems; however, their contribution to in vivo NO formation has yet to be quantified. It is likely that these reactions are minor even under hypoxic conditions. Quantification is currently a very difficult task, in particular, because NO is a compound that is easily utilized in various side reactions. Some of the side reactions are harmful to the cell (e.g. tyrosine nitrosylation of proteins), while S-nitrosylation of proteins or glutathione can function in a regulatory manner, such as promoting the initiation of programmed cell death (Mur et al., 2006). A specific NO-scavenging reaction has been linked to the operation of hypoxically induced Hb (Igamberdiev and Hill, 2004).
NITRITE AS AN ALTERNATIVE ELECTRON ACCEPTOR OF COX
Anaerobic use of nitrite as a terminal electron acceptor by mitochondria has been observed in some fungi, such as Fusarium (Kobayashi et al., 1996) and in ciliate protists (Finlay et al., 1983). These mitochondria use a nitrite reductase, which derives electrons from the cytochrome c or ubiquinone pool. Some fungal mitochondria can reduce not only nitrate and nitrite but also NO (Kobayashi et al., 1996) but they usually lack nitrous oxide reductase activity and N2O is the final denitrification product. In Fusarium oxysporum, cytochrome c549 serves as an electron donor for both nitrite reductase and cytochrome oxidase while formate dehydrogenase is involved in supplying electrons to the ubiquinone pool (Takaya et al., 2003). In the bacterium Paracoccus denitrificans, an NO reductase activity is inhibited by antimycin A and myxothiazol as the bc1 complex is proton translocating, while nitrite reductase is associated with the cytochrome complex cd1 (Carr et al., 1989). Most fungi can denitrify only nitrite with N2O as a major product of denitrification and a few such as Fusarium oxysporum and Gibberella fujikuroi also use nitrate (Watsuji et al., 2003).
Nitrite reduction by the mammalian (Kozlov et al., 1999), algal (Tischner et al., 2004) and plant (Planchet et al., 2005) mitochondria is strongly anaerobic and it is likely that it does not involve additional enzymes associated with the mitochondrial electron transport chain. Root mitochondria can produce NO using nitrite and NADH (Gupta et al., 2005; Planchet et al., 2005) and become a significant source of anaerobic NO. Kinetic characteristics of the process of mitochondrial nitrite reduction are not known because of the difficulties of measuring low fluxes of nitrite and NO under anaerobic conditions.
Aerobically, NO can be converted by COX to nitrite (Cooper, 2002; Pearce et al., 2002). Under anaerobic conditions the reaction can be reversed (Paitian et al., 1985) and nitrite may become a source of NO at the cytochrome oxidase site (Castello et al., 2006). Cytochrome c oxidase contains three redox-active metal sites: CuA, haem a and a haem a3/CuB binuclear centre (Cooper, 2002). The CuA and Fea catalyse electron transfer from the substrate (cytochrome c) to the catalytic site, where Fea3CuB binuclear centre catalyses reduction of oxygen to water. The oxygen concentrations at which half-maximal reduction of haem a + a3 occurs is sensitive to the energy state and respiratory rate. Thus, in state 4 and state 3 respiration, the Km values are 78 µm and 160 µm, respectively. In contrast, the Km of the copper site is 75 µm and is independent of both the energy state and the respiratory rate (Hoshi et al., 1993). Such values of oxygen affinity for COX would allow operation of some oxygenic respiration at low oxygen concentrations.
NO can be a complicating factor affecting COX oxygen saturation due to the sensitivity of the oxidase to NO at both the haem and copper sites of the complex (Cooper, 2002; Mason et al., 2006). This is a factor in plants since NO accumulates under anoxia (Dordas et al., 2003). The effect of NO on COX when oxygen is a terminal electron acceptor can be different under anaerobic conditions when nitrite, forming NO, acts as the terminal electron acceptor. NO can bind to either the ferrous haem or the cupric copper of COX, but not both at the same time (Mason et al., 2006). The affinity of the COX ferrous haem for NO is about 100 times greater than that of the oxidized copper for NO, making reactions dependent on the ferrous haem, such as oxygenic respiration, more sensitive to NO (Mason et al., 2006). Initially, NO formation was considered slow and non-physiological, but it has been confirmed to be operative in mammalian and yeast (Castello et al., 2006), algal (Tischner et al., 2004) and higher plant (Planchet et al., 2005; Gupta et al., 2005; Stoimenova et al., 2007) mitochondria. The very low affinity of COX for nitrite determined initially (Castello et al., 2006) is likely not to have taken into consideration the observation of Brunori et al. (2006) that when the binuclear centre iron is oxidized and copper is reduced, affinity increases many fold.
Oxygen can be reduced by COX only when both iron and copper are reduced, with the reaction being competitively inhibited by NO competing with oxygen at the binuclear centre:
The affinity (KD) of NO for the oxygen-binding ferrous haem site is 0·2 nm.
NO interacts with either ferrous haem iron or oxidized copper, but not both simultaneously. The non-competitive interaction with oxidized copper results in oxidation of NO to nitrite and behaves kinetically as if NO has an apparent affinity of 28 nm at low levels of NO; significant binding to copper can occur without inhibition of oxygen binding (Mason et al., 2006). NO binds rapidly to cupric CuB but also reduces it to the cuprous state, producing nitrite, which is subsequently released from the binuclear centre (Torres et al., 2000).
For the reverse reaction, the plausible form of the binuclear centre for reduction of nitrite would be CuB+Fea33+:
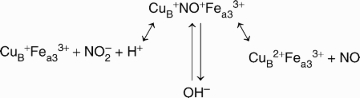
This process can be linked to proton translocation as with any reduction process where electrons have to be transferred into the catalytic centre, composed of haem a3 and CuB (Jancura et al., 2006). Contrary to previous suggestions that proton pumping is linked exclusively to the oxidative phase, Ruitenberg et al. (2002) reported that proton pumping during the reductive phase can occur when it is preceded by an oxidative phase. Thus, nitrite reduction can precede the proton pumping process when COX copper is reduced from cytochrome c and can also contribute to proton pumping when COX copper is oxidized in the course of NO formation.
There is not a great deal known about possible nitrite reduction by complex III and AOX and more information is required about the kinetic properties of these reactions. Salicylhydroxamic acid inhibits mitochondrial NO production from nitrite (Tischner et al., 2004; Gupta et al., 2005; Planchet et al., 2005). This suggests that AOX is capable of nitrite:NO reduction and may be functional under anaerobic conditions. Since salicylhydroxamic acid is not specific and can affect other proteins, including peroxidases, this interpretation should be treated with caution. The AOX reaction with oxygen is not inhibited by NO (Millar and Day, 1996). Furthermore, the affinity for oxygen is low (Km of 10–25 µm) and cannot provide sufficient oxygenic respiration under anoxic conditions.
MITOCHONDRIAL SUBSTRATE OXIDATION UNDER ANAEROBIC CONDITIONS
The previous sections present evidence for the operation of mitochondrial electron transport during anaerobiosis. What is the evidence for the oxidation of mitochondrial substrates under the same conditions?
There are two or more externally facing rotenone-insensitive dehydrogenases on the inner membrane of plant mitochondria (Rasmusson et al., 2008). They correspond to two detectable Ca2+-dependent activities, one specific to NADH and another to NADPH and distinguished by sensitivity to diphenyleneiodonium (Roberts et al., 1995). Nitrogen supply affects expression of both dehydrogenase activities (Escobar et al., 2006). Mitochondrial oxidation of cytosolic NADH and NADPH occurring via these dehydrogenases does not result in proton gradient formation at the site of electron transport from NAD(P)H to ubiquinone (Møller, 1997). Genetic and biochemical data (Rasmusson et al., 2008) suggest the possibility of the existence of even a higher number of external dehydrogenases.
Anaerobic NAD(P)H oxidation and ATP synthesis is insensitive to rotenone, suggesting that complex I is not involved (Stoimenova et al., 2007). The high nucleotide Km and Ca2+ dependence of externally facing NADH and NADPH dehydrogenases (Møller, 1997) suggests that they would have a major function when extramitochondrial NAD(P)H and Ca2+ concentrations are elevated, as observed during hypoxia (Subbaiah et al., 1998). Furthermore, NO stimulates the release of Ca2+ from mitochondria (Richter, 1997), while external NADH and NADPH oxidation by mitochondria plays an important role in seed germination under hypoxic conditions (Logan et al., 2001). This indicates a primary role of external NADH and NADPH oxidation in anaerobic mitochondria. It should be noted that one internal dehydrogenase activity is also Ca2+ dependent (NADPH dehydrogenase) (Møller and Rasmusson, 1998; Bykova and Møller, 2001).
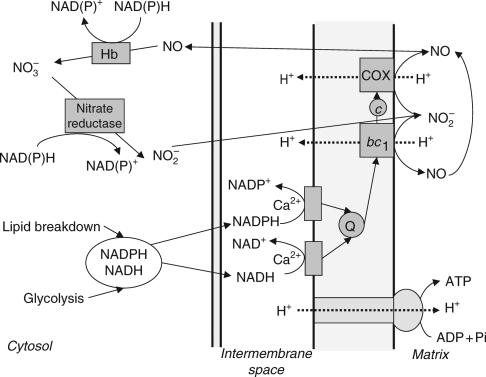
Figure 1 shows oxidation of cytosolic NADH and NADPH by mitochondrial, externally faced, Ca2+-dependent dehydrogenases. Operation of complexes III and IV, with nitrite as terminal acceptor (at the COX site), can result in proton pumping and can be linked to an observed ATP synthesis (Stoimenova et al., 2007). NO can diffuse to the cytosol and be converted to nitrate by hypoxia-induced (class 1) Hb, having an extremely high avidity to oxygen. Nitrate is reduced by nitrate reductase to nitrite and the cycle is repeated.
Fig. 1.
Anaerobic operation of plant mitochondria. Externally facing dehydrogenases oxidize NADH and NADPH and transfer electrons to ubiquinone (Q). At levels of oxygen below saturation of cytochrome c oxidase (COX), nitrite produced in the cytoplasm serves as an alternative electron acceptor at sites of complexes III (bc1) and IV (COX). NO formed in this reaction is converted in the cytosol by hypoxically induced haemoglobin (Hb) to nitrate NO3−. The latter is reduced to nitrite (NO2−) by nitrate reductase. ATP is synthesized due to proton pumping at the COX site. Modified from Stoimenova et al. (2007).
Calcium elevation can significantly influence metabolic fluxes and substrate oxidations under anoxic conditions. Ca2+, released from mitochondria during hypoxia possibly as a consequence of decrease of cytosolic pH (Subbaiah et al., 1998), is a major signalling molecule in the hypoxic response. It moves against an electrochemical gradient via the H+-Ca2+ antiport, using energy provided by the downhill influx of H+ into the mitochondria. The required pH change for the antiport is 0·5 units, and such a decrease occurs within a few minutes of the onset of anoxia (Gibbs and Greenway, 2003). Ca2+, amongst its many effects in the plant cell, regulates external NADH and NADPH dehydrogenases, internal NADPH dehydrogenase, glutamate decarboxylase and NAD kinase. The mechanism of Ca2+ release by mitochondria is unclear but it has been proposed that the shift in adenylate kinase equilibrium due to lower ATP synthesis may result in changes in equilibrium Mg2+ concentrations and this, in turn, affects calmodulin and the regulation of Ca2+ pores (Igamberdiev and Kleczkowski, 2003).
One of the substrates accumulated under hypoxia is succinate. Its accumulation is higher in hypoxia-resistant species increasing after 48 h of hypoxic treatment from 0·2 to 3·0 µmol g−1 fresh weight in rice and from 0·4 to 5·1 µmol g−1 fresh weight in Echinochloa (Menegus et al., 1989). It can appear as the final product of γ-aminobutyric acid (GABA) shunt. It could also be synthesized from fumarate, a reaction common in microorganisms, where it is catalysed by fumarate reductase. The ability of succinate dehydrogenase to display fumarate reductase activity has not been verified in plant mitochondria. The reaction is thermodynamically possible and a portion of electron flow from NADH could be directed to fumarate. Succinate can reduce NAD by reversing electron flow through complex I and possibly other dehydrogenases if electron flow to oxygen is limited (Rustin and Lance, 1991). The reverse of this reaction would be reduction of fumarate. The flow is regulated by the protonmotive force, ATP and ADP levels (Affourtit et al., 2001a).
There are a number of changes that take place under hypoxia in relation to malate metabolism. NAD-malic enzyme (NAD-dependent malate dehydrogenase decarboxylating) activity increases several fold in hypoxic maize root tips (Edwards et al., 1998). Malate can be formed via an alternative root of glycolytic fermentation through phosphoenolpyruvate and oxalacetic acid and potentially can participate in further reduction via reverse fumarase and succinate dehydrogenase activities. Increased pyruvate formation from malate is possible in line with a decrease in flux via the TCA cycle and an increase of fermentation. Its formation by NAD-malic enzyme can contribute to supply of a substrate for alanine biosynthesis and thus be linked to nitrate consumption under hypoxic conditions. Increased alanine synthesis may be linked to the utilization of GABA via its transamination with pyruvate (Miyashita and Good, 2008). Accumulation of alanine during hypoxia exceeds accumulation of lactate in hypoxically pre-treated maize seedlings by several-fold, indicating its importance during adaptation to low oxygen (Xia and Roberts, 1994).
Formate oxidation in hypoxic mitochondria is also activated. Formate dehydrogenase is an enzyme strongly induced under hypoxic conditions (Bykova et al., 2003) and may be involved in alternative pyruvate conversion. Formate oxidation will not lead to accumulation of toxic products, and the NADH produced could be oxidized via the mitochondrial electron transport chain. Alternative sources of formate could be serine degradation or decomposition of cell walls (Kreuzwieser et al., 1999), which may accompany aerenchyma formation.
Substrate oxidation under hypoxia raises the redox potential, and efficient mechanisms to reduce it are necessary for successful adaptation and survival in low-oxygen environments. The use of nitrite as an alternative electron acceptor can represent such a mechanism, if it is accompanied by efficient scavenging of NO.
ROLE OF HAEMOGLOBIN
We would contend that class 1 Hbs are proteins of major significance in maintaining mitochondrial electron flow under hypoxia. We suggest that these Hbs act to: (a) bind oxygen at extremely low solution oxygen concentrations; (b) react with NO, produced under anoxia, to form nitrate ion and methaemoglobin (metHb). The removal of NO allows nitrite and NAD(P)H-driven ATP synthesis to proceed without inhibition of cytochrome oxidase by NO. The oxidation of NAD(P)H by this mitochondrial electron flow and the regeneration of Hb from metHb contributes to the improved redox status of the anoxic cell, while the generated ATP increases the cell energy status.
The expression of an Hb gene accompanying hypoxia was first demonstrated in barley (Taylor et al., 1994). With an O2 dissociation constant in the range of 2–3 nm for this type of Hb (Arredondo-Peter et al., 1997; Duff et al., 1997; Trevaskis et al., 1997) it would remain oxygenated at extremely low oxygen concentrations. Hb induction is observed in response to nitrate (Nie and Hill, 1997), nitrite and NO treatment (Ohwaki et al., 2005), implicating Hb expression with these nitrogenous compounds. An anoxia-induced Hb gene is induced by a disruption of ATP synthesis (Nie and Hill, 1997) and triggered by Ca2+ release (Nie et al., 2006). Although the plant Hb is absent from mitochondria and located in the cytosol and nucleus (Hebelstrup et al., 2007), its importance in the maintenance of mitochondrial function is evident (Nie and Hill, 1997; Sowa et al., 1998).
There is abundant evidence that a primary function of class 1 Hb is NO scavenging (Dordas et al., 2003; Igamberdiev et al., 2004: Perazzolli et al., 2004, 2006). There is also strong evidence of a connection between NO turnover and the maintenance of redox and energy levels in the plant cell (Sowa et al., 1998; Dordas et al., 2003; Igamberdiev et al., 2004, 2006a, b; Stoimenova et al., 2007). The sequence of reactions involved in NO scavenging under hypoxic conditions has been termed the Hb/NO cycle (Igamberdiev and Hill, 2004). Under this proposed pathway, NO is oxygenated to nitrate by oxyHb, which, in the process, is oxidized to the ferric Hb(Fe3+) state (Fig. 1). To maintain the cycle, Hb(Fe2+) must be regenerated. A special enzyme possessing ferric Hb (metHb) reductase activity was purified from barley roots and identified as a cytosolic monodehydroascorbate reductase (Igamberdiev et al., 2006a).
Perazzolli et al. (2004, 2006) propose an alternative mechanism for Hb scavenging of NO, involving nitrosylation of an Hb cysteine residue as an intermediate in the reaction. This is based on estimation of S-nitrosylation in arabidopsis Hb, a molecule that has two cysteine residues. This mechanism is difficult to reconcile with the class 1 Hb of barley, which has only a single cysteine residue that is involved in an intermolecular disulfide bond to form the barley Hb dimer (Bykova et al., 2006). Furthermore, NO scavenging by a mutated barley Hb (Cys79 replaced by Ser) was unaffected by the mutation.
Deoxyhaemoglobins, like COX, can reduce nitrite to NO. However, the deoxy form of class 1 Hb exists at oxygen tensions several hundred times lower than necessary to saturate COX and are, therefore, not physiologically relevant in NO formation. Class 1 Hbs are, therefore, efficient NO scavengers at any physiologically relevant oxygen tensions (Grubina et al., 2007).
The rate of NAD(P)H-dependent NO conversion by the Hb/NO cycle in alfalfa root cultures (Igamberdiev et al., 2004) is comparable to the activity of alcohol dehydrogenase induced under hypoxic treatment (Dordas et al., 2003). NO degradation in this pathway would relieve inhibition of mitochondrial electron transport by NO. The reversible reaction between NO and nitrite catalysed by COX under hypoxic conditions will always be shifted towards NO formation because of the high redox potential in the electron transport chain (Castello et al., 2006) and NO can significantly accumulate (Dordas et al., 2003). In fact, in hypoxic conditions, maize cells overexpressing Hb exhibit a lower alcohol dehydogenase activity compared with control and to lines underexpressing Hb (Sowa et al., 1998). One potential reason for this could be more intensive operation of the NO scavenging cycle, which uses NAD(P)H to reduce metHb (Igamberdiev and Hill, 2004). Lower NADH/NAD and NADPH/NADP ratios in plants overexpressing Hb (Igamberdiev et al., 2004) support the operation of such a cycle, which, in a certain sense, replaces alcohol dehydrogenase activity for recycling NADH and can reduce the rate of glycolytic fermentation by 25 % in maize cell cultures (Sowa et al., 1998).
NO is claimed to control oxygen levels by inhibiting COX (Borisjuk et al., 2007), thus avoiding complete anoxia. By this mechanism, O2 concentration is maintained preventing its complete depletion. It is unlikely that this mechanism operates under physiological conditions; however, as class 1 Hbs, because of their binding kinetics, they would strongly outcompete cytochrome oxidase for oxygen. The existence of an Hb molecule with an extremely high avidity to oxygen provides a definite advantage to the anoxic cell for removal of NO by oxygenation.
THE ROLE OF ASCORBATE UNDER HYPOXIA AND ANOXIA
Ascorbate is likely to be the primary compound that is involved in reduction of metHb under hypoxic and anoxic conditions. As we showed earlier (Igamberdiev et al., 2006a), reduction of metHb in plants occurs via monodehydroascorbate reductase-mediated ascorbate reduction of metHb. Ascorbate levels in Hb-overexpressing plants are always higher than in plants down-regulating Hb (Igamberdiev et al., 2006b), supporting the premise that this compound has a role in maintenance of the Hb/NO cycle. Ascorbate alone can reduce metHb at a slow rate but is limited by formation of the strong oxidant monodehydroascorbate (or ascorbate free radical, AFR). The removal of AFR by monodehydroascorbate reductase drives the reaction strongly towards metHb reduction (Igamberdiev et al., 2006a).
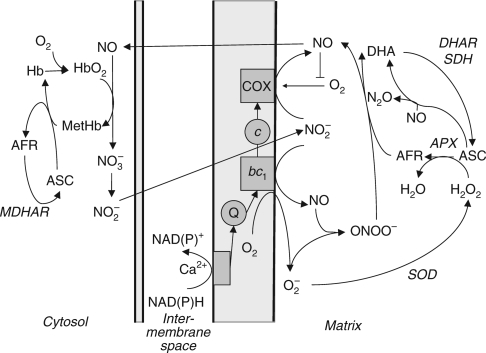
The importance of the ascorbate/glutathione cycle under hypoxic conditions is not only related to reduction of metHb. Another important process is the removal of peroxynitrite. NO formation by COX and superoxide formation at mitochondrial complex III, under conditions where COX transfer of electrons to oxygen is inhibited by low oxygen and NO, will lead to generation of peroxynitrite (ONOO−) (Fig. 2). Reactive oxygen species (ROS), particularly hydrogen peroxide, are increased under hypoxic conditions on plasma membranes (Blokhina et al., 2001) due to increases in the redox level and activation of plasmalemmal NADPH dehydrogenase (Blokhina et al., 2000) and operation of the Rop-signal transduction pathway (Fukao and Bailey-Serres, 2004). In mitochondria, ROS can be utilized immediately in the reaction with NO, forming ONOO−. Protein nitrosylation by ONOO− is toxic in mammalian cells, but the effect is not considered as harmful in plant cells. COX, in the reduced form, has peroxynitrite reductase activity forming NO and possibly H2O2 (Sharpe and Cooper, 1998). Oxidation of ONOO− to NO2 by COX (in the oxidized form) has also been shown (Pearce et al., 2002) but direct scavenging of ONOO− by AFR is probably the more active pathway (Barone et al., 2003). High concentrations of ascorbate in plant cells facilitates this reaction and can also contribute to O2− scavenging, H2O2 scavenging (via ascorbate peroxidase) and NO reduction to N2O (Alegria et al., 2004). The ability of plants to synthesize ascorbate may be the reason why ONOO− is less toxic in plant cells (Delledonne et al., 2001; Beligni et al., 2002).
Fig. 2.
Possible role of reactions of the ascorbate/glutathione cycle in nitric oxide metabolism. Ascorbate can participate in the reduction of metHb in cytosol, in a reaction coupled to monodehydroascorbate reductase. In mitochondria, ascorbate can be involved in scavenging of reactive oxygen species (H2O2), conversion of NO to nitrous oxide (N2O), scavenging of peroxynitrite (ONOO−) via AFR. Inhibition of oxygen consumption by NO leads to the maintenance of O2 levels sufficient for Hb operation. Synthesis of ascorbate from galactono-γ-lactone and non-enzymatic reduction of nitrite by ascorbate are not shown. Abbreviations: ASC, ascorbate; AFR, ascorbate free radical (monodehydroascorbate); DHA, dehydroascorbate; SOD, superoxide dismutase; APX, ascorbate peroxidase; SDH, succinate dehydrogenase; DHAR, dehydroascorbate reductase; MDHAR, monodehydroascorbate reductase.
Ascorbate and especially AFR are involved in scavenging of ONOO− to NO and thus resupplying NO again to the cell. By preventing ONOO− formation, ascorbate serves as a modulator of mitochondrial apoptotic signalling, in a similar fashion to glutathione (Hancock et al., 2001). It is known that NO prevents release of cytochrome c while ONOO− enhances it (Brown and Borutaite, 2001). Ascorbate is therefore expected to act intracellularly as a major peroxynitrite antagonist (Kirsch and de Groot, 2000). O2−, but only in the high millimolar range, can also be scavenged by ascorbate (Jackson et al., 1998) Furthermore the reaction with NO is 1000 times faster (Scarpa et al., 1983). The rate of peroxynitrite scavenging reaction catalysed by COX (Sharpe and Cooper, 1998; Pearce et al., 2002) is slower compared with that of ascorbate/AFR (Barone et al., 2003). While glutathione also breaks down ONOO−, its role is probably predominant in animal cells since high concentrations of ascorbate in plant cells allow this compound and its oxidized derivative (AFR) to be the primary peroxynitrite scavenger.
Ascorbate is synthesized from galactono-γ-lactone by galactono-γ-lactone dehydrogenase that supplies electrons directly to cytochrome c (Bartoli et al., 2000). The rate of this reaction may increase under hypoxia and contribute to reduction of cytochrome c facilitating reduction of nitrite by COX. Ascorbate can be recycled from its oxidized forms not only in the reactions catalysed by the enzymes of the ascorbate/glutathione cycle but also via the mitochondrial electron transport chain (Li et al., 2002). Succinate, accumulating under hypoxia, can be used for dehydroascorbate reduction at the level of complex II (Szarka et al., 2007). Ascorbate, thus, serves two functions relative to nitrogen oxide reactions in plants: it contributes to the production of NO, and; it breaks down ONOO−. Ascorbate free radical for the latter function can be formed spontaneously from oxidation of ascorbate or from the reaction of oxidized haemproteins that become available in anoxia. Ascorbate can also participate in non-enzymatic reduction of nitrite to NO at low pH. This reaction may be physiologically relevant upon hypoxic pH decrease and especially in aleurone layers of germinating seeds (Bethke et al., 2004). Dehydroascorbate formed in this reaction can be recycled via reactions of the ascorbate/glutathione cycle or by the mitochondrial electron transport chain (Fig. 2).
ANAEROBIC AMINO ACID METABOLISM AND THE GABA SHUNT
Nitrogen from nitrate accumulates in alanine, GABA, glutamate and other amino acids under anaerobiosis (Reggiani et al., 1995). Alanine aminotransferase in the cytoplasm increases 4-fold in anaerobic barley roots and the production of alanine may be comparable to or even exceed that of ethanol in some species, without consumption of NADH in the reaction (Smith and ap Rees, 1979). Nitrite-driven mitochondrial metabolism will oxidize glycolytically produced NADH via mitochondrial external dehydrogenases and shift glycolytic fermentation of pyruvate to oxidation within the mitochondria. Pyruvate may be metabolized through reactions of the TCA cycle but the major product found is alanine, with the possibility of formation of branched-chain amino acids such as valine and leucine (Sato et al., 2002), which are derived from alanine.
Isocitrate dehydrogenase activity is strongly influenced by the redox state of mitochondrial pyridine nucleotides (Igamberdiev and Gardeström, 2003). The limited oxidation capacity for intramitochondrial NADH under hypoxic conditions will result in citrate efflux and formation of 2-oxoglutarate in the cytosol. 2-Oxoglutarate is the main precursor of glutamate, which is formed actively under hypoxic conditions. Induction of glutamate decarboxylase in hypoxia, triggered by elevation of Ca2+, leads to active decarboxylation of glutamate, forming GABA (Shelp et al., 1999). GABA accumulates at later stages, when metabolism begins to fail, while alanine is the earlier product (Roberts et al., 1992). The ability to regulate pH by alanine depends on the source of the amino group (Greenway and Gibbs, 2003) and the contribution of GABA to pH regulation has been well documented (Reid et al., 1985). The Hb/NO cycle itself may not contribute to pH regulation (Libourel et al., 2006); however, it may contribute to pH regulation indirectly via modification of the redox status of the cell (Igamberdiev and Gardeström, 2003), altering isocitrate dehydrogenase activity, eventually affecting the turnover of glutamate to GABA. Thus, hypoxic accumulation of GABA results from reduced TCA cycle activity when a high NADH/NAD ratio triggers stimulation of a bypass of the 2-oxoglutarate dehydrogenase reaction (Shelp et al., 1999).
GABA can be metabolized not only to succinate via the GABA shunt, but also can initiate a pathway of secondary metabolism resulting in formation of glucosides (Liu and Castelfranco, 1970). There is a possibility of a pathway resulting in formation of isosuccinimide-β-glucoside and further ethyl-β-glucoside in pea seedlings. This pathway may link GABA to cell wall biosynthesis and reconstruction and to utilization of ethanol that is accumulated anaerobically (Liu and Castelfranco, 1970). It is possible that aerenchyma formation in hypoxia is linked to cell wall biosynthesis via GABA metabolism. GABA interferes with ethylene, auxin and Ca2+ signalling pathways by down-regulating the expression of 14-3-3 proteins (Lancien and Roberts, 2006). GABA can function in concert with ethylene (Reggiani, 2006) which is known as a regulator of aerenchyma formation probably via interference with NO levels and Hb expression (Manac'h-Little et al., 2005).
GABA can undergo a transamination reaction with pyruvate, forming alanine and succinic semialdehyde (Shelp et al., 1999). Glyoxylate can substitute for pyruvate in this reaction. There have been reports that succinic semialdehyde dehydrogenase activity prevents ROS formation (Bouché et al., 2003) and that the conversion of succinic semialdehyde to gamma-hydroxybutyrate utilizes NADH and, by this, contributes to redox regulation under hypoxia (Breitkreuz et al., 2003).
Hypoxic metabolism of glycine may be related to the importance of the glyoxylate pool in redox regulation, as alanine accumulation has relevance in regulating the pyruvate pool (Igamberdiev et al., 1991). Proline is a stress amino acid which can also be accumulated under hypoxia (Reggiani et al., 1988). It is formed from glutamate and protects cells from the osmotic stress.
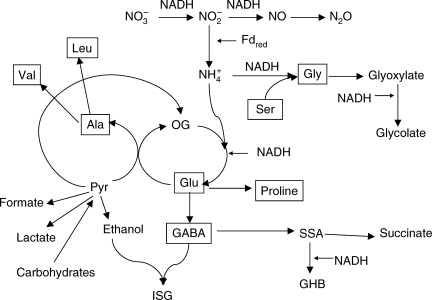
Pathways of nitrogen metabolism under hypoxia are summarized in Fig. 3.
Fig. 3.
Pathways of amino acid turnover in hypoxic conditions. The scheme illustrates connections of nitrite reduction to fermentation pathways operating under hypoxia. The connection of glycolytic fermentation and nitrite reductase-catalysed formation of ammonia occurs mainly via alanine production. Other pathways include lactate, ethanol and formate formation, a link from pyruvate to 2-oxoglutarate (OG) via partial TCA cycle, and the formation of γ-aminobutyric acid (GABA). GABA can be further converted in mitochondria to succinic semialdehyde (SSA) and then to succinate or γ-hydroxybutyrate (GHB). It can be metabolized via the reaction with ethanol to isosuccinimide-β-glycoside (ISG). Activation of glycine metabolism results in reduction of glyoxylate and formation of glycolate.
DIFFERENCES IN HYPOXIA TOLERANCE AND MITOCHONDRIAL FUNCTION
The observed differences in hypoxia tolerance can be explained in part by differences in mitochondrial plasticity. Nitrite-dependent ATP synthesis is more stable in rice than in barley (Stoimenova et al., 2007). This may be connected with a more efficient system of NO scavenging and lower susceptibility of mitochondrial electron transport to NO poisoning. It is evident that hypoxia tolerance is a complex process which includes resistance to pH change, more efficient fermentation, more effective NO scavenging and more efficient use of nitrite as an alternative electron acceptor. It also includes avoidance mechanisms such as aerenchyma formation and formation of adventitious roots at the base of shoots (Drew et al., 2000). Mitochondria play a key role in these processes and preserving mitochondrial functionality is one of major features of hypoxia-tolerant plants (Vartapetian et al., 2003). There is evidence of intact mitochondria remaining until the later stages of programmed cell death during aerenchyma formation (Evans, 2004).
CONCLUSIONS
Recent evidence suggests it would be worthwhile to re-visit the role of mitochondria in the adaptation of plant cells to hypoxia. The generation of NO by mitochondria under anoxic conditions, the ability to generate ATP from this reaction and the role of class 1 Hbs in maintaining this reaction provide the background evidence for pursuing this topic.
An important aspect of future work in this area should be the relationship between the above events and the products of carbon and amino acid metabolism downstream of pyruvate.
ACKNOWLEDGEMENTS
This work was supported by the grants of the Natural Sciences and Engineering Research Council of Canada to R.D.H. and A.U.I.
LITERATURE CITED
- Affourtit C, Krab K, Leach GR, Whitehouse DG, Moore AL. New insights into the regulation of plant succinate dehydrogenase: on the role of the protonmotive force. Journal of Biological Chemistry. 2001;a 276:32567–32574. doi: 10.1074/jbc.M103111200. [DOI] [PubMed] [Google Scholar]
- Affourtit C, Krab K, Moore AL. Control of plant mitochondrial respiration. Biochimica et Biophysica Acta. 2001;b 1504:58–69. doi: 10.1016/s0005-2728(00)00239-5. [DOI] [PubMed] [Google Scholar]
- Alegria AE, Sanchez S, Quintana I. Quinone-enhanced ascorbate reduction of nitric oxide: role of quinone redox potential. Free Radical Research. 2004;38:1107–1112. doi: 10.1080/10715760400009852. [DOI] [PubMed] [Google Scholar]
- Arredondo-Peter R, Hargrove MS, Sarath G, Moran JF, Lohrman J, Olson JS, Klucas RV. Rice haemoglobins: gene cloning, analysis and oxygen-binding kinetics of a recombinant protein synthesized in Escherichia coli. Plant Physiology. 1997;115:1259–1266. doi: 10.1104/pp.115.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone MC, Darley-Usmar VM, Brookes PS. Reversible inhibition of cytochrome c oxidase by peroxynitrite proceeds through ascorbate-dependent generation of nitric oxide. Journal of Biological Chemistry. 2003;278:27520–27524. doi: 10.1074/jbc.M304129200. [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiology. 2000;123:335–344. doi: 10.1104/pp.123.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL. Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiology. 2002;129:1642–1650. doi: 10.1104/pp.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Badger MR, Jones RL. Apoplastic synthesis of nitric oxide by plant tissues. The Plant Cell. 2004;16:332–341. doi: 10.1105/tpc.017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina OB, Virolainen E, Fagerstedt KV, Hoikkala A, Wahala K, Chirkova TV. Antioxidant status of anoxia-tolerant and -intolerant plant species under anoxia and reaeration. Physiologia Plantarum. 2000;109:396–403. [Google Scholar]
- Blokhina OB, Chirkova TV, Fagerstedt KV. Anoxic stress leads to hydrogen peroxide formation in plant cells. Journal of Experimental Botany. 2001;52:1179–1190. [PubMed] [Google Scholar]
- Borisjuk L, Macherel D, Benamar A, Wobus U, Rolletschek H. Low oxygen sensing and balancing in plant seeds: a role for nitric oxide. New Phytologist. 2007;176:813–823. doi: 10.1111/j.1469-8137.2007.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botrel A, Magne C, Kaiser WM. Nitrate reduction, nitrite reduction and ammonium assimilation in barley roots in response to anoxia. Plant Physiology and Biochemistry. 1996;34:645–652. [Google Scholar]
- Bouché N, Fait A, Bouchez D, Møller SG, Fromm H. Mitochondrial succinic-semialdehyde dehydrogenase of the gamma-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proceedings of the National Academy of Sciences of the USA. 2003;100:6843–6848. doi: 10.1073/pnas.1037532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreuz KE, Allan WL, Van Cauwenberghe OR, Jakobs C, Talibi D, Andre B, et al. A novel gamma-hydroxybutyrate dehydrogenase: identification and expression of an Arabidopsis cDNA and potential role under oxygen deficiency. Journal of Biological Chemistry. 2003;278:41552–41556. doi: 10.1074/jbc.M305717200. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide, mitochondria, and cell death. IUBMB Life. 2001;52:189–195. doi: 10.1080/15216540152845993. [DOI] [PubMed] [Google Scholar]
- Brunori M, Forte E, Arese M, Mastronicola D, Giuffrè A, Sarti P. Nitric oxide and the respiratory enzyme. Biochimica et Biophysica Acta. 2006;1757:1144–1154. doi: 10.1016/j.bbabio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Bykova NV, Møller IM. Involvement of matrix NADP turnover in the oxidation of NAD+-linked substrates by pea leaf mitochondria. Physiologia Plantarum. 2001;111:448–456. doi: 10.1034/j.1399-3054.2001.1110404.x. [DOI] [PubMed] [Google Scholar]
- Bykova NV, Stensballe A, Egsgaard H, Jensen ON, Møller IM. Phosphorylation of formate dehydrogenase in potato tuber mitochondria. Journal of Biological Chemistry. 2003;278:26021–26030. doi: 10.1074/jbc.M300245200. [DOI] [PubMed] [Google Scholar]
- Bykova NV, Igamberdiev AU, Ens W, Hill RD. Identification of an intermolecular disulfide bond in barley haemoglobin. Biochemical and Biophysical Research Communications. 2006;347:301–309. doi: 10.1016/j.bbrc.2006.06.091. [DOI] [PubMed] [Google Scholar]
- Carr GJ, Page MD, Ferguson SJ. The energy-conserving nitric oxide reductase system in Paracoccus denitrificans: distinction from the nitrite reductase that catalyses synthesis of nitric oxide and evidence from trapping experiments for nitric oxide as a free intermediate during denitrification. European Journal of Biochemistry. 1989;179:683–692. doi: 10.1111/j.1432-1033.1989.tb14601.x. [DOI] [PubMed] [Google Scholar]
- Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Cooper CE. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends in Biochemical Sciences. 2002;27:33–39. doi: 10.1016/s0968-0004(01)02035-7. [DOI] [PubMed] [Google Scholar]
- Couee I, Defontaine S, Carde JP, Pradet A. Effects of anoxia on mitochondrial biogenesis in rice shoots – modification of in organello translation characteristics. Plant Physiology. 1992;98:411–421. doi: 10.1104/pp.98.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalber A, Shoun H, Ullrich V. Nitric oxide reductase (P450(nor)) from Fusarium oxysporum. Journal of Inorganic Biochemistry. 2005;99:185–193. doi: 10.1016/j.jinorgbio.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proceedings of the National Academy of Sciences of the USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordas C, Hasinoff BB, Igamberdiev AU, Manac'h N, Rivoal J, Hill RD. Expression of a stress-induced haemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. The Plant Journal. 2003;35:763–770. doi: 10.1046/j.1365-313x.2003.01846.x. [DOI] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends in Plant Sciences. 2000;5:123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Duff SMG, Wittenberg JB, Hill RD. Expression, purification, and properties of recombinant barley (Hordeum sp.) haemoglobin: optical spectra and reactions with gaseous ligands. Journal of Biological Chemistry. 1997;272:16746–16752. doi: 10.1074/jbc.272.27.16746. [DOI] [PubMed] [Google Scholar]
- Edwards S, Nguyen BT, Do B, Roberts JKM. Contribution of malic enzyme, pyruvate kinase, phosphoenolpyruvate carboxylase, and the Krebs cycle to respiration and biosynthesis and to intracellular pH regulation during hypoxia in maize root tips observed by NMR imaging and gas chromatography-mass spectrometry. Plant Physiology. 1998;116:1073–1081. doi: 10.1104/pp.116.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar MA, Geisler DA, Rasmusson AG. Reorganization of the alternative pathways of the Arabidopsis respiratory chain by nitrogen supply: opposing effects of ammonium and nitrate. The Plant Journal. 2006;45:775–788. doi: 10.1111/j.1365-313X.2005.02640.x. [DOI] [PubMed] [Google Scholar]
- Evans DE. Aerenchyma formation. New Phytologist. 2004;161:35–49. [Google Scholar]
- Fan TW, Higashi RM, Lane AN. An in vivo 1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Archives of Biochemistry and Biophysics. 1988;266:592–606. doi: 10.1016/0003-9861(88)90292-5. [DOI] [PubMed] [Google Scholar]
- Fan TW, Higashi RM, Frenkiel TA, Lane AM. Anaerobic nitrate and ammonium metabolism in flood-tolerant rice coleoptiles. Journal of Experimental Botany. 1997;48:1655–1666. [Google Scholar]
- Finlay BJ, Span ASW, Harman JMP. Nitrate respiration in primitive eukaryotes. Nature. 1983;303:333–336. [Google Scholar]
- Fox TC, Kennedy RA. Mitochondrial enzymes in aerobically and anaerobically germinated seedlings of Echinochloa and rice. Planta. 1991;184:510–514. doi: 10.1007/BF00197900. [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. Plant responses to hypoxia – is survival a balancing act? Trends in Plant Science. 2004;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology. 2003;30:1–47. doi: 10.1071/PP98095. [DOI] [PubMed] [Google Scholar]
- Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. Journal of Biological Chemistry. 2000;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- Greenway H, Gibbs J. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology. 2003;30:999–1036. doi: 10.1071/PP98096. [DOI] [PubMed] [Google Scholar]
- Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, et al. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhaemoglobin. Journal of Biological Chemistry. 2007;282:12916–12927. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. Journal of Experimental Botany. 2005;56:2601–2609. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Desikan R, Neill SJ. Does the redox status of cytochrome c act as a fail-safe mechanism in the regulation of programmed cell death? Free Radical Biology and Medicine. 2001;31:697–703. doi: 10.1016/s0891-5849(01)00646-3. [DOI] [PubMed] [Google Scholar]
- Hebelstrup KH, Igamberdiev AU, Hill RD. Metabolic effects of haemoglobin gene expression in plants. Gene. 2007;398:86–93. doi: 10.1016/j.gene.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Hazeki O, Tamura M. Oxygen dependence of redox state of copper in cytochrome oxidase in vitro. Journal of Applied Physiology. 1993;74:1622–1627. doi: 10.1152/jappl.1993.74.4.1622. [DOI] [PubMed] [Google Scholar]
- Huang X, von Rad U, Durner J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta. 2002;215:914–923. doi: 10.1007/s00425-002-0828-z. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Gardeström P. Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in mitochondria and cytosol of pea leaves. Biochimica et Biophysica Acta. 2003;1606:117–125. doi: 10.1016/s0005-2728(03)00106-3. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Hill RD. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. Journal of Experimental Botany. 2004;55:2473–2482. doi: 10.1093/jxb/erh272. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Kleczkowski LA. Membrane potential, adenylate levels and Mg2+ are interconnected via adenylate kinase equilibrium in plant cells. Biochimica et Biophysica Acta. 2003;1607:111–119. doi: 10.1016/j.bbabio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Ivanov BF, Nichugovskaya VD, Shevchenko LV. Intermediates of peroxisomal metabolism under conditions of oxygen deficit and carbon dioxide excess. Soviet Plant Physiology. 1991;38:673–679. [Google Scholar]
- Igamberdiev AU, Seregélyes C, Manac'h N, Hill RD. NADH-dependent metabolism of nitric oxide in alfalfa root cultures expressing hemoglobin. Planta. 2004;219:95–102. doi: 10.1007/s00425-003-1192-3. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Baron K, Manac'h N, Stoimenova M, Hill RD. The haemoglobin/nitric oxide cycle: involvement in flooding stress and effects on hormone signalling. Annals of Botany. 2005;96:557–564. doi: 10.1093/aob/mci210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igamberdiev AU, Bykova NV, Hill RD. Scavenging of nitric oxide by barley haemoglobin is facilitated by a monodehydroascorbate reductase-mediated ascorbate reduction of methaemoglobin. Planta. 2006;a 223:1033–1040. doi: 10.1007/s00425-005-0146-3. [DOI] [PubMed] [Google Scholar]
- Igamberdiev A, Stoimenova M, Seregelyes C, Hill RD. Class-1 haemoglobin and antioxidant metabolism in alfalfa roots. Planta. 2006;b 223:1041–1046. doi: 10.1007/s00425-005-0145-4. [DOI] [PubMed] [Google Scholar]
- Jackson TS, Jr, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circulation Research. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- Jancura D, Antalik M, Berka V, Palmer G, Fabian M. Filling the catalytic site of cytochrome c oxidase with electrons: reduced CuB facilitates internal electron transfer to heme a3. Journal of Biological Chemistry. 2006;281:20003–20010. doi: 10.1074/jbc.M602066200. [DOI] [PubMed] [Google Scholar]
- Kirsch M, de Groot H. Ascorbate is a potent antioxidant against peroxynitrite-induced oxidation reactions. Evidence that ascorbate acts by re-reducing substrate radicals produced by peroxynitrite. Journal of Biological Chemistry. 2000;275:16702–16708. doi: 10.1074/jbc.M909228199. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matsuo Y, Takimoto A, Suzuki S, Maruo F, Shoun H. Denitrification, a novel type of respiratory metabolism in fungal mitochondrion. Journal of Biological Chemistry. 1996;271:16263–16267. doi: 10.1074/jbc.271.27.16263. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Letters. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Schnitzler JP, Steinbrecher R. Biosynthesis of organic compounds emitted by plants. Plant Biology. 1999;1:149–159. [Google Scholar]
- Lancien M, Roberts MR. Regulation of Arabidopsis thaliana 14-3-3 gene expression by γ-aminobutyric acid. Plant, Cell and Environment. 2006;29:1430–1436. doi: 10.1111/j.1365-3040.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- Li X, Cobb CE, May JM. Mitochondrial recycling of ascorbic acid from dehydroascorbic acid: dependence on the electron transport chain. Archives of Biochemistry and Biophysics. 2002;403:103–110. doi: 10.1016/S0003-9861(02)00205-9. [DOI] [PubMed] [Google Scholar]
- Libourel IGL, van Bodegom PM, Fricker MD, Ratcliffe RG. Nitrite reduces cytoplasmic acidosis under anoxia. Plant Physiology. 2006;142:1710–1717. doi: 10.1104/pp.106.088898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-Y, Castelfranco P. The biosynthesis of ethyl-β-glucoside in extracts of pea seedlings. Plant Physiology. 1970;45:424–428. doi: 10.1104/pp.45.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DC, Millar AH, Sweetlove LJ, Hill SA, Leaver CJ. Mitochondrial biogenesis during germination in maize embryos. Plant Physiology. 2001;125:662–672. doi: 10.1104/pp.125.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manac'h-Little N, Igamberdiev AU, Hill RD. Haemoglobin expression affects ethylene production in maize cell cultures. Plant Physiology and Biochemistry. 2005;43:485–489. doi: 10.1016/j.plaphy.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mason MG, Nicholls P, Wilson MT, Cooper CE. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proceedings of the National Academy of Sciences of the USA. 2006;103:708–713. doi: 10.1073/pnas.0506562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattana M, Coraggio I, Bertani A, Reggiani R. Expression of the enzymes of nitrate reduction during the anaerobic germination of rice. Plant Physiology. 1994;106:1605–1608. doi: 10.1104/pp.106.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Chersi A, Fronza G. Differences in the anaerobic lactate-succinate production and in the changes of cell sap pH for plants with high and low resistance to anoxia. Plant Physiology. 1989;90:29–32. doi: 10.1104/pp.90.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Day DA. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Letters. 1996;398:155–158. doi: 10.1016/s0014-5793(96)01230-6. [DOI] [PubMed] [Google Scholar]
- Millar AH, Bergensen FJ, Day DA. Oxygen affinity of terminal oxidases in soybean mitochondria. Plant Physiology and Biochemistry. 1994;32:847–852. [Google Scholar]
- Miyashita Y, Good AG. Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant and Cell Physiology. 2008;49:92–102. doi: 10.1093/pcp/pcm171. [DOI] [PubMed] [Google Scholar]
- Møller IM. The oxidation of cytosolic NAD(P)H by external NAD(P)H dehydrogenases in the respiratory chain of plant mitochondria. Physiologia Plantarum. 1997;100:85–90. [Google Scholar]
- Møller IM, Rasmusson AG. The role of NADP in the mitochondrial matrix. Trends in Plant Science. 1998;3:21–27. [Google Scholar]
- Morard P, Silvestre J, Lacoste L, Caumes E, Lamaze T. Nitrate uptake and nitrite release by tomato roots in response to anoxia. Journal of Plant Physiology. 2004;161:855–865. doi: 10.1016/j.jplph.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Carver TLW, Prats E. NO way to live: the various roles of nitric oxide in plant-pathogen interactions. Journal of Experimental Botany. 2006;57:489–505. doi: 10.1093/jxb/erj052. [DOI] [PubMed] [Google Scholar]
- Nie XZ, Hill RD. Mitochondrial respiration and haemoglobin gene expression in barley aleurone tissue. Plant Physiology. 1997;114:835–840. doi: 10.1104/pp.114.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie XZ, Durnin DC, Igamberdiev AU, Hill RD. Cytosolic calcium is involved in the regulation of barley haemoglobin gene expression. Planta. 2006;223:542–549. doi: 10.1007/s00425-005-0094-y. [DOI] [PubMed] [Google Scholar]
- Ohwaki Y, Kawagishi-Kobayashi M, Wakasa K, Fujihara S, Yoneyama T. Induction of class-1 non-symbiotic haemoglobin genes by nitrate, nitrite and nitric oxide in cultured rice cells. Plant and Cell Physiology. 2005;46:324–331. doi: 10.1093/pcp/pci030. [DOI] [PubMed] [Google Scholar]
- Paitian NA, Markossian KA, Nalbandyan RM. The effect of nitrite on cytochrome oxidase. Biochemical and Biophysical Research Communications. 1985;133:1104–1111. doi: 10.1016/0006-291x(85)91250-1. [DOI] [PubMed] [Google Scholar]
- Pearce LL, Kanai AJ, Birder LA, Pitt BR, Peterson J. The catabolic fate of nitric oxide: the nitric oxide oxidase and peroxynitrite reductase activities of cytochrome oxidase. Journal of Biological Chemistry. 2002;277:13556–13562. doi: 10.1074/jbc.M109838200. [DOI] [PubMed] [Google Scholar]
- Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, et al. Arabidopsis nonsymbiotic haemoglobin AHb1 modulates nitric oxide bioactivity. The Plant Cell. 2004;16:2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazzolli M, Romero-Puertas MC, Delledonne M. Modulation of nitric oxide bioactivity by plant haemoglobins. Journal of Experimental Botany. 2006;57:479–488. doi: 10.1093/jxb/erj051. [DOI] [PubMed] [Google Scholar]
- Planchet E, Kaiser WM. Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: a comparison using abiotic and biotic NO sources. Journal of Experimental Botany. 2006;57:3043–3055. doi: 10.1093/jxb/erl070. [DOI] [PubMed] [Google Scholar]
- Planchet E, Gupta KJ, Sonoda M, Kaiser WM. Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. The Plant Journal. 2005;41:732–743. doi: 10.1111/j.1365-313X.2005.02335.x. [DOI] [PubMed] [Google Scholar]
- Porterfield DM, Kuang A, Smith PJ, Crispi ML, Musgrave ME. Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Canadian Journal of Botany. 1999;77:1439–1446. [PubMed] [Google Scholar]
- Rasmusson AG, Geisler DA, Møller IM. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion. 2008;8:47–60. doi: 10.1016/j.mito.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Reggiani R. A role for ethylene in low-oxygen signaling in rice roots. Amino Acids. 2006;30:299–301. doi: 10.1007/s00726-006-0274-5. [DOI] [PubMed] [Google Scholar]
- Reggiani R, Cantú CA, Brambilla I, Bertani A. Accumulation and interconversion of amino acids in rice roots under anoxia. Plant and Cell Physiology. 1988;29:981–987. [Google Scholar]
- Reggiani R, Bertini F, Mattana M. Incorporation of nitrate nitrogen into amino acids during the anaerobic germination of rice. Amino Acids. 1995;9:385–390. doi: 10.1007/BF00807275. [DOI] [PubMed] [Google Scholar]
- Reid RJ, Loughman BC, Ratcliffe RG. 31P NMR measurements of cytoplasmic pH changes in maize root tips. Journal of Experimental Botany. 1985;36:889–897. [Google Scholar]
- Richter C. Reactive oxygen and nitrogen species regulate mitochondrial Ca2+ homeostasis and respiration. Bioscience Reports. 1997;17:53–66. doi: 10.1023/a:1027387301845. [DOI] [PubMed] [Google Scholar]
- Roberts JKM, Hooks MA, Miaullis AP, Edwards S, Webster C. Contribution of malate and amino acid metabolism to cytoplasmic pH regulation in hypoxic maize root tips studied using nuclear magnetic resonance spectroscopy. Plant Physiology. 1992;98:480–487. doi: 10.1104/pp.98.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TH, Fredlund KM, Møller IM. Direct evidence for the presence of two external NAD(P)H dehydrogenases coupled to the electron transport chain in plant mitochondria. FEBS Letters. 1995;373:307–309. doi: 10.1016/0014-5793(95)01059-n. [DOI] [PubMed] [Google Scholar]
- Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. Journal of Experimental Botany. 2002;53:103–110. [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H. Legume embryos develop in a hypoxic environment. Journal of Experimental Botany. 2002;53:1099–1107. doi: 10.1093/jexbot/53.371.1099. [DOI] [PubMed] [Google Scholar]
- Ruitenberg M, Kannt A, Bamberg E, Fendler K, Michel H. Reduction of cytochrome c oxidase by a second electron leads to proton translocation. Nature. 2002;417:99–102. doi: 10.1038/417099a. [DOI] [PubMed] [Google Scholar]
- Rustin P, Lance C. Succinate-driven reverse electron transport in the respiratory chain of plant mitochondria: the effects of rotenone and adenylates in relation to malate and oxaloacetate metabolism. Biochemical Journal. 1991;274:249–255. doi: 10.1042/bj2740249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Harada T, Ishizawa K. Stimulation of glycolysis in anaerobic elongation of pondweed (Potamogeton distinctus) turions. Journal of Experimental Botany. 2002;53:1847–1856. doi: 10.1093/jxb/erf036. [DOI] [PubMed] [Google Scholar]
- Scarpa M, Stevanato R, Viglino P, Rigo A. Superoxide ion as active intermediate in the autoxidation of ascorbate by molecular oxygen: effect of superoxide dismutase. Journal of Biological Chemistry. 1983;258:6695–6697. [PubMed] [Google Scholar]
- Sharpe MA, Cooper CE. Interaction of peroxynitrite with mitochondrial cytochrome oxidase: catalytic production of nitric oxide and irreversible inhibition of enzyme activity. Journal of Biological Chemistry. 1998;273:30961–30972. doi: 10.1074/jbc.273.47.30961. [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Bown AW, McLean MD. Metabolism and functions of gamma-aminobutyric acid. Trends in Plant Science. 1999;4:446–452. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- Smart DR, Bloom AJ. Wheat leaves emit nitrous oxide during nitrate assimilation. Proceedings of the National Academy of Sciences of the USA. 2001;98:7875–7878. doi: 10.1073/pnas.131572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, ap Rees T. Pathways of carbohydrate fermentation in the roots of marsh plants. Planta. 1979;146:327–334. doi: 10.1007/BF00387805. [DOI] [PubMed] [Google Scholar]
- Sowa AW, Duff SMG, Guy PA, Hill RD. Altering haemoglobin levels changes energy status in maize cells under hypoxia. Proceedings of the National Academy of Sciences of the USA. 1998;95:10317–10321. doi: 10.1073/pnas.95.17.10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta. 2007;226:465–474. doi: 10.1007/s00425-007-0496-0. [DOI] [PubMed] [Google Scholar]
- Stöhr C, Strube F, Marx G, Ullrich WR, Rockel P. A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta. 2001;212:835–841. doi: 10.1007/s004250000447. [DOI] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Mitochondrial contribution to the anoxic Ca2+ signal in maize suspension-cultured cells. Plant Physiology. 1998;118:759–771. doi: 10.1104/pp.118.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarka A, Horemans N, Kovacs Z, Grof P, Mayer M, Banhegyi G. Dehydroascorbate reduction in plant mitochondria is coupled to the respiratory electron transfer chain. Physiologia Plantarum. 2007;129:225–232. [Google Scholar]
- Takaya N, Kuwazaki S, Adachi Y, Suzuki S, Kikuchi T, Nakamura H, et al. Hybrid respiration in the denitrifying mitochondria of Fusarium oxysporum. Journal of Biochemistry (Tokyo) 2003;133:461–465. doi: 10.1093/jb/mvg060. [DOI] [PubMed] [Google Scholar]
- Taylor ER, Nie XZ, MacGregor AW, Hill RD. A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Molecular Biology. 1994;24:853–862. doi: 10.1007/BF00014440. [DOI] [PubMed] [Google Scholar]
- Tischner R, Planchet E, Kaiser WM. Mitochondrial electron transport as a source for nitric oxide in the unicellular green alga Chlorella sorokiniana. FEBS Letters. 2004;576:151–155. doi: 10.1016/j.febslet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Torres J, Sharpe MA, Rosquist A, Cooper CE, Wilson MT. Cytochrome c oxidase rapidly metabolises nitric oxide to nitrite. FEBS Letters. 2000;475:263–266. doi: 10.1016/s0014-5793(00)01682-3. [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Watts RA, Andersson C, Llewellyn D, Hargrove MS, Olson JS, et al. Two haemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghaemoglobins. Proceedings of the National Academy of Sciences of the USA. 1997;94:12230–12234. doi: 10.1073/pnas.94.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin AF, Svistunenko DA, Mikoyan VD, Serezhenkov VA, Fryer MJ, Baker NR, Cooper CE. Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. Journal of Biological Chemistry. 2004;279:24100–24107. doi: 10.1074/jbc.M312601200. [DOI] [PubMed] [Google Scholar]
- Vartapetian BB, Andreeva IN, Generozova IP, Polyakova LI, Maslova IP, Dolgikh YI, et al. Functional electron microscopy in studies of plant response and adaptation to anaerobic stress. Annals of Botany. 2003;91:155–172. doi: 10.1093/aob/mcf244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watsuji T, Takaya N, Nakamura A, Shoun H. Denitrification of nitrate by the fungus Cylindrocarpon tonkinense. Bioscience, Biotechnology and Biochemistry. 2003;67:1115–1120. doi: 10.1271/bbb.67.1115. [DOI] [PubMed] [Google Scholar]
- Xia JH, Roberts J. Improved cytoplasmic pH regulation, increased lactate efflux, and reduced cytoplasmic lactate levels are biochemical traits expressed in root tips of whole maize seedlings acclimated to a low-oxygen environment. Plant Physiology. 1994;105:651–657. doi: 10.1104/pp.105.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Shimoji H, Ohshiro Y, Sakihama H. Inhibitory effects of nitric oxide on oxidative phosphorylation in plant mitochondria. Nitric Oxide. 2001;5:261–270. doi: 10.1006/niox.2001.0353. [DOI] [PubMed] [Google Scholar]