Abstract
Background and Aims
When root-zone O2 deficiency occurs together with salinity, regulation of shoot ion concentrations is compromised even more than under salinity alone. Tolerance was evaluated amongst 34 accessions of Hordeum marinum, a wild species in the Triticeae, to combined salinity and root-zone O2 deficiency. Interest in H. marinum arises from the potential to use it as a donor for abiotic stress tolerance into wheat.
Methods
Two batches of 17 H. marinum accessions, from (1) the Nordic Gene Bank and (2) the wheat belt of Western Australia, were exposed to 0·2 or 200 mol m−3 NaCl in aerated or stagnant nutrient solution for 28–29 d. Wheat (Triticum aestivum) was included as a sensitive check species. Growth, root porosity, root radial O2 loss (ROL) and leaf ion (Na+, K+, Cl−) concentrations were determined.
Key Results
Owing to space constraints, this report is focused mainly on the accessions from the Nordic Gene Bank. The 17 accessions varied in tolerance; relative growth rate was reduced by 2–38 % in stagnant solution, by 8–42 % in saline solution (aerated) and by 39–71 % in stagnant plus saline treatment. When in stagnant solution, porosity of adventitious roots was 24–33 %; salinity decreased the root porosity in some accessions, but had no effect in others. Roots grown in stagnant solution formed a barrier to ROL, but variation existed amongst accessions in apparent barrier ‘strength’. Leaf Na+ concentration was 142–692 µmol g−1 d. wt for plants in saline solution (aerated), and only increased to 247–748 µmol g−1 d. wt in the stagnant plus saline treatment. Leaf Cl− also showed only small effects of stagnant plus saline treatment, compared with saline alone. In comparison with H. marinum, wheat was more adversely affected by each stress alone, and particularly when combined; growth reductions were greater, adventitious root porosity was 21 %, it lacked a barrier to ROL, leaf K+ declined to lower levels, and leaf Na+ and Cl− concentrations were 3·1–9-fold and 2·8–6-fold higher, respectively, in wheat.
Conclusions
Stagnant treatment plus salinity reduced growth more than salinity alone, or stagnant alone, but some accessions of H. marinum were still relatively tolerant of these combined stresses, maintaining Na+ and Cl− ‘exclusion’ even in an O2-deficient, saline rooting medium.
Key words: Aerenchyma, combined salinity and waterlogging, leaf Cl−, leaf K+, leaf Na+, radial O2 loss, salt tolerance, salinity–waterlogging interaction, sea barleygrass, waterlogging tolerance, wheat, wild Triticeae
INTRODUCTION
Salinity is an increasing problem for crop production in many regions of the world (Szabolcs, 1994). Large areas of saline agricultural land are also prone to waterlogging, so crops and pastures tolerant of both stresses are required (Barrett-Lennard, 2003). Hordeum marinum is a wild species in the Triticeae that could be used in wide hybridizations with wheat to develop a more salt- and waterlogging-tolerant cereal (Colmer et al., 2005, 2006; Islam et al., 2007). H. marinum grows in salt marshes (von Bothmer et al., 1995) and shows tolerance to waterlogging (McDonald et al., 2001; Garthwaite et al., 2003) and salinity (Garthwaite et al., 2005). These earlier studies, however, only evaluated one or two accessions and did not assess responses to combined waterlogging plus salinity.
Salinity (i.e. high NaCl) reduces plant growth by osmotic stress and ion toxicity (Greenway and Munns, 1980). Osmotic stress is caused by high concentrations of Na+ and Cl− that decrease soil water potential and thus impede water uptake by roots, whereas ion toxicity occurs when Na+ and/or Cl− accumulate in tissues to a level that inhibits metabolism and growth and/or if low K+/Na+ ratio occurs in the cytoplasm (Greenway and Munns, 1980; Flowers and Dalmond, 1992; Munns, 2005). Tolerance of non-halophytes, like wheat, to salinity depends on a number of traits (listed in Colmer et al., 2005), foremost amongst which are an ability to restrict Na+ and Cl− uptake and to sequester into vacuoles these ions when they do enter the tissues (Munns, 2005; Munns and Tester, 2008). Salt tolerance in H. marinum, at least in aerobic rooting conditions, is associated with the ability to ‘exclude’ (i.e. restrict the rate of entry of) Na+ and Cl− from young leaves, the maintenance of adequate K+ concentrations and the production of glycinebetaine (Garthwaite et al., 2005; Islam et al., 2007).
Waterlogging results in low O2 concentrations (hypoxic) or even zero O2 (anoxia) in the soil, as well as accumulation of potentially toxic compounds from anaerobic metabolism by soil microorganisms (Ponnamperuma, 1984). In the case of wheat, reduced growth and nutrient uptake has largely been attributed to O2 deficits in roots (Trought and Drew, 1980), as inhibition of respiration results in a severe energy crisis (Gibbs and Greenway, 2003). Tolerance to waterlogging relies on the development of adventitious roots with aerenchyma and a barrier to radial O2 loss (ROL), and/or by induction of anaerobic metabolism (Armstrong, 1979; Jackson and Drew, 1984; Setter and Waters, 2003; Gibbs and Greenway, 2003). H. marinum forms adventitious roots that contain aerenchyma and a barrier to ROL (McDonald et al., 2001; Garthwaite et al., 2003).
When waterlogging occurs together with salinity the combined effects can be particularly detrimental to sensitive species (Barrett-Lennard, 2003), although some halophytes can tolerate the combination of waterlogging and salinity (Colmer and Flowers, 2008). Combined salinity and waterlogging increased greatly the concentrations of Na+ and Cl− in shoots of wheat, as compared with salinity alone (Barrett-Lennard, 1986), and such increases also occur in other species (Barrett-Lennard, 2003), but not in some ‘wetland’ halophytes (Colmer and Flowers, 2008). Moreover, waterlogging (Wiengweera and Greenway, 2004) or salinity (Greenway and Munns, 1980; Maathuis and Amtmann, 1999) alone can reduce K+ uptake in plants; thus, when salinity and root hypoxia occur together, K+ uptake is reduced even further (Drew et al., 1988).
The relatively high tolerance of H. marinum to salinity and waterlogging (to date evaluated separately; Garthwaite et al., 2003, 2005) and the ability to hybridize H. marinum with wheat (Colmer et al., 2006; Islam et al., 2007) prompted us to evaluate a diverse collection of H. marinum accessions for tolerance of these stresses alone, and in combination. The objectives were to: (1) test the hypothesis that H. marinum, a species with superior root aeration and Na+ and Cl− ‘exclusion’ (references cited above), would be more tolerant to combined root-zone O2 deficiency and salinity than wheat; and (2) determine whether H. marinum accessions differ in tolerance to waterlogging, salinity and these stresses combined. Growth, root porosity, profiles of ROL and leaf ion concentrations were evaluated for plants in controlled environment experiments.
MATERIALS AND METHODS
Thirty-four accessions of H. marinum were evaluated in two batches of 17; the first batch was from the Nordic Gene Bank and the second was from the wheat belt of Western Australia; due to space constraints, the results for the first batch of 17 accessions (Experiment 1, accessions from the Nordic Gene Bank) are reported in full, whereas only selected results are reported for the second batch (Experiment 2, accessions from the wheat belt of Western Australia). Wheat (‘Chinese Spring’) was included in both experiments as a salt- and waterlogging-sensitive check species.
Experiment 1: accessions from the Nordic Gene Bank
Plant materials
Seventeen diploid accessions of sea barleygrass (Hordeum marinum) and one hexaploid wheat (Triticum aestivum ‘Chinese Spring’) were used. The accessions consisted of nine H. marinum spp. marinum and eight H. marinum spp. gussoneanum from different parts of the world (Table 1). Seeds were from the Nordic Gene Bank (R. von Bothmer, Swedish Agricultural University, Alnarp). All accessions were grown in quarantine, during which ploidy was determined in root tip squashes (all confirmed as diploids; A. K. M. R. Islam, University of Adelaide), and the seeds released were used in our experiments.
Table 1.
Porosity in adventitious roots of 17 accessions of Hordeum marinum and one wheat (Triticum aestivum ‘Chinese Spring’, CS) grown in non-saline aerated or stagnant nutrient solution, or in saline (200 mol m−3 NaCl) aerated or stagnant nutrient solution (Experiment 1; accessions from the Nordic Gene Bank; country of origin and accession codes are listed in the table)
| Adventitious root porosity (% gas volume per unit root volume) |
||||||
|---|---|---|---|---|---|---|
| Non-saline |
Saline |
|||||
| Species/accession code | Subspecies | Country of origin | Aerated | Stagnant | Aerated | Stagnant |
| H21 | marinum | Spain | 18 ± 4 | 28 ± 3 | 21 ± 3 | 17++ |
| H87 | marinum | Jordan | 19 ± 2 | 28 ± 3 | 13++ | 14++ |
| H90 | marinum | Greece | 14 ± 2 | 25 ± 3 | 19 ± 3 | 19 ± 7+ |
| H109 | marinum | Greece | 18 ± 3 | 26 ± 5 | 19 ± 4 | 27 ± 5 |
| H522 | marinum | Spain | 18 ± 3 | 25 ± 5 | 27 ± 2 | 27 ± 5+ |
| H524 | marinum | Spain | 20 ± 3 | 24 ± 3 | 21 ± 4 | 24 ++ |
| H546 | marinum | Spain | 25 ± 2 | 25 ± 5 | 21 ± 7+ | n.a. |
| H547 | marinum | Spain | 15 ± 7 | 24 ± 5 | 32 ± 4+ | n.a. |
| H559 | marinum | Spain | 17 ± 3 | 26 ± 5 | 18 ± 4 | 18++ |
| H155 | gussoneanum | Greece | 17 ± 3 | 29 ± 4 | 22 ± 2 | 21++ |
| H160 | gussoneanum | Portugal | 22 ± 2 | 24 ± 3 | 16 ± 3 | 25 ± 6+ |
| H162 | gussoneanum | Portugal | 23 ± 1+ | 33 ± 1+ | n.a. | n.a. |
| H563 | gussoneanum | Spain | 22 ± 3 | 26 ± 2 | 19 ± 4 | 31 ± 4 |
| H608 | gussoneanum | Greece | 25 ± 4 | 31 ± 3 | 14 ± 8 | 29 ± 3+ |
| H823 | gussoneanum | Bulgaria | 19 ± 3 | 25 ± 5 | 12 ± 5 | 26 ± 4 |
| H826 | gussoneanum | Turkey | 20 ± 3 | 30 ± 6 | 18 ± 3 | 29 ± 5+ |
| H1966 | gussoneanum | USA | 24 ± 4 | 27 ± 4 | 20 ± 4 | 27 ± 7+ |
| Wheat | CS | – | 8 ± 5 | 21 ± 5 | 1 ± 1 | 17 ± 4 |
| Mean | (not with CS) | – | 20 | 27 | 21 | 21 |
| l.s.d. | (not for CS) | – | 6·0* | n.s. | n.s. | n.s. |
Porosity was measured using 100–150-mm roots. Values are the means of three replicates (unless stated) ± s.e. Wheat was not included in the statistical analysis. The l.s.d. (5 % level) refers to the influence of accession within treatments. In some treatments, some accessions did not produce enough roots in the 100–150-mm length class for a reliable measurement, so some have two replicates (indicated by +), one replicate (++) or data are not available (n.a.). *P < 0·05; ***P < 0·001; n.s., not significant.
Experimental design
The experiment was carried out in a controlled environment room with photoperiod of 12 h, irradiance 400–500 µmol quanta m−2 s−1 photosynthetically active radiation at plant height, temperature 20/15 °C and relative humidity 60/80 % (light/dark). Four root-zone treatments were imposed: aerated non-saline (control), aerated saline (200 mol m−3 NaCl), stagnant non-saline and stagnant plus saline (200 mol m−3 NaCl). The experimental design was: 4 treatments × 17 accessions (plus wheat as a sensitive check) × 3 replicates, in a completely randomized block design. Due to space limitations in the controlled environment room the experiment was carried out as a series of three complete blocks staggered over time. Pots were completely randomized within each block, and to minimize the effects of possible environmental gradients within the controlled environment room, the pots were re-randomized weekly.
Plant culture
Seeds were surface-sterilized with 0·04 % (w/v) sodium hypochlorite, rinsed with deionized water, imbibed in aerated 0·5 mol m−3 CaSO4 overnight, and then transferred to plastic mesh floating on aerated tenth-strength nutrient solution (full-strength composition given below) in the controlled environment room, all in darkness for the first 4 d. The composition of nutrient solution at full strength was: (mol m−3): K+, 5·95; Ca2+, 4·0; Mg2+, 0·4; NH4+, 0·625; Na+, 0·2; NO3−, 4·375; SO42−, 4·4; H2PO4−, 0·2; H4SiO4−, 0·1; and the micronutrients (mmol m−3) Cl−, 50; B, 25; Mn, 2; Zn, 2; Ni, 1; Cu, 0·5; Mo, 0·5; Fe-EDTA, 50. The solution also contained 2·5 mol m−3 MES (2-[N-morpholino] ethanesulfonic acid) and the pH was adjusted to 6·5 using KOH (to give the final K+ concentration listed above). FeSO4 at 5 mmol m−3 was supplied during the second week to prevent symptoms of slight Fe-deficiency that can occur if not routinely added (observations from a preliminary experiment). During the treatment period (see below) an additional 2·5 mol m−3 NH4NO3 was included in all solutions (cf. Wiengweera et al., 1997; Rubinigg et al., 2002). All chemicals used were of analytical grade.
When seedlings were 4 d old, the nutrient solution was changed to quarter-strength, and seedlings were exposed to light. Seven-day-old seedlings were transplanted into 4·5-L pots containing full-strength nutrient solution. The solution in all pots was thereafter renewed every 7 d. Four pots of each accession were established for each block, and each pot contained three plants.
Treatments commenced when plants were 17 d old (2·0–2·5-leaf stage, Haun, 1973). In pots assigned to the saline treatments, NaCl was stepped up by 50 mol m−3 every 12 h, to a final concentration of 200 mol m−3. The Ca2+ concentration in the nutrient solution was 4 mol m−3 (see above), so the Na+/Ca2+ ratio approximated that in seawater (approx. 50:1), which is also similar to the ratio in the habitat of H. marinum in south-western Australia. The importance of avoiding unrealistically low external Ca2+ concentrations in nutrient solutions used for experiments on plant responses to salinity has been summarized elsewhere (Greenway and Munns, 1980; Cramer, 2002; Munns and Tester, 2008). After reaching 200 mol m−3 NaCl in the pots assigned to the saline treatment (all plants now 19 d old), a hypoxic pre-treatment was imposed in pots assigned to the stagnant treatments (i.e. stagnant non-saline and saline) by flushing the nutrient solution in these pots with N2 until the O2 concentration was approx. 0·03 mol m−3; these pots were then left overnight without bubbling. The next day, the nutrient solution in these stagnant treatment pots was replaced with deoxygenated stagnant nutrient solution containing 0·1 % (w/v) agar (non-saline or with 200 mol m−3 NaCl). The dilute agar prevents convective movements in the solution (Wiengweera et al., 1997). The pots assigned to the aerated treatments (non-saline or with 200 mol m−3 NaCl) also received new nutrient solution but without agar. Solution in pots assigned to aerated treatments continued to be bubbled with air.
Harvest procedure
An initial harvest of one plant was taken from each pot immediately prior to the stagnant treatment being imposed, leaving two plants per pot. One of these two remaining plants per pot was used for measurements of radial O2 loss (ROL) from adventitious roots (after 25–28 d of treatments; see below), and the other for a final harvest taken after 28–29 d of treatments. At harvests, roots and the ‘stem’ base were rinsed three times, for 10 s each time, in deionized water. Plants were divided into leaves, ‘stems’ (i.e. leaf sheaths), seminal roots and adventitious roots. Leaves were separated into three classes: youngest fully expanded leaf (YFEL), other green leaves and dead leaves. Samples were dried at 65 °C for 72 h and then dry masses were recorded. Whole-plant relative growth rate (RGR) was calculated from the dry weight data at the initial and final harvests, using the formula given by Hunt (1978).
Tissue ion analyses
Concentrations of Na+, K+ and Cl− were determined for the YFEL and for the bulk sample of other green leaves. Oven-dried samples were ground with a ball mill and ions were extracted in 500 mol m−3 HNO3 by shaking for 48 h at 20–25 °C. Na+ and K+ were determined in dilutions of the extracts using a flame photometer (PFP7, Jenway, Essex, UK) and Cl− using a Buchler-Cotlove Chloridometer (Buchler Instruments, Model 4-2000, NJ, USA). Plant reference material was also analysed and values obtained for Na+, K+ and Cl− were, respectively, 102, 102 and 97 % of expected. Data were not adjusted.
ROL from adventitious roots
ROL measurements were taken only for plants grown in stagnant treatments (i.e. stagnant nutrient solution, without or with NaCl). Time did not permit ROL measurements also to be taken for roots of plants grown in aerated treatments, but these second plants in each aerated pot were also removed at the time ROL measurements were taken, to ensure the same plant numbers per pot across all treatments. ROL was measured along intact adventitious roots that had no, or only few, laterals. Intact plants (after 25–28 d of treatments) were transferred to a 20 °C controlled temperature room. The shoot of each plant was in air and the root/shoot junction was inserted into a rubber lid so that the root system was immersed in a clear Perspex chamber containing deoxygenated stagnant full-strength nutrient solution with 0·1 % agar (w/v), and depending on the treatment either without or with 200 mol m−3 NaCl. ROL from adventitious roots (approx. 90–110 mm) was measured at various positions, using root-sleeving O2 electrodes (i.d. 2·25 mm, height 5·0 mm) fitted with guides to keep each root near the centre of the electrode (Armstrong and Wright, 1975; Armstrong, 1994). One intact root was measured for each of three plants, providing three replicates.
Aerenchyma in adventitious roots
Cross-sections were taken using a hand-held razor blade, at 50 mm behind the tip of selected adventitious roots. The roots used were those from the ROL measurements, and also roots of a similar length from the plants in aerated treatments removed at the time of ROL measurements (explained above). The cross-sections were examined using a light microscope (Olympus BH-2, USA, Inc.) and photographed using a digital camera (Nikon coolpix 4500, Osaka, Japan). Aerenchyma percentage in cross-sections was quantified using a public domain image analysis program (Image J, version 1·24o, Millersville, PA, USA).
Adventitious root porosity
Porosity (% gas volume/root volume) of adventitious roots was measured using the method described by Raskin (1983) and the equations as modified by Thomson et al. (1990). Measurements were taken for adventitious roots 100–150 mm in length.
Experiment 2: accessions from the wheat belt of Western Australia
Plant materials
A second set of 17 diploid accessions of sea barleygrass (Hordeum marinum) and one hexaploid wheat (Triticum aestivum ‘Chinese Spring’) were evaluated. The accessions were collected by K. A. Shepherd (WA Herbarium) from the wheat belt of Western Australia; sites were saline and prone to waterlogging, and between 20 °24′ and 34 °34′S and 114 °51′ and 118 °56′E. Single heads were collected, seeds were raised in a glasshouse and all were confirmed as diploids in root tip squashes by A. K. M. R. Islam (University of Adelaide). Experimental design, plant culture and all procedures were as described above for Experiment 1.
Statistical analyses
Data were analysed by calculating means, standard errors, regression and analysis of variance (ANOVA), where appropriate, using GenStat 10 for Windows statistical software (VSN International). Significant differences refer to P < 0·05.
RESULTS
Experiment 1: accessions from the Nordic Gene Bank
Growth
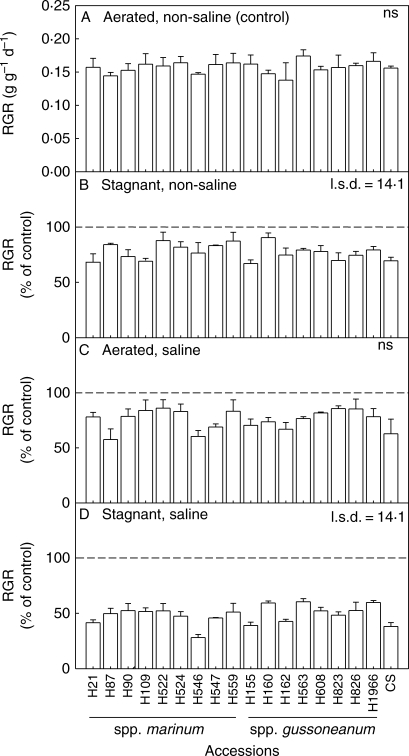
Whole-plant RGR did not differ amongst the 17 H. marinum accessions when in aerated non-saline nutrient solution (range 0·14–0·17 g g−1 d−1), and was similar to the RGR of wheat (0·16 g g−1 d−1; Fig. 1). The stagnant non-saline treatment reduced RGR of the H. marinum accessions to 68–89 % of the aerated controls, and for wheat to 70 % of the control. The aerated saline treatment reduced RGR of the H. marinum accessions to 58–86 % of the controls, and for wheat to 63 % of the control. The combined stagnant plus saline treatment reduced RGR of the H. marinum accessions to 29–61 % of the controls, and for wheat to 39 % of the control. The two subspecies of H. marinum (spp. marinum and spp. gussoneanum) did not differ in their mean reductions in RGR in response to the various treatments.
Fig. 1.
Relative growth rate (RGR) of whole plants for 17 accessions of Hordeum marinum and one wheat genotype (‘Chinese Spring’, CS) in (A) aerated non-saline nutrient solution (control). RGR as a percentage of controls for plants in nutrient solutions with treatments: (B) deoxygenated stagnant agar, non-saline; (C) aerated 200 mol m−3 NaCl; and (D) deoxygenated stagnant agar plus 200 mol m−3 NaCl. Values are means of three replicates ± s.e. The l.s.d. refers to the influence of accession in the different treatments at the 5 % level; ns = not significant. Triticum aestivum ‘Chinese Spring’ (CS) was not included in the statistical analysis. Experiment 1; accessions from the Nordic Gene Bank.
Root porosity and aerenchyma
Adventitious root porosity of the H. marinum accessions was 15–25 % when in aerated non-saline solution (P < 0·01), whereas in wheat, root porosity was only 8 % (Table 1). In stagnant non-saline solution, root porosity increased by 1·1- to 1·6-fold for the H. marinum accessions, but in wheat it increased 2·6-fold. Nevertheless, the highest root porosity was 33 % in accession H162, whereas porosity only reached 21 % in roots of wheat. Salinity in aerated solution resulted in root porosity values of 12–32 %; the mean value across all accessions did not differ from in aerated non-saline conditions, but in some accessions porosity decreased and in others it increased (Table 1). In stagnant medium, addition of 200 mm NaCl resulted in the mean root porosity across all accessions decreasing from 27 to 21 %; it decreased in some accessions but was not affected in others (Table 1).
Aerenchyma was also quantified in cross-sections taken at 50 mm behind the root tip. Small amounts of constitutive aerenchyma (up to 9 %) were evident in the roots of the H. marinum accessions when in aerated non-saline solution, and even wheat had 3 % aerenchyma (data not shown). In stagnant non-saline solution, root aerenchyma increased by 1·3- to 11·0-fold for the H. marinum accessions, and in wheat by 5·7-fold; in stagnant saline solution the amounts of aerenchyma were not different to those in stagnant non-saline conditions (exceptions being reductions in accessions H87, H547, H559). These results (data not shown) were consistent with those for root porosity (Table 1).
Ion concentrations in the youngest fully-expanded leaf
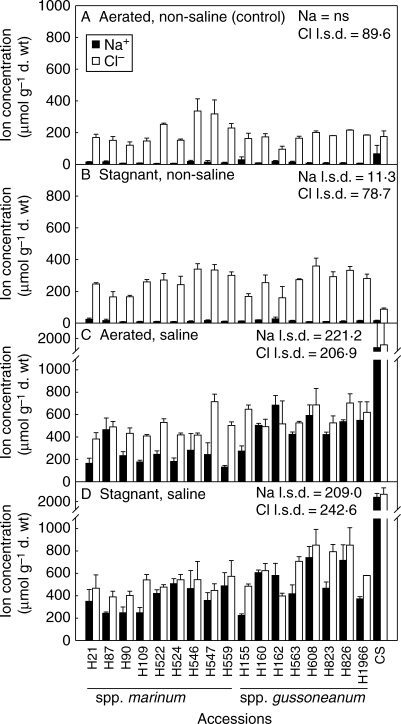
Leaf Na+ concentration was low and did not differ amongst the H. marinum accessions when in aerated non-saline solution; Na+ was also low in wheat (Fig. 2). The aerated saline treatment resulted in substantial increases in Na+ in all H. marinum accessions, but the increase was much larger for wheat (P < 0·001). Leaf Na+ concentrations in the H. marinum accessions exposed to 200 mol m−3 NaCl in aerated solution ranged from 138 to 692 µmol g−1 d. wt, whereas in wheat it was 1729 µmol g−1 d. wt. Stagnant non-saline treatment had little effect on leaf Na+ in comparison with the aerated non-saline controls. In the combined stagnant plus saline treatment leaf Na+ increased above that in the aerated saline treatment only in some of the accessions (Fig. 2). The leaf Na+ concentration in H. marinum accessions in the stagnant plus saline treatment ranged from 250 to 725 µmol g−1 d. wt, whereas in wheat it had increased to 2260 µmol g−1 d. wt. In spp. gussoneanum, leaf Na+ was, on average, 2·1-fold higher than for spp. marinum, for plants in the aerated saline treatment (P < 0·001); in the combined stagnant plus saline treatment, leaf Na+ in spp. gussoneanum was only 1·4-fold higher than in spp. marinum (P = 0·054).
Fig. 2.
Na+ (closed bars) and Cl− (open bars) concentrations in the youngest fully expanded leaf for 17 accessions of Hordeum marinum and one wheat genotype (CS) grown in nutrient solution with treatments of: (A) aerated non-saline (control); (B) deoxygenated stagnant agar, non-saline; (C) aerated 200 mol m−3 NaCl; and (D) deoxygenated stagnant agar plus 200 mol m−3 NaCl. Values are the means of three replicates ± s.e. The l.s.d. refers to the influence of accession in different treatments at the 5 % level; ns = not significant. Triticum aestivum ‘Chinese Spring’ (CS) was not included in the statistical analysis. Experiment 1; accessions from the Nordic Gene Bank.
Leaf Cl− concentrations in the H. marinum accessions when in aerated non-saline solution were, on average, 11·6-fold higher than those for Na+, whereas in wheat Cl− was only 2·5-fold higher than Na+ (Fig. 2). In stagnant non-saline solution, leaf Cl− concentrations were similar to those in plants in the aerated non-saline treatment. In the aerated saline treatment, leaf Cl− increased significantly in all accessions of H. marinum and even more so in wheat (P < 0·01). In aerated saline solution, leaf Cl− in the H. marinum accessions ranged from 389 to 723 µmol g−1 d. wt, whereas in wheat it was 1819 µmol g−1 d. wt. In the combined stagnant plus saline treatment, leaf Cl− was generally not affected in the H. marinum accessions (range 398–860 µmol g−1 d. wt) compared with in aerated saline treatment, whereas for wheat it increased to 2415 µmol g−1 d. wt. In spp. gussoneanum, leaf Cl− was, on average, 1·2-fold higher than for spp. marinum for plants in the aerated saline treatment (P < 0·05), and in combined stagnant plus saline treatment, leaf Cl− was 1·4-fold higher in spp. gussoneanum than in spp. marinum (P < 0·05).
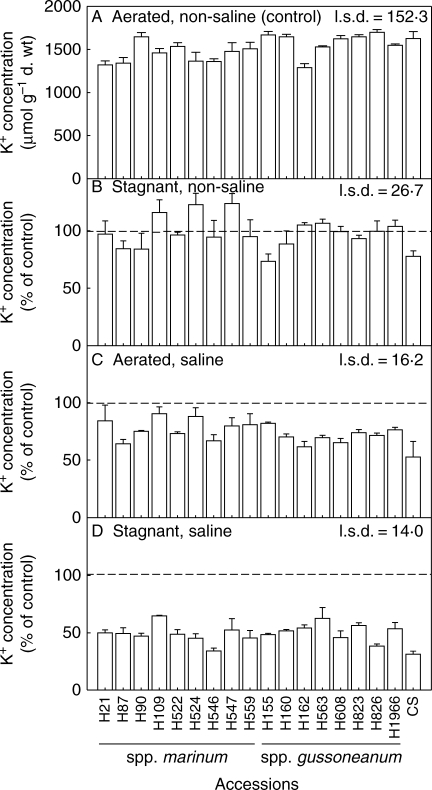
Leaf K+ concentration differed amongst the H. marinum accessions when in aerated non-saline solution (P < 0·001), ranging from 1299 to 1709 µmol g−1 d. wt (Fig. 3); in wheat it was 1637 µmol g−1 d. wt. Growth in the aerated saline solution decreased the K+ in all H. marinum accessions to 65–91 % of the values in controls, and in wheat to only 53 % of the control. In combined stagnant plus saline treatment, leaf K+ decreased further for H. marinum accessions, to be 34–64 % of the control values; and in wheat it was also decreased further to 31 % of the control (P < 0·001). There was no overall difference in K+ concentration between spp. marinum and spp. gussoneanum when in the saline treatments.
Fig. 3.
K+ concentration in the youngest fully expanded leaf for 17 accessions of Hordeum marinum and one wheat genotype (CS) when grown in (A) aerated non-saline nutrient solution (control). Responses to treatments are given as percentages of controls for: (B) deoxygenated stagnant agar, non-saline; (C) aerated 200 mol m−3 NaCl; and (D) deoxygenated stagnant agar plus 200 mol m−3 NaCl. Values are the means of three replicates ± s.e. The l.s.d. refers to the influence of accession in different treatments at the 5 % level. Triticum aestivum ‘Chinese Spring’ (CS) was not included in the statistical analysis. Experiment 1; accessions from the Nordic Gene Bank.
The treatment effects and differences amongst accessions in ion concentrations in the other bulked green leaves (data not shown) showed the same general trends as for those described above and shown in Figs 2 and 3 for the YFEL.
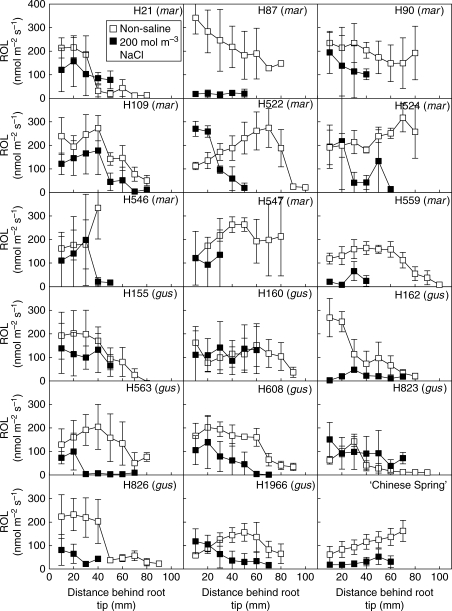
Patterns of ROL from roots
Adventitious roots of all H. marinum accessions, except H546, formed either a ‘tight’ or a ‘partial’ barrier to ROL when grown in stagnant non-saline solution (Fig. 4). The rates of ROL at 10 mm behind the root tip were up to 19-fold higher than the values at basal root zones (typically 70–90 mm behind the root tip). The result for H546, however, is uncertain as measurements could not be taken beyond 40 mm behind the tip, due to the presence of lateral roots; moreover, if small laterals were present at 40 mm this might also explain the apparent increase in ROL at this position. By contrast, with the H. marinum accessions, wheat showed a 2·6-fold higher ROL at the basal zones, compared with 10 mm behind the root tip, indicating the absence of (or only a ‘slight’) a barrier to ROL.
Fig. 4.
Rates of radial O2 loss (ROL) along adventitious roots for 17 accessions of Hordeum marinum and one wheat genotype (‘Chinese Spring’, CS), when in an O2-free medium with shoots in air. Plants were grown in stagnant deoxygenated nutrient solution that was either non-saline or contained 200 mol m−3 NaCl for the final 25–28 d. Measurements were taken in freshly prepared solutions of the same compositions as the growth medium, at 20 °C. Lengths of roots measured were 109 ± 3 mm (non-saline) and 89 ± 3 mm (200 mol m−3 NaCl). mar = H. marinum spp. marinum; gus = H. marinum spp. gussoneanum. Values are means ± s.e. of three replicates. Experiment 1; accessions from the Nordic Gene Bank.
For roots of plants grown in the stagnant plus saline treatment, compared with those in the non-saline stagnant treatment, ROL at 10 mm behind the root tip was generally lower in all accessions of H. marinum, and also in wheat; exceptions were accessions H522, H546 and H1966. Nevertheless, general patterns of ROL along the adventitious roots were similar for plants in stagnant non-saline and stagnant saline treatments; the exception again being H546, which did form a ‘tight’ barrier to ROL in basal regions when in the combined stagnant plus saline treatment (Fig. 4). Profiles of ROL measured for roots of plants from the stagnant plus saline treatment were often restricted by how far behind the tip the electrode could be positioned, due to formation of many long root hairs, or in some instances lateral roots, towards the basal zones of roots.
Experiment 2: accessions from the wheat belt of Western Australia
The 17 H. marinum accessions collected from Western Australia were used in this experiment. Whole-plant RGRs of these accessions when in aerated non-saline solution (0·14–0·17 g g−1 d−1) were similar to those of the accessions used in Experiment 1. All treatment effects followed the trends as described in Experiment 1 (data not shown). Measurements of ROL along the adventitious roots showed that accessions WA6, WA23 and WA11 formed a ‘tight’ barrier and all other accessions formed a ‘partial’ barrier to ROL, when in stagnant treatments (data not shown). For plants in non-saline stagnant solution, ROL at 10 mm behind the root tip was up to six-fold higher than at the basal root zones (70–90 mm behind the root tip). Roots of plants in stagnant plus saline solution, when compared with those in the stagnant non-saline treatment, had 48–98 % of ROL at 10 mm behind the tip (data not shown; exceptions: WA5, 1·5-fold higher and WA25, two-fold higher). As the leaf ion data for H. marinum when exposed to salinity, and particularly salinity in combination with a stagnant root zone, are of particular interest (see Introduction), these data are presented in Fig. 5.
Fig. 5.
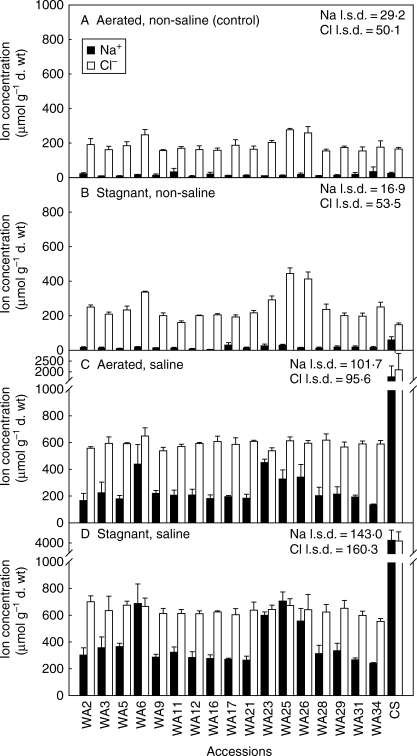
Na+ (closed bars) and Cl− (open bars) concentrations in the youngest fully expanded leaf for 17 accessions of Hordeum marinum (collected from Western Australia) and one wheat genotype, grown in nutrient solutions with treatments of: (A) aerated non-saline (control); (B) deoxygenated stagnant agar, non-saline; (C) aerated 200 mol m−3 NaCl; and (D) deoxygenated stagnant agar plus 200 mol m−3 NaCl. Values are the means of three replicates ± s.e. The l.s.d. refers to the influence of accession in different treatments at the 5 % level. Triticum aestivum ‘Chinese Spring’ (CS) was not included in the statistical analysis. Experiment 2; accessions from Western Australia.
When in aerated non-saline solution, Na+ in the YFEL was low and did not differ amongst the H. marinum accessions (Fig. 5). NaCl treatment increased the leaf Na+ in all H. marinum accessions (P < 0·001) and it increased even more in wheat. In aerated saline solution, leaf Na+ in the H. marinum accessions ranged from 142 to 457 µmol g−1 d. wt, whereas in wheat it was 1809 µmol g−1 d. wt. In combined stagnant plus saline treatment, leaf Na+ in the H. marinum accessions was 243–711 µmol g−1 d. wt, whereas in wheat it had increased to 4179 µmol g−1 d. wt.
In aerated non-saline solution, Cl− in the YFEL of the H. marinum accessions was, on average, 9·2-fold higher than Na+ concentrations; in wheat it was 5·7-fold higher (Fig. 5). The aerated saline treatment increased leaf Cl− in all the accessions of H. marinum (P < 0·001), with the range of concentrations being 544–625 µmol g−1 d. wt; by contrast, in wheat Cl− was 2132 µmol g−1 d. wt. The combined stagnant plus saline treatment had no significant effect on leaf Cl− in the H. marinum accessions (range 561–707 µmol g−1 d. wt), whereas leaf Cl− increased even further to 4146 µmol g−1 d. wt in wheat.
K+ concentrations in the YFEL differed amongst the H. marinum accessions when grown in aerated non-saline solution (P < 0·05); the range was 1466–1659 µmol g−1 d. wt (data not shown). In wheat, leaf K+ was 1737 µmol g−1 d. wt (data not shown). In the aerated saline solution, leaf K+ in the H. marinum accessions declined to 77–92 %, whereas in wheat it was 41 % of the control. In combined stagnant plus saline treatment, leaf K+ in the H. marinum accessions decreased to 41–62 % of the controls and in wheat it was only 23 % of the control (data not shown).
Relationships between growth and leaf ion concentrations amongst 34 H. marinum accessions, when in saline or in saline plus stagnant solutions (Experiments 1 and 2)
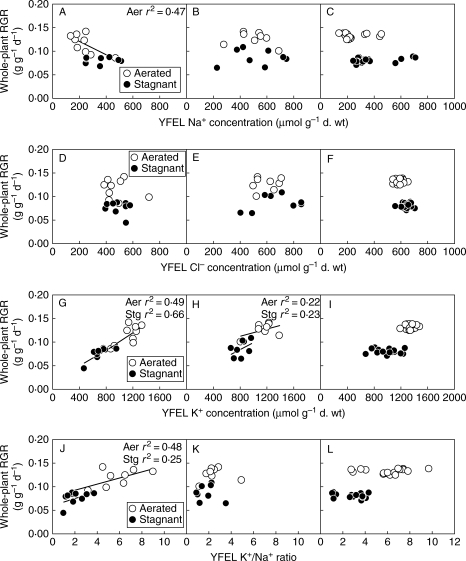
Using regression analyses, possible relationships were explored between plant growth in saline conditions and leaf concentrations of Na+, Cl−, K+ and K+/Na+ ratio, for all 34 accessions of H. marinum when in aerated or stagnant solutions (Fig. 6). Leaf Na+ concentration only showed a negative relationship (r2 = 0·47 in aerated solution) with growth in saline conditions for H. marinum spp. marinum (Fig. 6A); no such relationship was evident for H. marinum spp. gussoneanum (Fig. 6B) or for the H. marinum accessions from Western Australia (Fig. 6C). Leaf Cl− concentration did not show any significant relationship with growth (Fig. 6D–F). Leaf K+ concentration showed a positive relationship (r2 = 0·49 in aerated and 0·66 in stagnant solution) with growth in saline conditions for H. marinum spp. marinum (Fig. 6G), whereas these positive relationships were much weaker (r2 = 0·22 and 0·23) for H. marinum spp. gussoneanum (Fig. 6H) and absent for the H. marinum accessions from Western Australia (Fig. 6I). Consistent with the descriptions above, when leaf K+/Na+ ratio was considered, it showed a positive relationship (r2 = 0·48 in aerated and 0·25 in stagnant solution) with growth in saline conditions for H. marinum spp. marinum (Fig. 6J), but no such relationship was evident for H. marinum spp. gussoneanum (Fig. 6K) or for the H. marinum accessions from Western Australia (Fig. 6L). Relationships against growth as a percentage of non-saline controls were also evaluated (data not shown), but these had r2 values even lower than those shown in Fig. 6 for regressions using actual RGR in the various conditions.
Fig. 6.
Scatter plots exploring the relationships between whole plant relative growth rates (RGR) in saline conditions (aerated or stagnant) versus ion concentrations in the youngest fully expanded leaf (YFEL) of all 34 Hordeum marinum accessions Experiments 1 and 2; accessions from the Nordic Gene Bank and from Western Australia. The various panels show leaf Na+ (A–C), Cl− (D–F), K+ (G–I) and K+/Na+ ratio (J–L) of nine H. marinum spp. marinum (A, D, G, J), eight H. marinum spp. gussoneanum (B, E, H, K) (all from the Nordic Gene Bank) and 17 H. marinum accessions collected from Western Australia (C, F, I, L). Plants were grown in nutrient solution plus 200 mol m−3 NaCl in aerated or in deoxygenated stagnant 0·1 % agar. Linear regression lines and r2 values are presented for significant relationships; in all other cases r2 ≤ 0·15. Aer = aerated nutrient solution with 200 mol m−3 NaCl; Stg = stagnant 0·1 % agar nutrient solution with 200 mol m−3 NaCl. Data for the accessions from the Nordic Gene Bank were from Figs 1–3. Data for accessions from Western Australia were from Fig. 5 (Na+ and Cl−) or not previously shown (RGR and K+).
DISCUSSION
Saline regions are often also prone to transient waterlogging, so that tolerance of these stresses combined is essential for plants on these areas (Barrett-Lennard, 2003). For several crops, including wheat (e.g. Barrett-Lennard, 1986), waterlogging together with salinity decreases the capacity for ‘exclusion’ of Na+ and Cl− from shoots and growth is severely reduced (Barrett-Lennard, 2003), but some halophytes can tolerate combined waterlogging and salinity (Colmer and Flowers, 2008). The present experiments confirmed the sensitivity of wheat (‘Chinese Spring’) to root-zone O2 deficiency plus salinity, and identified several H. marinum accessions as being relatively tolerant of these stresses combined (Fig. 1). Nevertheless, growth of H. marinum was, as expected, still significantly reduced by 200 mol m−3 NaCl in stagnant solution; the most tolerant accession had an RGR in the combined stresses of 61 % of the control. Of particular significance, however, was that H. marinum, unlike wheat, was able to maintain low leaf Na+ and Cl− concentrations when in the stagnant plus saline treatment, so that on average, concentrations in H. marinum were only 25 % of those in wheat (Figs 2 and 5). Several of the H. marinum accessions also suffered less of a decline in leaf K+ concentration, compared with wheat (Fig. 3). The smaller additional impact of the stagnant plus saline treatment, compared with saline alone (i.e. aerated), on growth and leaf ion concentrations in H. marinum, compared with wheat, is consistent with H. marinum being more waterlogging (McDonald et al., 2001; Garthwaite et al., 2003) and salt (Garthwaite et al., 2005; Islam et al., 2007) tolerant than wheat. The present study confirmed these reputations for tolerance to the individual stresses, and extended this knowledge by demonstrating tolerance of H. marinum to combined stagnant plus saline conditions.
Salinity tolerance in non-halophytes is generally associated with an ability to ‘exclude’ Na+ and Cl− from leaves, and to tolerate the ions that eventually accumulate (Greenway and Munns, 1980; Tester and Davenport, 2003; Munns, 2005; Colmer et al., 2005; Munns and Tester, 2008). Exposure to salinity and root-zone O2 deficiency can result in substantial increases in shoot Na+ and Cl− concentrations above the levels in aerated saline conditions, for many plant species (Barrett-Lennard, 2003), whereas in some halophytes such increases did not occur (Colmer and Flowers, 2008). In waterlogging-sensitive plants, such as wheat, root hypoxia probably impedes respiration and the energy deficit would reduce the capacity for ion transport across cell membranes (cf. Greenway and Gibbs, 2003), so that control of net Na+ uptake is compromised in hypoxic plus saline conditions (Barrett-Lennard, 2003). Previous studies on wheat, with NaCl at 60–150 mol m−3 (7- to 42-d treatments), have shown that when root-zone hypoxia was also imposed, shoot Na+ and Cl− increased 1·4- up to 7-fold, compared with plants in aerated saline treatments (Barrett-Lennard, 1986; Akhter et al., 1994; Barrett-Lennard et al., 1999; Saqib et al., 2005). In the present study, the 1·3-fold increase in leaf Na+ concentration for wheat in 200 mol m−3 NaCl in stagnant, rather than aerated, solution, was at the lower end of the range of increases (namely up to 7-fold) described previously for wheat (preceding sentence). However, in our experiments, wheat in the aerated 200 mol m−3 NaCl treatment already contained high levels of leaf Na+ and Cl−, so that the scope was small for further increases in ion concentrations when the stagnant conditions were also imposed. This is similar to other sensitive species; for example, increases in Na+ uptake by maize resulting from root-zone O2 deficiency were more at moderate (e.g. 12·5-fold at 100 mol m−3) than at high (e.g. 1·5-fold at 200 mol m−3) external NaCl (Drew et al., 1988). Nevertheless, when in saline plus stagnant solution leaf Na+ and Cl− concentrations in wheat were, respectively, 3·1–9-fold higher and 2·8–6-fold higher, compared with H. marinum, demonstrating the relatively poor Na+ and Cl− ‘exclusion’ in wheat when in combined salinity and root-zone hypoxia.
The ability to exclude Na+ and Cl− from shoots, for wheat in a hypoxic and saline root zone, has been suggested to be related to the capacity to form aerenchyma in roots. For example, in two wheat genotypes, leaf Na+ increased 1·4-fold for SARC-6 and 1·8-fold for MH-97 in hypoxic saline compared with aerated saline treatment, and adventitious roots of SARC-6 contained up to eight-fold higher aerenchyma (Saqib et al., 2005). For H. marinum in the stagnant plus saline treatment, leaf Na+ only showed modest increases (average 1·2-fold), and leaf Cl− hardly increased (average 1·07-fold), as compared with levels in plants in the aerated saline treatment (Figs 2 and 5). Waterlogging tolerance in H. marinum is, at least partially, associated with development of adventitious roots containing aerenchyma and a barrier to ROL (present study; McDonald et al., 2001; Garthwaite et al., 2003). By contrast, wheat tends to produce fewer adventitious roots, with slightly less aerenchyma, but also lacking a barrier to ROL (present study; McDonald et al., 2001). Amounts of aerenchyma, alone, however, clearly did not determine the capacity for maintaining regulation of leaf Na+ (and Cl−) in an O2-deficient rooting medium in the present study. Several H. marinum accessions had similar root porosity to that in ‘Chinese Spring’ wheat (Table 1), but had much lower leaf Na+ and Cl− concentrations (Fig. 2). Moreover, no correlations were found between root porosity and leaf Na+ or Cl− concentrations, or with growth, of the H. marinum accessions, when in the stagnant plus saline treatment (data not shown). Possible reasons for poor correlations of root porosity even with waterlogging tolerance alone have been discussed by Garthwaite et al. (2003). In addition to forming aerenchyma, H. marinum forms a barrier to ROL in basal root zones, a feature absent in wheat (Fig. 4). In roots of H. marinum the barrier is formed by putative suberin deposits in cell walls in the hypodermal layer (Garthwaite et al., 2008); suberin deposits were also associated with barrier formation in roots of Phragmites australis (Soukup et al., 2007). Future research should determine whether the barrier to ROL assists regulation of ion ‘exclusion’ in roots of H. marinum and other wetland species. The barrier to ROL has O2-conserving benefits so that root aeration is improved (Armstrong, 1979; Colmer, 2003), and an improved O2 status in distal root parts would be expected to benefit energy status and hence ion regulation (cf. Barrett-Lennard, 2003). Moreover, the barrier to ROL is an anatomical feature considered to block the apoplastic pathway (e.g. rice: Ranathunge et al., 2003, 2004; and P. australis: Soukup et al., 2007), and thus the barrier might also benefit regulation of ion uptake via a possible influence on radial movement of water and ions in the apoplast of roots.
Together with Na+ (and Cl−) ‘exclusion’, higher tissue K+/Na+ ratio also contributes to salt tolerance in the Triticeae (Gorham, 1993; Dvorak et al., 1994; Garthwaite et al., 2005; Chen et al., 2007). Leaf K+/Na+ in the H. marinum accessions was, on average, 1·1–6·5 in aerated saline treatment and 0·9–3·7 in stagnant plus saline treatment (calculated from Figs 2 and 3). In wheat, leaf K+/Na+ was 0·5 in aerated saline treatment and only 0·2 in stagnant plus saline treatment. Using regression analyses, possible relationships were explored between plant growth in saline conditions and leaf concentrations of Na+, Cl−, K+ and K+/Na+ ratio, for all 34 accessions of H. marinum when in aerated or stagnant solutions (Fig. 6). Although relationships have been described between salt tolerance and leaf Na+ concentrations (e.g. durum wheat; Munns and James, 2003) or leaf K+/Na+ (e.g. barley, presented as Na+/K+; Chen et al., 2007), a lack of such relationships has also been noted (e.g., leaf Na+ in bread wheat; Genc et al., 2007). The present study shows that relationships between leaf ions and salt tolerance can also differ between subspecies; leaf Na+ concentration showed a negative relationship (r2 = 0·47 in aerated solution) with growth in saline conditions for H. marinum spp. marinum (Fig. 6A), but no such relationship was evident for H. marinum spp. gussoneanum (Fig. 6B) or for the H. marinum accessions from Western Australia (Fig. 6C). The lack of simple relationships between one (or few) trait(s) and salt tolerance is consistent with the view that several traits contribute to tolerance (e.g. for wheat: Colmer et al., 2005; Munns and Tester, 2008). Moreover, Genc et al. (2007) concluded for bread wheat that genotypes differ not only in capacity for ion ‘exclusion’, but presumably also differed for ‘tissue tolerance’ to the amount of ions that can accumulate in leaves before these are damaged; such genotypic differences probably also occur in H. marinum.
In summary, this study of 34 accessions of H. marinum has identified accessions within this species as being relatively tolerant to combined saline plus stagnant root zones. Compared with wheat, H. marinum is better able to regulate leaf Na+ and Cl− concentrations when in saline conditions, even when the root zone is also stagnant (Figs 2 and 5). There was, however, significant variation amongst the H. marinum accessions in leaf Na+ and Cl−, both in aerated and in stagnant saline solutions (Figs 2 and 5). On average, spp. marinum tended to maintain lower leaf Na+ and Cl− than spp. gussoneanum, although the best accessions from the two subspecies did not differ (Fig. 2). As noted by Garthwaite et al. (2005), Na+ ‘exclusion’ by H. marinum appears to be superior to that by other salt-tolerant ‘wild’ species in the Triticeae, such as Thinopyrum elongatum (Greenway and Rogers, 1963) and Thinopyrum bessarabicum (Gorham et al., 1986). This superior capacity for Na+ ‘exclusion’, as well as good Cl− ‘exclusion’, maintained even at high salinity (Garthwaite et al., 2005) and also when in O2-deficient conditions (Figs 2 and 5), makes H. marinum a candidate for use in wide hybridizations with wheat to develop a cereal for salt-affected land (Colmer et al., 2005, 2006; Islam et al., 2007).
ACKNOWLEDGEMENTS
We thank R. von Bothmer (Swedish Agricultural University, Alnarp) for providing accessions of H. marinum from the Nordic Seed Bank; A. K. M. R. Islam for kindly growing the seeds in quarantine and checking the ploidy of these and of accessions from Western Australia; K. A. Shepherd for collecting accessions in Western Australia; K. Frost for help during harvests; N. L. Teakle for comments on a draft of the manuscript; and the Grains Research and Development Corporation (GRDC) of Australia, via the CRC for Plant-based Management of Dryland Salinity (now Future Farm Industries CRC), for supporting this research.
LITERATURE CITED
- Akhtar J, Gorham J, Qureshi RH. Combined effect of salinity and hypoxia in wheat (Triticum aestivum L.) and wheat-Thinopyrum amphiploids. Plant and Soil. 1994;166:47–54. [Google Scholar]
- Armstrong W. Aeration in higher plants. Advances in Botanical Research. 1979;7:226–332. [Google Scholar]
- Armstrong W. Polarographic oxygen electrodes and their use in plant aeration studies. Proceedings of the Royal Society of Edinburgh. 1994;102B:511–527. [Google Scholar]
- Armstrong W, Wright EJ. Radial oxygen loss from roots: the theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiologia Plantarum. 1975;35:21–26. [Google Scholar]
- Barrett-Lennard EG. Effect of waterlogging on the growth and NaCl uptake by vascular plants under saline conditions. Reclamation and Revegetation Research. 1986;5:245–261. [Google Scholar]
- Barrett-Lennard EG. The interaction between waterlogging and salinity in higher plants: causes, consequences and implications. Plant and Soil. 2003;253:35–54. [Google Scholar]
- Barrett-Lennard EG, Ratingen P, van Mathie MH. The developing pattern of damage in wheat (Triticum aestivum L.) due to the combined stresses of salinity and hypoxia: experiments under controlled conditions suggest a methodology for plant selection. Australian Journal of Agricultural Research. 1999;50:129–136. [Google Scholar]
- von Bothmer R, Jacobsen N, Baden C, Jorgensen RB, Linde-Laursen I. Systematic and ecogeographic studies on crop genepools. 2nd edn. Vol. 7. Rome: International Plant Genetic Resources Institute; 1995. An ecogeographical study of the genus Hordeum. [Google Scholar]
- Chen Z, Zhou M, Newman IA, Mendham NJ, Zhang G, Shabala S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Functional Plant Biology. 2007;34:150–162. doi: 10.1071/FP06237. [DOI] [PubMed] [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment. 2003;26:17–36. [Google Scholar]
- Colmer TD, Flowers TJ. Flooding tolerance in halophytes. New Phytologist. 2008;179:964–974. doi: 10.1111/j.1469-8137.2008.02483.x. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Munns R, Flowers TJ. Improving salt tolerance of wheat and barley: future prospects. Australian Journal of Experimental Agriculture. 2005;45:1425–1443. [Google Scholar]
- Colmer TD, Flowers TJ, Munns R. Use of wild relatives to improve salt tolerance in wheat. Journal of Experimental Botany. 2006;57:1059–1078. doi: 10.1093/jxb/erj124. [DOI] [PubMed] [Google Scholar]
- Cramer GR. Sodium–calcium interactions under salinity stress. In: Lauchli A, Luttge U, editors. Salinity: environment – plants – molecules. Dordrecht: Kluwer Academic Publishers; 2002. pp. 205–228. [Google Scholar]
- Drew MC, Guenther J, Lauchli A. The combined effects of salinity and root anoxia on growth and net Na+ and K+-accumulation in Zea mays grown in solution culture. Annals of Botany. 1988;61:41–53. [Google Scholar]
- Dvorak J, Noaman MM, Goyal S, Gorham J. Enhancement of the salt tolerance of Triticum turgidum L. by the Kna1 locus transferred from the Triticum aestivum L. chromosome 4D by homoeologous recombination. Theoretical and Applied Genetics. 1994;87:872–877. doi: 10.1007/BF00221141. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Dalmond D. Protein synthesis in halophytes: the influence of potassium, sodium and magnesium in vitro. Plant and Soil. 1992;146:153–161. [Google Scholar]
- Garthwaite AJ, von Bothmer R, Colmer TD. Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Functional Plant Biology. 2003;30:875–889. doi: 10.1071/FP03058. [DOI] [PubMed] [Google Scholar]
- Garthwaite AJ, von Bothmer R, Colmer TD. Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl− into the shoots. Journal of Experimental Botany. 2005;56:2365–2378. doi: 10.1093/jxb/eri229. [DOI] [PubMed] [Google Scholar]
- Garthwaite AJ, Armstrong W, Colmer TD. Assessment of the O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytologist. 2008;179:405–416. doi: 10.1111/j.1469-8137.2008.02467.x. [DOI] [PubMed] [Google Scholar]
- Genc Y, McDonald GK, Tester M. Re-assessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant, Cell and Environment. 2007;30:1486–1498. doi: 10.1111/j.1365-3040.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology. 2003;30:1–47. doi: 10.1071/PP98095. [DOI] [PubMed] [Google Scholar]
- Gorham J. Genetics and physiology of enhanced K/Na discrimination. In: Randall PJ, editor. The Fourth International Symposium on genetic aspects of plant mineral nutrition. Canberra: Kluwer Academic Publishers; 1993. pp. 151–158. [Google Scholar]
- Gorham J, Forster BP, Budrewicz E, Wyn Jones RG, Miller TE, Law CN. Salt tolerance in the Triticeae: solute accumulation and distribution in an amphiploid derived from Triticum aestivum cv. Chinese Spring and Thinopyrum bessarabicum. Journal of Experimental Botany. 1986;37:1435–1449. [Google Scholar]
- Greenway H, Gibbs J. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology. 2003;30:999–1036. doi: 10.1071/PP98096. [DOI] [PubMed] [Google Scholar]
- Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annual Review of Plant Physiology. 1980;31:149–190. [Google Scholar]
- Greenway H, Rogers A. Growth and ion uptake of Agropyron elongatum on saline substrates, as compared with a salt-tolerant variety of Hordeum vulgare. Plant and Soil. 1963;18:21–30. [Google Scholar]
- Haun JR. Visual quantification of wheat development. Agronomy Journal. 1973;65:116–119. [Google Scholar]
- Hunt R. Plant growth analysis. London: Edward Arnold Limited; 1978. [Google Scholar]
- Islam S, Malik AI, Islam AKMR, Colmer TD. Salt tolerance in a Hordeum marinum–Triticum aestivum amphiploid, and its parents. Journal of Experimental Botany. 2007;58:1219–1229. doi: 10.1093/jxb/erl293. [DOI] [PubMed] [Google Scholar]
- Jackson MB, Drew MC. Effects of flooding on growth and metabolism of herbaceous plants. In: Kozlowski TT, editor. Flooding and plant growth. New York: Academic Press; 1984. pp. 47–128. [Google Scholar]
- Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany. 1999;84:123–133. [Google Scholar]
- McDonald MP, Galwey NW, Colmer TD. Waterlogging tolerance in the tribe Triticeae: the adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant, Cell and Environment. 2001;24:585–596. [Google Scholar]
- Munns R. Genes and salt tolerance: bringing them together. New Phytologist. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Munns R, James RA. Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant and Soil. 2003;253:201–218. [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Ponnamperuma FN. Effect of flooding on soils. In: Kozlowski TT, editor. Flooding and plant growth. New York: Academic Press; 1984. pp. 9–45. [Google Scholar]
- Ranathunge K, Steudle E, Lafitte R. Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta. 2003;217:193–205. doi: 10.1007/s00425-003-0984-9. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Kotula L, Steudle E, Lafitte R. Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. Journal of Experimental Botany. 2004;55:433–447. doi: 10.1093/jxb/erh041. [DOI] [PubMed] [Google Scholar]
- Raskin I. A method for measuring leaf volume, density, thickness, and internal gas volume. HortScience. 1983;18:698–699. [Google Scholar]
- Rubinigg M, Stulen I, Elzenga JTM, Colmer TD. Spatial patterns of radial oxygen loss and nitrate net flux along adventitious roots of rice raised in aerated or stagnant solution. Functional Plant Biology. 2002;29:1475–1481. doi: 10.1071/FP02081. [DOI] [PubMed] [Google Scholar]
- Saqib M, Akhtar J, Qureshi RH. Na+ exclusion and salt resistance of wheat (Triticum aestivum) in saline-waterlogged conditions are improved by the development of adventitious nodal roots and cortical root aerenchyma. Plant Science. 2005;169:125–130. [Google Scholar]
- Setter TL, Waters I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant and Soil. 2003;253:1–34. [Google Scholar]
- Soukup A, Armstrong W, Schreiber L, Rochus F, Votrubová O. Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytologist. 2007;173:264–278. doi: 10.1111/j.1469-8137.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- Szabolcs I. Soils and salinization. In: Pessarakali M, editor. Handbook of plant and crop stress. New York: Marcel Dekker; 1994. pp. 3–11. [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CJ, Armstrong W, Waters I, Greenway H. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant, Cell and Environment. 1990;13:395–403. [Google Scholar]
- Trought MCT, Drew MC. The development of waterlogging damage in wheat seedlings (Triticum aestivum L.) 1. Shoot and root growth in relation to the changes in the concentrations of dissolved gases and solutes in the soil solution. Plant and Soil. 1980;54:77–94. [Google Scholar]
- Wiengweera A, Greenway H. Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. Journal of Experimental Botany. 2004;55:2121–2129. doi: 10.1093/jxb/erh232. [DOI] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H, Thomson CJ. The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany. 1997;80:115–123. [Google Scholar]