Abstract
Background and Aims
Flooding slows seed germination, imposes fatalities and delays seedling establishment in direct-seeded rice. This study describes responses of contrasting rice genotypes subjected to flooding or low oxygen stress during germination and discusses the basis of tolerance shown by certain cultivars.
Methods
In one set of experiments, dry seeds were sown in soil and either watered normally or flooded with 10 cm of water. Seedling survival and shoot and root growth were assessed and seed portions of germinating seedlings were assayed for soluble sugars and starch concentrations. The whole germinating seedlings were assayed for amylase and peroxidase activities and for ethylene production. Activities of enzymes associated with anaerobic respiration were examined and gene expression was analysed separately with seeds germinating under different amounts of dissolved oxygen in dilute agar.
Key Results
Flooding during germination reduced survival but to a lesser extent in tolerant genotypes. Starch concentration in germinating seeds decreased while sugar concentration increased under flooding, but more so in tolerant genotypes. Amylase activity correlated positively with elongation (r = 0·85 for shoot and 0·83 for root length) and with plant survival (r = 0·92). Tolerant genotypes had higher amylase activity and higher RAmy3D gene expression. Ethylene was not detected in seeds within 2 d after sowing, but increased thereafter, with a greater increase in tolerant genotypes starting 3 d after sowing. Peroxidase activity was higher in germinating seeds of sensitive genotypes and correlated negatively with survival.
Conclusions
Under low oxygen stress, tolerant genotypes germinate, grow faster and more seedlings survive. They maintain their ability to use stored starch reserves through higher amylase activity and anaerobic respiration, have higher rates of ethylene production and lower peroxidase activity as germinating seeds and as seedlings. Relevance of these traits to tolerance of flooding during germination and early growth is discussed.
Key words: Amylase, anoxia, crop establishment, direct-seeded rice, ethylene, flooding, germination, hypoxia, Oryza sativa
INTRODUCTION
Direct seeding is increasingly being practiced by rice farmers under both rainfed and irrigated conditions. The trend is the outcome of a scarcity of labour required for transplanting, simplicity and additional benefits associated with direct seeding (Sasaki, 1974). However, poor crop establishment remains a major obstacle facing large-scale adoption, particularly in areas prone to flooding after sowing or where fields are not levelled. However, flooding the soil after direct seeding can have a benefit by improving weed control, a major constraint in direct-seeded systems (Tuong et al., 2000). Enhanced tolerance of flooding during germination and early seedling growth could therefore help improve crop establishment and promote more widespread adoption of direct seeding. Breeding for better seed and seedling vigour and higher tolerance has been attempted previously (Yamauchi et al., 1993; Redoña and Mackill, 1996; Biswas and Yamauchi, 1997), but with limited success because highly tolerant donors were unavailable and knowledge of the physiological basis of tolerance was inadequate (Seshu et al., 1988; Ling et al., 2004).
Strong seed germination and seedling growth under flooded conditions necessitates above-normal tolerance of the low-oxygen conditions usually experienced during flooding. Seeds with carbohydrate reserves are generally more tolerant of hypoxia (low O2) or even anoxia (absence of O2) than seeds with fatty acid reserves (Al-Ani et al., 1985; Raymond et al., 1985). Among cereal seeds studied to date, only rice can germinate and extend its coleoptile under anoxia (Taylor, 1942; Ella and Setter, 1999). However, this ability is limited to shoot elongation and associated with a failure to develop further (Biswas and Yamauchi, 1997). Moreover, it was reported that anaerobically germinating rice seed has the complete set of enzymes needed for the degradation of starch and is able to utilize the breakdown products. Among four cereal crops, only rice was found to express and translate α-amylase mRNA under anoxia (Perata et al., 1992, 1993). Amylases are believed to play a major role in starch breakdown in cereal seeds (Murata et al., 1968) and some reports have indicated that rice seeds are capable of degrading starch during germination under anoxic conditions (Atwell and Greenway, 1987) into readily fermentable carbohydrates to generate energy required for growth of the germinating embryos. Starch is a major energy source for developing rice embryos and numerous studies have established the importance of α-amylases in starch degradation when O2 is limiting (Guglielminetti et al., 1995; Perata et al., 1997; Hwang et al., 1999). Amylase induction seems to be controlled by plant hormones or glucose starvation (Umemura et al., 1998; Loreti et al., 2003) and temporal and spatial patterns in amylase type specificity were reported by Kaneko et al. (2002). Several studies investigated the process of starch breakdown under aerobic conditions (Beck and Zeigler, 1989; Fincher, 1989), yet the importance of activities and levels of expression of starch-degrading enzymes under low oxygen stress has not been sufficiently established.
Under anoxia, carbohydrate catabolism shifts from aerobic to anaerobic pathways (alcoholic fermentation) for generating ATP for growth and maintenance processes. However, ATP production is 18-fold less efficient in this process than in aerobic respiration (Greenway and Setter, 1996). Nevertheless, an increased rate of anaerobic respiration still remains one of the most important mechanisms to alleviate the adverse effects of reduced ATP supply during oxygen deficiency, and several reports demonstrated its importance in plants (Avadhani et al., 1978; Jackson et al., 1982; Waters et al., 1991; Setter and Ella, 1994; Gibbs et al., 2000).
The plant hormone ethylene promotes rice seedling growth under flooded conditions and this flooding-induced elongation is one of the escape mechanisms that help submerged plants regain contact with air. This elongation is beneficial in early growth as elongation of coleoptiles (Ku et al., 1970) and mesocotyl (Suge, 1971), but a disadvantage for seedlings if flooding duration is short because of the tendency of elongating plants to lodge after the water recedes (Ella et al., 2003; Das et al., 2005). In rice, the elongation-promoting effect of ethylene is not limited to the coleoptile and mesocotyl of young seedlings and internodes of older plants, but also leaves (Jackson et al., 1987) and roots (Konings and Jackson, 1979). Several studies showed the importance of ethylene interactions with other plant hormones like abscisic acid, auxins, and gibberellins (GA) under low oxygen stress. However, the effects of these plant hormones are unpredictable because of various environmental factors, including O2 and CO2 levels, and temperature and flooding depth. In the absence of oxygen, ethylene, auxins and GA are typically inactive in rice (Raskin and Kende, 1984; Pegoraro et al., 1988); however, auxins can stimulate coleoptile elongation under aerobic conditions (Pegoraro et al., 1988). Carbon dioxide that accumulates in plant tissue in the absence of photosynthetic carbon fixation can also have promoting effects on elongation (Raskin and Kende, 1984; Suge and Kusanagi, 1975). Apparently, both synergistic and counteracting plant hormone interactions can be found in several growth systems of plants (Davies, 1995). A notable case of synergistic interaction is internode elongation in deepwater rice, in which ethylene promotes growth of internodes, causing rapid elongation in response to flooding (Suge, 1974; Metraux and Kende, 1983). Ethylene increased the responsiveness of the internode tissue to GA, and appears to do so by causing a reduction in the endogenous levels of ABA, a counteracting interaction because ABA is a potent antagonist of GA. Here, the extent of growth rate is determined by the relative levels of endogenous GA and ABA (Hoffmann-Benning and Kende, 1992). Moreover, ethylene was reported to enhance sucrose transport from the scutellum to the growing coleoptile in germinating rice seeds, in which sucrose is cleaved into glucose and fructose (Ishizawa and Esashi, 1988).
Rapid cell expansion is necessary for fast growth under flooding and low-oxygen stress. One of the enzymes observed to regulate cell elongation is peroxidase (EC 1.11.1), which is responsible for the assembly of lignins and proteins in the cell wall (Whitmore, 1978) and for the binding of ferulic acid to cell walls by the formation of diferuloyl cross-links to matrix polysaccharides (Fry, 1979). These activities subsequently reduce wall extensibility thereby restricting cell elongation. Higher peroxidase activities are closely associated with reduced expansion growth in plants such as mung bean (Goldberg et al., 1987) and peanut (Zheng and van Huystee, 1992). In their study on the effect of ethylene and ethylene inhibitors, Lee and Lin (1996) reported that ethylene enhanced coleoptile elongation but decreased peroxidase activities, especially the wall-bound forms, and the introduction of an ethylene action inhibitor blocked both ethylene-mediated coleoptile elongation and the decline in peroxidase activity. They suggested that the activity of peroxidases, especially the cell wall-bound form, is inversely related to the rate of elongation by rice coleoptiles.
Expansins are another group of proteins known to regulate cell wall expansion. These non-enzymic proteins are encoded by a large gene superfamily with at least two major divisions: α-expansins and β-expansins. Their role in cell extensibility has been reported in many crops, including rice (Huang et al., 2000). Transcript expression patterns of Os-EXP1, Os-EXP2, Os-EXP3 and Os-EXP4 genes were often highly correlated with elongation (Cho and Kende, 1997; Huang et al., 2000). Transcript profiling of anoxia-grown rice seedlings revealed that EXPA7 and EXPB12 are likely to be involved in rice coleoptile elongation under anoxia (Lasanthi-Kudahettige et al., 2007). Together with peroxidase, these proteins could be involved in regulating cell-wall growth and elongation during germination under low-oxygen stress.
Germination of rice seeds in paddy fields that are either waterlogged (Drew, 1990) or submerged (Setter et al., 1988) is one instance in which plant tissues are exposed to hypoxia or even anoxia. Evidence for genetic variation in rice responses to direct seeding into anoxic soils has been demonstrated before by Yamauchi et al. (1993), and also through recent screening work (A. M. Ismail et al., unpubl. res.). Traits associated with coleoptile elongation of pre-germinated seeds under anoxia has been investigated before (Setter et al., 1994); however, the bases of tolerance of low-oxygen stress during germination of rice seeds are not yet sufficiently understood. This study attempts to elucidate the physiological traits associated with tolerance of flooding during germination and early seedling growth using a set of genotypes previously identified as being highly tolerant of flooding during germination.
MATERIALS AND METHODS
Plant materials and experimental set-up
Rice genotypes contrasting in their tolerance of anaerobic conditions during germination were selected from a previous screening, and were evaluated in six different experiments. The first three experiments were conducted in a greenhouse, whereas the second three were conducted under laboratory conditions with temperature maintained at about 26 °C. In the greenhouse experiments, 25 grains of each genotype were dry seeded at about 1-cm depth in pots containing 4 L of finely ground field soil, followed by either normal watering (control) or flooding with about 10 cm of tap water until the end of each experiment. All greenhouse trials were conducted using a randomized complete block design with four replicates of pots each containing 25 seedlings.
In expt I, five tolerant (‘Khao Hlan On’, ‘Khaiyan’, ‘Kalonchi’, ‘Nanhi’ and ‘Cody’) and five intolerant (‘FR13A’, ‘IR22’, ‘IR28’, ‘IR29’ and ‘IR42’) genotypes were used for growth and survival measurements. Lengths of the longest shoot and root were measured in seedlings grown under either a control or in flooded soil, and plant survival was calculated based on the number of seedlings that emerged from floodwater. All measurements were made 21 d after sowing. The number of seedlings emerging from the water surface was recorded and survival was calculated as the percentage of surviving seedlings relative to the number of seeds used.
In expt II, four genotypes were used: two tolerant (‘Khaiyan’ and ‘Khao Hlan On’) and two intolerant (‘FR13A’ and ‘IR42’). This experiment monitored root and shoot growth, non-structural carbohydrate concentrations (starch and soluble sugars) in the remaining seed portion of the germinating seeds, and total amylase activity in the entire germinating seeds and seedlings. A sufficient number of pots was used within each replicate to provide adequate plant material for daily sampling during the first 8 d following the start of the experiment, during which period, germinating seeds and seedlings were kept completely submerged. Survival was determined 21 d after sowing as in expt I. In expt III, two genotypes were used, ‘Khao Hlan On’ and ‘IR42’. This experiment determined ethylene production by germinating seeds and seedlings during the first 9 d following seeding, under both flooded soil conditions, where seedlings were kept completely flooded until measurements were completed, or under control (aerated) soil conditions. In expt IV, peroxidase activity was determined in the entire germinating seeds of the genotypes used in expt II and with four biological replicates of each genotype. Interference was observed when samples were analysed from soil-grown seedlings, so this experiment was conducted in flasks containing sterile deoxygenated stagnant agar solution bubbled with N2 gas until the desired O2 level of hypoxic condition in 1-cm depth was attained (about 0·01 mol O2 m−3), and this level was maintained until measurements were completed. Fifty seeds were used per 75 mL of 1 % agar solution and shoot length was measured in seedlings grown in flasks to test its association with peroxidase activity.
Experiment V was conducted to examine the pattern of gene expression of three amylase and two sucrose synthase genes in germinating seeds under different levels of oxygen concentration. Seeds of the tolerant ‘Khaiyan’ and intolerant ‘IR42’ genotypes were surface-sterilized in 10 % sodium hypochlorite solution for 10 min, washed twice with sterile distilled water, and blotted dry on sterile filter paper prior to the start of the experiment. The three levels of O2 regime consisted of (1) aerated (0·25 mol O2 m−3), in which seeds were sown on wetted filter paper in Petri dishes; (2) hypoxic (0·03 mol O2 m−3), in which seeds were incubated in 0·1 % sterile agar solution in flasks and bubbled with N2 gas until the desired O2 levels were attained; and (3) anoxic (<0·003 mol O2 m−3), in which the 0·1 % sterile agar solution was continuously bubbled with N2 gas. Oxygen in the agar solution was monitored using a dissolved oxygen meter (YSI Environmental Meters model D0200, China). All treatments were conducted under darkness using four independent biological replicates of Petri dishes (aerated) or flasks (hypoxic and anoxic treatments) per genotype. Tissue sampling for RNA extraction was done at 0, 12, 24, 48 and 72 h after the start of seed incubation. The last trial (expt VI) was conducted to monitor the enzyme activity and gene expression patterns of alcohol dehydrogenase (ADH; alcohol : NAD+ oxidoreductase; EC 1.1.1) and pyruvate decarboxylase (PDC; EC 4.1.1.1). This experiment was conducted using the same genotypes and conditions as in expt V, with germinating seeds sampled at the same intervals for enzyme activities in air and under hypoxia, and for gene expression analyses under hypoxia. Gene expression under aerated and anoxic conditions was determined on samples collected 24, 48 and 72 h after the start of the experiment. In all cases, germinated seeds and seedlings were kept under their respective conditions until measurements were completed.
Carbohydrate analyses
Germinating seeds were harvested daily after dry seeding, and the remaining seed portion was separated and frozen in liquid N2, freeze-dried and weighed to obtain their dry weights. The dried samples were extracted in 80 % ethanol (v/v) and used for soluble carbohydrate analysis with anthrone reagent (Fales, 1951). The residue was then washed several times and used for starch analysis following the method of Setter et al. (1989). Briefly, starch was solubilized in boiling water for 3 h with further hydrolysis using amyloglucosidase (Sigma Chemicals, St Louis, MO, USA) and subsequently analysed for free sugars using glucose oxidase (Sigma Chemicals, Missouri, USA) as described by Kunst et al. (1988).
Amylase activity assay
Total amylase activity was measured following the method of Bernfeld (1955). Seedlings were harvested for enzyme extraction using 0·02 m NaHxPO4 buffer at pH 6·9 with 0·006 m NaCl and the crude extract was used in the assay. Starch was first converted to maltose as catalysed by amylase. The maltose produced was made to react with 3, 5-dinitrosalicylic acid, forming a coloured product with maximum absorption at 540 nm when reduced. The absorption values were read on a standard curve established with increasing amounts of maltose. Total protein concentration was determined following the Bradford method (Bradford, 1976), and the activity expressed in units per milligram protein. One unit of amylase activity is defined as μmoles maltose produced per minute and specific activity is expressed in terms of units per mg protein.
Ethylene production by germinating seeds and seedlings
Ethylene concentration was measured in dry seeds and in seedlings harvested after 3, 4, 6, 7 and 9 d after sowing in soil. Samples were placed in Erlenmeyer flasks and covered with a ‘Suba-seal’ rubber puncture cap for 2 h before the gas in the headspace was sampled for ethylene. The volume of the gaseous headspace above the seedlings was determined by filling the flask with water, and was taken to be the total volume of gaseous atmosphere containing ethylene produced by the seeds. Ethylene concentration was measured using a gas chromatograph (Hewlett Packard 5890 Series II Plus) isothermally at 30 °C oven temperature, 8·3 psi (0·6 mL min−1 helium carrier gas as mobile phase), equipped with an SPB-5 column (manufactured by Supelco, Bellefonte, PA, USA) as stationary phase (35 m long, internal diameter 0·25 mm, film thickness 0·25 µm) and flame ionization detector with the following gas flows: 400 mL min−1 air, 30 mL min−1 hydrogen and 30 mL min−1 helium make-up gas. The gas samples were injected manually using a 100 Series gas-tight Hamilton TLL (Teflon level lock) syringe. The ethylene standard used was 10 ppm produced by Matheson Tri-gas Micro Mat14 (USA) and supplied by Consolidated Industrial Gases Inc. (Philippines). Based on the linearity response of this standard, 150 µL of gas was used per injection. Ethylene production was expressed in terms of weight of ethylene per seedling per hour.
Peroxidase activity
Peroxidase activity was measured following the method of Matsunaka (1960). After 5 d in N2-bubbled stagnant agar solution, 25 seedlings were harvested and incubated in the dark with 25 mL of 20 ppm α-naphthylamine in 0·1 m sodium phosphate buffer (pH 7·0) at 25 °C from 3 to 6 h. The rate at which α-naphthylamine was consumed is indicative of peroxidase activity. After the incubation, an aliquot of 2 mL of the solution in the flask was mixed with 10 mL of distilled water, 1 mL of 1 % sulfanilic acid and 10 ppm of sodium nitrite solution. Optical absorption at 510 nm was read and the amount of the α-naphthylamine remaining after the incubation was measured against a standard curve. All the results were expressed on a dry-weight basis.
ADH and PDC activities
Crude protein was extracted from 100 mg of tissue by grinding in 600 µL of ice-cold extraction buffer (100 mm TES, pH 7·7; 2 mm MgCl2·6H2O; 1 mm EDTA; 1·25 % (w/v) Triton X-100; 4 mm dithiothreitol). The crude extract was centrifuged at 10 000 g for 10 min at 5 °C, and the extract was divided into three parts for the PDC assay (200 µL), ADH assay (100 µL), and total protein assay (200 µL). To the 200 µL extract, 66·67 µL 3 % bovine serum albumin, 53·3 µL 250 mm MES–NaOH and 16·6 µL 10 mm thiamine pyrophosphate chloride were added and the mixture was centrifuged at 10 000 g for 3 min. From the supernatant, 100 µL was taken and 12 µL of 500 mm TES, pH 8·0, was added to avoid a decrease in pH. Total PDC activity was analysed using the procedures described by Quimio et al. (2000). Briefly, the extract was incubated at 20 °C for 1 h prior to the assay. The reaction mixture consisted of 100 µL extract, 62·5 mm MES, 0·5 mm thiamine pyrophosphate chloride, 50 mm oxamate, 10 U ADH, and 0·17 mm NADH. To initiate the reaction, 10 mm of pyruvate was added and the coupled NADH oxidation was monitored at A340 at 30 °C for 10 min. Total ADH activity was analysed using the procedures described in Ella et al. (1993). To the 100 µL of crude extract, 66·67 µL of 3 % bovine serum albumin was added and samples were centrifuged at 10 000 g for 3 min. From the supernatant, 20 µL of extract was used for the reaction mixture with 51·8 mm TES and 0·17 mm NADH, and tubes were kept on ice. Acetaldehyde (10·02 mm) was added and ADH activity was monitored at A340 at 30 °C for 7 min. Bradford's method (Bradford, 1976) was used for total protein assay with bovine serum albumin as a standard. Analyses were made from four independent replicates for each time-point.
RNA extraction and reverse-transcript (RT-PCR) analyses
Total RNA was extracted using a modified protocol for starchy tissue (Stirn et al., 1995) and described in Vergara and Ismail (2007). Twenty dissected rice embryonic tissues with halves of the starchy endosperm removed (about 100 mg) were ground in liquid nitrogen using a mortar and pestle. One millilitre of 1 : 1 (v/v) extraction buffer (1 % sarcosyl, 100 mm Tris–HCl, 280 mm NaCl, 10 mm EDTA at pH 8·5 with 10 µL β-mercaptoethanol mL−1 buffer) : phenol mixture was added to 100 mg of ground samples in 2-mL tubes, vortexed well, and placed on ice for 10 min. Centrifugation was performed at 10 000 g for 5 min at 5 °C and the supernatant was collected into new tubes. One-half volume of chloroform : isoamyl alcohol (24 : 1, v/v) was added and mixed and the upper phase was collected after centrifugation at 10 000 g for 5 min. The upper phase was further extracted twice using phenol : chloroform (1 : 1 v/v), and nucleic acids were precipitated from the upper clear phase by adding 1/10 volume of 3 m sodium acetate and 2× volume of absolute ethanol and placed in ice. The supernatant was discarded after brief centrifugation at 10 000 g for 5 min at 5 °C and the pellet was resuspended in 50 µL of diethyl pyrocarbonate (DEPC)-treated water. The RNA was re-precipitated using 0·1 volume of 2·5 m lithium chloride and 2× volume of absolute ethanol. After centrifugation at 10 000 g for 10 min at 5 °C, the supernatant was discarded and the pellet was washed with 70 % ethanol–DEPC-treated water and finally dissolved in 50 µL of DEPC-treated water and stored at –20 °C. The quality and quantity of total RNA were analysed by gel visualization in a 1·5 % Tris–boric–EDTA–agarose gel stained with ethidium bromide and by spectrophotometric analyses (Beckman DU 600). Residual DNA digestion was performed using RQ1 RNase-free DNase (Promega, Madison, WI, USA). Semi-quantitative reverse-transcribed PCR (RT-PCR) reactions were carried out using SuperScript one-step RT-PCR with the Platinum Taq system (Invitrogen, Carlsbad, CA, USA) in a reaction mixture containing 50–75 ng RNA, 12·5 µL 2× buffer reaction mix, 0·25 mm each of forward and reverse primers, and 0·5 µL Taq mix, with the final volume completed to 20 µL using DEPC–water. The reaction mixture was run in the following conditions: cDNA synthesis at 46 °C for 30 min, denaturation at 94 °C for 2 min, followed by 26–32 cycles of denaturation at 94 °C for 30 s, annealing at 47–58 °C for 30 s, 72 °C for 2 min, and a final extension at 72 °C for 10 min. Primers, annealing temperatures and expected band sizes for α-amylases (RAmy3C, RAmy3D and RAmy3E), pyruvate decarboxylases (Pdc1, Pdc2) and alcohol dehydrogenases (Adh1 and Adh2) were as described by Fukao et al. (2006), while primers for RAmy1A and RAmy2A were from Hwang et al. (1999). Normalized transcript levels of a GAPDH expression pattern were used as a control.
Environmental characterization
In greenhouse experiments the mean relative humidity was 64 % and the air temperature was in the range 25–40 °C, with an average of 32·4 °C. On average, the floodwater pH was about 7·98 and the water temperature ranged from 25·7 °C at 0800 h to 36·4 °C at 1300 h, with a daily mean of about 30·1 °C. Light and oxygen levels underwater were not measured because of the shallow water depth used in these experiments (10 cm). Soil redox potential was measured in a separate set of pots planted to IR42, with the tip of a locally fabricated platinum electrode placed adjacent to the germinating seeds. The positive average corrected values of soil redox potential indicated that some O2 was present and that the layer of submerged soil where the seeds were sown was apparently hypoxic (data not shown).
Statistical analysis
Data from each experiment were subjected to analysis of variance (ANOVA) using IRRISTAT for Windows version 5.0 (International Rice Research Institute, 2005). Relationships between different attributes were studied using linear regression.
RESULTS
Seedling survival and growth
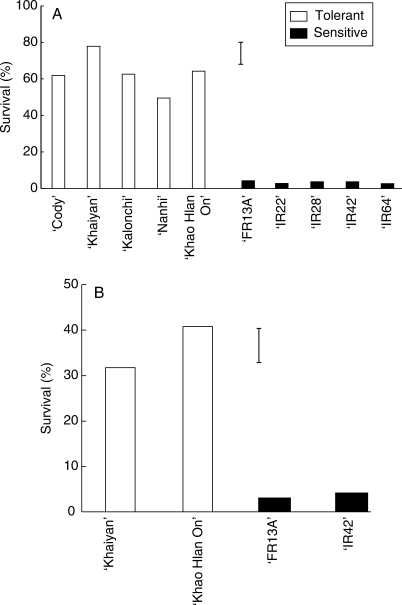
Tolerant and intolerant genotypes had the same seedling survival under control conditions, but survival of both groups decreased under flooding, with a substantially greater reduction in the intolerant genotypes (Fig. 1). Among the five tolerant genotypes, survival was highest in ‘Khaiyan’ and ‘Khao Hlan On’ (Fig. 1A), but was very low among the sensitive genotypes in both experiments (approx. 5 %).
Fig. 1.
Survival of flooded rice seedlings of (A) ten genotypes in expt I, (B) four genotypes in expt II, 21 d after seeding and flooding with 10 cm water. Vertical bars indicate l.s.d. at P = 0·05.
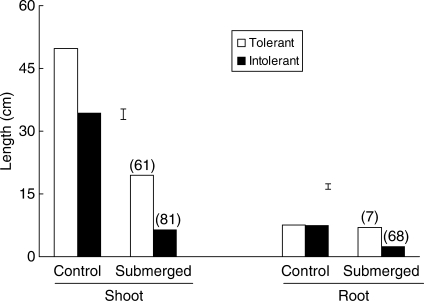
Under control conditions, the tolerant genotypes had longer shoots, but roots of both tolerant and intolerant genotypes were of similar length when measured 21 d after sowing (Fig. 2). Flooding decreased root and shoot lengths of both tolerant and intolerant genotypes, with a greater decrease in intolerant ones, amounting, on average, to an 81 % versus 61 % decrease for shoot lengths and a 68 % versus 7 % decrease for root lengths in sensitive and tolerant genotypes, respectively, at 21 d after seeding in the first experiment. Evidently, tolerant lines were able to maintain their root growth under hypoxia.
Fig. 2.
Shoot (longest leaf) and root lengths of seedlings of flooding-tolerant and -intolerant genotypes of rice measured 21 d following sowing under control and flooding in 10 cm of water. Data are means of ten genotypes in expt I. Values in parentheses are percentage reductions in length relative to the length in the control. Vertical bars indicate l.s.d. at P = 0·05.
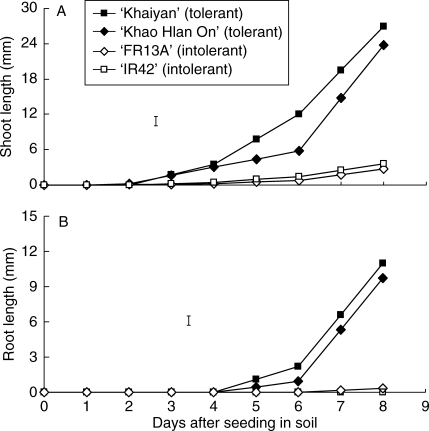
Experiment II evaluated the pattern of root and shoot growth of these contrasting genotypes over 8 d. Green shoots of both genotypes were observed at about 3 d following dry seeding under the control treatment, but when flooded, greening was delayed by 2–3 additional days in the tolerant genotypes and by 6–7 d in the sensitive genotypes (Fig. 3A). Shoot extension rate of the tolerant genotypes then increased with time after day 4, whereas that of the sensitive types remained slow through to the 8th day (Fig. 3A). Root growth showed delayed but similar trends compared with shoot growth in both tolerant and sensitive lines (Fig. 3B). In tolerant lines, roots started to appear earlier than in intolerant lines (at about 5 d after seeding) and their growth became faster and then linear beyond day 6. Conversely, roots of the sensitive lines did not appear until about 7–8 d following seeding.
Fig. 3.
Lengths of (A) shoots (longest leaf) and (B) roots of two flooding-tolerant and two flooding-sensitive rice genotypes following dry seeding and flooding in 10 cm of water in expt II. Vertical bars indicate l.s.d. at P = 0·05.
Non-structural carbohydrate concentrations in germinating seeds
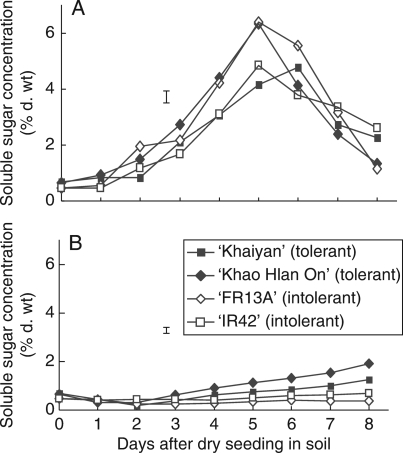
Soluble sugar concentration in seeds germinating under control and flooded conditions was assessed during the first 8 d after sowing (Fig. 4). Under control conditions, soluble sugars increased with time until the 5th day following sowing and then started to decrease through day 8, with similar patterns in both tolerant and sensitive lines (Fig. 4A). However, under flooding, soluble sugar concentration remained considerably lower in both tolerant and sensitive lines but became higher statistically in the tolerant line ‘Khao Hlan On’ after the 4th day and in ‘Khaiyan’ on the 8th day after sowing (Fig. 4B).
Fig. 4.
Changes in soluble sugar concentrations in germinating seeds of two flooding-tolerant and two flooding-sensitive rice genotypes sown in soil under either (A) control conditions or (B) flooding in 10 cm water in expt II. Vertical bars indicate l.s.d. at P = 0·05.
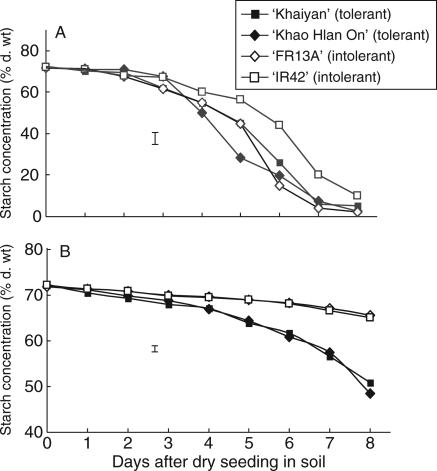
Under control conditions, starch concentration decreased progressively with time, reached a low level at day 8. No apparent differences were observed in the pattern of starch consumption between tolerant and sensitive lines (Fig. 5A). Under flooding, starch concentration remained high in sensitive genotypes, with only a slight reduction from day 3 to day 8 after seeding. Conversely, starch concentration in seeds of tolerant genotypes became significantly lower than that in sensitive lines as early as the second day after sowing, and then continued to decrease progressively, and at an increasing rate, through to day 8 (Fig. 5B). This obvious reduction in starch concentration in germinating seeds coupled with the increase in soluble sugars and consequent seedling growth of tolerant genotypes indicates that these genotypes maintain their ability to degrade stored carbohydrate and mobilize it for seed germination and seedling growth under hypoxia.
Fig. 5.
Changes in starch concentration in germinating seeds of two flooding-tolerant and two flooding-sensitive rice genotypes sown in soil under either (A) control conditions or (B) flooding in 10 cm water in expt II. Vertical bars indicate l.s.d. at P = 0·05.
Amylase activity
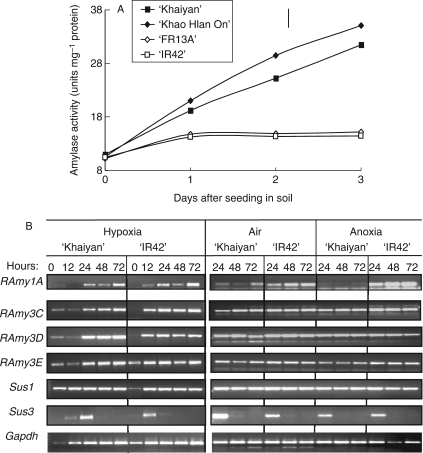
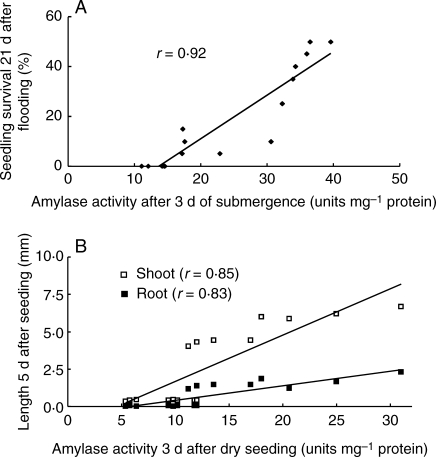
Low amylase activity was observed in the dry seeds of all genotypes. Amylase activity then increased progressively with time in seeds of tolerant genotypes germinating under flooded conditions, to reach a higher level at day 3. However, this increase was much slower in sensitive genotypes and was apparent only after the first day following sowing (Fig. 6A). Amylase activity in seeds germinating under flooding correlated positively with seedling survival (r = 0·92; Fig. 7A) and with seedling shoot (r = 0·85) and root (r = 0·83; Fig. 7B) lengths.
Fig. 6.
(A) Amylase activity in seeds germinating in flooded soil during the first 3 d after sowing in expt II (vertical bar indicates l.s.d. at P = 0·05) and (B) reverse-transcript (RT-PCR) analyses of amylases (RAmy1A, RAmy3C, RAmy3D and RAmy3E) and sucrose synthases (Sus1 and Sus3) using RNA extracted from germinating embryos of cultivars ‘Khaiyan’ and ‘IR42’ under hypoxia (0·03 mol O2 m−3), air (0·25 mol O2 m−3) and anoxia (<0·003 mol O2 m−3) treatments for 0, 12, 24, 48 and 72 h.
Fig. 7.
Association of amylase activity 3 d after sowing with (A) seedling survival measured 21 d after sowing and with (B) shoot and root length 5 d after sowing. Data are means of two tolerant cultivars (‘Khaiyan’ and ‘Khao Hlan On’) and two intolerant (‘FR13A’ and ‘IR42’) genotypes sown in soil and flooded in 10 cm water in expt II.
Expression of α-amylases and sucrose synthase genes
Gene expression analysis was performed using semi-quantitative RT-PCR, with RNA extracted from ‘Khaiyan’ (flooding-tolerant) and ‘IR42’ (flooding-intolerant) seeds germinating under different oxygen levels. In general, rice α-amylases were rapidly induced in germinating embryos (Fig. 6B). Expression of RAmy1A was barely visible at 0 h but increased from 12 h to 72 h of germination. The expression levels of RAmy1A were comparable in both tolerant and intolerant genotypes and no obvious differences were observed between hypoxic and aerated treatments. However, variation in expression levels of RAmy1A was observed under anoxia, with ‘Khaiyan’ showing a much lower level of expression. A difference in migration of RAmy1A RT-PCR products between ‘Khaiyan’ and ‘IR42’ were also noted, and, upon sequencing, a 27-bp deletion was found near the 3′ untranslated region in ‘IR42’. However, the 27-bp indel is not part of the terminal exon product. Whether this deletion has any functional role remains to be determined. RAmy2A was not expressed in any of the treatments (data not shown). RAmy3C was weakly expressed at 0 h in seeds of ‘Khaiyan’, and its expression level increased with time in both genotypes. There were no differences in RAmy3C expression levels between the different O2 treatments and similar patterns of expression were also observed for RAmy3E. Differential expression levels between tolerant and intolerant genotypes were more evident for RAmy3D, particularly from 24 h to 72 h of hypoxia. The tolerant genotype ‘Khaiyan’ showed 1·5-fold higher RAmy3D expression levels than ‘IR42’ during hypoxia and the degree of expression was also much stronger when compared with results from aerated and anoxic treatments (Fig. 6B).
Two sucrose synthases, Sus1 and Sus3, were strongly induced within 12 h to 24 h during germination under the different O2 regimes. Sus1 showed a continuous pattern of expression beyond 24 h, with no obvious differences between genotypes or treatments. Sus3 showed a strong initial induction after 24 h of germination but completely turned off at 48 h under all three O2 levels (Fig. 6B).
PDC and ADH activities and gene expression patterns
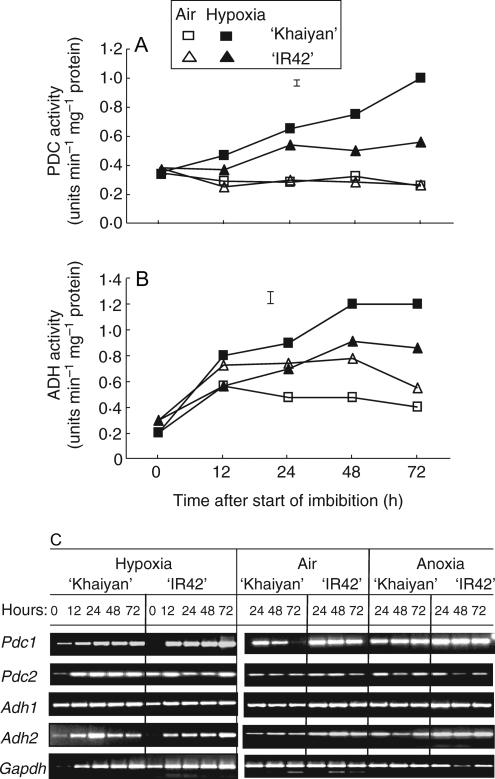
Activity of PDC in air remained low in germinating seeds of both tolerant ‘Khaiyan’ and intolerant ‘IR42’, but increased with time after 12 h of hypoxia, and with a substantially greater increase in germinating seeds of ‘Khaiyan’ than in seeds of ‘IR42’ (Fig. 8A). ADH activity increased in both genotypes upon imbibition, but was higher in germinating seeds of ‘Khaiyan’ from 12 h to 48 h under control conditions (Fig. 8B). However, ADH activity increased progressively with time in seeds germinating under hypoxia for the first 2 d, and with significantly greater activities in ‘Khaiyan’ than in ‘IR42’.
Fig. 8.
Enzyme activity of (A) pyruvate decarboxylase (PDC) and (B) alcohol dehydrogenase (ADH) in flooding-tolerant and flooding-sensitive genotypes during germination under aerated and hypoxic conditions. (C) Expression of Pdc and Adh in germinating seeds incubated in air, hypoxic or anoxic conditions. Data are from expt VI and vertical bars in (A) and (B) indicate l.s.d. at P = 0·05.
Transcripts of Pdc1, Pdc2, Adh1 and Adh2 genes were weakly present at 0 h and were strongly induced from 12 h to 72 h under hypoxia, air and anoxia (Fig. 8C). For Pdc1, there were no distinct differences between tolerant and intolerant lines under hypoxia, but expression levels appeared stronger during anoxia than in air or hypoxia for both genotypes. Under aerated conditions, Pdc1 also appeared to follow a gradual diminishing pattern from 24 h to 72 h. Pdc2 appeared to be present in all treatments, with stronger expression in germinating seeds of the tolerant cultivar from 24 h to 72 h during hypoxia. Adh1 and Adh2 were strongly expressed in all treatments, with no apparent differences between the two genotypes.
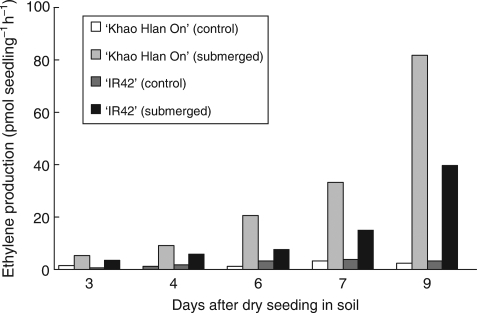
Ethylene production
No ethylene production was detected in dry seeds or after the first day following seeding, whereas very low levels were detected on the second day, with no apparent differences between genotypes (data not shown). Starting at day 3, ethylene production rates of seeds germinating in flooded soil increased progressively, with substantially higher levels in the tolerant line ‘Khao Hlan On’ (Fig. 9).
Fig. 9.
Ethylene production by rice seedlings after sowing and flooding in soil in expt III. Measurements were made 3, 4, 6, 7 and 9 d after sowing. Data for days 3 and 7 are based on one measurement per treatment. Data for day 9 are means of three replicate measurements (l.s.d. at P = 0·05 is 11).
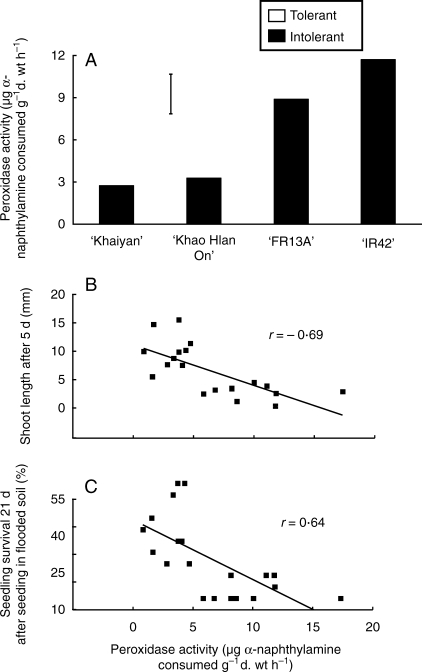
Peroxidase activity
Peroxidase activity in germinating seeds incubated under anoxia for 5 d was substantially lower in the tolerant genotypes than in the intolerant genotypes (Fig. 10A). This activity also correlated negatively with shoot length measured 5 d after sowing in flasks (r = 0·69; Fig. 10B), as well as with seedling survival 21 d after sowing in flooded soil (r = 0·64; Fig. 10C).
Fig. 10.
(A) Peroxidase activity in germinating rice seeds incubated in nitrogen-bubbled stagnant agar solution for 5 d to maintain hypoxic condition similar to flooded soil in expt IV. (B) Relationship of peroxidase activity with shoot length after 5 d in agar solution in expt IV, and (C) with seedling survival 21 d after sowing and flooding in soil in expt II. The vertical bar in (A) indicates l.s.d. at P = 0·05.
DISCUSSION
Through large-scale screening of diverse rice accessions and breeding lines, several rice genotypes with greater tolerance of flooding during germination were identified, and subsequent mapping identified six quantitative trait loci associated with tolerance (A. M. Ismail et al., unpubl. res.). The traits that are likely to be associated with tolerance were investigated using several contrasting genotypes, with seeds subjected to variable levels of oxygen during germination, ranging from aerated to anoxic conditions. Evidently, tolerance of flooding during germination is complex and involves numerous mechanisms, but it is clearly not associated with tolerance of complete submergence during the vegetative stage, as demonstrated by the sensitivity of FR13A, a landrace most tolerant of complete inundation during the vegetative stage (Ella et al., 2003; Jackson and Ram, 2003).
Survival of rice seedlings decreased significantly under flooded conditions, but to a much lower extent in the tolerant genotypes. Under flooding, growth of both shoot and root started earlier in tolerant genotypes and proceeded faster than growth in intolerant ones. Moreover, germinating seeds of tolerant genotypes showed a greater rate of reduction in starch concentration coupled with a slightly greater increase in soluble sugars during the first 8 d of flooding, and consequently faster seedling growth. The ability of tolerant genotypes to degrade starch into soluble sugars under low-oxygen stress probably plays a key role in their ability to survive and grow faster under these conditions.
Starch degradation is a complex biochemical process regulated by plant hormones and other metabolites such as sugar availability (Umemura et al., 1998; Loreti et al., 2003). In rice, amylase activity is highly induced during germination (Tanaka et al., 1970); however, this activity begins to appear even earlier, during seed maturation (Baun et al., 1970), and continues even in dry seeds to affect the quality of stored rice grains (Shin et al., 1985; Chrastil, 1990). This study highlighted the importance of amylase activity during germination and early seedling growth under flooded conditions. The results showed a strong positive correlation between seedling survival and amylase activity in germinating seeds (Fig. 7A). Moreover, tolerant genotypes had significantly higher survival and higher amylase activity than intolerant genotypes during flooding (Fig. 6A). The higher amylase activity during submergence is consistent with the faster growth observed in tolerant genotypes compared with intolerant ones, and is also illustrated by the strong positive correlations with shoot and root lengths during flooding (Fig. 7B). High amylase activity is also reflected through a faster rate of starch breakdown and higher soluble sugar concentrations in germinating seeds of tolerant lines during flooding (Figs 4 and 5), which presumably provided the substrates necessary for generating the energy required for growth and maintenance processes.
The activity of total amylases needed to break down starches was generally higher in tolerant cultivars under low-oxygen or flooded conditions than in intolerant ones (Fig. 6A). Analyses of transcriptional activity of specific rice α-amylases (RAmy1A, RAmy2A, RAmy3C, RAmy3D and RAmy3E) using semi-quantitative RT-PCR with total RNA extracted from germinating embryos of tolerant (Khaiyan) and intolerant (IR42) genotypes subjected to hypoxia, air or anoxia showed strong induction and expression of all of them except RAmy2A (Fig. 6B). In the first 3 d, rice seeds germinating under oxygen deficiency showed no differences in α-amylase transcript accumulation between tolerant and intolerant genotypes, except for RAmy3D, which is strongly expressed in ‘Khaiyan’ from 24 h to 72 h under hypoxia. RAmy3D was shown to be important for oligosaccharide degradation (Terashima et al., 1997) and is regulated by sugar availability (Loreti et al., 2003). Considering that α-amylase 3 constitutes approx. 60 % of total amylase mRNAs in rice cells starved of glucose (Lu et al., 1998), RAmy3D may play a significant role in tolerant cultivars during hypoxia and, together with other active α-amylases, may help promote rapid starch hydrolysis to fuel alcohol fermentation and sustain energy required during germination.
In the absence of oxygen, RAmy3D transcript levels were the same in germinating seeds of tolerant and intolerant genotypes, but RAmy1A was less expressed in tolerant lines. Hwang et al. (1999) also found that anoxia diminished the expression of Amy1A but up-regulated RAmy3 subfamilies in rice embryos. RAmy1A differs from RAmy3D by the presence of a GA-responsive element in its promoter region (Lu et al., 1998) and therefore is affected by the presence of GAs in epithelium layers (Kaneko et al., 2002). Terashima et al. (1997) also showed that the role of RAmy1A was mainly in soluble starch hydrolysis, whereas the barrel structure of RAmy3D helps to degrade complex oligosaccharides. Intolerant lines showing RAmy1A expression may still have sufficient endogenous GA levels for induction during anoxia, although GA action is likely to be arrested in anaerobic tissues. Different α-amylase activities under air, hypoxia and anoxia probably suggest that the ability of germinating rice seeds to sense sugar availability is a highly complex control mechanism involving several pathways of gene regulation that differ between tolerant and intolerant rice cultivars.
Sucrose is another major energy source for germinating embryos and can be hydrolysed to hexose via the sucrose synthase pathway or the invertase pathway. Under low-O2 stress, sucrose synthase (Sus) transcript levels increase while invertase decreases, suggesting that sucrose synthase is probably the principal enzyme that converts sucrose to phosphorylated hexoses under low-oxygen stress (Geigenberger, 2003). Comparison of the transcript expression pattern of sucrose synthase in tolerant and intolerant rice embryos showed no differences under aerated conditions or during hypoxia and anoxia. Sus1 was consistently induced during the first 72 h of treatments, whereas Sus3 expression was turned off after 24 h (Fig. 6B), suggesting that Sus1, but not Sus3, contributes to sucrose degradation during germination. The transient expression of Sus3 and induction of Sus1 may be considered a combination of a modulation and energy-saving strategy because the net flux needed to break down sucrose under low O2 is only one molecule of pyrophosphate compared with two molecules of ATP consumed by invertase and hexokinase (Zeng et al., 1999).
The alcohol fermentation pathway plays a dominant role under anaerobic conditions, in which ATP formation through mitochondrial oxidative phosphorylation is essentially inhibited. Maintenance of cellular metabolism and function is reduced because of the low efficiency of anaerobic respiration. Through aerobic respiration, one molecule of hexose equivalent produces up to 39 molecules of ATP, but only three molecules are generated from the glycolytic steps leading to alcohol fermentation. Plants adjust to low-oxygen stress by enhanced ethanol fermentation as reflected by increased PDC and ADH activities. The induction of Pdc and Adh transcripts under hypoxia and anoxia in the present experiments is consistent with previous results (Ricard and Pradet, 1989; Huq et al., 1999). However, the present data also suggested that, even under aerated conditions, germinating rice seeds probably experience hypoxia as evident by the induction of Pdc and Adh transcripts. Even in well-oxygenated surroundings (21 % v/v external oxygen), plant tissues that have high metabolic activity can become hypoxic and oxygen decreases sharply to about 1 % within the cutinized layer of the seed coat (Geigenberger, 2003). Alternatively, these genes could be induced by other factors even under aerated conditions such as high sugar concentrations as observed in developing and germinating tobacco pollens (Tadege and Kuhlemeier, 1997). Apparently, the present results from the Pdc and Adh transcription patterns may not explain differences in tolerance of flooding during germination, yet it has previously been shown that transgenic plants overexpressing PDC in rice has enhanced flooding tolerance (Quimio et al., 2000).
Measurements of total enzyme activities for PDC and ADH at different time-points during germination clearly revealed significant differences in activity levels under hypoxia and air, and between tolerant and intolerant genotypes. Progressive and higher activity levels were observed immediately after 24 h of hypoxia in tolerant genotypes, suggesting the significant role of this pathway in tolerance of anaerobic conditions during germination. The occurrence of low or baseline activity levels of PDC and ADH under aerated conditions and at zero-time has also been reported by Guglielminetti et al. (2001). Further studies are required to test whether post-transcriptional regulation might explain the lack of differences in gene expression under low-oxygen stress in spite of the clear differences in activities of both PDC and ADH enzymes.
This study also implicates ethylene as a potent regulator of seedling growth under flooded conditions. Ethylene production by dry seeds was not detected and at day 1 after dry seeding in soil, under both control and flooding conditions. Ethylene production rates were very slow in both tolerant and intolerant genotypes at day 2, but increased progressively afterwards, with significantly higher rates in seedlings of tolerant genotypes (Fig. 9). This delay in ethylene production suggests that the source of ethylene is probably the growing embryos under oxygen deficiency, and this is consistent with earlier reports, in which ethylene production was found to be more related to post-germination growth than to germination per se, and that ethylene production during the normal germination of intact rice seed just precedes the onset of seedling growth (developmental stage S1; Counce et al., 2000), but not the first visible stage of germination as indicated by pericarp splitting (Cohn and Hughes, 1986). Gianinetti et al. (2007) also reported that ethylene production in weedy (red) rice seeds is activated during germination and is involved in accelerating the germination process but not in the maintenance or breaking of dormancy to initiate germination.
Ethylene promotes mesocotyl and coleoptile elongation in rice seedlings (Ku et al., 1970; Satler and Kende, 1985) and higher ethylene levels could mean faster extension growth. During the initial screening and subsequent trials to confirm the tolerance of the genotypes selected for this study it was observed that tolerant genotypes emerged from the soil surface 3–4 d before the sensitive genotypes, and additionally they reach the floodwater surface 2–3 d earlier (A. M. Ismail et al., unpubl. res.). The fact that tolerant genotypes produced more ethylene than intolerant genotypes may explain their faster growth under flooding compared with the intolerant genotypes.
There was less peroxidase activity in tolerant seedlings grown in anoxic agar solution than in intolerant ones. This low activity could be related to ethylene-regulated growth as reported in the petioles of Ranunculus sceleratus that elongated faster and had lower peroxidase activity in response to ethylene (Horton, 1993). Lee and Lin (1996) reported a negative correlation between shoot elongation and peroxidase activity in anoxia-treated rice seedlings, similar to those in the present study (Fig. 6B). Ethylene is known to decrease peroxidase activity and eventually peroxidase-induced lignification and protein assembly in the cell wall (Waffenschmidt et al., 1993), as peroxidases have been recognized to catalyse the formation of diferuloyl cross-links to matrix polysaccharides (Fry, 1979). These activities of peroxidases may contribute to increased cell-wall rigidity and slow growth by cell elongation (Wardrop, 1964) as observed in tomato (Marigo and Boudet, 1980), mung bean (Goldberg et al., 1987) and peanut (Zheng and van Huystee, 1992). This might also hold true in germinating seedlings of intolerant rice genotypes in which the increased activity of peroxidases under low-oxygen stress correlated with slow seedling growth.
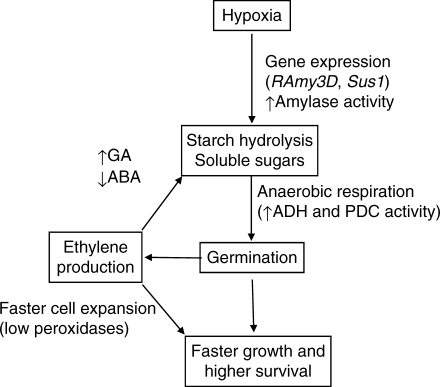
In summary, tolerance of flooding during germination in rice seems to involve several processes that warrant the supply of needed energy and ensure maintenance of seedling growth under the ensuing low-oxygen stress (Fig. 11). The ability to break down and utilise stored starch by maintaining gene expression (RAmy3D) and activity of amylases is probably the most critical step for survival and growth during hypoxia; the induction of the specific genes involved probably being signalled by severe sugar starvation in the tolerant genotypes. Soluble sugars can then be further catabolized through the anaerobic respiration pathway as shown by the increased activities of PDC and ADH, the key enzymes of the alcohol fermentation pathway. Upon germination, ethylene produced by the growing embryo may further promote cell expansion, possibly through down-regulation of the enzymes involved in enhanced cell-wall rigidity such as peroxidases. Ethylene can also augment further starch hydrolysis by reducing ABA synthesis while increasing synthesis and sensitivity to GA, both of which will enhance the activities of starch catabolic enzymes, particularly amylases. Further studies will elucidate the particular roles of ethylene and other regulatory processes in tolerance of flooding during germination.
Fig. 11.
Proposed mechanisms involved in enhancing germination and seedling growth under low-oxygen stress in rice. A detailed description is given in the Discussion.
ACKNOWLEDGEMENTS
The technical assistance of Lamberto Licardo, Melencio Apostol and Donna Holt is greatly appreciated. The authors also thank Dr Julia Bailey-Serres for helpful comments and discussion during this study and for providing some of the primers used in the gene expression analysis. We also thank Prof. Michael Jackson for critically reading the manuscript. This work was partially supported by a grant from the German Federal Ministry for Economic Cooperation and Development (BMZ).
LITERATURE CITED
- Al-Ani A, Bruzau F, Raymond P, Saint-Ges V, Leblanc JM, Pradet A. Germination, respiration, and adenylate energy charge of seeds at various oxygen partial pressures. Plant Physiology. 1985;79:885–890. doi: 10.1104/pp.79.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwell BJ, Greenway H. Carbohydrate metabolism of rice seedlings grown in oxygen-deficient solution. Journal of Experimental Botany. 1987;38:466–478. [Google Scholar]
- Avadhani PN, Greenway H, Lefroy R, Prior L. Alcoholic fermentation and malate metabolism in rice germinating at low oxygen concentrations. Australian Journal of Plant Physiology. 1978;5:15–25. [Google Scholar]
- Baun LC, Palmiano EP, Perez CM, Juliano BJ. Enzymes of starch metabolism in the developing rice grain. Plant Physiology. 1970;46:429–434. doi: 10.1104/pp.46.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E, Zeigler P. Biosynthesis and degradation of starch in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:95–117. [Google Scholar]
- Bernfeld P. Amylases, alpha and beta. Methods in Enzymology. 1955;1:149–152. [Google Scholar]
- Biswas JK, Yamauchi M. Mechanism of seedling establishment of direct-seeded rice (Oryza sativa L.) under lowland conditions. Botanical Bulletin of Academia Sinica. 1997;38:29–32. [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cho HT, Kende H. Expression of expansin genes is correlated with growth in deepwater rice. The Plant Cell. 1997;9:1661–1671. doi: 10.1105/tpc.9.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrastil J. Influences of storage on enzymes in rice grains. Journal of Agricultural and Food Chemistry. 1990;38:1198–1202. [Google Scholar]
- Cohn MA, Hughes JA. Seed dormancy in red rice. V. Response to azide, hydroxylamine, and cyanide. Plant Physiology. 1986;80:531–533. doi: 10.1104/pp.80.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counce PA, Keisling TC, Mitchell AJ. A uniform, objective, and adaptive system for expressing rice development. Crop Science. 2000;40:436–443. [Google Scholar]
- Das KK, Sarkar RK, Ismail AM. Elongation ability and non-structural carbohydrate levels in relation to submergence. Plant Science. 2005;168:131–136. [Google Scholar]
- Davies PJ. Plant hormones: physiology, biochemistry and molecular biology. Dordrecht: Kluwer Academic Publishers; 1995. pp. 1–35. [Google Scholar]
- Drew MC. Sensing soil oxygen. Plant, Cell & Environment. 1990;13:681–693. [Google Scholar]
- Ella ES, Setter TL. Importance of seed carbohydrates in rice seedling establishment under anoxia. Acta Horticulturae. 1999;504:209–216. [Google Scholar]
- Ella ES, Valdez AP, Reyes RV, Greenway H, Setter TL. Importance of several enzymes in limitation of alcoholic fermentation of rice under anoxia. Proceedings of the 6th Annual Meeting of the International Program on Rice Biotechnology; 1–5 February; Chiang Mai, Thailand. 1993. [Google Scholar]
- Ella ES, Kawano N, Yamauchi Y, Tanaka K, Ismail AM. Blocking ethylene perception during submergence reduced chlorophyll degradation and improved seedling survival in rice. Functional Plant Biology. 2003;30:813–819. doi: 10.1071/FP03049. [DOI] [PubMed] [Google Scholar]
- Fales FW. The assimilation and degradation of carbohydrates by yeast cells. Journal of Biological Chemistry. 1951;193:113–124. [PubMed] [Google Scholar]
- Fincher GB. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:305–346. [Google Scholar]
- Fry SC. Phenolic components of the primary cell wall and their possible role in the hormonal regulation of growth. Planta. 1979;146:343–351. doi: 10.1007/BF00387807. [DOI] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P. Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology. 2003;6:247–256. doi: 10.1016/s1369-5266(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Gianinetti A, Laarhoven LJJ, Persijn ST, Harren FJM, Petruzzelli L. Ethylene production is associated with germination but not seed dormancy in red rice. Annals of Botany. 2007;99:735–745. doi: 10.1093/aob/mcm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Morell S, Valdez A, Setter TL, Greenway H. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance for anoxia. Journal of Experimental Botany. 2000;51:785–796. [PubMed] [Google Scholar]
- Goldberg R, Liberman M, Mathieu C, Peirron M, Catesson AM. Development of epidermal cell wall peroxidase along the mung bean hypocotyls: possible involvement in the cell wall stiffening process. Journal of Experimental Botany. 1987;38:1378–1390. [Google Scholar]
- Greenway H, Setter TL. Is there anaerobic metabolism in submerged rice plants? A view point. In: Singh VP, Singh RK, Singh BB, Zeigler RS, editors. Physiology of stress tolerance in rice. Manila, Philippines: Narendra Deva University of Agriculture and Technology and International Rice Research Institute; 1996. pp. 11–30. [Google Scholar]
- Guglielminetti L, Yamaguchi J, Perata P, Alpi A. Amylolytic activities in cereal seeds under aerobic and anaerobic conditions. Plant Physiology. 1995;109:1069–1076. doi: 10.1104/pp.109.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Busillacchi H, Perata P, Alpi A. Carbohydrate-ethanol transition in cereal grains. New Phytologist. 2001;151:607–612. doi: 10.1046/j.0028-646x.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiology. 1992;99:1156–1161. doi: 10.1104/pp.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RF. Peroxidase, ethylene, and submergence-promoted growth of petiole of Ranunculus sceleratus L. Journal of Plant Physiology. 1993;141:690–693. [Google Scholar]
- Huang J, Takano T, Akita S. Expression of α-expansin genes in young seedlings of rice. Planta. 2000;211:467–473. doi: 10.1007/s004250000311. [DOI] [PubMed] [Google Scholar]
- Hwang YS, Thomas BR, Rodriguez RL. Differential expression of α-amylase genes during seedling development under anoxia. Plant Molecular Biology. 1999;40:911–920. doi: 10.1023/a:1006241811136. [DOI] [PubMed] [Google Scholar]
- Huq E, Harrington S, Hossain MS, Wen F, McCouch SR, Hodges TK. Molecular characterization of pdc2 and mapping of three pdc genes from rice. Theoretical and Applied Genetics. 1999;98:815–824. [Google Scholar]
- International Rice Research Institute. IRRISTAT for Windows Version 5.0. Makati City, Philippines: IRRI; 2005. [Google Scholar]
- Ishizawa K, Esashi Y. Action mechanism of ethylene in the control of sugar translocation in relation to rice coleoptile growth. I. Sucrose metabolism. Plant, Cell & Environment. 1988;29:131–141. [Google Scholar]
- Jackson MB, Ram PC. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Annals of Botany. 2003;91:227–241. doi: 10.1093/aob/mcf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Herman B, Goodenough A. An examination of the importance of ethanol in causing injury to flooded plants. Plant, Cell & Environment. 1982;5:163–172. [Google Scholar]
- Jackson MB, Waters I, Setter T, Greenway H. Injury to rice plants caused by complete submergence: a contribution by ethylene (ethene) Journal of Experimental Botany. 1987;38:1826–1838. [Google Scholar]
- Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiology. 2002;128:1264–1270. doi: 10.1104/pp.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings H, Jackson MB. A relationship between rates of ethylene production by roots and the promoting or inhibiting effects of exogenous ethylene and water on root elongation. Zeitschrift für Pflanzenphysiologie (Journal of Plant Physiology) 1979;92:385–397. [Google Scholar]
- Ku HS, Suge H, Rappaport L, Pratt HK. Stimulation of rice coleoptile growth by ethylene. Planta. 1970;90:333–339. doi: 10.1007/BF00386385. [DOI] [PubMed] [Google Scholar]
- Kunst A, Draeger B, Ziegenhorn J. Colorimetric methods with glucose oxidase and peroxidase. In: Bergermeyer HU, editor. Methods of enzymatic analysis. VI. Metabolites. I. Carbohydrates. Weinheim: Verlag-Chemie; 1988. pp. 178–185. [Google Scholar]
- Lee TM, Lin YH. Peroxidase activity in relation to ethylene-induced rice (Oryza sativa L.) coleoptile elongation. Botanical Bulletin of Academia Sinica. 1996;37:239–245. [Google Scholar]
- Lasanthi-Kudahettige R, Magneschi L, Loret E, et al. Transcript profiling of the anoxic rice coleoptile. Plant Physiology. 2007;144:218–231. doi: 10.1104/pp.106.093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Ming-yu H, Chun-ming W, Jian-min W. Quantitative trait loci and epistatic analysis of seed anoxia germinability in rice (Oryza sativa) Rice Science. 2004;11:238–244. [Google Scholar]
- Loreti E, Yamaguchi J, Alpi A, Perata P. Sugar modulation of α-amylase genes under anoxia. Annals of Botany. 2003;91:143–148. doi: 10.1093/aob/mcf117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CA, Lim EK, Yu SM. Sugar response sequence in the promoter of a rice α-amylase gene serves as a transcriptional enhancer. Journal of Biological Chemistry. 1998;273:10120–10131. doi: 10.1074/jbc.273.17.10120. [DOI] [PubMed] [Google Scholar]
- Marigo G, Boudet AM. Relations polyphenols-crossance: lignification et limitation de croissance chez Lycopersicum esculentum. Physiologia Plantarum. 1980;49:425–430. [Google Scholar]
- Matsunaka S. Studies on the respiratory enzyme systems of plants. I. Enzymatic oxidation of α-naphthylamine in rice plant root. Journal of Biochemistry. 1960;47:820–829. doi: 10.1093/oxfordjournals.jbchem.a127301. [DOI] [PubMed] [Google Scholar]
- Metraux JP, Kende H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiology. 1983;72:441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Akazawa T, Fukuchi S. Enzymic mechanism of starch breakdown in germinating rice seeds. I. An analytical study. Plant Physiology. 1968;43:1899–1905. doi: 10.1104/pp.43.12.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro R, Mapelli S, Torti G, Bertani A. Indole-3-acetic acid and rice coleoptile elongation under anoxia. Journal of Plant Growth Regulation. 1988;7:85–94. [Google Scholar]
- Perata P, Pozueta-Romero J, Akazawa T, Yamaguchi J. Effects of anoxia on starch breakdown in rice and wheat seeds. Planta. 1992;188:611–618. doi: 10.1007/BF00197056. [DOI] [PubMed] [Google Scholar]
- Perata P, Geshi N, Yamaguchi J, Akazawa T. Effect of anoxia on the induction of α-amylase in cereal seeds. Planta. 1993;191:402–408. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. Mobilization of endosperm reserves in cereal seeds under anoxia. Annals of Botany. 1997;79(Suppl. A):49–56. [Google Scholar]
- Quimio CA, Torrizo LB, Setter TL, et al. Enhancement of submergence tolerance in transgenic rice overproducing pyruvate decarboxylase. Journal of Plant Physiology. 2000;156:516–521. [Google Scholar]
- Raymond P, Al-Ani A, Pradet A. ATP production by respiration and fermentation, and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiology. 1985;79:9879–9884. doi: 10.1104/pp.79.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kende H. Regulation of growth in stem sections of deep water rice. Planta. 1984;160:66–72. doi: 10.1007/BF00392467. [DOI] [PubMed] [Google Scholar]
- Redoña ED, Mackill DJ. Genetic variation for seedling-vigor traits in rice. Crop Science. 1996;36:285–290. [Google Scholar]
- Ricard B, Pradet A. Anaerobic protein synthesis in different organs of germinating rice seeds. Plant Physiology and Biochemistry. 1989;27:761–768. [Google Scholar]
- Sasaki T. Studies on breeding for the germinability at low temperature of rice varieties adapted to direct sowing cultivation in flooded paddy field in cool region. Report of Hokkaido Prefectural Tokachi Agricultural Experimental Station. 1974;No. 24:1–90. [Google Scholar]
- Satler SO, Kende H. Ethylene and the growth of rice seedlings. Plant Physiology. 1985;79:194–198. doi: 10.1104/pp.79.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshu DV, Krishnasamy V, Siddique SB. Rice seed health. Los Baños, Philippines: International Rice Research Institute; 1988. Seed vigor in rice; pp. 315–329. [Google Scholar]
- Setter TL, Ella ES. Relationship between coleoptile elongation and alcoholic fermentation in rice exposed to anoxia. I. Importance of treatment conditions and different tissues. Annals of Botany. 1994;74:265–271. [Google Scholar]
- Setter TL, Kupkanchanakul T, Kupkanchanakul K, Bhekasut P, Wiengweera A, Greenway H. Symposium of the 1987 International Deepwater Rice Workshop. Los Baños, Philippines: International Rice Research Institute; 1988. Environmental factors in deepwater rice areas in Thailand: O2, CO2, and ethylene; pp. 55–66. [Google Scholar]
- Setter TL, Waters I, Wallace I, Bhekasut P, Greenway H. Submergence of rice. I. Growth and photosynthetic response to CO2 enrichment of floodwater. Australian Journal of Plant Physiology. 1989;6:251–263. [Google Scholar]
- Setter TL, Ella ES, Valdez AP. Relationship between coleoptile elongation and alcoholic fermentation in rice exposed to anoxia. II. Cultivar differences. Annals of Botany. 1994;74:273–279. [Google Scholar]
- Shin MG, Rhee JS, Kwon T-W. Effects of amylase activity on change in amylogram characteristics during storage of brown rice. Agricultural and Biological Chemistry. 1985;49:2505–2508. [Google Scholar]
- Stirn S, Mordhorst AP, Fuchs S, Lortz H. Molecular and biochemical markers for embryogenic potential and regenerative capacity of barley (Hordeum vulgare L.) cell cultures. Plant Science. 1995;106:195–206. [Google Scholar]
- Suge H. Stimulation of oat and rice mesocotyl growth by ethylene. Plant and Cell Physiology. 1971;12:831–837. [Google Scholar]
- Suge H. Synergistic action of ethylene with gibberellins in the growth of rice seedlings. Proceedings of Crop Science Society of Japan. 1974;43:83–87. [Google Scholar]
- Suge H, Kusanagi T. Ethylene and carbon dioxide: regulation of growth in two perennial aquatic plants, arrowhead and pondweed. Plant and Cell Physiology. 1975;16:65–72. [Google Scholar]
- Tadege M, Kuhlemeier C. Aerobic fermentation during tobacco pollen development. Plant Molecular Biology. 1997;35:343–354. doi: 10.1023/a:1005837112653. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ito T, Akazawa T. Enzymatic mechanism of starch breakdown in germinating rice seeds. III. α-Amylase isozymes. Plant Physiology. 1970;46:650–654. doi: 10.1104/pp.46.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL. Influence of oxygen tension on respiration, fermentation and growth of wheat and rice. American Journal of Botany. 1942;29:721–738. [Google Scholar]
- Terashima M, Hosono M, Katoh S. Functional roles of protein domains on rice α-amylase activity. Applied Microbiology and Biotechnology. 1997;47:364–367. doi: 10.1007/s002530050941. [DOI] [PubMed] [Google Scholar]
- Tuong TP, Pablico PP, Yamauchi M, Confesor R, Moody K. Increasing water productivity and weed suppression of wet-seeded rice: effect of water management and rice genotypes. Experimental Agriculture. 2000;36:71–89. [Google Scholar]
- Umemura T, Perata P, Futsuhara Y, Yamaguchi J. Sugar sensing and α-amylase repression in rice embryos. Planta. 1998;204:420–428. doi: 10.1007/s004250050275. [DOI] [PubMed] [Google Scholar]
- Vergara GV, Ismail A. Total RNA isolation from rice dry and germinating seeds for gene expression studies. International Rice Research Notes. 2007;32:35–36. [Google Scholar]
- Waffenschmidt S, Woessner JP, Beer K, Goodenough UW. Isodityrosine cross-linking mediates insolubilization of cell walls in Chlamydomonas. The Plant Cell. 1993;5:809–820. doi: 10.1105/tpc.5.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardrop AB. The structure and formation of the cell wall in xylem. In: Zimmerman MA, editor. The formation of wood in forest tree. New York, NY: Academic Press; 1964. pp. 87–134. [Google Scholar]
- Waters I, Morrell S, Greenway H, Colmer TD. Effects of anoxia on wheat seedlings. II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. Journal of Experimental Botany. 1991;42:1437–1447. [Google Scholar]
- Whitmore FW. Lignin-protein complex catalyzed by peroxidase. Plant Science Letters. 1978;13:241–245. [Google Scholar]
- Yamauchi M, Aguilar AM, Vaughan DA, Seshu DV. Rice (Oryza sativa L.) germplasm suitable for direct sowing under flooded soil surface. Euphytica. 1993;67:177–183. [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE. Rapid repression of maize invertases by low oxygen: invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiology. 1999;121:599–608. doi: 10.1104/pp.121.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, van Huystee RB. Peroxidase-regulated elongation of segments from peanut hypocotyls. Plant Science. 1992;81:47–56. [Google Scholar]