Abstract
Background and Aims
Oil pollution of wetlands is a world-wide problem but, to date, research has concentrated on its influences on salt marsh rather than freshwater plant communities. The effects of water-borne light oils (liquid paraffin and diesel) were investigated on the fresh/brackish wetland species Phragmites australis in terms of routes of oil infiltration, internal gas transport, radial O2 loss (ROL), underwater gas films and bud growth.
Methods
Pressure flow resistances of pith cavities of nodes and aerenchyma of leaf sheaths, with or without previous exposure to oil, were recorded from flow rates under applied pressure. Convective flows were measured from living excised culms with oiled and non-oiled nodes and leaf sheaths. The effect of oil around culm basal nodes on ROL from rhizome and root apices was measured polarographically. Surface gas films on submerged shoots with and without oil treatment were recorded photographically. Growth and emergence of buds through water with and without an oil film were measured.
Key Results
Internodes are virtually impermeable, but nodes of senesced and living culms are permeable to oils which can block pith cavity diaphragms, preventing flows at applied pressures of 1 kPa, natural convective transport to the rhizome, and greatly decreasing ROL to phyllospheres and rhizospheres. Oil infiltrating or covering living leaf sheaths prevents humidity-induced convection. Oil displaces surface gas films from laminae and leaf sheaths. Buds emerge only a few centimetres through oil and die.
Conclusions
Oil infiltrates the gas space system via nodal and leaf sheath stomata, reducing O2 diffusion and convective flows into the rhizome system and decreasing oxygenation of phyllospheres and rhizospheres; underwater gas exchange via gas films will be impeded. Plants can be weakened by oil-induced failure of emerging buds. Plants will be most at risk during the growing season.
Key words: Phragmites australis, oil pollution, convective flow, pressure flow resistance, phyllosphere oxygenation, rhizosphere oxygenation, underwater gas films, bud emergence, stomata, pith cavity diaphragms, leaf sheath aerenchyma, rhizome aeration
INTRODUCTION
Oil pollution of waterways and wetlands, originating from leakages from ships and pipelines, and the oily by-products of industries such as petrochemicals, desalination, tanning, and palm and olive oil production, is a world-wide problem, threatening not only animal life, but also the survival of plants which colonize coastal areas, marshes and the banks of estuaries, canals, rivers and lakes. Wetland species stabilize soils against erosion, provide habitats for wildlife, are important for human leisure activities and act as natural means of water purification (Mitsch and Gosselink, 1993). It is well known that fouling by light fuel oils can produce widespread death of salt marsh plants such as of Spartina alternifolia, S. patens (Alexander and Webb, 1985; Mendelssohn et al., 1990) and S. anglica (Baker, 1971). Oil covering shoot systems blocks stomata, seriously curtailing supplies of photosynthetic CO2 (Pezeshki and Delaune, 1993). Justin and Armstrong (1983) reported that oiling of shoot bases of Puccinellia festuciformis var. intermedia and P. maritima induced a decline in root radial O2 loss (ROL) to zero within <8 h due to the oil penetrating the internal gas transport pathways. Furthermore, transpiration and cooling of leaves are inhibited by oiling, and plants can become heat stressed (Pezeshki and Delaune, 1993). Oil infiltrating the soil may impede oxygen supplies and will degrade, with the production of intermediate toxins such as organic acids (Atlas, 1995), thus increasing stress on roots and underground buds (Armstrong and Armstrong, 1999).
To date, most research has concentrated on salt marsh plant communities; there is comparatively little information on effects of oil pollution on fresh water vegetation (Pezeshki et al., 2000). This study examined the effects of water-borne films of two light oils, liquid paraffin and diesel oil, on the common reed, Phragmites australis, a major colonizer and purifier of fresh and brackish wetlands, an important source of fodder, matting and roofing thatch, and widely used as a means of phytodepuration (Brix and Schierup, 1989; Brix, 1997) including some oily effluents, e.g. from olive oil production, were examined. The plant's competetiveness, ability to colonize deep waters (Brix et al., 1992; Vretare, 2002), efficacy in aerating underground rhizomes and roots, and oxygenating rhizospheres (Armstrong and Armstrong, 1990; Armstrong et al., 1992; Brix et al., 1996) and phyllospheres (Armstrong et al., 2006) are enhanced by generating pressurized throughflows of air (convections or ‘internal winds’) through shoots and rhizomes. The pith cavities of senesced culms from previous years provide the influx and efflux pathways for Venturi (wind)-induced convection (VIC), and the means of efflux for humidity-induced convection (HIC) (Armstrong et al., 1996b). Living leaf sheath stomata are the induction sites for HIC, the flow passing via leaf sheath aerenchyma channels and then through the culm and rhizome pith cavities, with venting via senesced culms. Living leaf sheaths and culm and rhizome pith cavities are also major conduits for diffusive aeration of underground parts. Gas films on submerged shoots also facilitate longitudinal and lateral gas exchange (Raskin and Kende, 1983; Beckett et al., 1988; Colmer and Pedersen, 2008). It was therefore important to examine possible pathways of oil penetration into both living and senesced culms. The hypotheses were tested that oil can (a) enter the plant's gas space system, increase resistance to pressure flow and reduce or prevent convective gas transport and diffusive aeration; (b) reduce radial O2 release from rhizome apices to phyllospheres and from roots to rhizospheres; (c) remove surface gas films from submerged parts of shoots; and (d) curtail growth of emerging shoots.
MATERIALS AND METHODS
Plant material and culture
Overwintered plants of Phragmites australis (Cav.) Steud. (shoot height 400–600 mm; rhizome length approx. 100 mm), which had been raised from seed the previous year, were secured with old culms emergent and rhizomes submerged in 1/4 strength Hoagland's solution in glass culture tubes (height = 400 mm; diameter = 50 mm) sheathed in black polythene to exclude the light. The plants were placed in a growth room to resume growth of roots, shoots and rhizomes; lighting was from the side to give an even distribution over the shoot systems (temperature = 18 °C, relative humidity = 35–55 %, photosynthetically active radiation = 80–100 µmol m−2 s−1). The Hoagland's solution was renewed every 2–3 d. Senesced and living culms (height approx. 2 m, basal diameter 5–10 mm) and apical portions of horizontal rhizome were collected from the banks of the Rivers Hull and Humber. On excision, the cut ends of living culms were immediately placed in water and used within 1 d; rhizome apices were stored in polythene bags at 1–3 °C, and used within a week.
Media
Experiments on polarography and growth were performed with roots and rhizomes submerged in nutrient agar, renewed every 2 d: 1/4 strength Hoagland's made up in 0·05 % (w/v) agar, previously de-oxygenated by bubbling with N2. Light oils are regarded as being more toxic to plants (Pezeshki et al., 2000); here liquid paraffin was used in most experiments, but, for those involving living nodes on excised parts which could senesce in the long term, diesel oil was used instead because of its quicker penetration.
Polarography
Platinum cathodes were used in conjunction with Ag–AgCl anodes, and oxygen was assayed polarographically according to Armstrong (1979). For measuring ROL to phyllosheres (ng O2 min−1), coiled Pt wire electrodes (wire diameter = 0·37 mm; length = 50 mm) were secured around vertical and horizontal rhizome apices (Armstrong et al., 2006). For measuring ROL to rhizospheres (ng O2 min−1), a cylindrical Pt cathode (diameter = 2·25 mm; length = 5 mm) was positioned around the sub-apical region of each root apex.
Effects of oil on pressure flow resistances (RPF) of segments of senesced and living culms and of living leaf sheaths
For these experiments, segments of culm (length = 110 mm) were used, each bearing a median node and pith cavity diaphragm, and, in the living culms, a portion of leaf sheath. After each RPF measurement, air flow or lack of flow was confirmed by placing the venting end in shallow water containing a few drops of detergent and observing any bubbling. Numbers in parentheses refer to treatment groups in Table 1.
Table 1.
Phragmites: pressure flow resistances (RPF) to air flow after 3 d, through lengths of senesced and living culm pith cavities and living leaf sheaths in relation to air, water, paraffin or diesel oil surrounding plant parts
| Treatment | RPF (MPa s m−3) |
|---|---|
| 1. Air around nodal stomata and internode (senesced culm)* | 59·3 ± 10·4a (pith cavity) |
| 2. Water around nodal stomata and internode (senesced culm)* | 59·6 ± 4·12a (pith cavity) |
| 3. Water around nodal stomata; oil around internode (senesced culm)* | 60·4 ± 4·5a (pith cavity) |
| 4. Oil around nodal stomata; internode in water (senesced culm)† | 10 270 to ∞b (pith cavity) |
| 5. Air around node and internode; ls sealed (living culm)* | 52·28 ± 7·9a (pith cavity) |
| 6. Water around node and internode; ls sealed (living culm)* | 59·3 ± 11·7a (pith cavity) |
| 7. Oil‡ around node, internode in water, ls sealed and emergent (living culm)† | ∞c (pith cavity) |
| 8. Air around living leaf sheath* | 19 092 ± 6900d (ls aerenchyma) |
| 9. Water around distal (upper) half of living leaf sheath* | 24 794 ± 7023d (ls aerenchyma) |
| 10. Distal (upper) part of living leaf sheath submerged in water, middle 5 mm in contact with surface oil; rest emergent† | ∞c (ls aerenchyma) |
* Applied pressure ≤120 Pa.
† Applied pressure = 1 kPa.
‡ Diesel oil, otherwise liquid paraffin used.
ls, leaf sheath.
abcd Means ± s.e. (n = 6–9); different letters indicate significant differences according to the Mann–Whitney rank sum test: P ≤ 0·01.
Pith cavity RPF of senesced culms
To determine if oil or water enters nodal stomata, and blocks pith cavities, 200 mm lengths of rubber tubing were connected to the ends of each culm portion and placed (1) in air, (2) with the nodal stomata and internode submerged in water, (3) with the nodal stomata in water and part of the internode in a surface layer of liquid paraffin, 5 mm thick, and (4) with the node in the oil layer and the internode in water. Prior to (4), care was taken to allow the nodes to dry in the air. Each treatment lasted 3 d. For the last three conditions, the free ends of the tubing were emergent. After days 1, 2 and 3 of each treatment, one end of each portion of culm was connected to a compressed air supply and to an air flow meter and pressure transducer (the latter two in parallel) to measure RPF.
Pith cavity RPF of living culms
The leaf sheaths were trimmed to 40 mm in length and their outer surface and cut ends sealed with Si-rubber to ensure that applied air flow was through the pith cavity and not through the sheaths. The samples were divided into three groups and the RPF of the pith cavity was measured as above: group (5) kept in air; group (6) after sealing the pith cavity at the opposite end to the leaf sheath, the internode, nodal stomata and 20 mm length of leaf sheath were submerged, but with 20 mm of leaf sheath emergent; group (7) similar to (6) but with the nodal stomata in contact with a layer of diesel oil 10 mm thick on the water surface and the leaf sheath emergent. For measuring RPF in groups (6) and (7) the samples were removed from the liquid, dried and the sealant removed from the pith cavity.
Living leaf sheath RPF
Each culm portion retained 55 mm of attached leaf sheath. For RPF measurement, the end of the pith cavity at the opposite end to the leaf sheath was sealed, so that attempts could be made to divert air from the pith cavity and thence through radial channels in the nodal culm wall and into the leaf sheath aerenchyma (Armstrong and Armstrong, 1988). Samples were divided into three groups: group (8) RPF was measured after 3 d in the air; group (9) prior to submergence the cut end of each leaf sheath was sealed with Si-rubber and the distal (upper) half of the sheath then submerged in water; group (10) similar to (9) but with the middle part of the leaf sheath in contact with a surface layer of liquid paraffin 5 mm thick. After 3 d, groups (9) and (10) were removed, dried, the leaf sheaths were trimmed to remove the sealant (but still keeping the pith cavity blocked at one end) and the RPF of leaf sheath aerenchyma measured as above.
Effects of oil on flow of air through excised rhizome apices
Freshly cut portions of rhizome apex (100 mm long) were each attached at the cut end to rubber tubing, while the free end (emergent during rhizome submergence) was connected in parallel to a compressed air supply and pressure transducer. Minimum pressures required just to force air bubbles out of the apex of each sample while under 20 mm depth of water were recorded 3 d after the rhizomes had been (a) in air, (b) submerged under 100 mm of water and (c) in the same depth of liquid paraffin. Before submergence in oil, the rhizomes were blotted with paper tissues and dried in the air for 2 h so that the oil would come into direct contact.
Effect of oil on HIC
Oiling of nodes
Freshly excised living culms (height = approx. 1·3 m; pith cavity basal diameter = approx. 5 mm) each had a narrow plastic tube (o.d. = 3 mm) inserted into the pith cavity, and were then placed with their cut ends in water (depth = 30 mm); the internal end of the tube was above the water surface to prevent flooding. The free emergent end of the tube was connected to a soap-film flow meter to measure humidity-induced convective flow rates during the day. Seven treatment culms were exposed to diesel oil by means of a sleeving plastic ‘cup’ (i.d. = 14 mm; height = 25 mm) containing water and a surface layer of oil approx. 10 mm thick, the latter being in contact with the stomata of the basal node and 5 mm of a healthy leaf sheath immediately above. Convective flow rates were measured on these and non-oiled controls over 5 d. On each culm the RPF values of the pith cavities of the basal node and two nodes above were measured on day 5.
Oiling of leaf sheaths
The leaf sheaths of ten healthy culms similar to those above were brushed with liquid paraffin; controls were not oiled; another set were sponged with water and for a fourth set of eight culms, the leaf sheaths of only the basal halves of the culms were oiled. The cut ends of the culms were in water and convective flows were measured as above after 5 min and over the following 5 d.
Effect of oil on ROL
ROL to the phyllospheres was determined with the rhizomes submerged and the plants (a) exposed for 4 d to a liquid paraffin layer 1 cm thick on the agar surface in contact only with non-stomatal basal regions of all shoots, including living and the previous year's senesced culms, (b) as in (a) but with the oil in contact for approx. 6 d with basal shoot nodes bearing stomata and basal parts of leaf sheaths, and (c) for 3 d with the oil layer removed. The experiment was repeated once.
ROL to rhizospheres was measured on separate plants, for the shoots firstly unoiled and then with basal nodes bearing stomata exposed to a 10 mm layer of liquid paraffin; the experiments lasted 1–2 d.
Effects of oil on surface gas films
Gas films on submerged parts of vertically positioned leaf laminae and sheaths were photographed before and after exposure (up to 36 h) to a layer of liquid paraffin, 5 mm deep, on the water surface. Camera positions and illumination were adjusted to record optimally the reflective properties of the gas films.
Effect of oil on emergence and growth of shoots
The plants used had developed new shoots (height = 10–30 cm) and roots and had at least one vertical rhizome growing, but not emergent. The basal parts of each shoot system lacking stomata were submerged to about 10 mm in nutrient agar; the plants were grown in culture tubes under growth room conditions, as above. Growth and time of emergence were recorded daily over 2 weeks on two sets of vertical rhizomes in: (a) controls with no oil film and (b) with a surface layer of liquid paraffin 5 mm thick on the medium surface.
RESULTS AND DISCUSSION
Effects of paraffin or diesel oil on RPF values of segments of senesced culms and living leaf sheaths
Senesced culms
Contact between nodal stomata, leaf sheaths or internodes and water, and contact between internodes and liquid paraffin had no effect on the RPF of pith cavities, compared with the non-submerged condition (approx. 60 MPa s m−3; Table 1, 1–4). However, when nodal stomata were exposed to oil, pith cavity RPF increased >170-fold within 2–3 d and in half of the samples became effectively infinite. The results indicate that oil only enters senesced culms through nodal stomata, passing via radial channels in the culm wall and blocking the pores of the pith cavity diaphragms. The latter was confirmed microscopically.
Living culms
Pith cavity RPF was unaffected by water around nodes, internodes and leaf sheaths. However, diesel oil readily entered nodal stomata and completely blocked the pith cavity diaphragms after 3 d (Table 1, 5–7); this was confirmed miroscopically. The oil no doubt spread across the culm wall via radial intercellular channels which are normally air-filled and thence into the pores of the nodal diaphragm. (Liquid paraffin took longer to penetrate, especially in young culms, where the radial channels seemed immature.)
Living leaf sheaths
Liquid paraffin readily entered leaf sheath stomata, completely blocking the aerenchyma channels, whereas exposure to water had no effect (Table 1, 8–10). The oil no doubt passed across the sheath via sub-stomatal cavities and intercellular spaces (Armstrong et al., 1996b).
Effects of oil on flow of air through excised rhizome apices
Resistances to flow of air through rhizome apices exposed to air or water were virtually the same, but after exposure to oil for 3 d values were doubled, indicating that oil penetrated the apices but water did not (Table 2). It has previously been shown that scale leaves around rhizome apices possess stomata (Armstrong et al., 2006), and it seems that the oil entered the gas space system by this means.
Table 2.
Phragmites: pressures (kPa) required to just force air out of rhizome tips under water in relation to previous immersion regime
| Immersion regime | Pressure (kPa) |
|---|---|
| Rhizome apices in air (3 d) | 1·0 ± 0·09a |
| Rhizome apices in water (3 d) | 1·03 ± 0·12a |
| Rhizome apices in liquid paraffin (3 d) | 2·14 ± 0·32b |
Apices were immersed in water while pressures were applied to see when bubbles first appeared. Means ± s.e. (n = 8); different letters indicate significant differences according to the Mann–Whitney rank sum test: P = 0·005.
Effect of oil on HIC
Oiling of nodes (diesel oil)
Convective flow rates were similar for controls and culms with oiled nodes during the first day; thereafter, flows from all the oiled culms gradually decreased and were zero by day 5 (Table 3). All control culms induced flows by the end of the experiment, but rates had decreased by 37 % by day 5, most probably because of some senescence of the sheaths. RPFs of basal node pith cavities of these culms showed that those exposed to oil were completely occluded, whereas those of the controls were normal (Table 3). It was also interesting that by day 5 the oil had spread and generally increased the pith cavity RPF of two nodes above the basal one. It was deduced that here, as found above (Table 1, 7), diesel oil can penetrate nodal diaphragms, and hence prevent convective flows to the underground rhizome. It also follows from the previous results (Table 1, 6) that, since water does not penetrate nodes, it would not prevent convective flow.
Table 3.
Phragmites, excised shoots: effects on convective flow rates and pressure flow resistances of oil around basal nodes or covering all leaf sheaths
| Basal node oiled | Non-oiled control | |
|---|---|---|
| Convective flow rate (cm3 min−1) prior to treatment | 0·33 ± 0·04a | 0·35 ± 0·05a |
| Convective flow rate (cm3 min−1) day 5 | 0b | 0·22 ± 0·04a |
| RPF (MPa s m−3) of pith cavity: node 1 (basal) day 5 | ∞x | 62·5 ± 6·38y |
| RPF (MPa s m−3) of pith cavity: node 2, day 5 | 328–∞ | 61·1 ± 3·3y |
| RPF (MPa s m−3) of pith cavity: node 3, day 5 | 128 ± 17z | 65·6 ± 4·7y |
| Leaf sheaths oiled | Non-oiled control | |
| Convective flow rate (cm3 min−1) prior to treatment | 0·25 ± 0·04a | 0·24 ± 0·05a |
| Convective flow rate (cm3 min−1) after 5 min. | 0b | 0·28 ± 0·06a |
Diesel oil was used for basal nodes and liquid paraffin for leaf sheaths.
Means ± s.e. (n = 7–10); different letters indicate significant differences according to t-test: P ≤0·007.
Oiling of leaf sheaths (liquid paraffin)
The complete oiling of all leaf sheaths immediately stopped convection (Table 3). Oil entering the stomata would prevent entry of air for humidity-induced convection. Oiling the leaf sheaths on the basal half of the shoots resulted in approx. 62 % reduction in flow rate (results not shown); the value was >50 % presumably because of the smaller surface area of the upper sheaths and the greater resistance in the train compared with those for the basal sheaths. Sponging the leaf sheaths with water resulted in only small (11 %), temporary reductions in flow rates, the shoots recovering within 30 min (results not shown).
These results support the first hypothesis.
Effect of oil on ROL
ROL to phyllospheres
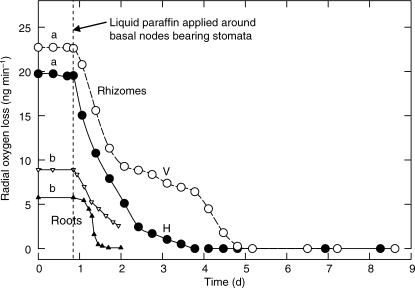
ROL from horizontal and vertical rhizome apices was appreciable both when non-stomatal parts of shoot bases were in air (not shown) and when they were exposed to oil (20–25 ng O2 min−1). However, when oil was applied to the lowest nodes bearing stomata, ROL declined to zero within 4 d (Fig. 1). Removal of the oil layer had no effect since ROL remained at zero.
Fig. 1.
Phragmites: effects of liquid paraffin around shoot bases on radial O2 loss (ROL) to phyllospheres and rhizospheres. Open and filled circles: ROL to phyllospheres around submerged vertical (V) and horizontal (H) rhizome apices, respectively. Upright and inverted triangles: ROL to rhizospheres. a = oil covering lowermost shoot nodes lacking stomata. b = non-oiled shoots. (These are typical results from three experiments.)
ROL to rhizosperes
Oil around shoot nodes bearing stomata also induced decreases in ROL from root apices (by at least 79 % per day for the roots measured: Fig. 1). Rates of decrease could no doubt have been smaller with large reserves of O2 in the rhizomes. These oil-induced decreases in ROL to phyllospheres and rhizospheres were doubtless due to oil entering nodal stomata, blocking the pith cavities at culm bases and thereby preventing convective and diffusive supplies of O2 from passing from emergent shoots to the rhizomes, and thence, by diffusion only, to root and rhizome apices. The results support the second hypothesis.
Effects of oil on surface gas films
An underwater gas film shows as a mirror-like surface due to double internal reflection. Oil spread along the surfaces of leaf laminae and sheaths displacing surface gas films as shown by the lack of reflections (Fig. 2), supporting the third hypothesis. Recently, Colmer and Pedersen (2007) demonstrated that surface gas films on submerged Phragmites shoots improve gas exchange and increase underwater photosynthesis by approx. 6- to 7-fold. It has been shown here and previously that surface oil can interrupt these gas films, suggesting that oil will reduce such underwater photosynthesis and respiration. Additionally, the oil could damage the photosynthetic apparatus as it penetrates the tissues; it was noted that exposure to diesel oil for 3 d had the effect of bleaching leaf sheaths.
Fig. 2.
Phragmites: Effects of liquid paraffin on gas film on submerged leaf lamina (A–C) and leaf sheath (D–E). (A) Lamina after 1 h in water, (B) lamina after 0·17 h with surface oil film (depth = 5 mm), (C) lamina after 36 h with oil film, (D) leaf sheath after 1 h in water, (E) leaf sheath after 1 h in liquid paraffin. Gas film shows as a mirror-like, reflective surface; note displacement of gas film by the oil and the spreading of oil along the emergent part of lamina in (C) and displacement of leaf sheath gas film in (E). Scale bars (A–C) = 4·4 mm; (D–E) = 1·56 mm.
Effect of oil on emergence and growth of shoots
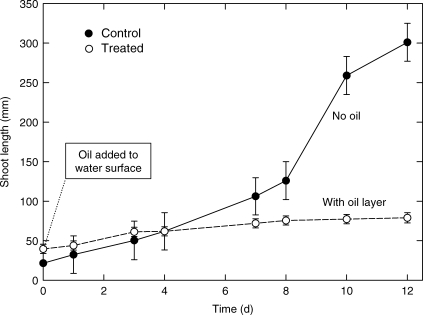
Control vertical rhizomes emerged after approx. 3·7 d from nutrient agar and showed accelerated growth, reaching an average height of 301 mm (243 mm above the medium) in 12 d (Fig. 3). Those with surface oil grew similarly while submerged in the agar, and emerged after approx. 2 d. However, after contact with the oil, they grew very slowly reaching an average height of 79 mm (only 27 mm above the medium) in 12 d, and then died. The results support the fourth hypothesis. It has been shown previously that scale leaves of rhizome apices have stomata and allow ROL from the internal gas space system to the phyllosphere (Armstrong et al., 2006); also, oil penetrates rhizome apices (Table 2). Therefore, it seems almost certain that oil penetrated scale leaf stomata, blocking gas spaces and probably damaging cell membranes (Van Overbeek and Blondeau, 1954) including those of the meristem. The results correlate with those of Mendelssohn et al. (1993), who reported reduced production of young shoots of S. alternifolia in oil-contaminated soil, and with those of Proffitt et al. (1995) on the oiling of mangrove seedlings. Moreover, death of emerging buds followed by prolonged tillering can deplete food reserves and weaken plants. It has been shown previously (Armstrong et al., 1996c) that resistances in the aeration system reduced or prevented growth of horizontal rhizome apices within a day.
Fig. 3.
Phragmites: effects of a surface layer of liquid paraffin (depth = 10 mm) on the growth of young shoots. Non-oiled control shoots were emergent after about 3·7 d. Treatment shoots emerged through the oil around day 2. n = 6; ± s.e.
CONCLUSIONS
In Phragmites, water-borne films of light oils such as liquid paraffin and diesel can spread over the shoot surface (Fig. 2) and enter stomatal pores of nodes and living leaf sheaths, passing through intercellular spaces to occlude major gas spaces, i.e. pith cavities and aerenchyma channels (Table 1), whereas water normally does not. These properties of oils are no doubt linked to their comparatively low surface tensions (light oils, 20–25 mNm−1; water, 79 mNm−1 at 20 °C) and to the oil wettability of epidermal waxes, cellulose and lignified cell walls. It has also been suggested that, similarly, oils can penetrate cells and disrupt cell membranes. Water-wetted stomatal surfaces were found to be resistant to the entry of oil, no doubt due to the immiscibility of oil and water. The diffusivity of O2 in light oils is approx. 4 × 10−5 times the value in air; hence it is not surprising that they can form effective barriers to the entry of atmospheric O2 into the plant and to its dispersal within the gas space system
During the winter, the underground parts of Phragmites are aerated principally by diffusion (especially via stubble and basal nodal stomata) and VICs via pith cavities of senesced culms, whereas during the growing season aeration is by these means together with diffusion and HIC via living leaf sheaths and culm pith cavities. Thus oil penetrating pith nodal diaphragms of both living and senesced culms and the oiling of living leaf sheaths will impede or prevent aeration of the underground parts depending on the number of culms affected. Once the oil has penetrated a nodal diaphragm of a culm's pith cavity or the aerenchyma of a leaf sheath, diffusion and convection (Table 3) by these routes appear to be prevented and the effects permanent. The persistence of an oil film must increase the number of nodes penetrated and the lengths of leaf sheath covered. It would appear that plants would be more seriously affected during the growing season, when potentially more stomata (including those of the leaf sheaths) could be exposed to the oil. However, even during the winter, occlusion by oil of a large proportion of stubble and culm basal nodes bearing stomata could seriously curtail aeration of underground organs and may lead to rhizome and root death. The strong decreases in phyllosphere and rhizosphere oxygenations by oiling basal nodes with stomata have been demonstrated here (Fig. 1). However, if pollution is not protracted, it is almost certain that some culms will be unaffected if the oil only comes into contact with internodal regions. There is a clear advantage of the Phragmites-type habit during the winter since a large proportion (approx. 95 %) of total lengths of senesced culms (internodes) above water are relatively impermeable to oil.
Another factor which could threaten the vigour and perhaps survival of the plant is the failure of young shoots to grow normally through an oil film (Fig. 3); this could induce prolonged tillering and depletion of food reserves. Also, the displacement or interruption of gas films by oil could impede underwater gas exchange. On leaf sheaths this could reduce aeration of underground parts; on laminae particularly, and to some degree on leaf sheaths, this would reduce photosynthesis. The effects could be particularly detrimental for plants growing in deep water.
Phragmites can tolerate certain degrees of sediment phytotoxicity due to Fe2+, Mn2+, sulfides and organic acids (Armstrong et al., 1996a) but this is related to effective oxygenation of rhizospheres. The latter is also important for inducing nitrification (Kirk and Kronzucker, 2005). In this respect, the convective aeration of rhizomes, which increases diffusion gradients for the aeration of root and rhizome apices, is particularly important for plants growing in deep water. Also, convective flows can increase the spread of the plant and the depths to which rhizomes and roots penetrate (Vretare, 2002). Therefore, phytotoxin tolerance, root survival and competetiveness could be threatened by oil pollution. It is possible that oily contaminants in artificial reed beds could reduce the plants' capacity to purify effluents, since this partly depends upon oxygenation of the rhizosphere regions (Brix, 1997). Some adverse effects of oil contamination of Phragmites are summarized in Fig. 4.
Fig. 4.
Some adverse effects and possible consequences of oil contamination on Phragmites australis.
ACKNOWLEDGEMENTS
We thank Mr Victor Swetez of the University of Hull Botanic Gardens for growing the Phragmites plants from seed.
LITERATURE CITED
- Alexander SK, Webb JW., Jr Seasonal response of Spartina alternifolia to oil. Proceedings, Oil Spill Conference (Prevention, Behaviour, Control, Cleanup); 25–28 February 1985; Los Angeles. American Petroleum Institute; 1985. pp. 355–357. [Google Scholar]
- Armstrong J, Armstrong W. Phragmites australis – a preliminary study of soil oxidizing sites and internal gas transport pathways. New Phytologist. 1988;108:373–382. [Google Scholar]
- Armstrong J, Armstrong W. Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (Cav.) Trin. ex Steud. New Phytologist. 1990;114:121–128. doi: 10.1111/j.1469-8137.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W. Phragmites die-back: toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytologist. 1999;142:201–217. [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM. Phragmites australis: Venturi- and humidity-induced convections enhance rhizome aeration and rhizosphere oxidation. New Phytologist. 1992;120:197–207. [Google Scholar]
- Armstrong J, Afreen-Zobayed F, Armstrong W. Phragmites die-back: sulphide- and acetic acid-induced bud and root death, lignifications, and blockages within the aeration and vascular systems. New Phytologist. 1996;a 134:601–614. doi: 10.1111/j.1469-8137.1996.tb04925.x. [DOI] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM, Halder JE, Lythe S, Holt R, Sinclair A. Pathways of aeration and the mechanisms and beneficial effects of humidity- and Venturi-induced convections in Phragmites australis (Cav.) Trin. ex Steud. Aquatic Botany. 1996;b 154:177–197. [Google Scholar]
- Armstrong J, Armstrong W, Van der Putten W. Phragmites die-back: bud and root death, blockages within the aeration and vascular systems and the possible role of phytotoxins. New Phytologist. 1996;c 133:399–413. doi: 10.1111/j.1469-8137.1996.tb04925.x. [DOI] [PubMed] [Google Scholar]
- Armstrong J, Jones RE, Armstrong W. Rhizome phyllosphere oxygenation in Phragmites and other species in relation to redox potential, convective gas flow, submergence and aeration pathways. New Phytologist. 2006;172:719–731. doi: 10.1111/j.1469-8137.2006.01878.x. [DOI] [PubMed] [Google Scholar]
- Armstrong W. Aeration in higher plants. In: Woolhouse HWW, editor. Advances in botanical research. Vol. 7. London: Academic Press; 1979. pp. 225–332. [Google Scholar]
- Atlas RM. Petroleum biodegradation and oil spill bioremediation. Marine Pollution Bulletin. 1995;31:178–182. [Google Scholar]
- Baker JM. Refinery effluent. In: Cowell EB, editor. The ecological effects of oil pollution on littoral communities. Barking, UK: Applied Science Publishers; 1971. pp. 33–43. [Google Scholar]
- Beckett PM, Armstrong W, Justin SHFW, Armstrong J. On the relative importance of convective and diffusive gas-flows in plant aeration. New Phytologist. 1988;110:463–468. [Google Scholar]
- Brix H. Do macrophytes play a role in constructed treatment wetlands? Water Science and Technology. 1997;35(5):11–17. [Google Scholar]
- Brix H, Schierup H-H. The use of aquatic macrophytes in water pollution control. Ambio. 1989;18:100–107. [Google Scholar]
- Brix H, Sorrel BK, Orr PT. Internal pressurisation and convective gas flow in some emergent freshwater macrophytes. Limnology and Oceanography. 1992;37:1420–1433. [Google Scholar]
- Brix H, Sorrel BK, Schierup H-H. Gas fluxes achieved by in situ convective flow in Phragmites australis. Aquatic Botany. 1996;54:151–163. [Google Scholar]
- Colmer TD, Pedersen O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist. 2008;177:918–926. doi: 10.1111/j.1469-8137.2007.02318.x. [DOI] [PubMed] [Google Scholar]
- Justin SHFW, Armstrong W. Oxygen transport in the salt-marsh genus Puccinellia with particular reference to the diffusive resistance of the root–shoot junction and the use of liquid paraffin as a diffusive barrier in plant studies. Journal of Experimental Botany. 1983;34:980–986. [Google Scholar]
- Kirk GJD, Kronzucker HJ. The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Annals of Botany. 2005;96:639–646. doi: 10.1093/aob/mci216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelssohn IA, Hester MW, Sasser C, Fischel M. The effect of a Louisiana crude oil discharge from a pipeline break on the vegetation of a Southeast Louisiana brackish marsh. Oil and Chemical Pollution. 1990;7:1–15. [Google Scholar]
- Mendelssohn IA, McKee KL, Kong T. An evaluation of indicators of wetland vegetation stress and their relationship to biological endpoints. Washington, DC: Office of Exploratory Research, US EPA; 1993. [Google Scholar]
- Mitsch WJ, Gosselink JG. Wetlands. New York: Van Nostrand Reinhold; 1993. [Google Scholar]
- Pezeshki SR, Delaune RD. Effect of crude oil on gas exchange functions of Juncus roemerianus and Spartina alternifolia. Water, Air and Soil Pollution. 1993;68:461–468. [Google Scholar]
- Pezeshki SR, Hester MW, Lin Q, Nyman JA. The effects of oil spill and clean-up on dominant US Gulf Coast marsh macrophytes: a review. Environmental Pollution. 2000;108:129–139. doi: 10.1016/s0269-7491(99)00244-4. [DOI] [PubMed] [Google Scholar]
- Proffitt E, Devlin DJ, Lindsey M. Effects of oil on mangrove seedlings grown under different environmental conditions. Marine Pollution Bulletin. 1995;30:788–793. [Google Scholar]
- Raskin I, Kende H. How does deepwater rice solve its aeration problem? Plant Physiology. 1983;72:447–454. doi: 10.1104/pp.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overbeek J, Blondeau R. Mode of action of phytotoxic oils. Weeds. 1954;3:55–65. [Google Scholar]
- Vretare Strand V. The influence of ventilation systems on water depth penetration of emergent macrophytes. Freshwater Biology. 2002;47:1097–1105. [Google Scholar]