Abstract
Background and Aims
Previous measurements of conifer alkaloids have revealed significant variation attributable to many sources, environmental and genetic. The present study takes a complementary and intensive, common garden approach to examine genetic variation in Pinus ponderosa var. ponderosa alkaloid production. Additionally, this study investigates the potential trade-off between seedling growth and alkaloid production, and associations between topographic/climatic variables and alkaloid production.
Methods
Piperidine alkaloids were quantified in foliage of 501 nursery seedlings grown from seed sources in west-central Washington, Oregon and California, roughly covering the western half of the native range of ponderosa pine. A nested mixed model was used to test differences among broad-scale regions and among families within regions. Alkaloid concentrations were regressed on seedling growth measurements to test metabolite allocation theory. Likewise, climate characteristics at the seed sources were also considered as explanatory variables.
Key Results
Quantitative variation from seedling to seedling was high, and regional variation exceeded variation among families. Regions along the western margin of the species range exhibited the highest alkaloid concentrations, while those further east had relatively low alkaloid levels. Qualitative variation in alkaloid profiles was low. All measures of seedling growth related negatively to alkaloid concentrations on a natural log scale; however, coefficients of determination were low. At best, annual height increment explained 19·4 % of the variation in ln(total alkaloids). Among the climate variables, temperature range showed a negative, linear association that explained 41·8 % of the variation.
Conclusions
Given the wide geographic scope of the seed sources and the uniformity of resources in the seedlings' environment, observed differences in alkaloid concentrations are evidence for genetic regulation of alkaloid secondary metabolism in ponderosa pine. The theoretical trade-off with seedling growth appeared to be real, however slight. The climate variables provided little evidence for adaptive alkaloid variation, especially within regions.
Key words: Pinus ponderosa var. ponderosa; Pinaceae; 2,6-disubstituted piperidine alkaloids; secondary products; geographic variation; progeny study; plant defense; Growth–Differentiation Balance Hypothesis; PRISM
INTRODUCTION
Pinus ponderosa produces a suite of piperidine alkaloids in most tissues (Tallent et al., 1955; Stermitz et al., 1994; Tawara et al., 1995; Gerson and Kelsey, 1998). Biosynthesis involves several pathways and intermediates leading to one predominant and stable end-product (pinidine) in mature foliage (Leete and Juneau, 1969; Leete et al., 1975; Tawara et al., 1993, 1995). Alkaloid variation due to combined environmental and genetic effects, as measured in an observational field study of ponderosa pine, is dependent on site to the extent that alkaloids may be entirely absent (Gerson and Kelsey, 1998). Environmental influences on ponderosa pine alkaloids were documented in a field fertilization study that showed the potential for nitrogen availability to influence alkaloid variation (Gerson and Kelsey, 1999a). To our knowledge, the genetic basis for intraspecific variation in conifer alkaloid production has not yet been explored. Intraspecific variation of other types of alkaloids in disparate plant genera have exhibited moderate (van Dam and Vrieling, 1994) to high levels of heritability, more so, for example, than terpenes of Pinus (Table 2 in Berenbaum and Zangerl, 1992).
Table 2.
Likelihood ratio tests for random effects on ln(total alkaloid concentration) in ponderosa pine foliage from seedlings grown in a common garden
| Model | Full | Reduced | χ2 | P |
|---|---|---|---|---|
| Family(region) | 971·3 | 977·2 | 5·8552 | 0·0155 |
| Row-plot[family(region)] | 971·3 | 979·7 | 8·3616 | 0·0038 |
| Bed | 971·3 | 984·9 | 13·5648 | 0·0002 |
As secondary metabolic products with 9-carbon:1-nitrogen structures, piperidine alkaloid synthesis may involve trade-offs with tissue growth and maintenance (Herms and Mattson, 1992; Stamp, 2003). Allocation among primary and secondary products can be influenced by environmental variables that are controlled in the common garden environment. With no herbivory, and sufficient light, water and nutrients (i.e. moderate to high resource availability and minimal intertree competition), the Growth–Differentiation Balance Hypothesis (GDBH) would predict alkaloid production to be negatively correlated with biomass production (Stamp, 2003). Furthermore, a pine seedling that has invested much into tissue growth may effectively dilute its alkaloid endowment. Intraspecific variation in growth traits in ponderosa pine are correlated with, if not adaptive to, environmental characteristics such as precipitation and temperature (e.g. Jenkinson, 1980; Sorenson et al., 2001; Kitzmiller, 2005). Thus, environmental variation has the potential to influence alkaloid variation, either directly or indirectly through effects on growth traits.
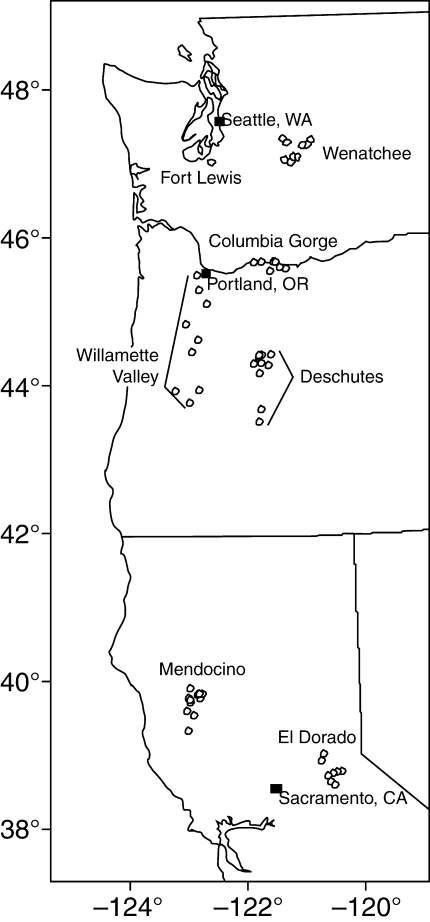
The research presented here takes advantage of a common garden study originally designed to describe geographic patterns of genetic variation in growth and phenology traits of Pinus ponderosa. Coincidentally, it provided an excellent opportunity to quantify specifically genetic variation of alkaloid concentration in a large number (500 + ) of ponderosa pine seedlings. The seven regions covered in this study were selected to represent a range of latitudes, elevations and climates within the historic range of the nominate form of ponderosa pine (Oliver and Ryker, 1990). They encompass roughly 9 ° of latitude and 2·5 ° of longitude in the western USA (Washington, Oregon and northern California) (Fig. 1). Within each region, open-pollinated seeds were collected from nine parent trees and grown in a nursery located in the Willamette Valley region. At the end of the seedlings' third growing season, alkaloids were quantified in current-year needles, heights and diameters were measured, and then they were destructively sampled to measure root and shoot biomass. With this information, it was possible to establish the baseline (genetic) alkaloid endowment for ponderosa pine under optimal nutrition and growth, and to characterize the variation within and among disparate geographical areas. Having growth data available on the same experimental units also allows intraspecific relationships for alkaloid concentrations vs. height, diameter, root and shoot growth to be described and metabolite allocation theory to be tested. Additionally, the broad geographical range of seed sources incorporated in this study enables possible associations between the climates (i.e. temperature and precipitation regimes) to which the parent trees were subjected and the alkaloid productivity of their offspring to be investigated. This study also documents the ubiquity of alkaloids within ponderosa pine, and helps to address the question of how much sampling is needed to characterize the alkaloid profile of a pine species. Qualitative profiles of alkaloids vary substantially among Pinus species and may be useful for chemotaxonomic purposes (Tallent et al., 1955; Gerson and Kelsey, 2004; but see Stermitz et al., 2000). Bioassays have indicated toxic, teratogenic, antifeedant and antimicrobial properties of the conifer alkaloids (Eisner et al., 1986; Schneider et al., 1991; Tawara et al., 1993; Stermitz et al., 1994; Eriksson, 2006); however, an ecologically relevant purpose or function for these constitutive compounds has yet to be found (Kamm, 1998; Gerson and Kelsey, 2002).
Fig. 1.
Map of parent tree locations within the seven regions studied.
In summary, the study design enabled the following primary questions to be addressed. (a) Are there genetic differences in foliar alkaloid concentrations among regions and among families within regions? (b) How is the variation in alkaloids distributed across the hierarchical levels of the study? (c) Is there a (negative) relationship between alkaloid and biomass production in ponderosa pine? (d) Are alkaloid concentrations associated with climate characteristics at seed source locations?
MATERIALS AND METHODS
Study design
Pinus ponderosa Dougl. ex Laws var. ponderosa (Oliver and Ryker, 1990) seedlings from open-pollinated seed collected from seven regions within Washington, Oregon and northern California, USA (Fig. 1), were grown for 3 years in a common garden of raised nursery beds in the Willamette Valley, Oregon. Within each region, seeds were collected from nine parent trees. The locations of parent trees and their associated seed banks are detailed in the Supplementary Data (available online). In the nursery, row-plots were planted with four seeds from a single parent tree. Sixty-three row-plots (one per parent tree) were randomly distributed in a complete block design that was replicated over four raised beds. Seedlings were well watered and fertilized during the first half of the summer, with declining water and fertilizer at the end of the growing season to promote bud-set. After the third growing season (late November), healthy needles from the current year were sampled from two seedlings per row-plot and analysed separately (as row-plot sub-samples) for alkaloids. Dry mass of foliage was recorded. Sampled seedlings then were harvested and oven-dried for root and shoot biomass measurements, reincorporating the weight of tissues taken for alkaloid analysis. In summary, the study design entailed 7 regions × 9 parent tree seed sources per region × 2 seedling offspring per parent tree row-plot × 4 nursery bed replications, providing a total of 504 observations; however, three were lost to seedling mortality.
Regions
For the purposes of this study, ‘region’ is defined as the broad geographic location of a group of parent (seed) trees (Fig. 1). Within each region, parent trees occurred at a range of latitude, longitude and elevation (Table 1); however, mean elevations of parents in three regions [Columbia Gorge (CG), 190 m; Fort Lewis (FL), 115 m; and Willamette Valley (WV), 172 m] were distinctly lower than the means for the other four regions [Deschutes (DS), 1155 m; Eldorado (EL), 1200 m; Mendocino (MN), 1219 m; and Wenatchee (WN), 1119 m]. East of the Cascade Crest, Wenatchee, Columbia Gorge and Deschutes have continental climates (average temperatures below 0 °C in the coldest months). The temperate climates of the other regions have varying degrees of maritime influence: Fort Lewis is located on lowlands south of Puget Sound; Willamette Valley and Mendocino are in the Pacific Coast Ranges; and Eldorado lies on the western slope of the Sierra Nevada, north-east of San Francisco Bay.
Table 1.
Physiographic range of parent tree (i.e. seed source) locations (nine trees per region)
| Region | Latitude range | (–)Longitude range | Elevation (m) |
|---|---|---|---|
| Fort Lewis (FL) | 47·03–47·05 °N | 122·53–122·55 °W | 108–119 |
| Wenatchee (WN) | 47·03–47·36 | 120·57–121·11 | 687–1721 |
| Columbia Gorge (CG) | 45·58–45·70 | 121·11–121·72 | 61–735 |
| Willamette Valley (WV) | 43·78–45·51 | 122·64–123·22 | 61–288 |
| Deschutes (DS) | 43·54–44·44 | 121·43–121·75 | 854–1391 |
| Mendocino (MN) | 39·36–39·93 | 122·78–123·01 | 995–1514 |
| Eldorado (EL) | 38·60–39·02 | 120·36–120·70 | 1047–1410 |
Piperidine alkaloid quantification
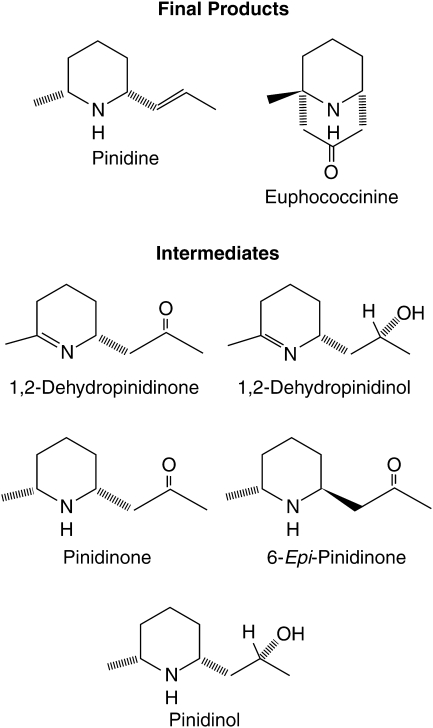
Methodology followed Gerson and Kelsey (1999b). Briefly, a 0·5 g sample of oven-dried, ground foliage was extracted in aqueous acid then centrifuged; the supernatant was basified and the alkaloids partitioned into chloroform using an Extrelut® solid-phase column. The eluate was reduced, an internal standard added, and the solution increased to a known volume. The following piperidine alkaloids (Fig. 2) were identified and quantified using gas chromatography–mass spectrometry (GC-MS): pinidine; pinidinone; 6-epi-pinidinone; pinidinol; 1,2-dehydropinidinone; 1,2-dehydropinidinol; euphococcinine; and RT 6·80. Pinidine is the primary end-product of alkaloid biosynthesis in ponderosa pine, and euphococcinine is an alternative end-product (Tawara et al., 1993, 1995). The remaining alkaloids listed are intermediates. Although it has never been officially described, RT (retention time) 6·80 very probably is 1,6-dehydropinidinone (Todd, 1994), based on the molecular ion (153 m/z) and mass spectrum. Another unknown, probable alkaloid with molecular ion 167 m/z, was observed but not incorporated in the data analysis (because it has not been properly described, and was a minor, sporadic component). The presence of the 6-epi form of pinidinone is interesting because, heretofore, only cis-2,6-disubstituted piperidines have been found in Pinus species (Tawara et al., 1993; Stermitz et al., 1994). Both isomers of pinidinone occurred sporadically at low concentrations in a quarter to a third of the samples.
Fig. 2.
Structures of piperidine alkaloids quantified in extracts of Pinus ponderosa foliage.
Statistical analyses
Differences in alkaloid concentrations among regions, and among families within regions
SAS MIXED procedure (SAS Institute, Inc., 1996) was used to fit a linear model with nested treatments (parent nested within region, and row-plot nested within parent). The REML method was specified, along with the KR option for appropriate denominator degrees of freedom. ‘Region’ was modelled as a fixed effect, selected based on characteristics of interest. ‘Parent’ (or ‘family’) was a random effect nested within region; parent trees were selected randomly in the field. Half-sibling seedlings grown from a single parent seed tree constitute a ‘family’. Half-sibling seedlings were grouped into row-plots in the nursery beds, hence row-plots are the experimental units and seedlings are sub-samples of the row-plots. ‘Row-plot’ is a random effect nested within parent within region, and ‘bed’ is a random block effect. No crossed-block effects were assumed. Parallel analyses were done for pinidine and total alkaloid concentrations as response variables. Ranges of the alkaloid variables were large, and the data followed log-normal distributions. Natural log transformations of pinidine and total alkaloid concentrations yielded normally distributed residuals and constant variance.
For the effect of region, SAS Type 3 F-tests and least-squares (LS) means are reported. The 95 % confidence intervals for the regional means are presented graphically; the intervals are uneven because upper and lower limits, as well as the means, were back-transformed. Comparisons between ranked means were made with Tukey–Kramer adjustments. Helmert contrasts were constructed to compare groups of regions by longitude and elevation. For the random effects, likelihood ratio tests were constructed (Tao et al., 2002). BLUPs (best linear unbiased predictors) of parent within region are presented because parent within region is a random effect. These were obtained with SAS Estimate statements.
Distribution of alkaloid variation
Variation in alkaloid concentrations was partitioned into the following sources: among experimental blocks (i.e. nursery beds); among regions; among families (within regions); among row-plots (within families within regions); and residual error. The latter includes seedling to seedling variation, as well as experimental error. These variance components were estimated by SAS MIXED procedure, with the Type I estimation method option. The response variable was total alkaloids. All sources (including region) were modelled as random effects for the purpose of computing variance estimates.
Relationship between seedling growth and alkaloid production
The following growth variables were measured: height, diameter, stem dry mass and root dry mass. Diameter was taken at 1 mm above the root collar. Annual height and diameter growth increments were calculated for the year seedlings were sampled. Simple linear regression models employing the growth variables individually were run with the SAS GLM procedure to evaluate whether any of the variables were associated with total alkaloid concentrations (as the dependent variable). Family means (n = 63) were used because observations on half-sibling seedlings lack independence. Mean total alkaloid concentrations were transformed to natural logs as residuals plots exhibited non-constant variance. Added variable plots were constructed to test whether adding multiple growth variables to the model would account for more of the variation.
Relationship between source climate and alkaloid production
Climate characteristics specific to each (n = 63) parent tree location were obtained from the Parameter-Elevation Regressions on Independent Slopes Model (PRISM), as detailed in St Clair et al. (2005). Variables are defined as follows: ANNAVT, annual average temperature; ANNPRE, annual precipitation; APRPRE, April precipitation; FFPRE, precipitation during the frost-free period; FRSTFREE, length of frost-free period; JULARID, ratio of July precipitation to July average temperature (+10); RNGAVT, temperature range between highest and lowest monthly average; SPRFRST, Julian date of last 0 °C day in spring; SUMMAXP, summer maximum precipitation; SUMMAXT, summer maximum temperature; WINMAXP, winter maximum precipitation; and WINMINT, winter minimum temperature. Climate variables were evaluated individually as independent variables in simple linear regression models using the SAS GLM procedure. For the dependent variable, total alkaloid means for families of seedlings from corresponding parent tree locations were used. Natural log transformations of the alkaloid means were used to address non-constant variance in the residuals.
RESULTS
Total alkaloid and pinidine concentrations as response variables of mixed models
Regional effects
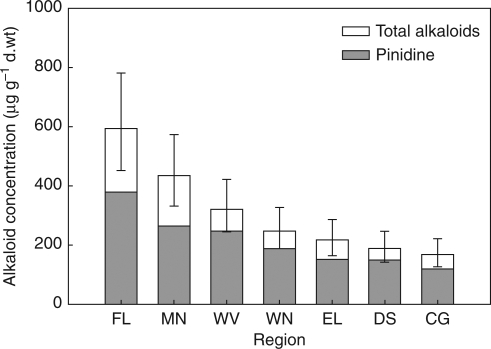
Significant differences in alkaloid concentrations exist among the seven regions (6,56 d.f.; F = 20·56; P < 0·0001), indicating a genetic basis for intraspecific variation in ponderosa pine alkaloids. Back-transformed LS mean concentrations of total alkaloids are presented in Fig. 3. Results were similar for differences in pinidine concentrations (6,56 d.f.; F = 21·86; P < 0·0001). The Mendocino mean was relatively lower, closer to the Willamette Valley mean, and the Eldorado and Deschutes means were more similar, but the rank order of the regional means was identical. One distinction is apparent: although the range of pinidine concentrations in Mendocino and Willamette Valley seedlings was similar, the Mendocino seedlings had higher total alkaloid concentrations. On the other hand, the total alkaloid concentrations of Deschutes seedlings appeared to be comprised of relatively high proportions of pinidine.
Fig. 3.
Least-squares means for the fixed effect of region on total alkaloids and pinidine (error bars indicate 95 % confidence intervals).
With Tukey–Kramer adjustments, none of the proximal pairs of total alkaloid means (adjacent bars in Fig. 3) was different (all Padj > 0·1). However, mean alkaloid concentrations in the Fort Lewis region were significantly higher than in the Willamette Valley region (55·7 d.f., t = 4·28, Padj = 0·001). Also, the Mendocino mean was higher than that of Wenatchee (55·7 d.f., t = 3·91, Padj = 0·005), and the Willamette Valley mean was higher than that of Deschutes (55·3 d.f., t = 3·74, Padj = 0·008), but not Eldorado (55·7 d.f., t = 2·72, Padj = 0·112). Among the four lowest means, there were no significant differences (all Padj > 0·1). Coincidentally, these four regions (WN, CG, DS and EL) all lie east of –122 °W longitude, whereas the three highest means were for the western regions (FL, WV and MN). The east–west contrast between these groups was significant (1, 55·6 d.f.; F = 96·85; P < 0·0001). The contrast between low elevation regions (CG, FL and WV) and high elevation regions (DS, EL, MN and WN) also was significant (F = 7·37; P = 0·009).
Within-region family effects
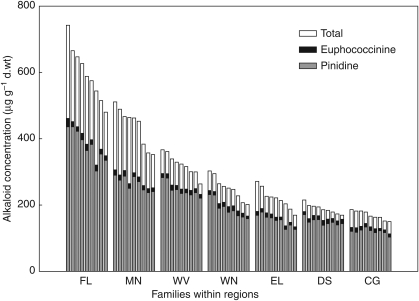
Likelihood ratio tests revealed significant differences in total alkaloid concentrations among families within regions, as well as for the other random effects in the mixed model (Table 2). The BLUPs for each parent were back-transformed and graphed for total alkaloids, euphococcinine and pinidine (Fig. 4). Each vertical bar represents one of nine families of seedlings grown from parents within the region. Overlaying the graphs with the same y-scale illustrates the proportion of the two final products, pinidine and euphococcinine, in the total alkaloid concentration.
Fig. 4.
Best linear unbiased predictors (BLUPs) for the effect of family within region. End-product bars (pinidine and euphococcinine) are stacked within total alkaloids.
Variance components
Residual error was the largest component of variation in total alkaloid concentrations (Table 3), which represents seedling to seedling variation within row-plots, in addition to measurement error. Among the remaining sources of variation, regional effects was by far the largest and 4·6-fold higher than at the level of families.
Table 3.
Variance estimates partitioned by source, for the response: total alkaloid concentration in ponderosa pine foliage from seedlings grown in a common garden
| Source of variation | d.f. | Expected mean square | Variance component | Percentage of total variation |
|---|---|---|---|---|
| Bed | 3 | σe2 + 1·99σw2 + 0·01σp/r2 + 0·01σr2 + 125·25σb2 | σb2, 1638 | 1·7 |
| Region | 6 | σe2 + 1·99σw2 + 7·95σp/r2 + 71·57σr2 | σr2, 31 629 | 33·6 |
| Family(region) | 56 | σe2 + 1·99σw2 + 7·95σp/r2 | σp/r2, 6903 | 7·3 |
| Row-plot[family(region)] | 186 | σe2 + 1·99σw2 | σw2, 10 464 | 11·1 |
| Residual error | 249 | σe2 | σe2, 43 421 | 46·2 |
Growth variables and alkaloid production
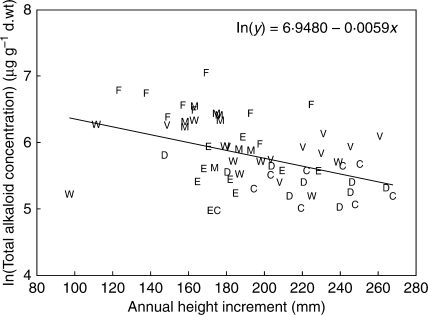
Regressions of ln(mean total alkaloid concentration) on the family means of the growth variables resulted in negative slope estimates for every variable; however, slopes for seedling diameter and current-year diameter growth increment were not significantly different from zero (Table 4). All slope estimates appear to be very small because the y-axis is on a log scale. Coefficients of determination were low; at best, height increment explained 19·4 % of the variation. Overall height accounted for 15 %, and the biomass variables for <10 %. All the growth variables are correlated with each other, some more (e.g. root and stem biomass, 0·89) than others (e.g. height increment and root biomass, 0·68). Adding the root biomass variable, or the diameter increment variable, into the model with height increment failed to explain any additional variation in alkaloid concentrations. Thus, using slope and intercept estimates for the regression of ln(total alkaloids) on height increment (Fig. 5) and back-transforming the regression equation, the strongest association found between seedling growth and alkaloid production took the form: total alkaloid concentration = 1041·1e(−0·0059 × height increment). With this model, if height increment doubles from 100 to 200 mm, total alkaloid concentration drops from 577 to 320 µg g−1, a decrease of 55·5 %. Unfortunately, confidence intervals for the alkaloid concentrations are quite large.
Table 4.
Results from simple linear regressions of ln(family means for total alkaloids) vs. family means for each growth variable (n = 63)
| 95 % confidence limits |
|||||
|---|---|---|---|---|---|
| Variable | P | R2 | Slope estimate | Lower | Upper |
| Seedling height | 0·002 | 0·149 | –0·0029 | –0·0047 | –0·0011 |
| Height increment | <0·001 | 0·194 | –0·0059 | –0·0090 | –0·0028 |
| Seedling diameter | 0·070 | 0·053 | –0·0009 | –0·0018 | 0·0001 |
| Diameter increment | 0·114 | 0·040 | –0·0014 | –0·0032 | 0·0004 |
| Stem biomass | 0·034 | 0·072 | –0·0002 | –0·0005 | –0·00002 |
| Root biomass | 0·018 | 0·089 | –0·0009 | –0·0017 | –0·0002 |
| Total biomass | 0·025 | 0·079 | –0·0002 | –0·0004 | –0·00003 |
Fig. 5.
Total alkaloid family means on a natural log scale plotted against current-year height increment means by family. Letters indicate the region of the parental seed source: C, Columbia Gorge; D, Deschutes; E, Eldorado; M, Mendocino; W, Wenatchee; V, Willamette Valley. Regression equation n = 63 families.
Climate variables and alkaloid production
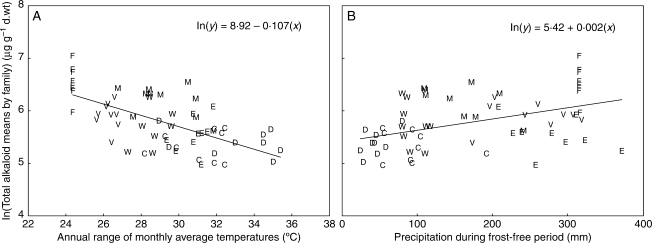
Among the 12 measures of climate, range of monthly average temperatures (RNGAVT) had the highest R2 (Table 5) and the only convincing relationship (Fig. 6A). Regressions of six other variables were significant (P < 0·05), but only the plots of ln(mean total alkaloids) vs. FFPRE (Fig. 6B) and SUMMAXP were linear and not splintered into regional groups. Both variables are measures of growing season precipitation and had small, positive slopes.
Table 5.
Results of simple linear regression of ln(total alkaloid family means) on climate characteristics at parent tree locations (n = 63)
| Variable | R2 | P |
|---|---|---|
| ANNAVT | 0·027 | 0·202 |
| ANNPRE | 0·068 | 0·039 |
| APRPRE | 0·049 | 0·081 |
| FFPRE | 0·197 | <0·001 |
| FRSTFREE | 0·125 | 0·005 |
| JULARID | 0·055 | 0·066 |
| RNGAVT | 0·418 | <0·001 |
| SPRFRST | 0·140 | 0·003 |
| SUMMAXP | 0·116 | 0·006 |
| SUMMAXT | 0·044 | 0·100 |
| WINMAXP | 0·038 | 0·128 |
| WINMINT | 0·183 | 0·001 |
Fig. 6.
Total alkaloid family means on a natural log scale vs. (A) average temperature range at parent tree location (RNGAVT), and (B) amount of precipitation during frost-free period at parent tree location (FFPRE). C, Columbia Gorge; D, Deschutes; E, Eldorado; M, Mendocino; W, Wenatchee; V, Willamette Valley.
DISCUSSION
The large differences among regions in alkaloid concentrations indicated significant intraspecific genetic variation in ponderosa pine. In the present study, the highest total mean alkaloid concentrations occurred in the northwestern Fort Lewis region, and the lowest in the Columbia Gorge (less than a third of the Fort Lewis mean). Although these two regions were the most disparate in alkaloids, geographically they are relatively close to one another. Overall, a large difference was found between higher alkaloid concentrations in the western regions (FL, WV and MN) and lower concentrations in the eastern regions. For an explanation of such a major biochemical distinction across a large geographic space, it is necessary first to look to the evolutionary history of ponderosa pine in western North America. Piperidine alkaloid production appears to be an ancestral trait, as it occurs most commonly in the genera Pinus and Picea (Tallent et al., 1955; Schneider et al., 1991; Tawara et al., 1993; Stermitz et al., 1994, 2000), which are thought to be relatively basal in the phylogeny of the Pinaceae (Price et al., 1998; Wang et al., 2000). Variation in such a trait may reflect migration, geographic isolation and genetic drift over time, in addition to adaptation to environmental drivers. Pinus ponderosa is considered to have originated in northern latitudes (Millar, 1998), but unfortunately its paleo record is very sparse within the present study area: there is one occurrence in the central Sierra Nevada approx 12 000 BP (MacDonald et al., 1998), and two glacial-age possibilities from Battle Ground Lake, Washington and Kings Canyon, California (Norris et al., 2006). During the last glacial maxima some 20 000 years ago, ice sheets would have covered the Fort Lewis area, and perhaps Wenatchee. Deschutes and Eldorado were near or directly impacted by mountain glaciation. Modern populations in these regions would certainly have migrated there more recently, whereas the Willamette Valley and Mendocino populations may be older. Much of the northwestern climate roughly 35 000–15 000 years BP may have been unsuitable for ponderosa, with the exception of along the coastal margins (Norris et al., 2006). This history could explain the similarly high alkaloid characteristic of these western regions, and leads one to suspect the Fort Lewis population was founded post-glacially from western sources. The crest of the Cascade mountain range and the large Central Valley of California could have slowed east–west gene flow and facilitated differentiation between western and eastern regions.
Over the years, the range of ponderosa pine has been dissected in somewhat different ways based on commonalities in various traits such as growth, phenology, chemistry and allozymes. Early on, Wells (1964a, b) developed progeny–environment relationships using ponderosa pine sources comparable with the present Wenatchee, Willamette Valley, Deschutes, Mendocino and Eldorado regions. Within P. ponderosa var. ponderosa, he distinguished a ‘California ecotype’ (including both MN and EL) apart from a ‘North Plateau ecotype’ (including WN and DS). His Willamette Valley sources were notably different from the other Pacific Northwest sources, in some ways more closely resembling California progeny, and so were not included in either division. Regarding the monoterpene composition of xylem resin (Smith, 1977, 2000; Sturgeon, 1979; Conkle and Critchfield, 1988), chemical regions or zones for ponderosa pines appear to agree fairly well with Wells' morphologically based distinctions. In an extensive comparison, Smith (1977, 2000) mapped the entire ponderosa species by monoterpene chemotypes. Three of the regions in the current study (DS, MN and EL) overlapped his plots, and a fourth in Washington state was between Fort Lewis and Wenatchee. Unfortunately, he had no data points for Willamette Valley, and he placed that area in an expansive ‘Cascade-Northern’ region that also encompassed part of P. ponderosa var. scopulorum. Southwestern Oregon and all except southern California were separated into a ‘Sierra-Pacific’ region which would include both of the California regions in the present study (MN and EL). Sturgeon (1979) then focused on the transition zone between the two subdivisions (Cascade-Northern/North Plateau vs. California/Sierra-Pacific) in the vicinity of the OR–CA border, where he confirmed higher proportions of several monoterpenes in the California/Sierra-Pacific zone. Conkle and Critchfield (1988) reviewed the multiple lines of investigation on intraspecific variation in P. ponderosa var. ponderosa and decided Willamette Valley ponderosa probably represented a northern extension of the Pacific region. They settled on a ‘Pacific race’ incorporating most of California plus Oregon west of the Cascades (including the present EL, MN and WV regions), and a ‘North Plateau race’ for central and eastern Oregon and Washington (including the present DS, CG and WN regions) plus areas further north and east. To some extent, the pattern of alkaloid variation observed in this common garden study corresponds to the distinctions between the Pacific and North Plateau races of Conkle and Critchfield (1988), in as far as it coincides with the present east–west differences. The primary lack of fit is the Eldorado region in California. Although located in their Pacific race, it has alkaloid levels comparable with the eastern regions (DS, CG and WN) that lie within their North Plateau race. The Fort Lewis region was not included in their race maps. It is located in a somewhat isolated pocket of ponderosa pine in northwestern Washington. As the highest ranked alkaloid producer in the present study, with Mendocino, second, and Willamette Valley, third, the Fort Lewis region appears to be an even further northward extension of the Pacific race. There was no north–south trend or progression within the western, nor the eastern, regions. The present study encompasses most of the native range in latitude of P. ponderosa var. ponderosa, short several degrees north and south, but variation at the margins remains possible and, perhaps, likely. For terpenes in field-collected foliage of ponderosa pine, Von Rudloff and Lapp (1991) reported no significant north–south differences; they also described P. ponderosa var. ponderosa as remarkably uniform in terms of leaf oil composition. For alkaloids in Duboisia myoporoides from northern and southern Australia, Gritsanapan and Griffin (1991) found inconsistent variation with latitude. North–south clines in genetic variation have been found, however, for Pinus strobus and Picea glauca in Canada (Li et al., 1997a, b).
Elevation is typically considered in progeny studies as a potential explanatory variable for trait variation. A preliminary report from Carey and Wink (1994) showed a negative relationship between altitude and alkaloid production in Lupinus argenteus grown in a greenhouse from seed collected at high and low elevations. In their case, the genetically based elevation effect was attributed to a history of selective pressure by herbivores more prevalent at lower elevations. The present examination of the effect of parent tree elevation also indicated an inverse relationship: as a group, low elevation (<200 m) regions had a significantly higher mean alkaloid concentration than the high elevation (>1200 m) group. Yet, there were large inconsistencies within the comparison: Columbia Gorge, with low mean elevation, had the lowest alkaloid mean; conversely, Mendocino, with high mean elevation, had the second highest alkaloid mean (see Table 1 and Fig. 3). As far as elevation is a proxy for temperature, the wide range of latitude among the regions studied may have contributed to the inconsistencies.
Seven of the climate variables examined were functions of temperature (ANNAVT, FRSTFREE, JULARID, RNGAVT, SPRFRST, SUMMAXT and WINMINT). Only one of these, the annual range between highest and lowest monthly average temperatures (i.e. RNGAVT), appeared to relate significantly to alkaloid concentrations. Across the broader geographic area of this study, total alkaloids were higher where temperature ranged less; however, this negative relationship did not hold within regions (see Fig. 6A). Presumably, local factors take precedence at the smaller scale. Overall, the magnitude of the difference between temperature extremes is key. No indication was found that high or low extremes alone (i.e. SUMMAXT and WINMINT) correlated with alkaloid concentrations.
Precipitation, more specifically the timing of it, has been identified as a distinguishing variable between the ranges of P. ponderosa var. ponderosa and P. ponderosa var. scopulorum (Wells, 1964b; Norris et al., 2006). Further, within P. ponderosa var. ponderosa, two precipitation regimes have been delineated: primarily winter moisture; and winter + ‘summer’ (growing season) moisture. West of the Cascades and Sierra Nevada, lower elevation and proximity to the Pacific Ocean extend the growing season into early spring when significant precipitation as rain occurs. The high alkaloid, western regions (FL, WV and MN) are well within this winter + ‘summer’ precipitation area, and the low alkaloid, eastern regions (DS, CG, WN and EL) are in or near the primarily winter precipitation (as snow) area. Thus, the seasonality of precipitation map of Norris et al. (2006) coincides better with the present pattern of alkaloid levels than other maps derived from growth traits or terpene chemistry. Regressions of five source-specific metrics of precipitation failed, however, to bear out this apparent, positive association between growing season moisture and higher alkaloid levels. The variable, FFPRE, a measure of precipitation during the frost-free period, was expected to best reflect growing season moisture. It did yield the highest R2 among the precipitation variables (19·7 %), but it could not be concluded from these data that there is a positive relationship (Fig. 6B). Ralphs and Gardner (2001) suspected that higher soil moisture from summer precipitation on natural populations of tall larkspur (Delphinium barbeyi) was responsible for higher toxic alkaloid concentrations, compared with duncecap larkspur (D. occidentale). However, reciprocal common garden tests of the two larkspur species showed no inherent differences in toxic alkaloids. Water availability has been generally thought to be negatively associated with alkaloid production in plants (Herms and Mattson, 1992). Water restriction has the potential to favour alkaloid synthesis by limiting growth, thereby increasing availability of substrates for allocation to secondary metabolism (Herms and Mattson, 1992); or rather, the mechanism may be increased leaf nitrogen (with comparable allocation) leading to higher alkaloid contents (Hoft et al., 1996). Duration of drought is also a factor, and elimination of water stress can reduce alkaloid concentrations (Krejsa et al., 1987).
There are differences in alkaloid concentrations among families within regions, but not as great as differences among regions. One-third of the total observed variation was distributed at the regional level, while only 7 % occurred at the family level (see Table 3). In comparison with the piperidines in the present study, constitutive pyrrolizidine alkaloid concentrations varied considerably among full-sibling families of houndstongue (Cynoglossum officinale) and heritability was significant (van Dam and Vrieling, 1994) (variance components were not stated). In a study of bush lupine (Lupinus arboreus), Adler and Kittelson (2004) observed large differences among collection sites and lesser differences among maternal families in terms of seed alkaloids, but no maternal effect on leaf alkaloids in progeny. For ponderosa pine located in the Deschutes region and eastern Oregon, a range of growth and phenology traits exhibited generally equal or higher variance among families within location compared with variance among locations (Sorensen and Weber, 1994). This lack of differentiation between two regions east of the Cascade Crest fits with the present observations, but over the entire scope of this study ponderosa pine appears to be rather highly differentiated with respect to the alkaloid trait. Li et al. (1997a) suggest that high regional variance in a number of Pacific Northwest conifers may be a product of environmental heterogeneity, compared with species with more northern and eastern (Canadian) distributions. Eastern and western white pine (Pinus strobus and P. monticola, respectively) are noted exceptions in their respective geographic areas (Li et al., 1997b).
Resource availability to the seedlings was designed to be uniform in the common garden, so the basic, source-driven GDBH was not directly tested in this study. Rather, a demand-side model of the GDBH (as in Lerdau et al., 1994) may be applied. Under this model, allocation to secondary products is subject to competing (genetically determined), phenologically driven demand for biomass. In the present study, the observed variation in total foliar alkaloid concentrations was only partially attributable to growth traits (seedling height growth, in particular). As theory would predict, alkaloid concentrations were negatively correlated with the growth variables. These variables all related linearly to the natural log of alkaloid concentrations (e.g. Fig. 5), or as inverse exponentials to untransformed alkaloid concentrations. No linear combinations of growth variables were found that would explain any more of the variation in alkaloids beyond that which was accounted for by current-year height increment (i.e. 19·4 %). Among the growth measures available, the annual increments best represent allocation of resources during the same period of time that total alkaloids accumulated in current-year foliage, so it may be noteworthy that height growth increment showed the strongest inverse relationship to alkaloids. On the other hand, diameter increment was the least significant among them.
The growth vs. alkaloid relationships described above are applicable across the broad geographic scope of the study, but in similar regressions within regions (n = 9), slopes were not detectably different from zero. In other ways, associations involving growth traits are not necessarily maintained over the range of this study. In southern Oregon, Sorenson et al. (2001) found that size traits in ponderosa pine (including height, diameter and biomass) responded strongly to elevation in the North Plateau race, but not in the Pacific race. In California, Jenkinson (1980) observed large differences in the timing of root growth among ponderosa seedlings from sources along an elevational transect within the Eldorado region. The seedlings in the present study were harvested in the autumn, possibly favouring higher root biomass in families from lower elevations. Confounding factors such as this probably contribute to variation in the data. For example, growth potential of ponderosa pine is inversely associated with seed source elevation, but temperature may be the real controlling factor (Kitzmiller, 2005). Differing precipitation patterns at the seedlings' sources (as previously discussed) is another potentially confounding factor likely to affect growth potential, if not alkaloid production directly. Orians et al. (1996) detected no correlation between plant growth parameters and heritable leaf chemistry (phenolic glycosides), despite controlling for significant block effects. They postulated that a time lag between measurements or nutrient limitations to growth could have influenced their findings (not likely to be issues in the present study). Others (van Dam and Vrieling, 1994) have seen genetically based, intraspecific variation in alkaloid levels with no correlation to plant biomass. Finally, the GDBH allocates resources between primary and secondary productivity, and although this study provided a solid quantitation of primary productivity in terms of growth, only the alkaloid component of secondary productivity was measured. As piperidine alkaloid concentrations are a minor component compared with terpenes and other secondary defence chemicals of ponderosa pine, no more than a partial accounting for the variation should be expected.
Most analyses of conifer alkaloids have been done with mature trees, and variation with tree maturation is not well documented. Other types of plants are known to have different levels of alkaloids at different life stages (e.g. Ralphs et al., 1997). For Picea pungens, Todd et al. (1995) provided a systematic comparison of alkaloids in young seedlings and mature trees, and noted qualitative and quantitative changes with age, but overall variation eclipsed any general age effect, if there was one. In another study, alkaloid profiles of whole ponderosa pine seedlings traced for 1 month past germination showed increasing, then decreasing, concentrations of intermediates (Tawara et al., 1995). Previously, generally higher alkaloid concentrations have been observed in Willamette Valley saplings compared with mature trees in the Deschutes region (Gerson and Kelsey, 1999a, b, 2004), raising the question of whether these differences were attributable to tree maturity, site quality or genetics. In the present study, total alkaloid concentrations in Deschutes region seedlings were low, as were concentrations measured in previous studies on mature ponderosa pine trees from the same region (even after fertilization) (Gerson and Kelsey, 1998, 1999a). This provides some evidence that foliar alkaloid concentrations are similar for young and old trees from the same region.
With respect to qualitative differences in alkaloid profiles (as opposed to quantitative variation in total alkaloid concentrations), variation is substantial among Pinus species (Tallent, 1955; Tawara et al., 1993; Stermitz et al., 1994; Gerson and Kelsey, 2004), to the extent that euphococcinine, rather than pinidine, is the dominant alkaloid in some species. In contrast, within ponderosa pine, little intraspecific qualitative variation that could be sorted into chemotypes was observed. Intraspecific differences in alkaloid profiles of other plants do occur with geography (Wink and Carey, 1994), and there was some evidence for this in the stacked bars of Fig. 4. The Fort Lewis and Mendocino seedlings had slightly lower proportions of pinidine in the total, otherwise the differences within and among regions were primarily a matter of quantity rather than composition. Euphococcinine, the secondary end-product of alkaloid biosynthesis in ponderosa pine, appeared consistently in very minor proportions (3–6 %) across the regions. This same alkaloid is synthesized de novo by a bean beetle (Epilachna varivestis) for deployment as a feeding deterrent (Eisner et al., 1986), but the minor quantities found in ponderosa pine suggest euphococcinine has not been selected for. Latta et al. (2003) suggested that trade-offs in production of particular monoterpenes within the total pool can occur where resources are limited, and this situation would lead to negative correlations between terpenes. They did find strong negative correlations for three major monoterpenes of field-sampled ponderosa pine. In the present case, however, log-transformed pinidine and euphococcinine concentrations related weakly, but positively with one another (R2 = 13·1 %, simple linear regression, P < 0·001) within the total alkaloid pool. From this, it is inferred that composition and quantity of alkaloids were not resource-constrained, indeed as the controlled experiment in nursery beds was designed to be.
The findings of this study are based on one common garden located in the Willamette Valley region. Interactions between seed source and test locations can occur for ponderosa growth traits (Kitzmiller, 2005; Zhang and Cregg, 2005), thus it is might be possible to find different degrees of variation in alkaloids, or even different rankings of regional means at reciprocal garden locations. Adler and Kittelson (2004) found that population-level variation in bush lupine alkaloid profiles depended on destination site. In a comparison of alkaloids in two species of Delphinium (Ralphs and Gardner, 2001), a significant difference occurred at one garden location, but not the other, in one of two years, and was attributed to differences in precipitation. Climate at the Willamette Valley study site is mesic and seedlings were well watered, and a range of alkaloid production was observed, so there is no compelling reason to suspect that the common garden environment was limiting. However, it would be hazardous to predict the same piperidine alkaloid variation from the same ponderosa pine sources grown in a common garden in a continental–montane environment, for example. With the exception of nitrogen availability, it is not known what environmental variables may affect the expression of alkaloid-producing genes in conifers.
Of 501 seedlings sampled, every one had measurable quantities of piperidine alkaloids. Alkaloid-free ponderosa pine trees have been discovered in the Deschutes region (Gerson and Kelsey, 1998), but the absence was attributable, at least partially, to nitrogen limitations (Gerson and Kelsey, 1999b). None of the Deschutes families in the present study lacked alkaloids, further suggesting that those alkaloid-free trees were exhibiting environmental rather than genetic constraints. Within other conifer species, absence of alkaloids has been observed even though nutritional status appeared to be adequate (Gerson and Kelsey, 2004). Genetic manipulation of Lupinus and Solanum species can result in alkaloid-free plants (Wink, 1988), so this degree of variation is certainly possible within species. For quantitative purposes, the present common garden study demonstrated that sampling from a wide geographic range is necessary to capture intraspecific variation, though a relatively small sampling of trees provides an adequate qualitative profile. Most importantly, high tree to tree variation should be expected.
In summary, significant genetic variation was found in foliar alkaloids of ponderosa pine in a seedling common garden study. Seedlings from sources in the Coast Ranges and Puget lowland along the western extent of the species range exhibited the highest concentrations. Seedlings from sources in or east of the Cascade/Sierra Nevada mountain ranges had relatively low alkaloid levels, and there were no north–south trends. The regional pattern of alkaloid variation generally fit with race maps previously developed from phenological traits and terpene chemistry, with the notable exception of the distinct difference between the two regions in California. The amount of variation among families within regions was much smaller than the regional variation. Overall, alkaloid variation was quantitative, rather than qualitative: the end-product, pinidine, predominated throughout the scope of this study. Alkaloid quantities were negatively associated with growth variables, especially height increment, in support of a phenologically driven, demand-side allocation model of growth vs. differentiation. Among climate and topographic variables at parental seed sources, average temperature range appeared to relate inversely to alkaloid concentrations, and very low precipitation was associated with low alkaloid concentrations. However, no single explanatory variable was very convincing, and relationships for some variables appeared to be influenced by what part of the geographic range was under consideration.
SUPPLEMENTARY DATA
Supplementary Data are available at Annals of Botany online, giving details of parent tree seed source identification and locations (latitude, longitude, elevation), and seed bank locations.
ACKNOWLEDGEMENTS
We thank Manuela Huso (Oregon State University) and Nancy Mandel (PNW Research Station) for help with the statistical analyses; their guidance was greatly appreciated. We also thank Jeff Riddle (PNW Research Station) for caring for the seedlings in the common garden and providing the climate data, and Ken Vance-Borland (Oregon State University) for creating the map in Fig. 1.
LITERATURE CITED
- Adler LS, Kittelson PM. Variation in Lupinus arboreus alkaloid profiles and relationships with multiple herbivores. Biochemical Systematics and Ecology. 2004;32:371–390. [Google Scholar]
- Berenbaum MR, Zangerl AR. Genetics of secondary metabolism and herbivore resistance in plants. In: Rosenthal GA, Berenbaum MR, editors. Herbivores, their interactions with secondary plant metabolites. San Diego: Academic Press Inc; 1992. pp. 415–438. [Google Scholar]
- Carey DB, Wink M. Elevational variation of quinolizidine alkaloid contents in a lupine (Lupinus argenteus) of the Rocky Mountains. Journal of Chemical Ecology. 1994;20:849–857. doi: 10.1007/BF02059582. [DOI] [PubMed] [Google Scholar]
- Conkle MT, Critchfield WB. Genetic variation and hybridization of ponderosa pine. In: Baumgartner DM, Lotan JE, editors. Ponderosa Pine: The Species and its Management, Symposium Proceedings. Spokane, WA: 1988. pp. 27–43. [Google Scholar]
- van Dam NM, Vrieling K. Genetic variation in constitutive and inducible pyrrolizidine alkaloid levels in Cynoglossum officinale L. Oecologia. 1994;99:374–378. doi: 10.1007/BF00627751. [DOI] [PubMed] [Google Scholar]
- Eisner T, Goetz M, Aneshansley D, Ferstandig-Arnold G, Meinwald J. Defensive alkaloid in blood of Mexican bean beetle (Epilachna varivestis) Experientia. 1986;42:204–207. doi: 10.1007/BF01952471. [DOI] [PubMed] [Google Scholar]
- Eriksson C. Isolation, synthesis and structure–activity relationships of antifeedants against the pine weevil. Sundsvall: Mid Sweden University; 2006. Hylobius abietis. PhD Thesis. [Google Scholar]
- Gerson EA, Kelsey RG. Variation of piperidine alkaloids in ponderosa (Pinus ponderosa) and lodgepole pine (P. contorta) foliage from central Oregon. Journal of Chemical Ecology. 1998;24:815–827. [Google Scholar]
- Gerson EA, Kelsey RG. Piperidine alkaloids in nitrogen fertilized ponderosa pine. Journal of Chemical Ecology. 1999;a 25:2027–2039. [Google Scholar]
- Gerson EA, Kelsey RG. Foliar storage and extraction methods for quantitative analysis of piperidine alkaloids from ponderosa pine (Pinus ponderosa) Phytochemical Analysis. 1999;b 10:322–327. [Google Scholar]
- Gerson EA, Kelsey RG. Piperidine alkaloids in sitka spruce with varying levels of resistance to white pine weevil (Coleoptera: Curculionidae) Journal of Economic Entomology. 2002;95:608–613. doi: 10.1603/0022-0493-95.3.608. [DOI] [PubMed] [Google Scholar]
- Gerson EA, Kelsey RG. Piperidine alkaloids in North American Pinus taxa: implications for chemosystematics. Biochemical Systematics and Ecology. 2004;32:63–74. [Google Scholar]
- Gritsanapan W, Griffin WJ. Alkaloid variation within Duboisia myoporoides. Phytochemistry. 1991;30:2667–2669. [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: to grow or defend. Quarterly Review of Biology. 1992;67:283–335. [Google Scholar]
- Hoft M, Verpoorte R, Beck E. Growth and alkaloid contents in leaves of Tabernaemontana pachysiphon Stapf (Apocynaceae) as influenced by light intensity, water and nutrient supply. Oecologia. 1996;107:160–169. doi: 10.1007/BF00327899. [DOI] [PubMed] [Google Scholar]
- Jenkinson JL. Improving plantation establishment by optimizing growth capacity and planting time of western yellow pines. Research Paper PSW-154. Albany, CA: US Department of Agriculture, Forest Service, Pacific Southwest Research Station; 1980. [Google Scholar]
- Kamm C. Spruce budworm larval processing of piperidine alkaloids from spruce needles. Journal of Chemical Ecology. 1998;24:1153–1160. [Google Scholar]
- Kitzmiller JH. Provenance trials of ponderosa pine in northern California. Forest Science. 2005;51:595–607. [Google Scholar]
- Krejsa BB, Rouquette FM, Jr, Holt EC, Camp BJ, Nelson LR. Alkaloid and nitrate concentrations in pearl millet as influenced by drought stress and fertilization with nitrogen. Agronomy Journal. 1987;79:266–270. [Google Scholar]
- Latta RG, Linhart YB, Snyder MA, Lundquist L. Patterns of variation and correlation in the monoterpene composition of xylem oleoresin within populations of ponderosa pine. Biochemical Systematics and Ecology. 2003;31:451–465. [Google Scholar]
- Leete E, Juneau KN. Biosynthesis of pinidine. Journal of the American Chemical Society. 1969;91:5614–5618. doi: 10.1021/ja01048a031. [DOI] [PubMed] [Google Scholar]
- Leete E, Lechleiter JC, Carver RA. Determination of the ‘starter’ acetate unit in the biosynthesis of pinidine. Tetrahedron Letters. 1975;44:3779–3782. [Google Scholar]
- Lerdau M, Litvak M, Monson R. Plant chemical defense: monoterpenes and the growth-differentiation balance hypothesis. Tree. 1994;9:58–61. doi: 10.1016/0169-5347(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Li P, Beaulieu J, Bousquet J. Genetic structure and patterns of genetic variation among populations in wastern white spruce (Picea glauca) Canadian Journal of Forest Research. 1997;a 27:189–198. [Google Scholar]
- Li P, Beaulieu J, Daoust G, Plourde A. Patterns of adaptive genetic variation in eastern white pine (Pinus strobus) from Quebec. Canadian Journal of Forest Research. 1997;b 27:199–206. [Google Scholar]
- MacDonald GM, Cwynar LC, Whitlock C. The late Quaternary dynamics of pines in northern North America. In: Richardson DM, editor. Ecology and biogeography of. Pinus. Cambridge: Cambridge University Press; 1998. pp. 122–136. [Google Scholar]
- Millar CI. Early evolution of pines. In: Richardson DM, editor. Ecology and biogeography of Pinus. Cambridge: Cambridge University Press; 1998. pp. 69–91. [Google Scholar]
- Norris JR, Jackson ST, Betancourt JL. Classification tree and minimum-volume ellipsoid analyses of the distribution of ponderosa pine in the western USA. Journal of Biogeography. 2006;33:342–360. [Google Scholar]
- Oliver WW, Ryker RA. Ponderosa pine (Pinus ponderosa Dougl. ex Laws) In: Burns RM, Honkala BH, editors. Silvics of North America: 1. Conifers. Agriculture Handbook 654. Washington, DC: US Department of Agriculture, Forest Service; 1990. pp. 413–424. [Google Scholar]
- Orians CM, Roche BM, Fritz RS. The genetic basis for variation in the concentration of phenolic glycosides in Salix sericea: an analysis of heritability. Biochemical Systematics and Ecology. 1996;24:719–724. [Google Scholar]
- Price RA, Liston A, Strauss SH. Phylogeny and systematics of Pinus. In: Richardson DM, editor. Ecology and biogeography of Pinus. Cambridge: Cambridge University Press; 1998. pp. 49–68. [Google Scholar]
- Ralphs MH, Gardner DR. Alkaloid levels in duncecap (Delphinium occidentale) and tall larkspur (D. barbeyi) grown in reciprocal gardens: separating genetic from environmental influences. Biochemical Systematics and Ecology. 2001;29:117–124. doi: 10.1016/s0305-1978(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Ralphs MH, Manners GD, Pfister JA, Gardner DR, James LF. Toxic alkaloid concentration in tall larkspur species in the western U.S. Journal of Range Management. 1997;50:497–502. [Google Scholar]
- von Rudloff E, Lapp MS. Chemosystematic studies in the genus Pinus. VII. The leaf oil terpene composition of ponderosa pine, Pinus ponderosa. Canadian Journal of Botany. 1991;70:374–378. [Google Scholar]
- SAS Institute Inc. SAS/STAT Software: changes and enhancements through release 6·11. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- Schneider MJ, Montali JA, Hazen D, Stanton CE. Alkaloids of Picea. Journal of Natural Products. 1991;54:905–909. [Google Scholar]
- Smith RH. Monoterpenes of ponderosa pine xylem resin in western United States. Technical Bulletin 1532. Washington, DC: US Department of Agriculture, Forest Service; 1977. [Google Scholar]
- Smith RH. Xylem monoterpenes of pines: distribution, variation, genetics, function. General Technical Report PSW-GTR-177. Albany, CA: US Department of Agriculture, Forest Service; 2000. [Google Scholar]
- Sorensen FC, Weber JC. Genetic variation and seed transfer guidelines for ponderosa pine in the Ochoco and Malheur National Forests of central Oregon. PNW Research Paper 468. Portland, OR: US Department of Agriculture, Forest Service; 1994. [Google Scholar]
- Sorensen FC, Mandel NL, Aagaard JE. Role of selection versus historical isolation in racial differentiation of ponderosa pine in southern Oregon: an investigation of alternative hypotheses. Canadian Journal of Forest Research. 2001;31:1127–1139. [Google Scholar]
- Stamp N. Out of the quagmire of plant defense hypotheses. Quarterly Review of Biology. 2003;78:23–55. doi: 10.1086/367580. [DOI] [PubMed] [Google Scholar]
- St Clair JB, Mandel NL, Vance-Borland KW. Genecology of Douglas fir in western Oregon and Washington. Annals of Botany. 2005;96:1199–1214. doi: 10.1093/aob/mci278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stermitz FR, Tawara JN, Boeckl M, Pomeroy M, Foderaro TA, Todd FG. Piperidine alkaloid content of Picea (spruce) and Pinus (pine) Phytochemistry. 1994;35:951–953. [Google Scholar]
- Stermitz FR, Kamm CD, Tawara JN. Piperidine alkaloids of spruce (Picea) and fir (Abies) species. Biochemical Systematics and Ecology. 2000;28:177–181. [Google Scholar]
- Sturgeon KB. Monoterpene variation in ponderosa pine xylem resin related to western pine beetle predation. Evolution. 1979;33:03–814. doi: 10.1111/j.1558-5646.1979.tb04736.x. [DOI] [PubMed] [Google Scholar]
- Tallent WH, Stromberg VL, Horning EC. Pinus alkaloids. The alkaloids of P. sabiana Dougl. and related species. Journal of the American Chemical Society. 1955;77:6361–6364. [Google Scholar]
- Tawara JN, Blohkin A, Foderaro TA, Stermitz FR, Hope H. Toxic piperidine alkaloids from pine (Pinus) and spruce (Picea) trees. New structures and a biosynthetic hypothesis. Journal of Organic Chemistry. 1993;58:4813–4818. [Google Scholar]
- Tawara JN, Stermitz FR, Blokhin AV. Alkaloids of young ponderosa pine seedlings and late steps in the biosynthesis of pinidine. Phytochemistry. 1995;39:705–708. [Google Scholar]
- Tao J, Littel R, Patetta M, Truxillo C, Wolfinger R. Mixed model analyses using the SAS system, course notes. Cary, NC: SAS Institute Inc; 2002. [Google Scholar]
- Todd FG. Potentially toxic compounds of Convolvulacae and piperidine alkaloids of. Fort Collins: Colorado State University; 1994. Picea. PhD Thesis. [Google Scholar]
- Todd FG, Stermitz FR, Blokhin AV. Piperidine alkaloid content of Picea pungens (Colorado blue spruce) Phytochemistry. 1995;40:401–406. [Google Scholar]
- Wang X-Q, Tank DC, Sang T. Phylogeny and divergence times in Pinaceae: evidence from three genomes. Molecular Biology and Evolution. 2000;17:773–781. doi: 10.1093/oxfordjournals.molbev.a026356. [DOI] [PubMed] [Google Scholar]
- Wells OO. Geographic variation in ponderosa pine. I. The ecotypes and their distribution. Silvae Genetica. 1964;a 13:89–103. [Google Scholar]
- Wells OO. Geographic variation in ponderosa pine. II. Correlation between progeny performance and characteristics of the native habitat. Silvae Genetica. 1964;b 13:125–132. [Google Scholar]
- Wink M. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theoretical and Applied Genetics. 1988;75:225–233. [Google Scholar]
- Wink M, Carey DB. Variability of quinolizidine alkaloid profiles of Lupinus argenteus (Fabaceae) from North America. Biochemical Systematics and Ecology. 1994;22:663–669. [Google Scholar]
- Zhang J, Cregg BM. Growth and physiological responses to varied environments among populations of Pinus ponderosa. Forest Ecology and Management. 2005;219:1–12. [Google Scholar]