Abstract
Background and Aims
Flowering phenology is a potentially important component of success of alien species, since elevated fecundity may enhance invasiveness. The flowering patterns of invasive alien plant species and related natives were studied in three regions with Mediterranean-type climate: California, Spain and South Africa's Cape region.
Methods
A total of 227 invasive–native pairs were compared for seven character types across the regions, with each pair selected on the basis that they shared the same habitat type within a region, had a common growth form and pollination type, and belonged to the same family or genus.
Key Results
Invasive alien plant species have different patterns of flowering phenology from native species in the three regions. Whether the alien species flower earlier, later or at the same time as natives depends on the climatic regime in the native range of the aliens and the proportion of species in the invasive floras originating from different regions. Species invading at least two of the regions displayed the same flowering pattern, showing that flowering phenology is a conservative trait. Invasive species with native ranges in temperate climates flower earlier than natives, those from Mediterranean-type climates at the same time, and species from tropical climates flower later. In California, where the proportion of invaders from the Mediterranean Basin is high, the flowering pattern did not differ between invasive and native species, whereas in Spain the high proportion of tropical species results in a later flowering than natives, and in the Cape region early flowering than natives was the result of a high proportion of temperate invaders.
Conclusions
Observed patterns are due to the human-induced sympatry of species with different evolutionary histories whose flowering phenology evolved under different climatic regimes. The severity of the main abiotic filters imposed by the invaded regions (e.g. summer drought) has not been strong enough (yet) to shift the flowering pattern of invasive species to correspond with that of native relatives. It does, however, determine the length of the flowering season and the type of habitat invaded by summer-flowering aliens. Results suggest different implications for impacts at evolutionary time scales among the three regions.
Key words: Biological invasions, flowering phenology, genetic inertia, Cape Floristic Region, California, Spain, Mediterranean-type ecosystems, water availability, climatic origin
INTRODUCTION
The timing of sexual reproduction is a critically important determinant of plant reproductive success. Flowering at the optimum time ensures fecundity and good development of seeds and fruits (Mazer, 1987). Flowering phenology is mediated by the interaction of internal factors (Murfet, 1977; Putterill et al., 2004) with external environmental signals such as temperature (Hollister et al., 2005), daylength (Imaizumi and Kay, 2006) or drought (Fox, 1990a). In general, plant species in their native ranges have coupled the sensitive flowering period to the optimal climatic conditions through natural selection, thus maximizing their reproductive success. The main selective factors acting upon flowering phenology differ between ecosystems. In Mediterranean-type ecosystems (MTEs), which occur in five widely separated regions of the world (Cowling et al., 1996), summer drought and rainfall variability (Cowling et al., 2005) modulate the flowering plant response. Drought is one of the most limiting factors for vegetative growth and flower development (Mitrakos, 1980; Roche et al., 1997). Flowering is concentrated in spring and autumn in most native plants in MTEs, which can be interpreted with reference to avoidance of summer water stress (Orshan, 1989; Johnson, 1993; Castro-Díez and Montserrat-Martí, 1998; Perez-Latorre and Cabezudo, 2002).
Rainfall variability plays an important role in the start and length of flowering phenology in these ecosystems. In less predictable regimes there is selection for a largely plastic response of start of flowering in order to cope with the uncertain moisture conditions of spring; this also occurs in other seasonally dry ecosystems (Borchert et al., 2004). Climate-change studies focused on responses of wide-ranging plant species occurring along latitudinal gradients corroborate the idea of high phenological plasticity in fluctuating environments (Arft et al., 1999; Parmesan, 2006). However, phylogenetic and genetic inertia of flowering phenology imposes limits to this plasticity (Rathcke and Lacey, 1985; Herrera, 1992). Consequently, plasticity of flowering, measured as the length of temporal internal plant sensitivity to flower development, is a conservative trait, since it has a genetic base (Ausin et al., 2005), and plant species may be unable to shift their timing of flowering when they are introduced into a new region.
Widespread introductions of plant species to areas outside their natural ranges provide the opportunity to gain new insights into the importance of flowering phenology as a component of success of alien species in a new region, since enhanced fecundity appears to be an important trait associated with invasiveness (Pyšek and Richardson, 2007). To be a successful invader, introduced plants must first cope with the abiotic filters imposed by the new region and then reproduce (Richardson et al., 2000); this requires them to flower at the appropriate time of year, which will depend on the plant's requirements. Flowering phenology has been shown to be fairly flexible in within-alien comparisons. For example, successful invaders generally display early flowering or long blooming periods (Goodwin et al., 1999; Pyšek et al., 2003), since the chance of acquiring improved fitness via effective pollination visits is increased. On the other hand, late, short flowering gives insufficient time for completion of the life cycle or results in a shorter time for pollination, reducing opportunities for fruit and seed development (Roche et al., 1997). In the case of alien–native comparisons, many authors have found that invasive alien species flower earlier than natives (Cadotte and Lovett-Doust, 2001; Lake and Leishman, 2004). Others have found that alien species that flower later than natives are more abundant (Celesti-Grapow et al., 2003; Lloret et al., 2005). Exhibiting a different flowering pattern compared with native species may be more frequent in those alien species which have evolved under climatic conditions markedly different from those of the invaded region. This premise is based on the following argument: if plant species maintain their genetic inertia of timing of flowering when they are introduced in a new ecosystem, different flowering phenology between invasive and native species may occur as a direct result of different strategies of reproduction selected by evolution. On the other hand, invasive species with the same climatic conditions in their native and invaded ecosystems will not show any difference in flowering phenology.
MTEs probably provide the best opportunity to test this hypothesis, since they have been severely affected by invasions of introduced (alien) plant species (Groves and di Castri, 1991). Many studies have sought reasons for differential success of different alien plant species in the different MTE regions [see Lloret et al. (2005) for the Mediterranean Basin; Rejmánek and Randall (1994) for California; Jimenez et al. (2008) and Sax (2002) for California and central Chile; and Richardson and Cowling (1992) and Richardson et al. (1992) for the Cape region of South Africa]. The fate of introduced species has clearly been influenced by many factors, including numerous inherent features of the different regions and differences in cultural links between the regions and colonial powers, which shaped the magnitude, timing and nature of early introductions and dissemination within regions. In addition, recent socio-economic developments and human-mediated modification of landscapes have also driven further introductions and their dissemination within the regions (Wilson et al., 2007).
This study examines the flowering phenology of invasive alien species in three different regions with Mediterranean-type climate. The following questions were addressed. (a) Does the flowering phenology of invasive alien species differ from that of native species? (b) Are there differences between regions? (c) Is the flowering phenology of invasive alien species explained by the climate in their regions of origin?
MATERIALS AND METHODS
Climatic characteristic of selected regions
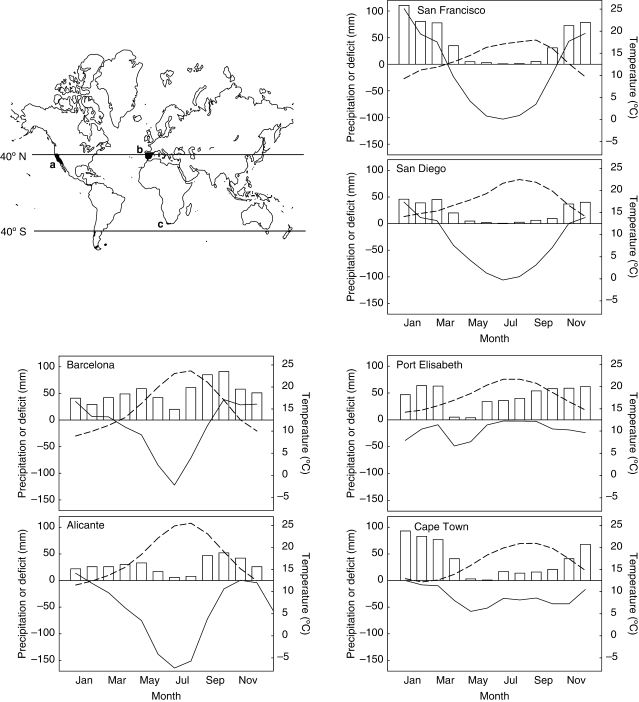
Three MTE regions were selected to represent a gradient of summer drought and rainfall reliability severity among regions of the world with this climatic regime (Cowling et al., 2005). The California region has the lowest summer precipitation [San Francisco (SF) = 4·9 mm, San Diego (SD) = 4·8 mm] and a high water deficit in this season (SF = −296·4 mm, SD = −298·5 mm), the Cape region has a relative high summer precipitation [Cape Town (CT) = 47 mm, Port Elizabeth (PE) = 110 mm] and the lowest water deficit (CT = −96·0 mm, PE = −13·7 mm; Fig. 1). The Spanish Mediterranean region falls somewhere between these two regions, although with a remarkable variability in summer rainfall along latitudinal and coast–inland gradients. Water deficit was calculated as the difference between the precipitation and the potential evapotranspiration in each month. In this sense, potential evapotranspiration was calculated using the method of Jensen et al. (1990). This method is considered the most accurate from latitudes 0° to 60°. It takes into account the latitude of the studied region, mean of the maximum and minimum temperatures, mean altitude and total irradiance considering the number of hours of sun. Climatic data for a 30-year period were used; for California and the Cape region, data were obtained from NOAA (1961–1990), and Spanish data were obtained from the national meteorological institute for the same period (INM 1971–2000).
Fig. 1.
Climatic characteristic of the three Mediterranean-type ecosystems studied: (a) California, (b) Spain and (c) the Cape region of South Africa. The three regions represent a gradient of summer drought severity. California has the driest and the Cape region has the mildest summers. Climatic charts of two different localities in each region illustrate this gradient. Columns represent the precipitation; solid lines the temperature; and dashed lines the water deficit in each month. Charts of the southern hemisphere localities (Cape region) have been modified to show drought between June and July for clearer comparison with Northern Hemisphere localities.
Species selection and data compilation
To standardize between regions, the data set comprised introduced plant species that were clearly invasive (sensu Pyšek et al., 2004), with clear impact on the native ecosystems (transformer species, sensu Richardson et al., 2000). Three major sources were used to compile the lists of invaders: Invasive plants of California's wildlands (Bossard et al., 2000), Atlas de las plantas alóctonas invasoras en España (Sanz Elorza et al., 2004) and The complete guide to declared weeds and invaders in South Africa (Henderson, 2001). For California, all species listed by Bossard et al. (2000) were included, since the criteria used by these authors for inclusion of species in their book match those in the present study. For Spain, all listed species were selected, except those alien plants that are invasive only in the Canary Islands (non-Mediterranean climate) and those that are naturalized but not invasive sensu (Pyšek et al., 2004). For South Africa, all species listed by Henderson (2001) with mapped occurrence in the Cape Floristic Region were included. A total of 227 alien species were selected (see Supplementary Data, available online).
Each of the selected species was coded for seven characters (Table 1), using primarily information from the sources mentioned above. Climatic origin of the invasive species in their former native range was considered important since plants have a genetic inertia on flowering development due to climatic conditions under which they evolved (Rathcke and Lacey, 1985; Herrera, 1992). Four main habitats that are representative of invaded habitats across the regions were selected, as differences in timing of flowering are sometimes explained by habitat conditions rather than different flowering strategies (Thies and Kalko, 2004). Growth form was selected because environmental variables that affect flowering differ for woody and herbaceous plants (Arft et al., 1999; Post and Stenseth, 1999). Pollination type was considered important because different flowering strategies have been documented for animal- and wind-pollinated plants (Rathcke and Lacey, 1985). Finally, data on the start, duration and end of flowering were also compiled.
Table 1.
List of characters for which data were scored and used in ecological pair construction and invasive–native comparisons.
| Character | Character state |
|---|---|
| Climatic origin | Tropical, temperate, Mediterranean type-ecosystems (MTE) |
| Habitat type | Disturbed areas, coastal areas, lakes and rivers, shrub and woodland |
| Growth form | Woody, herbaceous, climber |
| Start, end and length of flowering of invasive and native species in the three MTEs | January to December (months)* |
| Pollination type | Animal, wind |
* Flowering times for the Cape region were transformed to the Northern Hemisphere calendar.
In order to compare characters of invasive species with those of native plants with similar ecological requirements each invasive species was paired with one closely related native species based on four criteria: (1) within each pair, the native must be recorded in the region where the alien species is invasive; (2) native and invasive alien species must share the same habitat type – to be potential competitors; (3) the two species must belong to the same growth form and pollination type; and finally (4) the two species must belong to the same genus or family, to obtain phylogenetic independent contrasts (Ackerly, 2000). Native species with small distribution ranges and those listed in any IUCN threatened category were excluded. Criterion 4 was only realized for California and the Cape region since there were large phylogenetic differences between invasive and native floras in Spain. Thus, phylogenetic relatedness was taken into account a posteriori in Spain. In this case, total phylogenetic distances were collected for each species through the angiosperm plant phylogenetic supertree described by Soltis et al. (2000) and modified by Bremer et al. (2003). Currently, these studies are the most highly resolved and strongly supported topology obtained for angiosperms. Next, tests were conducted to determine whether the differences in the start and the end of flowering between invasive and native species were influenced by a phylogenetic relationship between each pair of species. Analysis of covariance (ANCOVA) testing for differences in flowering time demonstrated no phylogenetic effects on the results due to the native species selection for construction of the ecological pairs (start of flowering, F = 0·23 P = 0·632; end of flowering, F = 1·21 P = 0·274). In this sense, the phylogenetic relationship of the Spanish pairs was the covariable calculated as the mean of phylogenetic distance to the first common ancestor of both of the paired species.
Characteristics of Californian native species as well as their flowering phenology were collated from the Online Interchange for California Floristics (2007), based on The Jepson manual of higher plants of California (Hickman, 1993). For Spain, native plant characters were collated from the Flora Ibérica. Plantas vasculares de la Península Ibérica e Islas Baleares (todos los vols) (Castroviejo, 1986–2005). Unfortunately, accounts of some Spanish native species are yet to be published in the Flora Ibérica. Characters for these species were compiled from regional floras such as Flora of Western Andalucía (Valdés et al., 1987) and Flora of Catalonia (Bolòs and Vigo, 1984–2001). Because the information was obtained from three different sources, differences in flowering onset and cessation in 31 species common among floras were tested with a one-way analysis of variance (ANOVA). No differences were found either in the start (F = 7·7E-4, P = 0·978) or in the end of flowering time (F = 0·723, P = 0·402). Finally, Goldblatt and Manning (2000) provided the best reference on the required information for the native plants of the Cape region.
Statistical analyses
Chi-square tests were applied to test for differences between the exotic floras of the three regions in the spectra of climatic origin, life form and type of invaded habitat. An orthogonal general lineal model (GLM) for unbalanced designs was used to test for significant variables affecting differences in the start, end and length of flowering between native and invasive species. Categorical predictors were the invaded Mediterranean regions plus those used to create invasive–native pairs (growth form, pollination type and invaded habitat). Pairwise Watson–William F-tests for dependent samples in circular statistic were performed to test for differences in flowering phenology between: (a) all invasive alien and native species pairs in the three regions; (b) those species pairs in each region where the alien invasive species shared the same climatic origin or pollination type; (c) those species pairs that are animal-pollinated and for which the invaders share the same climatic origin; and (d) differences in flowering phenology between invasive alien species present in at least two different regions. These analyses were performed with the ORIANA package (Kovach, 1994). In all circular analyses, flowering phenology data followed a Von Mises distribution (circular version of normal distribution) so no transformation was needed. t-tests for paired samples were performed to test for differences in the length of flowering between invasive alien and native species. SPSS 12·0 (SPSS, Inc.) was used for non-circular statistic analysis.
RESULTS
Characteristic of invaders
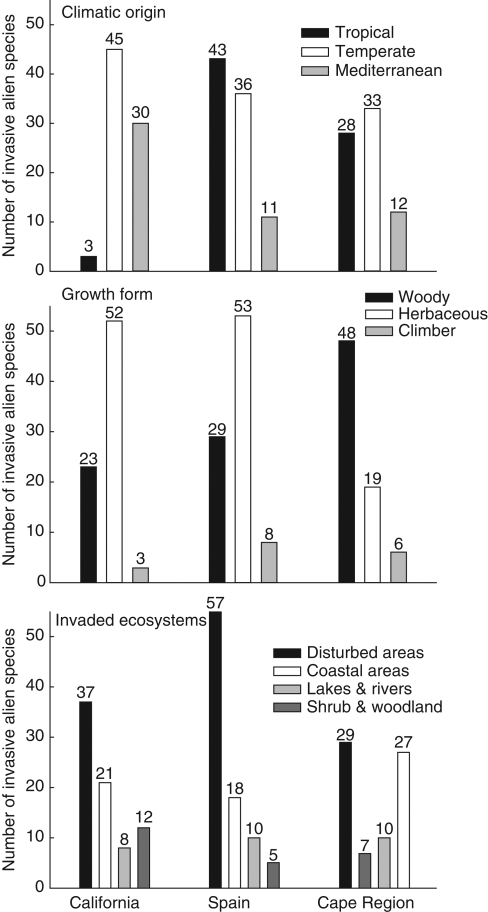
Invasive species in the three MTEs showed different patterns of climatic origin, growth form and invaded habitats (Fig. 2). The invasive flora of California had the smallest proportion of tropical species and a high proportion of invaders with Mediterranean and temperate origin. Spain and the Cape region had almost the same proportion of Mediterranean invaders (approx. 15 % of species). However, the alien flora of the Cape region showed a higher proportion of temperate species, while in Spain tropical species were more abundant. Herbaceous plants were the principal growth form in the invasive floras of California and Spain, and disturbed areas had the highest percentages of invasive species. However, a higher proportion of the invasive flora in the Cape region was made up by woody plants, and invaded habitats were mostly natural shrubland. The proportion of climbers is similarly low in the three MTEs.
Fig. 2.
Proportions of invasive species according to their climatic origin, growth form and invaded ecosystem in the three Mediterranean climate regions.
Differences in flowering phenology between invasive and native species
Differences in the start of flowering between invasive and native species were significantly influenced by the invaded region and by the interaction between region and pollination type (Table 2). These differences in the start were generally lower in California than in Spain and the Cape region. In addition, wind-pollinated species had higher differences than animal-pollinated species in California, whereas in the Cape region the pattern was the opposite. Differences in the end of flowering were significantly influenced by the interaction between region and growth form (Table 2). In this sense, only invasive climbers in California had lower differences in the end of flowering compared with the invasive climbers in Spain and the Cape Region. Finally, differences in the length of flowering varied significantly depending of the invaded region, being shorter in California (Tables 2 and 3).
Table 2.
Results of a general lineal model (GLM) of the differences in the start, end and length of flowering phenology (dependent variables) between invasive and native species pairs, for region (California, Spain and the Cape region of South Africa), growth form, habitat invaded and pollination type as categorical predictors (see Table 1)
| Start of flowering |
End of flowering |
Length of flowering |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | d.f. | F | P | d.f. | F | P | d.f. | F | P |
| Region (R) | 2 | 5·233 | <0·01 | 2 | 2·974 | 0·053 | 2 | 8·949 | <0·001 |
| Growth form (GF) | 2 | 1·998 | 0·138 | 2 | 0·144 | 0·866 | 2 | 1·422 | 0·244 |
| Pollination type (PT) | 1 | 0·002 | 0·963 | 1 | 0·411 | 0·522 | 1 | 2·129 | 0·146 |
| Habitat type (HT) | 3 | 0·93 | 0·427 | 3 | 0·359 | 0·783 | 3 | 0·166 | 0·919 |
| R × GF | 4 | 1·147 | 0·335 | 4 | 2·822 | <0·05 | 4 | 0·701 | 0·592 |
| R × PT | 2 | 3·506 | <0·05 | 2 | 2·99 | 0·052 | 2 | 0·187 | 0·83 |
| GF × PT | 2 | 0·647 | 0·525 | 2 | 0·299 | 0·742 | 2 | 0·85 | 0·429 |
| R × HT | 6 | 1·722 | 0·117 | 6 | 1·449 | 0·198 | 6 | 1·305 | 0·256 |
| GF × HT | 6 | 1·157 | 0·331 | 6 | 0·596 | 0·733 | 6 | 0·332 | 0·92 |
| PT × HT | 3 | 1·355 | 0·258 | 3 | 0·41 | 0·746 | 3 | 0·833 | 0·477 |
Three and higher order interactions are not showed for clarity and because they were not significant. To perform this analysis, flowering times for the Cape region were transformed to the Northern Hemisphere calendar (i.e. January–July). Significant values of P are indicated in bold.
Table 3.
Mean values of flowering phenology parameters between invasive and native plant species according to climatic origin and pollination type in the three Mediterranean climate regions
| Parameter | All species | Tropical | Temperate | Mediterranean | Animal-pollinated | Wind-pollinated |
|---|---|---|---|---|---|---|
| California | (n = 78) | (n = 3) | (n = 45) | (n = 30) | (n = 60) | (n = 18) |
| Start of flowering | ||||||
| Invasive | 29 April | 17 May | 19 May | 25 March | 11 April | 19 May |
| Native | 5 May | 17 February | 19 May | 26 April | 10 May | 3 May |
| F-value | 0·63 ns | 0·64 ns | 1·22E-04 ns | 4·83* | 2·44 ns | 0·88 ns |
| End of flowering | ||||||
| Invasive | 21 July | 16 October | 13 August | 6 June | 24 July | 23 July |
| Native | 22 July | 11 August | 6 August | 13 July | 8 August | 6 July |
| F-value | 9·10E-4 ns | 1·31 ns | 0·13 ns | 4·62* | 0·73 ns | 1·04 ns |
| Flowering duration (months) | ||||||
| Invasive | 4·1 | 6·7 | 3·9 | 3·9 | 4·2 | 3·6 |
| Native | 3·9 | 6·0 | 3·6 | 3·6 | 3·9 | 3·2 |
| t-value | 0·67 ns | 0·36 ns | 1·20 ns | 0·67 ns | 0·98 ns | 1·27 ns |
| Spain | (n = 90) | (n = 43) | (n = 36) | (n = 11) | (n = 67) | (n = 23) |
| Start of flowering | ||||||
| Invasive | 4 June | 14 June | 2 June | 20 May | 30 May | 30 May |
| Native | 18 April | 9 April | 16 April | 2 May | 19 April | 9 April |
| F-value | 21·85*** | 27·82*** | 11·48*** | 0·42 ns | 11·09*** | 11·63*** |
| End of flowering | ||||||
| Invasive | 28 September | 2 October | 8 October | 5 September | 27 September | 28 September |
| Native | 4 September | 3 September | 2 September | 7 September | 18 August | 24 September |
| F-value | 3·72* | 3·88* | 4·42* | 0·01 ns | 4·02* | 0·04 ns |
| Flowering duration (months) | ||||||
| Invasive | 4·8 | 4·7 | 4·8 | 5·5 | 4·8 | 4·6 |
| Native | 5·3 | 5·9 | 5·2 | 4·9 | 4·9 | 5·7 |
| t-value | –1·47 ns | –2·53** | –0·93 ns | 0·54 ns | –0·44 ns | –2·27* |
| Cape region | (n = 73) | (n = 28) | (n = 33) | (n = 12) | (n = 53) | (n = 20) |
| Start of flowering | ||||||
| Invasive | 15 September | 29 October | 27 August | 8 September | 11 September | 26 September |
| Native | 5 November | 3 November | 14 November | 22 September | 12 November | 22 October |
| F-value | 22·20*** | 0·05 ns | 37·52*** | 1·56 ns | 21·21*** | 2·53 ns |
| End of flowering | ||||||
| Invasive | 25 January | 4 April | 10 December | 9 February | 25 January | 22 January |
| Native | 13 January | 26 January | 5 February | 8 March | 15 January | 5 January |
| F-value | 0·51 ns | 3·97* | 1·26 ns | 0·61 ns | 0·33 ns | 0·25 ns |
| Flowering duration (months) | ||||||
| Invasive | 5·2 | 6·1 | 4·5 | 5·2 | 5·1 | 5·4 |
| Native | 4·3 | 4·4 | 4·0 | 5·1 | 4·1 | 4·8 |
| t-value | 2·59** | 2·96** | 1·16 ns | 0·86 ns | 2·51* | 0·86 ns |
Circular mean values were transformed to days of the year for easier interpretation.
Watson–Williams F-values and t-test values are given: *P < 0·05; **P < 0·01; ***P < 0·001; ns, P > 0·05.
Variation of flowering phenology of invasive species between regions
The flowering length of invaders was positively correlated with the climatic conditions of the three regions. Invasive species flower for longer periods where the summer precipitation is higher. Thus, invasive alien plants in the Cape region bloom over 5·2 months, in Spain over 4·8 months and in California over 4·1 months on average. Overall, no differences in flowering length were found between invasive–native pairs, except in the Cape region where invasive species flower for longer than natives (Table 3). When considering the climatic origins of invasive species, only tropical plants showed different patterns between the invaded region and the length of flowering. In Spain, tropical invaders flowered over a shorter period than the natives, whereas invaders of tropical origin in the Cape region flowered for longer than the natives (Table 3).
For different regions, invasive species flowered earlier, later or at the same time as co-occurring natives. In California, the start and the end of the flowering period was similar for invasive alien and native species. However, when the comparison only included those pairs where the invasive had a Mediterranean origin, invaders started flowering 1 month earlier and finished 1 month earlier than natives. In contrast, in Spain invasive species started and ended flowering later than native species. This result was true for those species pairs where the alien has either tropical or temperate origin, but not for the Mediterranean group (Table 3). Timing of flowering of tropical invasive species in Spain and California showed the same pattern. This suggests that a displacement of flowering phenology may also occur in the latter region. However, no significant differences were found, probably due to the small sample size. In the Cape region, invasive species flowered earlier than natives, due to the early onset of flowering of invaders of temperate origin (Table 3). Tropical species ended flowering later than their native pairs, but no differences were found when the comparison was conducted with the full set of native species. Although native species showed a large variation in their spring onset of flowering, the flower development corresponded with those months with a mean temperature of 18 °C and with relatively low water deficits (Fig. 1, Table 3).
Finally, the 28 species that are invasive in at least two regions showed no displacement of flowering phenologies, either for the initiation (F = 0·11, P = 0·745) or for the cessation of flowering (F = 0·22, P = 0·638). Overall, these results suggest that the differences in flowering phenology of invasive species are due to the differences in climatic origin of invaders rather than the particular species composition of the invasive flora.
Animal-pollinated species and climatic origin
Animal-pollinated invasive species displayed the same pattern as for the entire invasive–native comparison (Table 3, Fig. 3). This means that in California they had the same flowering phenology as native species, in Spain they started and finished their flowering later, and in the Cape region they started their flowering earlier, while invasive and native species finished at the same time. When comparing between regions, different climatic origins of animal-pollinated invaders showed differences in the onset of flowering. In California, Mediterranean invaders started flowering earlier than temperate invaders (Julian day Mediterranean species = 89, Julian day temperate species = 127; F = 4·65, P < 0·05). In Spain, tropical invaders started later than temperate invaders, but these differences were not significant (Julian day temperate species = 132, Julian day tropical species = 162; F = 3·56, P = 0·065). In the Cape region, tropical invasive species started significantly later than temperate ones (Julian day temperate species = 233, Julian day tropical species = 307; F = 20·65, P < 0·001) and also than Mediterranean ones (Julian day Mediterranean species = 232, Julian day tropical species = 307; F = 7·2, P < 0·01).
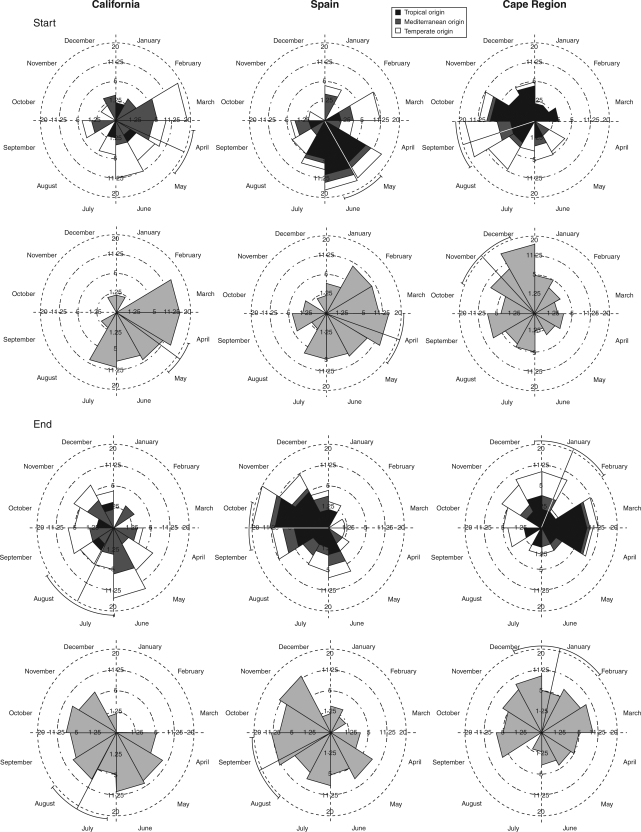
Fig. 3.
Circular histograms for the start and the end of flowering in animal-pollinated invasive species and corresponding native species (see text). The triangles represent the number of species that flower in that month. Black areas indicate alien species of tropical origin; white areas indicate species with temperate origin; and dark grey areas show species with Mediterranean origin. Native species are shown separately in light grey below. Means and s.d. are also shown.
Invasive alien species had a different end of flowering in relation to their climatic origin. In California, tropical invaders finished flowering later than invaders from the Mediterranean (Julian day Mediterranean species = 181, Julian day tropical specis = 285; F = 4·6, P < 0·05). In Spain, no differences between groups were found for the offset of flowering. Lastly, in the Cape region, temperate invaders finished flowering earlier than tropical (Julian day temperate species = 338, Julian day tropical species = 76; F = 27·1, P < 0·001) or Mediterranean invaders (Julian day temperate species = 338, Julian day Mediterranean species = 72; F = 7·1, P < 0·01).
In summary, these differences suggest that a segregation of timing of flowering is occurring depending on the climatic origin of invasive species. Temperate invaders start flowering first, followed by the Mediterranean invaders and then the tropical invaders.
DISCUSSION
The three Mediterranean climate regions dealt with here occur along a gradient of summer drought severity, and their invasive floras differ in terms of the proportion of growth forms, their climatic origins and the habitats most invaded. Depending on the region selected, invasive species flowered earlier, later or at the same time as natives. Thus, a different flowering phenology pattern between groups is context dependent. It must be taken into account that, for a different timing of flowering between invasive and native species, two events must co-occur: (1) a small proportion of invasive species have to belong to the same climatic origin as the invaded region, i.e. Mediterranean climate; and (2) climatic and habitat conditions must minimize summer drought to allow invasive plants to survive. Related to the former premise, species tend to show a genetic inertia for the time of flowering because flowering phenology is an adaptive trait selected to avoid unfavourable climatic conditions in the regions where the plants evolved (Fox, 1990b; Herrera, 1992; Johnson, 1993). In this sense, invasive species maintain the same flowering phenology when they are introduced to regions with the same climatic characteristics. Twenty-eight invasive species shared between at least two regions showed the same flowering phenology in both invaded regions, providing support for this idea. In general, invasive species from the Mediterranean flowered predominantly in spring, whereas tropical invaders continued flowering further into summer. On the other hand, temperate aliens flowered in early spring (in the Cape region) or in summer (in Spain) depending on whether they are woody or herbaceous species.
Recent studies have highlighted the importance of studying historical factors (e.g. the links between regions and colonial powers or human-mediated modifications to landscapes) as these factors are thought to shape the composition and magnitude of introductions (Lockwood et al., 2007; Wilson et al., 2007). Such anthropogenic factors may also influence the biotic interactions between invasive and native species as can occur with animal-pollinated plants. For example, no difference was noted in flowering phenology between invasive and native species in California, because the proportion of invaders from the Mediterranean Basin is high. This is due to California's historical links with Europe and especially with Spain as a colonial power (Bancroft, 1890). As both groups flower at the same time, they may compete for pollinators (Lopezaraiza-Mikel et al., 2007). Competition for pollinators is thought to be an important form of disruption of plant–animal interactions caused by invasive species (Traveset and Richardson, 2006). However, in the Cape region and in Spain, flowering phenology of invaders was different from that of natives, since the proportion of invaders from Mediterranean climate regions is small. A high proportion of invaders of temperate and tropical origin in the Cape region are attributable to two events. From the 17th to the 19th century the current South Africa and thus the Cape region was a European colony. The influence and trade with countries such as The Netherlands and especially the UK increased the rate of deliberate introductions (Henderson, 2001). Temperate alien species were introduced from Europe or other European colonies such as Australia (e.g. Hypericum perforatum from Europe, Acacia species from Australia). On the other hand, more recently, tropical species (e.g. Araujia sericifera, Passiflora caerulea) have also been deliberately introduced for horticulture (Henderson, 2001). Although the introductions of alien species in both historical situations were for different reasons, the ecological result is convergent. Invasive species flower at a different time compared with the natives, filling an empty temporal niche. Flowering at a different time compared with natives may be an advantage for invasive species. It increases sexual fitness due to avoidance of pollen limitation and competition for pollinators with natives (Sargent and Ackerly, 2008). In contrast to the situation in the Cape region, most of the invasive plants in Spain were introduced accidentally with the trade of plants for agricultural purposes (Lloret et al., 2005). Tropical summer weeds invading croplands and disturbed areas highlight the importance of the Spanish past linked to their American colonies.
The reason for some invaders flowering in summer (the least favourable period for flower development in MTEs) is due to the type of habitat they invade. Disturbed areas are generally the most susceptible to invasion (Lake and Leishman, 2004; Cadotte et al., 2006). Some disturbed habitats such as irrigated summer croplands and riparian habitats seldom experience water stress, allowing invasive plants to survive the summer drought in Mediterranean-type climates (Lake and Leishman, 2004). The importance of disturbed areas as a microenvironment for avoiding abiotic filters of the invaded region depends on the severity of summer drought. In California and Spain, where summer drought is intense, most of the species on the lists invade disturbed areas. In the Cape region, however, where summer drought is relatively mild, invasive species seem less limited by drought and can invade natural areas (Fig. 2).
Climatic and habitat environmental conditions can also influence the growth form of invaders and thus the length of flowering phenology of invasive species (Castro-Diez et al., 2003). For example, disturbed areas have the advantage of minimizing abiotic unfavourable conditions, but limit the type of growth form that can invade. Annuals and short-lived plants are better adapted to rapid changes and disturbance conditions of this type of habitat (Grime, 1974). These types of invaders which can complete their life cycles in a few months showed a short flowering period associated with their short-lived cycle. Mainly herbaceous invaders of tropical origin in Spain (e.g. Datura stramonium, Xanthium strumarium) illustrate this situation. They show significantly shorter flowering periods than natives (Table 1). On the other hand, tropical invaders in the Cape region are mainly woody species that invade natural areas and flower longer than natives (Tables 1 and 2, and Fig. 2).
Previous studies have shown that successful invaders generally display early flowering or long blooming periods (Goodwin et al., 1999; Pyšek et al., 2003). Also, in alien–native comparisons, many authors have found that invasive alien species flower earlier than natives (Cadotte and Lovett-Doust, 2001; Lake and Leishman, 2004). Those results suggest that invasive species capitalize on an early blooming strategy to increase their reproductive success since the chance to acquire improved fitness via effective pollination visits is also increased (Goodwin et al., 1999; Pyšek et al., 2003). This idea is supported by other authors who have found that late, short flowering for pollination reduced opportunities for fruit and seed development of alien species (Roche et al., 1997). However, the present results show that early flowering is not the only reproductive strategy for successful invaders. They can also flower at the same time or later than native species and be successful. Therefore, the possible different flowering phenology is mainly a consequence of different nature, historical and human factors that drive the reproductive relationship between groups. If this argument is correct, the same alien plant flowering phenological pattern should be found in regions with homogenous environmental conditions and the same history of introductions. This seems to apply for regions within the Mediterranean Basin. Dominance of summer flowering among invasive species in Spain (Table 3) is in agreement with previous results found for Italy (Celesti-Grapow et al., 2003) and Mediterranean Islands (Lloret et al., 2005).
Most invasion ecology studies relate traits of alien species to their capacity to invade, with the overall aim of unravelling aspects of the invasion process and aiming to predict future invasions. However, not all the observed plants traits identified as being associated with invasiveness in aliens really confer invasiveness, since other causes often underlie the observed pattern. This seems to be the case with flowering phenology. Although several studies have found a positive relationship between flowering phenology of aliens and their invasiveness potential (Goodwin et al., 1999; Cadotte and Lovett-Doust, 2001; Pyšek et al., 2003; Lake and Leishman, 2004), flowering phenology of invasive species and the possible differences relative to natives is only a consequence of different history of human-orchestrated introductions. The results of this study proved that under the same climatic conditions in three widely separated regions, invasive alien species do not display a common flowering phenology pattern. Instead, they flower earlier, later or at the same time as native species depending on the climatic regime in the region where they evolved.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consists of a checklist of the 227 plant species (alien–native comparisons) in the three Mediterranean-type regions, California, Spain and the Cape region of South Africa.
ACKNOWLEDGEMENTS
Thanks are due to Margarita Costa-Tenorio for help with the identification of appropriate native–invasive species pairs in Spain, and to Anna Traveset, Montserrat Vilà and Petr Pyšek for helpful discussions. Additionally, we thank Laura Celesti-Grapow and two anonymous referees for their helpful revision of this manuscript. Financial support was provided by the Spanish Ministry for Education and Science (grants RASINV, GL2004-04884-C02 02/BOS as part of the coordinate project RINVE, and CGL2007-61873/BOS). D.M.R. acknowledges support from the DST-NRF Centre of Excellence for Invasion Biology and the Hans Sigrist Foundation.
LITERATURE CITED
- Ackerly DD. Taxon sampling, correlated evolution, and independent contrasts. Evolution. 2000;54:1480–1492. doi: 10.1111/j.0014-3820.2000.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Arft AM, Walker MD, Gurevitch J, et al. Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecological Monographs. 1999;69:491–511. [Google Scholar]
- Ausin I, Alonso-Blanco C, Martinez-Zapater JM. Environmental regulation of flowering. International Journal of Developmental Biology. 2005;49:689–705. doi: 10.1387/ijdb.052022ia. [DOI] [PubMed] [Google Scholar]
- Bancroft HH. The works of Hubert Howe Bancroft. History of California to 1890. 1890 Vol. 14. [Google Scholar]
- Bolòs O, Vigo J. Flora dels Països Catalans. Barcelona: Barcino; 1984–2001. [Google Scholar]
- Borchert R, Meyer SA, Felger RS, Porter-Bolland L. Environmental control of flowering periodicity in Costa Rican and Mexican tropical dry forests. Global Ecology and Biogeography. 2004;13:409–425. [Google Scholar]
- Bossard CC, Randall JM, Hoshovsky MC. Invasive plants of Californiás wildlands. Berkeley, CA: University of California Press; 2000. [Google Scholar]
- Bremer B, Bremer K, Chase MW, et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Cadotte MW, Lovett-Doust J. Ecological and taxonomic differences between native and introduced plants of southwestern Ontario. Ecoscience. 2001;8:230–238. [Google Scholar]
- Cadotte MW, Murray BR, Lovett-Doust J. Ecological patterns and biological invasions: using regional species inventories in macroecology. Biological Invasions. 2006;8:809–821. [Google Scholar]
- Castro-Díez P, Montserrat-Martí G. Phenological pattern of fifteen Mediterranean phanerohytes from Quercus ilex communities of NE-Spain. Plant Ecology. 1998;139:103–112. [Google Scholar]
- Castro-Diez P, Montserrat-Martí G, Cornelissen JHC. Trade-offs between phenology, relative growth rate, life form and seed mass among 22 Mediterranean woody species. Plant Ecology. 2003;166:117–129. [Google Scholar]
- Castroviejo S. Flora Ibérica. Plantas Vasculares de la Península Ibérica e Islas Baleares (todos los vols) Madrid: Real Jardín Botánico-CSIC; 1986–2005. [Google Scholar]
- Celesti-Grapow L, Di Marzio P, Blasi C. Temporal niche separation of the alien flora of Rome. In: Child LE, Brock JH, Brundu G, et al., editors. Plant invasions: ecological threats and management solutions. Leiden: Backhuys; 2003. pp. 101–111. [Google Scholar]
- Cowling RM, Rundel PW, Lamont BB, Arroyo MK, Arianoutsou M. Plant diversity in Mediterranean-climate regions. Trends in Ecology and Evolution. 1996;11:362–366. doi: 10.1016/0169-5347(96)10044-6. [DOI] [PubMed] [Google Scholar]
- Cowling RM, Ojeda F, Lamont BB, Rundel PW, Lechmere-Oertel R. Rainfall reliability, a neglected factor in explaining convergence and divergence of plant traits in fire-prone mediterranean-climate ecosystems. Global Ecology and Biogeography. 2005;14:509–519. [Google Scholar]
- Fox GA. Components of flowering time – variation in a desert annual. Evolution. 1990;a 44:1404–1423. doi: 10.1111/j.1558-5646.1990.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Fox GA. Drought and the evolution of flowering time in desert annuals. American Journal of Botany. 1990;b 77:1508–1518. [Google Scholar]
- Goldblatt P, Manning J. Cape plants: a conspectus of the Cape flora of South Africa. Strelitzia. 2000;9:1–743. [Google Scholar]
- Goodwin BJ, McAllister AJ, Fahrig L. Predicting invasiveness of plant species based on biological information. Conservation Biology. 1999;13:422–426. [Google Scholar]
- Grime JP. Vegetation classification by reference to strategies. Nature. 1974;250:26–31. [Google Scholar]
- Groves RH, Di Castri F. Biogeography of Mediterranean invasions. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Henderson L. Alien weeds and invasive plants: a complete guide to declared weeds and invaders in South Africa. Pretoria: Agriculture Research Council; 2001. [Google Scholar]
- Herrera CM. Individual flowering time and maternal fecundity in a summer-flowering Mediterranean Shrub – making the right prediction for the wrong reason. Acta Oecologica-International Journal of Ecology. 1992;13:13–24. [Google Scholar]
- Hickman JC. The Jepson manual of higher plants of California. Berkeley, CA: University of California Press; 1993. [Google Scholar]
- Hollister RD, Webber PJ, Bay C. Plant response to temperature in Northern Alaska: implications for predicting vegetation change. Ecology. 2005;86:1562–1570. [Google Scholar]
- Imaizumi T, Kay SA. Photoperiodic control of flowering: not only by coincidence. Trends in Plant Science. 2006;11:550–558. doi: 10.1016/j.tplants.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Jensen ME, Burman RD, Allen RG. Evapotranspiration and irrigation water requirements. ASCE manual and reports on engineering practice 70. Amsterdam: Elsevier; 1990. [Google Scholar]
- Jimenez A, Pauchard A, Cavieres LA, Marticorena A, Bustamante RO. Do climatically similar regions contain similar alien floras? A comparison between the mediterranean areas of central Chile and California. Journal of Biogeography. 2008;35:614–624. [Google Scholar]
- Johnson SD. Climatic and phylogenetic determinants of flowering seasonality in the Cape Flora. Journal of Ecology. 1993;81:567–572. [Google Scholar]
- Kovach WL. Oriana for Windows, ver 2·01. UK: Kovach Computing Services; 1994. [Google Scholar]
- Lake JC, Leishman MR. Invasion success of exotics in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biological Conservation. 2004;117:215–226. [Google Scholar]
- Lloret F, Medail F, Brundu G, Camarda I, Moragues E, Rita J, Lambdon P, Hulme PE. Species attributes and invasion success by alien plants on Mediterranean islands. Journal of Ecology. 2005;93:512–520. [Google Scholar]
- Lockwood J, Hoopes M, Marchetti M. Invasion ecology. Oxford: Blackwell Publishing; 2007. [Google Scholar]
- Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J. The impact of an alien plant on a native plant–pollinator network: an experimental approach. Ecology Letters. 2007;10:539–550. doi: 10.1111/j.1461-0248.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Mazer SJ. The quantitative genetics of life-history and fitness components in Raphanus raphanistrum L. (Brassicaceae) – Ecological and evolutionary consequences of seed-weight variation. American Naturalist. 1987;130:891–914. [Google Scholar]
- Mitrakos KA. A theory for Mediterranean plant life. Acta Oecologica. 1980;1:245–252. [Google Scholar]
- Murfet IC. Environmental interaction and the genetics of flowering. Annual Review of Plant Physiology. 1977;28:253–278. [Google Scholar]
- Orshan G. Plant pheno-morphological studies in Mediterranean type ecosystems. Dordrecht: Kluwer Academic Publishers; 1989. [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics. 2006;37:637–669. [Google Scholar]
- Pérez-Latorre AV, Cabezudo B. Use of monocharacteristic growth forms and phenological phases to describe and differentiate plant communities in Mediterranean-type ecosystems. Plant Ecology. 2002;161:231–249. [Google Scholar]
- Post E, Stenseth NC. Climatic variability, plant phenology, and northern ungulates. Ecology. 1999;80:1322–1339. [Google Scholar]
- Putterill J, Laurie R, Macknight R. It's time to flower: the genetic control of flowering time. Bioessays. 2004;26:363–373. doi: 10.1002/bies.20021. [DOI] [PubMed] [Google Scholar]
- Pyšek P, Richardson DM. Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W, editor. Biological invasions. Berlin: Springer; 2007. pp. 97–125. [Google Scholar]
- Pyšek P, Sadlo J, Mandak B, Jarosik V. Czech alien flora and the historical pattern of its formation: what came first to Central Europe? Oecologia. 2003;135:122–130. doi: 10.1007/s00442-002-1170-7. [DOI] [PubMed] [Google Scholar]
- Pyšek P, Richardson DM, Rejmánek M, Webster GL, Williamson M, Kirschner J. Alien plants in checklist and floras: towards better communication between taxonomist and ecologist. Taxon. 2004;53:131–143. [Google Scholar]
- Rathcke B, Lacey EP. Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics. 1985;16:179–214. [Google Scholar]
- Rejmánek M, Randall JM. Invasive plants in California: summary and comparison with other areas in North America. Madrono. 1994;41:161–177. [Google Scholar]
- Richardson DM, Cowling RM. Why is mountain fynbos invasible and which species invade? In: Van Wilgen BW, Richardson DM, Kruger FJ, van Hensbergen HJ, editors. Fire in South African mountain fynbos. Berlin: Springer-Verlag; 1992. pp. 161–181. [Google Scholar]
- Richardson DM, Macdonald IAW, Holmes PM, Cowling RM. Plant and animal invasions. In: Cowling RM, editor. The ecology of fynbos: nutrients, fire and diversity. Cape Town: Oxford University Press; 1992. pp. 271–308. [Google Scholar]
- Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Panetta FD, West CJ. Naturalization and invasion of alien plants: concepts and definitions. Diversity and Distributions. 2000;6:93–107. [Google Scholar]
- Roche CT, Thill DC, Shafii B. Prediction of flowering in common crupina (Crupina vulgaris) Weed Science. 1997;45:519–528. [Google Scholar]
- Sanz-Elorza M, Dana-Sanchez D, Sobrino-Vesperinas E. Atlas de las plantas alóctonas invasoras en España. Madrid: Ministerio de Medio Ambiente; 2004. [Google Scholar]
- Sargent RD, Ackerly DD. Plant–pollinator interactions and the assembly of plant communities. Trends in Ecology and Evolution. 2008;23:123–130. doi: 10.1016/j.tree.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Sax DF. Native and naturalized plant diversity are positively correlated in scrub communities of California and Chile. Diversity and Distributions. 2002;8:193–210. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Thies W, Kalko EKV. Phenology of neotropical pepper plants (Piperaceae) and their association with their main dispersers, two short-tailed fruit bats, Carollia perspicillata and C. castanea (Phyllostomidae) Oikos. 2004;104:362–376. [Google Scholar]
- Traveset A, Richardson DM. Biological invasions as disruptors of plant reproductive mutualisms. Trends in Ecology and Evolution. 2006;21:208–216. doi: 10.1016/j.tree.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Valdés B, Talavera S, Fernández-Galiano E. Flora vascular de Andalucía Occidental (3 volúmenes) Barcelona: Ketres; 1987. [Google Scholar]
- Wilson JRU, Richardson DM, Rouget M, Procheş S, Amis MA, Henderson L, Thuiller W. Residence time and potential range: crucial considerations in modelling plant invasions. Diversity and Distributions. 2007;13:11–22. [Google Scholar]