Abstract
Background and Aims
The mechanisms involving light control of vitamin C content in fruits are not yet fully understood. The present study aimed to evaluate the impact of fruit and leaf shading on ascorbate (AsA) accumulation in tomato fruit and to determine how fruit sugar content (as an AsA precursor) affected AsA content.
Methods
Cherry tomato plants were grown in a glasshouse. The control treatment (normally irradiated fruits and irradiated leaves) was compared with the whole-plant shading treatment and with leaf or fruit shading treatments in fruits harvested at breaker stage. In a second experiment, the correlation between sugars and AsA was studied during ripening.
Key Results
Fruit shading was the most effective treatment in reducing fruit AsA content. Under normal conditions, AsA and sugar content were correlated and increased with the ripening stage. Reducing fruit irradiance strongly decreased the reduced AsA content (−74 %), without affecting sugars, so that sugar and reduced AsA were no longer correlated. Leaf shading delayed fruit ripening: it increased the accumulation of oxidized AsA in green fruits (+98 %), whereas it decreased the reduced AsA content in orange fruits (−19 %), suggesting that fruit AsA metabolism also depends on leaf irradiance.
Conclusions
Under fruit shading only, the absence of a correlation between sugars and reduced AsA content indicated that fruit AsA content was not limited by leaf photosynthesis or sugar substrate, but strongly depended on fruit irradiance. Leaf shading most probably affected fruit AsA content by delaying fruit ripening, and suggested a complex regulation of AsA metabolism which depends on both fruit and leaf irradiance and fruit ripening stage.
Key words: Ascorbate, fruit quality, irradiance, shading, Solanum lycopersicon, sugars, tomato, vitamin C
INTRODUCTION
The positive impact of irradiance on tomato fruit ascorbate (AsA) content has been known about for a long time (Hamner et al., 1945; Murneek et al., 1954; Venter, 1977; see reviews by Davey et al., 2000; Smirnoff and Wheeler, 2000; Dumas et al., 2003). Transferring plants from shade to sunshine increased fruit vitamin C content by up to 66 %, and the reverse effect was observed when transferring plants from sunshine to shade (Hamner et al., 1945). A linear relationship between tomato fruit AsA content and cumulated radiation during fruit development has been described in tomato fruit by shading plants for different lengths of time (Venter, 1977). These results were confirmed by shading plants with netting (0, 35, 51 or 63 % shade), which decreased fruit AsA content (El-Gizawi et al., 1993), indicating that light intensity rather than light photoperiod duration influenced AsA accumulation. Later, Davies and Hobson (1981) reported a strong impact of light and photosynthesis on AsA content in tomato fruits, suggesting that photosynthesis and AsA biosynthesis might be linked.
A correlation between sugars and AsA content has been well described in fruits since the first shading experiments by Mc Collum (1946) and could be linked to the AsA biosynthetic pathway and the role of sugars as substrates for AsA synthesis (Wheeler et al., 1998). Smirnoff and Pallanca (1996) also found a linear relationship between AsA and soluble carbohydrate content in barley leaves, which corroborates the hypothesis of a regulation of AsA biosynthesis by its sugars precursors. However, this relationship is not as clear in non-photosynthetic organs (Pallanca and Smirnoff, 1999). Moreover, the use of tomato ripened off-vine has been helpful in confirming that there was also a local response to irradiance at the fruit level: AsA accumulation increased under increased fruit irradiance (Gautier et al., 2008). Nevertheless, Giovanelli et al. (1999) observed that tomato fruit AsA content was lower during post-harvest ripening compared with fruits ripened on the vine. This indicated that AsA metabolism might be dependent on leaf to fruit transport. AsA is a small molecule and is likely to move from the leaves to the fruits with sucrose in the phloem, even though AsA transport in the phloem has not yet been shown in tomato and the contribution of AsA transport compared with AsA synthesis within the fruit can be negligible, as was reported in blackcurrant (Hancock et al., 2007). Franceschi and Tarlyn (2002) have shown that [14C]AsA loaded into leaves was found in phloem and sink organs such as flowers, in Arabidopsis thaliana, Medicago sativa and Impatiens walleriana. Similarly, Tedone et al. (2004) showed, using an EDTA exudation technique, that AsA was transported via the phloem from the leaves to potato tubers; they also found a correlation between AsA content in phloem exudates and foliar AsA content. AsA precursors could also move from the leaves to the fruits via phloem flux, as was found in the phloem of potato tuber (Tedone et al., 2004), so that AsA synthesis in potato tubers might be boosted by the l-galactono-1,4-lactone concentration reaching the potato. AsA content in tomato fruit could thus be modulated according to (a) leaf irradiance triggering changes in phloem influx of AsA or precursors to the fruits; or (b) fruit irradiance, as several enzymes linked to AsA metabolism are upregulated by light. Several studies have confirmed light-upregulated expression of mRNAs such as GDP-d-mannose pyrophosphorylase (GMPase; Tabata et al., 2002 in tobacco leaves) and l-galactono-γ-lactone dehydrogenase (GLDH; Tabata et al., 2002; Tamaoki et al., 2003 in Arabidopsis thaliana), despite this not being found in tobacco leaves (Pignocchi et al., 2003).
The present work investigated interactions between fruit development stage and changes in light environment at the fruit or leaf level, or at both fruit and leaf levels, and their effects on fruit AsA content. The experiments explored the impact of leaf shading on fruit AsA content, to respond to the question of a hypothetical transport of vitamin C or precursors from leaves to fruits which could enhance AsA biosynthesis in the fruit. As strong changes in AsA occurred during ripening, this study mainly focuses on fruit at mature green, breaker and orange stages.
MATERIALS AND METHODS
Experiment 1
Plant growth
Cherry tomato plants (Solanum lycopersicon ‘West Virginia 106’) were grown in a greenhouse in Avignon (Southern France, 44°N). On 10 August 2006, seeds were sown in Petri dishes containing Murashige and Skoog (1962) medium, and seedlings were transplanted on 25 August into 7 cm diameter pots containing potting soil (H21 Tref, Tref EGO Substrates BV, Moerdijk, The Netherlands). Plants with five growing leaves were transplanted on 13 September into 5 L pots containing potting soil (P3 Tref, Tref EGO Substrates BV) in a (24·3 × 8·5 m = 206·55 m2) compartment of a multispan Venlo-type greenhouse, N–S oriented. The plants were arranged in N–S-oriented double rows of 74 plants, which created a density of 1·8 plants m−2. Plant nutrition and chemical pest and disease control were in accordance with commercial practices. Water was supplied to the plants using a drip irrigation system to maintain 20–30 % drainage. Flowers were mechanically pollinated three times a week. Inflorescences were each pruned to ten flowers after anthesis to obtain ten fruits per truss and limit fruit size heterogeneity among trusses. All plant side shoots were removed as they appeared.
Shading treatments
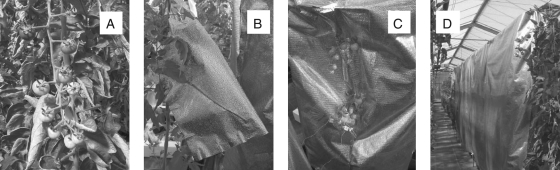
Four treatments were compared combining natural daylight and shading with a perforated silver screen which reflected 71 % of incident radiation (Lee Filter: no. 271; Andover, Hampshire, UK; Fig. 1): (A) IF-IL, control without any shading (irradiated fruits and irradiated leaves); (B) SF-SL, total shading (whole plant, i.e. shaded fruits and shaded leaves under a perforated silver screen); (C) SF-IL, shaded fruits and irradiated leaves (trusses were covered with a perforated silver screen); and (D) IF-SL, irradiated fruits and shaded leaves (leaves and stem were covered with a perforated silver screen).
Fig. 1.
Photographs illustrating the four types of treatment. (A) Control – irradiated fruits and leaves (IF-IL); (B) shaded fruits and irradiated leaves (SF-IL); (C) irradiated fruits and shaded leaves (IF-SL); (D) total shading (fruits and leaves shaded: SF-SL).
These shading treatments mostly affected irradiance and had a low impact on fruit temperature due to the reflective properties and the perforations of the film. Moreover, as 71 % of the incident light was reflected, leaf shading was likely to affect plant photosynthesis and carbon flux to the fruits.
Three plants were randomly assigned to each of the four different light treatments. Shading treatments were initiated on 2 November, 20 d after the mean anthesis date of the first seven flowers of truss number 3.
Fruit sampling
From 14 November to 11 December, twice a week, cherry tomato fruits at breaker stages were harvested at midday on trusses 3–5 (greenish to yellow or pale orange pericarp with light orange locular tissue) and were partitioned into three replicates per treatment. Each replicate corresponds to a sample of at least four tomatoes. Following harvest, fruits were cut on ice, seeds and gel were discarded, and pericarp tissue was frozen in liquid nitrogen and stored at −80 °C before blending in liquid nitrogen.
Experiment 2
Plant growth
Experiment 2 took place in Spring 2007 using similar materials and methods to those previously described for expt 1. Sowing took place on 16 January, and plants with five growing leaves were transplanted on 29 January and then placed in the greenhouse in 5 L pots on 23 February; 32 plants were randomly assigned to the four shading treatments (eight plants per treatment).
Shading treatment: interaction between fruit shading and fruit developmental stage
Fruit shading of trusses 6 started on 23 April for 21 d. On 15 May, fruits from different plants and treatments were harvested at midday according to their developmental stage to obtain 4–5 replicates per treatment. Fruit developmental stage was expressed as days post-anthesis (DPA): 28 DPA corresponded to mature green fruits (external fruit colour coordinate a = −11), 32 DPA to fruits at breaker stage (a = −5·8) and 36 DPA to fruits with orange external coloration (a = 28·7). Following harvest, fruit weight was determined, fruits were then cut on ice similarly to expt 1 and pericarps were frozen in liquid nitrogen and stored at −80 °C until blending in liquid nitrogen.
Shading treatment: interaction between leaf or whole-plant shading and fruit developmental stage
Whole-plant shading (SF-SL) and leaf shading only (IF-SL, fruits of truss 5 or 6 exposed to natural daylight and shaded leaves) were initiated on 9 May and lasted for 6 d to limit any developmental delay linked to leaf shading which could distort the treatment comparison as AsA and sugar content strongly depend on fruit developmental stage. After 6 d of shading, fruits were harvested at midday according to their stage of development from 28 to 36 DPA to obtain four replicates per treatment and per stage of development, as was previously described for shaded fruits.
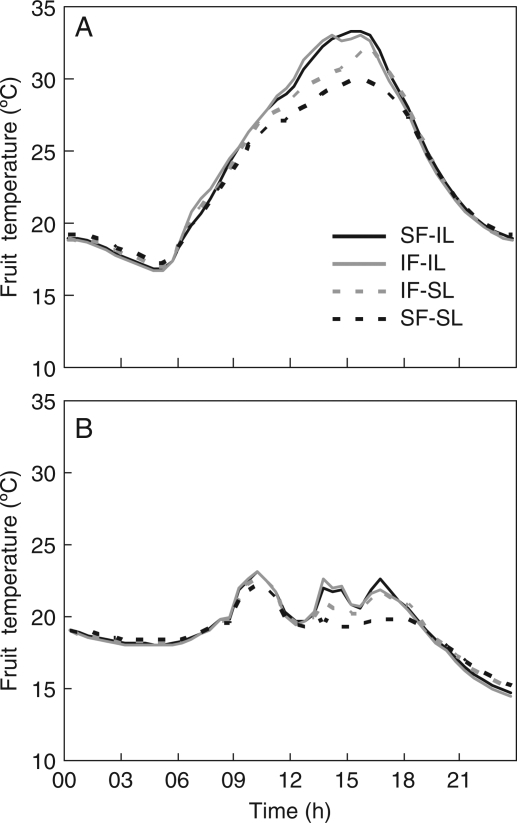
During expt 2, fruit temperature was measured every minute on 4–9 fruits per treatment with very fine thermocouples (0·2 mm copper–constantan). Thermocouples were inserted to a depth of 2 mm into the upper exposed side of fruits of the sixth truss in order to measure the impact of shading on fruit temperature. Temperature measurements were averaged and stored every 30 min on a delta logger (Delta-T DL2e, Delta-T Devices Ltd, Cambridge, UK). Evolution of fruit temperature during a sunny (12 April) or a cloudy day (14 April) is shown in Fig. 2. Fruit shading by itself had no impact on fruit temperature (comparison of SF- IL with IF- IL), but leaf shading (IF-SL) or whole-plant shading (SF-SL) reduced fruit temperature during the diurnal period. Thus, in the leaf shading treatment, despite the reflective properties of the shading filter, shading leaves could result in reduced light reaching the fruit. Due to a lower fruit temperature in the leaf shading treatment (SF-SL or IF-SL), these treatments were only applied for 6 d to limit the impact on fruit developmental stage. In contrast, as the fruit shading treatment had no effect on fruit temperature, fruit shading lasted for 21 d.
Fig. 2.
Impact of the different shading treatments on fruit temperature recorded on a sunny day (A) and a cloudy day (B). Data shown are means of temperature measurements made on four different plants per treatment during ripening of fruits of the sixth truss on 12 April (A) and 14 April (B) during expt 2. Copper–constantan thermocouples (0·2 mm) were inserted 2 mm into the upper exposed side of the fruits. Temperature measurements were made every minute and averaged every 30 min. SF-IL, shaded fruits and irradiated leaves; IF-IL, control, irradiated fruit and leaves; IF-SL, irradiated fruits and shaded leaves; SF-SL, total shading (fruits and leaves shaded).
Two complementary shading experiments were carried out. In expt 1, fruits at breaker stage were harvested to compare the respective impact of leaf, fruit or both leaf and fruit shading on fruit composition and reveal the most effective shading treatment. In the second experiment, the interaction between the shading treatment and fruit ripening was studied. The correlation between fruit soluble sugars and fruit AsA was studied during ripening under normal conditions (no shading) or fruit shading (which was the most effective shading treatment in reducing AsA). The second objective of expt 2 was to confirm that leaf shading also had a small impact on fruit AsA content by the comparison of fruit harvested from plants grown under normal conditions, leaf shading or whole-plant shading.
Fruit analyses
During expt 2, external fruit colour was characterized near the pistil scar by a Minolta Chroma meter (CR 300, Minolta, France SA) using the LAB colour space (Hunter colour coordinates L, a and b; L = lightness, a ranging from green to red, b ranging from blue to yellow). Fruit skin colour was expressed as a/b, which better relates to colour variation during tomato ripening as previously used in Giovanelli et al. (1999). As fruit dry matter content decreased during ripening and with shading, the decision was made to express fruit sugar and AsA content per unit dry weight.
Assays of total and reduced AsA content were carried out as previously described (Stevens et al., 2006) on material conserved at −80 °C. Briefly, tomato tissue was ground in liquid nitrogen, and 1 g of powder was homogenized with 600 µL of ice-cold 6 % trichloroacetic acid (TCA). Samples were centrifuged for 15 min at 16 000 g at 4 °C. A 20 µL aliquot of the supernatant was used in each assay. The AsA standards were prepared fresh: a solution of 1 mg mL−1 sodium AsA was diluted in 6 % TCA to give a concentration in 20 µL of 0, 5, 10, 15, 20 and 30 nmol, allowing a standard curve of absorption values of between 0 and 1 to be generated after addition of the appropriate reagents (below). Two assays were carried out on each sample, one to measure the total AsA [including addition of 5 mm dithiothreitol (DTT)] and one to quantify the reduced AsA content (omission of DTT from the assay). A 20 µL aliquot of each sample or standard was distributed into at least two wells (for two repetitions) of a 96-well microplate and mixed with 20 µL of 5 mm DTT (total AsA assay) or 0·4 m phosphate buffer pH 7·4 (reduced AsA assay). The plate was incubated at 37 °C for 20 min. A 10 µL aliquot of N-ethylmaleimide (total AsA assay) or 0·4 m phosphate buffer pH 7·4 (reduced AsA assay) was added and mixed followed by the addition of 80 µL of colour reagent (see below). After incubation at 37 °C for 50 min, the absorbance was read at 550 nm using the multiskan Ascent MP reader (Labsystems, Thermo Fisher Scientific, Courtaboeuf, France). The colour reagent was made up as follows: solution A = 31 % orthophosphoric acid, 4·6 % (w/v) TCA and 0·6 % (w/v) iron chloride (FeCl3); solution B = 4 % 2,2-dipyridyl (w/v; made up in 70 % ethanol). Solutions A and B were mixed 2·75 parts (A) to 1 part (B). The standard curve obtained from the standard solution values allowed calculation of the AsA concentration of the samples after correction for the quantity of water introduced by the tomato fruit sample. Additional assays were carried out with ascorbate oxidase on samples (shaded and unshaded fruits from green immature to red ripe stages) to check the impact of other compounds such as phenolics on Fe3+ reduction and therefore the specificity of the assay used. These assays confirmed that at least 95 % of the absorbance in the samples is specific for AsA: after ascorbate oxidase addition a background absorbance of between 0 and 5 % remained.
Sugars and acids were extracted as described in Gomez et al. (2002). Briefly, the soluble sugars and acids were extracted at 4 °C from 5 mg of freeze-dried fruit powder. First, 1 mL of a methanol/water solution (1 : 1 v/v) was added, then 0·3 mL of chloroform. Samples were shaken for 30 min at 4 °C and centrifuged (5 min at 16 000 g at 4 °C). A 0·8 mL aliquot of the methanol/water supernatant was recovered, evaporated under vacuum (Speed-Vac) and stored at −20 °C until measurement of soluble sugars or acids. For starch measurements, 1 mL of methanol was added to the tube containing chloroform and fruit powder, and the tube was shaken for 20 min before centrifugation (5 min at 16 000 g at 4 °C). The supernatant was discarded and the pellet was used for starch assay. Starch was dispersed by autoclaving for 2 h (120 °C) and then hydrolysed for 1·5 h at 56 °C by addition of amyloglucosidase solution. The glucose released by starch hydrolysis was measured as described previously (Gomez et al., 2007) using 150 µL of diluted extract, 100 µL of a solution containing ATP, NAD and 20 µL of a solution containing glucose-6-phosphate dehydrogenase and hexokinase.
For measurement of sugars or acids, samples were homogenized with 800 µL of water at 4 °C for 10 min before adding 5 mg of PVPP (polyvinylpolypyrrolidone). After 30 min, PVPP and phenolics were removed by centrifugation (5 min at 16 000 g at 4 °C), and sugars and acids were assayed from the supernatant. Sugars were estimated using the micro-method described in Gomez et al. (2007). Glucose, fructose and sucrose concentrations were successively quantified by enzymatic assays measuring the production of NADH directly in each well at 340 nm using the multiskan Ascent MP reader. Samples were diluted to obtain a final concentration <0·066 g L−1 for each sugar. A 150 µL aliquot of extract and 100 µL of a solution (buffer pH 7·6, containing ATP and NAD) were loaded into each well and the absorbance was measured before addition of 20 µL of a solution containing glucose phosphate dehydrogenase, hexokinase and ammonium sulfate solution. After 2 h, when the reaction was completed, a second absorbance measurement was performed. The increase in absorbance between the two readings was due to the formation of NADH and was proportional to the transformation of glucose in the extract. A 20 µL aliquot of a solution containing phosphoglucoisomerase was then added. A third absorbance measurement was carried out 2 h later, when the reaction was completed: the increase in absorbance was proportional to the initial fructose content in the extract. The addition of 20 µL of a solution containing β-fructosidase produced fructose and glucose from the sucrose present in the extract, which was determined from the absorbance measurement, performed 3 h later, when the reaction was completed. Acids were determined from enzymatic assays adapted from a citric acid kit (Boehringer Mannheim, ref kit 0 139 076) and an l-malic acid kit (Boehringer Mannheim ref kit 0 139 068) by measuring the disappearance (for citrate) or the appearance (for malate) of NADH. For the citrate assay, 100 µL of a reaction mixture was put into each well followed by 180 µL of sample. The reaction mixture was prepared from 12 mL of 0·6 m glycylglycine buffer (pH 7·8, containing 0·1 m l-glutamate) mixed with 23 µL of l-malate dehydrogenase (5 g L−1), 102 µL of lactate dehydrogenase (5 g L−1) and 5 mg of NADH. The absorbance was measured at 340 nm before addition of 20 µL of a solution containing 48 mg of citrate lyase diluted in 3 mL of water. The plate was incubated at room temperature and regularly shaken for 2 h, before reading the absorbance. For the malate assay, the reaction mixture consisted of 100 µL of 0·6 m glycylglycine buffer (pH 10), 20 µL of a 27·13 mm NAD solution, 20 µL of glutamate oxalate transaminase (66·7 mg L−1) and 100 µL of the sample. The absorbance was measured at 340 nm before the addition of 20 µL of l-malate dehydrogenase solution (33·3 mg L−1). After homogenization, the plate was incubated for 1·5 h at room temperature before reading at 340 nm.
Statistical analysis
Analyses of variance considering the factor ‘shading treatments’ (both experiments) and ‘fruit developmental stage’ (second experiment) and their interactions (second experiment) were performed with the XLSTAT 2007 software (XLSTAT, Addinsoft FRANCE, Paris, France), and significant differences among treatments were assessed using a Tukey test at 5 %. Pearson correlation coefficients were estimated using XLSTAT software, and a significant correlation corresponded to a P-value <5 %.
RESULTS
Impact of leaf and /or fruit shading on fruit composition at breaker stage
Leaf shading (IF-SL) decreased fruit dry matter content (−18 %) and modified dry matter composition in tomato harvested at breaker stage (Table 1). The concentration of different sugars was strongly reduced by leaf shading: glucose (−39 %), fructose (−23 %), sucrose (−33 %) and starch (−37 %), and, in contrast, malic and citric acids strongly increased (by 91 and 49 %, respectively). Fruit shading only (SF-IL) had no effect on fruit dry matter content and composition in soluble sugars, starch, citric and malic acids. The combination of leaf and fruit shading (SF-SL) was not different from the leaf shading treatment with regard to sugars, malic or citric acid accumulation. Consequently, fruit composition in terms of soluble sugars, starch, malic and citric acids was strongly influenced by leaf irradiance but not by the fruit microclimate.
Table 1.
Impact of shading (leaves, fruits or both) during expt 1 on mean fruit composition (± s.e.) at breaker stage expressed per 100 g dry weight
| Control IF-IL (irradiated fruit–irradiated leaves) | SF-IL (shaded fruit–irradiated leaves) | IF-SL (irradiated fruit–shaded leaves) | SF-SL (shaded fruit–shaded leaves) | Shading impact (Pr > F) | |
|---|---|---|---|---|---|
| Dry matter (g) | 7·7 ± 0·3a | 7·8 ± 0·3a | 6·3 ± 0·3b | 6·6 ± 0·3b | 0·0001 |
| Glucose (g 100 g−1 d. wt) | 17·1 ± 0·7a | 17·9 ± 1·2a | 10·4 ± .9b | 11·9 ± 0·6b | 0·0001 |
| Fructose (g 100 g−1 d. wt) | 16·4 ± 0·9a | 18·6 ± 1·4a | 12·6 ± 1·7b | 12·6 ± 0·4b | 0·001 |
| Sucrose (g 100 g−1 d. wt) | 9·6 ± 0·2a | 9·5 ± 0·6a | 6·4 ± 0·4b | 7·2 ± 0·2b | <0·0001 |
| Starch (g 100 g−1 d. wt) | 3·2 ± 0·7ab | 4·1 ±0·9a | 2·0 ± 1·1b | 1·7 ± 0·3b | 0·02 |
| Malic acid (g 100 g−1 d. wt) | 1·60 ± 0·03b | 1·78 ± 0·08b | 3·06 ± 0·60a | 2·41 ± 0·30ab | 0·003 |
| Citric acid (g 100 g−1 d. wt) | 7·2 ± 0·1b | 8·1 ± 0·7b | 10·7 ± 0·7a | 10·5 ± 1·0a | 0·001 |
The objective of this experiment was to determine the most effective shading treatment that reduces fruit ascorbate content at breaker stage. Four treatments were compared: fruits grown under normal conditions (irradiated fruits and irradiated leaves, IF-IL); fruits grown under shading (shaded fruits, irradiated leaves, SF-IL); fruits harvested on plants with shaded leaves (irradiated fruits shaded leaves, IF-SL); and fruits harvested on plants receiving global shading (shaded fruits and shaded leaves, SF-SL). Results in the same line with the same superscript were not significantly different (P < 0·05) according to the classification obtained by the Tukey test.
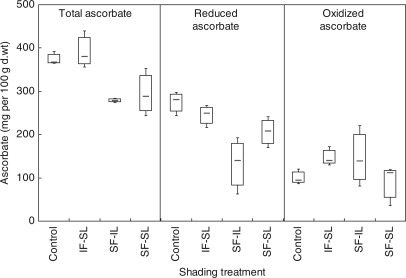
Total fruit AsA showed a totally different pattern from the major acids: it significantly decreased (P < 0·0001) under whole-plant shading (SF-SL, −21 %) or fruit shading (SF-IL, −26 %) but not under leaf shading (IF-SL, Fig. 3). Fruit shading had the strongest impact on the reduced AsA content (−52 %), in comparison with whole-plant shading (−25 %) or leaf shading (−11 %, not significant). The oxidized form, dehydroascorbic acid (DHA), was either not affected by fruit shading or the global shading treatment, or slightly increased under the leaf shading treatment. It is concluded that the reduced AsA content in fruit at breaker stage mostly depended on fruit microclimate rather than leaf microclimate.
Fig. 3.
Impact of shading on fruit ascorbate content and its partitioning as the reduced and oxidized forms in fruits harvested at breaker stage on plants grown under normal conditions (control: irradiated fruits and leaves, IF-IL), with shaded fruits and irradiated leaves (SF-IL), with irradiated fruits and shaded leaves (IF-SL) or under total shading (fruits and leaves shaded: SF-SL). Boxplots showed the median, the lower and upper quartile and the lower and upper limits according to Tukey.
Impact of fruit shading on AsA and sugar content in tomato fruit during ripening
The impact of fruit shading (the most effective treatment in reducing AsA) on both soluble sugars and AsA during fruit ripening was studied. Fruit shading did not impact on fruit ripening rate as assessed by the change in external fruit coloration from green to red: the coordinate a increased from −11·2 in shaded fruit at 28 DPA (−11 for control fruit), to −5·5 at 32 DPA (−5·8 for control fruit) and 25·2 at 36 DPA (28·7 for control fruit).
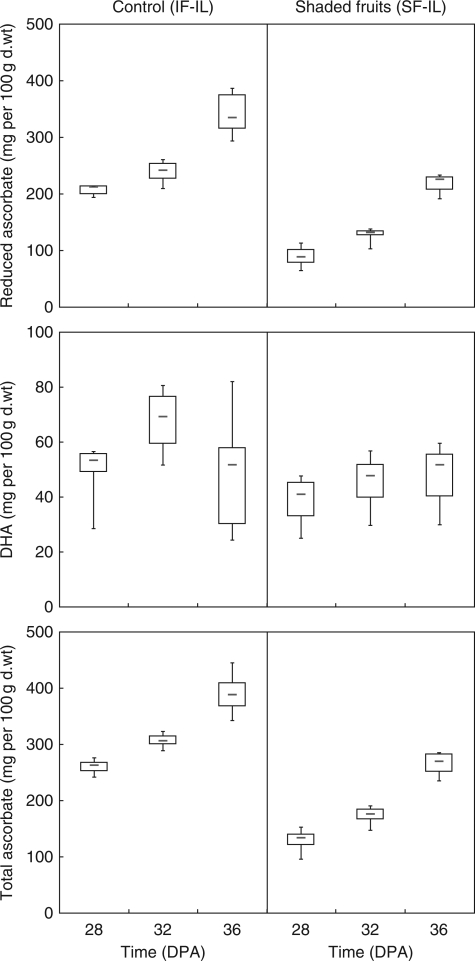
During ripening, soluble sugars slightly increased in fruits grown under normal conditions as well as in shaded fruits, mostly due to an increase in fructose (Table 2). Total AsA content also increased (+63 % in control and +106 % in shaded fruits, Fig. 4). This was mostly due to the increased content of the reduced AsA form (+78 % in control and +145 % in shaded fruits), whereas DHA content was not significantly modified.
Table 2.
Impact of fruit shading and ripening on fruit soluble sugars content
| Mature green (28 DPA) |
Breaker (32 DPA) |
Orange (36 DPA) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | SF-IL | Control | SF-IL | Control | SF-IL | Shading | Fruit stage | Interaction | |
| Glucose (g 100 g−1 d. wt) | 16·9 ± 1·1a | 16·8 ± 1·8a | 17·0 ± 1·7a | 16·3 ± 0·8a | 17·3 ± 1·0a | 16·6 ± 1·6a | 0·41 | 0·9 | 0·92 |
| Fructose (g 100 g−1 d. wt) | 16·6 ± 1·1b | 17·2 ± 1·1ab | 18·1 ± 1·7ab | 17·3 ± 0·3ab | 19·9 ± 1·8ab | 20·2 ± 2·3a | 0·94 | 0·002 | 0·64 |
| Sucrose (g 100 g−1 d. wt) | 8·1 ± 0·5a | 8·4 ± 0·7a | 8·3 ± 0·9a | 8·3 ± 0·1a | 10·4 ± 2·7a | 8·8 ± 0·8a | 0·39 | 0·08 | 0·32 |
During expt 2, fruits were harvested according to their developmental stage (expressed as days post-anthesis), which correspond to fruits at mature green, breaker and orange stage to de-correlate ascorbate and sugar contents. The details of the shading treatment are given in the footnotes of Table 1. Data are means ± s.e. Results in the same line with the same superscript were not significantly different (P < 0·05) according to the classification obtained by the Tukey test.
Fig. 4.
Impact of fruit shading (SF-IL) on fruit ascorbate content and its partitioning as the reduced and oxidized forms in fruits harvested at mature green, breaker and red stage. Boxplots showed the median, the lower and upper quartile and the lower and upper limits according to Tukey.
Similarly to what was observed during expt 1, fruit shading did not affect fruit sugar content, but it lowered the reduced AsA (−45 %) and DHA (−16 %) content and consequently total vitamin C content (Fig. 4). For all fruit ripening stages (mature green, breaker and orange), fruit shading decreased the reduced AsA content. In contrast, DHA content decreased with shading at mature green (−18 %) and breaker stage (−34 %) but not at orange stage (interaction between shading and developmental stage: P = 0·08).
During ripening, reduced AsA and soluble sugars (AsA precursors) were significantly correlated (r = 0·74; P = 0·006) in tomato fruits grown under normal conditions. In contrast, there was no correlation between DHA and soluble sugar contents (P = 0·96). Since reduced AsA was the predominant form of AsA, a significant correlation was also found between total AsA and soluble sugars (r = 0·69, P = 0·01). For shaded fruits, there was no longer a significant correlation between reduced AsA and sugars (r = 0·49, P = 0·10), nor between DHA and sugars (P = 0·46), or total AsA and sugars (P = 0·17).
Comparison of leaf shading and whole-plant shading during ripening on fruit AsA and sugar content
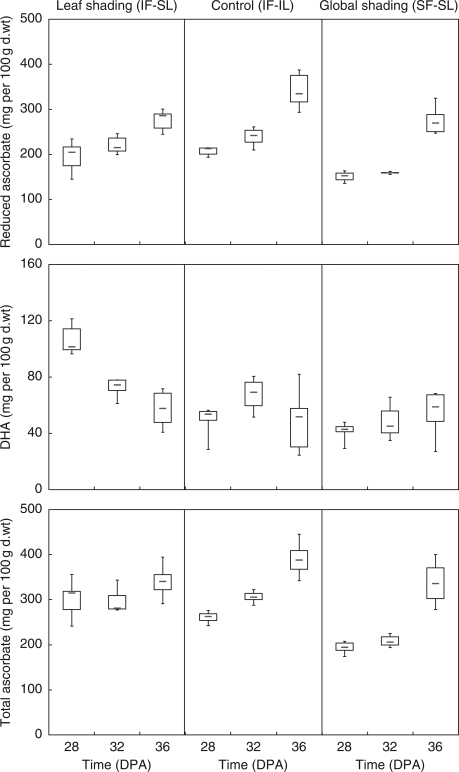
Leaf shading reduced fruit sugar content due to lower glucose, fructose and sucrose content (Table 3). The stronger effect of global shading (SF-SL) compared with leaf shading (IF-SL) on reduced AsA content confirmed that fruit irradiance was the most important regulating factor (Fig. 5). There was a strong interaction between fruit ripening stage and the shading treatment on reduced AsA and DHA content (P < 0·0001). Leaf shading did not affect reduced AsA content but it increased DHA by 127 % in mature green fruits (28 DPA, Fig. 5).
Table 3.
Interaction between fruit developmental stage and leaf shading [global plant shading (SF-SL) or leaf shading only (IF-SL)] on fruit soluble sugar content (expressed in g 100 g−1 d. wt)
| Mature green (28 DPA) |
Breaker (32 DPA) |
Orange (36 DPA) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SF-SL | IF-SL | Control | SF-SL | IF-SL | Control | SF-SL | IF-SL | Shading | Fruit stage | Interaction | |
| Glucose | 16·9 ± 1·1a | 13·9 ± 0·6bc | 12·8 ± 1·9c | 17·0 ± 1·7a | 13·4 ± 0·4bc | 14·4 ± 1·9abc | 17·3 ± 1·0a | 15·7 ± 0·5abc | 16·0 ± 1·0ab | <0·0001 | 0·006 | 0·16 |
| Fructose | 16·6 ± 1·1ab | 13·7 ± 0·8b | 17·7 ± 2·4a | 18·1 ± 1·7a | 13·3 ± 0·6b | 18·0 ± 2·9a | 19·9 ± 1·8a | 17·9 ± 1·2a | 17·1 ± 1·1ab | 0·001 | 0·006 | 0·02 |
| Sucrose | 8·1 ± 0·5ab | 6·8 ± 0·3b | 7·8 ± 0·8ab | 8·3 ± 0·9ab | 6·5 ± 0·3b | 8·4 ± 0.3ab | 10·4 ± 2·7a | 8·1 ± 0·5ab | 8·0 ± 0·6ab | 0·003 | <0·04 | 0·23 |
Data are means ± s.e. Results in the same line with the same superscript were not significantly different (P < 0·05) according to the classification obtained by the Tukey test.
Fig. 5.
Impact of leaf (IF-SL) or whole-plant shading (SF-SL) on fruit ascorbate content and its partitioning as the reduced and oxidized forms in fruits harvested at mature green, breaker and red stage. Boxplots showed the median, the lower and upper quartile and the lower and upper limits according to Tukey.
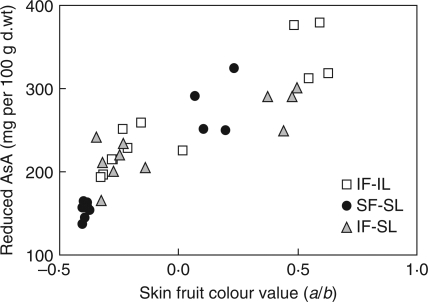
Leaf shading (IF-SL) for 6 d delayed the appearance of red fruit skin coloration (external fruit colour coordinate, a = 19·3 compared with 28·7 for the control 36 DPA). Thus, despite the short-term duration of the leaf shading treatment, the fruit ripening was delayed. This might be related to lower fruit temperature (Fig. 2), but also to reduced photosynthesis as a consequence of leaf shading. To analyse better the interactions between AsA metabolism and fruit ripening stage, fruit skin colour value was used instead of DPA to describe the fruit ripening stage. This showed that the ripening delay triggered by total shading was responsible for the lower reduced AsA content (Fig. 6).
Fig. 6.
Relationship between reduced AsA content and fruit ripening stage, defined by the skin colour value a/b, in control fruits (IF-IL), fruits from plants with shaded leaves (IF-SL) or fruits from totally shaded plants (SF-SL).
DISCUSSION
The aim of the present study was to determine the respective impact of fruit and leaf irradiance on fruit AsA content. Light-controlled regulation of fruit AsA content could be perceived at the fruit level by a modification of the synthesis, recycling or catabolism of AsA, but also at the leaf level, triggering changes in AsA and sugar synthesis and then in their phloem transport to the fruits. Using shading treatments allowed discrimination between the impact of leaf and fruit shading on fruit AsA content. It was shown that light received by the fruit has more of an impact on the reduced AsA content in fruit than light received by the plant.
The fact that tomatoes harvested at a mature green stage and left to ripen maintain their reduced AsA content (Jimenez et al., 2002) indicated that tomato fruits possess the different enzymes related to AsA metabolism (synthesis, recycling, Ahn et al., 2002; Jimenez et al., 2002). The impact of irradiance on AsA-related genes or enzymes has not been studied in fruit, but several studies reported upregulation by light of the expression of genes involved in AsA metabolism in leaves, even if some data are conflicting: only ascorbate oxidase and cytosolic ascorbate peroxidase mRNAs are light regulated in tobacco leaves (Pignocchi et al., 2003) and mRNAs for GMPase (Tabata et al., 2002) and GLDH (Tabata et al., 2002; Tamaoki et al., 2003) are light regulated in tobacco and Arabidopsis thaliana. Similarly, several enzymes involved in AsA metabolism showed increased activity when irradiance increased: the GDP-l-galactose phosphorylase appeared to be a key regulatory point of light regulation of the AsA pool in Arabidopsis leaves (Dowdle et al., 2007), in combination with enzymes involved in AsA recycling such as MDHAR (monodeyhydroascorbate reductase) and DHAR (dehydroascorbate reductase; Chen, 2004). Consequently, shading fruits may trigger downregulation of gene expression or enzyme activities involved in AsA metabolism.
The total AsA and carbohydrate pools are strongly correlated in barley leaves exposed to light (Smirnoff and Pallanca, 1996). The transition from low light to high light and back to low light suggested that increasing irradiance increased AsA synthesis and AsA turnover. In the present experiment, the link between AsA and sugar content during fruit ripening was less obvious for shaded fruits. A decrease in fruit AsA content under shading was unlikely to be due to a substrate limitation of AsA biosynthesis, as fruit shading did not impact on fruit sugar content. Decreased fruit AsA content could also not be explained by a downregulation of the expression of genes related to AsA biosynthesis and metabolism by sugars, as was shown in broccoli florets (Nishikawa et al., 2005); sucrose feeding of broccoli plants without leaves and roots suppressed the loss of AsA, and genes related to AsA metabolism in chloroplasts were upregulated by sucrose feeding. Thus sugar levels may act as a signal (reviewed by Smeekens, 2000) and may consequently affect AsA-related gene expression. Another explanation for the correlation between sugars and leaf AsA was proposed by Yabuta et al. (2007): recent studies in A. thaliana leaves suggested that the correlation could be related to the photosynthetic electron flux and independent of sugars. Consequently, the correlation between sugar and AsA content usually described in fruits might be linked to the existing correlation between fruit irradiance and leaf irradiance under natural conditions and not to the dependence of AsA biosynthesis on the availability of its substrate.
The impact of leaf shading clearly reduced fruit sugar content, but its effect on AsA content appears to be more complex as it interacted with fruit ripening stage. Leaf shading had no effect on reduced AsA content in fruits harvested 28 DPA, but it increased DHA and total AsA content. A different pattern was observed in fruits harvested at an orange stage: the reduced AsA content decreased and DHA content was not modified, so that total AsA was reduced. Higher DHA content in fruits harvested 28 DPA and lower reduced AsA content in fruit harvested 36 DPA could be linked to a delay in fruit ripening triggered by leaf shading; indeed a delay in the appearance of fruit external red coloration was observed with leaf shading and DHA has been shown to decrease during tomato ripening while reduced AsA increased (Gautier et al., 2008). This delay in fruit ripening could be linked to the lower fruit sugar content which may trigger downregulation of genes related to AsA biosynthesis and metabolism (Nishikawa et al., 2005).
The present data underlined the complexity of AsA content regulation which depends on irradiance reaching both leaves and fruits, and probably interacts with reactive oxygen species (ROS) production during ripening (Jimenez et al., 2002) and with changes in AsA-related enzyme activities such as ascorbate oxidase (Yahia et al., 2001) and ascorbate peroxidase (Ahn et al., 2002) during ripening.
It is concluded that the correlation usually observed between sugars and AsA content is the consequence of independent mechanisms: on one hand, leaf irradiance has an impact on photosynthesis and sugar transport to the fruits, and on the other hand fruit irradiance has an impact on AsA metabolism. The present data confirmed that both the leaf and fruit environment may have an impact on fruit AsA content. Fruit shading may directly reduce AsA synthesis and AsA metabolism globally so that the total AsA, reduced AsA and DHA contents decreased. The impact of leaf shading was not so obvious and not in contradiction to long-distance transport by the phloem of sugars, AsA or AsA precursors to the fruit. Leaf shading may trigger a delay in fruit ripening, delaying DHA degradation and accumulation of reduced AsA. Both light reaching the fruits and the leaves and the ripening stage controlled AsA metabolism; consequently further analyses of the regulation of AsA-related enzymes by fruit and leaf irradiance and fruit developmental stage will further the understanding of regulation of AsA metabolic.
ACKNOWLEDGEMENTS
We thank Emmanuel Botton and Claude Courbet for plant management and fruit harvesting, and Emilie Rubio, Patricia Robert, Doriane Bancel and Hassana Bouhala for starch, sugar, acid and AsA assays.
LITERATURE CITED
- Ahn T, Schofield A, Gopinadhan P. Changes in antioxidant enzyme activities during tomato fruit development. Physiology and Molecular Biology of Plants. 2002;8:241–249. [Google Scholar]
- Chen Z, Gallie DR. The ascorbic acid redox state controls guard cell signaling and stomatal movement. The Plant Cell. 2004;16:1143–1162. doi: 10.1105/tpc.021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MW, van Montagu M, Inze D, et al. Plant l-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture. 2000;80:825–860. [Google Scholar]
- Davies JN, Hobson GE. The constituents of tomato fruit – the influence of environment, nutrition, and genotype. Critical Reviews in Food Science and Nutrition. 1981;15:205–280. doi: 10.1080/10408398109527317. [DOI] [PubMed] [Google Scholar]
- Dowdle J, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. The Plant Journal. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- Dumas Y, Dadomo M, Di Lucca G, Grolier P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. Journal of the Science of Food and Agriculture. 2003;83:369–382. [Google Scholar]
- El-Gizawi AM, Abdallah MMF, Gomaa HM, Mohamed SS. Effect of different shading levels on tomato plants. 2. Yield and fruit quality. Acta Horticulturae. 1993;323:349–354. [Google Scholar]
- Franceschi VR, Tarlyn NM. l-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiology. 2002;130:649–656. doi: 10.1104/pp.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier H, Diakou-Verdin V, Bénard C, et al. How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? Journal of Agriculture and Food Chemistry. 2008;56:1241–1250. doi: 10.1021/jf072196t. [DOI] [PubMed] [Google Scholar]
- Giovanelli G, Lavelli V, Peri C, Nobili S. Variation in antioxidant components of tomato during vine and post-harvest ripening. Journal of the Science of Food and Agriculture. 1999;79:1583–1588. [Google Scholar]
- Gomez L, Bancel D, Rubio E, Vercambre G. The microplate reader: an efficient tool for the separate enzymatic analysis of sugars in plant tissues – validation of a micro-method. Journal of the Science of Food and Agriculture. 2007;87:1893–1905. [Google Scholar]
- Gomez L, Rubio E, Auge M. A new procedure for extraction and measurement of soluble sugars in ligneous plants. Journal of the Science of Food and Agriculture. 2002;82:360–369. [Google Scholar]
- Hamner KC, Bernstein L, Maynard LA. Effects of light intensity, day length, temperature, and other environmental factors on the ascorbic acid content of tomatoes. Journal of Nutrition. 1945;29:85–97. [Google Scholar]
- Hancock RD, Walker PG, Pont SDA, et al. l-Ascorbic acid accumulation in fruit of Ribes nigrum occurs by in situ biosynthesis via the l-galactose pathway. Functional Plant Biology. 2007;34:1080–1091. doi: 10.1071/FP07221. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Creissen G, Kular B, et al. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 2002;214:751–758. doi: 10.1007/s004250100667. [DOI] [PubMed] [Google Scholar]
- Mc Collum J. Effect of sunlight exposure on the quality of constituents of tomato fruits. Proceedings of the American Society for Horticultural Science. 1946;48:413–416. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Murneek AE, Maharg L, Wittner SH. Ascorbic acid (vitamin C) content of tomatoes and apples. University of Missouri Agricultural Experiment Station Research Bulletin. 1954;568:3–24. [Google Scholar]
- Nishikawa F, Kato M, Hyodo H, Ikoma Y, Sugiura M, Yano M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. Journal of Experimental Botany. 2005;56:65–72. doi: 10.1093/jxb/eri007. [DOI] [PubMed] [Google Scholar]
- Pallanca JE, Smirnoff N. Ascorbic acid metabolism in pea seedlings. A comparison of d-glucosone, l-sorbosone, and l-galactono-1,4-lactone as ascorbate precursors. Plant Physiology. 1999;120:453–461. doi: 10.1104/pp.120.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH. The function of ascorbate oxidase in tobacco. Plant Physiology. 2003;132:1631–1641. doi: 10.1104/pp.103.022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. Sugar-induced signal transduction in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Pallanca JE. Ascorbate metabolism in relation to oxidative stress. Biochemical Society Transactions. 1996;24:472–478. doi: 10.1042/bst0240472. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Critical Reviews in Plant Sciences. 2000;19:267–290. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- Stevens R, Buret M, Garchery C, Carretero Y, Causse M. Technique for rapid, small-scale analysis of vitamin C levels in fruit and application to a tomato mutant collection. Journal of Agricultural and Food Chemistry. 2006;54:6159–6165. doi: 10.1021/jf061241e. [DOI] [PubMed] [Google Scholar]
- Tabata K, Takaoka T, Esaka M. Gene expression of ascorbic acid-related enzymes in tobacco. Phytochemistry. 2002;61:631–635. doi: 10.1016/s0031-9422(02)00367-9. [DOI] [PubMed] [Google Scholar]
- Tamaoki M, Mukai F, Asai N, et al. Light-controlled expression of a gene encoding l-galactono-gamma-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Science. 2003;164:1111–1117. [Google Scholar]
- Tedone L, Hancock RD, Alberino S, Haupt S, Viola R. Long-distance transport of l-ascorbic acid in potato. BMC Plant Biology. 2004;4:16. doi: 10.1186/1471-2229-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter F. Solar radiation and vitamin C content of tomato fruits. Acta Horticulturae. 1977;58:121–127. [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Mieda T, Rapolu M, et al. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. Journal of Experimental Botany. 2007;58:2661–2671. doi: 10.1093/jxb/erm124. [DOI] [PubMed] [Google Scholar]
- Yahia EM, Contreras-Padilla M, Gonazalez-Aguilar G. Ascorbic acid content in relation to ascorbic acid oxidase activity and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. Lebensmittel-Wissenschaft Und-Technologie-Food Science and Technology. 2001;34:452–457. [Google Scholar]