Abstract
Background and Aims
The long co-existence of broomrapes and their hosts within the same environment has culminated in a strong adaptation and effective parasitism. As a first step of specialization in the parasitic process, seed receptors of parasitic plant species vary in their ability to recognize compounds released by their hosts. This work aims to investigate potential patterns for the reception requirements needed to activate germination within Orobanche and Phelipanche species.
Methods
Induction of the germination of seeds of nine Orobanche and Pheliphanche species by root exudates of 41 plant species was studied and subjected to biplot multivariate analysis.
Key Results
A high level of specialization in root exudate recognition was found in Orobanche densiflora, O. gracilis and O. hederae, which germinated almost exclusively in contact with root exudates from the plants they infect in nature. At the opposite extreme, Phelipanche aegyptiaca, P. ramosa and O. minor were highly generalist, germinating when in contact with the root exudates of most plant species. Orobanche crenata, O. cumana and O. foetida showed intermediate behaviour.
Conclusions
A universal germination stimulant for all broomrape species has not being identified to date. The synthetic stimulant GR24 is active against most of the weedy broomrape species, but fails with the non-weedy species tested in this study and with the very recent weedy species O. foetida. In addition, germination behaviour of broomrape species depends on the crop plant tested. Weedy broomrapes with a broad host spectrum respond better to the different exudates released by a wide range of crops and wild species than do non-weedy broomrapes, which have a narrow host spectrum and are more restricted to their host range. Root exudates of many plant species were active in stimulating germination of seeds of Orobanche and Phelipanche species for which they are not described as hosts, representing interesting examples of potential trap crops.
Key words: Xenognosis, broomrape, root exudate, germination, Orobanche, Phelipanche
INTRODUCTION
Parasitism on plants is a co-evolutionary mechanism developed by about 4000 species (approximately 1 % of angiosperms; Nickrent et al., 1997) that has been repeatedly manifested during angiosperm evolution as an independent event in 17 plant families (Kuijt, 1969; Atsatt, 1983), from the poles to the dry and humid tropics (Molau, 1995). These plants have become adapted to use the resources from autotrophic plants through different levels of parasitism.
All levels of trophism can be found in the Scrophulariaceae family, from holoparasitism (Orobanche and Phelipanche), to hemiparasitism (either obligate, e.g. Striga, or facultative, e.g. Triphysaria), although the majority of species in this family are full autotrophs (e.g. Antirrhinum; Yoder, 1999). Obligate parasites are generally more evolved and host-specific than facultative parasites (Estabrook and Yoder, 1998). Facultative parasites have a rather generalist behaviour, with a very broad host range; for example, in the genus Rhinanthus the host range includes at least 50 species in 18 families, and in Pedicularis it includes 80 species in 35 monocot and dicot families (Gibson and Watkinson, 1989). Conversely, the host range of obligate parasites such as Orobanche and Phelipanche is, in general, narrow (Schneeweiss, 2007). Most of the species of the genus Orobanche such as O. densiflora, O. gracilis and O. hederae are botanical wonders, parasitizing perennial wild species in nature and having a very narrow host range. However, a few species have become weedy, having specialized to parasitize crops in human-disturbed ecosystems, and have adapted to parasitize a rather broad range of host species. Phelipanche aegyptiaca (syn. O. aegyptiaca), O. cernua, O. crenata, O. minor and P. ramosa (syn. O. ramosa) have specialized to parasitize a wide range of crops since antiquity (Parker and Riches, 1993; Joel et al., 2007). In addition to these, some Orobanche species have become weedy only relatively recently, e.g. O. cumana and O. foetida, which preserve their host specialization.
The angiosperm parasitic strategy generally succeeds by co-ordinating early developmental stages with chemical signals from the hosts (Smith et al., 1990), termed xenognosins. Parasitic Scrophulariaceae sense their host plant through the recognition of secondary metabolites released by its roots (Yoder, 2001), which activate developmental programs such as germination, growth of the radicle towards the host root, attachment-organ development and the creation of bridge tissue connecting the vascular tissues of the host and parasite.
Several compounds from secondary metabolism have been described as having germination-stimulatory activity, most of them belonging to the strigolactones but also isoflavanones and sesquiterpene lactones (Fischer et al., 1989; Pérez-de-Luque et al., 2000; Keyes et al., 2001; Bouwmeester et al., 2003; Tsanuo et al., 2003). The events responsible for xenognostic perception that induce parasitic germination are assumed to be a receptor-mediated signalling mechanism that could be analogous to plant hormone perception through receptors (Wigchert et al, 1999; Matusova et al., 2005). The redundancy of host molecules that can serve as xenognosins contributes to the overall broad host range of parasitic plants (Yoder, 2001). Usually, exudates of a single plant contain more than one compound with independent stimulatory activity on parasitic seed germination (Awad et al., 2006). More than one compound can be specifically recognized in the root exudates of a single plant by different broomrape species (Fernández-Aparicio et al., 2008a).
In the present work, we studied the germination of conditioned seeds of nine different broomrape species induced by the root exudates of 41 autotrophic plant species belonging to 12 botanical families. The root exudates of each species were assayed separately in each broomrape species in order to identify a potential recognition pattern in broomrape species. This may help to discriminate between the specificity requirements of broomrape species and open a route to understanding the complex interactions between the signal and receptor that trigger germination in each broomrape species, and help to identify the different chemicals involved.
MATERIALS AND METHODS
Plant material
Root exudates were collected from 41 autotrophic plant species belonging to 12 families (Table 1). Seeds of nine broomrape species were collected and used for the germination experiments. The weedy species assayed were Orobanche crenata, collected on faba bean in Spain; O. cumana, collected on sunflower in Spain; O. foetida, collected on faba bean in Tunisia; O. minor, collected on red clover in Chile; Phelipanche aegyptiaca, collected on chickpea in Israel; and P. ramosa, collected on tobacco in Spain. In addition, some non-weedy species were included for comparison: O. densiflora, collected on Lotus creticus in Spain; O. gracilis, collected on Retama sphaerocarpa in Spain; and O. hederae, collected on ivy in France.
Table 1.
Families, species and cultivars used to obtain the root exudates tested on each broomrape species
| Family | Plant species | Cultivar (or source of material) |
|---|---|---|
| Alliaceae | Onion (Allium cepa L.) | ‘Prebosa’ |
| Apiaceae | Coriander (Coriandrum sativum L.) | Landrace |
| Parsley (Petroselinum sativum Hoffm.) | Landrace | |
| Araliaceae | Ivy (Hedera helix L.) | Leafy stem cuttings |
| Asteraceae | Sunflower (Helianthus annuus L.) | ‘Peredovic’ |
| Brassicaceae | Rapeseed (Brassica napus L.) | ‘Iris’ |
| White mustard (Sinapis alba L.) | Spanish population | |
| Cucurbitaceae | Cucumber (Cucumis sativa L.) | ‘Ashley’ |
| Melon (Cucumis melo L.) | ‘Piel de sapo’ | |
| Squash (Cucurbita pepo L.) | ‘Virginia 3’ | |
| Chenopodiaceae | Fodder beat (Beta vulgaris L.) | ‘893’ |
| Fabaceae | Astragalus lusitanicus Lam. | Spanish population |
| Barrel medic (Medicago truncatula Gaertn.) | ‘Mt-51’ | |
| Berseem (Trifolium alexandrinum L.) | ‘Tigri’ | |
| Chickpea (Cicer arietinum L.) | ‘CA-3026’ | |
| Cowpea (Vigna unguiculata L.) | Egyptian landrace | |
| Faba bean (Vicia faba L.) | ‘Prothabon’ | |
| Fenugreek (Trigonella foenum-graecum L.) | Tunisian landrace | |
| Grass pea (Lathyrus sativus L.) | ‘Lisa’ | |
| Lentil (Lens culinaris Medik.) | ‘L-1172’ | |
| Lotus japonicus Regel. | ‘Gifu’ | |
| Narbon bean (Vicia narbonensis L.) | ‘Vn-271’ | |
| Pea (Pisum sativum L.) | ‘Messire’ | |
| Prickly scorpion's-tail (Scorpiurus muricatus L.) | Wild population | |
| Retama sphaerocarpa L. | Wild population | |
| Scorpiurus vermiculatus L. | Wild population | |
| Sulla (Hedysarum coronarium L.) | ‘Grimaldi’ | |
| Common vetch (Vicia sativa L.) | ‘Mezquita’ | |
| White lupin (Lupinus albus L.) | ‘Giza 2’ | |
| Linaceace | Flax (Linum usitatissimum L.) | ‘Symphonia’ |
| Malvaceace | Cotton (Gossypium hirsutum L.) | ‘Fotini’ |
| Poaceae | Barley (Hordeum vulgare L.) | ‘Aspen’ |
| Maize (Zea mays L.) | ‘Pioneer’ | |
| Oat (Avena sativa L.) | ‘Cory’ | |
| Rye (Secale cereale M. Bieb.) | Landrace | |
| Shorgum (Sorghum bicolor (L) Moench) | Landrace | |
| Common millet (Panicum miliaceum L.) | Landrace | |
| Triticale (xTriticosecale) | ‘Peñarroya’ | |
| Durum wheat (Triticum durum L.) | ‘Meridiano’ | |
| Solanaceae | Tobacco (Nicotiana tabacum L) | Landrace |
| Tomato (Lycopersicon esculentum Mill.) | ‘Tres cantos’ |
Conditioning of broomrape seeds
The broomrape seeds belonging to the nine species were surface-sterilized with formaldehyde, rinsed thoroughly with sterile distilled water and dried for 60 min in a laminar air flow cabinet. Batches of approx. 150 seeds of each broomrape species were placed separately on 120 discs of 2-cm diameter glass fibre filter paper (GFFP) moistened with 250 µL of sterile distilled water and incubated in the dark at 20 °C for 11 d in order to promote the necessary conditioning for germination (Fernández-Aparicio et al., 2008a).
Collection of root exudates
Sixty seeds per host-plant species were surface-sterilized with 2 % sodium hypochlorite containing 0·02 % (v/v) Tween 20 for 5 min and then rinsed thoroughly with sterile distilled water. Seeds were sown in sterile perlite in aseptic conditions (20 °C, 12/12 h day/night, 200 µmol m−2 s−1). Plants received Hoagland's nutrient solution twice per week (Hoagland and Arnon, 1950) for 15 d after emergence above the perlite surface. After that, the plants were removed from the perlite and the roots were carefully washed and immersed for 2 d in flasks of distilled water in groups of 20 plants, thus allowing them to release root exudates. Three replicates consisting of 20 plants each were used per treatment. Due to the different root growth patterns of each species tested, the volume of water contained in the flasks was adjusted in order to get the same final proportion of grams of fresh root weight per millilitre of root solution. The root solution (i.e. the water substrate in which the plants were grown for 2 d) containing the root exudate was collected, filtered with sterile gauze and the total root material contained in each flask was weighed. In order to allow valid comparisons between species, the root solution from each flask was then diluted with sterile distilled water to achieve a ratio of 0·06 g fresh weight of root per millilitre of water.
Germination bioassay
After the conditioning period of the broomrape seeds, the GFFP discs were dried in a laminar air flow cabinet to remove moisture. One aliquot of 125 µL from each replicate of the root solution was applied to each of three replicate discs. The synthetic germination stimulant GR24 at 100 ppm (Johnson et al., 1976) was used as a positive control and sterile distilled water was included for each broomrape species as a negative control. Seeds were then stored in the dark at 20 °C for 7 d. Germination was scored for each disc by determining the number of germinated seeds out of a subsample of 100 seeds per disc using a stereoscopic microscope. Seeds were considered as germinated when the radicle was visible through the seed coat.
Observations and statistical analysis
Germination assays were performed with three replicates per treatment using a completely randomized design. Germination capability of each broomrape species (B) was calculated as the mean and standard deviation of germination percentage of each broomrape species taken into account (1) all root exudates tested in each broomrape species (x̄ ± S), and (2) only the root exudates with activity on each broomrape species (x̄a ± Sa). The variation coefficient (CV) was calculated for each broomrape species as S/x̄ to allow comparison of the germination response between broomrape species.
Analysis of variance was conducted on the percentage of broomrape germination observed, with the following as factors: the species' capability to germinate (B), the inductor effect achieved by the root exudate from each autotrophic plant species (Re), and their interaction (Re × B). Data were approximated to a normal frequency distribution by means of square-root transformation.
In an attempt to describe the interactions found between the responses of each broomrape species when confronted by the signals released from the roots of each autotrophic species, a two-dimensional biplot termed ReReB was constructed. This ReReB biplot, derived from the GGE biplot methodology, was originally developed for analysing multi-environment trial data (Yan et al., 2000; Yan, 2001) because it can be equally applied to all types of two-way data that assume an entry × tester structure (Yan, 2001). The stimulatory activity of root exudates of each autotrophic plant species was assumed as an entry and the ability of each broomrape species to germinate was assumed as a tester. The interaction (Re × B) between a root exudate's activity (Re) and a broomrape's ability to germinate (B) refers to the variation of the broomrape germination response that can not be explained only by the species' origin of the root exudate (Re) or by the broomrape's ability to germinate (B), but by their interaction. By using the ReReB biplot we have been able to recognize groups of autotrophic species whose exudates produce the same inductor pattern on broomrapes species with similar recognition behaviour.
The ReReB biplot was constructed using the first two principal components (PC1 and PC2; also referred to as primary and secondary effects, respectively) derived from subjecting the centred data for the broomrape species' capacity to germinate to singular value decomposition (Yan, 2002). Singular value partitioning involves the use of a scaling factor, f, to modify the exudate and broomrape scores. We chose the most straightforward variant called symmetric scaling (f = 0·5) since this has most of the properties associated with other scaling methods. Plant and broomrape species were displayed in the same plot. Analyses were performed using a SAS (SAS, 1996) program for graphing GGE biplots developed by Burgueño et al. (2003).
RESULTS
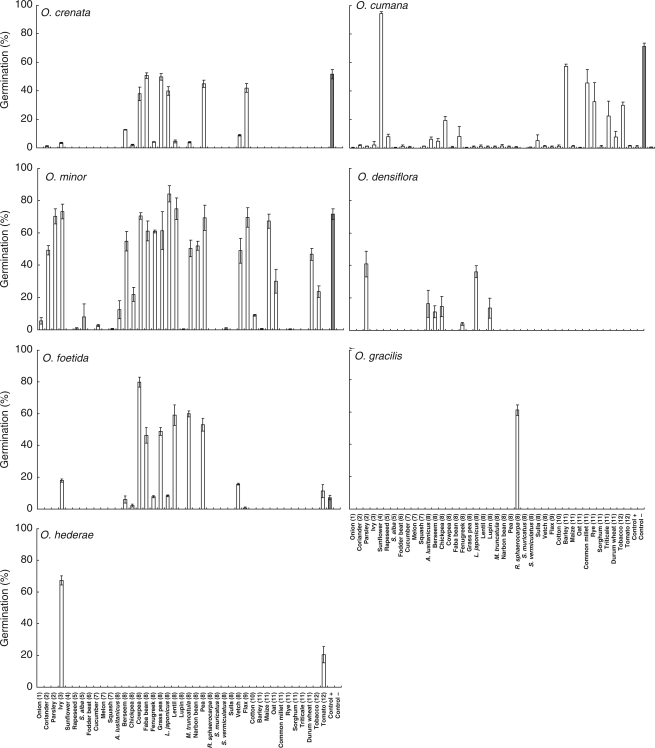
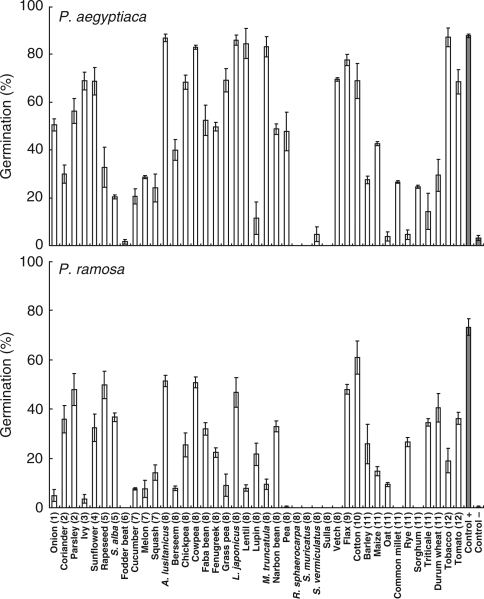
Application of GR24 had a significant effect on broomrape germination overall (ANOVA, P < 0·001). The weedy species P. aegyptiaca, P. ramosa, O. cumana, O. minor and O. crenata were highly stimulated by GR24 (89, 73, 71, 71 and 52 % of seeds germinated, respectively; Figs 1 and 2). However, the non-weedy species O. densiflora, O. gracilis and O. hederae did not respond to GR24 at the concentration tested. Interestingly, the weedy population of O. foetida had a negligible response to GR24 (7·2 %), grouping with the non-weedy species. Low but significant (<5 %) levels of spontaneous germination (i.e. sterile distilled water only as a negative control) were observed for P. aegyptiaca and O. cumana; no spontaneous germination was observed in other species.
Fig. 1.
Germination percentage of seeds of seven Orobanche species stimulated by root exudates released from 41 species of autotrophic plants belonging to the following families. (1) Alliaceae: onion (Allium cepa L.); (2) Apiaceae: coriander (Coriandrum sativum L.), parsley (Petroselinum sativum Hoffm.); (3) Araliaceae: ivy (Hedera helix L.); (4) Asteraceae: sunflower (Helianthus annuus L.); (5) Brassicaceae: rapeseed (Brassica napus L.), Sinapis alba; (6) Chenopodiaceae: fodder beat (Beta vulgaris L.); (7) Cucurbitaceae: cucumber (Cucumis sativa L.), melon (Cucumis melo L.), squash (Cucurbita pepo L.); (8) Fabaceae: Astragalus lusitanicus Lam., berseem (Trifolium alexandrinum L.), chickpea (Cicer arietinum L.), cowpea (Vigna unguiculata L.), faba bean (Vicia faba L.), fenugreek (Trigonella foenum-graecum L.), grass pea (Lathyrus sativus L.), Lotus japonicus Regel., lentil (Lens culinaris Medik.), lupin (Lupinus albus L.), Medicago truncatula Gaertn. (barrel medic), narbon bean (Vicia narbonensis L.), pea (Pisum sativum L.), Retama sphaerocarpa L., Scorpiurus muricatus L., S. vermiculatus L., sulla (Hedysarum coronarium L.), vetch (Vicia sativa L.); (9) Linaceae: flax (Linum usitatissimum L.); (10) Malvaceae: cotton (Gossypium hirsutum L.); (11) Poaceae: barley (Hordeum vulgare L.), maize (Zea mays L.), oat (Avena sativa L.), common millet (Panicum miliaceum L.), rye (Secale cereale M. Bieb.), sorghum (Sorghum bicolor (L.) Moench), triticale (× Triticosecale), durum wheat (Triticum durum L.); (12) Solanaceae: tobacco (Nicotiana tabacum L.), tomato (Solanum lycopersicum L.). The synthetic stimulant GR24 and water were used as positive and negative controls, respectively. Error bars represent the mean ± s.e.
Fig. 2.
Germination percentage of seeds from two Pheliphanche species stimulated by root exudates released from 41 species of autotrophic plants, as detailed in Fig. 1. The synthetic stimulant GR24 and water were used as positive and negative controls, respectively. Error bars represent the mean ± s.e.
There were significant differences in germination due to the broomrape species tested (ANOVA, P < 0·001), due to the plant species from which the root exudate was obtained (P < 0·001), and due to their interaction (P < 0·001). Different degrees of specialization for recognition of exudate by the broomrape species were observed. Some species were highly specialist, with seeds that germinated only in presence of exudates of a few plants, although reaching very high levels of germination with these plants (e.g. O. cumana, O. hederae and O. densiflora). Others were highly generalist, with seeds that germinated in the presence of exudates from the majority of species tested (e.g. P. aegyptiaca, P. ramosa and O. minor).
In the group of highly specialized broomrape species there were both weedy (O. cumana) and non-weedy species such as O. densiflora and O. hederae, which have a very narrow host range in nature. The very noxious weedy species O. cumana is known to infect sunflower only (Joel et al., 2007). Sunflower did indeed induce the highest level of germination in O. cumana (94·2 %), even higher than GR24 (see above), but exudates from some other plant species induced some levels of germination, for example Astragalus lusitanicus (6·2 %), berseem (5·0 %), triticale (7·7 %), rapeseed (8·0 %), faba bean (8·2 %), chickpea (19·4 %) and, remarkably, pearl millet (32·5 %), sorghum (22·5 %), wheat (30·1 %), oat (45·6 %) and cotton (57·2 %). The germination level induced by plant species active on O. cumana resulted in a mean value of germination of x̄a = 31·7 % (Table 2); however, the total mean value (reached with all root exudates included in the work) was x̄ = 9·7%.
Table 2.
General responses of each broomrape species to root exudates released by all the autotrophic plant species tested in the study
| Broomrape species | Max germination value (induced by) | Mean value of germination, x̄a ± S (calculated only with active exudates) | Mean value of germination, x̄ ± S (total root exudates) | CV |
|---|---|---|---|---|
| P. aegyptiaca | 88·8 (GR24) | 50·5 ± 28·0 | 45·6 ± 30·4 | 0·66 |
| P. ramosa | 73·2 (GR24) | 28·7 ± 18·9 | 23·7 ± 20·2 | 0·85 |
| O. crenata | 51·6 (GR24) | 22·4 ± 21·1 | 8·9 ± 17·2 | 1·92 |
| O. cumana | 94·2 (sunflower) | 31·7 ± 30·9 | 9·7 ± 21·1 | 2·17 |
| O. densiflora | 40·9 (parsley) | 19·6 ± 15·4 | 3·4 ± 9·8 | 2·84 |
| O. foetida | 79·8 (cowpea) | 29·6 ± 26·0 | 10·6 ± 21·0 | 1·97 |
| O. gracilis | 0·0 (–) | 0·0 ± 0·0 | 0·0 ± 0·0 | – |
| O. hederae | 67·3 (ivy) | 43·9 ± 26·4 | 2·2 ± 11·0 | 5·00 |
| O. minor | 84·0 (L. japonicus) | 48·0 ± 26·0 | 31·3 ± 31·0 | 0·99 |
The non-weedy species O. densiflora germinated in the presence of root exudates from L. japonicus (36·2 %) but also with exudates from non-host species such as parsley (40·9 %), A. lusitanicus (16·4 %), chickpea (14·6 %), lupin (13·7 %), berseem (11·4 %) and fenugreek (4·0 %). Orobanche hederae germinated in the presence of root exudates from its host, ivy (67·2 %), but also with exudates from tomato (20·5 %). Orobanche gracilis germinated only in presence of the root exudates from its host, Retama sphaerocarpa (61·2 %; Fig. 1).
At the opposite extreme, it was found that species such as P. aegyptiaca and P. ramosa germinated in the presence of root exudates from the majority of the plant species tested (89·5 and 84·2 % respectively; Table 2). The mean germination was higher in P. aegyptiaca (x̄ = 45·6 %; x̄a = 50·5 %) than in P. ramosa (x̄ = 23·7 %; x̄a = 28·7 %). The third broomrape found to have more generalist behaviour was O. minor, which geminated with root exudates from about two-thirds of the species tested, although O. minor had a higher mean germination (x̄ = 31·3 %; x̄a = 48·0 %) than P. ramosa. The maximum germination achieved with root exudates in these three broomrape species were as follows: 88·1 % of P. aegyptiaca seeds germinated with root exudates of tobacco; 60·9 % of P. ramosa seeds with cotton; and 84·0 % of O. minor seeds with L. japonicus (Figs 1 and 2). The synthetic stimulant GR24 induced the highest germination for P. aegyptiaca (88·8 %) and P. ramosa (73·2 %); however, L. japonicus induced even higher germination (84·0 %) in O. minor than was achieved with GR24 (73·2 %; Table 2; Figs 1 and 2).
Orobanche crenata and O. foetida germinated in the presence of root exudates from about one-third of the plants studied (mainly legumes), with a range of induction of germination of 1·3–50·5 % for O. crenata (minimum–maximum values for coriander and faba bean, respectively) and 0·7–79·8 % for O. foetida (flax and cowpea). The mean germination values calculated both with total root exudates tested in this work (x̄) and only the active root exudates for each species (x̄a) gave values of 8·9 and 22·4 % for O. crenata and 10·6 and 29·6 % for O. foetida, respectively. The total mean germination of these species was high in the presence of the root exudates from cowpea, faba bean, grass pea, lentil and pea. In addition, non-legumes such as flax induced high levels of germination in O. crenata seeds (42·0 %; Fig. 1) but low in O. foetida seeds (0·7 %). In contrast, Medicago truncatula induced high germination in O. foetida (59·1 %) but low germination in O. crenata (3·9 %). Similarly, tomato induced moderate levels of germination in O. foetida (11·3 %) but not in O. crenata. Root exudates from ivy induced moderate germination in O. foetida (18·0 %) but low germination in O. crenata (3·5 %). Low or no germination was induced by the remaining legumes such as chickpea, and others poorly infected by these species such as lupin, vetch and narbon bean. The rest of the species included in this work did not show any stimulatory activity on O. crenata and O. foetida.
Similarly, the plant species varied in their germination-stimulatory activity towards broomrape, ranging from species that did not induce germination of any of the broomrapes studied (e.g. fodder beat, sulla), to species that induced germination in a wide number of broomrape species (e.g. cowpea, flax, L. japonicus, faba bean).
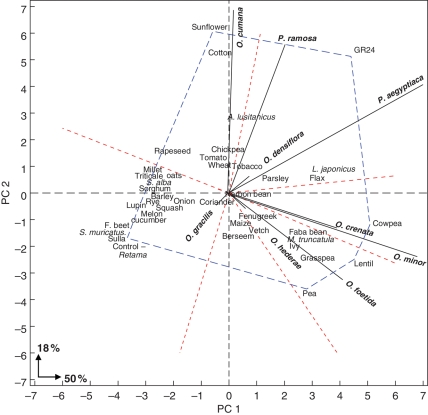
The mean inductor effect (Re) of the autotrophic plants from which the root exudates were obtained is visualized in the ReReB biplot (Fig. 3) approximated to the PC1; the stability of this root exudate as a general inductor for all broomrapes is visualized approximated to PC2. A universal germination inductor for all broomrapes would have the highest mean performance (inducting the maximum germination) and a general effect (on all broomrapes species). Such an ideal inductor would be defined as having the greatest vector length on the positive PC1 axis and zero ReB interaction, represented by a zero value on the PC2 axis. This ideal inductor does not exist among the plants tested here, but those species situated close to this ideal point would have root exudates with relatively more efficient and general inductor effects than the others. Cowpea was found to stimulate P. aegyptiaca (83·9 %), P. ramosa (50·8 %), O. crenata (37·9 %), O. foetida (79·8 %) and O. minor (70·4 %), and would have equalled the inductor effect of GR24 on broomrape species but for the fact that it did not also induce seeds of O. cumana. The next most effective inducer species was L. japonicus, which stimulated P. aegyptiaca (86·9 %), P. ramosa (46·7 %), O. crenata (4·6 %), O. densiflora (36·2 %), O. foetida (8·4 %) and O. minor (84·0 %). It is interesting to note that L. japonicus is used as a model species in genomic studies. This species was followed by flax, which induced the germination of P. aegyptiaca (78·5 %), O. ramosa (48·0 %), O. crenata (42·0 %), O. foetida (0·7 %) and O. minor (69·5 %). Medicago truncatula (also used as model plant) also had very effective root exudates, stimulating P. aegyptiaca (84·1 %), P. ramosa (9·5 %), O. crenata (3·9 %), O. foetida (59·8 %) and O. minor (50·3 %). The ability of these species to induce germination was similar to that of the synthetic inductor GR24, with the only exception that they did not induce O. cumana. They also differed from GR24 in induction ability with regard to O. foetida and, in the case of L. japonicus, to O. densiflora. Faba bean stimulated P. aegyptiaca (53·1 %), P. ramosa (32·0 %), O. crenata (50·6 %), O. foetida (46·3 %) and O. minor (61·1 %), and also a low percentage of O. cumana seeds (8·2 %). This latter value was similar to the mean germination (x̄) recorded for O. cumana with all the root exudates tested (9·7 %); faba bean stimulated the same number of broomrape species as GR24.
Fig. 3.
ReReB biplot: inductor effect of root exudates (Re) released by 41 autotrophic plant species × broomrape germination (B). For full explanation see text.
The polygon shown on the ReReB biplot (Fig. 3) was created by connecting the points for the autotrophic plant species that are furthest away from the biplot origin in such a way that all the other plant species are contained within the polygon. The six red lines radiating from the origin are perpendicular to the sides of the polygon (or an extension in the case of the pea–lentil side) and divide the polygon into six sectors; the nine broomrape species included in this work fall into five of them. The species positioned at the apex of each sector produced the root exudate that had the maximum inductor effect on the broomrapes that fall within that sector. Thus, GR24 had the maximum inductor effect on P. aegyptiaca and P. ramosa, which fall within the sector created by the lines perpendicular to the sides adjacent to GR24 apex; and sunflower had the maximum induction effect on O. cumana, the one broomrape species that falls within the sector created by the sunflower apex. Note that only 68 % of the ReB variation is explained by the biplot, so such statements about positioning of species are not entirely validated by the original data; arguably, however, the pattern displayed by the biplot may be more robust than the individual data points in the raw data because the biplot is based on all data points (Mohammadi et al., 2007). For example, the biplot reveals that lentil root exudate was the maximum inductor for O. foetida and that cowpea root exudate was the maximum inductor for O. minor and O. crenata; however, according to the raw data cowpea root exudate was the maximum inductor for O. foetida, GR24 for O. crenata, and L. japonicus for O. minor. GR24 is displaced to the upper-right quadrant in which P. ramosa and P. aegyptiaca are located because of a vector length with a high positive value on the PC1 axis (i.e. high mean inductor effect) and a high positive value on the PC2 axis (i.e. high ReB interaction, indicating high instability in inducing broomrape germination across all species). This is because the induction effect of GR24 varies with species, being high for P. aegyptiaca, P. ramosa and O. crenata, but being lower for O. foetida than the exudates from cowpea, lentil and pea. Lotus japonicus root exudate was the highest inductor for O. minor but it was poorer for O. crenata and O. foetida than the exudates from pea, lentil and cowpea. In addition, L. japonicus highly stimulated germination in P. aegyptiaca, P. ramosa and O. densiflora. These effects displaced L. japonicus to the upper-right quadrant inside the sector in which O. densiflora, P. aegyptiaca and P. ramosa are located
Interestingly, the interaction found by ANOVA between the potential to induce germination by each root exudate and the germination percentage of each broomrape species (Re × B) was highly significant. An example of this interaction is the induction of germination of P. aegyptiaca and P. ramosa seeds by root exudates from rye (4·6 and 26·5 %, respectively) and lentil (85·5 and 7·9 %, respectively).
In general, legumes tended to stimulate germination in P. aegyptiaca, O. crenata, O. foetida and O. minor, whilst cereals tended to stimulate P. aegyptiaca, P. ramosa, O. cumana and O. minor. Apiaceae and Solanaceae stimulated P. aegyptiaca, P. ramosa and O. minor. Brassicaceae had similar effects to the Cucurbitaceae, stimulating O. aegyptiaca and P. ramosa. Onion, a member of the Alliaceae family, mainly stimulated P. aegyptiaca. The effects of barley were very similar to those of the Cucurbitaceae, and the ability to induce germination observed for sunflower was very similar to that for cotton. The one member of the Chenopodiaceae family, fodder beet, did not stimulate any of the broomrapes tested; a similar absence of germination was also observed with root exudates from the legumes sulla and Scorpiurus muricatus.
DISCUSSION
The stimulatory effect of GR24 generally assumed for all broomrape species (Mangnus et al., 1992; Pérez-de-Luque et al., 2000) is clarified in this work, which shows that GR24 is not effective on some species. In general, it seems that GR24 had a better stimulatory effect on weedy than non-weedy species; however, there were some exceptions to this tendency. For example, O. foetida, despite being weedy, did not respond to the stimulatory effect of GR24. This could be explained because of the recent development of this species as a weedy parasitic plant (Kharrat et al., 1992), with a host specialization process still developing (Román et al., 2007; Vaz Patto et al., 2008). In addition, we can not assert that all non-weedy species do not respond to GR24 because work in our laboratory has shown that species such as P. nana (Fernández-Aparicio et al., 2008b) and P. mutelii (M. Fernández-Aparicio, unpubl. res.) respond to GR24 at the same high levels as weedy species.
Regarding root exudates, it can be hypothesized that each plant species releases several compounds in the exudate profile, each with differential stimulatory activity to different broomrape species and which vary in the amount in which they are released or in their efficiency. An alternative explanation could be that in addition to common inducing compounds being released by each plant, other compounds are released that have inhibitory or synergistic effects on some broomrape species but not on others (Whitney and Carstein, 1981; Evidente et al., 2007; Fernández-Aparicio et al., 2008a). The results support the idea that abundant and unidentified compounds released by host and non-hosts could be responsible for the induction of germination of the weedy broomrapes with a moderate-to-broad host range (P. aegyptiaca, P. ramosa, O. crenata, O. foetida and O. minor). Weedy species showed a higher ability for xenognostic recognition than non-weedy species. In natural ecosystems, host predictability could be relatively high due to the perennial nature of the wild hosts and the stability of the species integrated into the ecosystem. In contrast, human-disturbed ecosystems produce higher selection pressures: the annual nature of the hosts, the manipulated life cycle that synchronizes all plants together, and the rotation of crop stands make host predictability for weedy broomrapes much lower. This could create selection pressure for weedy broomrapes to improve their recognition system in order to adapt themselves to the high instability of human-created agro-ecosystems, i.e. increasing the sensitivity of the broomrapes' receptors to recognize a higher number of molecules and at a lower concentration. The associated danger of ‘suicidal’ germination due to more sensitive receptors could be compensated for by the extremely high broomrape seed bank produced in agricultural soils in comparison with natural ecosystems, in which the number of seeds per unit volume of soil is much lower. Thus, ‘suicidal’ germination is relatively more costly for a non-weedy species.
Exceptions to this rule are demonstrated by O. cumana and O. foetida, which have become weedy only relatively recently and preserve their host specialization. Orobanche cumana, which parasitizes sunflower, was an autochthonous species from Eastern Europe and Central Asia where it grew on Artemisia species. With the introduction of sunflower as a new crop in Eastern Europe in 19th century, O. cumana encountered a new host and became weedy and spread to other areas along with the spread of the sunflower crop (Pujadas-Salvá and Velasco, 2000). Orobanche foetida is widely distributed in the western Mediterranean as a non-weedy species (Pujadas-Salvá, 1999) but recently extended its range from that of the wild host and was thus described as a threat to faba bean crops in the Beja region of Tunisia (Kharrat et al., 1992).
The germination of O. foetida was found to be stimulated by a large number of species (more than one-third of those tested), some of which are already known as hosts. It is notable that S. muricatus, a reported host for non-weedy populations of O. foetida (Pujadas-Salvá, 1999), did not induce germination in the weedy population of O. foetida. Again, this could be explained because of the recent development of this population from Beja, Tunisia, as weedy, probably via selection from natural populations in response to particular germination stimulants from the crops.
Orobanche hederae demonstrated germination induced by its host, ivy, but also by the non-host tomato. Similarly, O. densiflora showed germination induced by its host Lotus, but also by the non-hosts parsley, A. lusitanicus, chickpea, lupine, berseem and fenugreek. This may be an indication of the presence of compounds with some degree of activity for these species. If these compounds share structural and/or chemical traits with those that demonstrate activity for some of the other weedy broomrapes, then this would be worthy of further investigation.
The results from cereals such as durum wheat, which stimulated high germination of O. minor, is remarkable and in agreement with Ross et al. (2004). Similarly, oat stimulated P. aegyptiaca, O. minor and O. cumana. In general, all cereals induced germination of some broomrape species. We found that the barley cultivar used in this study stimulated P. ramosa but did not induce germination of O. crenata, which contradicts the suggestion of barley as a trap crop for O. crenata (Parker and Riches, 1993), although we can not exclude a greater effect by other barley cultivars.
Akiyama et al. (2005) have recently contributed to the intriguing question of why host plants release substances that allow infection by a parasite. They discovered that strigolactones are branching factors for mycorrhizal fungi, and have an important function in symbiotic establishment. It seems that host plants do not signal to the parasite but to the symbiont, and parasitic plants ‘eavesdrop’ on this communication and become adapted to recognize these cues (Bouwmeester et al., 2007). However, it is also possible that both these obligate mutualists, initially mycorrhizal fungi at the time of plant species' adaptation to life on land and, later in evolution, parasitic plants, have independently developed the same principle of action in order to survive. They both invade the host in response to the wide set of compounds available in the rhizosphere from secondary metabolism and released by the autotrophic plants as external signals. However, there are plants such as Arabidopsis and rapeseed that allow broomrape parasitism but do not allow mycorrhizal invasion (Bouwmeester et al., 2007). Based on the report that strigolactones are branching factors for mycorrhizal fungi (Akiyama et al., 2005), we would expect higher induction of germination in plant species known to be colonized by mycorrhizal fungi. However, we did not observe a clear pattern in the induction of broomrape germination between plant species known to be colonized by mycorrhizal fungi (most of those tested) and those that do not (Brassicaceae and Chenopodiaceae). Further investigation is thus needed in order to understand the ecological role of germination stimulants in non-mycorrhizal plants.
The use of conventional trap crops, described frequently as a cultural method for broomrape control, is, in our opinion, of limited efficacy, due to the large amount of seeds present in infested agricultural soils combined with the moderate levels of germination that are induced only near each root, and also because of the lack of continuity of cultivation of most potential trap crops in the same area. However, the use of cereals could be a solution, because of the high frequency with which they are cultivated and their importance for human consumption. Hence breeding of cereal germplasm for the maximum induction effect on germination of broomrape seeds could be of great interest. Introducing such selected varieties with high inductor potentials for ‘suicidal’ broomrape germination into rotations in infected soils could thus shorten the time necessary between host crops.
In conclusion, it can be asserted that a universal germination stimulant for all broomrape species has not been identified to date. The synthetic stimulant GR24 is active against most of the weedy broomrape species, but it failed with the non-weedy species tested in this study and with the very recent weedy species O. foetida. In addition, the germination behaviour of broomrape species depends on the crop plant tested, and weedy broomrapes with a broad host spectrum respond better to different crop exudates than do non-weedy broomrapes with a narrow host spectrum. Identification of the genes in plants that control the production of germination stimulants for the most harmful broomrapes would make it possible to introduce and over-express such genes in non-host crops with high agronomic interest. By using molecular techniques it could be possible, for example, to create a wheat variety that strongly releases compounds that cause germination in all weedy species of broomrapes without their subsequent attachment being possible, thus eliminating the parasitic seeds within a large radius of the roots and clearing the infestation of the soil within a few years of cultivation.
LITERATURE CITED
- Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal banching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Atsatt PR. Host-parasite interactions in higher plants. In: Lang OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopedia of plant physiology. vol. 12C. Berlin: Springer-Verlag; 1983. pp. 519–535. New Series. [Google Scholar]
- Awad AA, Sato D, Kusumoto D, Kamioka H, Takeuchi Y, Yoneyama K. Characterization of strigolactones, germination stimulants for the root parasitic plants Striga and Orobanche, produced by maize, millet and sorghum. Plant Growth Regulation. 2006;48:221–227. [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. Secondary metabolite signalling in host–parasitic plant interactions. Current Opinion in Plant Biology. 2003;6:358–364. doi: 10.1016/s1369-5266(03)00065-7. [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Bécard G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends in Plant Science. 2007;12:224–230. doi: 10.1016/j.tplants.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Burgueño J, Crossa J, Vargas M. SAS programs for graphing GE and GGE biplots. México: CIMMYT, Biometrics and Statistics Unit; 2003. [Google Scholar]
- Estabrook EM, Yoder JI. Plant–plant communications: rhizosphere signalling between parasitic angiosperms and their hosts. Plant Physiology. 1998;116:1–7. [Google Scholar]
- Evidente A, Fernández-Aparicio M, Andolfi A, Rubiales D, Motta A. Trigoxazonane, a monosubstituted trioxazonane by Trigonella foenum-graecum root exudate, inhibiting agent of Orobanche crenata seed germination. Phytochemistry. 2007;68:2487–2492. doi: 10.1016/j.phytochem.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Fischer NH, Weidenhamer JD, Bradow JM. Dihydroparthenolide and other sesquiterpene lactones stimulate witchweed germination. Phytochemistry. 1989;28:2315–2317. [Google Scholar]
- Fernández-Aparicio M, Andolfi A, Evidente A, Pérez-de-Luque A, Rubiales D. Fenugreek root exudates with Orobanche species specific seed germination stimulatory activity. Weed Research. 2008;a 48:163–168. [Google Scholar]
- Fernández-Aparicio M, Pérez-de-Luque A, Prats E, Rubiales D. Variability of interactions between barrel medic (Medicago truncatula) genotypes and Orobanche species. Annals of Applied Biology. 2008;b 153:117–126. [Google Scholar]
- Gibson CC, Watkinson AR. The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia. 1989;78:401–406. doi: 10.1007/BF00379116. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Berkeley, CA: University of California Experimental Station; 1950. Circular No. 347. [Google Scholar]
- Joel DM, Hershenhorn J, Eizenberg H, et al. Biology and management of weedy root parasites. In: Janick J, editor. Horticultural Reviews Vol. 33. New York: John Wiley & Sons; 2007. pp. 267–349. [Google Scholar]
- Johnson AW, Rosebery G, Parker C. A novel approach to Striga and Orobanche control using synthetic germination stimulants. Weed Research. 1976;16:223–227. [Google Scholar]
- Keyes WJ, Taylor JV, Apkarian RP, Lynn DG. Dancing together. Social controls in parasitic plant development. Plant Physiology. 2001;127:1508–1512. [PMC free article] [PubMed] [Google Scholar]
- Kharrat M, Halila MH, Linke KH, Haddar T. First report of Orobanche foetida Poiret on faba bean in Tunisia. FABIS Newsletter. 1992;30:46–47. [Google Scholar]
- Kuijt J. Parasitic plants. Berkeley, CA: University of California Press; 1969. [Google Scholar]
- Mangnus EM, Stommen PLA, Zwanenburg B. A standardized bioassay for evaluation of potential germination stimulants for seeds of parasitic weeds. Journal of Plant Growth Regulation. 1992;11:91–98. [Google Scholar]
- Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiology. 2005;139:920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi R, Haghparast R, Aghaee M, Rostaee M, Pourdad SS. Biplot analysis of multi-environment trials for identification of winter wheat mega-environments in Iran. World Journal of Agricultural Sciences. 2007;3:475–480. [Google Scholar]
- Molau U. Reproductive ecology and biology. In: Press MC, Graves JD, editors. Parasitic plants. London: Chapman & Hall; 1995. pp. 1–13. [Google Scholar]
- Nickrent D, Duff FJ, Colwell AE, et al. Molecular phylogenetic and evolutionary studies of parasitic plants. In: Soltis DE, Soltis PS, Doyle JJ, editors. Molecular systematics of plants II. DNA sequencing. Boston, MA: Kluwer Academic Publishers; 1997. pp. 211–241. [Google Scholar]
- Parker C, Riches CR. Parasitic weeds of the world: biology and control. Wallingford, UK: CAB International; 1993. [Google Scholar]
- Pérez-de-Luque A, Galindo JCG, Macías FA, Jorrín J. Sunflower sesquiterpene lactone models induce Orobanche cumana seed germination. Phytochemistry. 2000;53:45–50. doi: 10.1016/s0031-9422(99)00485-9. [DOI] [PubMed] [Google Scholar]
- Pujadas-Salvá AJ. Species of the family Orobanchaceae parasitic of cultivated plants and its relatives growing on wild plants, in the south of the Iberian Peninsula. In: Cubero JI, Moreno MT, Rubiales D, Sillero JC, editors. Resistance to broomrape, the state of the art. Andalucía, Spain: Junta de Andalucía; 1999. pp. 187–193. [Google Scholar]
- Pujadas-Salvá AJ, Velasco L. Comparative studies on Orobanche cernua L. and O. cumana Wallr. (Orobanchaceae) in the Iberian Peninsula. Botanical Journal of the Linnean Society. 2000;134:513–527. [Google Scholar]
- Román B, Satovic Z, Alfaro C, et al. Host differentiation in Orobanche foetida Poir. Flora. 2007;202:201–208. [Google Scholar]
- Ross KC, Colquhoun JB, Mallory-Smith CA. Small broomrape (Orobanche minor) germination and early development in response to plant species. Weed Science. 2004;52:260–266. [Google Scholar]
- SAS Institute. SAS/STAT software: changes and enhancements through release 6.11. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Schneeweiss GM. Correlated evolution of life history and host range in the nonphotosynthetic parasitic flowering plants Orobanche and Phelipanche (Orobanchaceae) Journal of Evolutionary Biology. 2007;20:471–478. doi: 10.1111/j.1420-9101.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- Smith CE, Dudley MW, Lynn DG. Vegetative–parasitic transition control and plasticity in Striga development. Plant Physiology. 1990;93:208–221. doi: 10.1104/pp.93.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanuo MK, Hassanali A, Hooper AM, et al. Isoflavanones from the allelopathic aqueous root exudate of Desmodium uncinatum. Phytochemistry. 2003;64:265–273. doi: 10.1016/s0031-9422(03)00324-8. [DOI] [PubMed] [Google Scholar]
- Vaz Patto MC, Díaz-Ruiz R, Satovic Z, Román B, Pujadas-Salvá AJ, Rubiales D. Genetic diversity of Moroccan populations of Orobanche foetida: evolving from parasitising wild hosts to crop plants. Weed Research. 2008;28:179–186. [Google Scholar]
- Whitney PJ, Carstein C. Chemotropic response of broomrape radicles to host roots exudates. Annals of Botany. 1981;48:919–921. [Google Scholar]
- Wigchert SCM, Kuiper E, Boelhouwer GJ, Nefkens GHL, Verkleij JAC, Zwanenburg B. Dose–response of seeds of the parasitic weeds Striga and Orobanche toward the synthetic germination stimulants GR24 and Nijmegen 1. Journal of Agriculture Food Chemistry. 1999;47:1705–1710. doi: 10.1021/jf981006z. [DOI] [PubMed] [Google Scholar]
- Yan W. GGEbiplot—a windows application for graphical analysis of multienvironment trial data and other types of two-way data. Agronomy Journal. 2001;93:1111–1118. [Google Scholar]
- Yan W. Singular value partitioning for biplot analysis of multi-environment trial data. Agronomy Journal. 2002;94:990–996. [Google Scholar]
- Yan W, Hunt LA, Sheng Q, Slavnics Z. Cultivar evaluation and mega-environment investigation based on the GGE biplots. Crop Science. 2000;40:597–605. [Google Scholar]
- Yoder JI. Parasitic plant response to host plant signals: a model for subterranean plant–plant interactions. Current Opinion in Plant Biology. 1999;2:65–70. doi: 10.1016/s1369-5266(99)80013-2. [DOI] [PubMed] [Google Scholar]
- Yoder JI. Host-plant recognition by parasitic Scrophulariaceae. Current Opinion in Plant Biology. 2001;4:359–365. doi: 10.1016/s1369-5266(00)00185-0. [DOI] [PubMed] [Google Scholar]