Abstract
Background and Aims
Although several methods of sampling and storing floral nectar are available, little information exists on sampling and storing nectar from flowers with low nectar volumes. Methods for sampling and storing nectar from the flowers of species with low floral nectar volumes (<1 µL) were investigated using the flowers of Eucalyptus species.
Methods
Sampling with microcapillary tubes, blotting up with filter paper, washing and rinsing were compared to determine masses of sugars recovered and differences in sugar ratios. Storage methods included room temperature, refrigeration and freezing treatments; the addition of antimicrobial agents benzyl alcohol or methanol to some of these treatments was also evaluated. Nectar samples were analysed using high-performance liquid chromatography and the masses of sucrose, glucose and fructose in each sample were determined.
Key Results
Masses of sugars varied significantly among sampling treatments, but the highest yielding methods, rinsing and washing, were not significantly different. A washing time of 1 min was as effective as one of 20 min. Storage trials showed that the sugar concentration measurements of nectar solutions changed rapidly, with the best results achieved for refrigeration with no additive (sucrose and fructose were stable for at least 2 weeks). Sugar ratios, however, remained relatively stable in most treatments and did not change significantly across 4 weeks for the methanol plus refrigerator and freezing treatments, and 2 weeks for the refrigeration treatment with no additive.
Conclusions
Washing is recommended for nectar collection from flowers with low nectar volumes in the field (with the understanding that one wash underestimates the amounts of sugars present in a flower), as is immediate analysis of sugar mass. In view of the great variation in results depending on nectar collection and storage methods, caution should be exercised in their choice, and their accuracy should be evaluated. The use of pulsed amperometric detection, more specific than refractive index detection, may improve the accuracy of nectar sugar analysis.
Key words: Eucalyptus, flower with small nectar volume, nectar collection, nectar sampling, nectar storage, sugar ratio
INTRODUCTION
Methods to measure the volume, sugar contents and energy value of nectar are used in the study of many ecological processes (Dungan et al., 2004), in particular the study of plant– animal interactions (Bolten and Feinsinger, 1978; Kearns and Inouye, 1993), and can permit the calculation of carrying capacity for nectarivores (Petit and Pors, 1996). Sugar composition may also affect visitor preference or association (Hainsworth and Wolf, 1976; Bolten and Feinsinger, 1978; Martínez del Rio et al., 1992; Schondube and Martínez del Rio, 2003). Several methods are available to sample nectar, and selecting the best one can be difficult (Lloyd et al., 2002). The effectiveness of a chosen technique is influenced by floral morphology, nectar characteristics and sampling regime (Bolten and Feinsinger, 1978; Kearns and Inouye, 1993; Lloyd et al., 2002). While many papers have been published on the sampling of floral nectar, a paucity of information exists regarding sampling from flowers that produce low nectar volumes (<1 µL), and also on the conditions under which the resulting nectar samples should be stored.
Lloyd et al. (2002) described a qualitative method for visual assessment of nectar volume that did not involve the removal of nectar from flowers; however, this method did not allow for quantitative analysis of the sugars in the nectar. Methods described to sample nectar from flowers, which allow for such analysis, include: (a) soaking up the nectar with filter paper wicks (McKenna and Thomson, 1988; Kearns and Inouye, 1993; Corbet, 2003); (b) drawing up the nectar in microcapillary tubes, microsyringes or micropipettes (Collins and Newland, 1986; McKenna and Thomson, 1988; Kearns and Inouye, 1993; Lanza et al., 1995; Corbet, 2003; Tschapka, 2004); (c) agitating (washing) the flower in a stoppered tube of distilled water (Käpylä, 1978; Grünfeld et al., 1989); (d) rinsing flowers with distilled water (Núñez, 1977; Mallick, 2000); (e) centrifuging individual flowers or inflorescences (Swanson and Shuel, 1949; Armstrong and Paton, 1990); and (f) removing nectar with power-driven aspirators (Armstrong and Paton, 1990).
The concentration of a nectar solution is important in the energetics of foraging (Heinrich, 1975). The sugar concentration of nectar can be measured in the field using a hand-held refractometer (Bolten and Feinsinger, 1978; Bond and Brown, 1979; Collins and Newland, 1986; Kearns and Inouye, 1993; Mallick, 2000; Dungan, et al. 2004), or analysed in a laboratory using techniques such as chromatography or spectrophotometry. High-performance liquid chromatography (HPLC) is an easy and accurate way to qualify and quantify specific sugars and other chemicals within a nectar sample (Kearns and Inouye, 1993).
Where nectar volumes are small (<1 µL), and measurements of the volume and concentration of that nectar are not required, nectar can be blotted up from flowers with small pre-dried and weighed wicks of filter paper (McKenna and Thomson, 1988; Kearns and Inouye, 1993; Corbet, 2003). The change in mass of the paper after redrying can provide an estimate of the mass of sugars in the nectar (Dungan et al., 2004). The filter paper may also be soaked in a solvent, and the mass and composition of solutes can be analysed using techniques including chromatography or colorimetric analysis (McKenna and Thomson, 1988). The filter paper wick method does not, however, provide measurements of nectar volumes (McKenna and Thomson, 1988). The volume of nectar in flowers is a limiting factor in nectar intake by nectarivores and should be reported in nectar investigations (Bolten et al., 1979).
Microcapillary tubes and refractometers are commonly used in the field for measuring, respectively, the volume and sugar concentration of relatively abundant floral nectars (Collins and Newland, 1986; McKenna and Thomson, 1988; Corbet, 2003). Nectar is removed from a flower using a fixed-bore tube, and the volume is measured by determining the length of the liquid column within the tube. The nectar is then applied to a refractometer to measure the sugar concentration (Corbet, 2003). Micropipettes and microsyringes can be used instead of microcapillary tubes to achieve similar results (Kearns and Inouye, 1993).
While the microcapillary tube and refractometer methods are useful for sampling the nectar of many plant species, they are less effective where floral nectar is highly viscous, or is produced in low (<1 µL) volumes (Käpylä, 1978; McKenna and Thomson, 1988; Mallick, 2000; Manetas and Petropoulou, 2000; Corbet, 2003; Dungan et al., 2004; Birtchnell and Gibson, 2006). All available nectar cannot be extracted with microcapillary tubes (Stephanou et al., 2000; Manetas and Petropoulou, 2000), and probing may damage the nectary tissue and introduce cell contents into the nectar (Willmer, 1980). Additionally, it is difficult to measure low volumes of nectar with hand-held refractometers, except those with extremely sensitive prisms (McKenna and Thomson, 1988). Even if a reading can be obtained using a hand-held refractometer, non-sugar constituents such as amino acids can contribute to the refractive index and skew calculations of nectar energy content based on sugar concentration (Inouye et al., 1980).
While the nectar sampling studies mentioned previously examine methods of sampling floral nectar from flowers, few studies have examined the methods of storing these samples and the impact of bacterial and fungal infection on nectar storage life. Freezing (e.g. Lanza et al., 1995) could be an effective storage method, although it is not always a practical method in a field situation, as could refrigeration (Herbert and Calder., 1983; Erhardt and Baker, 1990) and/or the addition of antimicrobial agents.
This study was conducted to determine the most effective techniques for sampling and storing nectar from flowers with low nectar volumes. The information obtained from these studies will be of use to researchers investigating the sugar contents of the nectar of plant species with low floral nectar volumes.
MATERIALS AND METHODS
Plant species used
Methods for the collection and storage of floral nectar were compared using flowers from four species of Eucalyptus flowering on the grounds of the Mawson Lakes campus of the University of South Australia. Because few flowering trees were available for each of the three experiments (washing duration, sampling and storage), a different species of eucalypt was used for each. The flowers of a yellow gum (Eucalyptus leucoxylon ssp. leucoxylon) were used to test the effects of agitating and soaking flowers for different lengths of time. Swamp mallet (Eucalyptus spathulata) flowers were used for the comparison of sampling methods. The flowers of a coral gum (Eucalyptus torquata; Fig. 1) were used to test storage of nectar solutions, which were refrigerated or were left at room temperature (with and without the addition of benzyl alcohol), and those of a mallee salt gum (Eucalyptus sargentii ssp. fallens) were used to test storage of frozen nectar samples and those to which methanol had been added.
Fig. 1.
Eucalyptus torquata (coral gum) flowers.
Effect of washing duration
To evaluate the effect of longer washing durations on nectar sugar collection from individual flowers, three treatments were established. In each treatment, sets of five flowers were removed from the plant and agitated in 2 mL of distilled water for 1 min. The flowers in the first treatment were not soaked; other flowers were soaked for 19 min (second treatment, total 20 min) and 59 min (third treatment, total 60 min). Washing durations >10 min were chosen because Käpylä (1978) found that varying washing duration between 0·5 and 10 min did not affect the mass of sugars removed from rinsed flowers.
Sampling experiment
To compare the effects of nectar collection methods on nectar sugar mass recovery, flowers of similar maturity (open and showing no sign of senescence) were selected from a single plant. Flowers from a single plant, and of similar maturity, were selected because floral nectar sugar ratios may vary between individuals (Devlin et al., 1987; Hodges, 1993; Petanidou et al., 1996), and with the age of flowers (Petanidou et al., 1996). Nectar was sampled from these flowers using four commonly employed methods: (1) microcapillary tubes (Collins and Newland, 1986; McKenna and Thomson, 1988; Kearns and Inouye, 1993; Corbet, 2003); (2) filter paper wicks (McKenna and Thomson, 1988; Kearns and Inouye, 1993; Corbet, 2003); (3) washing in a known volume of distilled water (Käpylä, 1978; Grünfeld et al., 1989); and (4) rinsing with successive rinses of a known volume of distilled water (Núñez, 1977; Mallick, 2000).
All samples were collected concurrently, as nectar sugar ratios may vary depending on collection time (Petit and Freeman, 1997). Nectar volumes were too low for effective extraction using gravimetric techniques, microsyringes or micropipettes, and these methods were not considered further.
Microcapillary tubes
Seven flowers were sampled from the target plant using fixed-bore 0·25-µL microcapillary tubes (Drummond Scientific Company, Broomal, PA, USA). Nectar was drawn into the microcapillary tubes by means of capillary action. The volume of withdrawn nectar was then determined by measuring the column of nectar within the tube and calculating the proportion of the entire column (0·25 µL) filled. The nectar in the microcapillary tube was then rinsed into 2 mL of distilled water.
Filter paper wicks
Seven flowers were sampled from the target plant using filter paper wicks. Equilateral triangles of Whatman Number 1 filter paper, with sides 1 cm in length, were prepared. The filter paper was held with forceps and, in turn, each corner was applied to the nectaries. Each triangle of filter paper was then placed in a stoppered 30-mL vial containing 2 mL of distilled water, soaked for 15 min, and then agitated for approx. 1 min until the paper was reduced to pulp.
Washing in distilled water
The nectar from each of seven flowers was sampled from the target plant using a washing method. Flowers were cut from the plant, then each flower was placed in a 30-mL vial and 2 mL of distilled water were added using a micropipette. The vial was sealed and each vial was agitated manually for 1 min.
To determine how much of the sugar was removed in the initial wash, each flower was then removed from the vial and placed in a fresh vial containing 2 mL of water. This process was repeated one additional time for a total of three washes, and the resulting solutions analysed to determine what proportion of the total sugar was collected in each wash. For each flower, the amount of each sugar removed per wash was expressed as a percentage of the total sugar mass removed for all washes (Mallick, 2000). The sugars from these additional washes were not, however, used for the sampling comparisons, as multiple washes are not practical in a field situation.
Rinsing with a known volume of distilled water
Seven flowers were sampled from the target plant using a rinsing method. A flower was inverted over a 30-mL vial and four successive rinses of distilled water (0·5 mL) were expelled over the floral nectaries using a micropipette. This method did not require the removal of flowers from the plant. It was expected that rinsing was the most accurate sampling method because, unlike the washing method, sugars potentially leaking from the cut surface on the pedicel would not be represented; it would also be more likely to remove all the nectar from a flower than would other methods.
Comparison of methods for the storage of nectar solutions
Storage methods for nectar solutions were compared over a 28-d period. The aim of this experiment was to determine whether refrigeration and/or the addition of an antimicrobial agent, or freezing, would prolong the storage life of floral nectar and water solutions. Ethanol was not used as it interfered with the determination of fructose using the HPLC method employed for sugar analysis. Acetonitrile was investigated in a preliminary study, but was prone to contamination by ethanol.
To prepare enough sugar solution for all storage conditions, batches of 20 flowers from seven E. torquata were agitated in 50 mL (one flower per 2·5 mL) of water for 1 min. All flowers were collected within a 1-h period at noon. For each of four testing dates (days 0, 7, 14 and 28), the solution was divided into four vials, one for each storage condition. Sugar masses were not converted to mg per flower for this experiment. Solutions were filtered into 800-µL crimp-top vials and sealed with 8-mm aluminium ‘silver’ seals with PTFE/silicone liners (Grace Davison Discovery Sciences, Baulkham Hills, NSW, Australia). The storage conditions, using E. torquata, were: (a) refrigeration at 4 ºC; (b) refrigeration at 4 ºC with the antimicrobial agent benzyl alcohol (Hölzl, 2003) (one benzyl alcohol drop per mL of nectar solution); (c) room temperature (approx. 20 ºC); and (d) room temperature with one drop of benzyl alcohol per mL.
An additional experiment examined refrigeration with and without methanol and freezing, using the flowers of E. sargentii ssp. fallens. Because the flowers of this species are smaller than those of E. torquata, 200 mL of water and 100 flowers were used (one flower per 2 mL). The storage conditions were: (a) refrigeration at 4 ºC (analysed every day for 7 d); (b) refrigeration at 4 ºC with methanol (1 mL of methanol per 1 L of nectar solution; analysed at 0, 7, 14 and 28 d); and (c) freezer −8°C; analysed at 0, 7, 14 and 28 d).
To mimic a field collection situation, room temperature storage treatments were stored in a non climate-controlled room, and were subject to mild fluctuations in ambient temperature. Solutions were analysed using HPLC on day zero to provide control samples, and then 7, 14 and 28 d later. Only refrigeration with E. sargentii ssp. fallens was examined every day for a week, except day 2, during which an equipment problem was experienced.
HPLC analysis of nectar solutions
All solutions were filtered using a 0·45-µm nylon membrane into 7 × 40 mm, 800-µL crimp-top vials, sealed with 8-mm ‘silver’ seals with PTFE/silicone liners. Nectar volumes of individual flowers were too low (<1 µL) to be analysed with a hand-held sugar refractometer. Sugar analysis was carried out by injecting 10 µL of filtered sample using an LS-3200 autosampler (SGE, Ringwood VIC, Australia) and chromatographed using a BP-100 Carbohydrate Pb ++ Column, 300 × 7·8 mm (Benson Polymeric Inc., Reno, NV, USA) heated to 85 ºC using a column heating module. A Waters 600E solvent delivery system was used to deliver water as the solvent at a flow rate of 0·4 mL min−1. Detection was achieved using a Waters 410 refractive index detector heated to 40 ºC with sensitivity set at ×256. Data acquisition and handling were done using Baseline 815 software from Waters. The masses of three nectar sugars (sucrose, glucose and fructose) extracted from a single flower were determined using this method.
Data analysis
The statistical package SPSS® (Version 15·0) was used to conduct statistical analyses (α = 0·05). The unit of measurement provided in the HPLC output was in mg L−1; to convert this concentration to sugar mass per flower, the output was multiplied by 0·002 as 2 mL of distilled water were used to wash nectar from flowers. Where more than one flower was used, the output was divided by the number of flowers (except for storage, see above). Sugars collected using each sampling method and washing duration were compared using a one-way analysis of variance (ANOVA) after ensuring that the data were normally distributed using a Kolmogorov–Smirnov goodness-of-fit test. Least significant difference mean separation techniques were used to identify the locations of differences between sampling methods.
Sugar concentrations were examined across four test dates for E. torquata (days 0, 7, 14 and 28) using repeated-measures ANOVA (test day × sub-sample from the solution made with 20 flowers). In case of lack of sphericity, the Greenhouse–Geisser correction was used; repeated contrasts were used to compare each test day against the previous one (F-tests for contrasts are not presented). In addition, a χ2 contingency table was used to examine any differences in ratios of sugar between day 0 and later dates (counts consisted of units of sugar in mg). To remain conservative and highlight even small changes in sugar mass, the experiment-wise alpha level was not adjusted. Repeated-measures ANOVA with repeated contrasts (test day × sub-sample from the solution made with 100 flowers) was used to examine changes in sugar concentration among test days: 0, 1, 3, 4, 5, 6 and 7 in refrigerated samples without additive.
RESULTS
Effect of washing duration
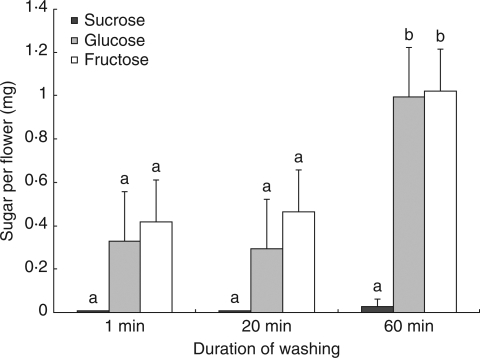
Significant differences were found in the masses of glucose [F(2,12) = 13·84, P = 0·001] and fructose [F(2,12) = 8·20, P = 0·006] among washing duration treatments; however, masses of sucrose did not vary significantly. Mean separation techniques showed no significant difference between the samples collected after 1 min of agitation in distilled water and those collected after 20 min of soaking (Fig. 2). The treatment that involved soaking for 60 min was significantly different from the other two treatments with increased masses of fructose (1 min, P = 0·003; and 20 min, P = 0·006) and glucose (1 min. P = 0·005; and 20 min. P < 0·001).
Fig. 2.
Mean sugar masses of sucrose, glucose and fructose recovered from E. leucoxylon ssp. leucoxylon flowers, using three durations of soaking in distilled water. The 1-min treatment was agitated for 1 min; the 20-min treatment was agitated for 1 min and soaked for 19 min; and the 60-min treatment was agitated for 1 min and soaked for 59 min. The error bars show the s.e. for each sugar; n = 5 for each treatment. Different letters indicate significant differences between treatments for each sugar considered separately.
Sampling experiment
Significant differences were detected for the masses of all sugars among the four sampling methods [sucrose, F(3,23) = 7·877, P = 0·001; glucose F(3,23) = 10·501, P < 0·001; fructose, F(3,23) = 10·265, P < 0·001]. Mean separation techniques were applied to determine the differences in sugar yields between pairs of treatments (Table 1).
Table 1.
Results of mean separation testing (least-significant difference after one-way ANOVA) for the differences in masses of sucrose, glucose and fructose in E. spathulata flowers among four sampling methods
| Significant differences |
|||||
|---|---|---|---|---|---|
| Sugar | Treatment | Microcapillary tube | Filter paper | Wash | Rinse |
| Sucrose | Microcapillary tube | – | – | 0·001 ↑ | 0·001 ↑ |
| Filter paper | – | – | 0·010 ↑ | 0·012 ↑ | |
| Wash | 0·001 ↓ | 0·010↓ | – | – | |
| Rinse | 0·001 ↓ | 0·012 ↓ | – | – | |
| Glucose | Microcapillary tube | – | 0·006 ↑ | <0·001 ↑ | <0·001 ↑ |
| Filter paper | 0·006 ↓ | – | – | – | |
| Wash | <0·001 ↓ | – | – | – | |
| Rinse | <0·001 ↓ | – | – | – | |
| Fructose | Microcapillary tube | – | 0·010 ↑ | 0·001 ↑ | <0·001 ↑ |
| Filter paper | 0·010 ↓ | – | – | 0·021 ↑ | |
| Wash | 0·001 ↓ | – | – | – | |
| Rinse | <0·001 ↓ | 0·021 ↓ | – | – | |
Values represent P-values and indicate significant differences between treatments. Arrows must be read by column only and indicate the direction of the difference (i.e. ↑= more, ↓= less).
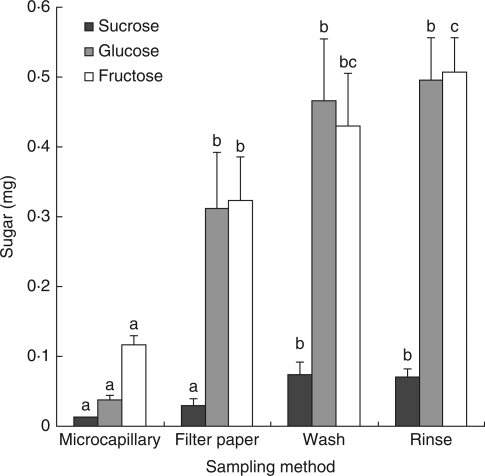
The microcapillary tube method collected the least sugar, yielding approximately four times less of all three sugars than the two highest yielding methods (Fig. 3). The only exception was sucrose, for which yields using filter paper triangles were not significantly different. The filter paper triangle method yielded significantly higher quantities of glucose and fructose than did the microcapillary tubes; it produced significantly less sucrose than did the washing or rinsing methods, and less fructose than the rinsing method (Table 1). Washing removed slightly lower quantities of sugar than did rinsing.
Fig. 3.
Mean masses of the sugars sucrose, glucose and fructose in the nectar recovered from the flowers of E. spathulata using four sampling methods. Error bars show s.e.; n = 7 for each treatment. Different letters indicate statistically significant differences between treatments for each sugar considered separately (see Table 1 for details). Note that the P-value between filter paper and rinse for glucose was 0·053.
The total volume of nectar sugar removed from a E. spathulata flower in each wash using three successive washes was also tested. The first two washes removed approx. 79% of the sucrose, 95% of the total glucose and 93% of the total fructose (Table 2). The proportion of the total sugar mass removed from flowers in the first two washes was lower than the 95% found by Mallick (2000) for Eucryphia lucida.
Table 2.
Mean percentages (± s.e.) of sugars removed from E. spathulata flowers using three successive washes in 2 mL of distilled water (n = 7 ).
| Sucrose (%) | Glucose (%) | Fructose (%) | |
|---|---|---|---|
| First wash | 60 ± 2·4 | 90 ± 3·4 | 82 ± 2·7 |
| Second wash | 19 ± 1·6 | 5 ± 0·8 | 11 ± 1·5 |
| Third wash | 21 ± 2·4 | 5 ± 3·0 | 7 ± 2·4 |
Storage of nectar solutions
The six storage treatments (refrigeration, refrigeration with benzyl alcohol or methanol, room temperature with and without benzyl alcohol, and freezer) all showed changes in sugar composition during the study period (Figs 4 and 5). After 1 week, the mass of at least one sugar had changed in all treatments.
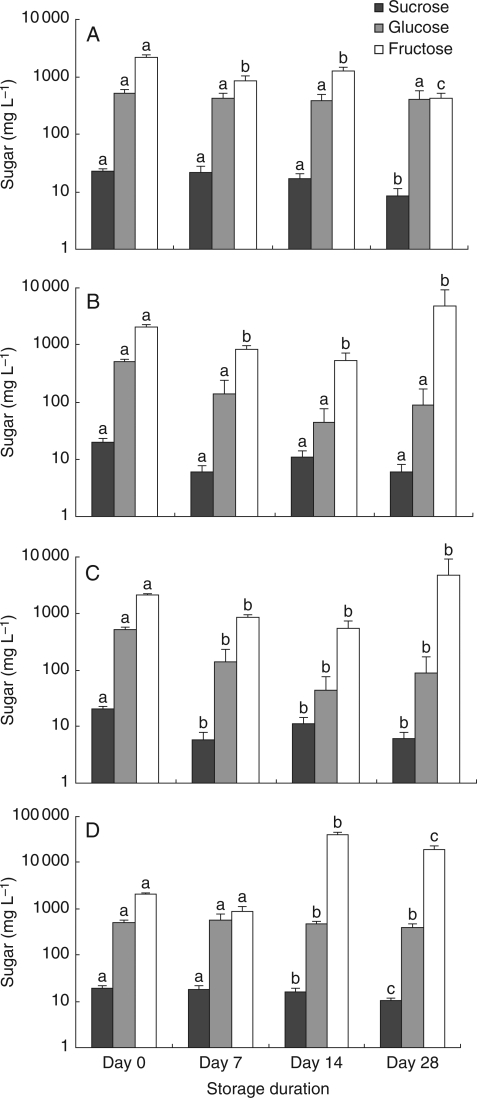
Fig. 4.
Mean concentration of sucrose, glucose and fructose in wash solutions of E. torquata for four nectar storage conditions over a 28-d period. Error bars show s.e. Sugar concentration in mg L−1 is expressed on a logarithmic scale. (A) Refrigerator, (B) refrigerator with benzyl alcohol, (C) room temperature, (D) room temperature with benzyl alcohol (note different vertical scale to A–C). Different letters indicate statistically significant differences between times of storage for each sugar considered separately.
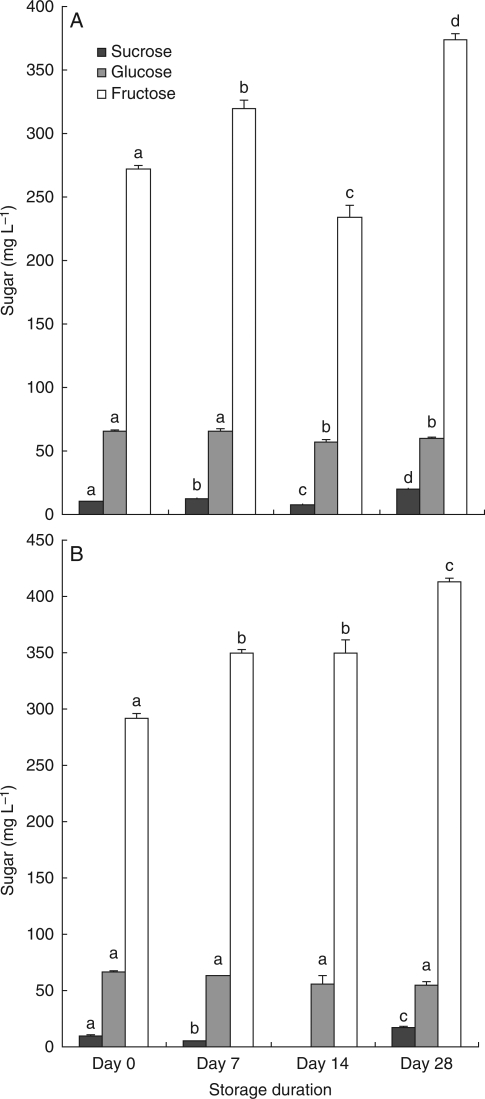
Fig. 5.
Mean concentration (mg L−1) of sucrose, glucose and fructose in wash solutions from flowers of E. sargentii ssp. fallens for two storage conditions over a 28-d period. Error bars show s.e. (standard errors for sucrose were generally too small to be visible at this scale). Sugar in mg is expressed on a logarithmic scale. (A) Freezer, (B) refrigerator with methanol. Different letters indicate statistically significant differences between times of storage for each sugar considered separately. Note that no data were recorded for sucrose in the refrigerator with methanol treatment on day 14.
Refrigerated or stored at room temperature, with and without the addition of benzyl alcohol
In the treatments that were refrigerated or stored at room temperature, involving E. torquata, benzyl alcohol did not prolong the life of any of the samples. Although the F-tests were not significant for all three sugars in the refrigerator–benzyl alcohol treatment because of the large standard errors (on a logarithmic scale, Fig. 4) and lack of sphericity for fructose, implying the use of reduced degrees of freedom, the mean concentration of fructose at day 14 was >10 times that at day 0 (2060 ± 198 mg L−1 vs. 22 000 ± 9500 mg L−1, respectively), indicating very large variability in measurements for this treatment. At room temperature with benzyl alcohol, the F-tests for all sugar concentrations were significantly different, with the same pattern of large means and standard errors for fructose at day 14 and day 28 compared with day 0 (Fig. 4). At day 28, the samples containing benzyl alcohol showed greatly increased sugar levels, particularly in the case of fructose, on the HPLC chromatograms. For example, at day 28, the mean fructose mass of the refrigerated samples that had been treated with benzyl alcohol was >40 times greater than those of the same treatment at day 14 (Fig. 4). Refrigeration without benzyl alcohol produced the best results in this set of experiments; however, the concentration of fructose had changed by day 7. Confidence intervals at day 0 for combined samples in the refrigerator (samples not yet refrigerated) and room temperature treatments were relatively large (sucrose = 17·3–27·8 mg L−1, glucose = 415–607 mg L−1 and fructose = 1810–2390 mg L−1), showing a perceptible error in measurement when sugar concentrations are high.
Frozen or treated with methanol
Using E. sargentii ssp. fallens, refrigeration with methanol and freezing at days 0, 7, 14 and 28 was also tested, and we found that glucose was stable in the methanol treatment, but sucrose and fructose had changed by day 7 (F = 397·913, d.f. = 2, P < 0·001 for the ANOVA; Fig. 5). Sucrose and fructose concentrations had changed by day 7 in the freezer treatment (F = 217·278, d.f. = 3, P < 0·001; F = 72·208, d.f. = 3, P < 0·001, respectively, for the ANOVA); glucose had changed between day 7 and day 14 (F = 8·651, d.f. = 3, P = 0·001 for the ANOVA). Overall it appears that refrigeration alone produces the best results in terms of sugar concentration, with relatively stable concentrations of glucose and sucrose for at least 2 weeks; sucrose and fructose had changed by day 7 in the freezer and refrigerator + methanol treatments.
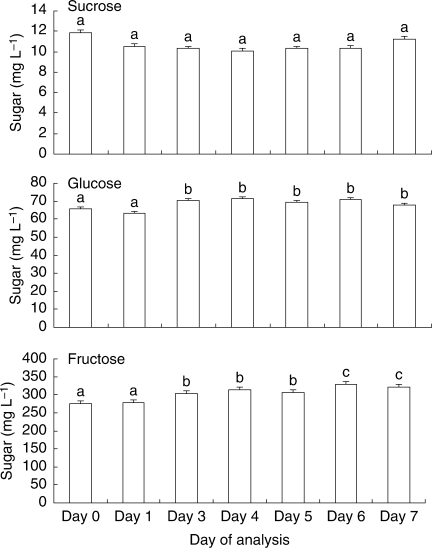
Further experimentation at a finer scale with E. sargentii ssp. fallens to detail changes in sugar concentration within 1 week using refrigerated samples without additive showed that the glucose and fructose concentrations had changed by day 3 (F = 4·638, d.f. = 6, P = 0·001; F = 21·330; d.f. = 2·625, P < 0·001, respectively); however, the concentration of these sugars did not follow a descending or ascending trend and an error in measurement may have been responsible for the differences found (Fig. 6). In fact, the confidence intervals that were calculated for day 0 for combined samples did not indicate a large error in measurement (sucrose = 10·65–11·78 mg L−1; glucose = 63·09–68·62 mg L−1; fructose = 267·73–280·13 mg L−1). The differences found between days in the 7-d trial for glucose and fructose, although statistically significant, may not have biological relevance.
Fig. 6.
Mean concentration (mg L−1) of sucrose, glucose and fructose in refrigerated nectar samples from E. sargentii ssp. fallens, with no additive, over a 7-d period. Error bars show s.e.; n = 7 for each treatment. Glucose and fructose concentrations had changed significantly by day 3. Different letters represent significant differences between adjacent bars only.
χ2 analysis for treatments involving four storage durations revealed that at day 7 the room temperature solution to which benzyl alcohol had been added showed significant changes in sugar ratios when compared with day 0 (χ2 = 8·42, d.f. = 2, P < 0·05). The difference in sugar ratios between day 0 and day 14 was strongly significant for the refrigerated solution to which benzyl alcohol was added (χ2 = 94·85, d.f. = 2, P < 0·01). The sugar ratios in the freezer treatment had not changed by day 28, nor had those in the refrigerator + methanol treatment; the remaining treatments experienced significant change by day 28 (refrigerator with no benzyl alcohol, χ2 = 13·58, d.f. = 2, P < 0·01; room temperature with no benzyl alcohol, χ2 = 37·40, d.f. = 2, P < 0·01). Until day 28, the sugar ratios are thus representative of those in nectar on the collection day when using the freezer or refrigerator + methanol storage treatment.
DISCUSSION
Significant variations were found in the effectiveness of the various methods for sampling and storing floral nectar from flowers with low nectar volumes. The results for floral nectar analysis show that: (a) it should be collected using a washing (1 min) or rinsing method (but only 60% of sucrose is recovered in the first wash, based on three washes, compared with 90% glucose and 82% fructose); (b) it should be refrigerated immediately after collection with no additive if sugar concentration is to be examined; (c) it should be analysed immediately for sugar mass if accuracy is needed; and (d) the sugar ratios remain stable for at least 14 d in the refrigerator and 28 d in the freezer and refrigerator + methanol treatments.
Nectar sampling
The 1-min agitation method was deemed to be the most suitable method for sampling in the field because it is more time efficient than is the 20-min treatment. The wash duration experiment showed that samples collected using this method are potentially prone to contamination by extrafloral sugars if soaked or agitated for too long. It is possible that the considerable increase in sugars in the 60-min washing treatment is a result of vascular and cellular fluid leaching from the cut surface on the pedicel during soaking, as was found by Herrera et al. (2006) in their investigation of the nectar sugar composition of the perennial herb Helleborus foetidus (Ranunculaceae). Sealing the cut surface with wax (D. W. Inouye, University of Maryland, pers. comm.) or surgical glue may obviate this problem. Conversely, the method may also remove more of the nectar sugars than do other methods, or nectaries may leak sugars because of osmotic stress. The first two 1-min washes of a flower were estimated to remove approx. 80% of the total sugars but, if leaching from the pedicel occurred, this number would overestimate the total amount of nectar sugar removed.
All sampling methods are likely to underestimate greatly the amounts of sugars present in flowers since one of the best methods only collects 60–90% of the sugars in the first wash, as estimated by three washes. This point needs to be taken into account for studies on energetics, for example. It was also noted that proportions of sugars extracted in each wash differ; sucrose leaching from the cells or phloem (Pate et al., 1985; De la Barrera and Nobel, 2004) may contribute to a relatively high percentage of this sugar recovered after the first wash. Sucrose is inverted to glucose and fructose in the nectaries by invertase (Pate et al., 1985). The rinsing and washing methods appear to be the most effective ways of determining the mass of sugars in the nectar of small flowers, although using distilled water may cause leaching of sugar from the flower's cells as a result of osmotic pressure. Washing or rinsing liquids simulating the saliva (at least its ionic strength and pH) of flower visitors may be more appropriate to understand how much sugar is available to them and their impact on nectar production. These considerations aside, the rinsing method yielded the highest masses of sugars, and, because it does not require flowers to be removed from the plant, it can be used for studies of nectar replenishment where repeated measurements are required. Also, this method does not introduce non-floral sugars into the nectar samples.
If we assume that rinsing is the most accurate sampling method because it removes the most nectar sugar, and is less likely to be contaminated with extrafloral sugars, washing, which is not significantly different, is the most practical field method. The washing method is a quick, easy method of estimating floral sugar in flowers with low nectar volumes and is not limited by nectar sugar concentration or flower structure. It is also less time consuming than is the rinsing method, and requires less equipment. Although it does not remove all sugar from the flower in the first extraction, it is the simplest and most effective method of quantifying nectar sugars in situations where small flowers, low floral nectar volumes or complex structures hinder the collection of nectar using other methods. This method is recommended where repeated measurements of nectar are not needed.
Blotting up nectar with a filter paper wick and microcapillary extraction methods were generally less effective methods of removing all sugars from a flower than were the rinsing and washing methods. Although removal of floral nectar using microcapillary tubes can provide an estimate of the nectar volume of a single flower (0·18 ± 0·016 µL for E. spathulata, n = 7), the results of the HPLC analysis show that this method is likely to underestimate floral nectar volume greatly.
It can be difficult to ensure that all nectar has been removed from flowers using filter paper or microcapillary tubes, particularly in species that have small flowers, low floral nectar volumes or complex structures. The filter paper and microcapillary methods require delicate movements, and make extraction of nectar from the flowers of such species difficult. These problems are compounded in difficult field conditions such as darkness or extreme weather (pers. obs.). Although some studies have found that microcapillary tubes and filter paper wicks are accurate methods for extracting nectar from flowers with low nectar volumes (McKenna and Thomson, 1988), the results of this study indicate that microcapillary tubes are less effective than are other methods for eucalypts.
In flowers with low nectar volumes, volume is difficult to estimate. Although microcapillary tubes are not as effective for sugar recovery as the other methods of sampling floral nectar sugars tested in this study, they may prove useful as tools to estimate nectar volume per flower. If sugar concentration collected on average by a microcapillary tube is known and we assume that this concentration represents the flower nectar, then total flower nectar volume can be extrapolated from sugar mass. For example, the mean nectar volume collected per flower using microcapillary tubes was 0·18 µL (± 0·016) for E. spathulata, and the mean total sugar mass was 0·17 mg (± 0·019; n = 7). The mean total sugar mass recovered with the rinse method was 1·07 mg (± 0·108; n = 7), so the mean volume per flower is estimated to be: (microcapillary nectar volume × sugar mass in rinse)/sugar mass in microcapillary tube, or 1·15 µL, >6 times more than estimated with the microcapillary tube alone.
Nectar storage
The results of the storage trials highlight the need to analyse nectar samples in a timely fashion. While the concentration of at least one sugar was significantly different by day 7 in all treatments, the ratios of sugars did not vary significantly before that day, and had not changed at day 28 in the freezer and refrigerator + methanol storage treatments, and day 14 in the refrigerator treatment. Thus, nectar solutions prepared for the investigation of sugar ratios may be stored for periods of at least 28 d if they are frozen, or refrigerated and treated with methanol, or 14 d if they are refrigerated with no additive.
For the examination of sugar concentrations, it is preferable to refrigerate samples with no additive. Over a 7-d trial period, statistically significant but numerically minor fluctuations were observed, which did not follow a trend. At a coarser (weekly) scale of analysis, no difference was found in both sucrose and glucose concentrations between day 0 and day 7 in the refrigerated samples, whereas other treatments did not perform as well (refrigeration with benzyl alcohol, although performing satisfactorily until day 7, was abandoned because of the large impact it had on fructose readings after that time). Storage conditions should be chosen depending on the objectives of the study. If very accurate sugar concentrations are needed, then immediate analysis of the samples is recommended; otherwise, samples may be stored for 7 d. Fructose concentration measurements tend to change more dramatically than those of sucrose and glucose, so rapid processing is imperative where exact measurements of this sugar are needed. It should be noted, however, that measurements may vary depending on the method of analysis.
The data shown in Fig. 4 indicate that the concentration of fructose in the sample had increased significantly after storage for 14 d, or longer, when benzyl alcohol had been added as a preservative. The only likely source of fructose is from the hydrolysis of sucrose, so such marked increases in masses are unlikely. Since the mass of sucrose in the samples is very small, hydrolysis of sucrose to produce fructose and glucose cannot account for the drastic increase in fructose after only 14 d of storage. It appeared that the addition of benzyl alcohol might be the cause of this increase in fructose. Oxidation of benzyl alcohol during storage may lead to formation of products such as benzaldehyde and/or benzoic acid; these compounds may co-elute with fructose during chromatographic analysis, resulting in anomalously high fructose concentration. To test this hypothesis, aqueous solutions of benzaldehyde and benzoic acid were chromatographed under the same conditions as those used for sugar analysis. The results of this experiment demonstrated that benzaldehyde elutes at a retention time very close to that of fructose. Since the amount of benzaldehyde, which must have resulted from oxidation of the benzyl alcohol added to the samples, is likely to be present in much higher concentration than fructose, it is probable that the fructose peak was missed and that the benzaldehyde peak was measured as fructose. This misinterpretation will have led to the perceived increases in the amount of fructose present in the storage samples to which benzyl alcohol was added.
Changes in the sugars are difficult to explain; microbial activity can create real changes in sugars and/or create other compounds that interfere in the assay, resulting in apparent changes that may not be real. If it is the case, pulsed amperometric detection (PAD) might overcome problems of apparent changes in sugar masses due to interferences, because of the specificity of this method as opposed to refractive index detection.
The results of nectar analysis can vary considerably depending on the sampling and storage methods chosen. To sample nectar from plants with low floral nectar volume in a field situation, it appears that the 1-min wash method is the most efficient, and that the sample should be refrigerated without additive. The samples should be analysed as soon as possible upon return to the laboratory, and preferably within a day.
ACKNOWLEDGEMENTS
We thank I. Ametov for her assistance in the laboratory, J. Slocombe, R. Aebi, D. Carver, and H. West for their help with obtaining equipment, and P. Rismiller, R. Sharrad, W. Scowcroft, D. Inouye, M. Sedgley and an anonymous reviewer for reviewing the manuscript; the latter inspired the ‘osmotic’ statement. This paper was part of D.M.'s Honours thesis in the programme of Applied Science (Hons) (Biodiversity, Environmental and Park Management). This work was supported by the National Geographic Society (8070-06 to S.P., with A. Sharp, and the collaboration of D.M. and R.S.); the Nature Foundation of South Australia (grant to S.P.); the Field Naturalists Society of South Australia (Lirabenda Endowment Fund Research Grant to D.M.); the Department for Environment and Heritage (South Australia; Northern and Yorke Region); the University of South Australia (UniSA) (travel); and the UniSA Division of Information Technology, Engineering and the Environment (Seed Funding to S.P. and R.S.; Summer Scholarship to Promising Undergraduate Student D.M.).
LITERATURE CITED
- Armstrong DP, Paton DC. Methods for measuring amounts of energy available from Banksia inflorescences. Austral Ecology. 1990;15:291–297. [Google Scholar]
- Birtchnell MJ, Gibson M. Long-term flowering patterns of melliferous Eucalyptus (Myrtaceae) species. Australian Journal of Botany. 2006;54:745–754. [Google Scholar]
- Bolten AB, Feinsinger P. Why do hummingbird flowers secrete dilute nectar? Biotropica. 1978;10:307–309. [Google Scholar]
- Bolten AB, Feinsinger P, Baker HG, Baker I. On the calculation of sugar concentration in flower nectar. Oecologia. 1979;41:301–304. doi: 10.1007/BF00377434. [DOI] [PubMed] [Google Scholar]
- Bond HW, Brown WL. The exploitation of floral nectar in Eucalyptus incrassata by honeyeaters and honeybees. Oecologia. 1979;44:105–111. doi: 10.1007/BF00346407. [DOI] [PubMed] [Google Scholar]
- Collins BG, Newland C. Honeyeater population changes in relation to food availability in the jarrah forest of Western Australia. Australian Journal of Ecology. 1986;11:63–76. [Google Scholar]
- Corbet SA. Nectar sugar content: estimating standing crop and secretion rate in the field. Apidologie. 2003;34:1–10. [Google Scholar]
- De la Barrera E, Nobel PS. Nectar: properties, floral aspects, and speculations on origin. Trends in Plant Science. 2004;9:65–69. doi: 10.1016/j.tplants.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Devlin B, Horton JB, Stephenson AG. Patterns of nectar production of Lobelia cardinalis. American Midland Naturalist. 1987;117:289–295. [Google Scholar]
- Dungan RJ, Beggs JR, Wardle DA. A simple gravimetric technique for estimating honeydew or nectar production. New Zealand Journal of Ecology. 2004;69:1306–1307. [Google Scholar]
- Erhardt A, Baker I. Pollen amino acids – an additional diet for a nectar-feeding butterfly? Plant Systematics and Evolution. 1990;169:111–112. [Google Scholar]
- Grünfeld E, Vincent C, Bagnara D. High performance liquid chromatography analysis of nectar and pollen of strawberry flowers. Journal of Agricultural and Food Chemistry. 1989;37:290–294. [Google Scholar]
- Hainsworth FR, Wolf LL. Nectar characteristics and food selection by hummingbirds. Oecologia. 1976;25:101–113. doi: 10.1007/BF00368847. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Energetics of pollination. Annual Review of Ecology and Systematics. 1975;6:139–170. [Google Scholar]
- Herbert SM, Calder WA. Sodium, potassium, and chloride in floral nectars: energy-free contributors to refractive index and salt balance. Ecology. 1983;64:339–402. [Google Scholar]
- Herrera C, Pérez R, Alonso C. Extreme intraplant variation in nectar sugar composition in an insect-pollinated perrenial herb. American Journal of Botany. 2006;93:575–581. doi: 10.3732/ajb.93.4.575. [DOI] [PubMed] [Google Scholar]
- Hodges SA. Consistent interplant variation in nectar characteristics of Mirabilis multiflora. Ecology. 1993;74:542–548. [Google Scholar]
- Hölzl W, Koppold J, Marquais-Bienwald S, Preuss A. Benzyl alcohol derivatives and their use as antimicrobial agents. 2003. Online patent: http://www.wipo.int/pctdb/en/wo/jsp?wo=2003078367&IA=EP200030002618&DISPLAY=STATUS .
- Inouye DW, Favre ND, Lanum JA, et al. The effects of nonsugar nectar constituents on estimates of nectar energy content. Ecology. 1980;61:992–996. [Google Scholar]
- Käpylä M. Amount and type of nectar sugar in some wild flowers in Finland. Annales Botanici Fennici. 1978;15:85–88. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Colorado: University Press of Colorado; 1993. [Google Scholar]
- Lanza J, Smith GC, Sack S, Cash A. Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia. 1995;102:113–119. doi: 10.1007/BF00333318. [DOI] [PubMed] [Google Scholar]
- Lloyd S, Ayre DJ, Whelan RJ. A rapid and accurate visual assessment of nectar production can reveal patterns of temporal variation in Banksia erecifolia (Proteaceae) Australian Journal of Botany. 2002;50:595–600. [Google Scholar]
- Mallick SA. Technique for washing nectar from the flowers of Tasmanian leatherwood (Eucryphia lucida: Eucryphiaceae) Austral Ecology. 2000;25:210–212. [Google Scholar]
- Manetas Y, Petropoulou Y. Nectar amount, pollinator visit duration and pollinator success in the Mediterranean shrub Cistus creticus. Annals of Botany. 2000;86:815–820. [Google Scholar]
- Martínez del Rio C, Baker HG, Baker I. Ecological and evolutionary implications of digestive processes: bird preferences and the sugar constituents of floral nectar and fruit pulp. Cellular and Molecular Life Sciences. 1992;48:544–551. [Google Scholar]
- McKenna MA, Thomson JD. A technique for sampling and measuring small amounts of floral nectar. Ecology. 1988;69:1306–1307. [Google Scholar]
- Núñez JA. Nectar flow by melliferous flora and gathering flow in Apis mellifera ligusta. Journal of Insect Physiology. 1977;23:265–275. [Google Scholar]
- Pate JS, Peoples MB, Storer PJ, Atkins CA. The extrafloral nectaries of cowpea (Vigna unguiculata (L.) Walp.). II. Nectar composition, origin of nectar solutes, and nectary functioning. Planta. 1985;166:28–38. doi: 10.1007/BF00397382. [DOI] [PubMed] [Google Scholar]
- Petanidou T, Van Laere AJ, Smets E. Change in floral nectar components from fresh to senescent flowers of Capparis spinosa (Capparidaceae), a nocturnally flowering Meditteranean shrub. Plant Systematics and Evolution. 1996;199:79–92. [Google Scholar]
- Petit S, Freeman CE. Nectar production of two sympatric species of columnar cacti. Biotropica. 1997;29:175–183. [Google Scholar]
- Petit S, Pors L. Survey of columnar cacti and carrying capacity for nectar-feeding bats on Curaçao. Conservation Biology. 1996;10:769–775. [Google Scholar]
- Schondube JE, Martínez del Rio C. Concentration-dependent sugar preferences in nectar-feeding birds: mechanisms and consequences. Functional Ecology. 2003;17:445–453. [Google Scholar]
- Stephanou M, Petropoulou Y, Georgiou O, Manetas Y. Enhanced UV-B radiation, flower attributes and pollinator behaviour in Cistus creticus: a Mediterranean field study. Plant Ecology. 2000;147:165–171. [Google Scholar]
- Swanson CA, Shuel RW. The centrifuge method for measuring nectar yield. Plant Physiology. 1949;25:513–520. doi: 10.1104/pp.25.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschapka M. Energy density patterns of nectar resources permit coexistence within a guild of Neotropical flower-visiting bats. Journal of Zoology. 2004;263:7–21. [Google Scholar]
- Willmer P. The effects of insect visitors on nectar constituents in temperate plants. Oecologia. 1980;47:270–277. doi: 10.1007/BF00346832. [DOI] [PubMed] [Google Scholar]