Abstract
Background
In contrast to C3 photosynthesis, the response of C4 photosynthesis to water stress has been less-well studied in spite of the significant contribution of C4 plants to the global carbon budget and food security. The key feature of C4 photosynthesis is the operation of a CO2-concentrating mechanism in the leaves, which serves to saturate photosynthesis and suppress photorespiration in normal air. This article reviews the current state of understanding about the response of C4 photosynthesis to water stress, including the interaction with elevated CO2 concentration. Major gaps in our knowledge in this area are identified and further required research is suggested.
Scope
Evidence indicates that C4 photosynthesis is highly sensitive to water stress. With declining leaf water status, CO2 assimilation rate and stomatal conductance decrease rapidly and photosynthesis goes through three successive phases. The initial, mainly stomatal phase, may or may not be detected as a decline in assimilation rates depending on environmental conditions. This is because the CO2-concentrating mechanism is capable of saturating C4 photosynthesis under relatively low intercellular CO2 concentrations. In addition, photorespired CO2 is likely to be refixed before escaping the bundle sheath. This is followed by a mixed stomatal and non-stomatal phase and, finally, a mainly non-stomatal phase. The main non-stomatal factors include reduced activity of photosynthetic enzymes; inhibition of nitrate assimilation, induction of early senescence, and changes to the leaf anatomy and ultrastructure. Results from the literature about CO2 enrichment indicate that when C4 plants experience drought in their natural environment, elevated CO2 concentration alleviates the effect of water stress on plant productivity indirectly via improved soil moisture and plant water status as a result of decreased stomatal conductance and reduced leaf transpiration.
Conclusions
It is suggested that there is a limited capacity for photorespiration or the Mehler reaction to act as significant alternative electron sinks under water stress in C4 photosynthesis. This may explain why C4 photosynthesis is equally or even more sensitive to water stress than its C3 counterpart in spite of the greater capacity and water use efficiency of the C4 photosynthetic pathway.
Key words: C3 and C4 photosynthesis, stomatal and non-stomatal limitation, high CO2, water stress
INTRODUCTION
Water stress is one of the most limiting environmental factors to plant productivity worldwide, and can be caused by both soil and atmospheric water deficits. The response of C3 photosynthesis to water stress has been well studied and reviewed, as indicated by the large number of research (e.g. Sharkey and Seemann, 1988; Ortiz-López et al., 1991; Cornic et al., 1992; Tezara et al., 1999; Cornic and Fresneau, 2002) and review (e.g. Cornic, 2000; Lawlor, 1995, 2002; Lawlor and Cornic, 2002; Flexas et al., 2004) articles published on this topic. In general, C3 photosynthesis is negatively affected by water stress measured as changes in leaf water potential (Ψleaf) or relative water content (RWC). In the early phase of water stress, when leaf RWC is still greater than 70 %, the decline in CO2 assimilation rates (A) is largely the result of reduced intercellular CO2 concentration (Ci) due to decreased stomatal conductance (g). Under these conditions, maximal photosynthetic capacity and quantum yield remain unaffected when measured under saturating irradiance and carbon dioxide concentration ([CO2]). In addition, photosynthetic inhibition usually recovers relatively quickly when plants are re-hydrated. If water stress persists and leaf RWC falls below 70 %, the loss of photosynthetic activity becomes increasingly less responsive to high [CO2] and A fails to recover to pre-stress values following the removal of water stress. The exact mechanisms underlying this non-stomatal phase, also termed metabolic inhibition, are diverse and less well understood (for more details and reviews on this topic, see Cornic, 2000; Lawlor and Cornic, 2002; Lawlor, 2002; Flexas et al., 2004; and references therein).
In contrast, the response of C4 photosynthesis to water stress has been less well studied. This is in spite of the fact that C4 plants make a significant contribution to the global carbon budget, and C4 crops, such as maize and sorghum, are pivotal to current and future global food security (Lloyd and Farquhar, 1994; Ehleringer et al., 1997; Brown, 1999; Pingali, 2001). Moreover, C4 plants predominate in hot, arid regions which are prone to frequent drought. This fact is likely to be exacerbated by global climate change in three main ways: (1) global warming and changes in precipitation patterns are likely to expose many ecosystems, including C4-dominated ones, to increasing soil and atmospheric water stresses (IPCC, 2007); (2) the impact of rising atmospheric [CO2] on the productivity of C4 plants is greatly influenced by soil water availability (Ghannoum et al., 2000, 2006); and (3) global warming may lead to an increase in the proportion of land area covered by C4 plants, especially in grasslands and rangelands (Archer, 1993; Henderson et al., 1994; Crimp et al., 2002). Therefore, it is important to understand how water stress influences the primary processes of CO2 fixation in C4 plants. In this article, I review the evidence related to the response of C4 photosynthesis to water stress and attempt to summarize the current state of understanding in this area, including the interaction of elevated [CO2] with the effects of water stress on C4 photosynthesis.
SIGNIFICANCE AND DISTRIBUTION OF THE C4 PHOTOSYNTHETIC PATHWAY
Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), the primary CO2-fixing enzyme in plants, has poor kinetic properties. Compared with other enzymes, Rubisco is a slow catalyst with a low affinity for its substrate CO2. Most importantly, Rubisco has a low ability to discriminate between molecular CO2 and O2 (Jordan and Ogren, 1981; Andrews and Lorimer, 1987). The latter feature is particularly problematic because O2 is the by-product of the light reactions of photosynthesis (Edwards and Walker, 1983) and is in high concentration in the atmosphere. By reacting RuBP with O2, Rubisco fixes less CO2 and initiates a series of reactions, photorespiration, which culminates in the release of CO2 back to the atmosphere (Edwards and Walker, 1983). Under the current atmospheric [CO2] and a temperature of 25 °C, photorespiration runs at about 20–30 % of photosynthesis in C3 leaves (Sage, 2001, 2004). With increasing temperature, photorespiration increases faster than photosynthesis (Jordan and Ogren, 1984; Sage and Kubien, 2007). The C4 photosynthetic pathway has evolved as an adaptation to high photorespiratory pressures resulting from various combinations of stresses which include low atmospheric [CO2], high temperature, aridity and/or salinity (Ehleringer et al., 1991, 1997; Sage, 2001, 2004; Tipple and Pagani, 2007). According to carbon isotope discrimination records, plant fossils and molecular taxonomy studies, it is likely that C4 plants formed a minor component of the world's flora for a long time before the recent expansion of C4 grasslands some 5–10 million years ago (Cerling, 1999; Kellogg, 1999; Sage, 2004). It is estimated that the C4 photosynthetic pathway has evolved independently some 45 times in three monocot and 16 dicot lineages (Kellogg, 1999; Sage et al., 1999; Sage, 2004).
Although C4 plants represent a mere 4 % of the world's flora, they contribute about 20 % of global primary productivity, mainly because of the high productivity of C4 grasslands (Lloyd and Farquhar, 1994; Ehleringer et al., 1997). The C4 photosynthetic pathway is strongly represented in the grass (Poaceae) family, comprising about 50 % of total grasses (Hattersley, 1992; Sage et al., 1999). C4 plants are grouped into three biochemical subtypes [NAD malic enzyme (NAD-ME), NADP malic enzyme (NADP-ME) and phosphoenolpyruvate carboxykinase (PCK)] following the major C4 acid decarboxylation enzyme in the bundle sheath (Hatch, 1987; Hattersley, 1992). The major C4 crops, such as maize, sugarcane and sorghum belong to the NADP-ME subtype. At the regional level, the geographic distribution of C4 grasses is strongly influenced by rainfall level. With decreasing rainfall (from 900 mm to 50 mm per annum), the abundance of NAD-ME grasses increases while that of NADP-ME grasses decreases. The distribution of PCK grasses is weakly correlated with rainfall gradient (Ellis et al., 1980; Hattersley, 1992; Taub, 2000). This distribution suggests that C4 grasses with different biochemical subtypes may have different water use efficiency (WUE) or drought tolerance. The first attribute has been validated with NAD-ME grasses having a greater whole-plant WUE under water stress than their NADP-ME counterparts (Ghannoum et al., 2002). However, there is no evidence suggesting that the three C4 biochemical pathways have different sensitivities to water stress. Hence, in the context of the current review, it is possible to discuss the effects of water stress on C4 photosynthetic metabolism in general.
THE CO2-CONCENTRATING MECHANISM IN C4 LEAVES
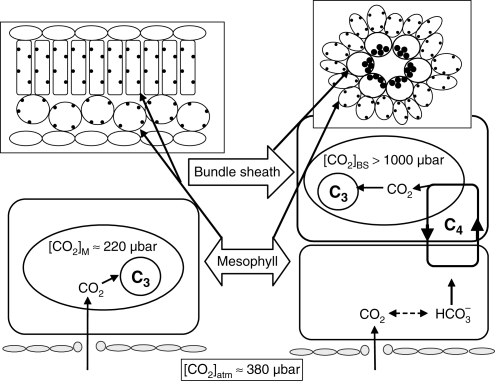
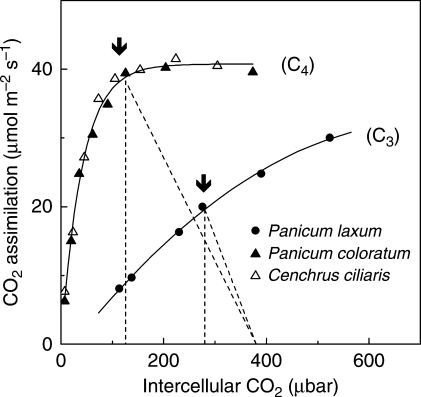
The key feature of C4 photosynthesis is the operation of a CO2-concentrating mechanism in the leaves of C4 plants, which consists of a series of biochemical and structural modifications around the ancestral C3 photosynthetic pathway (Hatch, 1987). Although there are many ways, biochemically and anatomically, of achieving C4 photosynthesis, the most common C4 syndrome in higher plants involves the operation of two photosynthetic cycles (C3 and C4) across two photosynthetic cell types (mesophyll and bundle sheath), which are arranged in concentric layers around the vascular bundle (Fig. 1; Hatch, 1987). The first steps of C4 photosynthesis occur in the mesophyll and involve the hydration of CO2 into bicarbonate, which reacts with phosphoenolpyruvate (PEP) with the aid of PEP carboxylase (PEPC) to produce oxaloacetate, a C4 acid, hence the terms C4 cycle and C4 photosynthesis. Oxaloacetate is converted into other C4 acids (malate, aspartate or alanine) which diffuse into the bundle sheath cells where they are decarboxylated, releasing CO2 for fixation by Rubisco and the rest of the C3 cycle. The C3 product of the decarboxylation reaction returns to the mesophyll, completing the C3 cycle (Fig. 1). The C4 cycle acts like a CO2-concentrating mechanism for two main reasons: (1) PEPC is faster than Rubisco and insensitive to O2; and (2) the bundle sheath cell wall presents a significant gaseous diffusion barrier (Hatch, 1987; Brown and Byrd, 1993). Consequently, the high [CO2] generated by the C4 CO2-concentrating mechanism in the bundle sheath leads to the suppression of apparent photorespiration in air as well as the saturation of C4 photosynthesis at a lower ambient [CO2] than for C3 plants (Fig. 2). In addition, photorespired CO2 is released within the bundle sheath, and either is refixed or contributes to increasing bundle sheath [CO2] ( [CO2]BS), which in turn, leads to reducing photorespiration. High [CO2]BS gives rise to the characteristic A/Ci curve of C4 leaves. Relative to C3 photosynthesis, the C4 A/Ci curve is characterized by abrupt saturation at a relatively low Ci (Fig. 2). This constitutes the basis of a number of advantages conferred by the C4, relative to C3, photosynthetic pathway, chief of which is higher WUE (Osmond et al., 1982; Long, 1999).
Fig. 1.
A simplified, schematic representation of C3 (left) and C4 (right) photosynthesis.
Fig. 2.
The response of CO2 assimilation rates (A) to intercellular CO2 concentration (Ci) in one C3 (Panicum laxum) and two C4 (Cenchrus ciliaris and Panicum coloratum) grasses. Gas exchange measurements were made at 30 °C and 1200 µmol quanta m–2 s–1. The dotted lines represent the slope of stomatal conductance, g = A/(Ca – Ci), where Ca and Ci are the ambient and intercellular [CO2], respectively. The arrows indicate A at the operational Ci (i.e. Ci at normal air [CO2]; O. Ghannoum, unpubl. res.).
C4 PHOTOSYNTHESIS AND WATER STRESS
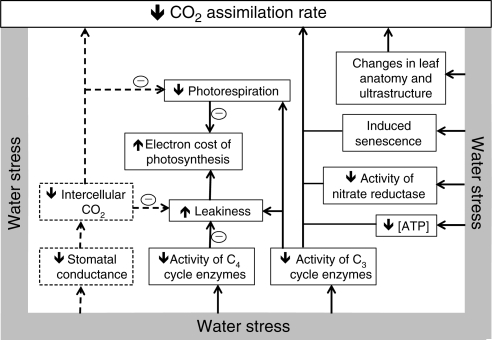
C3 and C4 photosynthesis share most of the fundamental photosynthetic processes such as the C3 cycle, light harvesting complexes and electron transport components. Hence, the two photosynthetic pathways may be expected to show, by and large, similar responses to water availability. Nevertheless, significant differences exist between the two photosynthetic types, which could make their response to water stress differ at a number of levels. A cursory examination of the literature reveals that the observed responses of C4 photosynthesis to water stress are as diverse as those reported for C3 photosynthesis. Some studies concluded that inhibition of C4 photosynthesis under water stress is mainly due to stomatal closure, while others concluded that non-stomatal factors play a major role (e.g. Lawlor and Fock, 1978; Becker and Fock, 1986; Loreto et al., 1995; Lal and Edwards, 1996; Saccardy et al., 1996; Maroco et al., 2000; Ghannoum et al., 2003; Marques da Silva and Arrabaça, 2004a; Ripley et al., 2007; Carmo-Silva et al., 2008). These studies used different C4 species subjected to different severities and methods of inducing water stress (e.g. withholding watering, using an osmotic agent or drying of detached leaves) and made photosynthetic measurements using different techniques (e.g. various gas exchange instruments or O2 electrodes) and under different conditions of light intensity and leaf temperature. Consequently, the different responses could be attributed to any combination of these factors. Therefore, there is a need to dissect the available evidence in order to draw a more comprehensive picture of the mechanisms underlying the response of C4 photosynthesis to water stress. These mechanisms are summarized in Fig. 3 and, as commonly argued in the literature, are divided into stomatal and non-stomatal factors. The stomatal factors refer to the downstream effects of CO2 limitation on photosynthetic activity. The non-stomatal factors encompass everything else, including the direct effects of reduced leaf and cellular water status on the activity of enzymes involved in the CO2 fixation and electron transport reactions, induction of early senescence, and changes to leaf anatomy and ultrastructure (Fig. 3).
Fig. 3.
Summary of the main effects of water stress on the photosynthetic parameters of C4 leaves. Stomatal and non-stomatal factors are indicated by dashed and continuous lines, respectively. The (–) sign indicates an effect in the opposite direction. The term leakiness (Φ) is defined as the fraction of CO2 fixed by PEPC which leaks out of the bundle sheath.
THE ROLE OF STOMATAL FACTORS IN THE INHIBITION OF C4 PHOTOSYNTHESIS UNDER WATER STRESS
Similarly to what has been reported in C3 plants, stomatal conductance of C4 plants decreases with declining leaf water status, and this invariably coincides with reduced photosynthetic rates (e.g. Kalapos et al., 1996; Maroco et al., 2000; Ghannoum et al., 2003; Carmo-Silva et al., 2008). The concomitant decline of A and g, particularly under mild water stress (i.e. for leaf RWC >70 %), has been interpreted in a causal way in C3 and C4 plants alike, based on four main lines of evidence: (1) reduced Ci, (2) recovery of A at high [CO2], (3) occurrence of photorespiration, and (4) recovery of A following re-hydration.
Intercellular CO2 of C4 plants subjected to water stress
Decreased Ci due to reduced stomatal conductance has been taken as a proof of CO2 limitation for C4 photosynthesis. The operation of a CO2-concentrating mechanism during C4 photosynthesis introduces additional layers of complexity to this otherwise straightforward argument. A closer look at the literature shows that Ci decreases only during the early phases of water stress as has been reported for maize (Becker and Fock, 1986; Lal and Edwards, 1996; Leakey et al., 2004), sorghum (Williams et al., 2001), sugarcane (Du et al., 1996), amaranthus (Lal and Edwards, 1996) and a non-crop C4 grass species (Marques da Silva and Arrabaça, 2004a). During the later stages of drought, it is often observed that Ci increases while A continues its decline (e.g. Becker and Fock, 1986; Du et al., 1996; Kalapos et al., 1996; Lal and Edwards, 1996). In contrast, some studies using various C4 plants reported no change in Ci under water stress (e.g. Saliendra et al., 1996, Ripley et al., 2007) or for most of the water stress period, with Ci increasing under severe stress at the end of the drying cycle (Kalapos et al., 1996; Lal and Edwards, 1996).
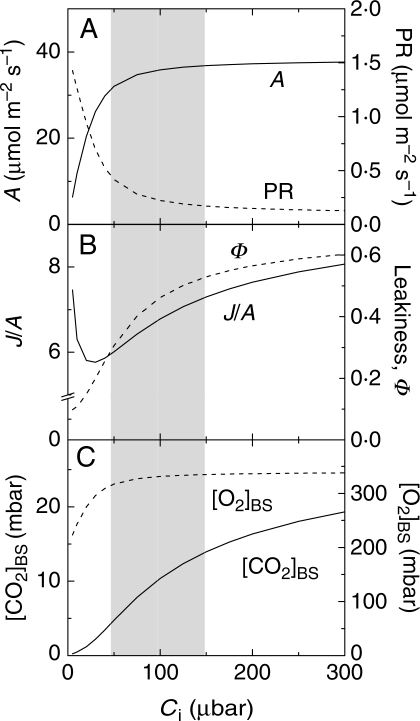
By raising [CO2] at the sites of Rubisco, the C4 CO2-concentrating mechanism serves to CO2-saturate A and virtually suppress photorespiration in normal air (Hatch, 1987). This is illustrated in Fig. 4 which uses the C4 model of von Caemmerer (2000) to simulate the response of some key photosynthetic parameters to Ci in a mature C4 leaf measured under optimal light and temperature. The shaded area highlights the range of Ci measured in well-watered and moderately water-stressed leaves (Fig. 4). The modelling predicts little change in A with Ci declining down to 50 µbar (Fig. 4A). Thus, based on our theoretical understanding, the CO2-concentrating mechanism endows C4 photosynthesis with a significant buffering capacity against short-term fluctuations in Ci down to a certain concentration, such as those usually observed in mildly water-stressed C4 leaves. This is supported by the results of Lal and Edwards (1996) who found that the initial decline in Ci, up to 50 % of control values, had no effect on A during the early phases of water stress in both maize and amaranthus. Hence, it may be concluded that during the early stages of water stress, stomatal closure may not always reduce Ci enough to cause a detectable decline in A. In addition to inter-species variations amongst C4 plants, whether or not a decline in Ci will elicit a reduction in A depends largely on growth and measuring conditions that influence the position of the operational Ci (i.e. Ci at normal air [CO2]; Fig. 2). For example, conditions of high irradiance and nutrition tend to shift the operational Ci down to the CO2-responsive part of the A/Ci curve (Ghannoum et al., 1997; Ghannoum and Conroy, 1998). In contrast, low irradiance tends to shift Ci to the flat part of the A/Ci curve, which necessitates a large decline in Ci before A is affected (e.g. Lal and Edwards, 1996). The interaction between environmental conditions (such as irradiance, nutrition, temperature) and the response of C4 photosynthesis to water stress has not yet received its due attention.
Fig. 4.
Modelling the response of C4 photosynthesis to intercellular [CO2], Ci, using the C4 photosynthesis model developed by von Caemmerer (2000). (A) CO2 assimilation rates (A, continuous line) and photorespiration (PR, dotted line); (B) the ratio of the rates of electron transport to CO2 assimilation (J/A, continuous line) and leakiness (Φ, dotted line); and (C) bundle sheath [CO2] ([CO2]BS, continuous line) and [O2] ([O2]BS, dotted line). The modelling simulates a mature C4 leaf with maximal PEPC and Rubisco activities of 120 and 40 µmol m–2 s–1, respectively; a bundle sheath conductance to CO2 per leaf area of 3 mmol m–2 s–1. Other parameters are similar to those described in table 2 of von Caemmerer and Furbank (1999). The shaded area represents the likely range of Ci experienced by well-watered and mildly water-stressed C4 leaves.
Recovery of photosynthetic rates by high [CO2] in C4 plants subjected to water stress
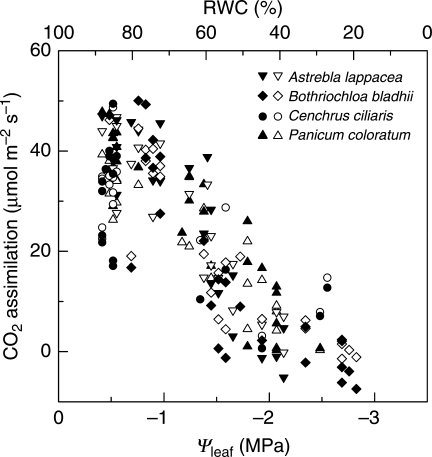
If C4 photosynthesis is limited by CO2 supply due to stomatal closure under water stress, then increasing [CO2] should restore A either fully or partially to pre-stress values. Surprisingly, very few studies (apart from the literature about CO2 enrichment, which is discussed in a later section) have attempted to specifically measure A under physiologically high [CO2]. In a study where four C4 grass species were exposed to a drying cycle, increased [CO2] up to 2500 ppm had no effect on A at any stage of the drying cycle in any of the four species (Fig. 5). Similar results were reported with three C4 grasses exposed to severe water stress and measured at a [CO2] of 1000 ppm (Carmo-Silva et al., 2008). When amaranthus was grown and measured at four [CO2] (18, 27, 35 and 70 Pa), elevated [CO2] alleviated slightly the negative impact of drought on A through the indirect effects of high [CO2] on Ψleaf, and only under mild but not severe water stress (Ward et al., 1999). Rather than adjusting ambient [CO2], Du et al. (1996) maintained Ci at control values. Their results showed that raising Ci in water-stressed sugarcane leaves enhanced A fully to control values for Ψleaf > –0·4 MPa, and partially for Ψleaf between –0·4 and –0·85 MPa. For Ψleaf < –0·85 MPa, Ci manipulations had no influence on A.
Fig. 5.
CO2 assimilation rates as a function of leaf relative water content (RWC) and water potential (Ψleaf) in four C4 grasses growing in a drying soil: Astrebla lappacea, Bothriochloa bladhii, Cenchrus ciliaris and Panicum coloratum. Measurements were made at 28 °C, 1000 µmol quanta m–2 s–1, and ambient [CO2] of either 350 (open symbols) or 2500 µbar (closed symbols). Adapted from Ghannoum et al. (2003).
It has been argued that a mere doubling or tripling of ambient [CO2] is not enough to overcome the stomatal limitation caused by water stress, and that very high [CO2] (>1 %) is needed in order to force CO2 to diffuse across the whole leaf surface and not just the near closed stomata. Super-saturating [CO2] may also be needed to overcome potential increases in mesophyll conductance in response to water stress (Cornic, 2000). For these reasons, some researchers used O2 electrodes to measure CO2-dependent rates of O2 evolution under super-saturating [CO2]. In some studies, the use of these high [CO2] overcame part of the inhibitory effects of water stress on O2 evolution rates (Saccardy et al., 1996; Marques da Silva and Arrabaça, 2004a), but not in others (Ghannoum et al., 2003). Super-saturating [CO2] was reported to restore O2 evolution rates to control values in only one instance, using slowly dehydrated maize leaves (Saccardy et al., 1996). A firmer conclusion regarding this line of evidence awaits further studies using more diverse C4 species under well-defined conditions.
Photorespiration in C4 plants subjected to water stress
In C3 plants, low Ci causes a decrease in A and an increase in the rate of photorespiration due to a decreased [CO2]:[O2] ratio at the sites of Rubisco. Increased photorespiration (e.g. due to reduced g under drought) causes an increase in the electron cost of CO2 fixation (J/A, the ratio of electron transport to CO2 assimilation rates), and indicates that A is CO2-limited. In C4 plants, the relationship between Ci, photorespiration and J/A is more complex (Fig. 4A and B). Photorespiration in C4 leaves remains very low under a range of environmental and genetic conditions, and runs at about 3·5–6 % of A (Lacuesta et al., 1997; Carmo-Silva et al., 2008). On the one hand, photorespiration may increase – from a very low base – with decreasing Ci without any measurable impact on A (Fig. 4A). This is because photorespired CO2 is most likely refixed within the bundle sheath before escaping to the atmosphere. The modelling results are supported by work on the oxygen sensitivity of C4 photosynthesis. In an early study using maize subjected to osmotic stress, Lawlor and Fock (1978) found that A changed little in response to increasing [O2] from 1·5 % to 21 %. The decline of A with Ψleaf was almost indistinguishable between 1·5 % and 21 % [O2] (Lawlor and Fock, 1978). In a recent study using three C4 grasses subjected to mild and severe water stress, Carmo-Silva et al. (2008) observed no changes in A with increasing [O2] above an optimum of 10 %, and estimated photorespiration rates were small under all water stress conditions (Carmo-Silva et al., 2008). On the other hand, if water stress were to reduce Rubisco activity independently of Ci, then both the carboxylation and oxygenation reactions of Rubisco would decrease in equal proportions. Accordingly, photorespiration is predicted to decrease rather increase under water stress (Fig. 3). This is in line with findings by Carmo-Silva et al. (2008). They found that photorespiration increased slightly between well-watered and moderate water-stress conditions, then decreased under severe water stress in two C4 grasses (Carmo-Silva et al., 2008). Consequently, while a small CO2-limitation may occur in the early phases of water stress, severe water stress tends to inhibit both photosynthesis and photorespiration in C4 plants (Fig. 3).
In contrast to the aforementioned works, Lal and Edwards (1996) reported increased J/A in maize and amaranthus exposed to water stress, and concluded that A was CO2-limited in these two C4 species under water stress (Lal and Edwards, 1996). In this study, the ambient [CO2] used for the low [CO2] comparison (fig. 4 in Lal and Edwards, 1996) was much lower than the Ci observed in the water-stressed leaves (fig. 1 in Lal and Edwards, 1996). Hence, the two situations, water stress and low Ci, were not comparable in their study. In the modelling example presented in Fig. 4, J/A showed a biphasic response to Ci (Fig. 4B). Below a Ci of ∼20 µbar, J/A increases with decreasing Ci due to increasing photorespiration (Fig. 4A and B). This is comparable to the low [CO2] and moderate water stress treatments in Lal and Edwards (1996) and Carmo-Silva et al. (2008), respectively. Above a Ci of ∼20 µbar, J/A increases with increasing Ci, which may be due to increased leakiness (Φ, the fraction of CO2 fixed by PEPC which leaks out of the bundle sheath). It should be noted that, although leakiness is predicted to increase with Ci (Fig. 4B), this was not confirmed experimentally (Henderson et al., 1992). Nevertheless, there is some evidence in the literature suggesting that leakiness increases under water stress (Bowman et al., 1989; Saliendra et al., 1996; Williams et al., 2001). Increased J/A as a result of increased leakiness could explain the water stress results of Lal and Edwards (1996). Conclusive testing of this proposition requires the use of sophisticated techniques such as on-line measurement of carbon and oxygen isotopes discrimination by mass spectrometry or tube diode laser.
Recovery of photosynthetic rates following re-hydration of C4 plants subjected to water stress
In addition to the aforementioned arguments, there remains one related to the recovery of A following re-hydration. Some studies reported that when plants which have been deprived of water for 3–10 d were re-hydrated, photosynthetic rates, measured in normal air, returned to near control values (i.e. well-watered plants) relatively quickly (Lal and Edwards, 1996; Saccardy et al., 1996; Foyer et al., 1998). This has been interpreted as proof that the photosynthetic capacity remains intact under water stress. However, most of these studies measured the recovery of photosynthetic rates using the C4 crop maize, which has been exposed to relatively mild stress such as withholding watering for several days (Lal and Edwards, 1996; Saccardy et al., 1996; Foyer et al., 1998). In a study using sorghum, recovery of A was only partial in response to re-hydration (Loreto et al., 1995). Hence, it is important to undertake these measurements using C4 species other than maize, exposed to different degrees of water stress. In these future studies, it is also important to distinguish whether the recovery of A occurs at the level of the same stressed leaf or the plant.
CONTRIBUTION OF NON-STOMATAL FACTORS TO THE INHIBITION OF C4 PHOTOSYNTHESIS UNDER WATER STRESS
As for stomatal factors, arguments related to non-stomatal inhibition of A are very similar to those advanced for C3 photosynthesis subjected to water stress (Lawlor, 2002). They include reduced activity of photosynthetic enzymes, decreased ATP concentration, inhibition of nitrate assimilation, induction of early senescence, and changes to the leaf anatomy and ultrastructure amongst others (Fig. 3 and Table 1). These metabolic factors have been reviewed recently by Lawlor (2002), Flexas and Medrano (2002) and Flexas et al. (2004). Therefore, in this review, my discussion is limited to evidence from the C4 literature for the operation of such factors under water stress. In particular, I focus on the main point of difference with C3 photosynthesis, which is the differential impact of water stress on the activity of C3 and C4 cycle enzymes.
Table 1.
A literature survey of the effects of water stress on the activity of selected enzymes in a number of C4 species
| Effect |
||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Assay basis | Stress indicator | Rubisco | PEPC | NADP-ME | PPDK | NR | Reference |
| Zea mays | Leaf area | Ψleaf = –1·17 MPa | ↓ by 20 % | ↓ by 30 % | ↓ by 33 % | ↓ by 98 % | Becker and Fock (1986) | |
| Chlorophyll | Ψleaf = –1·2 MPa | ↑ slightly | ↓ by 90 % | Foyer et al. (1998) | ||||
| Leaf area | RWC = 50 % | ↓ by 75 % | Lal and Edwards (1996) | |||||
| Leaf area | RWC = 50 % | ↔ | ↔ | Saccardy et al. (1996) | ||||
| Saccharum sp. | Leaf area | Ψleaf = –1·61 MPa | ↓ by 50 % | ↓ by 55 % | ↓ by 73 % | ↓ by 89 % | Du et al. (1996) | |
| Dry weight | Ψleaf = –1·36 MPa | ↔ | ↑ slightly | Saliendra et al. (1996) | ||||
| Amaranthus cruentus | Leaf area | RWC = 50 % | ↓ by 75 % | Lal and Edwards (1996) | ||||
| Setaria sphacelata | Total protein | RWC = 40 % | ↓ by 30 % | Marques da Silva and Arrabaça (2004a) | ||||
| Paspalum dilatatum | Dry weight | RWC = 50 % | ↔ | ↔ | Carmo-Silva et al. (2007) | |||
| Cynodon dactylon | RWC = 80 % | ↓ by 18 % | ↔ | |||||
| Zoysia japonica | RWC = 80 % | ↓ by 18 % | ↓ by 50 % | |||||
↑, increase; ↓, decrease; ↔, no significant change.
Impact of water stress on the activity of C3 and C4 cycle enzymes
A number of studies have reported significant changes in the activity of photosynthetic enzymes in C4 plants subjected to water stress (Table 1). For Rubisco, most studies reported decreased activity under water stress, while a couple of studies found no change (Table 1). In contrast, the response of the key C4 cycle enzymes appears to be less consistent, with some studies reporting a decrease in activity, while others report no change or even increased activity under water stress (Table 1). This makes it difficult to draw firm conclusions about the role of these enzymes in water stress-induced photosynthetic inhibition in C4 plants. This is further complicated by the fact that the literature offers only patchy data on a limited number of C4 species. Nevertheless, a number of observations can be made regarding this aspect. In particular, there seems to be a more consistent inhibition of the activity of C3 (e.g. Rubisco) than C4 (e.g. PEPC) cycle enzymes in response to water stress (Table 1). In other words, the available, albeit limited, data suggest that water stress may lead to a decrease in the activity ratio of C3/C4 cycle enzymes in C4 plants. This argument is supported by studies which reported increased leakiness in water-stressed C4 plants (Bowman et al., 1989; Saliendra et al., 1996). Increased leakiness may be caused by a number of factors, one of which is reduced activity of C3, relative to C4, cycle enzymes (von Caemmerer and Furbank, 1999). In particular, if the carboxylation activity decreases more than the decarboxylation activity, CO2 consumption will fall in the bundle sheath, leading to an increase in [CO2]BS. A greater [CO2]BS leads to a greater [CO2] gradient across the bundle sheath cell walls, and hence a greater leakage of CO2. In the study by Saliendra et al. (1996), increased leakiness was related to a decrease in Rubisco/PEPC activity ratio as a result of no change in Rubisco activity and a slight increase in PEPC activity. Bowman et al. (1989) concluded that a decrease in the C3/C4 activity ratio was the likely factor behind increased leakiness based on two main reasons. First, the changes in leakiness in response to water stress underwent diurnal fluctuations. This indicated that increased leakiness was caused by biochemical rather than anatomical factors (e.g. changes in the properties of bundle sheath cell wall and membranes). Secondly, there was a linear relationship between changes in leakiness and photosynthetic inhibition in response to water stress. This indicates that activities of C3 cycle enzymes are more sensitive to water stress, assuming that this cycle is limiting C4 photosynthesis (Bowman et al., 1989). The differential response of C3 and C4 cycle enzymes to water stress and their eventual impacts on leakiness in C4 plants is an important aspect which awaits further work.
Other non-stomatal factors
For C4 plants, there is good evidence indicating that nitrate assimilation and nitrate uptake are strongly reduced under water stress (Table 1; Becker and Fock, 1986; Foyer et al., 1998). This may explain the reported decreases in chlorophyll and protein content in a number of C4 species subjected to water stress (Du et al., 1996; Foyer et al., 1998; Marques da Silva and Arrabaça, 2004b; Carmo-Silva et al., 2007). The decrease in chlorophyll and protein contents under water stress may also be due to generalized protein degradation as a result of induced senescence as suggested by increased contents of amino acids (Becker and Fock, 1986). The induction of senescence under water stress – its timing and the factors which trigger it – is poorly understood.
Using light microscopy, Lal and Edwards (1996) observed ultra-structural distortions (e.g. changes in chloroplast position, distortion of intercellular spaces) in leaves of C4 species under water stress. Such changes may have significant impacts on CO2 diffusion inside the leaf as well as light penetration (Flexas et al., 2004). However, due to the lack of data, it is not possible to make much of this aspect at this stage. It is hoped that with the proliferation of more sophisticated microscopic and imaging techniques, especially those which allow the observation of live tissue, there will be more studies published on the effects of water stress on the anatomy of C3 and C4 leaves alike.
THE ROLE OF ALTERNATIVE ELECTRON SINKS IN C4 PHOTOSYNTHESIS EXPOSED TO WATER STRESS
Photorespiration results in the release of CO2 and NH3 into the atmosphere, and the consumption of ATP and other reducing equivalents. Consequently, photorespiration may act as an alternative electron sink in C3 plants exposed to water stress. By doing so, photorespiration can reduce the over-reduction of the photosynthetic electron transport chain (Osmond and Grace, 1995), and allow photosynthesis to recover more quickly after the removal of water stress. For C3 plants, there is some evidence showing that photorespiratory electron transport increases under mild to moderate water stress, thus maintaining electron flow (e.g. Cornic and Fresneau, 2002; Haupt-Herting and Fock, 2002). Such data is lacking for C4 plants. However, and as discussed earlier, photorespiration remains very low in C4 plants under a wide range of physiological conditions. Therefore, unlike the case for C3 photosynthesis, the scope for photorespiration acting as a protective electron sink is minimal during C4 photosynthesis exposed to water stress.
Another photosynthetic, alternative electron sink is the Mehler reaction, which involves the direct reduction of molecular O2 to superoxide radicals at photosystem I. Most studies involving C3 plants exposed to moderate water stress indicate that the contribution of the Mehler reaction to total photosynthetic electron flow decreases or remains unchanged (Cornic and Fresneau, 2002; Haupt-Herting and Fock, 2002). For example, in tomato, the percentage of photosynthetic electrons dissipated by the Mehler reaction decreased from 13 % to 6 % in control and water-stressed leaves, respectively, while the contribution of photorespiration increased from 23 % to 40 % under water stress (Haupt-Herting and Fock, 2002). Direct measurements of O2 exchange in leaves of well-watered C4 grasses showed that O2 uptake in the light depends on [CO2] and light intensity (Siebke et al., 2003). It was estimated that O2 uptake associated with the Mehler reaction represents about 18 % of total light-dependent O2 uptake in C4 leaves (Siebke et al., 2003). This is slightly greater than the rate of Mehler reaction measured in control C3 leaves, indicating that the Mehler reaction has a slightly greater capacity in C4 than C3 leaves. However, it is likely that the Mehler reaction is insensitive – or even slightly suppressed – by water stress in C4 as in C3 leaves. Although there are no comprehensive measurements of O2 exchange in C4 plants exposed to water stress, a couple of indirect lines of evidence support this conclusion. First, in a study where maize was exposed to mild drought, it was observed that the activities of ascorbate peroxidase and glutathione reductase – enzymes involved in hydrogen peroxide detoxification – were unaffected by drought (Brown et al., 1995). Secondly, in a review, Badger et al. (2000) argued that in higher plants excess light dissipation occurs mainly via non-radiative energy dissipation. Excess electron dissipation by Mehler O2 uptake is significant mainly in photosynthetic organisms lacking well-developed non-photochemical quenching mechanisms, such as cyanobacteria (Badger et al., 2000). The limited capacity of the Mehler reaction to act as a significant electron sink has been demonstrated by a study using tobacco with a genetically altered amount of Rubisco. Reducing the capacity for both photosynthesis and photorespiration in the transgenic, relative to the wild-type, plants did not lead to enhanced electron transport to free O2 (Ruuska et al., 2000). Consequently, the limited capacity for the Mehler reaction or photorespiration to act as significant alternative electron sinks may account for the strong correlation between CO2 assimilation and electron transport rates observed in C4 leaves under a wide range of environmental conditions (e.g. Oberhuber and Edwards, 1993).
INTERACTIVE EFFECTS OF ELEVATED [CO2] AND WATER STRESS ON C4 PHOTOSYNTHESIS
Due to unprecedented rates of fossil fuel burning and deforestation since the start of the industrial revolution, atmospheric [CO2] has been rising rapidly (IPCC, 2007). Understanding the effects of rising [CO2] on C4 plants is crucial given their significant contribution to the global carbon budget and food security. C4 plants were not expected to respond to high [CO2] because C4 photosynthesis is mostly CO2-saturated under current atmospheric [CO2] due to the operation of the CO2-concentrating mechanism. However, as more research was done on this topic, it became increasingly evident that C4 plants can accumulate more biomass at elevated [CO2], particularly if exposed to some form of water stress during growth. These findings were made consistently in controlled-environment and field studies alike (e.g. Samarakoon and Gifford, 1996; Seneweera et al., 1998, 2001; Wand et al., 1999; Wall et al., 2001; LeCain et al., 2003; Leakey et al., 2004, 2006). Ghannoum et al. (2000, 2006) reviewed the mechanism underlying the response of C4 plants to high [CO2]. They concluded that elevated [CO2] enhances biomass production in C4 plants predominantly via the indirect effects on stomatal conductance. By reducing leaf and hence canopy transpiration, high [CO2] leads to soil water conservation (Samarakoon and Gifford, 1996; Seneweera et al., 1998, 2001; Wall et al., 2001; LeCain et al., 2003; Leakey et al., 2006). In general, evidence from the literature about CO2 enrichment argues against a substantial role for stomatal limitation in the observed decline of C4 photosynthesis under water stress. In particular, high [CO2] does not directly alleviate the adverse effects of water stress on C4 photosynthesis (Ghannoum et al., 2003). The latter conclusion was supported by results from free air [CO2] enrichment (FACE) studies with the C4 crops sorghum and maize (Wall et al., 2001; Leakey et al., 2004, 2006) and open-top chamber experiments with the C4 grass Bouteloua gracili (LeCain et al., 2003). These experiments tested the interaction between elevated [CO2] and water stress on C4 photosynthesis in the field and, in the case of FACE, under natural growing conditions. A key advantage of these studies is that plants experience water stress at rates and severities normally experienced by field-grown plants, thus avoiding the need to get into discussions of whether drought occurred in a ‘realistic’ fashion in pots (or detached leaves). The other main advantage is that field studies allow for the impacts of soil feedbacks to be assessed. It is worth noting that some pot studies have attempted to measure changes in soil moisture with C4 plants, and reported similar results (e.g. Samarakoon and Gifford, 1996; Seneweera et al., 1998, 2001).
The FACE study undertaken in the North American Corn Belt with maize is particularly illuminating for the following main reasons: plants were grown in the field under rain-fed conditions; plants experienced both wet and dry seasons; and comprehensive diurnal and seasonal gas exchange and fluorescence analyses were carried out under growth conditions (Leakey et al., 2004, 2006). When the crop experienced a wet year due to above-average rainfall, A and all other measured photosynthetic parameters were not stimulated by high [CO2] at any stage of the day or season (Leakey et al., 2004). The failure of high [CO2] to affect A during the course of the day is particularly interesting because it reveals that the diurnal drifts in A – particularly those brought about by fluctuations in leaf-to-air vapour pressure deficit – in this C4 crop are not primarily stomatal in nature. Importantly, during the dry year, A was stimulated by elevated [CO2] only intermittently during the course of the season. This stimulation was associated with improved soil water content as a result of the consistent reductions in g at high [CO2] in maize (Leakey et al., 2004). In an open top chamber study using a C4 grass, Wall et al. (2001) reported a similar pattern of responses. Consequently, these studies indicate that when C4 plants experience drought in their natural environment, elevated [CO2] alleviates the effect of water stress almost entirely via the indirect effect of reduced stomatal conductance and subsequent improved soil moisture.
CONCLUSIONS
It is well-established that the physiological advantages, conferred by the higher photosynthetic efficiency of C4, relative to C3, photosynthesis under high light and temperature, are crucial for the ecological dominance of C4 plants in open, hot and arid environments (Osmond et al., 1982; Long, 1999). In particular, the presence of a CO2-concentrating mechanism in C4 leaves endows them with higher WUE than their C3 counterparts when compared under standard conditions (Osmond et al., 1982; Long, 1999). However, it remains questionable whether the higher WUE of C4, compared with C3, plants leads to greater tolerance to water stress. In this review, I argue that, although the C4 CO2-concentrating mechanism offers C4 photosynthesis a greater buffering capacity against CO2 shortages brought about by partial stomatal closure under water stress, the biochemistry of C4 photosynthesis is as – or even more – sensitive than that of C3 photosynthesis. The reasons are not clear. However, a greater sensitivity of the C3, relative to the C4, cycle emerges as a probable site of metabolic limitation under water stress.
ACKNOWLEDGEMENTS
I thank David Lawlor (Rothamsted Research, UK) for tirelessly encouraging me to write this review; and Susanne von Caemmerer (Australian National University, Australia) for assisting me in using her model of C4 photosynthesis.
LITERATURE CITED
- Andrews TJ, Lorimer GH. Rubisco: structure, mechanisms, and prospects for improvement. In: Hatch MD, Boardman NK, editors. The biochemistry of plants: a comprehensive treatise. New York, NY: Academic Press; 1987. pp. 131–218. Vol. 10. Photosynthesis. [Google Scholar]
- Archer S. Climate change and grasslands: a life-zone and biota perspective. In: Baker MJ, editor. Grasslands for our world. Wellington: SIR Publishing; 1993. pp. 396–402. [Google Scholar]
- Badger MR, von Caemmerer S, Ruuska S, Nakano H. Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and Rubisco oxygenation. Philosophical Transaction of the Royal Society of London, B. 2000;355:1433–1446. doi: 10.1098/rstb.2000.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TW, Fock HP. Effects of water stress on the gas exchange, the activities of some enzymes of carbon and nitrogen metabolism, and on the pool sizes of some organic acids in maize leaves. Photosynthesis Research. 1986;8:175–181. doi: 10.1007/BF00035247. [DOI] [PubMed] [Google Scholar]
- Bowman WD, Hubick KT, von Caemmerer S, Farquhar GD. Short-term changes in leaf carbon isotope discrimination in salt- and water-stressed C4 grasses. Plant Physiology. 1989;90:162–166. doi: 10.1104/pp.90.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH. Agronomic implications of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego, CA: Academic Press; 1999. pp. 473–507. [Google Scholar]
- Brown RH, Byrd GT. Estimation of bundle sheath cell conductance in C4 species and O2 insensitivity of photosynthesis. Plant Physiology. 1993;103:1183–1188. doi: 10.1104/pp.103.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PS, Knievel DP, Pell EJ. Effects of moderate drought on ascorbate peroxidase and glutathione reductase activities in mesophyll and bundle sheath cells of maize. Physiologia Plantarum. 1995;95:274–280. [Google Scholar]
- von Caemmerer S. Biochemical models of leaf photosynthesis. Melbourne: CSIRO Publishing; 2000. [Google Scholar]
- von Caemmerer S, Furbank RT. The modelling of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego, CA: Academic Press; 1999. pp. 173–211. [Google Scholar]
- Carmo-Silva AE, Soares AS, Marques da Silva J, Bernardes da Silva A, Keys AJ, Arrabaça MC. Photosynthetic responses of three C4 grasses of metabolic subtypes to water deficit. Functional Plant Biology. 2007;34:204–213. doi: 10.1071/FP06278. [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Powers SJ, Keys AJ, Arrabaça MC, Parry MAJ. Photorespiration in C4 grasses remains slow under drought conditions. Plant Cell and Environment. 2008 doi: 10.1111/j.1365-3040.2008.01805.x. doi: 10·1111/j.1365–3040·2008·01805.x. [DOI] [PubMed] [Google Scholar]
- Cerling TE. Paleorecords of C4 plants and ecosystems. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego, CA: Academic Press; 1999. pp. 473–507. [Google Scholar]
- Cornic G. Drought stress inhibits photosynthesis by decreasing stomatal aperture – not affecting ATP synthesis. Trends in Plant Science. 2000;5:187–188. [Google Scholar]
- Cornic G, Fresneau C. Photosynthetic carbon reduction and oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Annals of Botany. 2002;89:887–894. doi: 10.1093/aob/mcf064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornic G, Ghashghaie J, Genty B, Briantais JM. Leaf photosynthesis is resistant to a mild drought stress. Photosynthetica. 1992;27:295–309. [Google Scholar]
- Crimp SJ, Flood NR, Carter JO, Conroy JP, McKeon GM. Evaluation of the potential impacts of climate change on native pasture production – implications for livestock carrying capacity. 2002 Report to the Australian Greenhouse Office. [Google Scholar]
- Du YC, Kawamitsu Y, Nose A, Hiyane S, Murayama S, Muraya S, Wasano K, Uchida Y. Effects of water stress on carbon exchange rate and activities of photosynthetic enzyme in leaves of sugarcane (Saccharum sp.) Functional Plant Biology. 1996;23:719–726. [Google Scholar]
- Edwards G, Walker D, editors. C3, C4: mechanisms, and cellular and environmental regulation, of photosynthesis. Oxford: Blackwell Scientific Publications; 1983. [Google Scholar]
- Ehleringer JR, Sage RF, Flanagan LB, Pearcy RW. Climate change and the evolution of C4 photosynthesis. Trends in Ecology & Evolution. 1991;6:95–99. doi: 10.1016/0169-5347(91)90183-X. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. C4 photosynthesis, atmospheric CO2 and climate. Oecologia. 1997;112:285–299. doi: 10.1007/s004420050311. [DOI] [PubMed] [Google Scholar]
- Ellis RP, Vogel JC, Fuls A. Photosynthetic pathways and the geographic distribution of grasses in South Africa/Namibia. South African Journal of Science. 1980;76:307–314. [Google Scholar]
- Flexas J, Medrano H. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Annals of Botany. 2002;89:183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology. 2004;5:1–11. doi: 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Valadier M-H, Migge A, Becker TW. Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiology. 1998;117:283–292. doi: 10.1104/pp.117.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Conroy JP. Nitrogen deficiency precludes a growth response to CO2 enrichment in C3 and C4 Panicum grasses. Functional Plant Biology. 1998;25:627–636. [Google Scholar]
- Ghannoum O, von Caemmerer S, Barlow EWR, Conroy JP. The effect of CO2 enrichment and irradiance on the growth, morphology and gas exchange of a C3 (Panicum laxum) and a C4 (Panicum antidotale) grass. Functional Plant Biology. 1997;24:227–237. [Google Scholar]
- Ghannoum O, von Caemmerer S, Ziska LH, Conroy JP. The response of C4 plants to elevated CO2 partial pressure: a reassessment. Plant, Cell and Environment. 2000;23:931–942. [Google Scholar]
- Ghannoum O, von Caemmerer S, Conroy JP. The effect of drought on plant water use efficiency of 9 NAD-ME and 9 NADP-ME C4 grasses. Functional Plant Biology. 2002;29:1337–1348. doi: 10.1071/FP02056. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Conroy JP, Driscoll SP, Paul MJ, Foyer CH, Lawlor DW. Non-stomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytologist. 2003;159:835–844. doi: 10.1046/j.1469-8137.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Searson MJ, Conroy JP. Nutrient and water demands of plants under climate change. In: Newton PCD, Edwards G, Carran A, Niklaus P, editors. Agroecosystems in a changing climate. Vol. 12. Boca Raton, FL: CRC Press; 2006. pp. 55–85. Advances in Agroecology Series. [Google Scholar]
- Hatch MD. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta. 1987;895:81–106. [Google Scholar]
- Hattersley PW. C4 photosynthetic pathway variation in grasses (Poaceae): its significance for arid and semi-arid lands. In: Chapman GP, editor. Desertified grasslands: their biology and management. London: Academic Press; 1992. pp. 181–212. [Google Scholar]
- Haupt-Herting S, Fock HP. Oxygen exchange in relation to carbon assimilation in water-stressed leaves during photosynthesis. Annals of Botany. 2002;89:851–859. doi: 10.1093/aob/mcf023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SA, von Caemmerer S, Farquhar GD. Short-term measurements of carbon isotope discrimination in several C4 species. Functional Plant Biology. 1992;19:263–285. [Google Scholar]
- Henderson S, Hattersley PW, von Caemmerer S, Osmond CB. Are C4 pathway plants threatened by global climate change. In: Schultz E-D, Caldwell M, editors. Ecophysiology of photosynthesis. Vol. 100. New York, NY: Springer-Verlag; 1994. pp. 529–549. Ecological Studies. [Google Scholar]
- IPCC. Technical Summary. In: Solomon S, Qin D, Manning M, et al., editors. Climate change 2007: the physical science basis. Cambridge: Cambridge University Press; 2007. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- Jordan DB, Ogren WL. Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature. 1981;291:513–515. [Google Scholar]
- Jordan DB, Ogren WL. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase oxygenase – dependence on ribulosebisphosphate concentration, pH and temperature. Planta. 1984;161:308–313. doi: 10.1007/BF00398720. [DOI] [PubMed] [Google Scholar]
- Kalapos T, van den Boogaard R, Lambers H. Effect of soil drying on growth, biomass allocation and leaf gas exchange of two annual grass species. Plant and Soil. 1996;185:137–149. [Google Scholar]
- Kellogg EA. Phylogenetic aspects of the evolution of C4 photosynthesis. In: Sage RF, Monson RK, editors. The biology of C4 plants. San Diego, CA: Academic Press; 1999. pp. 411–444. [Google Scholar]
- Lacuesta M, Dever LV, Miñoz-Rueda A, Lea PJ. A study of photorespiratory ammonia production in the C4 plant Amaranthus edulis, using mutants with altered photosynthetic capacities. Physiologia Plantarum. 1997;99:447–455. [Google Scholar]
- Lal A, Edwards GE. Analysis of inhibition of photosynthesis under water stress in the C4 species Amaranthus cruentus and Zea mays: electron transport, CO2 fixation and carboxylation capacity. Functional Plant Biology. 1996;23:403–412. [Google Scholar]
- Lawlor DW. The effects of water deficit on photosynthesis. In: Smirnoff N, editor. Environment and plant metabolism: flexibility and acclimation. Oxford: BIOS Scientific Publishers; 1995. pp. 129–160. [Google Scholar]
- Lawlor DW. Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. Annals of Botany. 2002;89:871–885. doi: 10.1093/aob/mcf110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell and Environment. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Fock H. Photosynthesis, respiration, and carbon assimilation in water-stressed maize at two oxygen concentrations. Journal of Experimental Botany. 1978;29:579–593. [Google Scholar]
- Leakey ADB, Bernacchi CJ, Dohleman FG, Ort DR, Long SP. Will photosynthesis of maize (Zea mays) in the US Corn Belt increase in future [CO2] rich atmosphere? An analysis of diurnal courses of CO2 uptake under free-air concentration enrichment. Global Change Biology. 2004;10:951–962. [Google Scholar]
- Leakey ADB, Uribelarrea M, Ainsworth EA, Naidu SL, Rogers A, Ort DR, Long SP. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiology. 2006;140:779–790. doi: 10.1104/pp.105.073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCain DR, Morgan JA, Mosier AR, Nelson JA. Soil and plant water relations determine photosynthetic responses of C3 and C4 grasses in a semi-arid ecosystem under elevated CO2. Annals of Botany. 2003;92:41–52. doi: 10.1093/aob/mcg109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. Environmental responses. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego, CA: Academic Press; 1999. pp. 215–249. [Google Scholar]
- Loreto F, Tricoli D, Di Marco G. On the relationship between electron transport rate and photosynthesis in leaves of the C4 plant Sorghum bicolor exposed to water stress, temperature changes and carbon metabolism inhibition. Functional Plant Biology. 1995;22:885–892. [Google Scholar]
- Lloyd J, Farquhar GD. 13C discrimination during CO2 assimilation by the terrestrial biosphere. Oecologia. 1994;99:201–215. doi: 10.1007/BF00627732. [DOI] [PubMed] [Google Scholar]
- Maroco JP, Pereira JS, Chaves MM. Growth, photosynthesis and water-use efficiency of two C4 Sahelian grasses subjected to water deficits. Journal of Arid Environments. 2000;45:119–137. [Google Scholar]
- Marques da Silva J, Arrabaça MC. Photosynthesis in the water-stressed C4 grass Setaria sphacelata is mainly limited by stomata with both rapidly and slowly imposed water deficits. Physiologia Plantarum. 2004;a 121:409–420. [Google Scholar]
- Marques da Silva J, Arrabaça MC. Photosynthetic enzymes of the C4 grass Setaria sphacelata under water stress: a comparison between rapidly and slowly imposed water deficit. Photosynthetica. 2004;b 42:43–47. doi: 10.1078/0176-1617-01109. [DOI] [PubMed] [Google Scholar]
- Oberhuber W, Edwards GE. Temperature dependence of the linkage of quantum yield of photosystem II to CO2 fixation in C4 and C3 plants. Plant Physiology. 1993;101:507–512. doi: 10.1104/pp.101.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-López A, Ort DR, Boyer JS. Photophosphorylation in attached leaves of Helianthus annuus at low water potentials. Plant Physiology. 1991;96:1018–1025. doi: 10.1104/pp.96.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB, Grace SC. Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis. Journal of Experimental Botany. 1995;46:1351–1362. [Google Scholar]
- Osmond CB, Winter K, Ziegler H. Functional significance of different pathways of CO2 fixation in photosynthesis. In: Lange OL, Noble PS, Osmond CB, Ziegler H, editors. Encyclopedia of plant physiology, New Series. Vol. 12B. Berlin: Springer Verlag; 1982. pp. 479–547. [Google Scholar]
- Pingali PI. Meeting the World Maize Needs: Technological Opportunities and Priorities for the Public Sector. CIMMYT: Mexico City; 2001. CIMMYT 1999–2000 facts and trends. [Google Scholar]
- Ripley BS, Gilbert ME, Ibrahim DG, Osborne CP. Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. Journal of Experimental Botany. 2007;58:1351–1363. doi: 10.1093/jxb/erl302. [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Badger MR, Andrews TJ, von Caemmerer S. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. Journal of Experimental Botany. 2000;51:357–368. doi: 10.1093/jexbot/51.suppl_1.357. [DOI] [PubMed] [Google Scholar]
- Saccardy K, Cornic G, Brulfert J, Reyss A. Effect of drought stress on net CO2 uptake in Zea leaves. Planta. 1996;199:589–595. [Google Scholar]
- Sage RF. C4 plants. Encyclopedia of Biodiversity. 2001;1:575–598. [Google Scholar]
- Sage RF. The evolution of C4 photosynthesis. New Phytologist. 2004;161:341–370. doi: 10.1111/j.1469-8137.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- Sage RF, Kubien DS. The temperature response of C3 and C4 photosynthesis. Plant, Cell and Environment. 2007;30:1086–1106. doi: 10.1111/j.1365-3040.2007.01682.x. [DOI] [PubMed] [Google Scholar]
- Sage RF, Li M, Monson R. The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego, CA: Academic Press; 1999. pp. 551–584. [Google Scholar]
- Saliendra NZ, Meinzer FC, Perry M, Thom M. Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. Journal of Experimental Botany. 1996;47:907–914. [Google Scholar]
- Samarakoon AB, Gifford RM. Elevated CO2 effects on water use and growth of maize in wet and drying soil. Australian Journal of Plant Physiology. 1996;23:53–62. [Google Scholar]
- Seneweera SP, Ghannoum O, Conroy JP. High vapour pressure deficit and low soil water availability enhance shoot growth responses of a C4 grass (Panicum coloratum cv. Bambatsi) to CO2 enrichment. Functional Plant Biology. 1998;25:287–292. [Google Scholar]
- Seneweera SP, Ghannoum O, Conroy JP. Root and shoot factors contribute to the effect of drought on photosynthesis and growth of the C4 grass Panicum coloratum at elevated CO2 partial pressure. Functional Plant Biology. 2001;28:451–460. [Google Scholar]
- Sharkey TD, Seemann JR. Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiology. 1988;89:1060–1065. doi: 10.1104/pp.89.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebke K, Ghannoum O, Conroy JP, Badger MR, von Caemmerer S. Photosynthetic oxygen exchange in C4 grasses: the role of oxygen as electron acceptor. Plant, Cell and Environment. 2003;26:1963–1972. [Google Scholar]
- Taub DR. Climate and the US distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. American Journal of Botany. 2000;87:1211–1215. [PubMed] [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature. 1999;401:914–917. [Google Scholar]
- Tipple BJ, Pagani M. The early origins of terrestrial C4 photosynthesis. Annual Review of Earth and Planetary Sciences. 2007;35:435–461. [Google Scholar]
- Wall GW, Brooks TJ, Adam NR, Cousins A, Kimball BA, Pinter PJ, Jr, et al. Elevated atmospheric CO2 improved Sorghum plant water status by ameliorating the adverse effects of drought. New Phytologist. 2001;152:231–248. [Google Scholar]
- Wand SJE, Midgley GF, Jones MH, Curtis PS. Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a test of current theories and perceptions. Global Change Biology. 1999;5:723–741. [Google Scholar]
- Ward JK, Tissue DT, Thomas RB, Strain BR. Comparative responses of model C3 and C4 plants to drought in low and elevated CO2. Global Change Biology. 1999;5:857–867. [Google Scholar]
- Williams DG, Gempko V, Fravolini A, Leavitt SW, Wall GW, Kimball BA, et al. Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought. New Phytologist. 2001;150:285–293. [Google Scholar]