Abstract
Background
Herbivory reduces leaf area, disrupts the function of leaves, and ultimately alters yield and productivity. Herbivore damage to foliage typically is assessed in the field by measuring the amount of leaf tissue removed and disrupted. This approach assumes the remaining tissues are unaltered, and plant photosynthesis and water balance function normally. However, recent application of thermal and fluorescent imaging technologies revealed that alterations to photosynthesis and transpiration propagate into remaining undamaged leaf tissue.
Scope and Conclusions
This review briefly examines the indirect effects of herbivory on photosynthesis, measured by gas exchange or chlorophyll fluorescence, and identifies four mechanisms contributing to the indirect suppression of photosynthesis in remaining leaf tissues: severed vasculature, altered sink demand, defence-induced autotoxicity, and defence-induced down-regulation of photosynthesis. We review the chlorophyll fluorescence and thermal imaging techniques used to gather layers of spatial data and discuss methods for compiling these layers to achieve greater insight into mechanisms contributing to the indirect suppression of photosynthesis. We also elaborate on a few herbivore-induced gene-regulating mechanisms which modulate photosynthesis and discuss the difficult nature of measuring spatial heterogeneity when combining fluorescence imaging and gas exchange technology. Although few studies have characterized herbivore-induced indirect effects on photosynthesis at the leaf level, an emerging literature suggests that the loss of photosynthetic capacity following herbivory may be greater than direct loss of photosynthetic tissues. Depending on the damage guild, ignoring the indirect suppression of photosynthesis by arthropods and other organisms may lead to an underestimate of their physiological and ecological impacts.
Key words: Chlorophyll fluorescence imaging, thermography, plant–insect interactions, spatial patterns, autotoxicity, induced defences, jasmonates
INTRODUCTION
Insects consume vast quantities of plant biomass each year, but simply considering the amount of tissue removed may underestimate their impact on yield and ecosystem production. On average, herbivores remove approx. 15 % of primary production in terrestrial ecosystems, but complete removal is not uncommon in out-break years (Cyre and Pace, 1993). Similarly, insects consume approx. 14 % of total global agricultural output (Oerke and Dehne, 1997). This value is relatively low because of the widespread application of pesticides. In the absence of pesticides, losses would exceed 50 % for all major crops (Oerke and Dehne, 1997). Herbivore damage is assessed in agricultural fields by surveying the amount of tissue removed from foliage. This approach, however, assumes that the remaining leaf tissue functions normally. Many types of insect damage affect photosynthesis in undamaged tissues, and these ‘indirect’ effects on photosynthesis may be considerably greater than the direct removal of leaf area (Welter, 1989; Zangerl et al., 2002).
Insect herbivory, whether defoliation or by feeding on specific tissues (e.g. phloem or xylem), triggers a complex and interacting array of molecular and physiological responses in plants. These responses potentially reduce the photosynthetic capacity in remaining leaf tissues to a greater extent than the direct removal of photosynthetic surface area. For example, the removal of only 5 % of the area of an individual wild parsnip leaf by caterpillars reduced photosynthesis by 20 % in the remaining foliage (Zangerl et al., 2002), and the decline in photosynthesis in the remaining leaf tissue of an oak sapling was equal to the decrease in photosynthesis associated with the actual removal of leaf tissue (Aldea et al., 2006b). The mechanisms reducing photosynthesis in remaining leaf tissues are multifaceted, ranging from disruptions in fluid or nutrient transport to self-inflicted reductions in metabolic processes. However, the magnitude of these effects on photosynthesis and the underlying mechanisms are highly variable, depending in large part on the type of feeding damage and the mode of defence deployed by the plant under attack.
In this review, we build upon previous evaluations of the effects of insect herbivory on photosynthesis (Welter, 1989; Peterson and Higley, 2001) by examining feeding-induced spatial heterogeneity in photosynthesis across individual leaves. The application of fluorescence imaging techniques (Rolfe and Scholes, 1995; Baker et al., 2001) is providing new insight into how different damage guilds, including pathogens and insects, affect the component processes of photosynthesis. When combined with other imaging methods such as thermography, the use of reporter genes to follow transcription, and fluorescent dyes that track signalling compounds (e.g. Ca2+ ions, H2O2), the mechanisms responsible for altering photosynthesis in remaining tissues are being elucidated. The use of geographic image analysis as a tool for making quantitative comparisons of images representing different biological processes is discussed, as this method provides the capability to compile many layers of covariate information to reveal new mechanistic insights.
INDIRECT VERSUS DIRECT EFFECTS OF HERBIVORY ON PHOTOSYNTHESIS
Plant responses to arthropod herbivory traditionally have been assessed from the guild perspective, where different insect guilds are defined by their feeding mechanisms (Welter, 1989; Peterson and Higley, 2001). These guilds (e.g. chewing damage, piercing damage, etc.) were established in an effort to recognize ‘homogeneity in physiological response’ between different attacking agents (arthropods) that alter plant physiological processes in a similar manner (Higley et al., 1993). Using this guild approach, Welter (1989) examined an extensive body of literature across multiple guilds and found over 50 % of all plant–insect interactions resulted in a loss of photosynthetic capacity. Defoliation generally increases photosynthesis, whereas specialized cell-content feeding decreases photosynthesis. Since then, several studies have examined plant responses to different insect feeding guilds and even to different insects within guilds in an effort to develop models for predicting plant response to different feeding mechanisms (see Peterson and Higley, 2001).
A review of the recent literature is not entirely consistent with the conclusions stated by Welter (1989). Feeding on specialized tissues typically reduces photosynthesis, regardless of whether the attacked component is the phloem or xylem (Haile et al., 1999; Macedo et al., 2003a, b; Heng-Moss et al., 2006), the stem (Macedo et al., 2005, 2007) or general leaf fluids (Haile and Higley, 2003). There is some evidence indicating that increased photosynthesis occurs in the presence of phloem feeding, particularly when the annual photosynthesis rate is estimated (Dungan et al., 2007). In contrast, defoliation injury often does not alter photosynthetic capacity, within plant families (e.g. legumes) or between hardwoods and crops (Peterson et al., 1992, 1996, 2004); however, there are examples where defoliation reduced (Delaney and Higley, 2006) or increased photosynthesis (Turnbull et al., 2007).
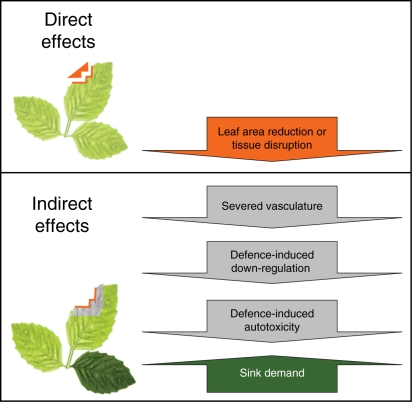
The removal of leaf tissue by herbivores represents a ‘direct’ reduction of photosynthetic capacity. The suppression of photosynthesis in remaining leaf tissue is defined by any one of a number of processes, including damage to the vasculature supplying that tissue, as an ‘indirect’ effect of herbivory. Arthropods damage xylem or phloem (Welter, 1989), which may alter water transport, stomatal aperture, and sucrose transport and loading, thereby reducing photosynthesis in remaining leaf tissue. Severing tissue vasculature alters leaf hydraulics, and, subsequently, nutrient or osmotica transport (Sack and Holbrook, 2006). If insect feeding is subtle enough to avoid outright cell rupture, modulation of nutrients sequestered by feeding will alter plant osmotica or sink/source relationships (Girousse et al., 2005; Dorchin et al., 2006). These effects also may be mediated by the plant's response. Insect attack, or even the perception of attack, can induce a myriad of defence-related responses while concomitantly reducing the expression of photosynthesis-related genes (Kessler and Baldwin, 2002). In instances where plant defences are constitutively expressed, the release of biocidal compounds against attackers may damage photosynthetic or homeostatic mechanisms vital for plant function (e.g. Zangerl et al., 2002). Indirect effects of herbivory were assigned to four classes: severed vasculature, altered sink demand, defence-related autotoxicity, and defence-induced down-regulation of photosynthesis (Fig. 1).
Fig. 1.
Conceptual model of the direct effect of herbivory (removal of leaf area) and the indirect effects of herbivore damage to foliage on photosynthesis in the remaining leaf tissues.
SEVERED VASCULATURE ALTERS PHOTOSYNTHESIS AND WATER BALANCE
Damage to leaf venation alters leaf hydraulic conductance thereby reducing stomatal conductance and photosynthesis. In the absence of alternative pathways for water transport, the consequences of damage to venation can persist for weeks after the initial injury and lead to leaf desiccation (Sack and Holbrook, 2006). Defoliation injury which severs venation indiscriminately or feeding on specific tissues, may physically obstruct fluid flow with insect mouthparts (stylets) or cell fragments and alter photosynthesis and water balance in remaining leaf tissue (Reddall et al., 2004; Delaney and Higley, 2006). In Glycine max (soybean) a form of defoliation (skeletonization) which removes patches of tissue reduced photosynthesis in remaining tissue on damaged leaves and on adjacent undamaged leaflets (Peterson et al., 1998). Interestingly, soybean increased carbon uptake rates and transpiration in remaining leaf tissue when one or two leaflets were completely lost (Suwignyo et al., 1995), but when leaf area removal (no patches) occurred to only part of a leaflet, CO2 uptake did not decrease in the remaining leaflet tissue (Peterson et al., 2004).
Aldea et al. (2005) confirmed that skeletonizing of soybean leaves by Japanese beetles substantially increased water loss from the cut edges. Damaging the interveinal tissue increased transpiration by 150 % for up to 4 d post-injury. While this uncontrolled water loss had no detectable effect on CO2 exchange, severed vasculature induced a short-lived (2 d) increase in photosynthetic efficiency (ΦPSII) in undamaged tissue of damaged leaves. The increase in ΦPSII without a corresponding increase in CO2 uptake suggests that insect damage transiently decoupled photosynthetic electron transport from carbon assimilation (Aldea et al., 2005). Severing veins and interveinal tissue alters the hydraulic construction of leaves by reducing resistance exponentially with increasing damage (Nardini and Salleo, 2005).
The effects of defoliation on photosynthesis seem to be less predictable than damage caused by other feeding guilds. In hardwoods, leaf gall and fungal damage consistently reduced ΦPSII at distances ≥1 cm from the point of direct damage, whereas defoliation resulted in only highly local reductions (<1 mm) in ΦPSII (Aldea et al., 2006b). With one exception, defoliation of soybean and Arabidopsis thaliana leaves caused only a minimal reduction in ΦPSII. When compared with the mild effect of feeding by larger 4th instar Trichoplusia ni larvae, damage by smaller 1st instars severely depressed ΦPSII, maximum photosynthetic efficiency, and nonphotochemical quenching (NPQ) in arabidopsis (Tang et al., 2006). The greater perimeter-to-area ratio of the numerous small holes produced by 1st instars compared with 4th instars may have promoted greater rates of water loss from the cut edges and a corresponding reduction in ΦPSII. That the reduction in ΦPSII could be reversed by exposing the leaf to higher concentrations of CO2, suggests that profligate water loss near cut edges reduced ΦPSII and increased NPQ by causing localized stomatal closure in the remaining undamaged leaf tissue.
HERBIVORY ALTERS SINK DEMAND
In instances where plants respond to herbivory with increased CO2 uptake, the mechanism typically is linked to compensation or an increase in the sink demand within the leaf. An extensive literature exists on photosynthetic compensation for arthropod herbivory (see Trumble et al., 1993); yet recent examples have highlighted previously uncharacterized compensatory responses. For some gall-forming insects, gall tissue itself increases photosynthesis relative to uninjured tissue. In Ilex aquifolium (holly), increased ΦPSII and electron transport rate enhanced photosynthesis (Retuerto et al., 2004) whereas a reduction in respiration in Acacia pycnantha galls contributed to an increase in net photosynthesis (Dorchin et al., 2006). While phloem feeding increased whole-canopy photosynthesis in beech trees, perhaps through a reduction in photosynthate build-up, the mechanism remains unclear and may be as simple as herbivore preference for hosts with higher rates of photosynthesis (Dungan et al., 2007).
In other galls of hardwoods, feeding damage reduced photosynthesis and altered water balance. Gall formation in red maple, pignut hickory and black oak reduced ΦPSII, but increased NPQ, indicating a down-regulation of the PSII reaction centres in the area around galls (Aldea et al., 2006b). A sharp reduction in leaf temperature near galls suggests that transpiration was greater and fluid and nutrient transport increased near the point of damage (Macfall et al., 1994). In contrast to gall-forming insects, a leaf-mining moth that lives enclosed within leaf tissue of apple trees, reduced carbon assimilation rates by decreasing transpiration (Pincebourd et al., 2006); however, the effects of this guild on plant physiology have yet to be evaluated using fluorescence and thermal imaging.
Defoliation also may increase photosynthesis by altering sink demand, but concerns over what and how remaining tissues were measured have been noted (Welter, 1989). By enclosing severed edges within gas exchange cuvettes or measuring treatment effects on leaves where adjacent leaves were removed (within-plant controls), the data may not accurately describe plant responses specific to the herbivory treatment. Despite these potential limitations, data suggest that defoliation, as well as removal of reproductive and other vegetative sinks, may improve photosynthesis in remaining leaf tissue by increasing carboxylation efficiency and the rate of RuBP regeneration (Layne and Flore, 1992; Holman and Oosterhuis, 1999; Thomson et al., 2003; Ozaki et al., 2004; Turnbull et al., 2007).
PLANT RESPONSES INDUCE AUTOTOXICITY
Plants invest in defences differently depending upon taxa, habitat, and resource availability (Fine et al., 2006), and many chemical defences are known for both model plant systems and across less-studied taxa (Coley and Barone, 1996; Berenbaum and Zangerl, 2008). Plants run the risk of autotoxicity because of the biocidal properties of many secondary compounds. Although in vivo studies of autotoxicity are limited, photosynthesis may be severely reduced for some species. For example, wild parsnip (Pastinaca sativa) contains an arsenal of defence compounds including furanocoumarins, which are photoactivated and biocidal against a variety of organisms (Arnason et al., 1991). Furanocoumarins are contained in oil tubes under positive pressure and bleed profusely from the wounding site (Gog et al., 2005). When herbivores sever these tubes, the release of furanocoumarins reduces ΦPSII and gas exchange at considerable distances from the actual point of insect damage (Zangerl et al., 2002; Gog et al., 2005).
The autotoxic effect of defensive compounds on photosynthesis is highly species specific. Essential oils derived from parsley (Petroselinum crispum), wild parsnip and rough lemon (Citrus jambhiri) reduce ΦPSII when applied to leaves of conspecifics; however, oils from parsley affected a 2-fold greater area than the other species (Gog et al., 2005). Baldwin and Callahan (1993) fed nicotine to two species of tobacco (Nicotiana sylvestris, N. glauca) that naturally synthesized this alkaloid as a defence (Kessler and Baldwin, 2002), and to two other solanaceous species lacking nicotine (Datura stramonium, Solanum lycopersicum). Photosynthetic rates declined in both species that synthesize nicotine but only in one that did not (S. lycopersicum). Priming plants with nicotine (simulated damage) prior to being fed reduced photosynthetic rates more than in damaged-unfed plants, linking nicotine toxicity to the reduction in photosynthesis. Reduced photosynthesis, in part, reduced total growth and fitness. Subsequently, plants producing nicotine constitutively or upon the induction of defence are likely to endure autotoxicity and reductions in fitness.
DEFENCE-INDUCED DOWN-REGULATION OF PHOTOSYNTHESIS-RELATED GENES
Jasmonates play a central role in regulating plant defence responses to herbivores. The mechanism by which herbivore-induced jasmonate synthesis promotes global reprogramming of defence gene expression and the regulation of this response have been reviewed recently (Howe and Jander, 2008). While jasmonates induce defences, they also inhibit growth and photosynthesis (Giri et al., 2006; Zavala and Baldwin, 2006; Yan et al., 2007).
Transcriptional analysis of plant–herbivore interactions revealed that photosynthesis-related genes are down-regulated after attack (e.g. Hui et al., 2003; Reymond et al., 2004); however, few studies have demonstrated the effects of herbivore attack on photosynthesis at the proteome and physiological levels. Attack by herbivores or pathogens reduces transcription of the primary enzyme responsible for carbon fixation, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBPCase; Hermsmeier et al., 2001; Hahlbrock et al., 2003; Hui et al., 2003). Using two-dimensional electrophoresis, Giri et al. (2006) observed that herbivory reduced the abundance of RuBPCase activase (RCA) in N. attenuata. RCA modulates the activity of RuBPCase (Portis, 1995), a key regulatory enzyme of photosynthetic carbon assimilation, by facilitating the removal of sugar phosphates (ribulose bisphosphate) that prevent substrate binding and carbamylation of the protein's active site.
The regulation of RCA content may optimize plant performance during attack. Reducing RCA protein and transcript levels by gene silencing, similar to elicited plants, decreases both net photosynthetic rates and nitrate assimilation in N. attenuata; these reductions in photosynthesis and nitrogen assimilation, in turn, reduced the rate of biomass accumulation (Giri et al., 2006). Since nitrogen and carbon metabolism are linked, crosstalk between signalling pathways that regulate nitrogen assimilation and carbon metabolism is expected (Schachtman and Shin, 2007). Either genetic or environmental manipulations that decrease photosynthesis also inhibit nitrate assimilation (Matt et al., 2002). These studies suggest that herbivore-induced reductions in RCA protein explain, at least in part, the decrease in photosynthetic rates in attacked leaves.
Partial defoliation of individual leaves by herbivores largely increases evapotranspiration via enhanced water loss from cut edges and produces leaf dehydration (Aldea et al., 2005), which not only reduces photosynthesis by causing stomata to close, but also by initiating senescence signalling (Lim et al., 2007). A number of genes are induced by endogenous abscisic acid (ABA) in response to dehydration through the synthesis of the regulating transcription factors MYC and MYB (Yamaguchi-Shinozaki and Shinozaki, 2006). Both MYC and MYB function as cis-acting elements which regulate transcription of dehydration-related genes (Abe et al., 1997). Transgenic plants overproducing MYC and MYB had higher osmotic stress tolerance, and microarray analysis indicated the presence of ABA- and jasmonic acid (JA)-inducible genes (Abe et al., 2003). In addition, AtMYC2 is a transcription factor that in arabidopsis functions in JA and JA–ethylene-regulated defence responses (Anderson et al., 2004; Boter et al., 2004; Lorenzo et al., 2004). It has been suggested that crosstalk occurs on AtMYC2 between ABA- and JA-responsive gene expression at the MYC recognition sites in the promoters, and that AtMYC2 is a common transcription factor of ABA and JA pathways in arabidopsis (Yamaguchi-Shinozaki and Shinozaki, 2006).
The lipoxygenase pathway is differentially induced depending on the attacking agent (Heidel and Baldwin, 2004; De Vos et al., 2005; Kempema et al., 2007), and the initiation of jasmonate signalling reduces photosynthesis and vegetative growth. Plants treated with methyl jasmonate develop shorter petioles than control plants (Cipollini, 2005), and arabidopsis mutants that accumulate higher JA concentrations have shorter petioles than wild-type (Bonaventure et al., 2007); these effects of JA on plant growth are modulated by the gene JASMONATE-ASSOCIATED1 (JAS1) (Yan et al., 2007). Moreover, herbivore-induced JA signalling suppresses regrowth and contributes to apical dominance (Zavala and Baldwin, 2006). It has been suggested that the slower growth and down-regulation of photosynthetic-related genes by herbivore elicitation may be required to free-up resources for defence-related processes (Baldwin, 2001). Herbivore attack produced rapid changes in sink–source relations and increased the allocation of sugars to roots in N. attenuata plants; this process is regulated by the β-subunit of SnRK1 (SNF1-related kinase) protein kinase, but is independent of jasmonate signalling (Schwachtje et al., 2006). It is not clear whether the change in carbon allocation affects photosynthetic rate, per se, but growth reduction would affect leaf expansion and total plant photosynthesis.
IMAGING METHODS APPLIED TO DAMAGED LEAVES
Chlorophyll fluorescence provides a non-invasive probe which quantifies the component processes related to photosynthetic electron transport and correlates with photosynthetic capacity measured by gas exchange. There are several comprehensive discussions of the theory behind calculating fluorescence parameters and how imaging has been applied to leaf-level physiology (Lenk et al., 2007), aided in crop production practices (Baker and Rosenqvist, 2004), or has been used to screen for stressors and circadian rhythms (Chaerle et al., 2007). High-resolution spatial maps of primary photosynthetic processes, including estimates of the rate of electron transport through PSII, energization of the thylakoid membrane and the quantum efficiency of PSII, not only provide direct estimates of the magnitude of damage but also provide insight into underlying mechanisms (Baker et al., 2001; Oxborough, 2004, 2005).
The mechanisms governing the spatial patterns of photosynthesis following herbivory can be explored further by examining the spatial correspondence of other processes. The ability to collect spatially resolved data for a wide range of molecular, physiological and biophysical processes is increasing dramatically (Chaerle and Van Der Straeten, 2000; Table 1). The damage to water-conducting xylem by chewing insects may generate localized water limitations (Tang et al., 2006). Insofar as these water limitations or other localized changes in leaf chemistry affect stomatal conductance, thermal imaging offers a powerful tool for mapping changes in temperature associated with variation in latent heat flux across leaf surfaces (Jones, 1999; Omasa and Takayama, 2003). With proper calibration, thermal maps can be converted directly into maps of stomata conductance (Jones, 2004; Bajons et al., 2005; Grant et al., 2006). However, because of intrinsic properties of thermal cameras as well as lateral heat transfer within leaves (Jones, 2004), the resolution of thermal images typically is lower than fluorescence images.
Table 1.
Representative physiological and molecular processes readily visualized in vivo using various imaging methods
| Parameter | Process | Wavelength | Reference | |
|---|---|---|---|---|
| Photosynthesis | Fv/Fm | Maximum quantum efficiency of PSII | 470/700 | Genty et al., 1989; Rolfe and Scholes, 1995; |
| NPQ | Non-photochemical energy dissipation | Oxborough, 2004 | ||
| ΦPSII | Quantum yield of electron transport | |||
| Water and energy status | Thermal | Transpiration, conductance | None/ >1200 | Jones, 1999; Omasa and Takayama, 2003 |
| MRI | Water transport | X-rays and microwaves | Gussoni et al., 2001, Clearwater and Clark, 2003 | |
| Tracers | Depends on tracer | Gaff and O-Ogoloa, 1971; Canny, 1990 | ||
| Leaf pigments | NDVI | Chlorophyll content | <700/750, 704 | Gamon and Surfus, 1999 |
| Red/green | Anthocyanin content | <700/600–699, 500–599 | ||
| PRI | Xanthophyll cycle | <700/531, 570 | ||
| Molecular interactions and cell environment | GFP | Gene expression, protein motility, organelle location, cellular pH | 485/509 | Buschmann et al., 2000; Dixit et al., 2006 |
| RFP | 490, 520, 563/583 | |||
| BFP | UV/440 | |||
| Defence compounds | Dyes | Reactive oxygen species, Ca2+ | 400–700 | Fryer et al., 2002; Maffei et al., 2004 |
| Metabolites | Beta emission | Carbohydrate/metabolite transport | Autoradiography | Minchin and Thorpe 2003; Thorpe et al., 2007 |
Where appropriate, the excitation and measurements wavelengths are noted (wavelength: excitation/measurement).
Modified from Aldea et al. (2006a).
The spatial pattern of other components of the photosynthetic machinery, including chlorophyll content and engagement of the xanthophyll cycle (Lichtenthaler et al., 1996; Gamon et al., 1997; Gitelson et al., 2005) are readily mapped with hyperspectral imaging (Chaerle and Van Der Straeten, 2000; Schuerger et al., 2003), though this has not yet been applied to variation within single leaves.
The construction of transgenic plants with the promoter region of a gene of interest connected to a ‘reporter gene’ permits monitoring of the spatial distribution of transcription, and markers for various organelles, subcellular structures, protein motility and the cellular environment (e.g. pH; Dixit et al., 2006). Genes for firefly luciferase or β-glucuronidase (de Ruijter et al., 2003) have been useful in this regard (Jefferson et al., 1987; Greer et al., 2002); intrinsically fluorescent proteins, such as green, blue and yellow fluorescent proteins, may be more useful partners for in vivo imaging studies because of their high quantum yield (Dixit et al., 2006).
In addition to the use of various tracers and dyes for mapping the movement of water, labeling defence compounds (reactive oxygen species) and following transmembrane signals (Ca2+), measurement of beta emissions from carbon isotopes by autoradiography provides a powerful technique for tracking the movement of carbohydrates and metabolites (Kawachi et al., 2006; Thorpe et al., 2007). Beta emissions from 11C are more useful for in vivo experiments than 14C, as the particles emitted from the former are short lived and more powerful, thus reducing the logistical problems of handling radioactive waste and providing the capability of penetrating thick plant tissues (Minchin and Thorpe, 2003).
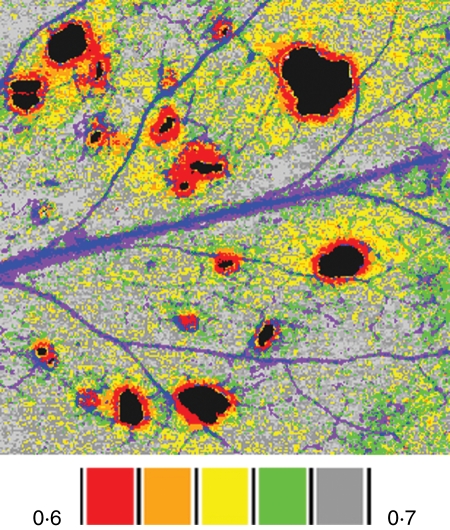
A wealth of information about how herbivory affects photosynthesis and other aspects of leaf physiology could be obtained by applying complementary imaging methods (Table 1) and, if they are applied to the same leaf in one experiment, could provide deeper insight into the mechanisms by which herbivory reduces photosynthesis in the remaining leaf tissue. Combining different images with different resolution is, however, challenging. One approach is to construct simple regressions between the values in aggregate pixels in one image with aggregate pixels in another image. West et al. (2005) applied this approach to an examination of the effect of stomatal patchiness (thermal image) on photosynthesis (fluorescence image). Deeper insight can be gained by applying methods of geographical image analysis to physiological data (Omasa and Takayama, 2003; Leinonen and Jones 2004; Aldea et al., 2006a). By registering and re-sampling images taken with different instruments, multiple images can be aligned precisely and expressed at a common resolution. Once aligned, new maps are generated that represent the composite information derived from the original separate images (Aldea et al., 2006a). The ‘image map’ of A. thaliana damaged by T. ni larvae (Fig. 2) revealed that immediately near holes, ΦPSII was greatly reduced and the gene coding for cinnamate-4-hydroxylase (C4H) was strongly induced (red areas). C4H is the first cytochrome P450 monooxygenase in the phenylpropanoid pathway and its induction near damaged areas suggests that a reorientation of metabolism toward defence may have contributed to the loss of photosynthetic efficiency near the cut edges. At greater distances from the edge, other factors contribute to the reduction in quantum efficiency as values of dark- adapted Fv/Fm and C4H expression are low.
Fig. 2.
False colour images of the location of damage classes surrounding holes in an arabidopsis leaf exposed to herbivory by Trichoplusia ni larvae. Transgenic Arabidopsis thaliana carried a cinnamate-4-hydroxylase (C4H) promoter and β-glucuronidase (GUS) reporter gene fusion. In A. thaliana, enzymes in the phenylpropanoid pathway may contribute to defence against pathogens; C4H is constitutively expressed in the veins of undamaged leaves and induced by wounding near the site of damage. The image was constructed by combining independent images of the same leaf of chlorophyll fluorescence (ΦPSII) and GUS staining for C4H activity using geographic image analysis software. The false-colour scale bars indicate the mean value of ΦPSII for each damage class. The veins shown in blue and purple were classes that were excluded from analysis because their high level of GUS staining was not related to herbivory. Data were generously provided by Dr Jennie Tang.
LIMITATIONS TO MEASURING GAS EXCHANGE SIMULTANEOUSLY WITH IMAGING
One of the major limitations to estimating herbivore-induced effects on photosynthesis is correctly characterizing CO2 diffusion and uptake within the leaf. Gas exchange measurements typically are used to generate a relationship between photosynthetic assimilation and internal [CO2] – the A/Ci response curve. This relationship assumes leaves have homogenous distribution of chloroplasts (for light absorption) and of stomata (for gas exchange; von Caemmerer, 2000). Heterogeneity across remaining leaf tissues caused by herbivory may compromise the utility of the A/Ci response curve. In addition, gas exchange chambers enclosing leaves reduce internal CO2 where gaskets overlay leaf area through shading-induced stomatal closure (Pieruschka et al., 2006). Diffusion of CO2 may also occur laterally, with respect to morphology, and may diffuse 2 mm in homobaric and up to 1 mm in heterobaric (compartmentalized) leaves (Pieruschka et al., 2006; Morison et al., 2007). Heterogeneity in photosynthesis caused by non-uniform CO2 uptake, in addition to lateral diffusion of CO2 within leaves, will interact with heterogeneity induced by feeding damage when scales are similar. For example, defoliation damage may reduce ΦPSII within a distance of 1–2 mm (Aldea et al., 2005, 2006b); however, CO2 diffusion through cut edges into damaged tissues and adjacent undamaged tissues, may increase Ci and alleviate the suppression or even enhance photosynthesis.
CONCLUSIONS
In many cases, arthropod damage reduces photosynthesis to a greater extent than would be predicted by the direct loss of leaf tissue. With the use of new imaging technologies we are beginning to understand how photosynthesis and water balance are modulated in undamaged tissue following herbivory. Connecting these alterations in physiology to changes in gene transcription and hormonal signalling will increase our ability to estimate whole-plant responses to herbivory, and will improve our estimates of the impact of herbivory on higher levels of biological organization, such as yield loss and assessments of overall ecosystem productivity.
Indirect alterations of photosynthesis have been identified across multiple plant systems and can be categorized by plant responses. Severed vasculature increases transpiration, reduces ΦPSII, and reduces NPQ, whereas sink demands of galls enhance transpiration. Photosynthesis is greatly reduced through the release of toxic secondary compounds or defences elicited by herbivore attack. Even the initiation of these defences triggers down-regulation of photosynthetic component processes or proteins. Despite these characterized indirect effects, investigations are lacking for some damage types (e.g. specialized cell content feeders) and their subsequent interactions with primary and secondary metabolite pools.
While we are closer to elucidating the mechanisms responsible for herbivore-induced alterations in photosynthesis and related processes in undamaged tissues, a complete understanding of how the indirect suppression of photosynthesis propagates away from the point of damage remains unknown. Genomic analyses of plants challenged by arthropods have revealed a trend for down-regulation of photosynthesis-related genes, but a closer look at transcriptional changes between and within feeding guilds has identified differential regulation of defence genes and overlap among damage guilds. A universal response to herbivory is the induction of the lipoxygenase pathway, but attacking agents differentially induce this pathway and corresponding jasmonate concentrations (Heidel and Baldwin, 2004; De Vos et al., 2005; Kempema et al., 2007). Differences in concentrations of defence signalling molecules may lead to differential down-regulation of photosynthesis genes. Already, the overlap in the magnitude of down-regulation has been noted between caterpillars and general cell content feeders compared with aphids (Voelckel et al., 2004), leading to species-specific regulation of different metabolic pathways (e.g. nitrogen metabolism by aphids). Subsequently, within-plant mechanisms underlying the indirect effect, and not the direct effect, may drive physiological responses in future plant–insect interactions.
ACKNOWLEDGEMENTS
We thank S. Davis, K. Teixeira and S. H. Woodard for constructive comments on an earlier version of this manuscript. We thank Dr Arthur Zangerl for the concept behind Fig. 1 and Dr Jennie Tang for conducting the experiment that produced Fig. 2. We acknowledge generous support from the Office of Science (BER), US Department of Energy, grant no. DE-FG02-04ER63489, and the National Science Foundation, grant no. IBN 0326053.
LITERATURE CITED
- Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. Role of Arabidopsis MYC and MYC homologs in drought- and abscisic acid-regulated gene expression. The Plant Cell. 1997;9:1859–1868. doi: 10.1105/tpc.9.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. The Plant Cell. 2003;15:63–78. doi: 10.1105/tpc.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldea M, Hamilton JG, Resti JP, Zangerl AR, Berenbaum MR, DeLucia EH. Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant, Cell & Environment. 2005;28:402–411. [Google Scholar]
- Aldea M, Frank TD, DeLucia EH. A method for quantitative analysis of spatially variable physiological processes across leaf surfaces. Photosynthesis Research. 2006;a 90:161–172. doi: 10.1007/s11120-006-9119-z. [DOI] [PubMed] [Google Scholar]
- Aldea M, Hamilton JG, Resti JP, Zangerl AR, Berenbaum MR, Frank TD, et al. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood samplings. Oecologia. 2006;b 149:221–232. doi: 10.1007/s00442-006-0444-x. [DOI] [PubMed] [Google Scholar]
- Arnason JT, Philogene BJR, Towers GHN. Phototoxins in plant-insect interactions. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: their interactions with secondary plant metabolites. New York, NY: Academic Press; 1991. pp. 317–341. [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, et al. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. The Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajons P, Klinger G, Schlosser V. Determination of stomatal conductance by means of infrared thermography. Infrared Physics and Technology. 2005;46:429–439. [Google Scholar]
- Baker NR, Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. Journal of Experimental Botany. 2004;55:1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- Baker NR, Oxborough K, Lawson T, Morison JIL. High resolution imaging of photosynthetic activities of tissues, cells and chloroplasts in leaves. Journal of Experimental Botany. 2001;52:614–621. [PubMed] [Google Scholar]
- Baldwin IT. An ecologically motivated analysis of plant-herbivore interactions in native tobacco. Plant Physiology. 2001;127:1449–1458. [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Callahan P. Autotoxicity and chemical defense: nicotine accumulation and carbon gain in solanaceous plants. Oecologia. 1993;94:534–541. doi: 10.1007/BF00566969. [DOI] [PubMed] [Google Scholar]
- Berenbaum MR, Zangerl AR. Facing the future of plant–insect interaction research: le retour a la ‘raison d'etre. Plant Physiology. 2008;146:804–811. doi: 10.1104/pp.107.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure G, Gfeller A, Proebsting WM, Hoerstensteiner S, Chetelat A, Martinoia E, et al. A gain of function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. The Plant Journal. 2007;49:889–898. doi: 10.1111/j.1365-313X.2006.03002.x. [DOI] [PubMed] [Google Scholar]
- Boter M, Ruiz-Rivero O, Abdeen A, Prat S. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes and Development. 2004;18:1577–1591. doi: 10.1101/gad.297704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann C, Langsdorf G, Lichtenthaler HK. Imaging of the blue, green and red fluorescence emission of plants: an overview. Photosynthetica. 2000;38:483–491. [Google Scholar]
- von Caemmerer S. Biochemical models of leaf photosynthesis. Victoria, Australia: CSIRO Publishing; 2000. [Google Scholar]
- Canny MJ. Tansley review no. 22 – what becomes of the transpiration stream? New Phytologist. 1990;114:341–368. doi: 10.1111/j.1469-8137.1990.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Chaerle L, Van Der Straeten D. Imaging techniques and the early detection of plant stress. Trends in Plant Science. 2000;5:495–501. doi: 10.1016/s1360-1385(00)01781-7. [DOI] [PubMed] [Google Scholar]
- Chaerle L, Leinonen I, Jones HG, Van Der Straeten D. Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. Journal of Experimental Botany. 2007;58:773–784. doi: 10.1093/jxb/erl257. [DOI] [PubMed] [Google Scholar]
- Cipollini D. Interactive effects of lateral shading and jasmonic acid on morphology, physiology, seed production, and defense traits in Arabidopsis thaliana. International Journal of Plant Science. 2005;166:955–959. [Google Scholar]
- Clearwater MJ, Clark CJ. In vivo magnetic resonance imaging of xylem vessel contents in woody lianas. Plant, Cell & Environment. 2003;26:1205–1214. [Google Scholar]
- Coley PD, Barone JA. Herbivory and plant defenses in tropical forests. Annual Review of Ecology and Systematics. 1996;27:305–335. [Google Scholar]
- Cyr H, Pace ML. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature. 1993;361:148–150. [Google Scholar]
- Delaney KJ, Higley LG. An insect countermeasure impacts plant physiology: midrib vein cutting, defoliation and leaf photosynthesis. Plant, Cell & Environment. 2006;29:1245–1258. doi: 10.1111/j.1365-3040.2006.01504.x. [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, et al. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiology. 2005;142:353–363. doi: 10.1104/pp.106.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Cyr R, Gilroy S. Using intrinsically fluorescent proteins for plant cell imaging. The Plant Journal. 2006;45:599–615. doi: 10.1111/j.1365-313X.2006.02658.x. [DOI] [PubMed] [Google Scholar]
- Dorchin N, Cramer MD, Hoffmann JH. Photosynthesis and sink activity of wasp-induced galls in Acacia pycnantha. Ecology. 2006;87:1781–1791. doi: 10.1890/0012-9658(2006)87[1781:pasaow]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dungan RJ, Turnbull MH, Kelly D. The carbon costs for host trees of a phloem-feeding herbivore. Journal of Ecology. 2007;95:603–613. [Google Scholar]
- Fine PVA, Miller ZJ, Mesones I, Irazuzta S, Appel HM, Stevens MHH, et al. The growth-defense tradeoff and habitat specialization by plants in Amazonian forests. Ecology. 2006;87:150–162. doi: 10.1890/0012-9658(2006)87[150:tgtahs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. Journal of Experimental Botany. 2002;53:1249–1254. [PubMed] [Google Scholar]
- Gaff DF, O-Ogola O. The use of nonpermeating pigments for testing the survival of cells. Journal of Experimental Botany. 1971;22:756–758. [Google Scholar]
- Gamon JA, Surfus JS. Assessing leaf pigment content and activity with a reflectometer. New Phytologist. 1999;143:105–117. [Google Scholar]
- Gamon JA, Serrano L, Surfus JS. The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia. 1997;112:492–501. doi: 10.1007/s004420050337. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Girousse C, Moulia B, Silk W, Bonnemain JL. Aphid infestation causes different changes in carbon and nitrogen allocation in alfalfa stems as well as different inhibitions of longitudinal and radial expansion. Plant Physiology. 2005;137:1474–1484. doi: 10.1104/pp.104.057430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri AP, Wunsche H, Mitra S, Zavala JA, Muck A, Svatos A, et al. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant's proteome. Plant Physiology. 2006;142:1621–1641. doi: 10.1104/pp.106.088781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelson AA, Viña A, Ciganada V, Rundquist DC, Arkebauer TJ. Remote estimation of canopy chlorophyll content in crops. Geophysical Research Letters. 2005;32:1–4. [Google Scholar]
- Gog L, Berenbaum MR, DeLucia EH, Zangerl AR. Autotoxic effects of essential oils on photosynthesis in parsley, parsnip, and rough lemon. Chemoecology. 2005;15:115–119. [Google Scholar]
- Grant OM, Caves MM, Jones HG. Optimizing thermal imaging as a technique for detecting stomatal closure induced by drought stress under greenhouse conditions. Physiologia Plantarum. 2006;127:507–518. [Google Scholar]
- Greer LF, III, Szalay AA. Imaging of light emission from the expression of luciferases in living cells and organisms: a review. Luminescence. 2002;17:43–74. doi: 10.1002/bio.676. [DOI] [PubMed] [Google Scholar]
- Gussoni M, Greco F, Vezzoli A, Osuga T, Zetta L. Magnetic resonance imaging of molecular transport in living morning glory stems. Magnetic Resonance Imaging. 2001;19:1311–1322. doi: 10.1016/s0730-725x(01)00468-4. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K, Bednarek P, Ciolkowski I, Hamberger B, Heise A, Liedgens H, et al. Non-self recognition, transcriptional reprogramming, and secondary metabolite accumulation during plant/pathogen interactions. Proceedings of the National Academy of Sciences of the USA. 2003;100:14569–14576. doi: 10.1073/pnas.0831246100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile FJ, Higley LG. Changes in soybean gas-exchange after moisture stress and spider mite injury. Environmental Entomology. 2003;32:433–440. [Google Scholar]
- Haile FJ, Higley LG, Ni X, Quisenberry SS. Physiological and growth tolerance in wheat to Russian wheat aphid (Homoptera: Aphididae) injury. Environmental Entomology. 1999;28:787–794. [Google Scholar]
- Heidel AJ, Baldwin IT. Microarray analysis of salicylic acid- and jasmonic acid-signaling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant, Cell & Environment. 2004;27:1362–1373. [Google Scholar]
- Heng-Moss T, Macedo T, Franzen L, Baxendale F, Higley L, Sarath G. Physiological responses of resistant and susceptible buffalograsses to Blissus occiduus (Hemiptera: Blissidae) feeding. Journal of Economic Entomology. 2006;99:222–228. doi: 10.1603/0022-0493(2006)099[0222:proras]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hermsmeier D, Schittko U, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera: Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiology. 2001;125:683–700. doi: 10.1104/pp.125.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley LG, Browde JA, Higley PM. Moving toward new understandings of biotic stress and stress interactions. In: Buxton DR, editor. International crop science I. Madison, WI: Crop Science Society of America; 1993. pp. 749–754. [Google Scholar]
- Holman EM, Oosterhuis DM. Cotton photosynthesis and carbon partitioning in response to floral bud loss due to insect damage. Crop Science. 1999;39:1347–1351. [Google Scholar]
- Howe GA, Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biology. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Hui DQ, Iqbal J, Lehmann K, Gase K, Saluz HP, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera: Sphingidae) and its natural host Nicotiana attenuata. V. Microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiology. 2003;131:1877–1893. doi: 10.1104/pp.102.018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant, Cell & Environment. 1999;22:1043–1055. [Google Scholar]
- Jones HG. Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. Advances in Botanical Research incorporating Advances in Plant Pathology. 2004;41:107–163. [Google Scholar]
- Kawachi N, Sakamoto K, Ishii S, Fujimaki S, Suzui N, Ishioka NS, et al. Kinetic analysis of carbon-11-labeled carbon dioxide for studying photosynthesis in a leaf using positron emitting tracer imaging system. Transactions of Nuclear Science. 2006;53:2991–2997. [Google Scholar]
- Kempema LA, Cui X, Holzer FM, Walling LL. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs: similarities and distinctions in responses to aphids. Plant Physiology. 2007;143:849–865. doi: 10.1104/pp.106.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Plant responses to insect herbivory: the emerging molecular analysis. Annual Review of Plant Biology. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- Layne DR, Flore JA. Photosynthetic compensation to partial leaf area reduction in sour cherry. Journal of American Society for Horticultural Science. 1992;117:279–286. [Google Scholar]
- Leiononen I, Jones HG. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. Journal of Experimental Botany. 2004;55:1423–1431. doi: 10.1093/jxb/erh146. [DOI] [PubMed] [Google Scholar]
- Lenk S, Chaerle L, Pfundel EE, Langsdorf G, Hagenbeek D, Lichtenthaler HK, et al. Multispectral fluorescence and reflectance imaging at the leaf level and its possible applications. Journal of Experimental Botany. 2007;58:807–814. doi: 10.1093/jxb/erl207. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Gitelson A, Lang M. Non-destructive determination of chlorophyll content of leaves of a green and an aurea mutant of tobacco by reflectance measurements. Journal of Plant Physiology. 1996;148:483–493. [Google Scholar]
- Lim PO, Kim HJ, Nam HG. Leaf senescence. Annual Review of Plant Biology. 2007;58:115–136. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R. JASMONATEINSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. The Plant Cell. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo TB, Bastos CS, Higley LG, Ostlie KR, Madhavan S. Photosynthetics responses of soybean to soybean aphid (Homoptera: Aphididae) injury. Journal of Economic Entomology. 2003;a 96:188–193. doi: 10.1093/jee/96.1.188. [DOI] [PubMed] [Google Scholar]
- Macedo TB, Higley LG, Ni X, Quisenberry S. Light activation of Russian wheat aphid-elicited physiological responses in susceptible wheat. Journal of Economic Entomology. 2003;b 96:194–201. doi: 10.1093/jee/96.1.194. [DOI] [PubMed] [Google Scholar]
- Macedo TB, Peterson RKD, Weaver DK, Morrill WL. Wheat stem sawfly, Cephus cintus Norton, impact on wheat primary metabolism: an ecophysiological approach. Environmental Entomology. 2005;34:719–726. [Google Scholar]
- Macedo TB, Weaver DK, Peterson RKD. Photosynthesis in wheat at the grain filling stage is altered by the wheat stem sawfly (Hymenoptera: Cephidae) injury and reduced water availability. Journal of Entomological Science. 2007;42:228–238. [Google Scholar]
- Macfall JS, Spaine P, Doudrick R, Johnson GA. Alterations in growth and water transport processes in fusiform rust galls of pine, determined by magnetic-resonance microscopy. Phytopathology. 1994;84:288–293. [Google Scholar]
- Maffei M, Bossi S, Spiteller D, Mithofer A, Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitated components. Plant Physiology. 2004;134:1–11. doi: 10.1104/pp.103.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt P, Krapp A, Haake V, Mock HP, Stitt M. Decreased Rubisco activity leads to dramatic changes of nitrate metabolism, amino acid metabolism and the levels of phenylpropanoids and nicotine in tobacco antisense RBCS transformants. The Plant Journal. 2002;30:663–677. doi: 10.1046/j.1365-313x.2002.01323.x. [DOI] [PubMed] [Google Scholar]
- Minchin PEH, Thorpe MR. Using short-lived isotope 11C in mechanistic studies of photosynthate transport. Functional Plant Biology. 2003;30:831–841. doi: 10.1071/FP03008. [DOI] [PubMed] [Google Scholar]
- Morison JIL, Lawson T, Cornic G. Lateral CO2 diffusion inside dicotyledonous leaves can be substantial: quantification in different light intensities. Plant Physiology. 2007;145:680–690. doi: 10.1104/pp.107.107318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Salleo S. Water stress-induced modifications of leaf hydraulic architecture in sunflower: co-ordination with gas exchange. Journal of Experimental Botany. 2005;56:3093–3101. doi: 10.1093/jxb/eri306. [DOI] [PubMed] [Google Scholar]
- Oerke E-C, Dehne H-W. Global crop production and the efficacy of crop protection – current situation and future trends. European Journal of Plant Pathology. 1997;103:203–215. [Google Scholar]
- Omasa K, Takayama K. Simultaneous measurement of stomatal conductance, non-photochemical quenching, and photochemical yield of photosystem II in intact leaves by thermal and chlorophyll fluorescence imaging. Plant Cell Physiology. 2003;44:1290–1300. doi: 10.1093/pcp/pcg165. [DOI] [PubMed] [Google Scholar]
- Oxborough K. Imaging of chlorophyll a fluorescence: theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. Journal of Experimental Botany. 2004;55:1195–1205. doi: 10.1093/jxb/erh145. [DOI] [PubMed] [Google Scholar]
- Oxborough K. Using chlorophyll a fluorescence imaging to monitor photosynthetic performance. In: Govindjee, Papageorgiou GC, editors. Chlorophyll fluorescence: a signature of photosynthesis. Dordrecht: Kluwer: Academic Press; 2005. pp. 409–428. [Google Scholar]
- Ozaki K, Saito H, Yamamuro K. Compensatory photosynthesis as a response to partial debudding in ezo spruce, Picea jezoensis, seedlings. Ecological Research. 2004;19:225–231. [Google Scholar]
- Peterson RKD, Higley LH. Biotic stress and yield loss. Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- Peterson RKD, Danielson SD, Higley LG. Photosynthetic responses of alfalfa to actual and simulated alfalfa weevil (Coleoptera: Curculionidae) injury. Environmental Entomology. 1992;21:501–507. [Google Scholar]
- Peterson RKD, Higley LG, Spomer SM. Injury by Hyalaphora cecropia (Lepidoptera: Saturniidae) and photosynthetic responses of apple and crabapple. Environmental Entomology. 1996;25:416–422. [Google Scholar]
- Peterson RKD, Higley LG, Haile FJ, Barrigossi JAF. Mexican bean beetle (Coleoptera: Coccinelidae) injury affects photosynthesis of Glycine max and Phaseolus vulgaris. Environmental Entomology. 1998;27:373–381. [Google Scholar]
- Peterson RKD, Shannon CL, Lenssen AW. Photosynthetic responses of legume species to leaf-mass consumption injury. Environmental Entomology. 2004;33:450–456. [Google Scholar]
- Pieruschka R, Schurr U, Jensen M, Wolff WF, Jahnke S. Lateral diffusion of CO2 from shaded to illuminated leaf parts affects photosynthesis inside homobaric leaves. New Phytologist. 2006;169:779–787. doi: 10.1111/j.1469-8137.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- Pincebourd S, Frak E, Sinoquet H, Regnard JL, Casas J. Herbivory mitigation through increased water-use efficiency in a leaf-mining moth-apple tree relationship. Plant, Cell & Environment. 2006;29:2238–2247. doi: 10.1111/j.1365-3040.2006.01598.x. [DOI] [PubMed] [Google Scholar]
- Portis AR. The regulation of rubisco by rubisco activase. Journal of Experimental Botany. 1995;46:1285–1291. doi: 10.1093/jxb/erm240. [DOI] [PubMed] [Google Scholar]
- Reddall A, Sadras VO, Wilson LJ, Gregg PC. Physiological responses of cotton to two-spotted spider mite damage. Crop Science. 2004;44:835–846. [Google Scholar]
- Retuerto R, Fernandez-Lema B, Rodriguez-Roiloa S, Obeso JR. Increased photosynthetic performance in holly trees infested by scale. Functional Ecology. 2004;18:664–669. [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. A conserved transcript pattern in response to a specialist and a generalist herbivore. The Plant Cell. 2004;16:3132–3147. doi: 10.1105/tpc.104.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe SA, Scholes JD. Quantitative imaging of chlorophyll fluorescence. New Phytologist. 1995;131:69–79. doi: 10.1111/j.1469-8137.1995.tb03056.x. [DOI] [PubMed] [Google Scholar]
- de Ruijter NCA, Verhees J, van Leewen W, van der Krol AR. Evaluation and comparison of GUS, LUC and GFP reporter system for gene expression studies in plants. Plant Biology. 2003;5:103–115. [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Shin R. Nutrient sensing and signaling: NPKS. Annual Review of Plant Biology. 2007;58:47–69. doi: 10.1146/annurev.arplant.58.032806.103750. [DOI] [PubMed] [Google Scholar]
- Schuerger AC, Capelle GA, Di Benedetto JA, Mao C, Thai CN, Evans MD, et al. Comparison of two hyperspectral imaging and two laser-induced fluorescence instruments for detection of zinc stress and chlorophyll concentration in bahia grass (Paspalum notatum Flugge.) Remote Sensing of Environment. 2003;84:572–588. [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proceedings of the National Academy of Sciences of the USA. 2006;103:12935–12940. doi: 10.1073/pnas.0602316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwignyo RA, Nose A, Kawamitsu Y, Tsuchiya M, Wasano K. Effects of manipulations of source and sink on carbon exchange rate and some enzymes of sucrose metabolism in leaves of soybean [Glycine max (L.) Merr.] Plant Cell Physiology. 1995;36:1439–1446. [Google Scholar]
- Tang JY, Zielinski RE, Zangerl AR, Crofts AR, Berenbaum MR, DeLucia EH. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. Journal of Experimental Botany. 2006;57:527–536. doi: 10.1093/jxb/erj032. [DOI] [PubMed] [Google Scholar]
- Thomson VP, Cunningham SA, Ball MC, Nicotra AB. Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia. 2003;134:167–175. doi: 10.1007/s00442-002-1102-6. [DOI] [PubMed] [Google Scholar]
- Thorpe MR, Ferrieri A, Herth MM, Ferrieri RA. 11C-imaging: methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate, and of photoassimilate even after proton transport is decoupled. Planta. 2007;226:541–551. doi: 10.1007/s00425-007-0503-5. [DOI] [PubMed] [Google Scholar]
- Trumble JT, Kolodny-Hirsch DM, Ting IP. Plant compensation for arthropod herbivory. Annual Reviews of Entomology. 1993;38:93–119. [Google Scholar]
- Turnbull TL, Adams MA, Warren CR. Increased photosynthesis following partial defoliation of field-grown Eucalyptus globulus is not caused by increased leaf nitrogen. Tree Physiology. 2007;27:1481–1492. doi: 10.1093/treephys/27.10.1481. [DOI] [PubMed] [Google Scholar]
- Voelckel C, Weisser WW, Baldwin IT. An analysis of plant-aphid interactions by different microarray hybridization strategies. Molecular Ecology. 2004;13:3187–3195. doi: 10.1111/j.1365-294X.2004.02297.x. [DOI] [PubMed] [Google Scholar]
- Welter SC. Arthropod impact on plant gas exchange. In: Bernays EA, editor. Insect–plant interactions. Boca Raton, FL: CRC Press; 1989. pp. 135–151. [Google Scholar]
- West JD, Peak D, Peterson JQ, Mott KA. Dynamics of stomatal patches for a single surface of Xanthium strumarium L. leaves observed with fluorescence and thermal images. Plant, Cell & Environment. 2005;28:633–641. [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. Responses and tolerance to dehydration and cold stresses. Annual Review of Plant Biology. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chetelat A, Reymond P, Pagni M, Dubugnon L, et al. A downstream mediator in the growth repression limb of the jasmonate pathway. The Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangerl AR, Hamilton JG, Miller TJ Crofts AR, Oxborough K, Berenbaum MR, et al. Impact of folivory on photosynthesis is greater than the sum of its holes. Proceedings of the National Academy of Sciences of the USA. 2002;99:1088–1091. doi: 10.1073/pnas.022647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT. Jasmonic acid signaling and herbivore resistance traits constrain regrowth after herbivore attack in Nicotiana attenuata. Plant, Cell & Environment. 2006;29:1751–1760. doi: 10.1111/j.1365-3040.2006.01551.x. [DOI] [PubMed] [Google Scholar]