Abstract
Background
In obligate Crassulacean acid metabolism (CAM), up to 99 % of CO2 assimilation occurs during the night, therefore supporting the hypothesis that CAM is adaptive because it allows CO2 fixation during the part of the day with lower evaporative demand, making life in water-limited environments possible. By comparison, in facultative CAM (inducible CAM, C3-CAM) and CAM-cycling plants drought-induced dark CO2 fixation may only be, with few exceptions, a small proportion of C3 CO2 assimilation in watered plants and occur during a few days. From the viewpoint of survival the adaptive advantages, i.e. increased fitness, of facultative CAM and CAM-cycling are not obvious. Therefore, it is hypothesized that, if it is to increase fitness, CAM must aid in reproduction.
Scope
An examination of published reports of 23 facultative CAM and CAM-cycling species finds that, in 19 species, drought-induced dark CO2 fixation represents on average 11 % of C3 CO2 assimilation of watered plants. Evidence is discussed on the impact of the operation of CAM in facultative and CAM-cycling plants on their survival – carbon balance, water conservation, water absorption, photo-protection of the photosynthetic apparatus – and reproductive effort. It is concluded that in some species, but not all, facultative and cycling CAM contribute, rather than to increase carbon balance, to increase water-use efficiency, water absorption, prevention of photoinhibition and reproductive output.
Key words: Facultative CAM, CAM-cycling, water, crassulacean acid metabolism, deficit
INTRODUCTION
Crassulacean acid metabolism (CAM), a form of CO2 assimilation which occurs in approx. 16 000 species of 328 genera in 33 families (Smith and Winter, 1996), has been extensively revised (Osmond, 1978; Ting, 1985; Winter, 1985; Winter and Smith, 1996; Cushman, 2001; among others). Briefly, in CAM plants CO2 uptake occurs during the night, when stomata open; CO2 is combined with phosphoenolpyruvate (PEP) through the action of PEP-carboxylase (PEPC) to yield oxaloacetate, which is reduced to malate. Malate is transported passively into the vacuole following the actively transported protons and malic acid accumulates during the night. Nocturnal acid accumulation and nocturnal stomatal aperture are the main diagnostic features of CAM. During the day malate is decarboxylated in the cytoplasm, providing ribulose-1,5-bisphoshate carboxylase/oxygenase (Rubisco) with one of its substrates during C3 photosynthesis.
The net outcome of the functioning of CAM is that CO2 is fixed with significant water saving relative to C3 photosynthesis, since the process occurs at times of lower evaporative demand and a larger air-leaf CO2 concentration gradient (Winter and Smith, 1996). This means that water-use efficiency (WUE), the ratio assimilation rate : transpiration rate, increases relative to a C3 plant. In the CAM plant, Plectranthus marrubioides, WUE increased nearly twice when air water vapour saturation deficit decreased five times, nocturnal CO2 gain remaining unchanged (Herppich, 1997). Transpiration ratio (the reciprocal of WUE) has been shown to be 3–10 times higher in CAM than in C3 plants (Kluge and Ting, 1978).
CAM may operate in different modes: (a) obligate CAM, with high nocturnal acid accumulation (ΔH+) and CO2 fixation; (b) facultative or inducible CAM, also known as C3-CAM, with a C3 form of CO2 fixation and nil ΔH+ in the non-induced state, and small nocturnal CO2 fixation and ΔH+ in the induced state; (c) CAM-cycling, with daytime CO2 fixation and ΔH+ but no nocturnal stomatal aperture; and (d) idling, with small ΔH+ and stomatal closure during the entire day and night in severely stressed plants (see Cushman, 2001; Fig. 1). Obligate CAM, in turn, can be differentiated between strong and weak CAM, on the basis of the magnitude of ΔH+ (for definition and examples, see Cushman and Bohnert, 1999; Winter and Holtum, 2002; Silvera et al., 2005; Holtum et al., 2007).
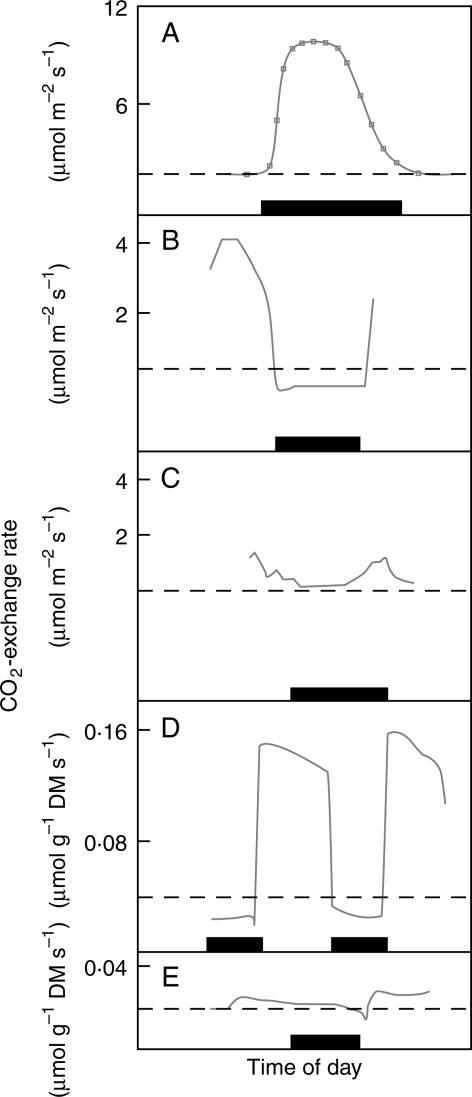
Fig. 1.
Daily gas-exchange of plants with different modes of CAM: (A) Obligate CAM in watered plants of Opuntia ficus-indica (Nobel and Hartsock, 1983); (B) C3 CO2-uptake in well-watered plants of the inducible-CAM species, Talinum triangulare (Herrera et al., 1991); (C) small dark CO2 fixation in plants of T. triangulare drought-stressed for 10 d (Herrera et al., 1991); (D) CAM-cycling in watered plants of Talinum calycinum (Martin et al., 1988); (E) CAM-idling in plants of Talinum calycinum drought-stressed for 3 d (Martin et al., 1988). The broken line indicates no net CO2-exchange; the dark bar on the abscissa indicates the duration of the dark period. The corresponding values of nocturnal acid accumulation were: (A) 85 µmol H+ cm−2 (droughted plants had a value of 16 µmol H+ cm−2); (B) 8 µmol H+ g−1 FM (0·2 µmol H+ cm−2); (C) 100 µmol H+ g−1 FM (2·0 µmol H+ cm−2); (D, E) 56 µmol H+ g−1 FM. Redrawn with permission from the authors.
Facultative CAM and CAM-cycling species typically grow on soil in semi-arid regions, on rocks, on tree branches, and, in general, in habitats where water deficit is frequent. It is widely accepted that CAM is an adaptive mechanism which optimizes water use under conditions of deficient supply; in order to be effective, an adaptation must increase fitness through increased individual survival, increased individual fecundity or both (Futuyma, 1986).
In facultative species, CAM may be induced by factors such as drought (Borland and Griffiths, 1990; Herrera et al., 1991; Olivares et al., 1984), salinity (Winter and von Willert, 1972), photoperiod (Brulfert et al., 1988), high photosynthetic photon flux (PPF) (Maxwell, 2002), nitrogen deficiency (Ota, 1988) and phosphorus deficiency (Paul and Cockburn, 1990), among others. Induction by external factors is generally rapid and reversible; in a classic example of plasticity, opposite leaves of Clusia minor may or may not perform CAM depending on the relative humidity of the air surrounding them (Schmitt et al., 1988); also, the CO2 exchange pattern of droughted plants of Talinum triangulare 24 h after re-watering reverted from idling to purely daytime assimilation (Herrera et al., 1991).
In perennial obligate CAM plants, such as cacti, up to 99 % of CO2 assimilation occurs during the night (Nobel, 1988), thus supporting the hypothesis that CAM is adaptive because it allows CO2 fixation during the part of the day with lower evaporative demand. Additionally, in many species of obligate CAM plants, watering enhances nocturnal CO2 fixation, as in the case of Opuntia basilaris (Hanscom and Ting, 1978). By comparison, the adaptive advantages, i.e. increased fitness, of facultative CAM and CAM-cycling are not obvious, since, even though it may be the sole means of CO2 acquisition in stressed plant, drought-induced dark CO2 fixation is either a small proportion of C3 CO2 assimilation in watered plants, as in Delosperma tradescantoiodes (Herppich et al., 1996) or a significant proportion but occurs only during a relatively short period (days), as in C. minor (Franco et al., 1990).
This review discusses examples of facultative CAM and CAM-cycling under water deficit and the implications of the functioning of these modes, distinguishing between traits associated mainly with survival or reproduction. Included will be some cases of CAM-cycling, since the relationship between increased WUE and fitness of individuals of species with this mode is, in my opinion (see also Martin, 1996), not fully understood. Also, in some reports authors seem to have had difficulty in distinguishing between facultative CAM and CAM-cycling [e.g. Martin et al. (1990, in reference to T. paniculatum as opposed to Güerere et al. (1996)], given the occurrence of ΔH+ together with very low nocturnal CO2 uptake under water deficit. Some of these cases may also be encompassed in the category of weak CAM. Obligate weak CAM is not touched because this mode may operate in watered plants and this review is centred on the question of CAM induction.
One of the criteria for assessing the operation of facultative CAM is the δ13C value of dry mass of photosynthesizing organs. The δ13C alone does not distinguish between field-grown C3 plants and plants that acquire up to one-third of their carbon through nocturnal CO2 fixation, as is the case for weak-CAM and facultative CAM plants (Winter and Holtum, 2002). Since the low dark CO2 fixation rates measured in the field may be at the end of the resolution of currently used portable infrared gas analysers, the limit between facultative CAM, CAM-cycling and weak CAM plants become blurry and modes are arguable.
In the rest of this review, the relationship between drought-induced CAM and carbon balance, reduction of respiratory CO2 loss, succulence, water saving, water absorption, photoprotection and reproduction will be examined, as well as the impact of the duration of induced CAM. Many authors have interpreted facultative CAM and CAM-cycling as mechanisms for avoiding respiratory CO2 losses, enabling water saving and extending the life cycle, hence favouring reproduction under stressful conditions. Evidence in support of these hypotheses is discussed below.
Aspects related to CAM induction by factors other than water deficit, such as photoperiod, mineral nutrition and submergence – important as they are – will not be covered here since they do not always imply stress. The emphasis will be on terrestrial plants exposed to environmental conditions that propitiate plant water deficit, with its consequences on carbon acquisition, water status and metabolism.
CARBON BALANCE
Eighty-three per cent of 23 facultative CAM and CAM-cycling species examined fix during the night in the field or in experiments with controlled water deficit only up to one-quarter, with a mean of 11 %, of the daily CO2 balance of watered plants (Table 1). The adaptive value of such a relatively small contribution to carbon budget has been questioned by Zotz (2002), who also proposed that a continuum between C3 and CAM exists, and considered that terminology may obscure our understanding of CAM. This is further supported by the observation that plants such as Sedum telephium (see Table 1) show either a very small or a very large proportion of the daily carbon balance fixed during the night, denoting extreme variability in CAM performance.
Table 1.
Maximum proportion of C balance contributed by nocturnal CO2 fixation relative to daytime carbon balance of watered plants and the relative carbon isotope composition (where available) of several facultative CAM species measured under controlled (C) and field (F) conditions
* Species are listed in order of increasing proportion of nocturnal carbon balance.
† Values of relative carbon balance are either reported in or calculated from publications.
‡ Values or δ13C are rounded-up averages of values reported under field (F) or controlled (C) conditions. na, Not available.
§ Where two references are given the first is for C balance, the second for δ13C.
In four of the species examined, dark CO2 fixation under water deficit represents from 39 % to 76 % of the carbon balance of watered plants (Table 1) but this process goes on for only a matter of days (Brulfert et al., 1988; Franco et al., 1990; Zotz and Winter, 1993). Therefore, the impact of CAM on growth of facultative plants is, in comparison with obligate CAM plants, only marginal. Nevertheless, a low dark CO2 fixation and/or recycling of respiratory CO2 may help maintain a positive carbon balance (or at least no C losses) under stress. Exceptions are the facultative species, Mesembryanthemum crystallinum (Bloom and Troughton, 1979) and M. nodiflorum (Winter and Troughton, 1978), in which, once induced, CAM is the sole CO2 fixation pathway and operates for a considerable length of time (months).
The great majority of species examined (Table 1) show low δ13C values that indicate little contribution of CAM to biomass production, but there are exceptions. In M. crystallinum dark CO2 fixation rate in the field satisfactorily accounted for relative growth rate and was higher than the photosynthetic rate of sympatric C3 species (Bloom and Troughton, 1979). Values of δ13C increased during the life cycle of M. crystallinum and M. nodiflorum from around −26 % to −14 ‰ (Winter and Troughton, 1978; Bloom and Troughton, 1979), highlighting the importance of nocturnal CO2 fixation for biomass production in these species.
REDUCTION OF RESPIRATORY CO2 LOSS
Recycling of respired CO2 during the night seems to be a universal role of CAM. Even though no nocturnal CO2 uptake may be observed, in facultative and cycling CAM species the imposition of water deficit results in maintenance or increases in ΔH+, indicating that an internal source of CO2 is being used for the carboxylation of PEP. A small respiration rate (<14 % of maximum daytime assimilation rate) or no nocturnal CO2 loss is found in facultative and CAM-cycling species, such as Sedum acre (Schuber and Kluge, 1981), Sedum pulchellum (Smith and Eickmeier, 1983; Martin et al., 1988), Cissus trifoliata (Olivares et al., 1984), T. triangulare (Herrera et al., 1991), Delosperma tradescantioides (Herppich et al., 1996) and Grahamia bracteata (Guralnick et al., 2008). Particularly under stressful conditions, recycling has been attributed the important role of reducing CO2 loss due to nocturnal respiration, which partially ameliorates damage to plant metabolism. Calculations made with published data on these species indicate that nocturnal CO2 loss of watered plants amounts to 6–20 % of diurnal balance. Since recycling of respiratory CO2 in droughted plants may be as high as 100 % (e.g. in T. triangulare; Herrera et al., 1991), this process is potentially saving on average 10 % CO2 that would otherwise be lost. In contrast, the value of recycling in watered CAM-cycling plants has been challenged by Martin (1996) on the grounds of the parity of energetic costs of daytime CO2 fixation versus recycling.
Internal recycling of respiratory CO2 may also contribute to growth. In plants of S. telephium subjected to water deficit under high or low PPF, an enhancement of CAM-cycling at high PPF not only helped water conservation but also allowed continued export of carbon to sinks and possibly continued growth (Borland, 1996). Under severe water deficit and low PPF which may limit photosynthesis, CAM served mainly as a maintenance mechanism providing a positive carbon balance through recycling of carbon skeletons at the expense of growth.
SUCCULENCE AND WATER SAVING
Survival may be increased in CAM plants thanks to increased WUE and succulence, which may delay dehydration. When four populations of the inducible species, Sedum wrightii, were compared, there was a correlation between leaf thickness and δ13C, the population with the thicker leaves showing the highest values of δ13C, and the population with the thinner leaves the lowest (Kalisz and Teeri, 1986). In contrast, in a survey of 214 orchid species, many of which showed obligate weak CAM (plants were watered daily), it was found that thick-leaved species had high δ13C and ΔH+ but there were exceptions in which thin leaves had high ΔH+ and the species with the thickest leaves, medium ΔH+ (Silvera et al., 2005). Re-examination of data provided by these authors shows that δ13C values grouped relative to a leaf thickness range of 0·2–4·0 mm in two clusters, one in the range of −18·1 to −11·8 ‰ and the other cluster in the range of −32·3 to −18·9 ‰, indicating that leaf thickness had apparently little to do with the degree of CAM. The authors acknowledge that anatomical studies are necessary to rule out that succulence is due to the presence of non-photosynthetic water-accumulating tissues.
In a study undertaken in the succulent Karoo – the very dry desert in South Africa and Namibia where most plants are succulent – the assumption that obligate CAM species have more succulent leaves than facultative CAM plants did not prove true; of three species studied, the least flexible one in the expression of CAM had the least succulent leaves (Veste et al., 2001). Examining the relationship between leaf anatomical traits and the operation of CAM, Nelson et al. (2005) found that leaves were thicker and with larger cells in CAM species than in C3 or C4 species, but in five out of 17 CAM species this variable did not differentiate them from C3 or C4 species; these CAM species with intermediate thickness were possibly C3-CAM. Facultative CAM is present mostly, with the possible exception of the arboreal genera Clusia, in which many species have a leaf hydrenchyma (Lüttge, 2007) and Pereskia (Edwards and Díaz, 2006), in ephemeral or deciduous perennial herbs and shrubs in which leaves tend to be less fleshy than in obligate CAM plants such as many Crassulaceae. The conclusion is that small succulence is not a characteristic feature of facultative CAM.
One variable that has proved useful in assessing the operation of CAM is the mesophyll succulence index, Sm, i.e. the ratio g leaf water : mg leaf chlorophyll (Kluge and Ting, 1978); a plant is considered CAM when Sm is higher than unit. In the inducible species C. trifoliata (Olivares et al., 1984), T. triangulare (Herrera et al., 1991) and T. paniculatum (Güerere et al., 1996), Sm was always higher than 1. The determination of Sm in the photosynthetic tissues of facultative CAM species should help establish the relationship between succulence and facultative CAM.
The operation of CAM is commonly induced by a few days of water deficit and CO2 fixation rapidly reverts to C3 upon watering (Herrera et al., 1991; Zotz and Winter, 1993). Under extended drought, the idling mode sets in. Thus, in the case of short duration of the CAM phase, the period during which a water-saving mechanism of carbon acquisition operates may be too short to contribute significantly to survival or leaf maintenance.
Potential water saving through the operation of CAM was only 5 % in plants of T. calycinum (Martin et al., 1988), whereas in S. telephium it ranged from 4 % to 77 % of daytime transpiration (Borland, 1996); in T. paniculatum water saved through recycling was between 5 and 12 times the amount lost by transpiration (Güerere et al., 1996). In contrast, Gravatt and Martin (1992) found no evidence of water conservation associated with CAM-cycling in several species of Sedum; similarly, Helliker and Martin (1997) found no significant differences in integrated WUE between a C3 and a C3-CAM species of Peperomia with similar life forms.
Morphological adaptations in CAM plants that contribute to increase WUE may be common to CAM and non-CAM species, thus obscuring the interpretation of the impact of CAM on WUE (Helliker and Martin, 1997). CAM induction ran parallel to increased leaf rolling in both T. triangulare (Herrera et al., 1991) and T. paniculatum (Güerere et al., 1996) and both mechanisms may have aided in the increased WUE. Unless manipulations of morphological characteristics, or comparison with congenerics lacking them, are carried out, distinguishing between their effect and those of CAM on plant performance (leaf duration, drought tolerance, among others) may lead to erroneous conclusions.
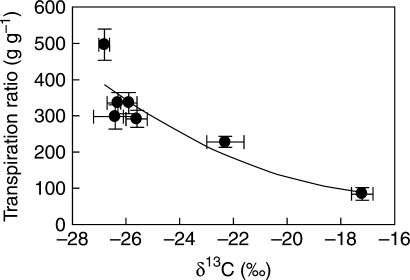
The increase in WUE due to the operation of CAM may range from nothing to 5-fold and a clear correlation between the magnitude of CAM induction and the resulting daily WUE has not proved universal in facultative species (Cushman and Borland, 2002). WUE was 6 (watered plants) and 12 (droughted plants) times higher during the night than the day in the obligate CAM species, Cissus quadrangularis (Virzo de Santo and Bartoli, 1996); in contrast, no difference in WUE was found between droughted plants of Cotyledon orbiculata (obligate CAM) and Othonna opina (C3), two succulents with similar growth form (Eller and Ferrari, 1997). These observations draw attention to the risks of over-generalizations. It is noteworthy, though, that in seven facultative species of Clusia, there was a high (r2 = 0·79) curvilinear negative regression between transpiration ratio (transpired water to biomass) and δ13C (Fig. 2), supporting the hypothesis that CAM increases WUE in facultative CAM species (Winter et al., 2005).
Fig. 2.
Relationship between transpiration ratio (mass H2O transpired : biomass) and carbon isotopic composition in several species of Clusia. Values are mean ± 1 s.e. The determination coefficient is r2 = 0·79. Data in Winter et al. (2005).
WATER ABSORPTION
CAM may not only serve for water saving but also for water collection. In droughted plants of the obligate CAM species Senecio medley-woodii, an increase in morning osmotic pressure and malate content correlated significantly with water uptake (Ruess et al., 1988). Also, accumulation of malate in the vacuole of the obligate CAM species Kalanchoë daigremontiana caused a reduction in leaf sap osmotic potential, which may favour water absorption (Smith and Lüttge, 1985). A nocturnal decrease of 2·2 MPa in leaf sap osmotic potential calculated from data of ΔH+ and sugar contents in C. minor (Lüttge, 2007) suggests that night-time water absorption is favoured by CAM.
Although examples of facultative CAM species similar to the findings in S. medley-woodii are not abundant in the literature, it is possible that the operation of CAM in facultative and CAM-cycling plants serves the same end. In C. minor, the proportion of daily sap flow occurring at night was strongly and positively correlated with the concentration of acid in sap taken from leaves at dawn, suggesting that the induction of CAM aided in water absorption through a decrease in leaf sap osmotic potential (Herrera et al., 2008).
PHOTOPROTECTION
Ever since Osmond (1982) suggested that internal recycling of respiratory CO2 might contribute to the protection of the photosynthetic machinery against high irradiance with closed stomata, an appreciable amount of evidence has been gathered, some of it in facultative CAM plants, in favour of this hypothesis.
Plants of C. minor grown under controlled conditions and subjected to water deficit for 16 d showed a significant, albeit small, decrease in maximum quantum yield of photosystem two (PSII; Fv/Fm = 0·80 down to 0·75), which was interpreted as a sign of chronic photoinhibition; this was reverted after 2 d of re-watering, highlighting the potential of CAM for photoprotection and recovery in this species (Mattos et al., 1999). Decreases in relative quantum yield of PSII (ϕPSII) and increases in the Stern–Volmer coefficient of non-photochemical quenching (NPQ) were pronounced in phases II and IV of CAM but did not occur on phase III, when decarboxylation of organic acids probably sustained a high intercellular CO2 concentration (Ci). The reduction in Fv/Fm was observed at a stage during the drought cycle when no CO2 fixation took place on phase IV and quenching by slow-relaxing components (qs) was highest.
When plants of the epiphytic bromeliads Guzmania lingulata (C3) and G. monostachia (CAM) without or with CAM induced by high PPF were compared, electron transport rate at midday suffered a decrease of 60 % (G. lingulata) and 30 % (G. monostachia in the C3 mode) relative to early morning and late afternoon values but remained nearly stable in G. monostachia in the CAM mode (Maxwell, 2002). These results lend support to the photoprotective role of internal recycling of CO2 produced by decarboxylation of acids when stomata are closed.
In T. triangulare, the operation of CAM appeared to contribute to the maintenance of photosynthetic capacity, since chlorophyll content remained unchanged under drought and photosynthetic rate was, a few hours after re-watering, even higher than at the beginning of drought (Herrera et al., 1991). Similarly, in plants of T. paniculatum droughted for 20 d, light-saturated photosynthetic rate and apparent quantum yield of PSII recovered to the values of watered plants after 24-h re-watering (Güerere et al., 1996). A high and positive correlation was found in droughted plants of T. triangulare between de-epoxidation state of xanthophylls, NPQ and ΔH+ (Pieters et al., 2003), supporting the hypothesis of the existence of a daytime sink for the products of photochemical activity when no external CO2 is fixed.
Although the possible relationship between zeaxanthin synthesis and ΔH+ is not clear from these results, it is tempting to speculate that it may be at the heart of a mechanistic explanation for the observed changes in these variables with drought. The operation of CAM in M. crystallinum inhibits the accumulation of zeaxanthin in guard cells, thus preventing stomatal opening in response to blue light (Tallman et al., 1997). The fact that CAM species close their stomata during the day may be the cause of increasing Ci and abscisic acid (ABA) (Tallman, 2004). Daytime decarboxylation combined with high rate of respiration due to high temperature would increase Ci and favour Calvin cycle activity in guard cell chloroplasts and consumption of NADPH, and prevent destruction of endogenous guard cell ABA, thus ensuring stomatal closure during the day (Tallman, 2004). This, together with the fact that zeaxanthin is a precursor of ABA (Tallman, 2004), could help link the observed lack of zeaxanthin synthesis in the guard cell chloroplast with the decrease in whole-leaf zeaxanthin in plants of T. triangulare in the idling mode.
Photoprotection through the xanthophyll cycle may not necessarily be more effective in CAM than in C3 species, as shown in a comparison of Clusia multiflora (obligate C3) and C. minor (Lüttge, 2007). Both species have the same life form and similar large, leathery leaves. Zeaxanthin content and NPQ were equally large in both species subjected to high PPF, precluding a specific role of CAM in energy dissipation. Nevertheless, leaves of C. multiflora became necrotic and died when plants were maintained for a long period under high PPF, whereas leaves of C. minor, by switching to CAM, did not.
Another aspect in which the operation of CAM can confer photoprotection is the processing of reactive oxygen species that appear when PSII is over-energized during phase III behind closed stomata with high PPF, low Ci, low NADP content and high intercellular O2 concentration (Niewiadomska and Borland, 2008). Plants of M. crystallinum with CAM induced by salinity and exposed to high ozone concentration in the air –in order to cause oxidative stress – showed no signs of oxidative damage, in contrast to plants operating in the C3 mode, which showed severe necrosis and a 31 % reduction in Fv/Fm; photoprotection in CAM plants was attributed to the up-regulation of CuZn-superoxide dismutase, one of the enzymes of the antioxidative response system (Hurst et al., 2004). When plants of a CAM-less mutant of M. crystallinum and the wild type were subjected to salinity, the activities of several isoforms of superoxide dismutase used as markers for the production of reactive oxygen species increased in both genotypes, but this increase was greatest in the mutant, indicating potentially smaller oxidative load in the wild type (Borland et al., 2006). However, a causal relationship between increased activity of enzymes of the antioxidative response system, the xanthophyll cycle and the operation of CAM awaits disclosure.
REPRODUCTION
The operation of CAM may be associated with an increase in fitness by increasing reproduction under drought. Exposing plants of M. crystallinum, with CAM induced, to CO2-free air during the night caused a reduction in both ΔH+ and reproductive output, suggesting a direct relationship between the operation of CAM and fecundity (Winter and Ziegler, 1992). Moreover, CAM-less mutants of this species had lower fecundity than the wild type (Cushman et al., 2008). In plants of M. nodiflorum (Sayed and Hegazy, 1994) both ΔH+ and reproductive output increased during summer, and CAM induction was interpreted as a strategy to extend resource allocation to reproductive biomass and maximize reproductive yield before the end of the life-span.
Similarly, in five CAM-cycling species of Talinum, the ratio droughted : watered plants for reproductive, but not for vegetative, biomass was correlated with ΔH+ (Harris and Martin, 1991). A high positive correlation was found in T. triangulare between ΔH+ and reproductive effort (reproductive mass : leaf mass) under both laboratory (Taisma and Herrera, 1998) and field conditions (Taisma and Herrera, 2003). Also, the fecundity of plants of T. triangulare, in which induction of CAM by drought was prevented by manipulation of the photoperiod, was drastically reduced relative to plants with CAM induced by drought under normal photoperiod (Herrera, 1999).
Another role of CAM induction by drought may be to provide an important source of energy for reproduction. Fruits of M. crystallinum exhibited higher δ13C values than leaves (−11 ‰ vs. −13 ‰), indicating that carbon used for inflorescence development was primarily fixed through CAM late in the growing season (Winter, 1985). In contrast, no difference was found in δ13C of fruits and leaves of plants of T. triangulare under natural drought, suggesting that the source of carbon for fruit set was not different from that for leaf production (Taisma and Herrera, 2003) and that the contribution of dark CO2 uptake to reproduction was as small as it was to carbon balance of leaves.
An increase in WUE of plants of T. triangulare subjected to natural water supply in the field, possibly due to the joint effect of the operation of CAM, leaf rolling and increased reflectance and angle, may help explain the observed increase in reproductive effort (Taisma and Herrera, 2003) and so far its is not possible to pinpoint any of the variables studied as causing increased reproduction.
DURATION OF INDUCED CAM DURING THE LIFE CYCLE
The period during which drought-induced nocturnal CO2 fixation occurs may be long (months), as in M. crystallinum (Bloom and Troughton, 1979), or short (hours to a few days), as in Clusia uvitana (Zotz and Winter, 1993), T. triangulare (Herrera et al., 1991), C. minor (Lee and Griffiths, 1987), S. nuttallianum (Martin and Jackson, 1986), S. acre and S. mitte (Schuber and Kluge, 1981). Inducible CAM as well as CAM-cycling may help extend the time the plant is physiologically active during prolonged drought (Martin and Jackson, 1986; Sayed and Hegazy, 1994). A frequently cited example of long-term functioning of induced CAM is that of M. crystallinum, in which plants begin exhibiting CAM as the dry summer season approaches and remain in this mode until the end of the life cycle. Evidence in favour of the adaptive character of the operation of CAM in plants of M. crystallinum and M. nodiflorum under dry and saline conditions is very convincing and the induction and operation of CAM by drought has been closely followed in the field (Winter and Troughton, 1978; Bloom and Troughton, 1979).
Nevertheless, many facultative CAM species, such as C. trifoliata, T. triangulare and T. paniculatum, are deciduous perennials which lose their leaves under extreme drought, yet show CAM after as little as 1 d under drought, and resort to idling after 10–20 d of drought. What is real life like for these species? For how long under natural conditions may induced CAM play its role in the prevention of the impairment of, or help optimize physiological performance under drought?
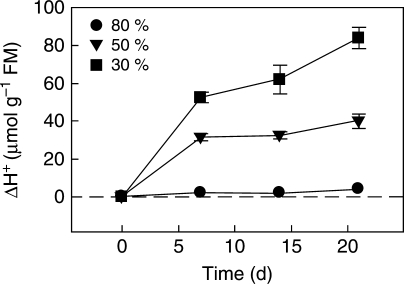
Species of Sedum and Talinum inhabit rock outcrops, and semi-arid as well as seasonally dry environments, and may experience short periods of drought followed by rains in an unpredictable manner. The possibility exists that they spend a significant length of time in the CAM mode, given an adequately low water soil content. Watering plants of T. triangulare to 80 % field capacity prevented the induction of CAM, whereas 50 % and 30 % field capacity increasingly promoted nocturnal acid accumulation; ΔH+ in the 50 % treatment tended to a plateau, whereas at 30 % it tended to increase with the duration of the dry period (Irazábal, 2005; Fig. 3). Similarly, low rates of nocturnal CO2 uptake were observed for 80 d in plants of S. pulchellum maintained at a soil water potential of −5·0 to −8·5 MPa but not at −1·5 to −4·0 MPa, when either no net loss or gain or respiration were observed during the night (Smith and Eickmeier, 1983).
Fig. 3.
Time-course of changes in nocturnal acid accumulation on a fresh mass basis in plants of the facultative CAM plant, Talinum triangulare, watered to 80 % (circles), 50 % (triangles) and 30 % (squares) field capacity. Values are mean ± 1 s.e. Modified from Irazábal (2005).
The above-described experiments may reflect the actual situation in the field: C3 photosynthesis under abundant rainfall, induced CAM at a moderate level in partly wet soil for a considerable length of time, and CAM idling in a dry soil until leaf shedding occurs. As in the case of C. minor, in which it is so plastic, CAM may in rooted individuals make a major contribution to biomass by operating for a longer period than short-term water-deficit experiments (Franco et al., 1990) or measurements on hemi-epiphytic individuals (Zotz and Winter, 1993) suggest.
Many of the data reported in the references in Table 1 were gathered in experiments done in pots to which watering was withheld and pots let to dry out. Clearly, operation of CAM in the field, with a very large rooting volume (equivalent to a very large ‘pot’ volume) and slow drying, continues for much longer than in pot experiments. This brings us to the issue of low δ13C values in facultative CAM plants.
With the possible exception of M. crystallinum, facultative CAM plants tend to show δ13C values which are more C3- than CAM-like, the extreme being found in one experiment on S. nuttallianum under controlled conditions, in which δ13C was −30 ‰ [but see comment by Martin and Jackson (1986) on the carbon isotope composition of the air in this experiment] and in the study of orquids in Panama, in which a δ13C value as low as −30·0 ‰ was associated with a significant ΔH+ (Silvera et al., 2005). Even in facultative species of Clusia, which may easily and reversibly shift from C3 to CAM in a matter of days throughout the seasons, values of δ13C much higher than −21 ‰ have not been found (Lüttge, 2007). Such low values indicate that carbon is mostly acquired through C3 photosynthesis and dark CO2 fixation makes only a small contribution to δ13C.
Values of δ13C as high as −14 ‰, typical of a full CAM plant, were found in low-altitude populations of S. wrightii, whereas those populations at higher altitudes tended towards −23 ‰, a more C3-like value (Kalisz and Teeri, 1986). This points to the possibility that the lowland populations spent more of their life cycle in the CAM mode than the highland ones, although the effect of altitude on air δ13C and Ci should be evaluated.
CONCLUSIONS AND FUTURE RESEARCH
In the great majority of facultative CAM and CAM-cycling species examined, dark CO2 fixation represented <30 % of the daily carbon balance of watered plants. Examination, by no means exhaustive, of evidence suggests that these modes of carbon acquisition have an important role in water balance, photo-protection and reproduction. In order to aid in our understanding of these complex forms of carbon acquisition and elucidate the potential of facultative CAM for biomass (vegetative as well as reproductive) production, the following are needed.
Experiments under controlled conditions. Subjecting to water deficit CAM-deficient mutants, such as those obtained for M. crystallinum (Cushman et al., 2008) or wild-type plants under environmental manipulations that prevent CAM induction, such as T. triangulare under interrupted dark periods (Herrera, 1999), and determining the impact of the lack of CAM operation on biomass maintenance/growth, photoprotection and reproduction.
Field studies. More long-term field studies are necessary on the performance of facultative, especially drought-deciduous, CAM plants when they remain for long periods under the induced state; determination of 24-h carbon balance, WUE, productivity, reproductive effort and survival in static cohorts of natural populations subjected to abiotic and biotic factors (Taisma and Herrera, 1998, 2003) could help clarify the role of induced CAM on fitness.
Among matters pending is whether facultative CAM and CAM-cycling merely represent an intermediate step between C3 and obligate CAM possessing neutral characters that neither promote nor hinder adaptation; this issue could be settled by extended research into the evolution of CAM.
ACKNOWLEDGEMENTS
Thanks are due to P. S. Nobel and C. E. Martin for permission to use their published material to draw Fig. 1, and to S. Irazábal for letting me use her data to draw Fig. 3.
LITERATURE CITED
- Bloom AJ, Troughton JH. High productivity and photosynthetic flexibility in a CAM plant. Oecologia. 1979;38:35–43. doi: 10.1007/BF00347822. [DOI] [PubMed] [Google Scholar]
- Borland AM. A model for the partitioning of photosynthetically fixed carbon during the C3-CAM transition in Sedum telephium. New Phytologist. 1996;134:433–444. [Google Scholar]
- Borland AM, Griffiths H. The regulation of CAM and respiratory recycling by water supply and light regime in the C3-CAM intermediate Sedum telephium. Functional Ecology. 1990;4:33–39. [Google Scholar]
- Borland AM, Laszlo IT, Leegood RC, Walker RP. Inducibility of crassulacean acid metabolism (CAM) in Clusia species; physiological/biochemical characterisation and intercellular localization of carboxylation and decarboxylation processes in three species which exhibit different degrees of CAM. Planta. 1998;205:342–351. [Google Scholar]
- Borland A, Elliott S, Patterson S, Taybi T, Cushman J, Pater B, et al. Are the metabolic components of crassulacean acid metabolism up-regulated in response to an increase in oxidative burden? Journal of Experimental Botany. 2006;57:319–328. doi: 10.1093/jxb/erj028. [DOI] [PubMed] [Google Scholar]
- Brulfert J, Kluge M, Glüçlü S, Queiroz O. Interaction of photoperiod and drought as CAM inducing factors in Kalanchoë blossfeldiana Poelln. cv. Tom Thumb. Journal of Plant Physiology. 1988;133:222–227. [Google Scholar]
- Castillo FJ. Antioxidative protection in the inducible CAM plant Sedum album L. following the imposition of severe water stress and recovery. Oecologia. 1996;107:469–477. doi: 10.1007/BF00333937. [DOI] [PubMed] [Google Scholar]
- Cushman JC. Crassulacean acid metabolism: a plastic photosynthetic adaptation to arid environments. Plant Physiology. 2001;127:1439–1448. [PMC free article] [PubMed] [Google Scholar]
- Cushman JC, Bohnert HJ. Crassulacean acid metabolism: molecular genetics. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:305–332. doi: 10.1146/annurev.arplant.50.1.305. [DOI] [PubMed] [Google Scholar]
- Cushman JC, Borland AM. Induction of Crassulacean acid metabolism by water limitation. Plant, Cell & Environment. 2002;25:295–310. doi: 10.1046/j.0016-8025.2001.00760.x. [DOI] [PubMed] [Google Scholar]
- Cushman JC, Agarie S, Albion RL, Elliot SM, Taybi T, Borland AM. Isolation and characterization of mutants of common ice plant deficient in crassulacean acid metabolism. Plant Physiology. 2008;147:228–238. doi: 10.1104/pp.108.116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw MJ, Carver KA, Lee JA. Changes in leaf water potential and CAM in Sempervivum montanum and Sedum album in response to water availability in the field. Oecologia. 1985;67:486–492. doi: 10.1007/BF00790018. [DOI] [PubMed] [Google Scholar]
- Earnshaw MJ, Carver KA, Charlton WA. Leaf anatomy, water relations and crassulacean acid metabolism in the chlorenchyma and colourless internal water-storage tissue of Carpobrotus edulis and Senecio ?mandraliscae. Planta. 1987;170:421–432. doi: 10.1007/BF00395036. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Díaz M. Ecological physiology of Pereskia guamacho, a cactus with leaves. Plant, Cell & Environment. 2006;29:247–256. doi: 10.1111/j.1365-3040.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- Eller BM, Ferrari S. Water use efficiency of two succulents with contrasting CO2 fixation pathways. Plant, Cell & Environment. 1997;20:93–100. [Google Scholar]
- Franco AC, Ball E, Lüttge U. Patterns of gas exchange and organic acid oscillations in tropical trees of the genus Clusia. Oecologia. 1990;85:108–114. doi: 10.1007/BF00317350. [DOI] [PubMed] [Google Scholar]
- Futuyma D. Evolutionary biology. Sunderland, MA: Sinauer Associates; 1986. [Google Scholar]
- Gravatt DA, Martin CE. Comparative ecophysiology of five species of Sedum (Crassulaceae) under well-watered and drought-stressed conditions. Oecologia. 1992;92:532–541. doi: 10.1007/BF00317845. [DOI] [PubMed] [Google Scholar]
- Güerere I, Tezara W, Herrera C, Fernández MD, Herrera A. Recycling of CO2 during induction of CAM by drought in Talinum paniculatum (Portulacaceae) Physiologia Plantarum. 1996;98:471–476. [Google Scholar]
- Guralnick LJ, Rorabaugh PA, Hanscom Z., III Influence of photoperiod and leaf age on crassulacean acid metabolism in Portulacaria afra (L.) Jacq. Plant Physiology. 1984;a 75:454–457. doi: 10.1104/pp.75.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnick LJ, Rorabaugh PA, Hanscom Z., III Seasonal shifts of photosynthesis in Portulacaria afra (L.) Jacq. Plant Physiology. 1984;b 76:643–646. doi: 10.1104/pp.76.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnick LJ, Cline A, Smith M, Sage RF. Evolutionary physiology: the extent of C4 and CAM photosynthesis in the genera Anacampseros and Grahamia of the Portulacaceae. Journal of Experimental Botany. 2008;59:1735–1742. doi: 10.1093/jxb/ern081. [DOI] [PubMed] [Google Scholar]
- Hanscom Z, Ting IP. Irrigation magnifies CAM-photosynthesis in Opuntia basilaris (Cactaceae) Oecologia. 1978;33:1–15. doi: 10.1007/BF00376993. [DOI] [PubMed] [Google Scholar]
- Harris FS, Martin CE. Plasticity in the degree of CAM-cycling and its relationship to drought stress in five species of Talinum (Portulacaceae) Oecologia. 1991;86:575–584. doi: 10.1007/BF00318325. [DOI] [PubMed] [Google Scholar]
- Helliker BR, Martin CE. Comparative water-use efficiencies of three species of Peperomia (Piperaceae) having different photosynthetic pathways. Journal of Plant Physiology. 1997;150:259–263. [Google Scholar]
- Herppich WB. Stomatal response to changes in air humidity are not necessarily linked to nocturnal CO2 uptake in the CAM plant Plectranthus marrubioides Benth. (Lamiaceae) Plant, Cell & Environment. 1997;20:393–399. [Google Scholar]
- Herppich WB, Midgley G, von Willert DJ, Veste M. CAM variations in the leaf-succulent Delosperma tradescantioides (Mesembryanthemaceae), native to southern Africa. Physiologia Plantarum. 1996;98:485–492. [Google Scholar]
- Herrera A. Effects of photoperiod and drought on the induction of CAM and the reproduction of plants of Talinum triangulare. Canadian Journal of Botany. 1999;77:1–6. [Google Scholar]
- Herrera A, Delgado J, Paraguatey I. Occurrence of Crassulacean acid metabolism in Talinum triangulare (Portulacaceae) Journal of Experimental Botany. 1991;42:493–499. [Google Scholar]
- Herrera A, Ballestrini C, Tezara W. Nocturnal sap flow in the C3-CAM species. Clusia minor. Trees – Structure and Function. 2008;22:491–497. [Google Scholar]
- Holtum JAM, Winter K, Weeks MA, Sexton TR. Crassulacean acid metabolism in the ZZ plant, Zamioculcas zamiifolia (Araceae) American Journal of Botany. 2007;94:1670–1676. doi: 10.3732/ajb.94.10.1670. [DOI] [PubMed] [Google Scholar]
- Hurst AC, Grams TEE, Ratajczak R. Effects of salinity, high irradiance, ozone, and ethylene on mode of photosynthesis, oxidative stress and oxidative damage in the C3/CAM intermediate plant Mesembryanthemum crystallinum L. Plant, Cell & Environment. 2004;27:187–197. [Google Scholar]
- Irazábal S. Balance estacional de carbono y distribución de asimilados en la especie CAM facultativa. Universidad Central de Venezuela; 2005. Talinum triangulare. Licenciado Thesis. [Google Scholar]
- Kalisz S, Teeri JA. Population-level variation in photosynthetic metabolism and growth in Sedum wrightii. Ecology. 1986;67:20–26. [Google Scholar]
- Kluge M, Ting IP. Crassulacean acid metabolism. Berlin: Springer-Verlag; 1978. [Google Scholar]
- Lee HSJ, Griffiths H. Induction and repression of CAM in Sedum telephium L. in response to photoperiod and water stress. Journal of Experimental Botany. 1987;38:187–192. [Google Scholar]
- Lüttge U. Clusia: a woody neotropical genus of remarkable plasticity and diversity. Heidelberg: Springer-Verlag; 2007. [Google Scholar]
- Martin CE. Recycling of CO2 via crassulacean acid metabolism. In: Winter K, Smith JAC, editors. Crassulacean acid metabolism. Berlin: Springer-Verlag; 1996. pp. 192–203. [Google Scholar]
- Martin CE, Jackson JL. Photosynthetic pathway in a midwestern rock outcrop succulent, Sedum nuttallianum Raf. (Crassulaceae) Photosynthesis Research. 1986;8:17–29. doi: 10.1007/BF00028473. [DOI] [PubMed] [Google Scholar]
- Martin CE, Zee AK. C3 photosynthesis and CAM in a Kansas rock outcrop succulent, Talinum calycinum (Portulacaceae) Plant Physiology. 1983;73:718–723. doi: 10.1104/pp.73.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Gravatt DA, Loeschen VS. Crassulacean acid metabolism in three species of Commelinaceae. Annals of Botany. 1994;74:457–463. [Google Scholar]
- Martin CE, Higley M, Wang W-Z. Ecophysiological significance of CO2-recycling via CAM in Talinum calycinum Engelm. (Portulacaceae) Plant Physiology. 1988;86:562–568. doi: 10.1104/pp.86.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Loeschen VS, Coke LB. Crassulacean acid metabolism in selected terrestrial succulents in southeastern Jamaica, including two species in the Commelinaceae. Oecologia. 1990;84:99–102. doi: 10.1007/BF00665601. [DOI] [PubMed] [Google Scholar]
- Mattos EA, Herzog B, Lüttge U. Chlorophyll fluorescence during CAM-phases in Clusia minor L. under drought stress. Journal of Experimental Botany. 1999;331:253–261. [Google Scholar]
- Maxwell K. Resistance is useful: diurnal patterns of photosynthesis in C3 and crassulacean acid metabolism epiphytic bromeliads. Functional Plant Biology. 2002;29:679–687. doi: 10.1071/PP01193. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Sage TL, Sage RF. Functional leaf anatomy of plants with crassulacean acid metabolism. Functional Plant Biology. 2005;32:409–419. doi: 10.1071/FP04195. [DOI] [PubMed] [Google Scholar]
- Niewiadomska E, Borland AM. Crassulacean acid metabolism: a cause or consequence of oxidative stress in planta? Progress in Botany. 2008;69:247–266. [Google Scholar]
- Nobel PS. Environmental biology of agaves and cacti. Cambridge: Cambridge University Press; 1988. pp. 45–50. [Google Scholar]
- Nobel PS, Hartsock TL. Relationships between photosynthetically active radiation, nocturnal acid accumulation, and CO2 uptake for a crassulacean acid metabolism plant, Opuntia ficus-indica. Plant Physiology. 1983;71:71–75. doi: 10.1104/pp.71.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares E, Urich R, Montes G, Coronel I, Herrera A. The occurrence of crassulacean acid metabolism in Cissus trifoliata L. (Vitaceae) Oecologia. 1984;61:358–362. doi: 10.1007/BF00379635. [DOI] [PubMed] [Google Scholar]
- Osmond CB. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology. 1978;29:379–414. [Google Scholar]
- Osmond CB. Carbon cycling and stability of the photosynthetic apparatus in CAM. In: Ting IP, Gibbs M, editors. Crassulacean acid metabolism. Rockville, MD: American Society of Plant Physiology; 1982. pp. 112–127. [Google Scholar]
- Ota K. Stimulation of CAM photosynthesis in Kalanchoë blossfeldiana by transferring to nitrogen-deficient conditions. Plant Physiology. 1988;87:454–457. doi: 10.1104/pp.87.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Cockburn W. The stimulation of CAM activity in Mesembryanthemum crystallinum in nitrate- and phosphate-deficient conditions. New Phytologist. 1990;114:391–398. doi: 10.1111/j.1469-8137.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Pieters AJ, Tezara W, Herrera A. Operation of the xanthophyll cycle and degradation of D1 protein in the inducible CAM plant, Talinum triangulare, under water deficit. Annals of Botany. 2003;92:393–399. doi: 10.1093/aob/mcg153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruess BR, Ferrari S, Eller BM. Water economy and photosynthesis of the CAM plant Senecio medley-woodii during increasing drought. Plant, Cell & Environment. 1988;11:583–589. [Google Scholar]
- Rundel PW, Esler KJ, Cowling RM. Ecological and phylogenetic patterns of carbon isotope discrimination in the winter-rainfall flora of the Richtersveld, South Africa. Plant Ecology. 1999;142:133–148. [Google Scholar]
- Sayed OH, Hegazy AK. Growth-specific phytomass allocation in Mesembryanthemum nodiiflorum as influenced by CAM induction in the field. Journal of Arid Environments. 1994;27:325–329. [Google Scholar]
- Schmitt AK, Lee HSJ, Lüttge U. The response of the C3-CAM tree, Clusia rosea, to light and water stress. I. Gas exchange characteristics. Journal of Experimental Botany. 1988;39:1581–1590. [Google Scholar]
- Schuber M, Kluge M. In situ studies on CAM in Sedum acre L. and Sedum mite Gil. Oecologia. 1981;50:82–87. doi: 10.1007/BF00378797. [DOI] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Winter K. Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Functional Plant Biology. 2005;32:397–407. doi: 10.1071/FP04179. [DOI] [PubMed] [Google Scholar]
- Smith JAC, Lüttge U. Day-night changes in leaf water relations associated with the rhythm of crassulacean acid metabolism in Kalanchoë daigremontiana. Planta. 1985;163:272–282. doi: 10.1007/BF00393518. [DOI] [PubMed] [Google Scholar]
- Smith JAC, Winter K. Taxonomic distribution of crassulacean acid metabolism. In: Winter K, Smith JAC, editors. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag; 1996. pp. 427–436. [Google Scholar]
- Smith TL, Eickmeier WG. Limited photosynthetic plasticity in Sedum pulchellum Michx. Oecologia. 1983;56:374–80. doi: 10.1007/BF00379715. [DOI] [PubMed] [Google Scholar]
- Tallman G. Are diurnal patterns of stomatal movement the result of alternating metabolism of endogenous guard cell ABA and accumulation of ABA delivered to the apoplast around guard cells by transpiration? Journal of Experimental Botany. 2004;55:1963–1976. doi: 10.1093/jxb/erh212. [DOI] [PubMed] [Google Scholar]
- Tallman G, Zhu J, Mawson BT, Amodeo G, Nouhi Z, Levy K, Zeiger E. Induction of CAM in Mesembryanthemum crystallinum abolishes the stomatal response to blue light and light-dependent zeaxanthin formation in guard cell chloroplasts. Plant and Cell Physiology. 1997;38:236–242. [Google Scholar]
- Taisma MA, Herrera A. A relationship between fecundity, survival and the operation of CAM in T. triangulare. Canadian Journal of Botany. 1998;7:1–8. [Google Scholar]
- Taisma MA, Herrera A. Drought under natural conditions affects leaf properties, induces CAM and promotes reproduction in plants of Talinum triangulare. Interciencia. 2003;28:1–6. [Google Scholar]
- Ting IP. Crassulacean acid metabolism. Annual Review of Plant Physiology. 1985;36:595–622. [Google Scholar]
- Ting IP, Bates L, Sternberg LO, Deniro MJ. Physiological and isotopic aspects of photosynthesis in Peperomia. Plant Physiology. 1985;78:246–249. doi: 10.1104/pp.78.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veste M, Herppich WB, von Willert DJ. Variability of CAM in leaf-deciduous succulents from the succulent Karoo (South Africa) Basic and Applied Ecology. 2001;2:283–288. [Google Scholar]
- Virzo de Santo A, Bartoli G. Crassulacean acid metabolism in leaves and stems of Cissus quadrangularis. In: Winter K, Smith JAC, editors. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag; 1996. pp. 216–229. [Google Scholar]
- Winter K. NaCl-induzierter Crassulaceensäurestoffwechsel bei einer weiteren Aizoaceae: Carpobrotus edulis. Planta. 1973;115:187–188. doi: 10.1007/BF00387783. [DOI] [PubMed] [Google Scholar]
- Winter K. Crassulacean acid metabolism. In: Barber J, Barker NR, editors. Photosynthetic mechanism and the environment. Amsterdam: Elsevier; 1985. pp. 329–387. [Google Scholar]
- Winter K, Gademann R. Daily changes in CO2 and water vapor exchange, chlorophyll fluorescence, and leaf water relations in the halophyte Mesembryanthemum crystallinum during the induction of crassulacean acid metabolism in response to high NaCl salinity. Plant Physiology. 1991;95:768–776. doi: 10.1104/pp.95.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology. 2002;129:1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Smith JAC. An introduction to crassulacean acid metabolism: biochemical principles and ecological diversity. In: Winter K, Smith JAC, editors. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin: Springer-Verlag; 1996. pp. 1–13. [Google Scholar]
- Winter K, Troughton JH. Carbon assimilation pathways in Mesembryanthemum nodiflorum L. under natural conditions. Zeitschrift für Pflanzenphysiologie. 1978;88:153–162. [Google Scholar]
- Winter K, von Willert DJ. NaCl-induzierter Crassulaceensäurestoffwechsel bei Mesembryanthemum crystallinum. Zeitschrift für Pflanzenphysiologie. 1972;67:166–170. [Google Scholar]
- Winter K, Ziegler H. Induction of crassulacean acid metabolism in Mesembryanthemum crystallinum increases reproductive success under conditions of drought and salinity stress. Oecologia. 1992;92:475–479. doi: 10.1007/BF00317838. [DOI] [PubMed] [Google Scholar]
- Winter K, Zotz G, Baur B, Dietz K. Light and dark CO2 fixation in Clusia uvitana and the effects of plant water status and CO2 availability. Oecologia. 1992;91:47–51. doi: 10.1007/BF00317239. [DOI] [PubMed] [Google Scholar]
- Winter K, Aranda J, Holtum JAM. Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism. Functional Plant Biology. 2005;32:381–388. doi: 10.1071/FP04123. [DOI] [PubMed] [Google Scholar]
- Zotz G. Categories and CAM – blurring divisions, increasing understanding? New Phytologist. 2002;156:6–8. [Google Scholar]
- Zotz G, Winter K. Short-term regulation of crassulacean acid metabolism activity in a tropical hemiepiphyte, Clusia uvitana. Plant Physiology. 1993;102:835–841. doi: 10.1104/pp.102.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]