Abstract
Brain inflammation is a complex cellular and molecular response to stress, injury or infection of the central nervous system (CNS) in attempt to defend against insults, clear dead and damaged neurons and return the CNS to a normal state. Inflammation in the CNS is driven by the activation of resident microglia, astrocytes and infiltrating peripheral macrophages, which release a plethora of anti- and pro-inflammatory cytokines, chemokines, neurotransmitters and reactive oxygen species. This inflammatory state inadvertently causes further bystander damage to neurons and produces both detrimental and favorable conditions for neurogenesis. Inflammatory factors have varying effects on neural progenitor cell (NPC) proliferation, migration, differentiation, survival and incorporation of newly born neurons into the CNS circuitry. The unique profile of inflammatory factors, which depends on the severity of inflammation, can have varying consequences on neurogenesis. Inflammatory factors released during mild acute inflammation usually stimulate neurogenesis; where as the factors released by uncontrolled inflammation create an environment that is detrimental to neurogenesis. This review will provide a summary of current progress in this emerging field and examine the potential mechanisms through which inflammation affects neurogenesis during neurological complications.
Keywords: Inflammation, cytokines, chemokines, neural stem/progenitor cell, neurodegenerative disorders
Introduction
Neurogenesis is the process of creating new neurons from neural stem/progenitor cells (Gage 2000; Emsley et al. 2005). The generation of neurons occurs primarily during development, but it is now accepted that neurogenesis occurs throughout adult life. In the adult mammalian central nervous system (CNS), neurogenesis occurs in two primary areas of the CNS. Neural progenitor cells (NPCs) in the subgranular zone (SGZ) of the dentate gyrus (DG) generate neurons in the hippocampus, and NPCs from the subventricular zone (SVZ) migrate along the rostral migratory stream to generate neurons in the olfactory bulb (Ming and Song 2005). In the healthy adult CNS, constitutive neurogenesis is involved in learning and memory as well as neural turnover in the olfactory bulb.
Though deficits resulting from brain injury and neurodegenerative disorders are primarily due to the damage and death of neurons, disruption of endogenous neurogenesis also contributes to neurological deficits and hinders the recovery from insults to the CNS (Krathwohl and Kaiser 2004a, b; Kaul 2008; Peng et al. 2008a; Waldau and Shetty 2008). Brian injury and neurodegenerative disorders are associated with acute and chronic brain inflammation. The relationship between brain inflammation and the regulation of neurogenesis has been well documented and remains the subject of intense investigation. Notably, recent collective evidence indicates that neurogenesis is affected during brain injury and neurodegenerative disorders by the dysregulation of cytokines, chemokines, neurotransmitters and reactive oxygen species caused by inflammation and mediated by activated macrophages, microglia and reactive astrocytes. This review provides an overview of recent progress on the research of chemokines, cytokines, neurotransmitters and reactive oxygen species and their roles in the regulation of neurogenesis during various pathological conditions, as well as the cells involved in propagating CNS inflammation. Understanding the involvement of inflammation in the pathogenesis of CNS diseases through changes in neurogenesis may potentially provide the opportunity to identify particular therapeutic targets for the treatment of neurodegenerative disorders and neuronal injury. This review utilized Pubmed literature searches to examine recent reports on the influence of inflammation on neurogenesis.
Neurogenesis
Neurogenesis occurs primarily during development; however, the formation of new neurons continues throughout life. Adult neurogenesis occurs primarily in the hippocampus from SGZ NPCs and in the olfactory bulb from NPCs in the SVZ, but neurogenesis also occurs in other areas throughout the CNS, albeit at lower levels (Taupin 2005). NPCs are found in many other regions of the CNS, including the neocortex (Magavi et al. 2000), spinal cord (Yamamoto et al. 2001; Yamamoto Si et al. 2001; Chen et al. 2004), tegmentum (Hermann et al. 2006), substantia nigra (SN) (Zhao et al. 2003), amygdala (Bernier et al. 2002) and brainstem (St-John 1998). The functions of these NPCs are unknown, but it has been speculated that they are waiting for some stimulus to induce neurogenesis; what these stimuli are, or how they stimulate is not yet understood. If these signals were elucidated, they could be used to induce neurogenesis in non-neurogenic regions by stimulating local NPCs or recruiting NPCs from neurogenic areas to other areas of the brain.
Multiple steps are involved in neurogenesis including proliferation, migration, differentiation, survival and integration of the newly formed neurons into the circuitry of the CNS (Ming and Song 2005). Neurogenesis is often defined as an enhancement of any of these steps; however, true neurogenesis requires that NPCs properly proliferate, migrate, differentiate, integrate and survive. In this review we will refer to increases in any of these steps as neurogenesis, even though this might not equate to successful neurogenesis.
Inflammation in the CNS
Inflammation is a complex cellular and molecular response to stress, injury or infection that attempts to defend against insults, to clear dead and damaged cells and to return the affected area to a normal state. Inflammation in the brain, however, is different from that in peripheral tissues in many ways such as initiation and sensitivity to inflammation. The brain is immune privileged due to its protection by the blood-brain barrier (BBB), which only allows certain molecules and cells to enter and exit. Due to the selective permeability of this barrier, only T cells, macrophages and dendritic cells can enter the CNS under normal physiological conditions (Hickey 1999). Following damage or exposure to pathogen, an inflammatory process is initiated by the activation of resident microglia and astrocytes as well as infiltrating peripheral macrophages and lymphocytes. Activated microglia, astrocytes, macrophages and lymphocytes release a plethora of anti- and pro-inflammatory cytokines (i.e. interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-18 (IL-18) and interleukin-6 (IL-6)), chemokines (i.e. stromal cell-derived factor-1 alpha (SDF-1α) and monocyte chemoattractant protein-1 (MCP-1)), neurotransmitters (i.e. glutamate) and reactive oxygen species (i.e. nitric oxide) (Table 1). These factors disrupt the BBB and recruit monocytes and lymphocytes to cross through the BBB to the site of inflammation (Hickey 1999; Lossinsky and Shivers 2004; Taupin 2008) in addition to recruiting resident microglia and stimulating astrogliosis. These newly recruited cells become activated and release more inflammatory factors, creating a positive feedback loop that results in neuronal damage and changes in neurogenesis (Das and Basu 2008). This process inadvertently causes further bystander damage to neurons and causes both detrimental and positive consequences to neurogenesis.
Table 1.
Cytokine ligand and receptor expression in the CNS
| Inflammatory Factor | Receptors | Inflammatory Factor Expression Cell Type |
References |
|---|---|---|---|
| IFN-γ | IFN-γ R | T-cells | Griffin 1997 |
| IFN-α | IFN-α R | Macrophage Microgila | Vezzani et al. 2002, Kast 2002, Peng et al. 2008 |
| IL-1β | IL-1β R | Macrophage Microgila | Peng et al. 2006, Peng et al. 2008, Zhao et al, 2001 |
| IL-18 | IL-18 R | Macrophage Microgila | Conti et al.1998 |
| IL-6 | IL-6 R | Macrophage Microgila | Monje et al. 2003, Nakanishi et al. 2007 |
| SDF-1α | CXCR4 CXCR7 | Astrocytes | Imitola et al. 2004, Peng et al. 2006 |
| MCP-1 | CCR2 | Macrophage Microgila Astrocytes | Conant et al. 1998, Cinque et al. 1998 |
In order to minimize this reciprocating cycle, reduce neuronal cell death and maintain the vulnerable microenvironment in the CNS, uncontrolled activation is avoided by increasing the threshold needed to initiate an immune response. Thus, the CNS requires higher levels of antigen or damage compared to the levels in the periphery to induce an inflammatory response (Matyszak 1998; Perry 1998). The severity of inflammation in the CNS varies from mild acute to uncontrolled chronic inflammation, and the profile of inflammatory factors differs between the two extremes, resulting in different affects on neurogenesis. This review will focus on the affects of inflammation on neurogenesis during neurodegenerative disorders and the pro- or anti-neurogenic roles of individual inflammatory factors.
Neurogenesis affected by neurodegenerative disorders
Ischemia
It is well established that inflammation plays a positive role in neurogenesis during ischemia. Multiple models of ischemic-induced inflammation have demonstrated an increase in neurogenesis (Liu et al. 1998; Takagi et al. 1999; Kee et al. 2001; Yagita et al. 2001; Zhang et al. 2001; Iwai et al. 2002; Choi et al. 2003; Iwai et al. 2003; Bingham et al. 2005; Darsalia et al. 2005; Tang et al. 2007). Transient global ischemia enhanced proliferation in the DG (Liu et al. 1998; Takagi et al. 1999; Kee et al. 2001; Yagita et al. 2001; Iwai et al. 2002; Choi et al. 2003; Bingham et al. 2005; Darsalia et al. 2005) and SVZ (Zhang et al. 2001; Arvidsson et al. 2002; Iwai et al. 2003; Tang et al. 2007) as shown by an increase in number of Bromodeoxyuridine (BrdU)-positive cells. BrdU-positive cells from the SGZ successfully migrated to the granular cell layer (Liu et al. 1998; Kee et al. 2001; Yagita et al. 2001; Iwai et al. 2002; Bingham et al. 2005) and cells from the SVZ migrated to the olfactory bulb, cortex (Zhang et al. 2001; Iwai et al. 2003) and striatum (Arvidsson et al. 2002; Darsalia et al. 2005). Moreover, the majority of the proliferating cells differentiated into neurons, as demonstrated by co-immunolabeling with BrdU and neuronal markers (Kee et al. 2001; Yagita et al. 2001; Arvidsson et al. 2002; Iwai et al. 2002; Bingham et al. 2005). It was recently demonstrated following ischemic damage in a rat model, new GABAergic and cholinergic neurons were generated and successfully integrated into the ischemic-damaged striatum neural circuitry, where these neurons form synapses with pre-existing neurons, fire action potentials and display spontaneous postsynaptic currents (Hou et al. 2008). Notably, treatment of an ischemic mouse model with the antidepressant flouxetine enhanced hippocampal neurogenesis by promoting the survival of newborn neurons and more importantly improved spatial cognitive function (Li et al. 2008b). Collectively, these recent reports demonstrated following ischemia newly born functional neurons can successfully incorporate into the CNS circuitry and improve cognitive function, supporting the potential of pro-neurogenic drugs as a tool to repair the damage caused by ischemia.
Status epilepticus
Electrically-induced status epilepticus results in neuronal loss, microglial activation and chronic inflammation; however, neurogenesis is also induced and new neurons survived for a substantial period of time (Bonde et al. 2006). Newly generated neurons labeled with BrdU during the first two weeks after status epilepticus induction were found in the hippocampus up to six months after status epilepticus (Bonde et al. 2006). There was a seven-fold increase in the number of BrdU+ mature neurons without a change in the total number of mature neurons, indicating that newborn neurons replaced dead granule cells (Bonde et al. 2006). One possible mechanism for status epilepticus-induced proliferation is through CREB-regulated production of insulin-like growth factor-1 (IGF-1) by activated microglia, which then activates the Erk MAPK pathway, resulting in NPC proliferation (Choi et al. 2008). The efficacy of seizure-induced neurogenesis depends on the severity of seizures (Iosif et al. 2008). Severe seizures in adult rats caused higher levels of cell proliferation in the DG than mild seizures; however less than 20% of new cells differentiated into neurons after severe seizures, whereas mild seizures resulted in more than 60% of new cells that differentiated into neurons (Iosif et al. 2008). This decreased efficiency of neurogenesis from severe seizure may be due to the production of factors such as the pro-inflammatory cytokine TNF-α, which inhibits neurogenesis via the activation of the TNF-α receptor 1 (TNF-R1) (Iosif et al. 2008). Further research into the specific inflammatory factors that enhance and inhibit neurogenesis could provide effective drug targets for the treatment of disorders such as seizures.
Mechanical damage
Neurogenesis is also enhanced following mechanical damage (Parent 2003). Cortical aspiration induced proliferation in the SVZ and increased the expression of the early neuronal marker polysialated neural cell adhesion molecule (PSA-NCAM) (Szele and Chesselet 1996). Traumatic brain injury stimulated proliferation in the DG, and newly generated neurons survived 3–4 weeks post-injury in the granule cell layer as demonstrated by co-labeling with BrdU or [3H]thymidine and the mature neuronal marker calbindin (Gould and Tanapat 1997; Dash et al. 2001). Similarly, acute spinal cord injury in mice induced NPC proliferation, migration, and differentiation to NeuN+ neurons (Ke et al. 2006). Data suggests astrocytes play a major role in inflammation that causes secondary damage after spinal cord injury. Transgenic expression of dominant negative inhibitor of κBα (IκBα) in astrocytes prevents activation of nuclear factor (NF)-κB and reduces secondary inflammation-mediated damage following spinal cord injury (Brambilla et al. 2005). Whether or not neurogenesis is changed in this model was not investigated, but it indicates the key role of astrocytes in brain inflammation. The inflammation-induced neurogenesis described above results from acute neurological disorders or damage, but certain inflammatory diseases with chronic yet controlled inflammation also stimulate neurogenesis.
Alzheimer’s disease
Alzheimer’s disease is a chronic inflammatory disease that has been shown to cause elevated levels of neurogenesis. Early neuronal differentiation markers TUC-4, doublecortin (Dcx) and PSA-NCAM were expressed at higher levels as compared to control in the hippocampus of patients with Alzheimer’s disease (AD) and in a mouse model of AD (Jin et al. 2004a; Jin et al. 2004b). Moreover, TUC-4 and Dcx were expressed in the GCL and CA1 region of Ammon’s horn, suggesting that hippocampal neurogenesis was induced by AD (Jin et al. 2004b). In AD-like neurodegenerative mouse models, cell proliferation was induced in the DG at early stages of disease onset (Chen et al. 2008; Gan et al. 2008). Newborn neurons migrated to the GCL (Chen et al. 2008; Gan et al. 2008) and hilus region; however, the newly generated neurons did not survive long term (Chen et al. 2008). Similarly, newborn neurons in the AD dentate gyrus did not fully mature, even though neuroproliferation was increased (Li et al. 2008a). This again emphasizes the fact that successful neurogenesis requires that newly born neurons are functionally integrated into the CNS circuitry and survive, and that proliferation or neural differentiation alone does not equate to an increase in successful neurogenesis.
It has been suggested that AD-induced neurogenesis may be partially due to AD-specific proteins as opposed to general inflammation. Amyloid-beta (Aβ) peptides, which are associated with the neurotoxicity of AD, have been linked to AD-induced neurogenesis in vitro (Lopez-Toledano and Shelanski 2007) and in vivo (Gan et al. 2008). Moreover, soluble amyloid precursor protein stimulates neuronal ERK signaling and is partially responsible for the increase in neurogenesis (Rohe et al. 2008). Nevertheless, the exact role of inflammation versus the AD-specific proteins remains to be further investigated.
Parkinson’s disease
Neurogenesis is increased in Parkinson’s disease as demonstrated by BrdU-positive proliferating cells in the mouse SN, the site of cell death in Parkinson’s disease (Zhao et al. 2003). In a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease NPCs successfully migrated to the SN, proliferated and differentiated into dopaminergic neurons as shown by tyrosine hydroxylase staining (Shan et al. 2006). However, another group demonstrated neurogenesis occurred in the striatum where dopaminergic projections innervate as a result of MPTP treatment alone, but only occurred in the SN where dopaminergic cell bodies are lost during Parkinson’s disease when MPTP mice were also treated with fibroblast growth factor-2 (FGF-2) (Peng et al. 2008b). Moreover, MPTP-treated Parkinsonian-like mice showed an increase of neurogenesis in the DG and SVZ; however, the newborn neurons only survived for a short period of time (4–6 days) (Jackson-Lewis and Przedborski 2007; Peng et al. 2008b). Suggesting chronic inflammation during Parkinson’s disease enhances proliferation and differentiation of NPCs to neurons, but inflammation may not provide an environment that is supportive of survival and/or incorporation of newly born neurons. Even though chronic inflammation may induce the proliferation and differentiation of NPCs, this does not equate to successful neurogenesis because the newly born neurons do not survive. Thus, further research is needed to specifically identify the inflammatory factors that promote neurogenesis as well as the factors that inhibit the survival and/or incorporation of newly born neurons into CNS circuitry.
Huntington’s disease
Patients inflicted with Huntington’s disease (HD) and an animal HD model demonstrated increased neurogenesis as compared to controls (Curtis et al. 2003; Tattersfield et al. 2004). Increased proliferation was observed in the human subependymal layer of the caudate nucleus (Curtis et al. 2005). Proliferating cell nuclear antigen (PCNA) staining showed that increased cell proliferation in the SVZ correlated with the severity of HD as shown by the patient’s pathological grade and number of CAG repeats in the HD gene (Curtis et al. 2003). The PCNA+ cells differentiated into immature neurons and astrocytes (Curtis et al. 2003). In a quinolinic acid lesion rat model of HD, BrdU-positive cell proliferation was increased in the SVZ (Tattersfield et al. 2004). The proliferating cells differentiated into Dcx+ immature neurons and the new neurons migrated to the lesion site in the striatum, where they matured into MAP2+ and NeuN+ neurons (Tattersfield et al. 2004). In another quinolinic acid lesion HD rat model, exogenous rat NPCs were transplanted into the lesioned striatum, where they survived and differentiated into astrocytes and NeuN+ neurons (Vazey et al. 2006). More noteworthy, the HD rats injected with exogenous NPCs into the lesioned striatum demonstrated enhanced motor performance as compared to sham-injected HD animals, suggesting that neurogenesis resulted in functional neurons that successfully integrated into the CNS in this model of inflammation (Vazey et al. 2006). Though it is known that neurogenesis is increased during HD, the molecular mechanisms behind this increased neurogenesis under the influences of HD are unknown. Recently it was reported that NPCs isolated from the SVZ of transgenic mice expressing CAG repeats in Hdh, the mouse homolog to the human huntingtin protein, had higher levels of neural differentiation compared to wild type. This suggests that mutant huntingtin may directly enhance neurogenesis in addition to inflammation-induced neurogenesis during HD (Lorincz and Zawistowski 2008).
Amyotrophic lateral sclerosis
Active neurogenesis occurs in the spinal cord of a mutant CuZn superoxide dismutase mouse model of amyotrophic lateral sclerosis (ALS) (Chi et al. 2006; Chi et al. 2007; Corti et al. 2007). In this ALS mouse model, endogenous NPCs proliferate, vigorously migrate and differentiate into mature neurons (Chi et al. 2006; Chi et al. 2007). This process may partially compensate for the loss of motor neurons but is not enough to sustain their population. This concept is supported by the transplantation of exogenous NPCs, which generates motor neuron-like cells, protects endogenous motor neurons, delays disease onset and prolongs survival (Corti et al. 2007).
HIV-1 associated dementia
HIV-1 associated dementia (HAD) is detrimental to neurogenesis. Fewer NPCs are thought to be present in the brains of patients with HAD as compared to those without dementia (Krathwohl and Kaiser 2004b). Injection of HIV-1-infected human macrophages into the basal ganglia of mice inhibited hippocampal neurogenesis (Poluektova et al. 2005). The attenuation of neurogenesis was shown by a decrease in PSA-NCAM+ and KI-67+ cells (Poluektova et al. 2005). Furthermore, our laboratory recently demonstrated NPC injected in conjunction with HIV-infected monocyte-derived macrophages enhanced astrocyte differentiation and decreased neuronal differentiation as compared to NPC injection alone; this effect is partially due to the dysregulation of TNF-α production by activated and infected macrophages (Peng et al. 2008a). In addition to the affects of HIV-mediated inflammation on neurogenesis, the HIV-envelope glycoprotein gp120 inhibits proliferation of NPCs (Peng et al. 2003; Okamoto 2007). NPC proliferation is inhibited by arresting cell-cycle progression at the G1 phase through activation of p38, MAPK-activated protein kinase 2 and Cdc25B/C (Okamoto 2007). Interestingly, NPCs are permissive to latent HIV-1 infection (Lawrence et al. 2004; Schwartz et al. 2007), but it is not yet known if or how this direct HIV-1 infection of NPCs influences neurogenesis.
Neurogenesis is clearly positively and negatively affected by inflammatory neurodegenerative disorders. In order to get a better understanding of the mechanisms for inflammatory-mediated neurogenesis, we must examine the role of individual inflammatory factors.
Pro-regenerative role of inflammatory factors
Effects of IFN-γ on neurogenesis
Phenotypic changes in neurogenesis associated with inflammatory diseases are partially through the regulation and production of cytokines. Several cytokines may influence neurogenesis, for example, the inflammatory cytokine interferon gamma (IFN-γ) is pro-neurogenic. IFN-γ promotes neural differentiation and neurite outgrowth of murine adult neural stem cells (Wong et al. 2004) and the human neuroblastoma Paju cell line (Song et al. 2005). This IFN-γ-induced neuronal differentiation may be through the JNK pathway as shown in the C17.2 neural stem cell line (Kim et al. 2007). Studies have shown the JNK pathway is required for neural differentiation from embryonic stem cells (Amura et al. 2005), PC12 cells (Zentrich et al. 2002) and P19 embryonal carcinoma cells (Wang et al. 2001). Though IFN-γ was shown to stimulate neuronal differentiation (Wong et al. 2004; Song et al. 2005) and induce NPC migration, it also inhibits NPC proliferation and survival of NPCs (Ben-Hur et al. 2003) calling attention to the dualistic effect of IFN-γ on neurogenesis.
Chemokines enhance neurogenesis
Chemokines are a family of small, secreted proteins that have well-established roles in leukocyte migration as well as influencing the migration, proliferation, differentiation and survival of NPCs. Numerous chemokines are expressed in the CNS including stromal cell-derived factor-1 alpha (SDF-1α, CXCL12), monocyte chemoattractant protein-1 (MCP-1 or CCL2), MIP-1α (CCL3), RANTES (CCL5) and IP-10 (CXCL10). In addition, chemokine receptors are widely expressed in NPCs, including CXCR4, CCR2, CCR5 and CX3CR1 (Ji et al. 2004; Ni et al. 2004; Peng et al. 2004; Tran et al. 2004; Widera et al. 2004). Migration of NPCs to the site of inflammation is a key step in neurogenesis during inflammatory disease; thus, the release of chemokines plays a vital role in the recovery from inflammatory-induced neuronal loss.
The SDF-1α receptors CXCR4 and newly identified CXCR7 are highly expressed on NPCs (Ni et al. 2004; Peng et al. 2004; Tran et al. 2004)(Peng et al., unpublished observation). Not only does SDF-1α induce the migration of NPCs, but SDF-1α also promotes survival of NPCs (Molyneaux et al. 2003); contrasting reports demonstrated SDF-1α promotes quiescence (Krathwohl and Kaiser 2004a) or proliferation of NPCs (Gong et al. 2006)(Wu et al, unpublished observation). The exact receptor (CXCR4 versus CXCR7) and the signaling pathways responsible for SDF-1α-induced proliferation are currently under intensive investigation by our laboratory. During inflammation, local astrocytes produce SDF-1α, recruiting NPCs to the site of inflammation (Imitola et al. 2004). Our laboratory demonstrated during the inflammatory state of HAD, macrophages produce IL-1β (Peng et al. 2006), a hallmark of brain inflammation (Rock et al. 2004), which induces the production of SDF-1α by astrocytes (Peng et al. 2006). SDF-1α has been shown to induce the migration of NPCs in vitro (Peng et al. 2004) and in vivo to areas of hypoxic-ischemia-induced inflammation via CXCR4 signaling pathways (Imitola et al. 2004; Kelly et al. 2004). The release of SDF-1α from sites of inflammation provides a signal for NPCs to migrate to the region of neuronal damage.
The chemokine MCP-1 is also upregulated in response to inflammation and induces the migration of NPCs. The pro-inflammatory factor TNFα increases the expression of MCP-1 in U373 human glioblastoma cells (Schwamborn et al. 2003). The MCP-1 receptor CCR2 is expressed by NPCs and MCP-1 recruits NPCs to the site of brain inflammation by binding to CCR2, and inducing their migration (Widera et al. 2004). In addition to the ability of MCP-1 to recruit NPCs to the site of inflammation, MCP-1 protects neurons against inflammatory damage caused by N-methyl-D-aspartate (NMDA)-mediated excitotoxicity and HIV-tat toxicity (Eugenin et al. 2003). Whether MCP-1 plays a role in the survival of NPCs remains to be determined.
Glutamate promotes neurogenesis
Cytokines and chemokines are not the only factors released in response to inflammation; for example, excitatory neurotransmitters are also released and result in neuronal loss. Excitotoxic neuronal death caused by increased extracellular glutamate is one consequence of brain inflammation; on the other hand, glutamate has been shown to induce neurogenesis. NMDA receptor activation induces neurogenesis and NMDA receptor blockade inhibits neurogenesis (Luk et al. 2003; Suzuki et al. 2006; Joo et al. 2007; Mochizuki et al. 2007). Although the intracellular signaling pathway for NMDA receptor-mediated neurogenesis has not been fully elucidated, it has been suggested that calcium entry via glutamate receptors leads to the activation of ERK signaling and CREB phosphorylation (Suzuki et al. 2006), which has been linked to neurogenesis (Zhu et al. 2006). Conversely, it has also been documented that NMDA receptor activation decreases hippocampal cell proliferation and NMDA receptor blockade increases hippocampal cell proliferation (Cameron et al. 1995; Gould and Tanapat 1997; Nacher et al. 2003). These contrary results certainly require further careful investigation to determine the exact role of NMDA receptor activation in neurogenesis. However, due to the relatively low expression of NMDA receptors on NPCs, the role of other glutamate receptors in the regulation of neurogenesis has recently become a topic of investigation.
Notably, glutamate-induced neurogenesis has also been linked to the activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors, an ionotropic glutamate receptor (Mackowiak et al. 2002; Bai et al. 2003; O'Neill et al. 2004). Our laboratory has recently reported that human NPCs contain calcium-permeable AMPA receptors with Q/R-unedited GluR2 subunits, due to the low expression of adenosine deaminase (ADAR2), the enzyme responsible for Q/R editing (Whitney et al. 2008). Activation of these calcium-permeable AMPA receptors directs the differentiation of NPCs to the neuronal lineage and increases dendritic arborization. AMPA-induced differentiation of NPCs to neurons can be prevented using an AMPA-specific antagonist, Joro spider toxin, and by overexpressing ADAR2, which results in Q/R editing of the GluR2 subunits and the expression of calcium-impermeable AMPA receptors (Whitney et al. 2008). This AMPA-induced neurogenesis may be a compensatory mechanism to counteract the glutamate-induced death of neurons during inflammatory neurological diseases.
Metabotropic glutamate (mGlu) receptors are important during development of the embryonic brain (Di Giorgi Gerevini et al. 2004; Lujan et al. 2005) and found in neurogenic regions of the adult brain. The mGlu5 and mGlu3 receptors seem to play a key role in neurogenesis. In cultured neurospheres, mGlu5 and mGlu3 blockade decreases cell proliferation and survival; moreover, there is an increase in cell proliferation upon activation of mGlu5 (Di Giorgi-Gerevini et al. 2005). Similarly, the SVZ and DG regions of the adult mouse brain show a decrease in proliferating NPCs when treated with antagonists to mGlu3 and mGlu5 (Di Giorgi-Gerevini et al. 2005). Mouse knockouts for mGlu5 seem to develop normally, but have impaired learning and also have a decrease in proliferating NPCs (Lu et al. 1997; Kinney et al. 2003).
Many studies have demonstrated the positive role of inflammation on neurogenesis. However, an increase in proliferation, migration or differentiation of NPCs resulting from inflammation does not necessarily equate to an increase in neurogenesis. Neurogenesis also requires the successful and proper integration of these newly formed neurons into the circuitry of the CNS and their long-term survival. Thus many of the examples of neurogenesis stated above may not represent fully successful and functional neurogenesis.
Inflammation suppresses neurogenesis
Inflammation is a complex process that both enhances and suppresses neurogenesis. In addition to the varied effects of mild acute versus uncontrolled chronic inflammation on neurogenesis, the discrepancies between the pro- and anti-neurogenic properties of inflammation may depend on the means by which microglia, macrophages and/or astrocytes are activated and the duration of inflammation. It has been suggested that microglia activated by inflammation inhibited neurogenesis; however, microglia activated by IL-4, or a low level of IFN-γ associated with T-helper cells, induced neurogenesis (Butovsky et al. 2006).
Activated microglia are a hallmark and driving force of brain inflammation. Lipopolysaccharide (LPS)-induced microglial activation strongly impairs basal hippocampal neurogenesis (Ekdahl et al. 2003) partially through the production of TNF-α (Liu et al. 2005); the effects of TNF-α will be discussed later in the paper. LPS-induced inflammation resulted in an 85% reduction of newborn neurons in the hippocampus; the degree of impaired neurogenesis correlated with the number activated microglia (Ekdahl et al. 2003), thus, indicating that uncontrolled inflammation is detrimental to neurogenesis. Suppression of activated microglia by minocycline treatment resulted in increased numbers of new neurons in the hippocampus, demonstrating the significance of activated microglia in the reduction of neurogenesis by inflammation (Ekdahl et al. 2003). Further, anti-inflammatory treatment with indomethacin restored neurogenesis that was diminished by irradiation-induced inflammation (Monje et al. 2003) and focal cerebral ischemia (Hoehn et al. 2005). This microglial inhibition of neurogenesis is mediated by activated, but not resting, microglia (Monje et al. 2003). The negative effect on neurogenesis by activated microglia is due to the production of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, as well as reactive oxygen species (Rock et al. 2004). Table 2, provides a summary of the inflammatory factors and their effects on neurogenesis.
Table 2.
Effects of inflammatory factors on neurogenesis
| Inflammatory Factor | Effects on Proliferation of NPCs |
Effects on Migration of NPCs |
Effects on Differentiation of NPCs | Effects on Neurite Growth |
Survival of NPCs and Newly-born Neurons |
References |
|---|---|---|---|---|---|---|
| IFN-γ | ↓ | ↑ | ↑ Neural Differentiation | ↑ | ↓ | Wong et al. 2004, Song et al. 2005, Kim et al. 2007, Ben-Hur et al. 2003 |
| IFN-α | ↓ or ↑ | ↑ |
↑ Astrocyte Differentiation ↓ Neural Differentiation(TNF-R1) ↑ Neural Differentiation(TNF-R2) |
↓ or ↑ | Liu et al. 2005, Peng et al.2008a, Ben-Hur et al.2003, Heldman et al. 2005, Cacci et al. 2005, losif et al. 2006 | |
| IL-1β | ↓ or ↑ | ↓ | Wang et al. 2007, Peng et al. 2008a | |||
| IL-18 | ↓ Neural Differentiation | Liu et al. 2005 | ||||
| IL-6 | ↓ |
↓ Neural Differentiation ↑ Astrocyte Differentiation |
↓ | Ekdahl et al.2003, Nakanishi et al. 2007, Valliers et al. 2002 | ||
| SDF-1α | ↓ or ↑ | ↑ | ↑ | Krathwohl and Kaiser 2004, Peng et al. 2004, Imitola et al. 2004, Kelly et al. 2004, Molyneaux et al. 2003 | ||
| MCP-1 | ↑ | ↑ | Widera et al. 2004, Eugenin et al. 2003 | |||
| Glutamate | ↓ or ↑ | ↑ Neural Differentiation | ↑ | Suzuki et al. 2006, Mochizuki et al. 2007, Joo et al.2007, Luk et al 2003, Nacher et al. 2003, Gould and Cameron 1997, Cameraon et al. 1995, Bai et al. 2003, Whitney et al.2008 | ||
| Nitric oxide | ↓ (nNOS) or ↑ (iNOS) |
↓ Neural Differentiation ↑ Astrocytes Differentiation |
↓ | Materredona et al. 2005, Romero-Grimaldi et al.2006, Torraglosa et al. 2007, Covacu et al. 2006, Ciani et al. 2006, Moreno-Lopez et al. 2004, Packer et al. 2003, Zhu et al. 2006, Zhu et al. 2003, Fritzen et al. 2007, Luo et al. 2007 |
Other cell types than microglia such as astrocytes play major roles in inflammation in the CNS. Astrocytes constitute the majority of glial cells in the CNS, vastly outnumbering microglia, monocytes and lymphocytes. Activated astrocytes release a plethora of inflammatory factors, growth factors and regulate extracellular levels of excitatory amino acids, which have both negative and positive effects on neurogenesis (Song et al. 2002; Blasko et al. 2004). Neuronal differentiation of adult rat NPCs increased tenfold when co-cultured with astrocytes, and both soluble and membrane-bound factors are responsible for this effect. In addition to directing the differentiation of NPCs to neurons, astrocyte co-culture also induced a twofold increase in NPC proliferation (Song et al. 2002). The role of astrocytes in brain inflammation and its consequence on neuronal injury and neurogenesis during various CNS disorders has recently been intensely studied and continues to be thoroughly investigated.
Effects and mechanisms of TNF-α on neurogenesis
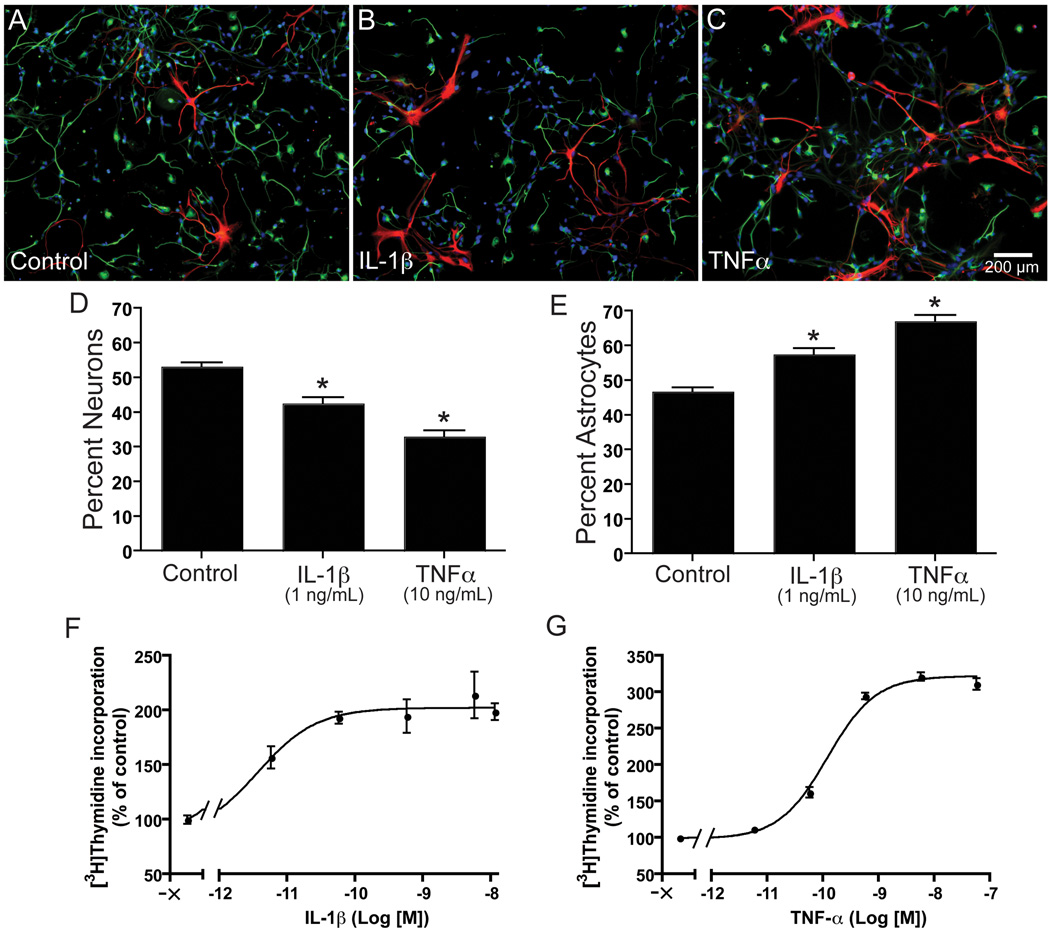
TNF-α is a pleiotropic pro-inflammatory cytokine that has been extensively investigated during neurodegenerative disorders. TNF-α is predominantly expressed by activated microglia, and to a lesser extent, astrocytes and neurons (Meda et al. 1995; Bezzi and Volterra 2001; Perry et al. 2002). TNF-α has been reported to have both pro-and anti-neurogenic properties, depending on different models, methods, and cell-derived regions (Wu et al. 2000; Ben-Hur et al. 2003; Iosif et al. 2006). Conditioned media from LPS-activated microglia and macrophages inhibited neuronal differentiation via the production of TNF-α (Liu et al. 2005; Peng et al. 2008a). Soluble TNF-α receptors and pentoxifylline, a TNF-α inhibitor, partially restored neuronal differentiation, demonstrating TNF-α is partially responsible for the anti-neurogenic effect of the LPS-conditioned media (Liu et al. 2005; Peng et al. 2008a). Furthermore, TNF-α promotes the proliferation of NPCs and induces differentiation of NPCs to astrocytes (Fig. 1C, F, G) (Wu et al. 2000; Peng et al. 2008a). However, others reported TNF-α inhibited proliferation and had no effect on differentiation but induced NPC migration (Ben-Hur et al. 2003). Moreover, treatment of a rat stroke model with anti-TNF-α antibody resulted in fewer new hippocampal and striatal neurons, suggesting that TNF-α can promote the survival of new neurons (Heldmann et al. 2005). However, TNF-α was shown to induce apoptosis of a post-mitotic NPC line (Cacci et al. 2005). These variations in observed effects of TNF-α on neurogenesis are likely due to differences in the models utilized. For example, conditioned media from microglia treated with LPS for 24 h reduced NPC survival and prevented neural differentiation, whereas media from 72 h LPS-treated microglia increased NPC survival and induced neural differentiation (Cacci et al. 2008). Moreover, the effects of TNF-α depend on which of the two known receptors is activated. TNF-α receptor (TNF-R) knockout demonstrated TNF-R1 activation reduced hippocampal neurogenesis in normal conditions and after status epilepticus, but TNF-R2 activation increased status epilepticus-induced neurogenesis (Iosif et al. 2006). These variations in the observed effects of TNF-α on NPCs necessitate further research on the role of TNF-α and its receptors in neurogenesis.
Figure 1. IL-1β and TNF-α reduce neural differentiation and enhance proliferation of human NPCs.
Human NPCs were cultured in neural basal medium supplemented with B27 alone (A), with IL-1β (1 ng/mL) (B) or TNFα (10 ng/mL) (C). Cells were stained for Map-2 (green), GFAP (red) and Hoechst (blue). Scale bar, 200 µm. The numbers of neurons were counted in ten pictures per condition and expressed as percent neurons (D) or percent astrocytes (E) *P < 0.001 vs. control. (F, G) NPCs were treated with different concentrations of TNF-α (G) or IL-1β (F) in X-vivo media with EGF for 6 days. Cell proliferation was measured by [3H]thymidine incorporation assay. Data is presented as a percentage of control, as mean ± SD. Results represent average of three donors.
TNF-α may inhibit neuronal differentiation through induction of neuronal cell death or by shifting differentiation of NPCs to astrocytes. Most new hippocampal neurons in the dentate gyrus die within weeks after epileptic insult through a caspase-mediated apoptotic mechanism (Ekdahl et al. 2001). Anti-inflammatory treatment with minocycline significantly improved the survival of these cells, demonstrating inflammation accompanying this brain insult is detrimental to newborn hippocampal neurons (Ekdahl et al. 2003). Specifically, increased production of TNF-α by microglia, which accompanies hippocampal inflammation (Vezzani et al. 2002), could be one of the major causes for the death of newly generated neurons. Furthermore, TNF-α released from microglia may be an important contributor to inflammation-induced death of newly formed hippocampal NPCs in the adult brain after insult (Cacci et al. 2005). However, data collected in our laboratory demonstrated TNF-α did not induce significant apoptosis of human NPCs or differentiated neurons and astrocytes (Peng et al. 2008a).
TNF-α-directed differentiation of NPCs to astrocytes may be through the regulation of basic helix-loop-helix (bHLH) transcription factors (Peng et al. 2008a). Activator-type bHLH factors such as Mash1 promote neural differentiation by forming a heterodimer with the ubiquitously expressed bHLH factor E47 and activate gene expression by binding to the E box, promoting the neuronal subtype specification. Whereas, Hes1 a repressor-type bHLH factor regulates maintenance of the stem cell pool and promotes astrogliogenesis by directly binding to promoters and repressing proneuronal genes such as Mash1, and by forming non-functional heterodimers with E47, inhibiting formation of Mash1-E47 heterodimers. Treatment of NPCs with TNF-α decreases Mash1 and increases Hes1 mRNA levels (Peng et al, unpublished observations), which may direct the differentiation of NPCs to astrocytes (Peng et al. 2008a). However, the exact mechanism through which TNF-α regulates these transcription factors and the process of TNF-α-induced astrogliogenesis must be further investigated.
TNF-α modulates NPC proliferation and cell cycle progression via I kappa B kinase/nuclear factor kappa B (IKK/NF-κB) signaling (Widera et al. 2006). TNF-α treatment of NPCs activates IKK-β, which leads to activation of NF-κB, resulting in the upregulation of Cyclin D1 (Widera et al. 2006). Cyclin D1 forms a complex with cyclin dependent kinase 4 (CDK4) that is necessary for cell cycle progression by promoting passage through the G1/S restriction point, thus promoting NPC proliferation (Ferguson et al. 2000; Widera et al. 2006). Our laboratory has demonstrated that TNF-α increases the expression of Cyclin D1 and increases the percentage of NPCs in the S-phase in a dose-dependant manner (Peng et al. 2008a).
Effects of IL-1β on neurogenesis
Proliferation of fetal rat NPCs was reduced by IL-1β in a dose-dependent manner, and this can be partially prevented using an IL-1 receptor antagonist (IL-1ra); however, the apparent inhibition of proliferation may be due to IL-1β-induced apoptosis of NPCs (Wang et al. 2007). IL-1β reduces proliferation and increases apoptosis of NPCs through the SAPK/JNK pathway (Wang et al. 2007). Conversely, our laboratory demonstrated IL-1β increases the proliferation of fetal human NPCs (Fig. 1 F), an effect that can be partially prevented with IL-1ra, and inhibits neural differentiation (Fig. 1 B, D) (Peng et al. 2008a). However, in a model of HAD, IL-1ra failed to prevent the increased proliferation of NPCs and reduced neurogenesis caused by conditioned media from HIV-1-infected and/or LPS-activated macrophages (Peng et al. 2008a). Similarly, a neutralizing antibody for IL-1β did not prevent the reduced neurogenesis caused by conditioned media from LPS-activated microglia (Liu et al. 2005). Moreover, IL-1ra had no effect on NPC differentiation (Wang et al. 2007). These findings support an earlier report that IL-1β had no significant effect on neurogenesis (Monje et al. 2003). The current data on IL-1β does not provide a concrete role of IL-1β in neurogenesis; thus, further research in this area is needed.
IL-6 and IL-18
One mechanism through which activated microglia inhibit neurogenesis is the production of the cytokine IL-6. Conditioned media from LPS-activated microglia decreased neuronal differentiation by nearly 50%, but neuronal differentiation was almost completely restored by an IL-6-blocking antibody (αIL-6) (Monje et al. 2003), indicating that IL-6 plays a major role in the reduction of neurogenesis during inflammation. IL-6, released by activated microglia, directs the differentiation of NPCs to astrocytes via the activation of the JAK/STAT and MAPK pathways (Nakanishi et al. 2007). In further support of the anti-neurogenic role of IL-6, an adult transgenic mouse model with chronic production of IL-6 by astrocytes demonstrated a dramatic reduction in hippocampal neurogenesis. Chronic IL-6 production reduced the proliferation, survival and differentiation of NPCs without having notable effects on neurons or astrocytes (Vallieres et al. 2002).
The proinflammatory cytokine interleukin-18 (IL-18) is produced at high levels by activated microglia (Conti et al. 1999). Treatment of NPCs with IL-18 reduced neural differentiation in a dose- and time-dependent manner (Liu et al. 2005). Clearly, the exact role of IL-18 in the regulation of neurogenesis requires further exploration.
Reactive oxygen and nitrogen species
Reactive oxygen and nitrogen species are significant factors in microglial-driven inflammation (Rock et al. 2004). Of these reactive oxygen and nitrogen species, nitric oxide (NO) is the most studied and understood. NPCs in the SVZ are anatomically in close proximity to NO-producing cells (Matarredona et al. 2005). This is imperative for NO signaling because it is highly reactive with a lifespan of only a few seconds. NO is associated with decreased neurogenesis as demonstrated by decreased proliferation of NPCs isolated from the SVZ (Matarredona et al. 2004). Moreover, NO inhibition by Nω-nitro-L-arginine methylester (L-NAME) increased NPC proliferation in the SVZ and DG (Romero-Grimaldi et al. 2006) and increased proliferation and neuronal differentiation in vitro (Matarredona et al. 2004). This NO-induced inhibition of proliferation may be linked to disruption of EGF receptor signaling. Increased proliferation in the SVZ from inhibition of nitric oxide synthase (NOS) was restricted to cells that expressed the EGF receptor (Romero-Grimaldi et al. 2006). Furthermore, NO-decreased proliferation may be via impaired tyrosine kinase activity of EGF receptor-induced activation of PI3-K/Akt pathway (Torroglosa et al. 2007). The induction of astrogliogenesis and inhibition of neuronal differentiation by NO may be linked to the decreased expression of the proneuronal gene neurogenin-2 (Covacu et al. 2006).
NO effects on neurogenesis are dependent on the developmental period and source of NO. Inhibition of NO during a time-restricted window of the first three postnatal days increased cerebellar proliferation in a rat model; however, NO inhibition at a later stage, three to seven days postnatal, had no effect on proliferation (Ciani et al. 2006). Moreover, rats with NO inhibition had greater numbers of BrdU-labeled neurons that survived up to 60 days as compared to control (Ciani et al. 2006). Contrary to this report, another group observed NO inhibition had no effect on cell proliferation in the SVZ and olfactory bulb of postnatal P1-P7 mice, but did enhance neurogenesis in the SVZ and olfactory bulb of adults (Romero-Grimaldi et al. 2008).
NO can be produced by multiple cell types and through multiple mechanisms, including neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS). NO produced by nNOS inhibits NPC neurogenesis (Packer et al. 2003; Moreno-Lopez et al. 2004; Zhu et al. 2006); whereas, NO from iNOS stimulates neurogenesis (Zhu et al. 2003). Proliferation was increased in the mouse DG by nNOS inhibition (Zhu et al. 2006) and in an nNOS−/− knockout mouse model (Zhu et al. 2006; Fritzen et al. 2007). Furthermore, survival of newly formed neurons was increased in nNOS−/− knockout mice as compared to the wild type (Fritzen et al. 2007). Transient ischemia results in an increase of iNOS immunoreactive interneurons and a decrease of nNOS in the hippocampus (Luo et al. 2007; Corsani et al. 2008). Ischemia-induced cell proliferation in the DG was increased by nNOS inhibition and in nNOS−/− knockout mice (Luo et al. 2007). Further, nNOS inhibition reduced ischemic injury and up-regulated iNOS expression in the ischemic hippocampus (Luo et al. 2007). CREB phosphorylation also varies according to which enzyme is used to create NO, increasing after inhibition of nNOS and decreasing following iNOS inhibition (Luo et al. 2007). nNOS inhibition had no effect on iNOS−/− knockout mice, suggesting ischemia induced hippocampal neurogenesis via decreased nNOS and an up-regulation of iNOS and CREB phosphorylation (Luo et al. 2007; Corsani et al. 2008). Depression induced by chronic mild stress in mice resulted in increased hippocampal nNOS expression and behavioral changes indicative of depression. nNOS−/− knockout and nNOS inhibition prevented depression and nNOS-impaired neurogenesis. Furthermore, disrupting hippocampal neurogenesis by 3’-azido-deoxythymidine (AZT) prevented the antidepressant effect of nNOS inhibition (Zhou et al. 2007). NO produced by nNOS in SVZ and olfactory bulb under normal physiological conditions inhibits NPC proliferation, possibly regulating the size of the precursor pool, and promotes neuronal differentiation as demonstrated by the delay in neuronal differentiation associated with NOS inhibition (Moreno-Lopez et al. 2004). Whereas, under inflammatory conditions a decrease in nNOS and an increase in iNOS may act as a mechanism to induce neurogenesis and function as a recovery mechanism to counteract the neuronal loss resulting from inflammation.
Chemokines may inhibit neurogenesis
Chemokines promote neurogenesis by recruiting NPCs to sites of inflammation and neural damage. However, chemokines also promote the progression of inflammation by recruiting resident microglia and peripheral macrophages to the sites of brain inflammation, resulting in further neural damage and death of neurons. These additional microglia and macrophages become activated and enhance the chronic inflammatory response, creating a positive feedback loop that results in uncontrolled chronic inflammation. Preventing these chemoattractant signals by chemokine receptor blockade (Ubogu et al. 2006) or anti-chemokine antibodies (Castellani et al. 2007) is a novel therapeutic approach for brain inflammation treatment.
Post-transcriptional modification of SDF-1 can eliminate its pro-neurogenic properties and possibly result in a highly neurotoxic byproduct. During HAD, HIV-1-infected macrophages secrete matrix metalloproteinase-2 (MMP-2), which cleaves the N-terminus of SDF-1, creating a cleaved form of SDF-1, SDF-1(5–67) (Zhang et al. 2003). This SDF-1 fragment no longer binds to CXCR4, thus losing its chemoattractant properties, and it is neurotoxic at high levels (Vergote et al. 2006), attenuating its positive role in neurogenesis.
Future therapeutic strategies
Two therapeutic strategies exist to enhance neurogenesis; NPCs can be transplanted or endogenous NPCs can be stimulated to proliferate, migrate and differentiate. A full understanding of the mechanisms which influence neurogenesis during inflammation may lead to the development of drugs that can induce neurogenesis from endogenous NPCs. The use of drugs that specifically block anti-neurogenic inflammatory factors such as TNF-α, IL-6 and IL-18, without preventing the pro-neurogenic role of inflammation could potentially increase levels of endogenous neurogenesis.
Exogenous NPCs for transplantation can be isolated from elective aborted fetuses or derived from embryonic stem/germ cell lines; however, these strategies face many ethical and host-versus-donor rejection issues. Recent studies have transdifferentiated stem cells from non-neural tissue including skin (Shih et al. 2005; Fernandes et al. 2006; Hunt et al. 2008), bone marrow (Somoza et al. 2008) and adipose tissue (Sago et al. 2008) to neural stem cells. These non-neuronal stem cells can be obtained through minimally- or non-invasive surgery, transdifferentiated into neuronal stem cells, amplified in culture and transplanted back into the patient. Non-neuronal-derived NPCs could be transplanted directly into the brain or injected intravenously, resulting in a small fraction that will cross the blood brain barrier to repopulate the damaged portion of the CNS (Fujiwara et al. 2004; Takeuchi et al. 2007; Guzman et al. 2008). Because individuals could use NPCs transdifferentiated from their own non-neuronal stem cells to create new self-neurons, host-versus-donor rejection would not be an issue.
A recent breakthrough report demonstrated in vivo reprogramming of adult mouse pancreatic exocrine cells into insulin-producing islet β-cells using adenoviral delivery of only three transcription factors Ngn3, Pdx1 and Mafa (Zhou et al. 2008). Exocrine cells were transdifferentiated into functional β-cells with gene and protein expression profiles similar to endogenous β-cells and are structurally indistinguishable from endogenous β-cells without entering a de-differentiated state (Zhou et al. 2008). This gives potential to the concept that perhaps astrocytes could be transdifferentiated into neurons or oligodendrocytes. Astrocytes are terminally differentiated proliferative cells that share the same immediate progenitor as neurons and oligodendrocytes, suggesting the concept of astrocytes transdifferentiation may be feasible. If the transcription factors and/or microRNAs responsible for neuronal or oligodendrocyte differentiation were completely elucidated, it may be theoretically possible to identify a combination of these factors that reprogram astrocytes into neurons or oligodendrocytes. If this concept could reach fruition, it would provide a relatively limitless source of new self-neurons or oligodendrocytes without rejection or delivery complications. Moreover, if the cells are directly reprogrammed instead of entering a de-differentiated state, the likelihood of these cells causing tumors is greatly reduced. It must be noted that the creation of new neurons or oligodendrocytes is only half of the story. In order for the creation of new neurons to result in successful neurogenesis, the new neurons must integrate into the CNS circuitry, be functional and survive. Thus, we must not only focus research on the generation of new neurons, but also investigate methods to enhance or ensure proper integration and survival of new neurons.
Conclusions
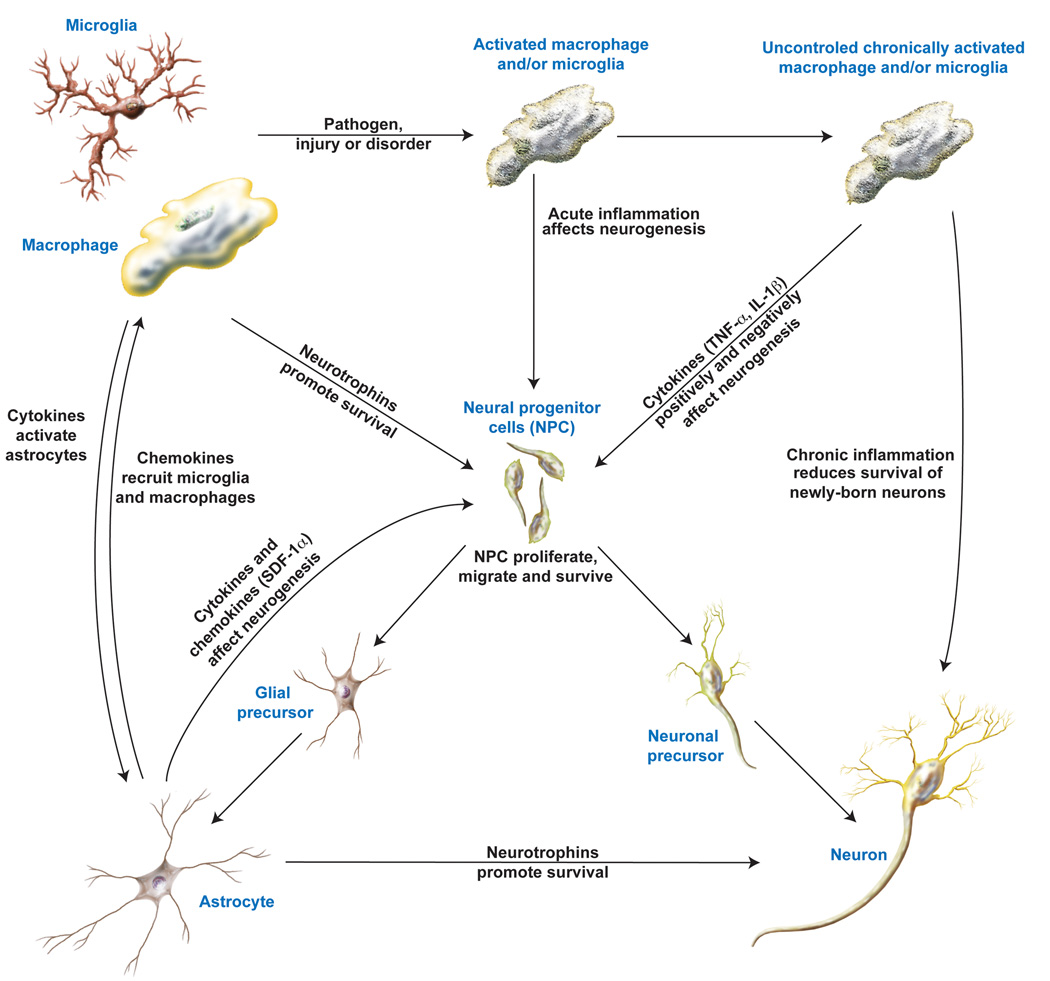
A controlled acute inflammatory response is necessary to eliminate any pathogen or insult to the CNS and clear away damaged and dead cells, returning the CNS to a normal state. Acute inflammation also promotes neurogenesis to replace the damaged CNS. However, uncontrolled chronic inflammation is detrimental to neurogenesis. Even if chronic inflammation stimulates one or more process of neurogenesis, such as proliferation, migration or differentiation, the chronic inflammatory environment does not allow for the survival of newborn neurons (Figure 2). More research is needed to identify methods to control chronic inflammation, identify drug targets to enhance inflammation-induced neurogenesis and prevent the death of newborn neurons and research must continue on regenerative stem cell therapies for neuronal replacement.
Figure 2. A proposed mechanism for the effects on neurogenesis by inflammation.
During neuronal injury, neurons produce chemokines, which recruit macrophage and microglia into the brain and to the site of injury. As these macrophage and microglia enter an environment of injury or inflammation, they become activated, subsequently releasing factors that promote neurogenesis. These factors include but are not limited to neurotrophins that may act directly to enhance the survival and proliferation of NPCs, and cytokines that activate astrocytes. These activated astrocytes then produce chemokines that promote the migration of NPCs to the site of injury and release neurotrophins that promote neuronal survival. Once the NPCs receive these migratory and neurotrophic signals, the NPCs can migrate, proliferate, and differentiate into neuronal or astrocyte precursors, which then mature into neurons and astrocytes that may integrate into the CNS circuitry. However, uncontrolled inflammation with chronically activated astrocytes have both pro- and anti-neurogenic effects and release factors that are detrimental to the survival of newly born neurons.
Acknowledgements
This work was supported in part by research grants by the National Institutes of Health: R01 NS 41858, R01 NS 61642, R21 MH83525, P01 NS043985 and P20 RR15635 to JZ and F31 NS062659 to NPW. We kindly acknowledge Mr. Matthew Beaver and Ms. Li Wu who provided technical support for this work. Ms. Julie Ditter, Johna Belling, Robin Taylor, Myhanh Che, Na Ly and Emilie Scoggins provided outstanding administrative support.
Abbreviations
- NPC
neural progenitor cell
- SGZ
subgranular zone
- DG
dentate gyrus
- SN
substantia nigra
- PSA-NCAM
polysialated neural cell adhesion molecule
- Dcx
doublecortin
- AD
Alzheimer’s disease
- Aβ
amyloid-beta
- FGF-2
fibroblast growth factor-2
- HD
Huntington’s disease
- PCNA
proliferating cell nuclear antigen
- ALS
amyotrophic lateral sclerosis
- IFN-γ
interferon gamma
- SDF-1α
stromal cell-derived factor-1 alpha
- MCP-1
monocyte chemoattractant protein-1
- HAD
HIV-associated dementia
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- ADAR2
adenosine deaminase acting on RNA 2
- mGlu
metabotropic glutamate
- LPS
Lipopolysaccharide
- bHLH
basic helix-loop-helix
- NF-κB
nuclear factor kappa B
- CDK4
cyclin dependent kinase 4
- IL-1ra
IL-1 receptor antagonist
- αIL-6
IL-6 blocking antibody
- IL-18
interleukin-18
- NO
nitric oxide
- L-NAME
Nω-nitro-L-arginine methylester
- nNOS
neuronal nitric oxide synthase
- iNOS
inducible nitric oxide
- AZT
3’-azido-deoxythymidine
- MMP-2
matrix metalloproteinase-2
References
- Amura CR, Marek L, Winn RA, Heasley LE. Inhibited neurogenesis in JNK1-deficient embryonic stem cells. Mol Cell Biol. 2005;25:10791–10802. doi: 10.1128/MCB.25.24.10791-10802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bai F, Bergeron M, Nelson DL. Chronic AMPA receptor potentiator ( LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology. 2003;44:1013–1021. doi: 10.1016/s0028-3908(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24:623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Volterra A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol. 2001;11:387–394. doi: 10.1016/s0959-4388(00)00223-3. [DOI] [PubMed] [Google Scholar]
- Bingham B, Liu D, Wood A, Cho S. Ischemia-stimulated neurogenesis is regulated by proliferation, migration, differentiation and caspase activation of hippocampal precursor cells. Brain Res. 2005;1058:167–177. doi: 10.1016/j.brainres.2005.07.075. [DOI] [PubMed] [Google Scholar]
- Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3:169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Bonde S, Ekdahl CT, Lindvall O. Long-term neuronal replacement in adult rat hippocampus after status epilepticus despite chronic inflammation. Eur J Neurosci. 2006;23:965–974. doi: 10.1111/j.1460-9568.2006.04635.x. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–425. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani ML, Bhattacharya K, Tagen M, Kempuraj D, Perrella A, De Lutiis M, Boucher W, Conti P, Theoharides TC, Cerulli G, Salini V, Neri G. Anti-chemokine therapy for inflammatory diseases. Int J Immunopathol Pharmacol. 2007;20:447–453. doi: 10.1177/039463200702000303. [DOI] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci U S A. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Nakajima A, Choi SH, Xiong X, Sisodia SS, Tang YP. Adult neurogenesis is functionally associated with AD-like neurodegeneration. Neurobiol Dis. 2008;29:316–326. doi: 10.1016/j.nbd.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Gan L, Luo C, Lien L, Liu R. Temporal response of neural progenitor cells to disease onset and progression in amyotrophic lateral sclerosis-like transgenic mice. Stem Cells Dev. 2007;16:579–588. doi: 10.1089/scd.2006.0120. [DOI] [PubMed] [Google Scholar]
- Chi L, Ke Y, Luo C, Li B, Gozal D, Kalyanaraman B, Liu R. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells. 2006;24:34–43. doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56:791–800. doi: 10.1002/glia.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Lee MY, Sung KW, Jeong SW, Choi JS, Park HJ, Kim ON, Lee SB, Kim SY. Regional differences in enhanced neurogenesis in the dentate gyrus of adult rats after transient forebrain ischemia. Mol Cells. 2003;16:232–238. [PubMed] [Google Scholar]
- Ciani E, Calvanese V, Crochemore C, Bartesaghi R, Contestabile A. Proliferation of cerebellar precursor cells is negatively regulated by nitric oxide in newborn rat. J Cell Sci. 2006;119:3161–3170. doi: 10.1242/jcs.03042. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. Aids. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Park LC, Calingasan NY, Kim Y, Kim H, Bae Y, Gibson GE, Joh TH. Cultures of astrocytes and microglia express interleukin 18. Brain Res Mol Brain Res. 1999;67:46–52. doi: 10.1016/s0169-328x(99)00034-0. [DOI] [PubMed] [Google Scholar]
- Corsani L, Bizzoco E, Pedata F, Gianfriddo M, Faussone-Pellegrini MS, Vannucchi MG. Inducible nitric oxide synthase appears and is co-expressed with the neuronal isoform in interneurons of the rat hippocampus after transient ischemia induced by middle cerebral artery occlusion. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Del Bo R, Nizzardo M, Nardini M, Donadoni C, Salani S, Fortunato F, Strazzer S, Bresolin N, Comi GP. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain. 2007;130:1289–1305. doi: 10.1093/brain/awm043. [DOI] [PubMed] [Google Scholar]
- Covacu R, Danilov AI, Rasmussen BS, Hallen K, Moe MC, Lobell A, Johansson CB, Svensson MA, Olsson T, Brundin L. Nitric oxide exposure diverts neural stem cell fate from neurogenesis towards astrogliogenesis. Stem Cells. 2006;24:2792–2800. doi: 10.1634/stemcells.2005-0640. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson J, Dragunow M, Connor B, Faull RL. The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington's disease human brain. Neuroscience. 2005;132:777–788. doi: 10.1016/j.neuroscience.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci U S A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36:1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Di Giorgi Gerevini VD, Caruso A, Cappuccio I, Ricci Vitiani L, Romeo S, Della Rocca C, Gradini R, Melchiorri D, Nicoletti F. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Res Dev Brain Res. 2004;150:17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Di Giorgi-Gerevini V, Melchiorri D, Battaglia G, Ricci-Vitiani L, Ciceroni C, Busceti CL, Biagioni F, Iacovelli L, Canudas AM, Parati E, De Maria R, Nicoletti F. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12:1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Mohapel P, Elmer E, Lindvall O. Caspase inhibitors increase short-term survival of progenitor-cell progeny in the adult rat dentate gyrus following status epilepticus. Eur J Neurosci. 2001;14:937–945. doi: 10.1046/j.0953-816x.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Ferguson KL, Callaghan SM, O'Hare MJ, Park DS, Slack RS. The Rb-CDK4/6 signaling pathway is critical in neural precursor cell cycle regulation. J Biol Chem. 2000;275:33593–33600. doi: 10.1074/jbc.M004879200. [DOI] [PubMed] [Google Scholar]
- Fernandes KJ, Kobayashi NR, Gallagher CJ, Barnabe-Heider F, Aumont A, Kaplan DR, Miller FD. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol. 2006;201:32–48. doi: 10.1016/j.expneurol.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Fritzen S, Schmitt A, Koth K, Sommer C, Lesch KP, Reif A. Neuronal nitric oxide synthase (NOS-I) knockout increases the survival rate of neural cells in the hippocampus independently of BDNF. Mol Cell Neurosci. 2007;35:261–271. doi: 10.1016/j.mcn.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Tanaka N, Ishida O, Fujimoto Y, Murakami T, Kajihara H, Yasunaga Y, Ochi M. Intravenously injected neural progenitor cells of transgenic rats can migrate to the injured spinal cord and differentiate into neurons, astrocytes and oligodendrocytes. Neurosci Lett. 2004;366:287–291. doi: 10.1016/j.neulet.2004.05.080. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gan L, Qiao S, Lan X, Chi L, Luo C, Lien L, Yan Liu Q, Liu R. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer's disease-like transgenic (pPDGF-APPSw,Ind) mice. Neurobiol Dis. 2008;29:71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, He X, Qi L, Zuo H, Xie Z. Stromal cell derived factor-1 acutely promotes neural progenitor cell proliferation in vitro by a mechanism involving the ERK1/2 and PI-3K signal pathways. Cell Biol Int. 2006;30:466–471. doi: 10.1016/j.cellbi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Griffin DE. Cytokines in the brain during viral infection: clues to HIV-associated dementia. J Clin Invest. 1997;100:2948–2951. doi: 10.1172/JCI119847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman R, Choi R, Gera A, De Los Angeles A, Andres RH, Steinberg GK. Intravascular cell replacement therapy for stroke. Neurosurg Focus. 2008;24:E15. doi: 10.3171/FOC/2008/24/3-4/E14. [DOI] [PubMed] [Google Scholar]
- Heldmann U, Thored P, Claasen JH, Arvidsson A, Kokaia Z, Lindvall O. TNF-alpha antibody infusion impairs survival of stroke-generated neuroblasts in adult rat brain. Exp Neurol. 2005;196:204–208. doi: 10.1016/j.expneurol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hermann A, Maisel M, Wegner F, Liebau S, Kim DW, Gerlach M, Schwarz J, Kim KS, Storch A. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells. 2006;24:949–964. doi: 10.1634/stemcells.2005-0192. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Hou SW, Wang YQ, Xu M, Shen DH, Wang JJ, Huang F, Yu Z, Sun FY. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39:2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- Hunt DP, Morris PN, Sterling J, Anderson JA, Joannides A, Jahoda C, Compston A, Chandran S. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2008;26:163–172. doi: 10.1634/stemcells.2007-0281. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, Kokaia Z, Lindvall O. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–1587. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- Iwai M, Sato K, Omori N, Nagano I, Manabe Y, Shoji M, Abe K. Three steps of neural stem cells development in gerbil dentate gyrus after transient ischemia. J Cereb Blood Flow Metab. 2002;22:411–419. doi: 10.1097/00004647-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Iwai M, Sato K, Kamada H, Omori N, Nagano I, Shoji M, Abe K. Temporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbils. J Cereb Blood Flow Metab. 2003;23:331–341. doi: 10.1097/01.WCB.0000050060.57184.E7. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci Lett. 2004;355:236–240. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc Natl Acad Sci U S A. 2004a;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci U S A. 2004b;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JY, Kim BW, Lee JS, Park JY, Kim S, Yun YJ, Lee SH, Lee SH, Rhim H, Son H. Activation of NMDA receptors increases proliferation and differentiation of hippocampal neural progenitor cells. J Cell Sci. 2007;120:1358–1370. doi: 10.1242/jcs.002154. [DOI] [PubMed] [Google Scholar]
- Kast RE. Feedback between glial tumor necrosis factor-alpha and gp120 from HIV-infected cells helps maintain infection and destroy neurons. Neuroimmunomodulation. 2002;10:85–92. doi: 10.1159/000065184. [DOI] [PubMed] [Google Scholar]
- Kaul M. HIV's double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–2494. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Chi L, Xu R, Luo C, Gozal D, Liu R. Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells. 2006;24:1011–1019. doi: 10.1634/stemcells.2005-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee NJ, Preston E, Wojtowicz JM. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res. 2001;136:313–320. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Kim K, Park HR, Mattson MP, Lee J. Interferon-gamma promotes differentiation of neural progenitor cells via the JNK pathway. Neurochem Res. 2007;32:1399–1406. doi: 10.1007/s11064-007-9323-z. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004a;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004b;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, Iqbal K, Grundke-Iqbal I. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008a;67:78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WL, Cai HH, Wang B, Chen L, Zhou QG, Luo CX, Liu N, Ding XS, Zhu DY. Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J Neurosci Res. 2008b doi: 10.1002/jnr.21829. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Lin HI, Tzeng SF. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005;1054:152–158. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Lopez-Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind) J Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- Lorincz MT, Zawistowski VA. Expanded CAG repeats in the murine Huntington's disease gene increases neuronal differentiation of embryonic and neural stem cells. Mol Cell Neurosci. 2008 doi: 10.1016/j.mcn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kennedy TE, Sadikot AF. Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J Neurosci. 2003;23:2239–2250. doi: 10.1523/JNEUROSCI.23-06-02239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CX, Zhu XJ, Zhou QG, Wang B, Wang W, Cai HH, Sun YJ, Hu M, Jiang J, Hua Y, Han X, Zhu DY. Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J Neurochem. 2007;103:1872–1882. doi: 10.1111/j.1471-4159.2007.04915.x. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, O'Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology. 2002;43:1–10. doi: 10.1016/s0028-3908(02)00066-7. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Matarredona ER, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Nitric oxide synthesis inhibition increases proliferation of neural precursors isolated from the postnatal mouse subventricular zone. Brain Res. 2004;995:274–284. doi: 10.1016/j.brainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Matarredona ER, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Role of nitric oxide in subventricular zone neurogenesis. Brain Res Brain Res Rev. 2005;49:355–366. doi: 10.1016/j.brainresrev.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Matyszak MK. Inflammation in the CNS: balance between immunological privilege and immune responses. Prog Neurobiol. 1998;56:19–35. doi: 10.1016/s0301-0082(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Takagi N, Kurokawa K, Kawai T, Besshoh S, Tanonaka K, Takeo S. Effect of NMDA receptor antagonist on proliferation of neurospheres from embryonic brain. Neurosci Lett. 2007;417:143–148. doi: 10.1016/j.neulet.2007.02.066. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C, Lehmann R. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130:4279–4286. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmerq TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24:273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Ni HT, Hu S, Sheng WS, Olson JM, Cheeran MC, Chan AS, Lokensgard JR, Peterson PK. High-level expression of functional chemokine receptor CXCR4 on human neural precursor cells. Brain Res Dev Brain Res. 2004;152:159–169. doi: 10.1016/j.devbrainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord. 2004;3:181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Kang Y, Christopher B, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 Decreases Adult Neural Progenitor Cell Proliferation via Checkpoint Kinase-Mediated Cell-Cycle Withdrawal and G1 Arrest. Cell Stem Cell. 2007;1:1–7. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci U S A. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Erichsen D, Zheng J. CXCR4 mediated signaling on neural progenitor cells: Relevance to HIV-1 associated dementia. 10th conference on Retroviruses and Opportunistic Infections; Foundation for Retrovirology and Human health; Boston, MA. 2003. [Google Scholar]
- Peng H, Whitney N, Wu Y, Tian C, Dou H, Zhou Y, Zheng J. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia. 2008a;56:903–916. doi: 10.1002/glia.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1 mediated CXCR4 signaling in rat and human cortical neural progenitor cells. Journal of Neuroscience Research. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Peng H, Erdmann N, Whitney N, Douz H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Xie L, Jin K, Greenberg DA, Andersen JK. Fibroblast growth factor 2 enhances striatal and nigral neurogenesis in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Neuroscience. 2008b doi: 10.1016/j.neuroscience.2008.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SW, Dewhurst S, Bellizzi MJ, Gelbard HA. Tumor necrosis factor-alpha in normal and diseased brain: Conflicting effects via intraneuronal receptor crosstalk? J Neurovirol. 2002;8:611–624. doi: 10.1080/13550280290101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohe M, Carlo AS, Breyhan H, Sporbert A, Militz D, Schmidt V, Wozny C, Harmeier A, Erdmann B, Bales KR, Wolf S, Kempermann G, Paul SM, Schmitz D, Bayer TA, Willnow TE, Andersen OM. Sortilin-related Receptor with A-type Repeats (SORLA) Affects the Amyloid Precursor Protein-dependent Stimulation of ERK Signaling and Adult Neurogenesis. J Biol Chem. 2008;283:14826–14834. doi: 10.1074/jbc.M710574200. [DOI] [PubMed] [Google Scholar]
- Romero-Grimaldi C, Moreno-Lopez B, Estrada C. Age-dependent effect of nitric oxide on subventricular zone and olfactory bulb neural precursor proliferation. J Comp Neurol. 2008;506:339–346. doi: 10.1002/cne.21556. [DOI] [PubMed] [Google Scholar]
- Romero-Grimaldi C, Gheusi G, Lledo PM, Estrada C. Chronic inhibition of nitric oxide synthesis enhances both subventricular zone neurogenesis and olfactory learning in adult mice. Eur J Neurosci. 2006;24:2461–2470. doi: 10.1111/j.1460-9568.2006.05127.x. [DOI] [PubMed] [Google Scholar]
- Sago K, Tamahara S, Tomihari M, Matsuki N, Asahara Y, Takei A, Bonkobara M, Washizu T, Ono K. In vitro differentiation of canine celiac adipose tissue-derived stromal cells into neuronal cells. J Vet Med Sci. 2008;70:353–357. doi: 10.1292/jvms.70.353. [DOI] [PubMed] [Google Scholar]
- Schwamborn J, Lindecke A, Elvers M, Horejschi V, Kerick M, Rafigh M, Pfeiffer J, Prullage M, Kaltschmidt B, Kaltschmidt C. Microarray analysis of tumor necrosis factor alpha induced gene expression in U373 human glioblastoma cells. BMC Genomics. 2003;4:46. doi: 10.1186/1471-2164-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, Kinzel N, Lawrence DM, Hazra R, Major EO. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol. 2007;13:274–283. doi: 10.1080/13550280701344975. [DOI] [PubMed] [Google Scholar]
- Shanx X, Chi L, Bishop M, Luo C, Lien L, Zhang Z, Liu R. Enhanced de novo neurogenesis and dopaminergic neurogenesis in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease-like mice. Stem Cells. 2006;24:1280–1287. doi: 10.1634/stemcells.2005-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DT, Lee DC, Chen SC, Tsaix RY, Huang CT, Tsai CC, Shen EY, Chiu WT. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005;23:1012–1020. doi: 10.1634/stemcells.2004-0125. [DOI] [PubMed] [Google Scholar]
- Somoza R, Conget P, Rubio FJ. Neuropotency of human mesenchymal stem cell cultures: clonal studies reveal the contribution of cell plasticity and cell contamination. Biol Blood Marrow Transplant. 2008;14:546–555. doi: 10.1016/j.bbmt.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Song JH, Wang CX, Song DK, Wangx P, Shuaib A, Hao C. Interferon gamma induces neurite outgrowth by up-regulation of p35 neuron-specific cyclin-dependent kinase 5 activator via activation of ERK1/2 pathway. J Biol Chem. 2005;280:12896–12901. doi: 10.1074/jbc.M412139200. [DOI] [PubMed] [Google Scholar]
- St-John WM. Neurogenesis of patterns of automatic ventilatory activity. Prog Neurobiol. 1998;56:97–117. doi: 10.1016/s0301-0082(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nelson AD, Eickstaedt JB, Wallace K, Wright LS, Svendsen CN. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 2006;24:645–653. doi: 10.1111/j.1460-9568.2006.04957.x. [DOI] [PubMed] [Google Scholar]
- Szele FG, Chesselet MF. Cortical lesions induce an increase in cell number and PSA-NCAM expression in the subventricular zone of adult rats. J Comp Neurol. 1996;368:439–454. doi: 10.1002/(SICI)1096-9861(19960506)368:3<439::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nozaki K, Takahashi J, Yodoi J, Ishikawa M, Hashimoto N. Proliferation of neuronal precursor cells in the dentate gyrus is accelerated after transient forebrain ischemia in mice. Brain Res. 1999;831:283–287. doi: 10.1016/s0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Natsume A, Wakabayashi T, Aoshima C, Shimato S, Ito M, Ishii J, Maeda Y, Hara M, Kim SU, Yoshida J. Intravenously transplanted human neural stem cells migrate to the injured spinal cord in adult mice in an SDF-1- and HGF-dependent manner. Neurosci Lett. 2007;426:69–74. doi: 10.1016/j.neulet.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Tang H, Wang Y, Xie L, Mao X, Won SJ, Galvan V, Jin K. Effect of neural precursor proliferation level on neurogenesis in rat brain during aging and after focal ischemia. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersfield AS, Croon RJ, Liu YW, Kells AP, Faull RL, Connor B. Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington's disease. Neuroscience. 2004;127:319–332. doi: 10.1016/j.neuroscience.2004.04.061. [DOI] [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis in the mammalian central nervous system: functionality and potential clinical interest. Med Sci Monit. 2005;11:RA247–RA252. [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int J Med Sci. 2008;5:127–132. doi: 10.7150/ijms.5.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25:88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Hughes SM, Connor B. Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington's disease. Exp Neurol. 2006;199:384–396. doi: 10.1016/j.expneurol.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Vergote D, Butler GS, Ooms M, Cox JH, Silva C, Hollenberg MD, Jhamandas JH, Overall CM, Power C. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci U S A. 2006;103:19182–19187. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Perego C, De Simoni MG. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43 Suppl 5:30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- Waldau B, Shetty AK. Behavior of neural stem cells in the Alzheimer brain. Cell Mol Life Sci. 2008;65:2372–2384. doi: 10.1007/s00018-008-8053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ikeda S, Kanno S, Guang LM, Ohnishi M, Sasaki M, Kobayashi T, Tamura S. Activation of c-Jun amino-terminal kinase is required for retinoic acid-induced neural differentiation of P19 embryonal carcinoma cells. FEBS Lett. 2001;503:91–96. doi: 10.1016/s0014-5793(01)02699-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Fu S, Wang Y, Yu P, Hu J, Gu W, Xu XM, Lu P. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol Cell Neurosci. 2007;36:343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Peng H, Erdmann NB, Tian C, Monaghan DT, Zheng JC. Calcium-permeable AMPA receptors containing Q/R-unedited GluR2 direct human neural progenitor cell differentiation to neurons. Faseb J. 2008 doi: 10.1096/fj.07-104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, Kaltschmidt C. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- Wong G, Goldshmit Y, Turnley AM. Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol. 2004;187:171–177. doi: 10.1016/j.expneurol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Wu JP, Kuo JS, Liu YL, Tzeng SF. Tumor necrosis factor-alpha modulates the proliferation of neural progenitors in the subventricular/ventricular zone of adult rat brain. Neurosci Lett. 2000;292:203–206. doi: 10.1016/s0304-3940(00)01472-5. [DOI] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol. 2001;172:115–127. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
- Yamamoto SiS, Nagao M, Sugimori M, Kosako H, Nakatomi H, Yamamoto N, Takebayashi H, Nabeshima Yi Y, Kitamura T, Weinmaster G, Nakamura K, Nakafuku M. Transcription Factor Expression and Notch-Dependent Regulation of Neural Progenitors in the Adult Rat Spinal Cord. J Neurosci. 2001;21:9814–9823. doi: 10.1523/JNEUROSCI.21-24-09814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentrich E, Han SY, Pessoa-Brandao L, Butterfield L, Heasley LE. Collaboration of JNKs and ERKs in nerve growth factor regulation of the neurofilament light chain promoter in PC12 cells. J Biol Chem. 2002;277:4110–4118. doi: 10.1074/jbc.M107824200. [DOI] [PubMed] [Google Scholar]
- Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM, Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 in HIV-1 encephalitis. J Neuroimmunol. 2001;115:182–191. doi: 10.1016/s0165-5728(00)00463-x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, Zhu XJ, Wang B, Xu JS, Zhu DY. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem. 2007;103:1843–1854. doi: 10.1111/j.1471-4159.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–229. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XJ, Hua Y, Jiang J, Zhou QG, Luo CX, Han X, Lu YM, Zhu DY. Neuronal nitric oxide synthase-derived nitric oxide inhibits neurogenesis in the adult dentate gyrus by down-regulating cyclic AMP response element binding protein phosphorylation. Neuroscience. 2006;141:827–836. doi: 10.1016/j.neuroscience.2006.04.032. [DOI] [PubMed] [Google Scholar]