Abstract
The influence of highly active antiretroviral therapy (HAART) upon hepatitis B virus (HBV) vaccine responses in HIV-infected individuals is unclear. After classification of vaccinees as non-responders (HBsAb <10 IU/L) or responders (HBsAb ≥10 IU/L) in our HIV cohort, multivariate logistic regression was used to assess factors associated with subsequent vaccine response. Of 626 participants vaccinated from 1988–2005, 217 (35%) were vaccine responders. Receipt of ≥3 doses of vaccine (OR 1.83, 95% CI 1.24–2.70), higher CD4 count at vaccination (OR 1.09, 95% CI 1.05–1.13 per 50 cells/μl increase), and use of HAART (OR 2.37, 95% CI 1.56–3.62) were all associated with increased likelihood of developing a response. However, only 49% of those on HAART at last vaccination responded, and 62% of those on HAART, with CD4 count ≥350, and HIV RNA <400 copies/mL responded. Compared to those on HAART with CD4 count ≥350, those not on HAART with CD4 count ≥350 had significantly reduced odds of developing a vaccine response (OR 0.47, 95% CI 0.30–0.70). While HAART use concurrent with HBV immunization was associated with increased probability of responding to the vaccine, the response rate was low for those on HAART. These data provide additional evidence of HAART benefits, even in those with higher CD4 counts, but also highlight the need for improving HBV vaccine immunogenicity.
Keywords: Hepatitis B vaccine, Hepatitis B virus, Human Immunodeficiency Virus, Highly active antiretroviral therapy, immunization
INTRODUCTION
The burden and clinical impact of hepatitis B virus (HBV) infection in individuals infected with human immunodeficiency virus (HIV) are significant.[1] The prevalence of serologic HBV infection in individuals with HIV has been reported to be approximately 40–80%, with an overall prevalence of chronic HBV of 5–10%.[1, 2] HBV co-infection in individuals with HIV increases liver-related morbidity and mortality, toxicity of antiretroviral therapy, and complicates treatment decisions.[3–5] For these reasons, HBV vaccination is recommended by consensus guidelines for all HIV-infected individuals without evidence of prior HBV infection or immunity.[6, 7]
Unlike some currently recommended vaccines, HBV vaccine has an established correlate of clinical protection, particularly in HIV-uninfected individuals where antibody to hepatitis B surface antigen (HBsAb) titer ≥10 IU/L has been shown to predict vaccine efficacy.[8] Similar to HIV-uninfected patients, we recently reported HIV-infected vaccine recipients with HBsAb ≥10 IU/L had lower risk of developing HBV infection compared to vaccine recipients with HBsAb <10 IU/L for at least seven years following vaccination.[9, manuscript submitted] However, in our cohort only 35% of vaccine recipients developed such a response, and several other studies have shown response rates to vaccination are indeed lower in HIV-infected individuals than in HIV-uninfected individuals.[10–12] Risk factors for vaccine non-response have included higher HIV RNA at the time of vaccination,[13, 14] anti-hepatitis C virus (HCV) positivity, [15, 16] and lower nadir CD4 cell count prior to vaccination.[17] Lower CD4 cell count at the time of vaccination has also been associated with lower vaccination response rates in some, but not all studies.[11, 13, 14, 16, 18]
Despite these investigations, little is known regarding the effect of highly active antiretroviral therapy (HAART) on responses to HBV vaccine. In one study evaluating the use of an adjuvant where all vaccinees had undetectable HIV RNA on HAART, 89% of subjects not receiving the adjuvant developed HBsAb ≥10 IU/L suggesting receipt of HAART at the time of vaccination may increase the rates of vaccine response although this study was not designed to answer this question.[19] Another investigation reported that of those receiving HAART at the time of vaccination, vaccine responders had been treated with HAART for a significantly longer duration prior to vaccination compared to nonresponders.[13] Additional unanswered questions include whether all patients would benefit from HAART at the time of vaccination or only those with lower CD4 cell counts. Several recent studies have described benefits from HAART in those with higher CD4 cell counts. Improved immunologic reconstitution and an increased probability of virologic suppression have been reported in those initiating HAART at higher CD4 cell counts, including >350 cells/μL.[20–22] In addition, CD4 cell count guided structured treatment interruption was recently shown to increase risk of death, opportunistic disease, and non-AIDS defining illness, such as cardiovascular disease, even among those with a CD4 cell count ≥350 cells/μL.[23] Consequently, use of HAART even in those with CD4 counts ≥350 cells/μL may be associated with other immunologic benefits, such as improved vaccine responses.
Therefore, we sought to determine factors associated with developing a response to HBV vaccine, including the use of HAART, in our well characterized prospectively followed multicenter cohort.
METHODS
The U.S. Military HIV Natural History Study (NHS) is an ongoing, continuous enrollment observational cohort of HIV-infected Department of Defense beneficiaries followed at seven military medical centers in the United States and has been previously described.[24] Enrolling since 1986, the NHS has over 4900 participants with signed, written consent. Approval for this research was obtained from the institutional review board at each participating site.
Participants were eligible for this study if they were (1) concurrently non-reactive for HBsAg and HBcAb initially following HIV diagnosis, (2) there was a documented date of HBV vaccination after the HIV seropositivity date, and (3) there was either a documented vaccine response 3–9 months after the last dose of vaccine or an available repository specimen within this time interval. Participants with history of HBV vaccination prior to HIV seropositivity were excluded. Neither the vaccine dose nor preparation was captured in the database. HAART was defined as combination antiretroviral therapy with at least three agents, similar to previous investigations.[24] The presence of an AIDS-defining illness was defined using 1993 Centers for Disease Control and Prevention criteria, [25] with the exception of isolated CD4 cell count <200 cells/μL. Cutaneous delayed-type hypersensitivity (DTH) responses were categorized as complete anergy, partial anergy, and normal/no anergy for reactions to 0/1, 2, or 3/4 intradermally placed antigens, respectively.[26] Infection with syphilis, N. gonorrhea, C. trachomatis, or genital herpes simplex virus was considered to be a sexually transmitted infection (STI).
For study participants without a documented vaccine response 3–9 months following vaccination, available repository specimens within the same time interval were tested for HBsAb (ETI-AB-AUK PLUS: Enzyme Immunoassay for the Detection of Antibody to Hepatitis B Surface Antigen (HBsAb),, DiaSorin Inc., Stillwater, MN) by the Department of Laboratory Diagnostics and Monitoring, Division of Retrovirology, Walter Reed Army Institute of Research, Rockville, MD. Non-response to vaccine was defined as HBsAb <10 IU/L and positive response was defined as HBsAb ≥10 IU/L. Subjects were then classified as non-responders or responders for statistical analyses.
Descriptive statistics were used to examine the characteristics of vaccine responders and non-responders. Medians were reported with inter-quartile ranges (IQR). Proportions were compared with chi-square tests; continuous valued variables were compared with general linear models or Kruskal-Wallis tests as appropriate. The probability of obtaining a protective vaccine response was investigated with univariate and multivariate logistic regression models. In additional analyses, we stratified subjects into clinically relevant categories based on their CD4 count and HAART use at the time of vaccination. For these categories, the proportions with a protective vaccine response were estimated with a normal approximation to the binomial distribution, and the likelihood of developing a protective vaccine response was assessed. Because some variables may have changed during the course of vaccination, including CD4 count, viral load, and HAART use, multivariate analyses for predictors of vaccine response and the analyses based on CD4 count and HAART use categories were conducted using two separate models: Model 1 denoted the analysis of factors at first vaccination and Model 2 denoted the analysis of factors at last vaccination. Finally, univariate and multivariate exploratory analyses were perfomed on the subset of participants who were on HAART at last HBV vaccination, adjusting for factors at last HBV vaccination. Factors captured for at least 75% of participants were included in all initial multivariate models. All final multiviariate models were adjusted for era of initial HBV vaccination (before 1996 versus 1996 or later). Odds ratios (OR) were reported with 95% confidence intervals (CI). All p-values were two-sided; all statistical analyses were performed using SAS software (version 9.1, Cary, NC).
RESULTS
Participant characteristics
A total of 626 participants met inclusion criteria and were included in the analysis. 217 (35%) were vaccine responders and 409 (65%) were non-responders. The date of administration of the first dose of vaccine ranged from 1988–2005. The median time from HIV seropositivity to vaccination was 254 days (IQR, 56–958). Subject characteristics at the time of vaccination overall and by subsequent vaccine response are shown in table 1. Sixteen percent (n=102) were on HAART at the time of initial vaccination, and 33% (n=206) were on HAART at last vaccination. Thirty-eight percent (58/172) of responders not on HAART at first vaccination initiated HAART prior to last vaccination compared to 15% (53/352) of non-responders (p <0.001) Overall, the median CD4 count was 490 cells/μL (IQR 350–655) at first vaccination and 515 cells/μL (IQR 373–682 ) at last vaccination. Sixty-one percent of vaccinees received ≥3 doses of vaccine.
Table 1.
Patient characteristics overall and by vaccine response.
| Vaccine Response |
||||
|---|---|---|---|---|
| Characteristic | N | Overall | HBsAb < 10 IU/L N = 409 | HBsAb ≥ 10 IU/L N = 217 |
| Age, median years (IQR) | 626 | 29.6 (25.1–35.1) | 29.8 (25.4–34.9) | 28.9 (24.4–35.3) |
|
| ||||
| No. male | 626 | 556 (89) | 370 (91) | 186 (86) |
|
| ||||
| Ethnicity | 626 | |||
| Caucasian | 286 (46) | 189 (46) | 97 (45) | |
| African American | 258 (41) | 167 (41) | 91 (42) | |
| Other | 82 (13) | 53 (13) | 29 (13) | |
|
| ||||
| BMI, median kg/m2 (IQR) | 503 | 25.3 (23.0–27.4) | 25.1 (22.9–27.3) | 25.5 (23.1–28.1) |
|
| ||||
| CD4 cell count at first vac., median cells/μL (IQR) | 519 | 490 (350–655) | 474 (337–620) | 537 (397–744) |
|
| ||||
| CD4 cell count at last vac., median cells/μL (IQR) | 591 | 515 (373–682) | 479 (328–630) | 592 (450–751) |
|
| ||||
| Nadir CD4 cell count, median cells/μL (IQR) | 529 | 416 (296–564) | 395 (287–546) | 440 (307–628) |
|
| ||||
| HIV RNA load at first vac., median log10 copies/mL (IQR) | 266 | 3.73 (2.43–4.46) | 3.97 (3.03–4.58) | 3.25 (2.30–4.04) |
|
| ||||
| HIV RNA load at first vac., copies/mL | 266 | |||
| <400 | 73 (27) | 31 (20) | 42 (38) | |
| ≥400 | 193 (73) | 123 (80) | 70 (62) | |
|
| ||||
| HIV RNA load at last vac., median log10 copies/mL (IQR) | 376 | 2.90 (2.07–4.02) | 3.30 (2.30–4.20) | 2.30 (1.40–3.60) |
|
| ||||
| HIV RNA load at last vac., copies/mL | 376 | |||
| <400 | 167 (44) | 75 (35) | 92 (56) | |
| ≥400 | 209 (56) | 138 (65) | 71 (44) | |
|
| ||||
| Prior AIDS-defining illness | 626 | 13 (2) | 9 (2) | 4 (2) |
|
| ||||
| Prior STI | 626 | 162 (26) | 117 (29) | 45 (21) |
|
| ||||
| Anti-HCV positive | 408 | 10 (3) | 8 (3) | 2 (1) |
|
| ||||
| Cutaneous DTH | 373 | |||
| Normal response | 288 (77) | 189 (75) | 99 (82) | |
| Partial response | 52 (14) | 40 (16) | 12 (10) | |
| Anergic | 33 (9) | 23 (9) | 10 (8) | |
|
| ||||
| Number of vaccinations | 626 | |||
| 1–2 | 241 (39) | 175 (43) | 66 (30) | |
| 3 | 240(38) | 150 (37) | 90 (41) | |
| 4–6 | 125 (20) | 75 (18) | 50 (23) | |
| ≥7 | 20 (3) | 9 (2) | 11 (5) | |
|
| ||||
| HAART at first vac. | 626 | |||
| No | 524 (84) | 352 (86) | 172 (79) | |
| Yes | 102 (16) | 57 (14) | 45 (21) | |
|
| ||||
| HAART at last vac. | 626 | |||
| No | 420 (67) | 304 (74) | 116 (53) | |
| Yes | 206 (33) | 105 (26) | 101 (47) | |
|
| ||||
| Year of first vaccination | 626 | |||
| Before 1996 | 307 (49) | 227 (56) | 80 (37) | |
| 1996 or later | 319 (51) | 182 (45) | 137 (63) | |
NOTE. All data are prior to or at time of receipt of first dose of vaccine unless otherwise indicated. Data are no. of patients (%) with data available, unless otherwise indicated. IQR, inter-quartile range; BMI, body mass index; STI, sexually transmitted infection; HCV, hepatitis C virus; DTH, delayed-type hypersensitivity; HAART, highly active antiretroviral therapy; vac., HBV vaccination.
Factors associated with vaccine response
Univariate analysis of factors associated with developing a vaccine response is shown in table 2. The final multivariate models for factors at first vaccination (Model 1) and factors at last vaccination (Model 2) are shown in table 3. In the final multivariate model of factors at last vaccination use of HAART at the time of vaccination (OR 2.37, 95% CI 1.56–3.62) and receipt of ≥3 doses (OR 1.83, 95% CI 1.24–2.70) were both independently associated with an increased probability of achieving a positive response. (Model 2, Table 3) Additionally, for every 50 cells/μL increase in CD4 cell count at the time of last vaccination the odds of developing a vaccine response increased by 9% (OR 1.09, 95% CI 1.05–1.13 per 50 cells/μL increase). Finally, female gender also was significantly associated with an increased likelihood of developing a response (OR 1.99, 95% CI 1.15–3.45). Results for factors at first vaccination were similar to those of factors at last vaccination. (Model 1, Table 3) Receipt of ≥4 doses of vaccine was not associated with increased rates of vaccine response compared to 3 doses of vaccine, and prior STI was not associated with vaccine response when included in any multivariate model, and therefore not included in the final models (data not shown). HIV RNA at the time of vaccination was not included in either final multivariate model because this test was not routinely performed prior to 1996, and subsequently unknown for many subjects. In addition, nadir CD4 cell count was not included in the models because it was found to be highly correlated with CD4 cell count at vaccination.
Table 2.
Univariate analysis for factors associated with developing a vaccine response of HBsAb ≥10 IU/L.
| Characteristic | N | Univariate OR (95% CI) | P |
|---|---|---|---|
| Age, per 10 year increase | 626 | 0.84 (0.66–1.05) | 0.13 |
|
| |||
| Gender | |||
| Male | 556 | Referent | |
| Female | 70 | 1.58 (0.96–2.62) | 0.07 |
|
| |||
| Ethnicity | |||
| Caucasian | 286 | Referent | |
| African American | 258 | 1.06 (0.75–1.51) | 0.74 |
| Other | 82 | 1.07 (0.63–1.77) | 0.81 |
|
| |||
| BMI, per 1 kg/m2 | 503 | 1.02 (0.98–1.07) | 0.34 |
|
| |||
| CD4 cell count at first vac., per 50 cells/μL increase | 519 | 1.07 (1.03–1.11) | <0.001 |
|
| |||
| CD4 cell count at last vac., per 50 cells/μL increase | 591 | 1.09 (1.06–1.13) | <0.001 |
|
| |||
| Nadir CD4 cell count, per 50 cells/μL decrease | 529 | 0.94 (0.91–0.98) | 0.004 |
|
| |||
| HIV RNA load at first vac., per 0.5 log10 increase | 266 | 0.79 (0.70–0.89) | <0.001 |
|
| |||
| HIV RNA load at last vac., per 0.5 log10 increase | 376 | 0.81 (0.74–0.88) | <0.001 |
|
| |||
| Prior AIDS-defining illness | 626 | 0.83 (0.22–2.60) | 0.77 |
|
| |||
| Prior STI | 626 | 0.65 (0.44–0.96) | 0.03 |
|
| |||
| Anti-HCV positive | 408 | 0.45 (0.07–1.83) | 0.32 |
|
| |||
| Cutaneous DTH | |||
| Normal response | 288 | Referent | |
| Partial response/Anergic | 85 | 0.67 (0.39–1.15) | 0.14 |
|
| |||
| Total no. vaccinations | |||
| 1–2 | 241 | Referent | |
| ≥3 | 385 | 1.71 (1.21–2.44) | 0.003 |
|
| |||
| HAART at first vac. | |||
| No | 524 | Referent | |
| Yes | 102 | 1.62 (1.05–2.48) | 0.03 |
|
| |||
| HAART at last vac. | |||
| No | 420 | Referent | |
| Yes | 206 | 2.52 (1.78–3.57) | <0.001 |
|
| |||
| Year of first vaccination | |||
| Before 1996 | 307 | Referent | |
| 1996 or later | 319 | 2.14 (1.52–2.99) | <0.001 |
NOTE. All data are prior to or at time of receipt of first dose of vaccine unless otherwise indicated. N, number of participants with data available; OR, odds ratio; CI, confidence interval; BMI, body mass index; STI, sexually transmitted infection; HCV, hepatitis C virus; DTH, delayed-type hypersensitivity; HAART, highly active antiretroviral therapy; vac., HBV vaccination.
Table 3.
Final multivariate models for factors associated with developing a vaccine response of HBsAb ≥10 IU/L adjusted for era of vaccination (before 1996 versus 1996 or later).
| Model 1 At First Vaccination | Model 2 At Last Vaccination | |||
|---|---|---|---|---|
| Characteristic | Multivariate OR (95% CI) | P | Multivariate OR (95% CI) | P |
| Age, per 10 year increase | 0.81 (0.61–1.07) | 0.14 | 0.79 (0.61–1.00) | 0.05 |
|
| ||||
| Gender | ||||
| Male | Referent | Referent | ||
| Female | 1.95 (1.09–3.50) | 0.02 | 1.99 (1.15–3.45) | 0.01 |
|
| ||||
| CD4 cell count, per 50 cells/μL increase | 1.08 (1.04–1.12) | <0.001 | 1.09 (1.05–1.13) | <0.001 |
|
| ||||
| No. vaccinations | ||||
| 1–2 | Referent | Referent | ||
| ≥3 | 2.18 (1.45–3.27) | <0.001 | 1.83 (1.24–2.70) | 0.003 |
|
| ||||
| HAART at vaccination | ||||
| No | Referent | Referent | ||
| Yes | 1.38 (0.80–2.38) | 0.25 | 2.37 (1.56–3.62) | <0.001 |
NOTE. OR, odds ratio; CI, confidence interval; HAART, highly active antiretroviral therapy.
HAART and vaccine response
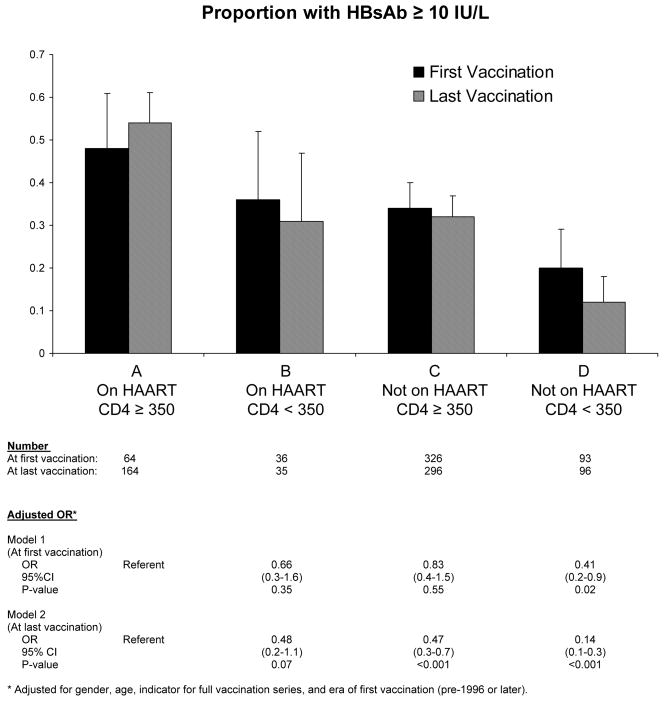
To evaluate the relationships of HAART use and CD4 cell count with vaccine response, participants were classified into four categories by CD4 cell count and HAART use at the time of vaccination and analyzed by two different models similar to above for first and last vaccination. (Figure 1) One hundred seven participants did not have a CD4 cell count available at time of first vaccination and were not included in Model 1. At last vaccination, 35 participants did not have a CD4 cell count available, and were subsequently not included in Model 2. The estimated proportions of participants with a protective response following last vaccination differed significantly among the groups defined at last vaccination (p < 0.001). Receipt of HAART at last vaccination was associated with an increased probability of developing a vaccine response regardless of CD4 category. (Figure 1) Among the 131 participants with CD4 cell count <350 cells/μL at last vaccination, 31% on HAART and 12% not on HAART had a protective response (p < 0.001); and among the 460 participants with CD4 cell count ≥350 cells/μL, 54% on HAART and 32% not on HAART developed a positive response (p < 0.001). From adjusted logistic regression models with categories formed by CD4 cell count and HAART use at time of last vaccination, those with CD4 cell count ≥350 cells/μL but not on HAART had a 53% reduced odds of developing a vaccine response compared to those with similar CD4 cell count, i.e. ≥350 cells/μL, and on HAART (adjusted OR 0.47, 95% CI 0.30–0.70). (Figure 1) Results analyzing HAART use and CD4 cell count at first vaccination were similar (Figure 1).
Figure 1.
Proportion with HBsAb ≥10 IU/L following vaccination by CD4 cell count and HAART status at the time of first dose or last dose of vaccine.
Finally, given the strong association of HAART with developing a vaccine response, exploratory analyses were performed for participants on HAART at last vaccination (N = 206). (Table 4) Of 206 participants on HAART at last vaccination, 101 (49%) developed HBsAb ≥10 IU/L. Median duration of HAART prior to vaccination was not significantly different between non-responders and responders, 16.1 months (IQR, 7.9 – 35.2) versus 18.0 months (IQR, 9.2 –46.3), respectively (p= 0.18). However, of the 200 participants with an available HIV RNA measurement at last vaccination, 81/145 (56%) with HIV RNA < 400 copies/ml responded compared to 19/55 (35%) of those with HIV RNA ≥400 copies/ml (p=0.007). Of the 95 individuals with a “best case” scenario (on HAART at last vaccination, with CD4 cell count ≥350 cells/μL, HIV RNA <400 copies/mL, and with receipt of ≥3 doses), 59 (62%) developed a response compared to 42/111 (38%) of those on HAART but lacking at least one of these other favorable characteristics (p <0.001). In multivariate analysis of those on HAART at last vaccination receipt of ≥3 doses remained associated with developing a response (OR 2.40, 95% CI 1.16–4.98) and higher HIV RNA load was associated with non-response (OR 0.60, 95% CI 0.41–0.88 per 1.0 log10 HIV RNA increase).
Table 4.
Univariate and multivariate analyses for factors at last vaccination associated with developing a vaccine response of HBsAb ≥10 IU/L in participants on HAART at last vaccination (n= 206).
| Characteristic | N | Univariate OR (95% CI) | P | Multivariate* OR (95% CI) | P |
|---|---|---|---|---|---|
| Age, per 10 year increase | 206 | 0.69 (0.49–0.95) | 0.03 | 0.63 (0.42–0.91) | 0.02 |
|
| |||||
| Gender | |||||
| Male | 184 | Referent | Referent | ||
| Female | 22 | 3.11 (1.16–8.29) | 0.02 | 4.85 (1.53–15.38) | 0.007 |
|
| |||||
| Ethnicity | |||||
| Caucasian | 108 | Referent | |||
| African American | 78 | 1.11 (0.62–1.99) | 0.73 | ||
| Other | 20 | 0.43 (0.15–1.20) | 0.11 | ||
|
| |||||
| BMI, per 1 kg/m2 | 190 | 0.99 (0.92–1.06) | 0.69 | ||
|
| |||||
| CD4 cell count, per 50 cells/μL increase | 199 | 1.08 (1.02–1.15) | 0.01 | 1.03 (0.96–1.11) | 0.36 |
|
| |||||
| Nadir CD4 cell count, per 50 cells/μL decrease | 203 | 1.07 (0.99–1.16) | 0.12 | ||
|
| |||||
| HIV RNA load, per 1.0 log10 increase | 200 | 0.58 (0.42–0.79) | <0.001 | 0.60 (0.41–0.88) | 0.008 |
|
| |||||
| Prior AIDS-defining illness | 206 | 0.32 (0.10–1.03) | 0.06 | ||
|
| |||||
| Prior STI | 206 | 1.05 (0.53–2.09) | 0.89 | ||
|
| |||||
| Anti-HCV positive | 206 | 0.77 (0.17–3.54) | 0.74 | ||
|
| |||||
| Cutaneous DTH | |||||
| Normal response | 129 | Referent | |||
| Partial response/Anergic | 49 | 0.75 (0.39–1.46) | 0.40 | ||
|
| |||||
| No. vaccinations | |||||
| 1–2 | 60 | Referent | Referent | ||
| ≥3 | 146 | 2.25 (1.21–4.20) | 0.01 | 2.40 (1.16–4.98) | 0.02 |
The multivariate model was adjusted by era of vaccination (before 1996 versus 1996 or later).
NOTE. All data are prior to or at time of receipt of last dose of vaccine unless otherwise indicated. N, number of participants with data available; OR, odds ratio; CI, confidence interval; BMI, body mass index; STI, sexually transmitted infection; HCV, hepatitis C virus; DTH, delayed-type hypersensitivity; HAART, highly active antiretroviral therapy; vac., vaccination.
DISCUSSION
As HBV vaccine responses have been associated with HBV infection risk in HIV-infected individuals in our cohort, [9, manuscript submitted] we investigated factors associated with developing an initial vaccine response of HBsAb ≥10 IU/L to improve vaccine administration practices as well as our understanding of which HIV-infected patients respond better to vaccine. Receipt of at least three doses of vaccine and use of HAART at the time of vaccination were both associated with developing HBsAb ≥10 IU/L following vaccination. In addition, these benefits were evident even in those with CD4 count ≥350 cells/μL. Furthermore, in participants on HAART, response rates were further increased in those with viral suppression. These data provide further support to an expanding body of evidence that HAART is beneficial for patients with higher CD4 cell counts in addition to those with advanced immunosuppression. However, the overall low response rates seen in our relatively healthy cohort, in an open access healthcare system, highlight the need for new strategies to improve HBV vaccine immunogenicity and delivery for HIV-infected patients.
While the importance of completing the vaccination series is intuitive, data suggest vaccination completion rates are poor in clinical practice.[17, 27] In one study of nine metropolitan HIV clinics, only 53% of patients receiving one dose of HBV vaccine went on to complete the series.[17] In that study, frequency of patient visits and clinical site were associated with administration of vaccine, but factors predictive of completing the vaccine series were not reported. We found a similar percentage of participants received ≥3 doses, 62%. As our patients receive medical treatment in the military healthcare system where vaccines and medications are provided free of charge, the low completion rate cannot be attributed to barriers such as lack of insurance coverage. Identification of factors associated with completion of the vaccine series may help improve vaccine delivery practices. Such information may be useful for other clinics as well, because factors other than insurance coverage have been shown to impact delivery of vaccines, including HBV vaccine.[17, 28] Until such data are known, clinics providing HBV vaccine to HIV-infected patients should consider instituting systems-based measures to ensure completion.
Our findings were consistent with prior investigations in both HIV-infected and HIV-uninfected patients which identified a number of factors associated with non-response to HBV vaccine, including male gender, increasing age, receipt of less than three doses, detectable HIV RNA load, lower CD4 count, and lower nadir CD4 count prior to vaccination.[6, 13, 14, 17, 18, 29–32] That anti-HCV positivity was not associated with non-response in our study, in contrast to previous reports[15, 16], likely reflects the low prevalence of HCV infection in our cohort.
While HAART use was associated with increased likelihood of obtaining a response, the response rates for participants on HAART were only approximately 50%. Although HAART improved response rates in those with both high and low CD4 cell counts, participants with low CD4 counts on HAART and those with high CD4 counts not on HAART demonstrated similar response rates of approximately 30%. Participants with low CD4 counts not on HAART demonstrated response rates below 20%. Such low response rates suggest that deferral of vaccination until after HAART initiation may be appropriate, particularly for those with low CD4 counts and not on HAART. In contrast, the response rate for those receiving ≥3 doses, with CD4 count ≥350 cells/μL, and on HAART with suppressed viral load was 62% suggesting this may be the optimal setting for HBV vaccine administration in this patient population. However, unless earlier HAART initiation is proven beneficial and widely adopted, more immunogenic HBV vaccines or vaccination strategies able to elicit responses in those without these optimal characteristics will likely be needed to reduce the burden of HBV-associated liver disease in this population. Two studies evaluating the use of higher doses of vaccine demonstrated that improvements in vaccine responses were marginal and limited to those with higher CD4 counts.[14, 33] Results using adjuvants with HBV vaccine in HIV-infected individuals have been mixed.[19, 34] Finally, revaccination following initiation of HAART in children has been shown to improve HBV vaccine response rates, suggesting that this approach may be beneficial in HIV-infected adults.[35]
Recent investigations indicate HIV-infected patients with CD4 counts ≥350 cells/μL may obtain clinical benefits from HAART. Results have shown associations between improved immunologic and virologic responses to therapy and initiation of HAART at higher CD4 cell counts, as well as a decreased risk of death, opportunistic illness, and non-AIDS defining events for patients whose therapy was not interrupted.[20, 22, 23, 36] In agreement with those results we found use of HAART in participants with CD4 count ≥350 cells/μL was associated with another potential benefit, an improved likelihood of achieving the known correlate of protection following HBV vaccination. While the impact of initiating HAART at higher CD4 counts upon death and morbidity remains unknown, such practice would also likely improve the percentage of patients achieving a response to HBV vaccine by simply increasing the number of patients on HAART at the time of vaccination.
The mechanisms by which HAART improves HBV vaccine responses are not known. In HIV-uninfected adults, non-response to HBV vaccine appears to result from inefficient antigen presentation and failure of early CD4 cell stimulation.[37, 38] Although the immunologic basis for HBV vaccine non-response in HIV-infected individuals has not been thoroughly studied, the effects of HAART on CD4 cell function are well characterized, including a reduction of HIV-induced immune activation.[39, 40] Indeed, ongoing non-specific immune activation from HIV is thought to be a central feature of HIV pathogenesis contributing to, if not causing, the immunologic dysfunction seen, including a reduced ability to respond appropriately to antigens.[41] While immune activation was not assessed in our study, use of HAART was associated with improved ability to respond to HBV vaccine. Furthermore, in those on HAART at the time of vaccination, HIV RNA load predicted the likelihood of developing a response, concordant with previous data showing immune activation in patients on HAART correlates with the degree of ongoing viral replication.[40, 42] In addition to improving initial HBV vaccine responses, HAART at the time of HBV immunization may affect long-term vaccine-induced protection by improving immune memory. Recent studies have shown partial restoration of the functional memory B cell pool with HAART[43] and T cell dependence of memory B cell function following HBV vaccination, [44] suggesting the use of HAART would be associated with improved maintenance of immune memory to HBV vaccine.
Our investigation has limitations. First, our cohort has some unique features, including diagnosis early after HIV infection due to frequent, routine military screening, open access to care in the military health system, and virtually no illicit drug use.[45] Because of these factors, our findings may not be generalizable to patients with more advanced HIV infection at the time of diagnosis or with other characteristics, such as intravenous drug use. However, these characteristics make our cohort an optimal setting for investigating HBV vaccine responses. In addition, administration of both HBV vaccine and HAART were not randomized, and therefore we are not able to conclude definitively that use of HAART directly resulted in improved HBV vaccine response rates. While the overall sample size was large, some comparisons were limited by the number of participants in some groups, such as those on HAART at the time of vaccination with CD4 count <350 cells/μL, or by the number of individuals with available data, such as viral load. Lastly, we were not able to evaluate the association between vaccine dose and responses, although guideline recommendations are generally followed at sites in our network, and the effects of higher doses of HBV vaccination in HIV-infected patients appear somewhat limited.[14, 33]
The principal findings from this study, the largest to date of HBV vaccine responses in HIV-infected patients, are important for vaccine delivery programs and clinicians. First, in order to maximize vaccine response rates and thus population benefit, emphasis must be placed not only upon providing HBV vaccine, but also on ensuring completion of the immunization series. Second, achievement of a vaccine response, the accepted correlate of clinical protection, was significantly more likely in those on HAART at the time of vaccination including the subset with relatively preserved CD4 cell counts, providing more evidence of the clinical and immunological benefits of HAART in those with high CD4 counts. However, the overall low response rates in our healthy cohort with open access to healthcare suggest additional strategies toward optimizing immunization practices and improving HBV vaccine immunogenicity are needed. Such practices could reduce the prevalence of HBV infection among patients with HIV, saving money and lives.
Acknowledgments
The authors would like to express our gratitude to the current members of the IDCRP HIV Working Group and the long line of military HIV researchers who have supported the HIV NHS and for the research coordinators and support staff for their countless hours of work. Most importantly, we would like to thank the patients for their participation, without which this research would not have been possible.
Support for this work was provided by the Infectious Disease Clinical Research Program (IDCRP) of the Uniformed Services University of the Health Sciences (USUHS). The IDCRP is a DoD tri-service program executed through USUHS and the Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF), in collaboration with HHS/NIH/NIAID/DCR through Interagency Agreement HU0001-05-2-0011. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting the views of the Departments of the Army, Navy, Air Force, or the Department of Defense. The authors have no commercial or other association that might pose a conflict of interest.
Footnotes
These data were presented in part at the 46th Annual Meeting of the Infectious Diseases Society of America, Washington, DC, Oct 24–28, 2008, Abstract H-2314.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soriano V, Puoti M, Bonacini M, Brook G, Cargnel A, Rockstroh J, et al. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV international panel. AIDS. 2005;19:221–40. [PubMed] [Google Scholar]
- 2.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. Journal of Infectious Diseases. 2003 Aug 15;188(4):571–7. doi: 10.1086/377135. [DOI] [PubMed] [Google Scholar]
- 3.Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Munoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 4.Sulkowski M, Thomas DL, Chaisson RE, Moore D. Hepatoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. Journal of the American Medical Association. 2000;283(1):74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 5.Levy V, Grant RM. Antiretroviral therapy for hepatits B virus-HIV-coinfected patients: promises and pitfalls. Clinical Infectious Diseases. 2006;43:904–10. doi: 10.1086/507532. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of Adults. MMWR. 2006;55:1–25. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Guidelines for preventing opportunistic infections among HIV-infected persons- 2002 Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR. 2002;51 (RR8):1–40. [PubMed] [Google Scholar]
- 8.Hadler SC, Francis DP, Maynard JE, Thompson SE, Judson FN, Echenberg DF, et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. New England Journal of Medicine. 1986;315:209–14. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- 9.Landrum ML, Huppler Hullsiek K, Ganesan A, Weintrob AC, Crum-Cianflone NF, Barthel RV, et al. Hepatitis B vaccination and risk of hepatitis B infection in HIV-infected Individuals. doi: 10.1097/QAD.0b013e32832cd99e. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannucci PM, Zanetti AR, Gringeri A, Tanzi E, Morfini M, Messori A, et al. Long-term immunogenicity of a plasma-derived hepatitis B vaccine in HIV seropositive and HIV seronegative hemophiliacs. Archives of Internal Medicine. 1989;149:1333–7. [PubMed] [Google Scholar]
- 11.Collier AC, Corey L, Murphy VL, Handsfield HH. Antibody to human immunodeficiency virus (HIV) and suboptimal response to hepatitis B vaccination. Annals of Internal Medicine. 1988;109:101–5. doi: 10.7326/0003-4819-109-2-101. [DOI] [PubMed] [Google Scholar]
- 12.Landrum ML, Dolan MJ. Routine vaccination in HIV-infected adults. Infect Dis Clin Pract. 2008;16:85–93. [Google Scholar]
- 13.Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable HIV plasma RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clinical Infectious Diseases. 2005;41:1045–8. doi: 10.1086/433180. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca MO, Pang LW, Cavalheiro N, Barone AA, Lopes MH. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard to a double dose. Vaccine. 2005;23:2902–8. doi: 10.1016/j.vaccine.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 15.Quaglio G, Talamini G, Lugoboni F, Lechi A, Venturini L, Jarlais DC, et al. Compliance with hepatitis B vaccination in 1175 heroin users and risk factors associated with lack of vaccine response. Addiction. 2002;97:985–92. doi: 10.1046/j.1360-0443.2002.00147.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghandi RT, Wurcel A, Lee H, McGovern B, Shopis J, Geary M, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. Journal of Infectious Diseases. 2005;191:1435–41. doi: 10.1086/429302. [DOI] [PubMed] [Google Scholar]
- 17.Tedaldi EM, Baker RK, Moorman AC, Wood KC, Fuhrer J, McCabe RE, et al. Hepatitis A and B Vaccination Practices for Ambulatory Patients Infected with HIV. Clinical Infectious Diseases. 2004;38:1478–84. doi: 10.1086/420740. [DOI] [PubMed] [Google Scholar]
- 18.Rey D, Krantz V, Partisani M, Schmitt MP, Meyer P, Libbrecht E, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161–5. doi: 10.1016/s0264-410x(99)00389-8. [DOI] [PubMed] [Google Scholar]
- 19.Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19:1473–9. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 20.Palella FJ, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Annals of Internal Medicine. 2003;138:620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Mocroft A, Phillips AN, Gatell J, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–13. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 22.Gras L, Kesselring AM, Griffiin JT, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. ATHENA, Netherlands National Observational Cohort Study. Journal of Acquired Immune Deficiency Syndrome. 2007;45:183–92. doi: 10.1097/QAI.0b013e31804d685b. [DOI] [PubMed] [Google Scholar]
- 23.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. Strategies for the management of antiretroviral therapy (SMART) study group. New England Journal of Medicine. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 24.Weintrob AC, Fieberg AM, Agan BK, Ganesan A, Crum-Cianflone NF, Marconi VC, et al. Increasing age at HIV seroconversion from 18 to 40 years is associated with favorable virologic and immunologic responses to HAART. J Acquir Immune Defic Syndr. 2008;49(1):40–7. doi: 10.1097/QAI.0b013e31817bec05. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41 Accessed Jan 4, 2008 at http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm. [PubMed]
- 26.Dolan MJ, Kulkarni H, Camargo JF, He W, Smith A, Anaya J, et al. CCL3L1 and CCR5 influence cell-mediated immunity and affect HIV-AIDS pathogenesis via viral entry-independent mechanisms. Nature Immunology. 2007;8(12) doi: 10.1038/ni521. [DOI] [PubMed] [Google Scholar]
- 27.Bailey CL, Smith V, Sands M. Hepatitis B vaccine: a seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults. International Journal of Infectious Diseases. 2008;12:e77–e83. doi: 10.1016/j.ijid.2008.05.1226. [DOI] [PubMed] [Google Scholar]
- 28.Ndiaye SM, Hopkins DP, Shefer AM, Hinman AR, Briss PA, Rodewald L, et al. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5S):248–79. doi: 10.1016/j.amepre.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Alper CA, Kruskall MS, Marcus-Bagley D, Craven DE, Katz AJ, Brink SJ, et al. Genetic prediction of nonresponse to hepatitis B vaccine. New England Journal of Medicine. 1989;321:708–12. doi: 10.1056/NEJM198909143211103. [DOI] [PubMed] [Google Scholar]
- 30.Weissman JY, Tsuchiyose MM, Tong MJ, Co R, Chin K, Ettenger RB. Lack of response to recombinant hepatitis B vaccine in nonresponders to the plasma vaccine. JAMA. 1988;260:1734–8. [PubMed] [Google Scholar]
- 31.Bertino JS, Tirrell P, Greenberg RN, Keyserling HL, Poland GA, Gump D, et al. A comparative trial of standard or high-dose S subunit recombinant hepatitis B vaccine versus a vaccine containing S subunit, pre-S1, and pre-S2 particles for revaccination of healthy adult nonresponders. Journal of Infectious Diseases. 1997;175:678–81. doi: 10.1093/infdis/175.3.678. [DOI] [PubMed] [Google Scholar]
- 32.Wood RC, MacDonald KL, White KE, et al. Risk factors for lack of detectable antibody following hepatitis B vaccination of Minnesota health care workers. JAMA. 1993;270:2935–9. [PubMed] [Google Scholar]
- 33.Cornejo-Juarez P, Volkow-Fernandez P, Escobedo-Lopez K, Vilar-Compte D, Ruiz-Palacios G, Soto-Ramirez LE. Randomized controlled trial of hepatitis B virus vaccine in HIV-1-infected patients comparing two different doses. AIDS Research and Therapy. 2006;3:9. doi: 10.1186/1742-6405-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overton ET, Kang M, Umbleja T, Alston-Smith BL, Bastow B, Koziel MJ, et al. ACTG 5220: Hepatitis B vaccine responses in HIV-infected persons using granulocyte-macrophage colony-stimulating factor (GM-CSF). 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and the 46th Annual Meeting of the Infectious Diseases Society of America; October 25–28, 2008; Washington, DC. Abstract G-395. [Google Scholar]
- 35.Lao-araya M, Puthanakit T, Aurpibul L, Sirisanthana T, Sirisanthana V. Antibody response to hepatitis B re-vaccination in HIV-infected children with immune recovery on highly active antiretroviral therapy. Vaccine. 2007;25:5324–9. doi: 10.1016/j.vaccine.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Lundgren JD, Babiker A, El-Sadr WM, Emery S, Grund B, et al. Inferior clinical outcome of the CD4 cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4 cell counts and HIV RNA levels during follow-up. Journal of Infectious Diseases. 2008;197:1145–55. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 37.Goncalves L, Albarran B, Salmen S, Borges L, Fields H, Montes H, et al. The nonresponse to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology. 2004;326:20–8. doi: 10.1016/j.virol.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 38.Egea E, Iglesias A, Salazar M, Morimoto C, Kruskall MS, Awdeh ZL, et al. The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J Exp Med. 1991;173:531–8. doi: 10.1084/jem.173.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anthony KB, Yoder C, Metcalf JA, DerSimonian R, Orenstein JM, Stevens RA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. Journal of Acquired Immune Deficiency Syndromes. 2003;33:125–33. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 40.Benito JM, Lopez M, Lozano S, Ballesteros C, Martinez P, Gonzalez-Lahoz J, et al. Differential upregulation of CD38 on different T-cell subsets may influence the ability to reconstitute CD4 T cells under successful highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2005;38:373–81. doi: 10.1097/01.qai.0000153105.42455.c2. [DOI] [PubMed] [Google Scholar]
- 41.Hunt PW. Role of immune activation in HIV pathogenesis. Current HIV/AIDS Reports. 2007;4:42–7. doi: 10.1007/s11904-007-0007-8. [DOI] [PubMed] [Google Scholar]
- 42.Karlson AC, Younger SR, Martin JN, Grossman Z, Sinclair E, Hunt PW, et al. Immunologic and virologic evolution during period of intermittent and persistent low-level viremia. AIDS. 2004;18:981–9. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]
- 43.Moir S, Malaspina A, Ho J, Wang W, DiPoto AC, O’Shea MA, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. Journal of Infectious Diseases. 2008;197:572–9. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 44.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24:572–7. doi: 10.1016/j.vaccine.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 45.Brodine SK, Starkey MJ, Shaffer RA, Ito SI, Tasker SA, Barile AJ, et al. Diverse HIV-1 subtypes and clinical, laboratory and behavioral factors in a recently infected US military cohort. AIDS. 2003;17:2521–7. doi: 10.1097/00002030-200311210-00016. [DOI] [PubMed] [Google Scholar]