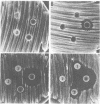
Abstract
Various factors affect the sensitivity of Neisseria gonorrhoeae to physiological levels of hydrophobic molecules. A total of 98 N. gonorrhoeae strains from rectal, cervical, and urethral cultures of homosexual men and heterosexual men and women were examined for their sensitivities to fecal lipids. Isolates were characterized according to cell envelope phenotype, auxotype, and protein I serogroup. Although cell envelope phenotype was an important factor in the resistance of this organism to fecal lipids (Mtr phenotype greater than wild type greater than Env phenotype), other factors were also of importance. AHU- strains (strains having a requirement for arginine, hypoxanthine, and uracil) uniformly exhibited a wild-type envelope phenotype but were as sensitive to fecal lipids as were Env strains. The protein I serogroup was not a factor in determining the sensitivity of wild-type envelope phenotype non-AHU- strains to fecal lipids. However, sexual preference and site of isolation were important factors. Wild-type envelope phenotype (non-AHU-) strains from homosexual men and heterosexual women were more resistant to fecal lipids than were similar isolates from heterosexual men. When these strains were compared by isolation site, it was observed that rectal isolates from homosexual men and heterosexual women were more resistant than were cervical isolates from heterosexual women or urethral isolates from heterosexual men. Urethral isolates from homosexual men were also more resistant to fecal lipids than were urethral isolates from heterosexual men. These data suggest that the host environment can select for increased resistance to hydrophobic molecules by an Mtr-independent mechanism. The basis for this Mtr-independent resistance is presently unknown, but it is likely that it involves an alteration of the target site(s) for fecal lipid inhibition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cacciapuoti A. F., Morse S. A. Glucose-6-phosphate dehydrogenase from Neisseria gonorrhoeae: partical characterization of the enzyme and inhibition by long-chain fatty acid acyl-coenzyme A derivatives. Can J Microbiol. 1980 Aug;26(8):863–873. doi: 10.1139/m80-151. [DOI] [PubMed] [Google Scholar]
- Cacciapuoti A. F., Wegener W. S., Morse S. A. Phospholipid metabolism in Neisseria gonorrhoeae: phospholipid hydrolysis in nongrowing cells. Lipids. 1979 Aug;14(8):718–726. doi: 10.1007/BF02533897. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Coleman R., Iqbal S., Godfrey P. P., Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979 Jan 15;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Sheu C. W., Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973 Feb 2;241(5388):321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarroll E. L., Muller P. J., Meyer E. A., Morse S. A. Lipid and carbohydrate metabolism of Giardia lamblia. Mol Biochem Parasitol. 1981 Feb;2(3-4):187–196. doi: 10.1016/0166-6851(81)90099-2. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Kolodkin A. B., Clark V. L., Tenover F. C., Young F. E. High correlation of the presence of methyladenine in Neisseria gonorrhoeae DNA with the AHU auxotype. Infect Immun. 1982 May;36(2):586–590. doi: 10.1128/iai.36.2.586-590.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith D. K., Morse S. A. Cross-linking analysis of Neisseria gonorrhoeae outer membrane proteins. J Bacteriol. 1980 Jul;143(1):182–187. doi: 10.1128/jb.143.1.182-187.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko P. G., Morse S. A. Neisseria gonorrhoeae cell envelope: permeability to hydrophobic molecules. J Bacteriol. 1981 Feb;145(2):946–952. doi: 10.1128/jb.145.2.946-952.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W., Schoolnik G. K., Falkow S. Genetic studies on Neisseria gonorrhoeae from disseminated gonococcal infections. Infect Immun. 1977 Oct;18(1):165–172. doi: 10.1128/iai.18.1.165-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Brown K. E., Morse S. A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect Immun. 1977 Aug;17(2):303–312. doi: 10.1128/iai.17.2.303-312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Morse S. A. Binding of progesterone to Neisseria gonorrhoeae and other gram-negative bacteria. Infect Immun. 1977 Apr;16(1):115–123. doi: 10.1128/iai.16.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980 Jan;26(1):13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Lysko P. G., McFarland L., Knapp J. S., Sandstrom E., Critchlow C., Holmes K. K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun. 1982 Aug;37(2):432–438. doi: 10.1128/iai.37.2.432-438.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Sawyer W. D., Haak R. A. Cross-linking analysis of the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):785–791. doi: 10.1128/iai.28.3.785-791.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Characterization of rabbit hyperimmune antisera by line-rocket immunoelectrophoresis for use in coagglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):17–26. doi: 10.1111/j.1699-0463.1980.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Blackman E., Sparling P. F. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol. 1974 Dec;120(3):1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F., Blackman E., Lewis E. Loss of low-level antibiotic resistance in Neisseria gonorrhoeae due to env mutations. J Bacteriol. 1975 Nov;124(2):750–756. doi: 10.1128/jb.124.2.750-756.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner P. J., Handsfield H. H., Holmes K. K. Low antibiotic resistance of gonococci causing disseminated infection. N Engl J Med. 1973 Jun 7;288(23):1221–1222. doi: 10.1056/NEJM197306072882308. [DOI] [PubMed] [Google Scholar]
- Willcox R. R. The rectum as viewed by the venereologist. Br J Vener Dis. 1981 Feb;57(1):1–6. doi: 10.1136/sti.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]