Abstract
Background
Improving the healthcare for patients with depression is a priority health policy across the world. Roughly, two major problems can be identified in daily practice: (1) the content of care is often not completely consistent with recommendations in guidelines and (2) the organization of care is not always integrated and delivered by multidisciplinary teams.
Aim
To describe the content and preliminary results of a quality improvement project in primary care, aiming at improving the uptake of clinical depression guidelines in daily practice as well as the collaboration between different mental health professionals.
Method
A Depression Breakthrough Collaborative was initiated from December 2006 until March 2008. The activities included the development and implementation of a stepped care depression model, a care pathway with two levels of treatment intensity: a first step treatment level for patients with non-severe depression (brief or mild depressive symptoms) and a second step level for patients with severe depression. Twelve months data were measured by the teams in terms of one outcome and several process indicators. Qualitative data were gathered by the national project team with a semi-structured questionnaire amongst the local team coordinators.
Results
Thirteen multidisciplinary teams participated in the project. In total 101 health professionals were involved, and 536 patients were diagnosed. Overall 356 patients (66%) were considered non-severely depressed and 180 (34%) patients showed severe symptoms. The mean percentage of non-severe patients treated according to the stepped care model was 78%, and 57% for the severely depressed patient group. The proportion of non-severely depressed patients receiving a first step treatment according to the stepped care model, improved during the project, this was not the case for the severely depressed patients. The teams were able to monitor depression symptoms to a reasonable extent during a period of 6 months. Within 3 months, 28% of monitored patients had recovered, meaning a Beck Depression Inventory (BDI) score of 10 and lower, and another 27% recovered between 3 and 6 months.
Conclusions and discussion
A stepped care approach seems acceptable and feasible in primary care, introducing different levels of care for different patient groups. Future implementation projects should pay special attention to the quality of care for severely depressed patients. Although the Depression Breakthrough Collaborative introduced new treatment concepts in primary and specialty care, the change capacity of the method remains unclear. Thorough data gathering is needed to judge the real value of these intensive improvement projects.
Keywords: quality improvement, depression, Breakthrough Collaborative, multidisciplinary teams, stepped care, care pathway
Introduction
Policies aiming to create an evidence-based mental healthcare system, offering appropriate care to patients and delivering better outcomes, have not been successful until now. According to the European Study of the Epidemiology of Mental Disorders (ESEMED) conducted in six western countries including the Netherlands, of all patients treated for an anxiety disorder or a depressive disorder, 57% were treated appropriately in secondary care and only 23% received the right treatment in primary care [1].
Major depressive disorder (MDD) [2] is a prevalent condition worldwide: 12 months- prevalence of MDD ranges from 4 to 10% and a lifetime prevalence of 15 to 17% [3–6]. In the Netherlands Mental Health Survey and Incidence Study (NEMESIS) [6, 7] a median duration of new depressive episode of 3 months was found, 63% of those with a new episode had recovered within 6 months and 76% in 12 months. Almost 20% of those affected had not recovered in 24 months [8]. Primary care is the key supplier of care to patients, because of the high prevalence of patients with depression or depressive feelings in general practice of around 21% [9]. Despite policy incentives to strengthen the capacities of primary care, general practitioners still refer more patients to a more expensive form of care in specialty care than to psychologists and social workers in primary care [9, 10].
Two depression guidelines are actually available to Dutch practitioners, recommending effective interventions for different subgroups of patients. The Multidisciplinary Guideline for Depressive Disorder, adopted in 2005 by a range of professional organizations in specialised mental health, and the depression standard, adopted by general practitioners in 2003 [11, 12]. Following depression guidelines can be of value to professionals as applying the effective interventions recommended in guidelines can lead to better outcomes for patients and to lower costs to society [13–18]. Unfortunately, the uptake of the depression guideline recommendations in Dutch daily practice has been slow. A study looking into evidence-based depression care in 1999, concluded that previous depression guideline editions were considered to be too globally formulated, giving insufficient tools to practitioners for decision support in daily practice [19–22]. Other implementation barriers can be related to characteristics of the professionals and the patients, and environmental factors such as a lack of support from peers or superiors, insufficient staff or time, and poor collaboration between professionals [18, 23].
The effective treatments proposed in the most recent depression guidelines, to be published in the Netherlands in the spring of 2009, range from less intensive interventions like psycho-education or self help intervention (individual or group courses), problem solving treatment (PST), and physical exercise (running therapy), to more intensive treatments such as cognitive behavioural therapy, pharmacotherapy and electro-convulsion therapy. Considering the heterogeneous course of MDD, the selection of the appropriate intervention and the organization of depression care needs to be built on careful timing and paced appropriately. Goals of treatment should be to avoid over-treatment in those with a favourable prognosis and to prevent the development of chronic symptoms in those depressed individuals with an unfavourable prognosis (under-treatment).
Over-treatment of minor and mild-major depressions is seen in general practice where antidepressant drugs are prescribed to 68% of the patients, regardless of the severity of depression [21, 24]. Also, antidepressants in many cases are prescribed over too long a period of time [25]. This is contrary to guideline recommendations and recent studies that advise less intensive treatments in mild cases because there is no additional effectiveness of antidepressant treatment over counseling alone [21, 26–28]. Less intensive treatment alternatives are insufficiently known and not made available or used by primary care professionals, despite the fact that they have been proven to be effective in recent randomised controlled trials in the Netherlands [29].
Under-treatment of patients with more severe symptoms, is caused by provider barriers including concerns about patient stigma, time pressures, inadequate knowledge about diagnostic criteria and treatment options, and a lack of psycho-social orientation. Also, poor recognition of depression by general practitioners has been reported; in one study 33% of cases were not diagnosed as depression or any other psychological disease. Moreover, patient-provider communication concerning pharmacotherapy can be improved [30]. Patient related causes include somatic presentation of depression by patients and resistance to a diagnosis of depression. Once pharmacotherapy is started, compliance is low. Up to 37% of patients stop taking medication too soon, after one or two prescriptions, whereas 15–45% stop psychotherapy treatment too early [19, 25]. System barriers include productivity pressures, limitations of mental health coverage, restrictions of specialists and treatments, the lack of a systematic method for detecting and managing depressed patients and inadequate continuity of care [22].
One of the methods to overcome barriers and improve the content and organization of care is the Breakthrough Series Collaborative, because of its ability to enhance the rate of diffusion of existing science into clinical practice, by using multi-institutional or multi-site work groups [31–35]. In this article, we present the content and results of a part of a large Breakthrough Collaborative project targeting better outcomes for patients suffering from depression. The information presented is directed at the improvements for adult patients in primary care. The collaborative was initiated by the Netherlands Institute of Mental Health and Addiction (http://www.trimbos.nl), operated from December 2006 to April 2008, and was funded by a national health insurers fund, as part of the depression initiative programme [36].
In the remainder of this article, we describe the problems in depression care targeted by the participants in this project, the improvement principles and goals, the improvement method, the methods used to collect and analyse the data, and the impact on key outcome and process indicators. In the discussion, the results are interpreted and compared to similar work, giving suggestions for future quality improvement projects.
Methods
Improvement principles and goals
A national expert team of depression opinion leaders and project coordinators was set in place. They developed a project plan, containing improvement principles, goals and suggestions for improvement ideas. The overall improvement principle was the implementation of a stepped care approach. In a stepped care approach evidence-based treatment options are ranked by their degree of intensity, looking at the impact on the patients life, the length of treatment, the setting (general practice or specialty care) and the costs, as well as combinations of these criteria [37–39]. Patients start to step in at the appropriate intensity level, which matches their (severity) profile. Stepped care models have the potential to improve efficiency and effectiveness of depression care [40–42]. Also, the implementation of a stepped care model can lead to better collaboration and integration, involving all partners across primary and secondary care, and making them aware of their individual contributions to the shared approach [38, 39, 42, 43].
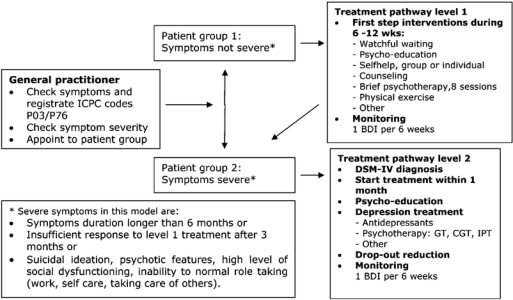
A pragmatic stepped care model was developed (Figure 1), consisting of a depression care pathway with two levels of treatment intensity: a first step treatment level for patients with mild depressive symptoms and a second step treatment level for patients with severe depressive symptoms.
Figure 1.
Stepped care depression model.
The stepped care model was based on previous projects in Dutch mental health care and on the (inter)national literature [37, 38]. Professionals applying all the elements of the stepped care depression model, needed to implement the following changes in their practices:
Stepped diagnostics. Depressive episodes were diagnosed as usual, with general practitioners using (patient group 1 in Figure 1) and patients with severe symptoms (patient group 2 in Figure 1) had to be made, based on a set of severity criteria (see box in left lower bottom of Figure 1). For severely depressed patients a DSM-IV assessment was indicated.
Stepped treatment. Implementation of a treatment pathway with two treatment levels: a first step level consisting of interventions for first, mild depressive episodes with a duration up to 3 months (treatment pathway level 1 in Figure 1) and a second step level mainly consisting of antidepressant medication and effective psychotherapeutic interventions (treatment pathway level 2 in Figure 1).
Monitoring and evaluation of the treatment plan. The course of symptoms and treatment progress were to be monitored in both pathways using the Beck Depression Inventory (BDI).
Derived from this stepped care model a set of SMART-goals was formulated; goals that are specific, measurable, attractive, realistic and timely (Table 1). These two instruments, the Stepped Care Depression Model and the set of SMART goals, provided the improvement teams with guidance for their improvement work. The teams made a selection of goals, developed additional local goals if they wished and implemented changes.
Table 1.
The SMART goals of the Depression Breakthrough Collaborative
| 1. Within 6 months of treatment, 80% of all new patients have a score of 10 or lower on the Beck Depression Inventory (BDI). Obligatory goal |
| 2. 80% of systematic follow-up visits is according to planning, meaning 1 visit every 6 weeks until the scores on the BDI is 10 or lower. Obligatory goal |
| 3. <10% of patients with non-severe symptoms receive antidepressants or psychotherapy as a first step treatment |
| 4. All patients with severe depressive symptoms start treatment within 1 month after diagnosis |
| 5. <20% of all patients with severe symptoms, treated with antidepressants, have dropped out of treatment within the first 3 months |
Breakthrough method
The Breakthrough method, developed by Berwick and colleagues at the Institute for Healthcare Improvement in Boston (http://www.ihi.org), was used as the model for change during the collaborative [44]. This method was chosen for various reasons. Firstly, Breakthrough Collaboratives are attractive projects, creating learning opportunities for professionals, offering them knowledge, a model for change and permitting them to spend time on testing changes and experimenting with new behaviour. Breakthrough Collaboratives can be especially useful for microsystem improvements, within small units of care delivery [45]. Secondly, these projects have become very popular over the last few years within the Dutch Ministry of Health, which has funded many in different health care settings. This positive reputation is only partly based on research literature. A recently published systematic review of quality improvement collaboratives showed that the underlying evidence is positive but limited, with modest effects on outcomes at best [35]. In mental healthcare, the Breakthrough method had rarely been applied and evaluated.
Breakthrough Collaboratives can be considered as a multifaceted implementation strategy. Central characteristics of all Breakthrough Collaboratives are: the use of guidelines, local multidisciplinary improvement teams consisting of professionals and a local team coordinator, a national expert team consisting of depression opinion leaders and national project coordinators, data collection and continuous feedback loops [34, 46]. In the Depression Breakthrough Collaborative a specific mix of these improvement strategies was offered to the participating teams (Table 2).
Table 2.
Improvement strategies offered during the Depression Breakthrough Collaborative
| • A network of multidisciplinary teams; |
| • An expert team, teaching the stepped care model; |
| • SMART goal setting, a set of indicators to monitor results and an Excel worksheet; |
| • A training for local team coordinators on the Breakthrough method and data collection; |
| • Four conference days for all improvement teams for exchange and learning; |
| • One conference day for local team coordinators for more intensive exchange with the expert team; |
| • Five meetings between local team coordinators, with the expert team present; |
| • Team visits of experts and national project coordinators; |
| • Telephone contact between local and national coordinators; |
| • Written feedback on improvement reports and data charts; |
| • A virtual network environment for exchange of best-practices, a Toolkit of instruments and treatment protocols, online discussions and links to relevant sites; |
| • A two-day training on problem solving treatment for professionals; |
| • A workshop workflow improvement. |
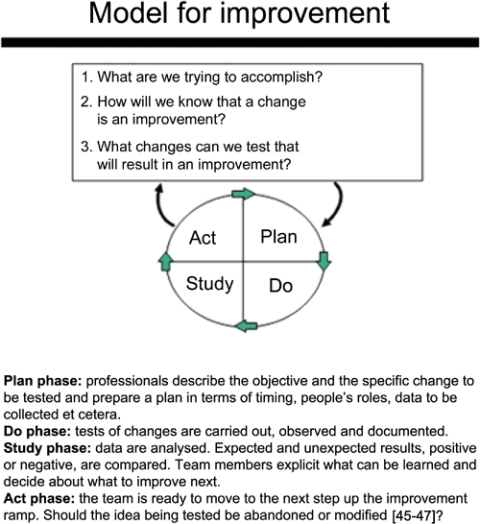
A central feature of the Breakthrough Collaboratives is continuous feedback loops according to the Nolan model (Figure 2). The model consists of two elements: three questions to focus the improvement work and a PLAN-DO-STUDY-ACT (PDSA) cycle. This model, originally developed by Langley and popularized by Nolan, provides an overarching framework for testing change ideas that are expected to make progressively more complex changes along an improvement ramp. Instead of focusing on changing the behaviour of individual providers, the focus is on gradually changing organizations into high performing (micro)systems of care [45–47].
Figure 2.
The Nolan model for improvement.
Data collection and analysis
Quantitative improvement data were collected by the professionals of the Breakthrough Collaborative’s teams. Measurements were derived from process and outcome indicators, developed by the national expert team to measure goal attainment on each of the SMART goals. Data were entered and processed in Excel by the local coordinators, who had received training to do so. Periodically, the local data were fed back to the teams for discussions and adaptation of improvement plans. Aggregation and analysis of all data was done by the expert team and data managers of the Trimbos Institute. To maintain privacy, patient data were made anonymous before being sent to be processed on a national level. In order to monitor the change over time, the team performances of process indicators were analyzed as repeated measures of three-monthly data. Teams that collected data throughout the improvement year had four terms of 3 months to demonstrate change. Other teams, starting to collect data only later, may have produced just three sets of data. In addition to the improvement data, qualitative data were collected from the local team coordinators, in the last stage of the project. For this purpose, a questionnaire was used, with items on: characteristics of the team, results according to the coordinator, strengths and weaknesses of the improvement method, influencing factors, spread and consolidation of results. Seven coordinators, reporting on 10 out of 13 teams, returned the completed questionnaire.
Results
A total of 13 teams participated in the project, consisting of 101 professionals and 15 managers or staff. The teams all had a multidisciplinary character, including at least one or more general practitioners, and a psychiatrist or a psychotherapist working in a specialized Mental Health Organization. In total, 39 general practitioners were involved, 14 primary care psychologists, 16 social workers, 11 specialised mental health nurses, 8 physiotherapists, 6 psychologists or psychotherapists and 7 psychiatrists. The smallest team consisted of 6 persons, the largest had 15 members. The teams all had a local team coordinator, responsible for supporting the professionals, managing communications within the national network, and pushing the local improvement process forward. Most of the team coordinators were staff employees in primary care support organizations called Regional Support Structures (Regionale Ondersteuning Structuur, ROS). Five hundred and forty-three adult patients were registered by the 13 teams during the improvement year. The inclusion ranged from 17 patients in the team with the lowest patient number and 93 patients in the team with the highest. All teams selected their goals for improvement (see Table 1). SMART goals 1 and 2 were obligatory for all teams, goal 3 and 4 were selected by 10 teams, goal 5 was selected by four teams.
Diagnostic skills
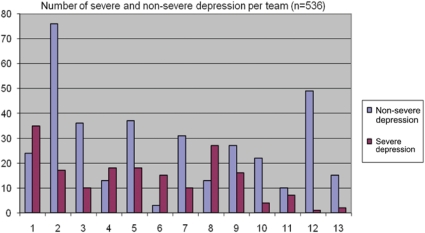
General practitioners were asked to differentiate between severely depressed and non-severely depressed patients. The label severe depression was considered appropriate if the patient previously had depressive symptoms lasting 6 months or longer, and/or showed an insufficient response to a former treatment and/or reported suicidal ideation, psychotic features or a high level of social malfunctioning. Out of the 543 patients registered during the project, 536 patients were diagnosed to have either non-severe or severe depressive symptoms (Figure 3). Overall 356 patients (66%) were considered non-severely depressed and 180 (34%) showed severe symptoms according to the general practitioners. Figure 3 also shows a large variability between the teams in the proportion of patients in each category, with the proportion of severely depressed patients ranging from 2% (team 12) to 83% (team 6). The team with the largest patient group (n=93) registered 76 non-severe depressed patients (82%) and 17 severe patients (18%).
Figure 3.
Number of severe and non-severe depression per team.
Stepped care approach
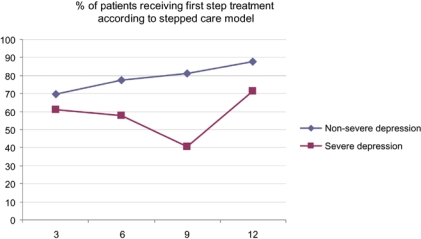
The overall goal of the improvement teams was the implementation of a stepped care model, a depression care pathway with two levels of treatment intensity: a first step treatment level for patients with non-severe depressive symptoms and a second step level for patients with severe depressive symptoms (Figure 4).
Figure 4.
Percentage of patients receiving first step treatment according to stepped care model.
The teams registered treatment data of a total of 514 patients, 346 (67%) patients with a non-severe depression and 168 (33%) patients with a severe depression. The overall mean percentage of the non-severe patient group receiving a first step treatment according to the stepped care model was 78%, ranging from 53% in the worst performing team to 100% in three best performing teams. The mean percentage of the severely depressed patient group was 57%, ranging from 25 to 100% between the teams. The patient groups were extremely small in certain teams, thus accounting for these wide ranges. Although the scores in the non-severe group did not reach the level of 90%, there was a positive trend towards this target. This is in line with the reports of the local team coordinators, indicating that general practitioners did learn to offer patients with few or mild symptoms a brief or first step intervention instead of antidepressant treatment, once these first step interventions were made available in primary care. According to the stepped care model, all patients with severe symptoms should have received psychotherapy or antidepressant treatment within 1 month, either in primary or in specialty care. Unfortunately, the improvement teams were not able to move good quality treatment for severely depressed patients close to the targeted 100%. In total 72 (43%) severely depressed patients did not receive antidepressant treatment or psychotherapy within 1 month or were offered treatment options of a too low intensity. This number includes 23 patients whom were referred to specialty care within 1 month, where they might have received proper treatment in time. The team coordinators indicated improvement in terms of a growing consciousness amongst professionals of the needs of severely depressed patients, better referral procedures and more attention to psychotherapy as an alternative for antidepressants.
Monitoring of depressive symptoms
The professionals were asked to monitor depressive symptoms with the BDI until recovery, defined as a BDI-score of 10 or lower. Table 3 shows that the teams succeeded in following around 70% of their patients during a period of 6 months. Repeated BDI monitoring by itself, was conceived to be very difficult to organize, especially since integration of the BDI measurements in existing ICT systems was lacking.
Table 3.
Depression symptoms at 6 months
| Non-severely depressed patients (n=91) | Severely depressed patients (n=50) | |
|---|---|---|
| Improved/recovered | 75 (82%)/27 (30%) | 44 (88%)/12 (24%) |
| Stable | 3 (3%) | 2 (4%) |
| Worse | 13 (17%) | 4 (8%) |
During the project, 477 patients received BDI monitoring at baseline, within 2 weeks after diagnosis. Four hundred and seventy-four patients (99%) scored more than 10, of whom 270 patients (57%) received a follow-up measurement within 3 months. Of this group 76 persons (28%) had recovered according to the BDI score of 10 or lower. Of the 194 non-recovered patients, 103 patients had another follow-up measurement at 6 months (53%). Of this group, another 28 patients (27%) had recovered, 75 patients (73%) had a BDI score higher than 10.
Overall, 91 non-severely depressed and 50 severely depressed patients had BDI monitoring at baseline and within 3 to 6 months. Eighty-two percent non-severely depressed patients improved during that period, of whom 30% recovered and 17% of the patients worsened with increased scores on the BDI. Of the severely depressed group, 88% of the patients improved, 24% recovered and 8% patients worsened.
Collaboration and integration
In addition to the data, the comments of the team coordinators on the project were asked in a questionnaire. All team coordinators indicated that the project had a positive impact on collaboration within primary care. Professionals grew to know each other during the project, and as a consequence developed a mutual language on depression care, a better understanding of the content and added value of each of the different competencies and a more reliable collaborative relationship. This was a good basis for a regionally shared approach and responsibility in depression care. Teams also reported better collaboration in daily practice. Collaboration improved in terms of easier and faster consultation of a psychiatrist or psychologist when the patient’s condition was unclear, better access to specialty care for primary care patients, and general practitioners staying better informed after referral. Improved collaboration was restricted to the professionals in the improvement teams, and did not really spread beyond this group.
Knowledge and guidelines
Another effect mentioned by the coordinators was improved knowledge of depression amongst the professionals and improved competence in terms of diagnosing and treating depressive symptoms. Some teams intensively discussed the guidelines at the start of the project, whereas other teams considered the Depression Breakthrough Collaborative as their knowledge base.
Strengths and weaknesses of the breakthrough method
The top-down goal setting appeared to be a success factor in primary care, general practitioners being in favour of practical tools, standards and clear instructions. Another successful element was the outcome monitoring using the BDI. Although hard to implement, it shifted the focus of professionals from their own clinical judgements to more objective results that could be shared with others. The Toolkit, describing the content of interventions in detail (number of sessions needed, topics to inform the patient about) served as a fidelity tool for correct development and implementation and as a basis for team discussions.
Weaknesses of the project, experienced by the team coordinators, were related to a mismatch between the project’s design and the primary care working culture. PDSA cycles were hard to apply and did not fit into the existing culture of primary care professionals, who were not used to discussing care processes and reflecting on results. Also the website, the main source of information and communication, was of no help to individual professionals, who were not used to virtual project environments. Other negative aspects of the project were the obligatory reports that needed to be sent to the national expert team and the changing planning of conference days and other happenings.
Influencing factors
Factors facilitating the project were: the presence of a strong local team coordinator, enthusiastic team members (particularly the general practitioner as the key player in the team), financial support for time spent on the project from an insurance company, and the embedment of the project within a broader quality improvement policy of the Mental Health Organization or primary care health centre. Most of the local team coordinators were employed by the so-called Regional Support Structures, rather new organizations in Dutch primary care, created by the Ministry of Health to help professionals improve the quality of care. Some of the health care insurance companies reimbursed general practitioners for the time spent on the project and paid for the team coordinator to support the team.
Factors hindering the project were: a lack of interest by the management, a lack of dedicated time for participating professionals, a lack of patients with new depressive symptoms in primary care during the project, and the short length of the project’s duration. Most teams felt the time frame of the project was too short for real change, especially in smaller teams, with only one general practitioner. Focusing on a longer change period and continuing improvement activities after the project’s formal ending, was the way most teams dealt with these frustrations.
Discussion
Thirteen multidisciplinary teams participated in the quality improvement project. In total 101 health professionals were involved, and 536 patients were diagnosed. Overall 356 patients (66%) were considered non-severely depressed and 180 (34%) patients showed severe symptoms. The mean percentage of non-severe patients treated according to the model was 78%, and 57% for the severely depressed patient group. Compared to numbers mentioned in the literature of 23% of patients with anxiety and depression receiving the right treatment in primary care, this could be considered as relatively high [1]. The proportion of non-severely depressed patients receiving the right first step treatment slightly improved during the project, but this was not the case for the severely depressed patients. The teams were able to monitor depression symptoms to a reasonable extent during a period of 6 months. Within 3 months, 28% of monitored patients had recovered, meaning a BDI score of 10 and lower, and another 27% recovered between 3 and 6 months. Collaboration between primary care and specialty care and within primary care improved but did not spread beyond the teams. The team coordinators indicated that a breakthrough, although still fragile, was being achieved in terms of professionals improving their knowledge of depression and depression guidelines, learning to use new and less intensive treatments in mild cases instead of antidepressant treatment and improving collaboration within and between the settings, so that access to specialty care for severely primary care improved.
In total, 39 practitioners identified 536 new cases, a mean of 14 patients per general practitioner. This is lower than expected, considering the national incidence rate of 24 patients in a general practice of 2300 subscribed patients, suggesting that the general practitioners did not identify all patients with depressive symptoms or did not include all patients who were identified [24]. The diagnostic performances suggest that the project served as a platform for general practitioners to change their behaviour and start to differentiate between severe and non-severe depressive symptoms. Whether this was done in a reliable way, reflecting the true proportions, is not clear. The large variability between the general practitioners suggests that, apart from epidemiological differences, several professional related factors could have influenced the diagnosis. For instance, the sensitivity of some of the general practitioners to picking up on mild or early depressive symptoms, and their ability to discuss their findings with the patient, could have been more or less developed. Also, a doctor feeling uncomfortable with a particular label and the corresponding treatment level could have adapted the treatment criteria to his own perception.
The monitoring indicators showed that the teams were able to monitor depression symptoms to a reasonable extent during the first 6 months of the treatment. This can be considered as a rather big improvement, considering the lack of routine, infrastructure and ICT support. When patients had stopped visiting the practices, possibly because of diminishing symptoms, continuous monitoring proved to be problematic.
The data suggest an improvement ramp pushing the quality of care for patients with non-severe depression forward. This in line with data from a previous Depression Breakthrough Collaborative that served as a pilot project. In that project, data of precollaborative treatment were compared to the improvement data, showing a very sudden drop in unnecessary antidepressant prescriptions for non-severely depressed patients from 61 to 11%, during the very first weeks of the collaborative [48]. In the current project, no pre-post trend can be shown, so nothing can be said about the actual change introduced during the collaborative.
The recovery rates are in line with the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, a naturalistic study showing that only one-third of patients achieves remission with initial treatment and that remission rates decline with successive treatment failures [49]. The results of our project, although not based on research data, confirm the suggestion derived from the scientific research into collaboratives, showing modest effects on outcomes at best [35].
Our project also builds on reports of other improvement work in depression care that show a positive impact on the quality of care and on patient outcomes. In the American version of the Depression Breakthrough Collaborative, the chronic care model was implemented, also based on the assumption that depression care is fragmented and that there is a gap between guideline recommended and actual care [50]. The change concepts considered to be essential in the American project turned out to be establishing and maintaining a patient register, care coordination, diagnostic assessment and pro-active follow-up. Factors facilitating that project were: the support of organizational leadership showing the essential role of the top management, and a small practice size [50, 51]. Some of the essential change concepts show overlap with the positive experiences in our project concerning diagnostic assessment and pro-active follow-up. Still, the stepped care approach, introducing different patient categories and corresponding treatment levels, with much attention to other than pharmacological approaches, can be considered distinctive and of relevance to international readership.
There are several limitations to this project. Firstly, registration of improvement indicators was hampered in various ways and the quality of data gathering during the project varied. Although some teams managed to collect most data for their patients, the overall database showed many missing values. A second limitation was the poor insight in the actual implementation of the interventions. The data are based on reports of the professionals; it is unclear whether patients actually received care according to the protocol or guidelines. Thirdly, the twelve months duration of the project; this may have been too short to measure any impact on the care processes.
It is clear that the information derived from these data does not pretend to serve as new, generalizable knowledge on causal mechanisms in healthcare, but as a mirror for reflection and discussion on processes of change in depression care. Quality improvement is a growing topic of interest to many managers and professionals in this sector, also stimulated by policy makers and insurance companies. Although changing depression care is on the agenda of many, the question of how to go about it is still unanswered. The data presented here may help to find some of the answers. Parallel to these quality improvement data, a quasi-experimental trial was conducted, comprising rigorous quantitative and qualitative process and outcome data-gathering on the patient, the professional and the team level, and a comparison between the collaborative study population and a care as usual group. The results of that study will be published from 2010 onwards.
Conclusions
A stepped care approach seems acceptable and feasible in primary care, introducing different levels of care for different patient groups. Although the Depression Breakthrough Collaborative introduced new treatment concepts in primary and specialty care, the change capacity of the method remains unclear. Thorough data gathering is needed to judge the real value of this intensive improvement project.
Acknowledgments
This project is part of the depression initiative of the Trimbos Institute that is supported by a grant from the Innovation Fund of Healthcare Insurers (Innovatiefonds Zorgverzekeraars). We wish to thank the professionals and project leaders in the Depression Breakthrough Collaborative teams for sharing their improvement experiences.
Contributor Information
Gerdien Franx, Trimbos Institute, Netherlands Institute of Mental Health and Addiction, P.O. Box 725, 3500 AS Utrecht, The Netherlands.
Jolanda A.C Meeuwissen, Trimbos Institute, Netherlands Institute of Mental Health and Addiction, P.O. Box 725, 3500 AS Utrecht, The Netherlands.
Henny Sinnema, Trimbos Institute, Netherlands Institute of Mental Health and Addiction, P.O. Box 725, 3500 AS Utrecht, The Netherlands.
Jan Spijker, Trimbos Institute, Netherlands Institute of Mental Health and Addiction, P.O. Box 725, 3500 AS Utrecht, The Netherlands.
Jochanan Huyser, Jellinek Mentrum, Amsterdam, The Netherlands.
Michel Wensing, IQ Healthcare, Radboud University Nijmegen Medical Centre, P.O. Box 9101, 114, 6500 HB Nijmegen, The Netherlands.
Jacomine de Lange, Trimbos Institute, Netherlands Institute of Mental Health and Addiction, P.O. Box 725, 3500 AS Utrecht, The Netherlands.
Reviewers
Christopher Dowrick, BA, MSc, MD, CQSW, FRCGP, FFPHM, Professor of Primary Medical Care, University of Liverpool, Liverpool, UK
David Perkins, Associate Professor, Director Centre For Remote Health Research, Broken Hill Department of Rural Health, University of Sydney, Broken Hill NSW, Australia
M.A. Prins, Drs, Junior-investigator, NIVEL the Netherlands Institute for Health Services Research, Utrecht, The Netherlands
References
- 1.Fernandez A, Haro JM, Martinez-Alonso M, Demyttenaere K, Brugha TS, Autonell J, et al. Treatment adequacy for anxiety and depressive disorders in six European countries. British Journal of Psychiatry. 2007 Feb;190:172–3. doi: 10.1192/bjp.bp.106.023507. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 3.Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Archives of General Psychiatry. 1993 Feb;50(2):85–94. doi: 10.1001/archpsyc.1993.01820140007001. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994 Jan;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 5.Offord DR, Boyle MH, Campbell D, Goering P, Lin E, Wong M, et al. One-year prevalence of psychiatric disorder in Ontarians 15 to 64 years of age. Canadian Journal of Psychiatry. 1996 Nov;41(9):559–63. doi: 10.1177/070674379604100904. [DOI] [PubMed] [Google Scholar]
- 6.Bijl RV, Ravelli A, van Zessen G. Prevalence of psychiatric disorder in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Social Psychiatry and Psychiatric Epidemiology. 1998 Dec;33(12):587–95. doi: 10.1007/s001270050098. [DOI] [PubMed] [Google Scholar]
- 7.Bijl RV, van Zessen G, Ravelli A, de Rijk C, Langendoen Y. The Netherlands Mental Health Survey and Incidence Study (NEMESIS): objectives and design. Social Psychiatry and Psychiatric Epidemiology. 1998 Dec;33(12):581–6. doi: 10.1007/s001270050097. [DOI] [PubMed] [Google Scholar]
- 8.Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) British Journal of Psychiatry. 2002 Sep;181:208–13. doi: 10.1192/bjp.181.3.208. [DOI] [PubMed] [Google Scholar]
- 9.Van’ t Land H, Grolleman J, Mutsaers K, Smits C. Trendrapportage GGZ 2008: deel 2: toegang en zorggebruik: basisanalyse. [Trendreport Mental Health Care 2008: part 2: admission and care utilization]. Utrecht: Trimbos-instituut; 2008. [in Dutch] [Google Scholar]
- 10.Van Balkom AJLM. Imago [Image] Tijdschrift voor Psychiatrie. 2005;47(12):825–6. [in Dutch] [Google Scholar]
- 11.Landelijke Stuurgroep Multidisciplinaire Richtlijnontwikkeling in de GGZ. Multidisciplinaire richtlijn depressie: richtlijn voor de diagnostiek en behandeling van volwassen cliënten met een depressie 2005. [Multidisciplinary guideline depression: guideline for diagnostics and treatment of adult clients with a major depressive disorder 2005]. Utrecht: Trimbos-instituut; 2005. [in Dutch] [Google Scholar]
- 12.Van Marwijk HWJ, Grundmeijer HGLM, Bijl D, Van Gelderen MG, De Haan M, Van Weel-Baumgarten EM, et al. NHG-Standaard Depressieve stoornis: eerste herziening. [NHG Standard Major Depressive Disorder: first revision]. Huisarts en Wetenschap. 2003;46(11):614–23. [in Dutch] [Google Scholar]
- 13.Andrews G, Issakidis C, Sanderson K, Corry J, Lapsley H. Utilising survey data to inform public policy: comparison of the cost-effectiveness of treatment of ten mental disorders. British Journal of Psychiatry. 2004 Jun;184:526–33. doi: 10.1192/bjp.184.6.526. [DOI] [PubMed] [Google Scholar]
- 14.Chisholm D, Sanderson K, Ayuso-Mateos JL, Saxena S. Reducing the global burden of depression: population-level analysis of intervention cost-effectiveness in 14 world regions. British Journal of Psychiatry. 2004 May;184:393–403. doi: 10.1192/bjp.184.5.393. [DOI] [PubMed] [Google Scholar]
- 15.Adli M, Bauer M, Rush AJ. Algorithms and collaborative-care systems for depression: are they effective and why? A systematic review. Biological Psychiatry. 2006 Jun;59(11):1029–38. doi: 10.1016/j.biopsych.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Bauer MS. A review of quantitative studies of adherence to mental health clinical practice guidelines. Harvard Review of Psychiatry. 2002 May-Jun;10(3):138–53. doi: 10.1080/10673220216217. [DOI] [PubMed] [Google Scholar]
- 17.Hodiamont PPG. Passende psychiatrische zorg: de waarde van richtlijnen bij depressie. [Appropriate psychiatric care: the value of guidelines for depression]. Tijdschrift voor Psychiatrie. 2001;42(3):173–83. [Google Scholar]
- 18.Grol R, Wensing M, Eccles M. Improving patient care: the implementation of change in clinical practice. Amsterdam: Elsevier; 2005. [Google Scholar]
- 19.Ormel J, Bartel M, Nolen WA. Onderbehandeling bij depressie; oorzaken en aanbevelingen. [Undertreatment of depression: causes and recommendations]. Nederlands Tijdschrift voor Geneeskunde. 2003;147(21):1005–9. [in Dutch] [PubMed] [Google Scholar]
- 20.Tiemeier H, de Vries WJ, van het LM, Kahan JP, Klazinga N, Grol R, et al. Guideline adherence rates and interprofessional variation in a vignette study of depression. Quality and Safety in Health Care. 2002 Sep;11(3):214–8. doi: 10.1136/qhc.11.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spies T, Mokkink HGA, De Vries Robbé P, Grol RPTM. Huisarts kiest vaak voor antidepressiva onafhankelijk van de ernst van de depressie. [GP often chooses antidepressants, irrespective of depression severity]. Huisarts en Wetenschap. 2004;47:364–7. [in Dutch] [Google Scholar]
- 22.Cepoiu M, McCusker J, Cole MG, Sewitch M, Belzile E, Ciampi A. Recognition of depression by non-psychiatric physicians—a systematic literature review and meta-analysis. Journal of General Internal Medicine. 2008 Jan;23(1):25–36. doi: 10.1007/s11606-007-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Medical Informatics and Decision Making. 2008 Sep 12;8:38. doi: 10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braspenning JCC, Schellevis FG, Grol RPTM. Tweede nationale studie naar ziekten en verrichtingen in de huisartspraktijk 4: kwaliteit huisartsenzorg belicht. [Second national study into diseases and performances in general practice 4: quality of GP care illustrated]. Utrecht/Nijmegen: NIVEL/WOK; 2004. [in Dutch] [Google Scholar]
- 25.DGV. Benchmark voorschrijven 2008. [Benchmark prescriptions 2008]. Utrecht: DGV; 2008. [in Dutch] [Google Scholar]
- 26.Spijker J, Bijl RV, de Graaf R, Nolen WA. Care utilization and outcome of DSM-III-R major depression in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatrica Scandinavica. 2001 Jul;104(1):19–24. doi: 10.1034/j.1600-0447.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Rijswijk E. Mental health problems in general practice: an explorative study on diagnosis and treatment. Nijmegen: Radboud Universiteit Nijmegen; 2005. [Google Scholar]
- 28.Hermens MLM. Antidepressant treatment for minor and mild-major depressive disorders in primary care. Amsterdam: Vrije Universiteit; 2005. [Google Scholar]
- 29.Smit F. Prevention of Depression. Amsterdam: Vrije Universiteit; 2007. [Google Scholar]
- 30.Volkers A, De Jong A, De Bakker D, Van Dijk L. Doelmatig voorschrijven van antidepressiva in de huisartspraktijk. [Efficient prescription of antidepressants in general practice]. Utrecht: NIVEL; 2005. [in Dutch] [Google Scholar]
- 31.Kilo CM. Improving care through collaboration. Pediatrics. 1999 Jan;103(1 Suppl E):384–93. [PubMed] [Google Scholar]
- 32.Pearson ML, Wu S, Schaefer J, Bonomi AE, Shortell SM, Mendel PJ, et al. Assessing the implementation of the chronic care model in quality improvement collaboratives. Health Services Research. 2005 Aug;40(4):978–96. doi: 10.1111/j.1475-6773.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Quarterly. 2004;82(4):581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ØVretveit J, Bate P, Cleary P, Cretin S, Gustafson D, McInnes K, et al. Quality collaboratives: lessons from research. Qualily and Safety in Health Care. 2002 Dec;11(4):345–51. doi: 10.1136/qhc.11.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schouten LM, Hulscher ME, van Everdingen JJ, Huijsman R, Grol RP. Evidence for the impact of quality improvement collaboratives: systematic review. British Medical Journal. 2008 Jun 28;336(7659):1491–4. doi: 10.1136/bmj.39570.749884.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Feltz-Cornelis CM, Henkelman ALCM, Walburg JA. Het Depressie Initiatief: depressie management in Nederland. [The Depression Initiative: depression management in the Netherlands]. Utrecht: Trimbos-instituut; 2006. [in Dutch] [Google Scholar]
- 37.Meeuwissen J, Van Weeghel J. Modulenboek bij het stepped care-programma voor depressie. [Modules for the stepped-care programme for depression]. Utrecht: Trimbos-instituut; 2003. [in Dutch] [Google Scholar]
- 38.Meeuwissen JAC, Van der Feltz-Cornelis CM, van Marwijk HW, Rijnders PB, Donker MC. A stepped care programme for depression management: an uncontrolled pre-post study in primary and secondary care in The Netherlands. International Journal of Integrated Care [serial online] 2008 Feb 21;8 doi: 10.5334/ijic.228. Available from: http://www.ijic.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meeuwissen JAC, Donker MCH. Minder is meer: stepped care in de geestelijke gezondheidszorg. [Less is more: stepped care in mental healthcare]. MGv: Maandblad Geestelijke volksgezondheid. 2004;59(11):904–15. [in Dutch] [Google Scholar]
- 40.Katon W, Von Korff M, Lin E, Simon G, Walker E, Unutzer J, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Archives of General Psychiatry. 1999 Dec;56(12):1109–15. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 41.Haaga DA. Introduction to the special section on stepped care models in psychotherapy. Journal of Consulting and Clinical Psychology. 2000 Aug;68(4):547–8. [PubMed] [Google Scholar]
- 42.Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Narrative literature review. British Journal of Psychiatry. 2005 Jan;186:11–7. doi: 10.1192/bjp.186.1.11. [DOI] [PubMed] [Google Scholar]
- 43.Von Korff M, Tiemens B. Individualized stepped care of chronic illness. Western Journal of Medicine. 2000 Feb;172(2):133–7. doi: 10.1136/ewjm.172.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berwick DM. Developing and testing changes in delivery of care. Annals of Internal Medicine. 1998 Apr 15;128(8):651–6. doi: 10.7326/0003-4819-128-8-199804150-00009. [DOI] [PubMed] [Google Scholar]
- 45.Nelson EC, Batalden PB, Godfrey MM. Quality by design: a clinical microsystems approach. San Francisco: Jossey-Bass; 2007. [Google Scholar]
- 46.Van Splunteren P, Van Everdingen J, Janssen S, Minkman M, Rouppe van de Voort M, Schouten L, et al. Doorbreken met resultaten: verbetering van de patiëntenzorg met de doorbraakmethode. [Breaking through with results: improvement of patient care using the Breakthrough method]. Assen: Koninklijke Van Gorcum; 2003. [in Dutch] [Google Scholar]
- 47.Langley GJ, Nolan KM, Norman CL, Provost LP, Nolan TW. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass Publishers; 1996. [Google Scholar]
- 48.Franx G, Spijker J, Huyser J, De Doelder P. Daling in depressie: doorbraakmethode brengt afname in overbehandeling teweeg. [Decrease in depression: breakthrough method leads to a decrease of over treatment]. Medisch contact. 2006;61(40):1592–5. [in Dutch] [Google Scholar]
- 49.Nelson JC. The STAR*D study: a four-course meal that leaves us wanting more. American Journal of Psychiatry. 2006 Nov;163(11):1864–6. doi: 10.1176/ajp.2006.163.11.1864. [DOI] [PubMed] [Google Scholar]
- 50.Katzelnick DJ, Von Korff M, Chung H, Provost LP, Wagner EH. Applying depression-specific change concepts in a collaborative breakthrough series. Joint Commission Journal of Quality and Patient Safety. 2005 Jul;31(7):386–97. doi: 10.1016/s1553-7250(05)31052-x. [DOI] [PubMed] [Google Scholar]
- 51.Meredith LS, Mendel P, Pearson M, Wu SY, Joyce G, Straus JB, et al. Implementation and maintenance of quality improvement for treating depression in primary care. Psychiatric Services. 2006 Jan;57(1):48–55. doi: 10.1176/appi.ps.57.1.48. [DOI] [PubMed] [Google Scholar]